Abstract
Objective:
Adult women with polycystic ovary syndrome (PCOS) and obesity have an 8-fold increased risk of developing type 2 diabetes (T2D). Our goal was to determine the incidence and risk factors for T2D in adolescents with PCOS and obesity.
Research Design and Methods:
Retrospective chart review of girls aged 11–21 years with confirmed PCOS (oligomenorrhea and hyperandrogenism) diagnosis between 7/2013–8/2018 and at least one follow-up visit and BMI >85%ile. T2D incidence, defined with an HbA1c ≥6.5%, was calculated. A nested case-control study with 1:3 matching by race, ethnicity, and BMI was performed to determine predictors of T2D diagnosis.
Results:
493 patients with PCOS (age 15.6±1.9 years, BMI 36.2±6.3 kg/m2) were identified with a follow-up of 1,018 person-years. 23 developed T2D (incidence 22.6/1,000 person-years) with diagnosis a median of 1.8 years (2 months to 5.5 years) after PCOS diagnosis. T2D risk was higher in girls with a prediabetes HbA1c (5.7–6.4%) [HR 14.6 (4.8–44.5)] and among Hispanic girls with an elevated HbA1c and alanine aminotransferase [HR 19.0 (3.7–97.2)] at the time of PCOS diagnosis. In the 1:3 matched cohort, T2D risk was 18.7 times higher [OR 18.66 (2.27–153.24)] for every 0.1% increase in HbA1c at the time of PCOS diagnoses.
Conclusions
Girls with PCOS and obesity have an 18-fold increase in T2D incidence compared to published rates in non-PCOS youth. Hispanic girls with elevated HbA1c and ALT are at particular risk. Due to the morbidity associated with youth onset T2D, these findings argue for better screening and prevention approaches in this population.
Keywords: Polycystic ovary syndrome, type 2 diabetes, obesity, adolescents
Introduction
Polycystic Ovary Syndrome (PCOS) affects 6–10% of women in the United States and upwards of 15% of obese women. PCOS is associated with many co-morbidities including insulin resistance (IR), type 2 diabetes (T2D), nonalcoholic fatty liver disease (NAFLD), obstructive sleep apnea (OSA), and decreased quality of life (1–7). Women with both PCOS and obesity are 2.5 times more likely to have IR (3,4,8,9). The conversion from IR to diabetes is more rapid in adult women with PCOS than in those without PCOS (10,11). A recent longitudinal study from Australia involving the largest cohort to-date estimated the incidence of T2D among adults with PCOS at 4.19/1,000 person years (PYs) versus 1.02/1,000 PYs for women without PCOS, an approximately 4-fold increase in risk (12). The incidence rates for T2D in women with PCOS increases proportionally with body mass index (BMI): 3.2/1,000 PYs in normal-weight, 4.7/1,000 PYs in overweight, and 8.8/1,000 PYs in obese women (12). Current screening recommendations acknowledge the increased risk of diabetes in women with PCOS and thus recommend universal screenings for T2D in women with PCOS every 1–3 years (1,13,14). Although similar screening guidelines exist for adolescent girls, there is little evidence informing the frequency of screening for T2D (1,15–18).
Youth-onset T2D is more severe and distinctively different than adult-onset diabetes. Youth who develop T2D have accelerated disease progression, higher rates of morbidity and earlier mortality than adults with diabetes (18–22). Further, typical therapies to treat T2D, such as lifestyle, metformin, insulin or thiazolidinediones, fail at a much higher rate in youth (19,21). T2D appears to preferentially impact adolescent females, and more than 50% of girls with T2DT2Dfail metformin therapy within two years, relative to 40% of adolescent boys or 10% of adults within five years (19). Thus, the need to develop tools and interventions to prevent development of youth-onset T2D, especially among adolescent girls is urgent.
The risk of T2D is increased among adult women with PCOS. Evidence-based data regarding T2D incidence among adolescent girls are lacking. Therefore, the two aims of this study are to determine the incidence of T2D in overweight and obese girls with PCOS and to identify genetic, metabolic, or hormonal predictors for T2D to better inform future screening and preventative efforts.
Methods
Data Collection
This study consisted of a retrospective chart review of the electronic medical record (EMR) at Children’s Hospital Colorado, a large tertiary care center with a 7-state referral area for pediatric diabetes management. This EMR system captures the data from at least 10 affiliated general pediatric practices and, thus, represents a large variety of patients. The Colorado Multiple Institutional Review Board approved the study. Data collection was designed to calculate the incidence and risk factors for T2D. Patient selection criteria for the initial EMR search patient selection included: 1) patients aged 11–21 years at time of PCOS diagnosis 2) at least 2 office visits, with at least one between 7/1/2013–8/15/2018, 3) PCOS identified using ICD 9/10 code of “polycystic ovaries”, and 4) excess weight identified by ICD9/10 “overweight or obese”. These data were chosen to coincide with the application of the Endocrine Society PCOS guidelines in clinical practice, as well as the widespread adoption in our area of LCMS/MS for the measurement of serum testosterone. From this initial cohort, manual data extraction was performed by a single researcher (JHB) to confirm that patients met criteria for inclusion in the final cohort. Overweight or obese was defined as BMI > 85th percentile at time of PCOS diagnosis. PCOS diagnosis was confirmed using Endocrine Society guidelines which were implemented 6 months prior to data collection (13), in particular 1) documented oligomenorrhea or age > 15 years with primary amenorrhea and 2) clinical or biochemical evidence of hyperandrogenism and 3) exclusion of any other cause for irregular menses or hyperandrogenism. T2D was defined per American Diabetes Association criteria (18). PCOS and T2D status were adjudicated by a board-certified pediatric endocrinologist (MCG). Patients with type 1 diabetes or transgender individuals receiving gender-affirming treatment with testosterone were excluded. Those with a diagnosis of T2D prior to the diagnosis of PCOS, or with simultaneous diagnosis of PCOS and diabetes were also excluded.
