Abstract
This study examines reported prevalence and trends in use of melatonin supplements among US adults from 1999 through 2018.
Exogenous supplementation of melatonin, one of the key hormones governing circadian rhythm, is indicated for treatment of circadian rhythm sleep disorders1 and, despite insufficient evidence,2 is widely used as an over-the-counter sleep aid. Evidence of antioxidant and anti-inflammatory properties of melatonin3 has prompted investigation of its therapeutic value in multiple diseases.4 Although melatonin is generally regarded as safe, adverse effects have been reported, and data on long-term use and high-dose use are scarce.5 The heterogeneity in over-the-counter formulations6 further supports the need for a broader understanding of consumption of exogenous melatonin in the population.
We examined reported prevalence and trends in use of melatonin supplements among US adults from 1999 through 2018. Because the recommended dosage of melatonin typically does not exceed 5 mg/d, we additionally evaluated prevalence and trends in use of greater than 5 mg/d of melatonin.
Methods
We used data from the 1999-2000 through 2017-2018 cycles of the National Health and Nutrition Examination Survey (NHANES), a series of cross-sectional, stratified, multistage probability sample surveys of the noninstitutionalized US population, with response rates ranging from 51.9% (2017-2018) to 84.0% (2001-2002). Participants younger than 20 years (n = 46 235) or without available dietary supplement data (n = 58) were excluded. For the analysis on greater than 5 mg/d of melatonin use, we additionally excluded those reporting melatonin use but without daily dose information (n = 2). The National Center for Health Statistics Institutional Review Board approved the NHANES protocol and all participants provided written informed consent. Data on dietary supplement use during the past 30 days and daily dose used when taking the supplements were collected through in-home interview, and to aid reporting, participants were required to show supplement containers.
For each survey cycle, we computed weighted prevalences and 95% CIs of melatonin use in the overall sample and across sex and age categories. Prevalences and 95% CIs of greater than 5 mg/d of melatonin use was also estimated. P values for linear and quadratic trends were calculated using linear regression modeling with survey cycle as a continuous variable. To determine if trends varied by demographic characteristics, interaction effects were assessed using weighted logistic regression. R version 4.0.1 (R Foundation) and SPSS version 20.0 (IBM Corp) were used for analysis, and a 2-sided P < .05 was considered statistically significant.
Results
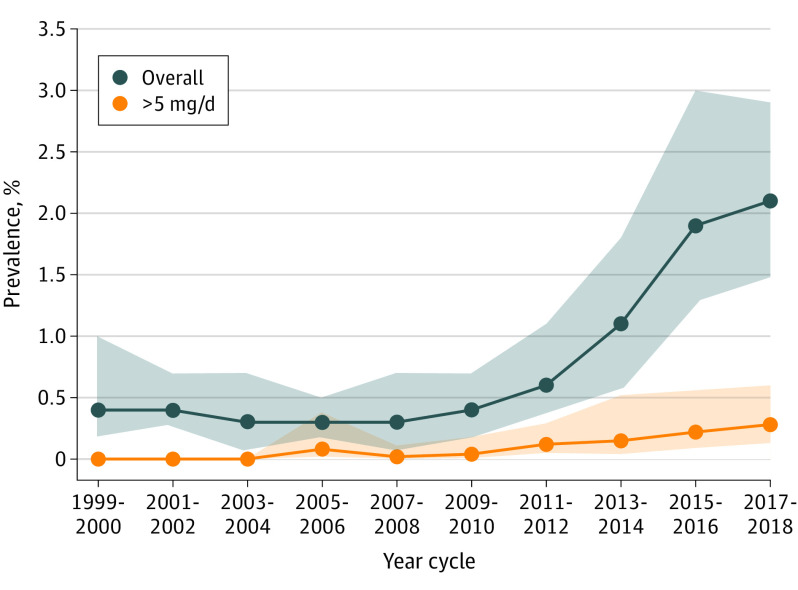
Data from 55 021 adults (mean age, 47.5 [SD, 17.1] years; 52.0% women) from 10 NHANES cycles were included. The number of participants included in each cycle ranged from 4865 to 6214 (Table), and supplement containers were verified by interviewers for 93.9% of self-reported melatonin users. The overall reported weighted prevalence of melatonin use increased from 0.4% (95% CI, 0.2%-1.0%) in 1999-2000 to 2.1% (95% CI, 1.5%-2.9%) in 2017-2018 (linear P = .004) (Figure), with an increase beginning in 2009-2010 (quadratic P < .001). Trends were similar across sex and age groups (interaction P > .05 for all) (Table).
Table. Reported Weighted Prevalence of Melatonin Use by Sex and Age Among US Adults, 1999-2018.
