Summary
Background
Shortages of component two of Sputnik V vaccine (rAd5) are delaying the possibility of achieving full immunisation. The immunogenic response associated with the use of alternative schemes to complete the scheme was not explored.
Methods
We did two non-inferiority randomized clinical trials with outcomes measures blinded to investigators on adults aged 21–65 years, vaccinated with a single dose of rAd26 ≥ 30 days before screening and no history of SARS-CoV-2. Participants were assigned (1:1:1:1:1) to receive either rAd5; ChAdOx1; rAd26; mRNA-1273 or BBIBP-CorV. The primary endpoint was the geometric mean ratio (GMR) of SARS-CoV-2 anti-spike IgG concentration at 28 days after the second dose, when comparing rAd26/rAd5 with rAd26/ChAdOx1, rAd26/rAd26, rAd26/mRNAmRNA-1273 and rAd26/BBIBP-CorV. Serum neutralizing capacity was evaluated using wild type SARS-CoV-2 reference strain 2019 B.1. The safety outcome was 28-day rate of serious adverse. The primary analysis included all participants who received ≥ 1 dose. The studies were registered with NCT04962906 and NCT05027672. Both trials were conducted in Buenos Aires, Argentina.
Findings
Between July 6 and August 3, 2021, 540 individuals (age 56·7 [SD 7·3]; 243 (45%) women) were randomly assigned to received rAd5 (n=150); ChAdOx1 (n=150); rAd26 (N=87); mRNAmRNA-1273 (n=87) or BBIBP-CorV (n=65). 524 participants completed the study. As compared with rAd26/rAd5 (1·00), the GMR (95%CI) at day 28 was 0·65 (0·51–0·84) among those who received ChAdOx1; 0·47 (0·34–0·66) in rAd5; 3·53 (2·68–4·65) in mRNA-1273 and 0·23 (0·16–0·33) in BBIBP-CorV. The geometric mean (IU/ml) from baseline to day 28 within each group increased significantly with ChAdOx1 (4·08 (3·07–5·43)); rAd26 (2·69 (1·76–4·11)); mRNA-1273 (21·98 (15·45–31·08)) but not in BBIBP-CorV (1·22 (0·80–1·87)).
Interpretation
Except for mRNA-1273 which proved superior, in all other alternatives non-inferiority was rejected. Antibody concentration increased in all non-replicating viral vector and RNA platforms.
Funding
The trials were supported (including funding, material support in the form of vaccines and testing supplies) by the Buenos Aires City Government.
Keywords: COVID-19, Randomised clinical trial, Public health, Vaccines
Research in context.
Evidence before this study
Russia's Sputnik V vaccine has proven its efficacy in a large clinical trial. By October 2021, at least 75 countries with an estimated population of 4 billion people have approved its emergency use. However, low production of the second component (rAd5) severely limits the possibility of immunising the population.
Although there is evidence for the efficacy of combining vaccines based on non-replicating viral vector platforms with those based on mRNA technology, a search of PubMed/Medline, EMBASE and Cochrane up to 10/1/2021 using the terms (COVID-19) AND (heterologous) AND (vaccin*) found a total of 129 papers of which only one referred to the Sputnik V vaccine and which corresponded to the phase III report of the original trial. A further search expanding to terms including 'COVID-2019,' 'severe acute respiratory syndrome coronavirus 2,' '2019- nCoV,' 'vaccination,' 'BNT,' 'AstraZeneca,' 'Moderna,' 'Janssen,' 'mRNA, ' 'adenovirus vector,' 'heterologous,' 'Sputnik V,' 'Sinopharm,'and 'boost' with Boolean operators and MeSH terms found no results addressing alternatives for replacement of component 2 of the Sputnik V vaccine.
Added value of this study
We report for the first time using two randomised clinical trials, the safety and immunogenicity of the replacement of the second component of Sputnik V using either non-replicating viral vectors, mRNA-based vaccines or inactivated virus platforms. At 28 days post-randomisation, all platforms but inactivated virus significantly increased anti-S and neutralizing antibody titres. The RNA platform was significantly superior and the inactivated virus platform significantly inferior to the combination of the two doses of Sputnik V. In the case of the non-replicative viral platforms, non-inferiority could not be rejected, although the use of these platforms was associated with a significant increase in antibody titres. No safety concerns were raised.