Key demographic and diagnostic information at the time of PCOS diagnosis were collected. Demographic information included: age, race, ethnicity, and insurance type (public, private, uninsured). Diagnostic information included: BMI value/percentile/Z-score, systolic blood pressure, HbA1c, alanine aminotransferase (ALT), testosterone concentration, personal history of obstructive sleep apnea (OSA), family history of a first-degree relative with PCOS, T2D, obesity, or OSA. Clinic sites of initial presentation for PCOS were categorized as Pediatric Endocrinology, Lifestyle Medicine, Adolescent Gynecology, Adolescent Medicine, and Other. Data on medications were collected where available.
To control for the variable duration of follow-up and further understand risk factors for PCOS beyond the effect of race/ethnicity, age and BMI, a secondary nested case-control (T2D-vs noT2D) evaluation was performed. Each T2D case was matched to three controls that did not develop T2D by 1) duration of follow-up (all three controls exceeded follow-up time for each case) 2) race/ethnicity, 3) age and 4) BMI at time of PCOS diagnosis. In this sub-cohort, the following variables were recorded at baseline: triglycerides, high-density lipoprotein (HDL), ALT, free testosterone, medication use including exogenous hormones and medications known to affect insulin sensitivity, and maternal history of gestational diabetes mellitus during any pregnancy.
Statistical Analysis
Data are presented as the mean ± standard deviation, median (25th %,ile 75th %ile) or number (percent). For descriptive statistics (Table 1), comparisons between PCOS only and PCOS developing T2D were performed with chi-square or Fisher’s exact or student’s t-test, as appropriate. As follow-up length varied per individual patient, the cohort was stratified into those above or below the median follow-up time, to determine whether follow-up time impacted the results. The incidence of diabetes was defined as the number of incident diabetes cases/1,000 PY of PCOS patients with longitudinal data. PY were calculated as the date of the last visit in the EMR system recorded where symptoms for T2D could be assessed minus the date of PCOS diagnosis, and in those with T2D, date of T2D diagnosis minus date of PCOS diagnosis. To examine factors related to time to development of diabetes, Kaplan-Meier curves were generated for 1) the entire longitudinal cohort, 2) the cohort stratified by race/ethnicity, HbA1c at time of PCOS diagnosis (normal <5.7 or prediabetes ≥5.7%−6.4%) and 3) the girls at highest-risk for T2D, defined as Hispanic ethnicity and both elevated HbA1c and ALT compared to those with neither or only one of HbA1c or ALT elevated. Hispanic ethnicity and ALT were selected for this model in addition to HbA1c after they were found to be related to T2D development in initial analysis. Comparisons were performed with the log-rank test, and group differences are summarized with the hazard ratio (HR) and associated 95% confidence interval. Within the nested case-control sub-study, conditional logistic regression was used to investigate the relationship between T2D development and potential predicting factors. Analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC, USA) and Prism 8 (GraphPad Software, San Diego, CA, USA).
Table 1.
Characteristics at time of PCOS diagnosis of longitudinal cohort by T2D status
| PCOS only n=470 |
PCOS + T2D n = 23 |
|
|---|---|---|
|
| ||
| Age at PCOS Diagnoses (years) | 15.7 ± 1.8 | 15.4 ± 2.1 |
|
| ||
| Age at diabetes diagnosis (years) | - | 17.5 ± 2.2 |
|
| ||
| Body Mass Index kg/m 2 | 35.9 ± 6.3 | 39.0 ± 5.8* |
|
| ||
| BMI Z-score | 2.15 ± 0.41 | 2.36 ± 0.31** |
|
| ||
| Race | ||
| African American | 50 (11%) | 5 (22%) |
| White | 366 (78%) | 17 (69%) |
| Other | 26 (5%) | 2 (9%) |
| Refused | 28 (6%) | 0 (0%) |
|
| ||
| Hispanic Ethnicity | 224 (47%) | 15 (65%)* |
|
| ||
| Insurance Type | ||
| Public | 267 (57%) | 17 (74%) |
| Private | 164 (34%) | 3 (13%) |
| None | 39 (9%) | 3 (13%) |
|
| ||
| Clinic Type | ||
| Endocrinology | 215 (46%) | 15 (65%) |
| Adolescent Gynecology | 66 (14%) | 1 (4%) |
| Lifestyle Medicine | 59 (13%) | 4 (17%) |
| Adolescent Medicine | 91 (19%) | 2 (9%) |
| Other | 39 (8.3%) | 1 (4%) |
|
| ||
| Past Medical History | ||
| Obstructive sleep apnea | 81 (17%) | 8 (35%) |
|
| ||
| Family History | ||
| PCOS | 44 (9%) | 2 (9%) |
| T2D | 111 (23%) | (35%) |
| Obesity | 113 (23%) | (39%) |
| Obstructive sleep apnea | 28 (6%) | 3 (13%) |
|
| ||
| HbA1c (%) | 5.4 ± 0.3 | 5.8 ± 0.4 *** |
|
| ||
| ALT (IU/L) | 38 ± 28 | 53 ± 36 * |
Data presented are mean ± standard deviation of the mean, or number (percent of the total). Significance is denoted as
p<0.001
p<0.01
p<0.05 when compared to PCOS only group.