| No.a | Prevalence of melatonin use, weighted % (95% CI) | P valueb | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1999-2000 (n = 4865) | 2001-2002 (n = 5401) | 2003-2004 (n = 5035) | 2005-2006 (n = 4973) | 2007-2008 (n = 5930) | 2009-2010 (n = 6214) | 2011-2012 (n = 5556) | 2013-2014 (n = 5767) | 2015-2016 (n = 5717) | 2017-2018 (n = 5563) | ||||
| Linear | Quadratic | ||||||||||||
| Sex | |||||||||||||
| Women | 175 | 0.5 (0.3-1.1) | 0.5 (0.3-0.9) | 0.3 (0.1-0.8) | 0.2 (0.1-0.4) | 0.5 (0.2-1.1) | 0.4 (0.1-0.9) | 0.6 (0.3-1.2) | 1.3 (0.8-2.0) | 2.0 (1.1-3.9) | 2.1 (1.4-3.3) | .005 | <.001 |
| Men | 121 | 0.4 (0.1-1.5) | 0.4 (0.1-1.1) | 0.2 (0.1-0.8) | 0.4 (0.2-0.8) | 0.2 (0.1-0.4) | 0.3 (0.1-0.8) | 0.6 (0.3-1.2) | 0.9 (0.3-2.5) | 1.8 (1.2-2.8) | 2.1 (1.1-3.7) | .006 | <.001 |
| Age group, y | |||||||||||||
| 20-44 | 87 | 0.3 (0.1-1.1) | 0.4 (0.2-0.7) | 0.3 (0.1-1.2) | 0.1 (0.01-0.7) | 0 | 0.4 (0.1-0.9) | 0.6 (0.3-1.2) | 0.8 (0.3-2.2) | 1.4 (0.8-2.4) | 1.4 (0.7-2.9) | NA | NA |
| 45-64 | 121 | 0.6 (0.2-1.7) | 0.6 (0.3-1.1) | 0.6 (0.3-1.1) | 0.6 (0.3-1.1) | 0.7 (0.3-1.8) | 0.2 (0.1-0.7) | 0.7 (0.3-1.5) | 1.1 (0.5-2.4) | 3.0 (1.5-5.9) | 2.9 (1.8-4.7) | .01 | .002 |
| ≥65 | 88 | 0.6 (0.2-1.8) | 0.4 (0.1-1.1) | 0.1 (0.01-1.2) | 0.3 (0.1-1.1) | 0.3 (0.1-1.3) | 0.6 (0.3-1.2) | 0.7 (0.2-2.2) | 1.6 (0.9-2.6) | 1.2 (0.5-2.7) | 2.1 (1.4-3.1) | .005 | <.001 |
Unweighted number of participants reporting melatonin use.
P values for linear and quadratic trends were calculated using linear regression modeling survey cycle as a continuous variable. P values for trend tests are listed as NA (not analyzable) when 1 or more survey cycle had no observed outcome.
Figure. Trends in Reported Weighted Prevalence of Overall Melatonin Use and Melatonin Use Greater Than 5 mg/d Among US Adults.
The unweighted numbers of participants using melatonin overall and using >5 mg/d of melatonin were 296 and 39, respectively. Shaded areas indicate 95% confidence intervals. P values for linear and quadratic trends were calculated using linear regression modeling survey cycle as a continuous variable. For overall use, linear P = .004 and quadratic P < .001; for use greater than 5 mg/d, linear P = .005 and quadratic P = .04. Prevalence trends for melatonin use greater than 5 mg/d were estimated from the 2005-2006 to 2017-2018 cycles.
Melatonin use of greater than 5 mg/d was not reported before 2005-2006. The reported prevalence of melatonin use greater than 5 mg/d increased from 0.08% (95% CI, 0.02%-0.38%) in 2005-2006 to 0.28% (0.13%-0.60%) in 2017-2018 (linear P = .005) (Figure).
Discussion
Among US adults, reported prevalence of melatonin supplement consumption significantly increased from 1999-2000 to 2017-2018 across all demographic groups. Although it remained very low, prevalence of self-reported use of greater than 5 mg/d of melatonin also increased over time. These estimates may raise safety concerns, especially given that the actual content of melatonin in marketed supplements may be up to 478% higher than the labeled content6 and that evidence supporting melatonin use for sleep disturbances is weak.2 The growing use of exogenous melatonin in the general population and its expanding therapeutic potential4 provide impetus for the acquisition of robust evidence of long-term safety of melatonin supplementation. Limitations include self-reported use of melatonin, although supplement containers were verified in nearly all participants. Because of the small number of melatonin users in some subgroups, results of stratified analyses should be interpreted with caution. Reliable estimates of trends in melatonin use across racial/ethnic groups cannot be provided. Also, reasons for use were not available in all cycles.
Section Editors: Jody W. Zylke, MD, Deputy Editor; Kristin Walter, MD, Associate Editor.
References
- 1.Auger RR, Burgess HJ, Emens JS, Deriy LV, Thomas SM, Sharkey KM. Clinical practice guideline for the treatment of intrinsic circadian rhythm sleep-wake disorders: advanced sleep-wake phase disorder (ASWPD), delayed sleep-wake phase disorder (DSWPD), non-24-hour sleep-wake rhythm disorder (N24SWD), and irregular sleep-wake rhythm disorder (ISWRD): an update for 2015: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2015;11(10):1199-1236. doi: 10.5664/jcsm.5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349. doi: 10.5664/jcsm.6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morvaridzadeh M, Sadeghi E, Agah S, et al. Effect of melatonin supplementation on oxidative stress parameters: a systematic review and meta-analysis. Pharmacol Res. 2020;161:105210. doi: 10.1016/j.phrs.2020.105210 [DOI] [PubMed] [Google Scholar]
- 4.Tordjman S, Chokron S, Delorme R, et al. Melatonin: pharmacology, functions and therapeutic benefits. Curr Neuropharmacol. 2017;15(3):434-443. doi: 10.2174/1570159X14666161228122115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besag FMC, Vasey MJ, Lao KSJ, Wong ICK. Adverse events associated with melatonin for the treatment of primary or secondary sleep disorders: a systematic review. CNS Drugs. 2019;33(12):1167-1186. doi: 10.1007/s40263-019-00680-w [DOI] [PubMed] [Google Scholar]
- 6.Erland LAE, Saxena PK. Melatonin natural health products and supplements: presence of serotonin and significant variability of melatonin content. J Clin Sleep Med. 2017;13(2):275-281. doi: 10.5664/jcsm.6462 [DOI] [PMC free article] [PubMed] [Google Scholar]