Abstract
Introduction:
Venous leg ulcers (VLU) embody the most severe stage of the broad spectrum of chronic venous disease. Approximately 40% of patients with VLU present with the underlying deep venous disease (DVD). Although the data are scarce, these deep venous disease-related VLU (DRV) are thought to have higher recurrence rates and a substantial economic burden. The objective of this study was to assess the economic burden of DRV across Australia, France, Germany, Italy, Spain, the UK, and the USA.
Methods:
A comprehensive literature review was undertaken to identify publications documenting the incidence and prevalence of VLU and DRV, medical resource utilization, and associated costs of DRV. Findings from this literature review were used to estimate the economic burden of illness, including direct medical costs over a 12-month interval following initial presentation of a newly formed DRV.
Results:
Total annual incidence of new or recurrent DRV in Australia, France, Germany, Italy, Spain, UK, and the US are estimated at 122,000, 263,000, 345,000, 253,000, 85,000, 230,000, and 643,000 events, respectively, in 2019. Incidence ranges from 0.73 to 3.12 per 1000 persons per year. The estimated annual direct medical costs for patients managed conservatively in these geographies total ~ $10.73 billion (USD) or $5527 per person per year.
Conclusion:
The availability of published data on the costs of VLU care varies widely across countries considered in this analysis. Although country-specific VLU practice patterns vary, there is a uniform pattern of high-cost care.
Keywords: burden of illness, healthcare costs, venous insufficiency, venous leg ulcers (VLU), wound care
Introduction
Chronic limb wounds reduce patients’ quality of life and pose a substantial economic and clinical burden to healthcare systems globally. 1 The costs of chronic wounds are estimated to represent 1–4% of total healthcare spending in developed countries.2,3 Venous leg ulcers (VLU) result in significant morbidity, prolonged disability, and substantial socioeconomic burden. 4 VLU account for 70% of all chronic leg ulcers and are associated with a recurrence rate of 60–70% at 10 years. 5 The deep venous system is responsible for 90% of the venous return to the right atrium from the lower extremities. 6 Venous abnormalities including obstruction or reflux can result in chronic venous disease and VLU.7–9 Concomitant factors such as obesity, elevated central venous pressures, 10 immobility, and reduced calf muscle pump function may exacerbate impaired venous return.
Recent clinical data and associated national guidelines suggest that early intervention for ulcers caused by superficial venous disease is cost-effective. 11 In contrast, insufficient data preclude similar recommendations for deep venous disease (DVD). The Early Venous Reflux Ablation (EVRA) trial evaluated 450 VLU patients with superficial venous disease. The study demonstrated that time to ulcer healing was shorter in the early-intervention group (compression therapy and early endovenous ablation) compared to the deferred intervention group (compression therapy alone with consideration for deferred ablation). Questions regarding differences in ulcer healing and recurrence rates due to deep obstruction remain, although a recent paper reports similar evidence regarding stenting in the deep veins. 12 As a result, data clarifying the costs of conservative management of VLU due to DVD are limited.
The lack of information on deep venous disease-related VLU (DRV) may underlie the inattention towards this morbid health condition. A 2018 study compared real-world wound healing rates from the US Wound Registry (USWR) with publicly reported rates across the US. 13 Although centers reported healing rates ranging from 80% to 90% (mean: 85%) within 2.7–16 weeks (mean: 4.3 weeks), USWR data showed that 44.1% of VLU were healed at 12 weeks. The disassociation between publicly reported wound healing rates and the real-world evidence creates confusion and exacerbates the underestimation of the overall disease burden.
The objective of this study is to identify and summarize the epidemiology and the current cost burden associated with the care for DRV, which includes reflux and obstruction from a payer perspective by country. Five European countries (UK, Germany, France, Italy, Spain), along with the US, building on Rice et al., 14 and Australia were selected for this analysis. These countries were selected because their overall health expenditure is high, and their resource utilization estimates are often available to see if the pattern of care is similar or varies across a variety of health systems.
Methods
Data sources and search strategy
Systematic literature searches were conducted using PubMed as shown in online Supplementary Table 1. Original research studies with cost, utilization, and burden-related findings pertaining specifically to VLU were included in this literature review.
Studies that did not provide data specific to the C6 population (with active VLU) based on the Clinical Etiologic Anatomic and Pathophysiologic (CEAP) classification 15 were excluded. Case reports, review articles, and editorials describing biochemical mechanisms and randomized controlled trials on non-standard-of-care interventions also were excluded from the initial abstract review.
Search results
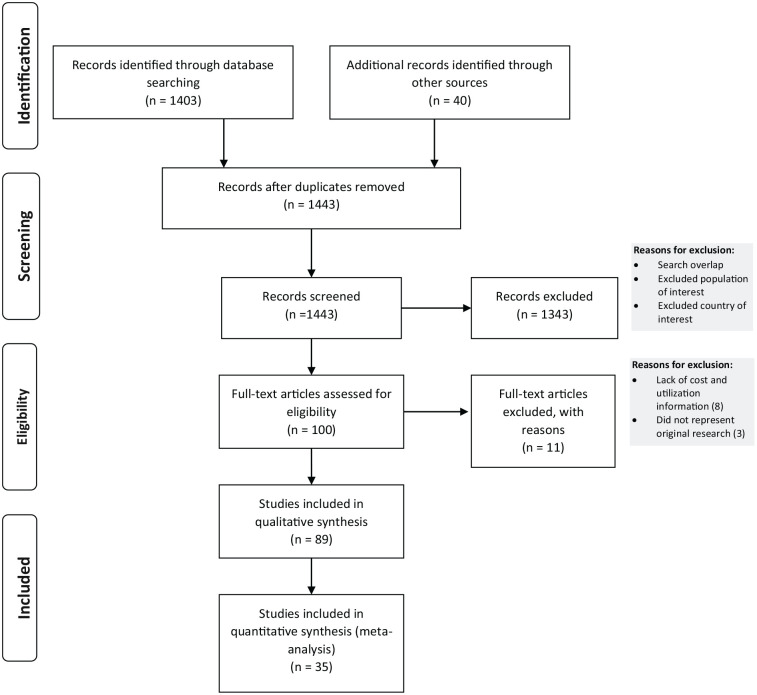
The flow diagram for identifying studies for detailed review is depicted in the PRISMA diagram 16 in Figure 1; further details are available in the online supplementary content.