Implications of all the available evidence
The results show that the second component of Sputnik V can be substituted with an mRNA platform. Although increases in antibody titres were observed using non-replicative viral platforms, these did not reach the established limit of non-inferiority. In a context of vaccine shortages and the emergence of new and more contagious viral mutations, these results contribute to decision-making by health agencies. Furthermore, this evidence suggests that a change of vaccine platform may be considered as an important option for additional boost programs.
Alt-text: Unlabelled box
Introduction
Argentina as well as at least 71 other countries around the world have relied on the Russian vaccine Sputnik V to play an important role in the COVID vaccination campaign. The efficacy and safety of the Russian vaccine - composed of a heterologous regimen of two non-replicating viral vectors rAd26 and rAd5 administered separately for a period of at least 21 days - is supported by a large clinical trial1 and the amount of supporting evidence on its efficacy is robust.2 Local implementation studies have confirmed effectiveness.3 However, the availability of the second component of the Sputnik V vaccine is critically low.4 This shortage means that the number of people waiting for the second component is steadily increasing and consequently the rate of immunisation with two doses is slow. In a context of uncertainty due to the arrival of more contagious variants, the need to vaccinate as many people as possible with two doses cannot be postponed.
Although there are clinical trials that studied the combination of non-replicative virus platforms with mRNA vaccines,5,6 these trials did not test the Sputnik V vaccine or complement its administration with other non-RNA platforms. In Argentina, the vaccination campaign also uses the vaccine produced by the Astra Zeneca Consortium/Oxford University (ChAdOx1), the inactivated virus vaccine produced by the Beijing Institute of Biological Products - People's Republic of China (BBIBP-CorV) and, recently, the US government-donated mRNA-1273 COVID-19 (Moderna Switzerland GmbH). All of these have been shown to be safe and effective in large clinical trials.1,7, 8, 9 Whether replacing the Sputnik V 2 component with any of these other vaccines would be safe and effective is still unknown.
Accordingly, we did two randomised controlled trials to determine whether the immune responses to alternative schedules to deal with Sputnik V shortage are noninferior to the standard regimen.
Methods
Study design
The NCT04983537 (Trial A) and NCT05027672 (Trial B) clinical trials were two, single-center, randomized, open, non-inferiority trials with primary outcome assessment blind to the researcher that were designed and conducted by the Ministry of Health of the City of Buenos Aires (Argentina). The trials were supported (including funding, material support in the form of vaccines and testing supplies) by the Buenos Aires City Government. The enrollment site was located at the Ministry of Health headquarters, where there is a vaccinatory with the capacity to perform the vaccine inoculations and process the blood samples of all volunteers. An institutional review board (Hospital Ricardo Gutierrez) reviewed and approved both trial protocols. The protocols of both clinical trials are provided in the Supplementary files.
Study population
Both clinical trials enrolled individuals of both sexes aged ≥ 21 and ≤ 65 years who had received a dose of the first component of Sputnik V (rAd26) vaccine at least 30 days before randomization and were awaiting a second dose. All participants had to meet the inclusion criteria and none of the exclusion criteria and provided written informed consent.
In Trial A (NCT04962906) participants were recruited if they had received the first dose of vaccine within a period of more than 30 days and less than or equal to 90 days. In Trial B (NCT05027672), the inclusion and exclusion criteria were the same as in the previous trial. However, in this second clinical trial, the participants had to have received the first dose within a period longer than 30 days, but with no upper limit. Thus, the only difference between the two trials was the maximum time elapsed since the first dose. This only difference between the two trials was made for reasons of feasibility. At the time of enrollment for Trial B - one month after the first trial - the number of people less than 90 days since the first dose was low due to the backlog of people still waiting for the second dose.
The main exclusion criteria for both clinical trials included a known history of COVID in the last 6 months; being pregnant or breastfeeding; use of systemic corticosteroids in the last 30 days; known history of allergy to any vaccine; history of anaphylaxis; known history of autoimmune disease; cancer treatment in the last 6 months; having any medical procedure scheduled that could jeopardize the 14th and 28th post-randomisation visits; and any disease or condition that, in the investigator's opinion, could jeopardize participation in the study.