Lifestyle Medicine is a referral T2D clinic. PCOS= polycystic ovary syndrome, T2D= type 2 diabetes, BMI = body mass index, ALT=alanine aminotransferase. BMI, HbA1c and ALT are from time of PCOS diagnosis.
Results
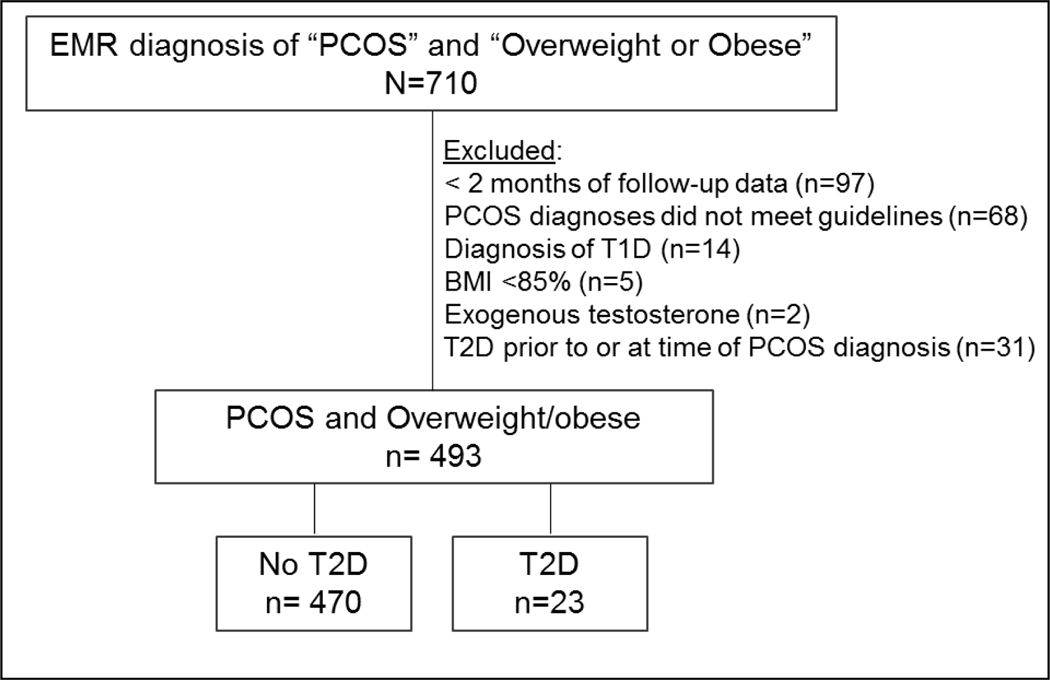
A total of 710 charts of overweight or obese girls with possible PCOS were identified from the initial EMR query (Figure 1). On manual chart review, 493 met criteria for inclusion. Of the 217 who were ineligible, reasons for exclusion were: 68 unable to confirm PCOS per guidelines; 97 with less than 2 months of follow-up data; 14 with type 1 diabetes; 5 not overweight or obese at time of PCOS diagnosis; 2 receiving testosterone treatment for transgender therapy. Due to the nature of our research question, 31 cases of prevalent T2D and PCOS were also excluded; 12 diagnosed with PCOS and T2D simultaneously and 19 diagnosed with T2D prior to PCOS diagnosis. The final cohort (n=493) included 470 cases of PCOS and 23 cases of PCOS + T2D. The duration of observation ranged from 2 months - 8.5 years, with a total follow-up time of 1,018 PY.
Figure 1:
Identification and inclusion of participants
Numbers of patients initially identified and then the final cohort identified are shown.
The incidence of T2D among overweight or obese adolescents with PCOS was 22.6/1,000 PYs. The median time to diagnosis of T2D was 1.8 (0.5–3.1) years with a range of 2 months - 5.5 years after PCOS diagnosis. The median follow-up for the PCOS-only group was 1.5 (0.7–2.9) years with a range of 2 months - 8.5 years.