Figure 1.
Flow diagram of the study selection.
A total of 1403 citations were initially retrieved from search strategies 1 and 2. The literature search was supplemented with a manual search of 40 specialty society guidelines and health technology assessment reports related to the care of venous ulcer disease. We excluded 1343 studies after screening the titles and abstracts due to search overlap, the population of interest not being reported, countries beyond the scope of study, or other reasons as described above. Eight studies were excluded as they did not include detailed information on unit costs or utilization, and three that did not represent original research were also excluded. Of the 89 remaining articles, 18 were guidelines, which were used to assess treatment patterns, 36 contained relevant clinical endpoints that guided model structure, and 35 were then used to retrieve cost and utilization metrics to assess the burden of illness.
Rates of utilization of standard-of-care treatments for VLU were based on a review of 18 guidelines and available literature, as shown in online Supplementary Table 2. Study authors, who practice in each country included in the analysis, were consulted to assess the level of concordance between guideline recommendations and what is considered routine clinical practice in their respective countries. Specific consideration was made for country-level differences in practice based on guidelines; the 2014 guidelines on the ‘Management of Venous Leg Ulcers’ by the Society for Vascular Surgery and the American Venous Forum 4 were used to guide understanding of the overall approaches to management, whereas the ‘Wound Healing Society 2015 Update on Guidelines for Venous Ulcers’ 17 and the ‘S3-Guideline on Venous Leg Ulcer’ developed by the European Dermatology Forum guideline subcommittee and entitled ‘Diagnostics and Treatment of Venous Leg Ulcers’ served as key sources for detailed information on the conservative and topical management of VLU. 18
The five major cost categories assessed for conservative treatment of DRV included:
Practitioner costs: clinic visits, medical practitioner and nursing staff time, fees, and wages;
Inpatient hospitalization costs;
Compression therapy: the cost of compression systems;
Wound bed preparation, pain management products, and skin substitutes in the US;
Drug/medication costs: antibiotics, ointments, lotions, topical creams.
General agreement was noted across the various guidelines regarding the use of compression therapy in all countries examined. However, the type and level of compression varied by patient dexterity, mobility, preference, compliance, pain/comfort, cost, caregiver resources, and the size and shape of the leg. We were unable to find agreement in the use of dressings, bandages, debridement, topical cream, lotions, ointments, and medications for pain management. The US is the only market with a significant reported use of skin substitutes.
The results of the literature review were compiled to support the development of a computational model in Excel (Microsoft, Redmond, WA, USA). The model captured the direct medical burden for each cost category by multiplying the total number of patients receiving a particular treatment, the frequency of utilization, and the unit cost per resource for each component, which were then summed within a cost category to develop an average cost per patient for each category of healthcare resource.
Model inputs
Key parameters and general assumptions
Since the data on DRV epidemiology are limited, inputs for the analysis were derived from published literature, health technology assessments, government database documents, and expert opinion. Where country-specific data were not obtainable, available data from comparable countries in the analysis for resource utilization were used. For instance, reliable incidence estimates for VLU are available for Spain and the UK, but not Australia, France, Germany, Italy, and the US.19–22 The UK estimate of 7.8 per 1000 person-years in the general population was used for all European countries except for Spain.19,21 For the US, we derived an incidence value based on reliable prevalence data, rate of healing, and recurrence rates. 22 Prevalence estimates for VLU are widely reported across all countries included in this analysis, and range from 1.5% to 3% in the general population; prevalence in the US is the highest of all countries included, consistent with the higher prevalence of risk factors such as obesity associated with VLU in the US.18,19,23,24 Key incidence and recurrence parameters21,23 are shown in Table 1 and Table 2. DRV epidemiologic estimation is shown as a flow chart in Figure 2.
Table 1.
Incidence and recurrence parameters by country.
| Australia | France | Germany | Italy | Spain | UK | US | |
|---|---|---|---|---|---|---|---|
| Incidence (no prior history of DRV)a,b,c | 78,634 | 203,406 | 260,944 | 51,520 | 65,431 | 211,198 | 240,000 |
| Incidence per 1000 person-years | 3.12 | 3.12 | 3.12 | 0.85 | 1.40 | 3.12 | 0.73 |
| Recurrences d | 43,200 | 59,144 | 84,305 | 201,600 | 19,643 | 19,237 | 402,775 |
| Recurrences per 1000 person-years | 1.71 | 0.91 | 1.01 | 3.33 | 0.42 | 0.28 | 1.22 |
| Total Incidence and recurrence | 121,834 | 262,550 | 345,250 | 253,120 | 85,075 | 230,435 | 642,775 |
| Incident and recurred cases per 1000 person-years | 4.83 | 4.03 | 4.13 | 4.18 | 1.82 | 3.40 | 1.95 |
Note – 2019 population estimates used: Australia 25,203,198; France 65,194,261; Germany 83,636,045; Italy 60,510,637; Spain 46,736,776; UK 67,691,582; US 329,064,917.
According to Agale, 19 there were 3.5 VLU cases per 1000 individuals and according to Cheng, 21 the VLU incidence rate (annual) was 0.0121, elderly population. UK incidence estimates were used for Australia, France, Germany, and Italy.
According to Ito et al., 22 in the USA, 600,000 new cases of lower leg ulcers occur annually, and approximately 80% of them are reported to be caused by disturbances of the venous return.
Spain VLU incidence is between two and five new cases per 1000 people per year according to Gutiérrez Iglesias et al. 20
Reliable recurrence estimates were only available for Australia and Spain. Cheng et al. 21 and Rubio-Terrés and Dominguez-Gil 23 were used to estimate the recurrence for Australia and Spain, respectively. Australian estimates were used for France, Germany, Italy, UK, and US as they align with global recurrence estimates.