Randomization and masking
In Trial A individuals were randomized in a 1:1:1 ratio to receive the second component of the Sputnik V vaccine (rAd5), ChAdOx1 (Astra Zeneca) or BBIBP-CorV (SinoPharm).
In Trial B, participants were randomized in a 1:1:1:1 ratio to receive the second component of Sputnik V vaccine (rAd5), ChAdOx1 (Astra Zeneca), the first component of Sputnik V (rAd26) or mRNA-1273 COVID-19 (Moderna).
All participants were vaccinated by health-care personnel who were aware of group allocation but were not otherwise involved in trial procedures or data collection. The patients were also aware of the arm of the study to which they were assigned. The study investigators who performed all antibody measurements were blinded to the patients' arm of allocation. A randomisation list was prepared by the statistician responsible for the study, blind to the researchers. Patients were randomised by 1: 1: 1 in trial A and 1:1:1:1 in trial B allocation using stratified randomisation by randomised permuted blocks. Randomisation was stratified by age (people ≥ 60 and < 60 years) and by time since the first vaccination dose (≥ 45 and < 45 days).
Procedures
Both clinical trials were announced in the public mass media and the Government set up an enrollment registry on its web portal. From this registry, a random fraction was invited to participate through a telephone recruitment where the inclusion and exclusion criteria were checked. Participants who accepted the invitation were sent information by text message explaining the nature of the study and were invited to make a baseline visit (day 0). Of these, participants who passed the final eligibility assessment and provided written informed consent were randomly assigned to a study group. At the time of treatment assignment, all participants were effectively vaccinated, and blood was drawn to check for laboratory safety, anti-S antibody measurement and neutralizing antibody titers. During the baseline visit, participants were given a diary with instructions to record adverse events. The vaccines used in the trial included the rAd26/rAd5, consisting of two adenovirus vectors (recombinant Ad26 [rAd26] and Ad5 [rAd5]) both containing the gene coding for the SARS-CoV-2 glycoprotein; the Oxford-AstraZeneca vaccine COVID-19 (ChAdOx1) which uses as a vector a modified adenovirus containing the full-length codon-optimised coding sequence of SARS-CoV-2 spike protein along with a tissue plasminogen activator (tPA) leader sequence; the BBIBP-CorV (Sinopharm) vaccine, consisting of inactivated SARS-CoV-2 virus antigens; and the nucleoside-modified mRNA in the Moderna COVID‑19 Vaccine, which enables delivery of the nucleoside‑modified mRNA into host cells to allow expression of the SARS‑CoV‑2 Spike antigen.
Vaccines were administered by appropriately trained trial staff. Participants were observed for at least 30 min after vaccination.
All participants were contacted by telephone on days 1, 5, 10 and 20 after vaccination. During the telephone interview, trained investigators from the Ministry of Health collected information on the occurrence of solicited and unsolicited local or systemic adverse reactions. In addition, participants were invited for two in-person visits on days 14 and 28 post-vaccination. At the in-person visits, participants underwent a blood draw to measure antibody levels, a safety laboratory evaluation (hematocrit, hemoglobin, white blood cells, platelets, creatinine and liver functions test including bilirubin, aspartate transaminase (SGOT) and alanine transaminase (SGPT)), and neutralizing antibody titers. Anti S IgG SARS-CoV-2 was processed using a previously described enzyme-linked immunoabsorbent microplate assay (COVIDAR – Laboratorio Lemos SRL, Buenos Aires, Argentina) which has include the WHO International Standards, allowing the quantitation in international units (IU/ml).10 In addition, serum samples from all patients were also analized by a chemiluminescent microparticle immunoassay for the quantitative detection of IgG antibodies against the receptor binding domain (RBD) of the S1 subunit of the spike protein (CMIA, SARS-CoV-2 IgG II Quant Architect, Abbott Laboratories Inc.) which reports antibody levels using arbitrary units per milliliter (AU/ml).11 Both assays were performed following the manufacturer's instructions.