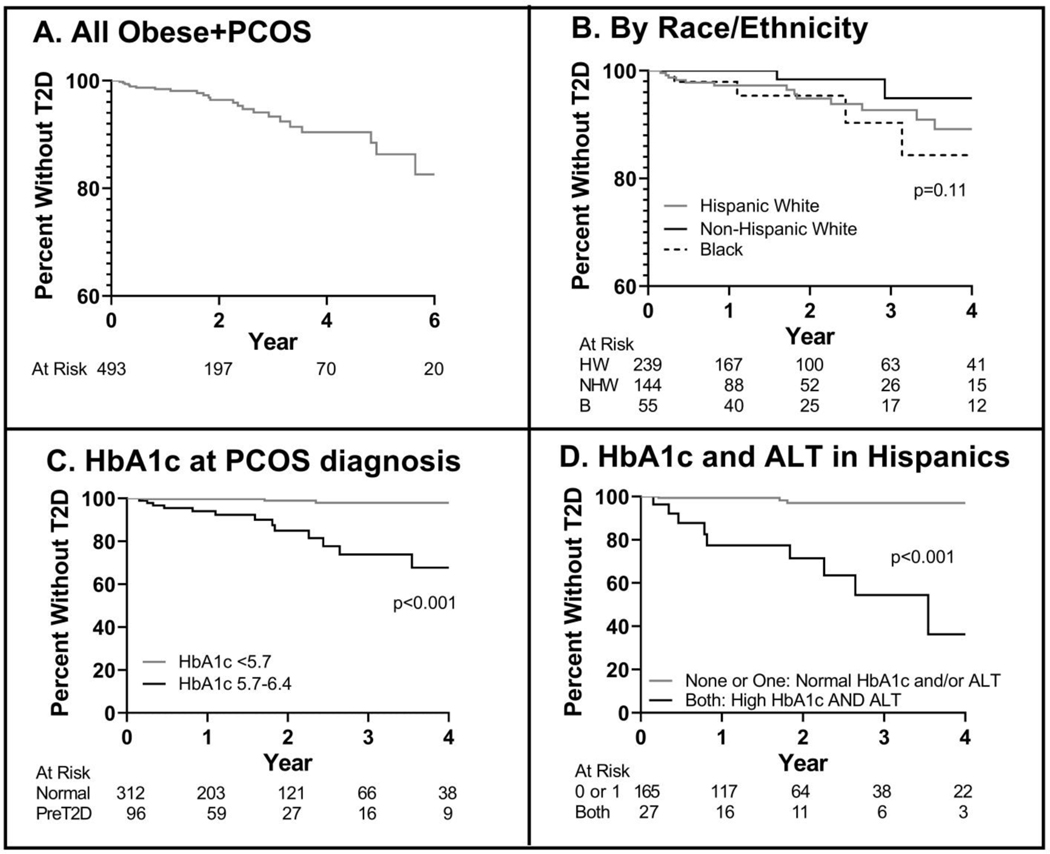
Both the PCOS only and PCOS+T2D groups were approximately 15 years of age at the time of PCOS diagnosis, and mean age of diagnosis of T2D was age 17 years (Table 1). Only 19 without T2D and 1 who developed T2D were > 18 years at time of PCOS diagnosis. The T2D group had a higher BMI (p=0.016) and BMI Z-score at time of PCOS diagnosis (p=0.006). Race was similar between groups, although those with T2D were more likely to be of Hispanic ethnicity (p=0.031). There was no difference in type of health insurance, personal history of OSA or family history of PCOS, T2D, obesity or OSA between the groups. Whereas there was no statistical difference in the initial clinic of presentation for PCOS, the rate of those seen in a primary care clinics (other/adolescent medicine) is < 25%, with most seen in endocrinology or gynecology clinics, and < 20% in specialty obesity referral clinics. When the cohort was stratified by length of follow-up, those with follow-up that was > than the median were on average 6 months younger, were more likely to receive care in the Adolescent Medicine Clinic and have an obese parent, and less likely to receive care in the Lifestyle Medicine Clinic. Otherwise, the PCOS only and PCOS+T2D groups were similar in terms of BMI Z-score, race/ethnicity, insurance status, rates of development of diabetes and HbA1c and ALT at diagnosis (data not shown). Medication data in terms of use of hormonal therapy or metformin could not be systematically quantified due to incomplete records that did not adequately document dose or adherence, multiple therapies tried over a duration of follow-up or contraception started by providers outside the EMR system. However, in a separate PCOS clinic analysis, we found that approximately 40% of girls with obesity and PCOS are prescribed metformin for management of PCOS (23).Time to development of T2D was estimated using a Kaplan-Meier (KM) survival curve (Figure 2A). Patients were stratified by Hispanic White, Non-Hispanic White and Non-Hispanic Black race/ethnicity (Figure 2B), with patients with no race/ethnicity reported or reported as Native American or Asian excluded for this analysis due to low numbers. There was no difference between the three KM curves divided by race/ethnicity (p=0.11). Patients were also stratified by HbA1c at the time of PCOS diagnosis (Figure 2C) as normal HbA1c (N=312) or prediabetes HbA1c (N=96). Sixty-five patients had only fasting or oral glucose tolerance test (OGTT) glucose samples collected and thus did not have a HbA1c at PCOS diagnosis. 24% of participants had a prediabetes-range HbA1c at the time of PCOS diagnosis, and they had a higher risk of developing T2D [HR 14.6 (4.8–44.5), p<0.0001]. In Figure 2D, the KM curves for the 27 Hispanic girls with both a prediabetes HbA1c and elevated ALT (defined as >32 IU/L) were significantly different, compared to the 165 Hispanic girls with neither or only one of elevated HbA1c and/or ALT [HR 18.9 (3.7–97.2); p<0.001].
Figure 2:
Kaplan-Meier Estimator of Survival without diabetes in Obese/Overweight Adolescents with PCOS
Kaplan-Meier curves are shown, with number at risk shown under the graphs. A) All participants with longitudinal data are shown B) Participants are stratified by race and ethnicity C) Patients are stratified by HbA1c category at time of PCOS diagnosis D) Hispanic patients with a HbA1c ≥ 5.7 % and ALT > 32 IU/L are compared to Hispanic girls with neither HbA1c or ALT elevated or just HbA1c OR ALT elevated.
Results from the case-control analysis are shown in Table 2. As designed, the cases of girls with PCOS who then developed T2D did not differ by age, BMI z-score, or race/ethnicity compared to the matched PCOS only cases. There was no statistical difference in length of follow-up between the groups (2.7±1.9 and 2.1±1.6 years in the PCOS only and PCOS + T2D groups, respectively). For every 1% increase in HbA1c at the time of PCOS diagnosis, there were 18.7 (2.3–153) higher odds of developing T2D. Other demographics and biomarkers were not different between the groups.