DRV, deep venous disease-related VLU; VLU, venous leg ulcer.
Table 2.
Incidence and recurrence original source inputs.
| Prevalence 18,19,23,24 | Incidence 19–22 | Recurrence 21,23 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | Original values (VLU) |
Model inputs (DRV) |
Reference | Country | Original values (VLU) |
Model inputs (DRV) |
Reference | Country | Original values (VLU) |
Model inputs (DRV) |
Reference |
| Australia | 0.0119 | 0.00476 | 21 | Australia | 0.0078 | 0.00312 |
19; UK estimates |
Australia | 0.22 | 0.0792 |
21; Australian estimates |
| France | 0.0063 | 0.00252 | 44 | France | 0.0078 | 0.00312 | France | 0.22 | 0.0792 | ||
| Germany | 0.007 | 0.0028 | 45 | Germany | 0.0078 | 0.00312 | Germany | 0.22 | 0.0792 | ||
| Italy | 0.0231 | 0.0093 | 46 | Italy | 0.0078 | 0.00312 | Italy | 0.22 | 0.0792 | ||
| Spain | 0.00156 | 0.00187 | 20 | Spain | 0.0035 | 0.0014 | 20 | ||||
| 0.00245 | 47 | Spain | 0.25 | 0.090 | 23 | ||||||
| 0.01 | 48 | ||||||||||
| UK | 0.002 | 0.000789 | 49 | UK | 0.0035 | 0.00312 | 19 | ||||
| 0.002 | 50 | 0.0121 | 21 | UK | 0.22 | 0.0792 | 21; Australian estimates | ||||
| 0.002 | 51 | ||||||||||
| US | 0.0085 | 0.0034 | Markov model based on incidence and recurrence estimates | US | 0.0018 | 0.00073 | 22 | US | 0.22 | 0.0792 | |
DRV, deep venous disease-related VLU; HAS, Haute Autorité de Santé; SIGN, Scottish Intercollegiate Guidelines Network; VLU, venous leg ulcer.
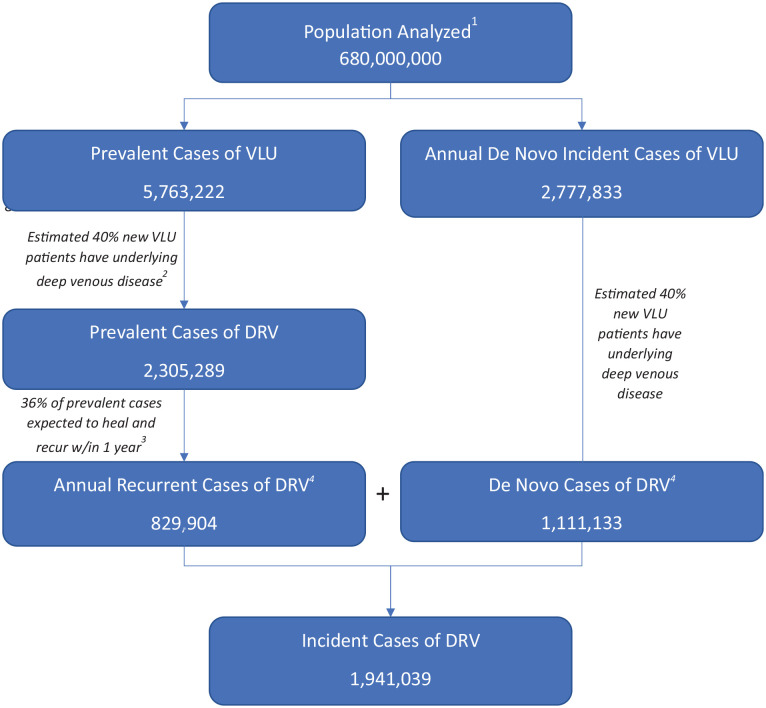
Figure 2.
Annual DRV epidemiologic burden estimation.
The cumulative population of seven countries (Australia, France, Germany, Italy, Spain, UK, and US) is 680,000,000. The prevalent cases of VLU are 5,763,222, and annual de novo incidence cases of VLU are 2,777,833. As an estimated 40% of new VLU patients have underlying DVD, the prevalent cases of DRV are 2,305,289, and annual recurrent cases of DRV are 829,904, assuming 36% of prevalent cases heal and recur within 1 year. Similarly, as an estimated 40% of new VLU patients have underlying DVD, 1,111,133 cases have de novo cases of DRV per year. In aggregate, there are 1,941,039 incident cases of DRV across all seven geographies per year.
1 Refers to the cumulative population of seven countries (Australia, France, Germany, Italy, Spain, UK, and US).
2,3Model assumptions (detailed explanation and reference provided in the text).
DRV, deep venous disease-related VLU; DVD, deep venous disease; VLU, venous leg ulcer.
In addition, all calculations are based on the following central assumptions from our literature review:
DRV are responsible for 40% of VLU;
60% of DRV are unhealed at 6 months with conservative treatment;
DRV recur at a higher rate (36% annually) than VLU without deep venous involvement;
Unhealed VLU are 4.5 times more expensive to manage than the cost of managing a patient with a healed wound;
The useful life of compression therapy systems is 3 months.
Detailed explanations of the studies supporting these assumptions are shown in the online Supplementary Table 3.24–32 Details regarding the exchange rates used are available in the supplementary content.