Serum neutralizing capacity was assessed using the ancestral SARS-CoV-2 reference strain 2019 B.1 (GISAID Accession ID: EPI_ISL_499083). Serum samples were heat-inactivated (56°C, 30 min) and serial dilutions from 1/2 to 1/8192 were incubated for 1h at 37°C with the ancestral variant of SARS-CoV-2 in DMEM 2% FBS. Fifty µl of the mixtures were then cultured with Vero cell monolayers for 1 h at 37°C (MOI = 0.01). The media was then removed and replaced by DMEM 2% FBS. After 72 h, cells were fixed with PFA 4% (4°C, 20 min) and stained with crystal violet solution in methanol. The cytopathic effect of the virus on the cell monolayer was then analyzed and the neutralization titer was defined as the highest serum dilution capable of preventing any cytopathic effect.
Outcomes
The primary endpoint was the geometric mean concentration ratio (calculated as the antilogarithm of the difference between the mean of the log10 transformed SARS-CoV-2 anti-spike IgG in the heterologous group and that in the homologous group (as the reference), at 28 days between the standard two-dose Sputnik V regimens versus that obtained with each of the alternative regimens.
We report the combined and specific rate of serious adverse events; defined as death for any reason, any life-threatening event or any event that require inpatient hospitalization at 28-day.
Secondary endpoints included the rate of adverse events of any type and those considered moderate or severe (those resulted in death, require either inpatient hospitalization or the prolongation of hospitalization, are life-threatening, result in a persistent or significant disability/incapacity or result in a congenital anomaly/birth defect) in each of the studies.
In addition, the study analyzes and reports total SARSCoV-2 anti-spike binding IgG concentration at 0, 14 and 28 days after randomization and antibody neutralization titres at days 0, 14, and 28.
Statistical analysis
Baseline patient characteristics were described using absolute counts and proportions for categorical variables; and means and standard deviations for numerical variables. To compare the differences, chi square tests were used for categorical variables and independent sample t-tests were used for numerical variables.
The primary analysis of SARS-CoV-2 anti-spike IgG was done at day 28 in a per-protocol analysis. The per-protocol analysis population consisted of all participants who had a result at 28 days and who did not have any protocol deviations (e.g., development of COVID during study participation).
The GMR was calculated as the antilogarithm of the difference between the mean of the log10 transformed SARS-CoV-2 anti-spike IgG in the heterologous groups and that in rAd26/rAd5 (as reference category). The GMRs were reported separately for participants with rAd26/rAd5 and those with each of the alternative platforms separately, with a one-sided 97.5% CI to adjust for multiple testing. The criterion for non-inferiority of alternative platforms compared with rAd26/rAd5 was for the lower limit of the one-sided 97·5% CI of the GMR to be greater than 0·67. Hence, the sample size was calculated considering for a non-inferiority analysis, with 90% power, one sided alpha error of 0.025, a SD for the Log10 antibody concentration of 0.3, and a lower limit of the GMR greater than 0.67 (according to WHO recommendations for vaccine non-inferiority trials), with a geometric mean ratio (GMR) between the rAd26/rAd5 and alternative platforms assumed to be one. Following these assumptions, the study needed to recruit at least 66 participants per group. Neutralizing antibody titers were analyzed after conversion to a logarithmic scale and differences in titer levels at 28 days were performed with the same procedures as described for anti-S antibody levels.
The alternative platforms were considered superior to rAd26/rAd5 if the lower limit of the two-sided 95% CI was greater than one, and the rAd26/rAd5 group was considered superior to the alternative treatments if the upper limit of the two-sided 95% CI was less than one. In the censored data reported below the lower limit of detection or lower limit of quantification were imputed with a value equal to one-fifth of the threshold before transformation. If a normal distribution could not be rendered after transformation, the Mann-Whitney U test was used. Correlations between different immunological outcomes were evaluated by Pearson correlation coefficients (data not shown).
Participants who received at least one dose of a study vaccine were included in the safety analysis. The proportion of participants with at least one safety event was reported by vaccine schedule. Fisher's exact test was used to compare the difference between groups if expected counts were less than 5 per cell. All statistical analyses were done using R version 4.1.0.
Role of the funding source
The funder, Ministerio de Salud de la Ciudad de Buenos Aires, designed and conducted the trial. Trial coordination, participant recruitment, and final data analysis were done by the researchers of the Ministerio de Salud de la Ciudad de Buenos Aires. Serum samples in which anti-spike antibody determinations were performed were analysed at the Hospital Francisco Javier Muñiz. Serum neutralising capacity assays were carried out at the Instituto de Investigaciones Biomédicas en Retrovirus y SIDA, Universidad de Buenos Aires, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Buenos Aires, Argentina.