Table 2.
Risk factors for T2D in BMI, age and race/ethnicity matched cohort
| Matched PCOS only n = 69 |
PCOS + T2D n = 23 |
Odds Ratio (95% CI) | |
|---|---|---|---|
| Age at PCOS Diagnosis (years) | 15.6 ± 1.8 | 15.6 ± 2.0 | |
| BMI Z-score | 2.3 ± 0.3 | 2.4 ± 0.3 | |
| Non-Hispanic White | 6 (8.7%) | 2 (8.7%) | |
| Duration of Follow-up Past Medical History | 2.7±1.9 | 2.1±1.6 | |
| Obstructive sleep apnea | 14 (22%) | 8 (35%) | 1.49 (0.19–1.47) |
| Family History | |||
| PCOS | 3 (5%) | 2 (9%) | 0.50 (0.06–4.04) |
| Diabetes | 15 (24%) | (35%) | 0.58 (0.21–1.63) |
| Obesity | 15 (24%) | (39%) | 0.41 (0.13–1.30) |
| Obstructive sleep apnea | 0 (0%) | (13%) | n/a* |
| Gestational Diabetes | 0 (0%) | (17%) | n/a* |
| Clinical Markers (at PCOS diagnosis) | |||
| HbA1c (%) | 5.4 ± 0.4 | 5.8 ± 0.4 | 18.7 (2.27–153.24) |
| Triglyceride (mg/dL) | 135 ± 53 | 168 ± 76 | 1.01 (1.00–1.03) |
| HDL (mg/dL) | 41 ± 11 | 38 ± 9 | 0.82 (0.66–1.03) |
| ALT (IU/L) | 40 ± 39 | 53 ± 36 | 1.00 (0.98–1.02) |
| Free Testosterone (ng/dL) | 10.4 ± 7.4 | 12.4 ± 14.0 | 1.00 (0.94–1.07) |
3:1 matching of PCOS only to PCOS+diabetes cases based on BMI, race/ethnicity, and age at PCOS diagnosis. Data presented are mean ± standard deviation or frequency (%). PCOS only served as reference group for regression analysis. A 1% increase in HbA1c at PCOS diagnosis reflected the 18.7 odds ratio.
Model did not converge.
Discussion
We report that overweight and obese girls with PCOS are at increased risk for T2D compared to published rates. With the most recent estimates of T2D incidence among overweight or obese adults with PCOS ranging from 4.7–8.8/1,000 PYs(12), the estimated incidence of T2D among overweight or obese adolescents with PCOS of 22.6/1,000 PYs is nearly 3-fold greater. Adolescent girls with obesity and PCOS are also at much higher risk than the general U.S. youth population (10–18 years) for whom the incidence rate of T2D is 0.13/1,000 PYs according to the SEARCH for diabetes in youth data from 2012 (24). From this SEARCH cohort, the rate of T2D in adolescents in Colorado was 0.08/1,000 PY, and 0.16/1,000 PY among adolescent females. However, only 32% of youth in the SEARCH study are overweight or obese, and thus these estimates are lower than would be seen with an only obese population (25). Data from the United Kingdom were categorized by overweight or obese status, and the incidence of T2D was 1.26/1,000 PYs in overweight or obese youth in 2014, although the racial and ethnic make-up of this cohort is different and carries a lower risk for T2D (26). Therefore, even compared to other overweight or obese youth, the incidence of T2D is still higher in girls with PCOS.
Several studies have been performed to measure rates of dysglycemia in adolescents with PCOS. These studies confirm that dysglycemia is relatively common and presents early in the development of PCOS. The prevalence of impaired glucose tolerance (IGT) was 25% in a Chinese study of 20 overweight or obese girls with PCOS and 24% in a larger Canadian study of 117 girls of similar weight(16,17). In a mixed BMI cohort of 28 girls with PCOS of similar racial/ethnic composition as our study, 32% had IGT (27). These rates of dysglycemia are nearly twice that reported for adolescent girls 13.4 % (10.8–16.5) included in a recent NHANES study (28). Our rates of pre-diabetes showed that early evidence of dysglycemia was common at 24%, similar to other adolescent PCOS cohorts and is related to T2D development.
Similar to other obese populations, several known risk factors for T2D were identified including Hispanic ethnicity, more severe obesity, existing dysglycemia and evidence of elevated transaminases as a marker of NAFLD(29,30). Youth of Hispanic ethnicity are known to have increased risk for T2D, and it thus appears that this risk remains regardless of PCOS status (24,28,30). A prediabetes range HbA1c at the time of PCOS diagnosis was predictive for the development of T2D although we do not have the data to know if this is more sensitive than the more time intensive OGTT. Whereas elevated ALT was not independently related to T2D risk, the conversion to T2D was very high in girls of Hispanic ethnicity with both an elevated ALT and HbA1c. This finding is consistent with the literature in adults suggesting that NALFD is a predictor of future T2D (31).