Cost and utilization of resources
Practitioner costs
Resource use
VLU care is provided by a wide range of providers, including general practitioners (GPs), specialists such as vascular surgeons, dermatologists, plastic surgeons, orthopedic surgeons, nurse practitioners (NPs), and community nurses. The granularity of information in the literature on the percentage of patients treated by each specialty and the frequency of visits is variable. For instance, in Australia, according to Edward et al., 33 (2013), patients may be treated by multiple practitioners; indeed, 91% of patients are treated by GPs, 35% are treated by medical specialists, and 31% by community nurses. VLU patients commonly visit GPs bi-weekly, specialists one to two times per week, and community nurses two to three times per week. Given the lack of published estimates in other countries, the authors, who are experienced clinicians and wound care experts, provided country-specific guidance. Based on this input, the consensus is that on average, patients visit clinics weekly. For the treatment setting, in Spain, 54% of visits for ulcer care are provided in health centers, and 46% occur in the home care setting. 23 Both costs and resource use specific to home care were only available for the US; the percentage of patients receiving home healthcare ranged from 10% to 50% and reportedly costs up to $11,365 (USD) per patient per year.32–35 Our model assumed that home healthcare is received by 50% of patients during the first 6 months and 50% of the remaining unhealed patients during the next 6 months.
Costs
Countries such as Australia, Italy, Spain, the UK, and the US report per unit resource use, whereas France and Germany only report total annual costs for practitioners and outpatient visits as shown in online Supplementary Table 4. For instance, in France, according to Lévy and Lévy, 36 the average annual 2019 practitioner cost per VLU patient was $756.46. Since per-visit costs were unavailable, annual costs were used for the model. In Germany, annual nurse practitioner costs for VLU patients are $1333.98, whereas the annual GP cost is $254.46. 37
In the US, detailed costs were assessed via Common Procedural Technology (CPT) codes for the management of the disease. Initial visit costs for a new DRV patient include physician evaluation (99203), facility (99213), debridement physician (11042), and debridement facility (11042) and are determined to be $74.88, $51.90, $63.16, and $128.84, respectively. Cost of an established clinic visit was determined using CPT codes 99212 (office or other outpatient visit for the evaluation and management of an established patient), 97597 (physician, debridement), and 97597 (facility, debridement) and was determined to be $45.77, $90.82, and $24.51, respectively. The annual cost of weekly recurring outpatient visits using CPT 99212 was determined to be $2380.04 ($45.77 per week for 52 weeks). Home healthcare costs (C2F2S1) for 1 weekly visit for a dressing change with a 60-day episode of care, for Medicare, was determined to be $2230. 38
Hospitalization
Resource use
Information on hospitalization rates for severe VLU patients was not available for all countries in our analysis. A hospitalization rate of 2.98% was extracted from Australian estimates based on a 2018 cost-effectiveness analysis by Cheng et al. 21 The study compared treatment under guideline-based optimal care, which assumes that 100% of patients use high-compression therapy, to usual care, which implies that only 50% of patients use high-compression therapy. Likewise, the model identified a 1.16% annual probability of hospitalization for optimal care and a 4.8% annual probability of hospitalization for usual care. Our model conservatively uses the average rate of hospitalization between these two models, which is 2.98%. Hospitalization costs between countries are highly variable and were driven by the source of the data and availability of information for this metric, as shown in online Supplementary Table 4. However, hospitalization costs had minimal impact on the total economic burden as they were presumed to impact only 2.98% of patients in our model.
Compression therapy
Resource use
Although compression therapy is the standard of care and the recommended first-line treatment for VLU patients according to clinical guidelines, evidence suggests that it is not widely used in practice. Reasons for low compression therapy utilization may be due to multiple factors, including lack of information, resources, poor compliance, and inadequate reimbursement. The evidence reviewed suggests that, in Australia, 40–60% of patients do not receive compression therapy. 21 In Germany, 31.1% of all VLU patients received no compression therapy, whereas in Spain, 54% of VLU patients were not given compression stockings. 20 Assessed countries used a variety of compression systems, including compression stockings and wraps, as shown in online Supplementary Table 4. Annual compression therapy costs range between $259.06 and $360, assuming the compression systems have a useful life of 3 months.
Wound care products, skin grafts or substitutes, and drugs/medications
Detailed information on wound care product costs was available for some countries, as shown in online Supplementary Table 4. Barnsbee et al. 38 reported that the weekly cost of primary/secondary dressings, bandages, topical medications (e.g., antimicrobial ointments), skincare items (e.g., barrier creams), cleansers, and other disposables was $51.52. In the US, information on dressings was not accessible via CPT codes as Medicare does not pay separately for dressing changes. It reimburses services as part of a billable evaluation and management or procedure that often occurs on the same date of service as the dressing change; it is thereby assumed that the cost of dressings and bandages is included under clinic visits and practitioner costs. In the outpatient setting, the cost of skin substitutes is determined to be $1729.57. CPT codes 15271 and 15272 were used to determine physician payment and hospital payment based on Ambulatory Payment Classifications (APC). In our experience, three applications of skin substitutes per patient are typically approved in the US.
The availability of information on drugs and prescription costs and utilization frequency is variable, as six acute episodes of 3 weeks in duration, and the prescription can be refilled. For Australia, dressing and drug costs were combined; product costs from Barnsbee et al. 38 were used. For France, the annual cost of medication for VLU patients is given as $515.67. It is presumed that this includes the cost of compression therapy, bandages/dressings, and other disposables, as Lévy and Lévy did not separately report these costs. 36 The annual drug costs in Germany for DRV patient care totals $1085.36. This sum is comprised of topical treatment, systematic treatment, and therapeutics costing $368.12, $148.70, and $275.40, respectively, and an additional $293.14 for prescription co-pays. For the US, the total cost per fill of prescription drugs (amitriptyline, gabapentin, and hydrocodone) 39 for management of pain associated with venous leg ulcers was $158.63. 39
Results
The annual direct medical, economic burden for DRV patients managed conservatively across all seven geographies is estimated to total $10.73 billion (USD), as shown in Figure 3. To assess the sensitivity of the results to our assumptions, we evaluated the impact of a 10% change in assumptions values. In this one-way sensitivity analysis, estimates regarding the useful life of compression therapy and the costs of VLU (either healed or unhealed) had minimal impact (± 0.1%) on the all-country total economic burden. In contrast, changes in the assumptions regarding the rate of DRV recurrence resulted in higher variability (± 11.8%).