Results
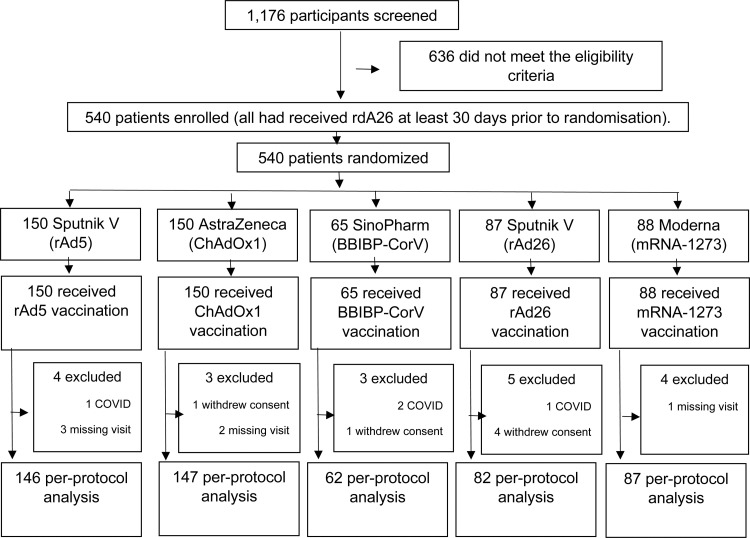
A total of 1176 participants were screened of whom 636 did not meet the eligibility criteria, and 540 participants were randomised into the two clinical trials (Figure 1). From July 6 to July 9, 2021, 192 people were enrolled in Trial A and from July 30 to August 3, 2021, a total of 348 people were recruited in Trial B. In total, 150 people were randomised to receive ChAdOx1, 88 to mRNA-1273 COVID-19, 65 to BBIBP-CorV, 87 to rAd26 and 150 to rAd5. A total of 524 participants were included in the per-protocol analysis (Figure 1). Three percent of the participants (n=16) could not be analysed on an intention-to-treat basis. Four participants developed COVID while participating in the study and 12 had no results at day 28 (6 due to withdrawal of informed consent and 6 due to missing the scheduled appointment).
Figure 1.
Flow chart of participants.
The mean age of participants was 56·7 years (SD 7·3), with 243 (45·0%) female participants. Baseline characteristics were well balanced across groups in both clinical trials (Table 1).
Table 1.
Baseline characteristics by clinical trial and overall.
| Trial A |
Trial B |
Both trials |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rAd26/ChAdOx1 | rAd26/BBIBP-CorV | rAd5/rAd26 | rAd26/ChAdOx1 | rAd26/mRNA-1273 | rAd26/rAd26 | rAd5/rAd26 | rAd26/ChAdOx1 | rAd26/mRNA-1273 | rAd26/BBIBP-CorV | rAd26/rAd26 | rAd5/rAd26 | |
| N | 64 | 65 | 63 | 86 | 88 | 87 | 87 | 150 | 88 | 65 | 87 | 150 |
| Age – mean (SD) | 56·3 (8) | 55·8 (8) | 55·5 (8) | 57·6 (7) | 57 (7) | 56·4 (7) | 57·5 (7) | 57 (7) | 57 (7) | 55·8 (8) | 56·4 (7) | 56·6 (8) |
| Weight – mean (SD) | 84·1 (20) | 78·6 (19) | 79·4 (19) | 78·9 (17) | 79·5 (19) | 82·1 (20) | 81 (18) | 81·1 (18) | 79·5 (19) | 78·6 (19) | 82·1 (20) | 80·4 (18) |
| Height– mean (SD) | 168 (9) | 168 (9) | 171 (9) | 169 (10) | 169 (10) | 170 (9) | 169 (9) | 169 (10) | 169 (10) | 168 (9) | 170 (9) | 170 (9) |
| Days from 1 dose– mean (SD) | 50 (12) | 50 (11) | 52 (12) | 74 (14) | 74 (16) | 74 (14) | 74 (14) | 63 (18) | 74 (16) | 50 (11) | 74 (14) | 65 (17) |
| Range from 1 dose– mean (SD) | 36-89 | 34-75 | 31-86 | 48-95 | 45-148 | 46-95 | 47-114 | 36-95 | 45-148 | 34-75 | 46-95 | 31-114 |
| ≥45 days from 1 dose– mean (SD) | 37 (58) | 38 (58) | 37 (59) | 86 (100) | 88 (100) | 87 (100) | 87 (100) | 123 (82) | 88 (100) | 38 (58) | 87 (100) | 124 (83) |