Current ADA guidelines recommend that women with PCOS and obesity be considered a high risk group and screened annually for development of T2D if HbA1c is ≥5.7%, or every 3 years if HbA1c is in the normal HbA1c range (32). ADA guidelines for obese youth call for screening every 1–3 years (18). T2D screening guidelines for adult women with PCOS are also every 1–3 years (1,15,25). In the TODAY study, T2D progression to failure of a single agent occurred within a year, confirming the rapid progression of T2D in youth (19). In girls with PCOS and additional risk factors, 2 years may be too long of an interval for early detection and intervention implementation, given that the mean time to T2D development in our cohort was approximately 21 months from PCOS diagnosis. Girls with PCOS and additional risk factors for T2D (i.e. Hispanic ethnicity, more severe obesity, family history of gestational diabetes, HbA1c ≥ 5.7%, ALT > 32 IU/L) may benefit from shorter screening intervals and more aggressive interventions to prevent progression to T2D.
Our risk predictors for T2D of HbA1c and ALT are also not part of the routine screening procedures for obese youth listed in the most recent PCOS guidelines (1). Whereas the Endocrine Society guidelines recommend an OGTT or an HbA1c for diabetes screening, more recent PCOS guidelines recommend repeated OGTT’s in the setting of obesity (1,13). However, our data indicate that a pre-diabetes HbA1c is predictive of future T2D in this patient cohort, and conversely, a normal HbA1c was overall reassuring against the development of future T2D. The choice to emphasize an OGTT in the PCOS guidelines is based on several studies which demonstrated dysglycemia with an OGTT in the setting of a “normal” HbA1c, and thus the concern that waiting for a change in HbA1c will miss early dysglycemia, and delay initiation of metformin therapy. However, metformin can also be prescribed without evidence of dysglycemia. Further study is needed to determine if HbA1c can be utilized for screening for dysglycemia in this patient population, as this test is cheaper, easier, less time intensive and more reproducible than an OGTT, especially in youth (33,34). Neither set of PCOS guidelines recommends routine screening for NAFLD, although the condition is discussed in the Endocrine Society guidelines. However, for this pediatric patient population with obesity, routine screening with ALT is recommended by American Academy of Pediatrics general obesity or North American Society of Pediatric Gastroenterology, Hepatology and Nutrition NAFLD guidelines (35,36).
Our study has several strengths and weaknesses. The main strength is the relatively large population of overweight or obese adolescents with PCOS. Unlike other studies that depended upon self-report of PCOS, T2D, and BMI, the cases of PCOS and T2D described here were all objectively measured and confirmed by a pediatric endocrinologist in accordance with Endocrine Society and ADA standards. Another strength is that the exact age at diagnosis of PCOS and T2D was known from the dates listed in the chart, and that longitudinal data on glycemic progression were available. Whereas age of diagnosis does not equal age of symptom development, this is still a useful marker for clinicians who are making a new diagnosis of PCOS. Limitations of this study include the single center scope, which limits the diversity of the population included, although our center does care for patients from a seven-state referral area. Given our geographic location, nearly half of the population included was of Hispanic ethnicity, primarily Mexican descent. An overrepresentation of adolescents of Hispanic ethnicity included in the study relative to the general population could inflate the T2D incidence observed, especially as it relates to the SEARCH cohort, which was 23.4% Hispanic (24). Because our center is a large T2D referral center with a multi-state catchment area, there may also be a referral bias that increases the prevalence of T2D. However, the proportion of initial appointments in the Lifestyle Medicine clinic were the same in the PCOS-only and the PCOS+T2D group, limiting the likelihood of significant referral bias affecting the incidence results, and our rates of pre-diabetes are similar to that seen in other PCOS cohorts. Most PCOS diagnoses were made in gynecology or endocrinology clinics, with less than 20% in primary care clinics. It is possible that the incidence is even higher than reported, as the median follow-up was 1.5 years, and if we had more years of follow-up on all patients we could have even more cases of T2D. Finally, an ideal comparator group of obese girls of a similar age without PCOS has not yet been published and frequent screening for T2D is not regularly advised in asymptomatic obese youth, so non-research clinical cohorts do not exist. An attempt of a chart review of girls with obesity to match these data would require thousands of patients, due to the low prevalence of diabetes, and even then, the results may be biased as repeated screening for T2D is often performed only when the clinician has a concern for T2D development.
There are several additional challenges inherent to any retrospective study of data collected for clinical care purposes. We acknowledge that the dependence upon ICD 9/10 codes for initial chart identification may mean that we missed cases eligible for inclusion. The quality of the data were also dependent on physicians drawing the relevant laboratory studies as part of their routine clinical practice, and, as a result, not every patient had every data point at every visit, especially ALT, which is not part of the 2018 international PCOS guidelines. Further, 17% did not have an HbA1c at PCOS diagnosis, but rather a different assessment of glycemia. The quality of the medication data varied, with little documentation on adherence to prescribed medications and we were thus unable to assess the role, if any, of metformin or hormonal therapy on T2D development. However, there may have been a bias in these data even if it were available, as in our practice, metformin is typically advised for those at higher metabolic risk (23). Finally, patients who were lost to follow-up could not be tracked for development of T2D and the number of patients in the cohort decreased after 2 years. However, there were few clinical differences between those with shorter or longer durations of follow-up.
Conclusion
The risk of T2D in overweight and obese youth with PCOS is more than two-fold greater than that of obese women with PCOS and more than 18-fold greater than that of the general overweight and obese adolescent population. Girls with an elevated HbA1c at time of PCOS diagnosis and Hispanic ethnicity are at particular risk. Future multi-center studies are needed to confirm the incidence and prevalence of T2D among overweight and obese adolescents with PCOS, to determine the rates among healthy-weight girls with PCOS, and to compare these rates to the general adolescent population. Due to the worse outcomes associated with youth-onset T2D, these findings should prompt concern for greater metabolic health implications and potentially more intensive screening protocols for overweight and obese adolescents with PCOS.