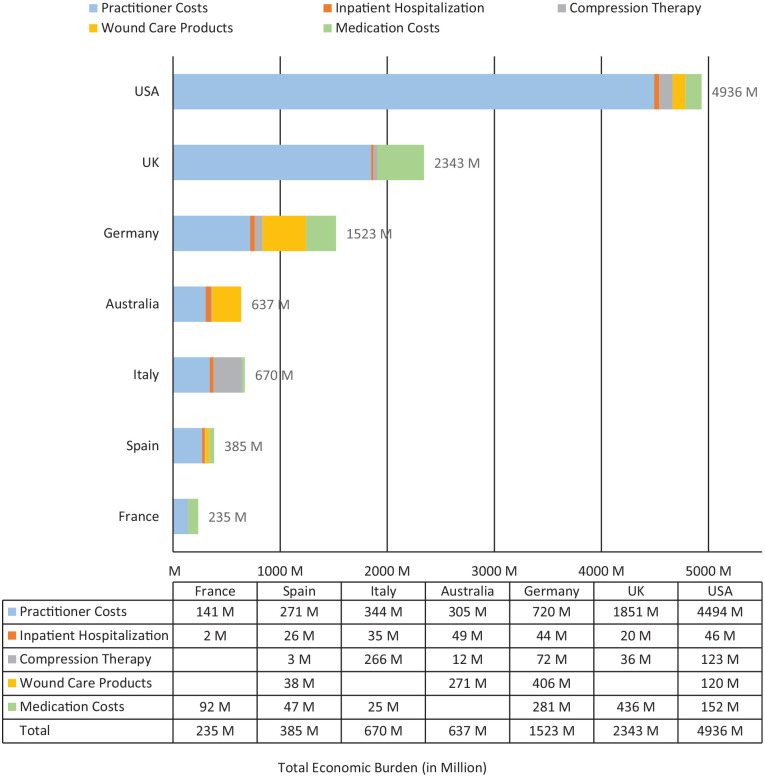
Figure 3.
All-country annual economic burden of illness estimates (total: $10.73 billion [USD]).
Compression therapy costs are not separately included for France. Wound care costs are not separately included for France, Italy, and the UK. Drug costs are not separately included for Australia. These costs are accounted for under other cost buckets. Skin substitute costs for the US are included under wound care products.
The economic burden and the percentage utilization of resources vary substantially between countries. Most of the costs related to DRV are attributable to the practitioner and clinic costs in the US, Australia, and the UK. In the remaining countries, costs are split between provider and other wound healing-related costs. For all countries except Italy, practitioner and clinic expenses are the most prominent cost drivers, ranging from 47% of costs in Germany to 91% of the costs in the US. Hospitalization costs range between 1% of total costs (France) to 8% (Australia). Compression therapy use ranges from 45% to 90%, and costs range from 1% of total costs in Spain to 40% in Italy, as shown in Figure 3.
The average annual costs of DRV care per patient per year (in USD) are $5226 for Australia, $894 for France, $4410 for Germany, $2467 for Italy, $4527 for Spain, $10,169 for the UK, and $7679 for the US. The direct medical, economic burden of DRV ranged from $0.24 billion (France) to $4.94 billion (US) and totaled US$10.73 billion in the geographies analyzed. This conservative figure represents nearly 0.21% (a fraction of the total spend on all chronic wounds) of the total $5.1 trillion healthcare spending across these countries, a significant expense. For comparison, global spending on HIV/AIDS from 2000 to 2015 totaled $562.6 billion, or about 0.39% of the $9.7 trillion global healthcare spend in 2015. 40
Discussion
Differences in practice patterns drive significant variation in the provision of VLU care across the world due, in part, to the availability of topical and interventional therapies. Although several studies have reported the resource utilization and the associated costs of VLU care, few have provided a detailed review of the burden of illness of VLU across multiple countries. Our report is the first study to provide such information. We estimate an overall annual cost of at least US$10.73 billion associated with conservative management of DRV in the seven geographies studied, a substantial expense. Prior research has established that patients with persistent, non-healing ulcers incur the highest costs of care. 41 The ability to provide early diagnosis and appropriate intervention to treat underlying vascular disease more quickly could potentially promote faster healing, thus decreasing the overall cost, a hypothesis worthy of further analysis. 32 In our model, if just 10% more patients were healed at 6 months (50% versus 40%), costs would be reduced by $511 million. If 30% more were healed (70%), $1.5 billion would be saved annually.
Given these high costs associated with conservative care, further evidence is needed in a variety of areas, particularly on the epidemiology, costs, and resource utilization of DRV. For our analysis, the total number of DRV patients per year is assessed as the number of incident cases as well as recurrent ones. Prevalent cases were excluded to avoid double-counting of patients. However, some patients have ulcers that persist for longer than a year and tend to incur the highest costs for care. 42 Moreover, both the incidence and prevalence of the disease is likely significantly underestimated in the available literature. To date, there is no definitive study that demonstrates superiority of deep venous revascularization over conservative management. The VLU subgroup analysis (depending on the size of this subgroup) of the ongoing National Institutes of Health (NIH) sponsored randomized controlled trial – the C-TRACT Trial, may provide some insights into VLU outcomes related to deep venous interventions. 43
Study limitations
Our approach likely underestimates the true burden of care, as prior research has suggested an average cost of treatment of VLU higher than what is estimated here.36,37 Although gross-costing methods have been used in the past to estimate the cost of VLU care in the US, 36 limitations in access to insurance claims and general ledger data necessitate a micro-costing approach for a multi-country analysis such as this one, an approach that may underestimate the total costs of care. For instance, as no cost estimates specific to DRV are available, we assumed that monthly costs and resource utilization for all VLU patients, both related to the superficial and DVD, are similar in patients from either venous disease category but in the same ulcer disease state (i.e., open ulcer or healed ulcer). We accounted for the higher costs associated with DRV by adjusting for the duration of treatment supported by evidence that points to longer time-to-healing and higher rates of recurrence in the DVD population. However, although our literature review confirmed the widespread use of home care in multiple geographies, cost estimates for home care are only included in the US. Costs of newer and potentially high-cost procedures, such as ultrasound-assisted debridement, more modern skin substitutes, compression pumps, and growth factor application, are not included due to high variability in their utilization. Furthermore, we could not include the additional indirect burden associated with productivity losses linked to patients and caretaker absenteeism resulting from VLU care, or the out-of-pocket expenses associated with care given inconsistent data on indirect costs. Although the current analysis is conservatively focused on deep venous obstruction (DVO), we recognize the additional burden of deep venous reflux (which may or may not be secondary to DVO) leading to DRV. Moreover, DVD resulting from obstruction or reflux manifests in other forms of severe venous disease such as venous eczema, lipodermatosclerosis, recurrent cellulitis, and recurrent venous thromboembolism, all of which carry an additional healthcare cost burden. Therefore, although this economic estimate of burden of illness is compelling, it is likely a conservative and Western representation of the total financial burden of DRV care, as the above-mentioned costs were not included in our analysis. Furthermore, our study was limited to PubMed. Database searches from other databases such as EMBASE may have revealed other articles.