Acknowledgements
JHB designed the study and data collection instruments, collected data, drafted the initial manuscript, and reviewed and revised the manuscript. JLK assisted with study design, data analysis, and reviewed and revised the manuscript. PZ reviewed and revised the manuscript. KJN, PZ, MMK, JSB assisted with study design and reviewed and revised the manuscript. LP designed the statistics, performed data analysis and reviewed and revised the manuscript. MCG conceptualized the study, assisted with study design, supervised data collection, performed analysis and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding Source: Dr. Cree-Green is supported by the National Institute of Diabetes and Digestive and Kidney Diseases grant K23DK107871.
This work was presented in poster form at the 2019 annual American Diabetes Association meeting.
Abbreviations:
- PCOS
polycystic ovary syndrome
- IR
insulin resistance
- T2D
type 2 diabetes
- PYs
person years
- ADA
American Diabetes Association
- IGT
impaired glucose tolerance
- EMR
electronic medical record
- BMI
body mass index
- HbA1c
hemoglobin A1c
- OSA
obstructive sleep apnea
- HDL
high density lipoprotein
- ALT
alanine aminotransferase
- GDM
gestational diabetes mellitus
- NHANES
National Health and Nutrition Examination Survey
Footnotes
Disclosure: Dr. Cree-Green has received research product from AminoCorp LLC and is on advisory board for NovoNordisk. The other authors have no potential conflicts of interest to disclose.
Data Availability:
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1.Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ, International PN. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):1602–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel SS, Truong U, King M, Ferland A, Moreau KL, Dorosz J, Hokanson JE, Wang H, Kinney GL, Maahs DM, Eckel RH, Nadeau KJ, Cree-Green M. Obese adolescents with polycystic ovarian syndrome have elevated cardiovascular disease risk markers. Vasc Med. 2017:1358863X16682107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cree-Green M, Rahat H, Newcomer BR, Bergman BC, Brown MS, Coe GV, Newnes L, Garcia-Reyes Y, Bacon S, Thurston JE, Pyle L, Scherzinger A, Nadeau KJ. Insulin Resistance, Hyperinsulinemia, and Mitochondria Dysfunction in Nonobese Girls With Polycystic Ovarian Syndrome. J Endocr Soc. 2017;1(7):931–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cree-Green M, Newcomer BR, Coe G, Newnes L, Baumgartner A, Brown MS, Pyle L, Reusch JE, Nadeau KJ. Peripheral insulin resistance in obese girls with hyperandrogenism is related to oxidative phosphorylation and elevated serum free fatty acids. American journal of physiology Endocrinology and metabolism. 2015;308(9):E726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cree-Green M, Bergman BC, Coe GV, Newnes L, Baumgartner AD, Bacon S, Sherzinger A, Pyle L, Nadeau KJ. Hepatic Steatosis is Common in Adolescents with Obesity and PCOS and Relates to De Novo Lipogenesis but not Insulin Resistance. Obesity (Silver Spring). 2016;24(11):2399–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barcellos CR, Rocha MP, Hayashida SA, Nery M, Marcondes JA. Prevalence of abnormalities of glucose metabolism in patients with polycystic ovary syndrome. Arquivos brasileiros de endocrinologia e metabologia. 2007;51(4):601–605. [DOI] [PubMed] [Google Scholar]
- 7.Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi MN, Group PCTS. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. The Journal of clinical endocrinology and metabolism. 2006;91(1):48–53. [DOI] [PubMed] [Google Scholar]
- 8.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38(9):1165–1174. [DOI] [PubMed] [Google Scholar]
- 9.Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Human reproduction update. 2010;16(4):347–363. [DOI] [PubMed] [Google Scholar]
- 10.Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in glucose tolerance over time in women with polycystic ovary syndrome: a controlled study. The Journal of clinical endocrinology and metabolism. 2005;90(6):3236–3242. [DOI] [PubMed] [Google Scholar]
- 11.Norman RJ, Masters L, Milner CR, Wang JX, Davies MJ. Relative risk of conversion from normoglycaemia to impaired glucose tolerance or non-insulin dependent diabetes mellitus in polycystic ovarian syndrome. Hum Reprod. 2001;16(9):1995–1998. [DOI] [PubMed] [Google Scholar]
- 12.Kakoly NS, Earnest A, Teede HJ, Moran LJ, Joham AE. The Impact of Obesity on the Incidence of Type 2 Diabetes Among Women With Polycystic Ovary Syndrome. Diabetes care. 2019. [DOI] [PubMed] [Google Scholar]
- 13.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK, Endocrine S. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2013;98(12):4565–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E, American Association of Clinical E, American College of E, Androgen E, Society P. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and Pcos Society Disease State Clinical Review: Guide to the Best Practices in the Evaluation and Treatment of Polycystic Ovary Syndrome - Part 2. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2015;21(12):1415–1426. [DOI] [PubMed] [Google Scholar]
- 15.Ibanez L, Oberfield SE, Witchel S, Auchus RJ, Chang RJ, Codner E, Dabadghao P, Darendeliler F, Elbarbary NS, Gambineri A, Garcia Rudaz C, Hoeger KM, Lopez-Bermejo A, Ong K, Pena AS, Reinehr T, Santoro N, Tena-Sempere M, Tao R, Yildiz BO, Alkhayyat H, Deeb A, Joel D, Horikawa R, de Zegher F, Lee PA. An International Consortium Update: Pathophysiology, Diagnosis, and Treatment of Polycystic Ovarian Syndrome in Adolescence. Hormone research in paediatrics. 2017;88(6):371–395. [DOI] [PubMed] [Google Scholar]