Our analysis followed PRISMA guidelines to identify countries where the most data are currently available. Even so, the need for model assumption highlights the dearth of reliable, comprehensive data on the epidemiology and costs of both VLU and DRV. We are also limited by a lack of uniformity in cost estimate data available across countries. Reliable incidence estimates were absent for Australia, France, Germany, and Italy. Thus, we used the incidence rate of the UK. Also, our analysis may overestimate the percentage of venous leg ulcers that are related to DVD. Although multiple analyses have reported that roughly 40% of patients with ulcers have deep venous abnormalities, this may be reflective of a generalized referral bias, in that patients included in such studies are more likely to have been referred for specialist treatment as a result of more difficult-to-treat disease. In addition, for the US, per unit costs were identified using 2019 CPT codes related to the disease, whereas for Italy and Spain, tariff and fee schedules, and other cost studies, were utilized. For Australia, Germany, the UK, and France, we relied on cost-effectiveness analyses reported in the literature. Australia, Germany, and the UK reported unit costs, and France reported annual costs. Studies used were published over 15 years, from 2002 (France) to 2018 (Australia), causing variations in our confidence on the reliability of the estimates across countries. Further, budget allocation varies among and within countries. Variations in reimbursement may lead to an underestimation of costs as out-of-pocket spending may not be uniformly captured in all countries; for instance, in Australia, compression garments are generally paid for by patients out-of-pocket. We acknowledge that the limitations associated with this lack of data impact our model, but we believe our comprehensive approach provides the best credible estimate, albeit a conservative one, to measure the burden of DRV care under the circumstances.
Conclusions
Our study demonstrates an annual direct healthcare expenditure of $10.73 billion (USD) associated with DRV care in the seven countries analyzed. We believe this is an underestimation of the actual costs related to DRV care and DVD in general. Although the reasons include lack of direct evidence of DRV-specific costs, heterogeneity in data, as well as inconsistent and inadequate reporting of the actual costs, we arrive at one consistent finding – VLU and DRV result in significant healthcare expenditure in all the countries analyzed.
Supplemental Material
Supplemental material, sj-pdf-1-vmj-10.1177_1358863X211028298 for An estimate of the economic burden of venous leg ulcers associated with deep venous obstruction by Raghu Kolluri, Marzia Lugli, Laurencia Villalba, Ramon Varcoe, Oscar Maleti, Fernando Gallardo, Stephen Black, Fannie Forgues, Michael Lichtenberg, Jordan Hinahara, Saranya Ramakrishnan and Josh Beckman in Vascular Medicine
Acknowledgments
The authors would like to acknowledge the late Susan Meyr for her input in conceptualizing the need for this study. She is recognized by the authors posthumously for her contributions.
Footnotes
Declaration of conflicting interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Raghu Kolluri, uncompensated consultant – Boston Scientific, Intervene, Medtronic, Philips IGT/Ultrasound, Vesper Medical, Intact Vascular; data and safety monitoring board (DSMB) – Thrombolex Inc. Marzia Lugli, no conflicts of interest. Laurencia Villalba, speaker – Boston Scientific, Philips; research grants – Bard, Pyramed. Ramon Varcoe, consultant – Abbott, Medtronic, BD Bard, Boston Scientific, Surmodics, InterVene, Intact Vascular. Oscar Maleti, no conflicts of interest. Fernando Gallardo, no conflicts of interest. Stephen Black, consulting and speaking fees – Cook, Medtronic, BSCI, BD Bard, Phillips-Volcano, Optimed; consulting fees – Veryan, Vesper, Vetex. Fannie Forgues, fees – workshop for Thuasne. Michael Lichtenberg, speaker honoraria and research grants – BD Bard, Boston Scientific, Cook Medical, Optimed. Jordan Hinahara, Boston Healthcare Associates – consulting fees for development of the manuscript. Saranya Ramakrishnan, Boston Healthcare Associates – consulting fees for development of the manuscript. Josh Beckman, consulting – Amgen, AstraZeneca, Sanofi, Janssen, JanOne, GlaxoSmithKline, Upperline Health; DSMB – Novartis.
Funding: This research was supported by funding from Philips Image Guided Therapy. Philips funded Boston Healthcare Associates for the data analysis and synthesis. All authors were involved in all aspects of the manuscript preparation and were uncompensated for the work.
ORCID iDs: Raghu Kolluri  https://orcid.org/0000-0002-4374-7513
https://orcid.org/0000-0002-4374-7513
Josh Beckman  https://orcid.org/0000-0001-8332-8439
https://orcid.org/0000-0001-8332-8439
Supplementary material: The supplementary material is available online with the article.
References
- 1. Epstein DM, Gohel MS, Heatley F, et al. Cost-effectiveness analysis of a randomized clinical trial of early versus deferred endovenous ablation of superficial venous reflux in patients with venous ulceration. Br J Surg 2019; 106: 555–562. [DOI] [PubMed] [Google Scholar]
- 2. Järbrink K, Ni G, Sönnergren H, et al. The humanistic and economic burden of chronic wounds: A protocol for a systematic review. Syst Rev 2017; 6: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sen CK. Human wounds and its burden: An updated compendium of estimates. Adv Wound Care (New Rochelle) 2019; 8: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O’Donnell TF, Passman MA, Marston W, et al. Management of venous leg ulcers: Clinical practice guidelines of the Society for Vascular Surgery® and the American Venous Forum. J Vasc Surg 2014; 60(2 Suppl): 3S–59S. [DOI] [PubMed] [Google Scholar]
- 5. Finlayson KJ, Parker CN, Miller C, et al. Predicting the likelihood of venous leg ulcer recurrence: The diagnostic accuracy of a newly developed risk assessment tool. Int Wound J 2018; 15: 686–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fitridge R, Thompson M. (eds). Mechanisms of vascular disease: A reference book for vascular specialists. University of Adelaide Press, 2011. [PubMed] [Google Scholar]
- 7. Youn YJ, Lee J. Chronic venous insufficiency and varicose veins of the lower extremities. Korean J Intern Med 2019; 34: 269–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marston W, Fish D, Unger J, et al. Incidence of and risk factors for iliocaval venous obstruction in patients with active or healed venous leg ulcers. J Vasc Surg 2011; 53: 1303–1308. [DOI] [PubMed] [Google Scholar]
- 9. Gohel MS, Heatley F, Liu X, et al. A randomized trial of early endovenous ablation in venous ulceration. N Engl J Med 2018; 378: 2105–2114. [DOI] [PubMed] [Google Scholar]
- 10. Kolluri R, Bashir R, Matros T, et al. Prevalence and predictors of elevated central venous pressure and obstructive sleep apnea in patients with lower extremity chronic venous disease. J Vasc Surg Venous Lymphat Disord 2020; 8: 775–782. [DOI] [PubMed] [Google Scholar]
- 11. National Institute for Health and Care Excellence (NICE). Varicose veins: diagnosis and management. Clinical guideline [CG168], www.nice.org.uk/guidance/cg168 (2013, accessed 22 April 2020). [PubMed]
- 12. Rognoni C, Lugli M, Maleti O, et al. Venous stenting for patients with outflow obstruction and leg ulcers: Cost-effectiveness and budget impact analyses. J Comp Eff Res 2020; 9: 705–720. [DOI] [PubMed] [Google Scholar]
- 13. Fife CE, Eckert KA, Carter MJ. Publicly reported wound healing rates: The fantasy and the reality. Adv Wound Care (New Rochelle) 2018; 7: 77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rice JB, Desai U, Cummings AKG, et al. Burden of venous leg ulcers in the United States. J Med Econ 2014; 17: 347–356. [DOI] [PubMed] [Google Scholar]
- 15. Eklöf B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders: Consensus statement. J Vasc Surg 2004; 40: 1248–1252. [DOI] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marston W, Tang J, Kirsner RS, et al. Wound healing society 2015 update on guidelines for venous ulcers. Wound Repair Regen 2016; 24: 136–144. [DOI] [PubMed] [Google Scholar]
- 18. Neumann HAM, Cornu-Thénard A, Jünger M, et al. Evidence-based (S3) guidelines for diagnostics and treatment of venous leg ulcers. J Eur Acad Dermatology Venereol 2016; 30: 1843–1875. [DOI] [PubMed] [Google Scholar]
- 19. Agale SV. Chronic leg ulcers: Epidemiology, aetiopathogenesis, and management. 2013; 2013: 413604. [Google Scholar]
- 20. Gutiérrez Iglesias A, Bayón Yusta JC, Quesada Ramos C, et al. Análisis coste efectividad de la terapia tópica de presión negativa para el tratamiento de las úlceras venosas de pierna, https://ulcerasfora.sergas.gal/Informacion/Documents/202/(Ministriodesanidadtratamientodelasúlcerasvenosas.pdf (2015, accessed 16 December 2019).
- 21. Cheng Q, Gibb M, Graves N, et al. Cost-effectiveness analysis of guideline-based optimal care for venous leg ulcers in Australia. BMC Health Serv Res 2018; 18: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ito T, Kukino R, Takahara M, et al. The wound/burn guidelines – 5: Guidelines for the management of lower leg ulcers/varicose veins. J Dermatol 2016; 43: 853–868. [DOI] [PubMed] [Google Scholar]
- 23. Rubio-Terrés C, Dominguez-Gil H. [Cost-effectiveness analysis of the treatment of patients with venous leg ulcers due to chronic venous insufficiency with micronized purified flavonoid fraction and compression therapy or compression therapy only] [in Spanish]. Rev Esp Econ Salud 2005; 487–494. [Google Scholar]
- 24. National Institute for Health and Care Excellence (NICE). Lower limb deep vein valve reconstruction for chronic deep venous incompetence. Interventional procedures guidance [IPG219], https://www.nice.org.uk/guidance/ipg219 (2007, accessed 23 April 2021).