- 16.Coles N, Bremer K, Kives S, Zhao X, Hamilton J. Utility of the Oral Glucose Tolerance Test to Assess Glucose Abnormalities in Adolescents with Polycystic Ovary Syndrome. Journal of pediatric and adolescent gynecology. 2016;29(1):48–52. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Chen X, He Z, Zhao X, Huang L, Yang D. Clinical and metabolic features of polycystic ovary syndrome among Chinese adolescents. Journal of pediatric and adolescent gynecology. 2012;25(6):390–395. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes A. 13. Children and Adolescents: Standards of Medical Care in Diabetes-2019. Diabetes care. 2019;42(Suppl 1):S148–S164. [DOI] [PubMed] [Google Scholar]
- 19.TODAY, Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, Arslanian S, Cuttler L, Nathan DM, Tollefsen S, Wilfley D, Kaufman F. A clinical trial to maintain glycemic control in youth with type 2 diabetes. The New England journal of medicine. 2012;366(24):2247–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynolds K, Saydah SH, Isom S, Divers J, Lawrence JM, Dabelea D, Mayer-Davis EJ, Imperatore G, Bell RA, Hamman RF. Mortality in youth-onset type 1 and type 2 diabetes: The SEARCH for Diabetes in Youth study. Journal of diabetes and its complications. 2018;32(6):545–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bacha F, Cheng P, Gal RL, Kollman C, Tamborlane WV, Klingensmith GJ, Manseau K, Wood J, Beck RW, for the Pediatric Diabetes C. Initial Presentation of Type 2 Diabetes in Adolescents Predicts Durability of Successful Treatment with Metformin Monotherapy: Insights from the Pediatric Diabetes Consortium T2D Registry. Hormone research in paediatrics. 2018;89(1):47–55. [DOI] [PubMed] [Google Scholar]
- 22.Kim G, Divers J, Fino NF, Dabelea D, Lawrence JM, Reynolds K, Bell RA, Mayer-Davis E, Crume T, Pettitt DJ, Pihoker C, Liu L. Trends in prevalence of cardiovascular risk factors from 2002 to 2012 among youth early in the course of type 1 and type 2 diabetes. The SEARCH for Diabetes in Youth Study. Pediatric diabetes. 2019;20(6):693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torres-Zegarra C, Sundararajan D, Benson J, Seagle H, Witten M, Walders-Abramson N, Simon SL, Huguelet P, Nokoff NJ, Cree-Green M. Care for Adolescents with PCOS: development and prescribing patterns of a multidisciplinary clinic Journal of pediatric and adolescent gynecology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, Imperatore G, Linder B, Marcovina S, Pettitt DJ, Pihoker C, Saydah S, Wagenknecht L, Study SfDiY. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. The New England journal of medicine. 2017;376(15):1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhupathiraju SN, Hu FB. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circulation research. 2016;118(11):1723–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbasi A, Juszczyk D, van Jaarsveld CHM, Gulliford MC. Body Mass Index and Incident Type 1 and Type 2 Diabetes in Children and Young Adults: A Retrospective Cohort Study. J Endocr Soc. 2017;1(5):524–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cree-Green M, Cai N, Thurston JE, Coe GV, Newnes L, Garcia-Reyes Y, Baumgartner AD, Pyle L, Nadeau KJ. Using simple clinical measures to predict insulin resistance or hyperglycemia in girls with polycystic ovarian syndrome. Pediatric diabetes. 2018;19(8):1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andes LJ, Cheng YJ, Rolka DB, Gregg EW, Imperatore G. Prevalence of Prediabetes Among Adolescents and Young Adults in the United States, 2005–2016. JAMA pediatrics. 2019:e194498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Love-Osborne KA, Sheeder JL, Nadeau KJ, Zeitler P. Longitudinal follow up of dysglycemia in overweight and obese pediatric patients. Pediatric diabetes. 2018;19(2):199–204. [DOI] [PubMed] [Google Scholar]
- 30.Andrisse S, Garcia-Reyes Y, Pyle L, Kelsey MM, Nadeau KJ, Cree-Green M. Racial and ethnic differences in metabolic disease in adolescents with obesity and polycystic ovary syndrome. J Endocr Soc. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantovani A, Byrne CD, Bonora E, Targher G. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-analysis. Diabetes care. 2018;41(2):372–382. [DOI] [PubMed] [Google Scholar]
- 32.American Diabetes A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes care. 2019;42(Suppl 1):S13–S28. [DOI] [PubMed] [Google Scholar]
- 33.Libman IM, Barinas-Mitchell E, Bartucci A, Robertson R, Arslanian S. Reproducibility of the oral glucose tolerance test in overweight children. The Journal of clinical endocrinology and metabolism. 2008;93(11):4231–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeckel CW, Weiss R, Dziura J, Taksali SE, Dufour S, Burgert TS, Tamborlane WV, Caprio S. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. The Journal of clinical endocrinology and metabolism. 2004;89(3):1096–1101. [DOI] [PubMed] [Google Scholar]
- 35.Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, Mouzaki M, Sathya P, Schwimmer JB, Sundaram SS, Xanthakos SA. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). Journal of pediatric gastroenterology and nutrition. 2017;64(2):319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barlow SE, Expert C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120 Suppl 4:S164–192. [DOI] [PubMed] [Google Scholar]