- 25. Alhalbouni S, Hingorani A, Shiferson A, et al. Iliac-femoral venous stenting for lower extremity venous stasis symptoms. Ann Vasc Surg 2012; 26: 185–189. [DOI] [PubMed] [Google Scholar]
- 26. Lawrence PF, Hager ES, Harlander-Locke MP, et al. Treatment of superficial and perforator reflux and deep venous stenosis improves healing of chronic venous leg ulcers. J Vasc Surg Venous Lymphat Disord 2020; 8: 601–609. [DOI] [PubMed] [Google Scholar]
- 27. Raju S, Neglen P. High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: A permissive role in pathogenicity. J Vasc Surg 2006; 44: 136–144. [DOI] [PubMed] [Google Scholar]
- 28. Neglén P, Hollis KC, Olivier J, et al. Stenting of the venous outflow in chronic venous disease: Long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg 2007; 46: 979–990. [DOI] [PubMed] [Google Scholar]
- 29. George R, Verma H, Ram B, et al. The effect of deep venous stenting on healing of lower limb venous ulcers. Eur J Vasc Endovasc Surg 2014; 48: 330–336. [DOI] [PubMed] [Google Scholar]
- 30. McDaniel HB, Marston WA, Farber MA, et al. Recurrence of chronic venous ulcers on the basis of clinical, etiologic, anatomic, and pathophysiologic criteria and air plethysmography. J Vasc Surg 2002; 35: 723–728. [DOI] [PubMed] [Google Scholar]
- 31. Wen-da W, Yu Z, Yue-xin C. Stenting for chronic obstructive venous disease: A current comprehensive meta-analysis and systematic review. Phlebology 2016; 31: 376–389. [DOI] [PubMed] [Google Scholar]
- 32. Olin JW, Beusterien KM, Childs MB, et al. Medical costs of treating venous stasis ulcers: Evidence from a retrospective cohort study. Vasc Med 1999; 4: 1–7. [DOI] [PubMed] [Google Scholar]
- 33. Edwards H, Finlayson K, Courtney M, et al. Health service pathways for patients with chronic leg ulcers: Identifying effective pathways for facilitation of evidence based wound care. BMC Heal Serv Res 2013; 13: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mostow EN, Haraway GD, Dalsing M, et al. Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: A randomized clinical trial. J Vasc Surg 2005; 41: 837–843. [DOI] [PubMed] [Google Scholar]
- 35. Ma H, O’Donnell TF, Rosen NA, et al. The real cost of treating venous ulcers in a contemporary vascular practice. J Vasc Surg Venous Lymphat Disord 2014; 2: 355–361. [DOI] [PubMed] [Google Scholar]
- 36. Lévy E, Lévy P. [The therapeutic attitudes of French doctors in the face of venous leg ulcers: diversity and induced costs] [in French]. J Mal Vasc 2001; 26: 39–44. [PubMed] [Google Scholar]
- 37. Purwins S, Herberger K, Debus ES, et al. Cost-of-illness of chronic leg ulcers in Germany. Int Wound J 2010; 7: 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barnsbee L, Cheng Q, Tulleners R, et al. Measuring costs and quality of life for venous leg ulcers. Int Wound J 2019; 16: 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carter MJ, Waycaster C, Schaum K, et al. Cost-effectiveness of three adjunct cellular/tissue-derived products used in the management of chronic venous leg ulcers. Value Heal 2014; 17: 801–813. [DOI] [PubMed] [Google Scholar]
- 40. Dieleman JL, Haakenstad A, Micah A, et al. Spending on health and HIV/AIDS: Domestic health spending and development assistance in 188 countries, 1995–2015. Lancet 2018; 391: 1799–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fife CE, Carter MJ, Walker D, et al. Wound care outcomes and associated cost among patients treated in US outpatient wound centers: Data from the US Wound Registry. Wounds 2012; 24: 10–17. [PubMed] [Google Scholar]
- 42. Melikian R, O’Donnell TF, Suarez L, et al. Risk factors associated with the venous leg ulcer that fails to heal after 1 year of treatment. J Vasc Surg Venous Lymphat Disord 2019; 7: 98–105. [DOI] [PubMed] [Google Scholar]
- 43. Vedantham S. C-TRACT Trial: Coming together to tackle chronic DVT. Endovascular Today, https://evtoday.com/articles/2019-july/c-tract-trial-coming-together-to-tackle-chronic-dvt (2019, accessed 22 April 2021).
- 44. Managing venous leg ulcers (excluding dressings). Haute Autorité de Santé website. Published Nov 30, 2006. https://www.has-sante.fr/jcms/c_459541/en/managing-venous-leg-ulcers-excluding-dressings (accessed 26 July 2021).
- 45. Rabe E, Pannier-Fischer F, Bromen K, et al. [Bonn vein study of the German Society for Phlebology] [in German]. Phlebologie 2003; 32: 1–14. [Google Scholar]
- 46. Mosti G, Gasperini S. Observations made on three patients suffering from ulcers of the lower limbs treated with Blue Light. Chronic Wound Care Manag Res 2018; 5: 23–28. [Google Scholar]
- 47. [Guide of Clinical Practice: Consensus on Vascular Ulcers and Diabetic Foot of the Spanish Association of Vascular Nursing and Wounds (AEEVH)], 3rd ed. [in Spanish] Spanish Association of Vascular Nursing and Wounds, 2017. https://aeevh.org/wp-content/uploads/2020/04/Guia-de-Practica-Clinica-web.pdf (accessed 23 April 2021).
- 48. Rumbo-Prieto JM, Arantón-Areosa L, Palomar-Llatas F, et al. Quality of clinical practice guidelines of lower extremity venous ulcers [in English, Spanish]. Enferm Clin (Engl Ed) 2018; 28: 49–56. [DOI] [PubMed] [Google Scholar]
- 49. Venous leg ulcer. National Health Service website. Last updated Jan 11, 2019. https://www.nhs.uk/conditions/leg-ulcer/ (accessed 26 July 2021).
- 50. Management of chronic venous leg ulcers. Health Improvement Scotland website. Published Aug 2010. https://www.sign.ac.uk/our-guidelines/management-of-chronic-venous-leg-ulcers/ (accessed 26 July 2021).
- 51. Graham ID, Harrison MB, Shafey M, et al. Knowledge and attitudes regarding care of leg ulcers. Survey of family physicians. Can Fam Physician 2003; 49: 896–902. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-vmj-10.1177_1358863X211028298 for An estimate of the economic burden of venous leg ulcers associated with deep venous obstruction by Raghu Kolluri, Marzia Lugli, Laurencia Villalba, Ramon Varcoe, Oscar Maleti, Fernando Gallardo, Stephen Black, Fannie Forgues, Michael Lichtenberg, Jordan Hinahara, Saranya Ramakrishnan and Josh Beckman in Vascular Medicine