| Sex female – n (%) | 28 (44) | 32 (49) | 24 (38) | 40 (47) | 42 (48) | 41 (47) | 36 (41) | 68 (43) | 42 (48) | 32 (49) | 41 (47) | 60 (40) |
| Sex male– n (%) | 36 (56) | 33 (51) | 39 (62) | 46 (53) | 46 (52) | 46 (53) | 51 (57) | 82 (55) | 46 (52) | 33 (51) | 46 (53) | 90 (60) |
| Current smoker– n (%) | 24 (37) | 12 (18) | 18 (29) | 30 (35) | 26 (29) | 26 (21) | 29 (33) | 54 (36) | 26 (30) | 12 (18) | 26 (21) | 47 (31) |
| Cancer diagnosis– n (%) | 1 (2) | 2 (3) | 4 (6) | 2 (2) | 5 (6) | 6 (7) | 5 (6) | 3 (2) | 5 (6) | 2 (3) | 6 (7) | 9 (6) |
| Respiratory disease– n (%) | 8 (12) | 9 (14) | 8 (13) | 8 (9) | 2 (2) | 7 (8) | 4 (5) | 16 (11) | 2 (2) | 9 (14) | 7 (8) | 12 (8) |
Among participants who were randomised to receive as a second dose RdA5, anti-S titers (IU/mL) went from 93 at day 0 to 508 at day 28 (GMC rate = 1.00 reference category). Anti-S titers went from 81 to 332 (GMC ratio (95%CI) = 0.65 (0.51 to 0.84)) in participants receiving ChAdOx1; from 97 to 119 (GMC ratio=0.23 (0. 17 to 0.33) among those randomised to BBIBP-CorV; from 90 to 241 (GMC ratio=0.47 (0.34 to 0.66) for those receiving rAd26 and from 82 to 1793 (GMC ratio= 3.53 (2.68 to 4.65) for participants receiving mRNA-1273 (Table 2). All alternative treatments were statistically inferior to the standard regimen of rAd26/rAd5, with the exception of the group assigned to receive mRNA-1273, which proved to be statistically superior.
Table 2.
Immune responses at baseline, 14 and 28 days after second dose.
| Randomization group | Baseline |
14 day |
28 day |
||||
|---|---|---|---|---|---|---|---|
| GM | GMR between groups | GM | GMR between groups | GM | GMR between groups | GMR within groups | |
| rAd26/rAd5 | 92·6 | 1 | 512·9 | 1 | 508·34 | 1 | 5·49 (4·16 - 7·24) |
| rAd26/ChAdOx1 | 81·4 | 0·88 (0.65 - 1.19) | 363.6 | 0·71 (0·55 - 0·90) | 332·31 | 0·65 (0·51 - 0·84) | 4·08 (3·07 - 5·43) |
| rAd26/BBIBP-CorV | 97·2 | 1·05 (0.72 - 1.54) | 128.5 | 0·25 (0·18 - 0·35) | 118·76 | 0·23 (0·17 - 0·33) | 1·22 (0·80 - 1·87) |
| rAd26/rAd26 | 89·6 | 0·97 (0.68 - 1.39) | 278.6 | 0·54 (0·40 - 0·73) | 241·31 | 0·47 (0·34 - 0·66) | 2·69 (1·76 - 4·11) |
| rAd26/mRNA-1273 | 81·6 | 0·88 (0.66 - 1.25) | 2465.9 | 4·81 (3·67 - 6·29) | 1792·87 | 3·53 (2·68 - 4·65) | 21·98 (15·54 - 31·08) |
GMR=Geometric mean ratio; GM=Geometric mean.
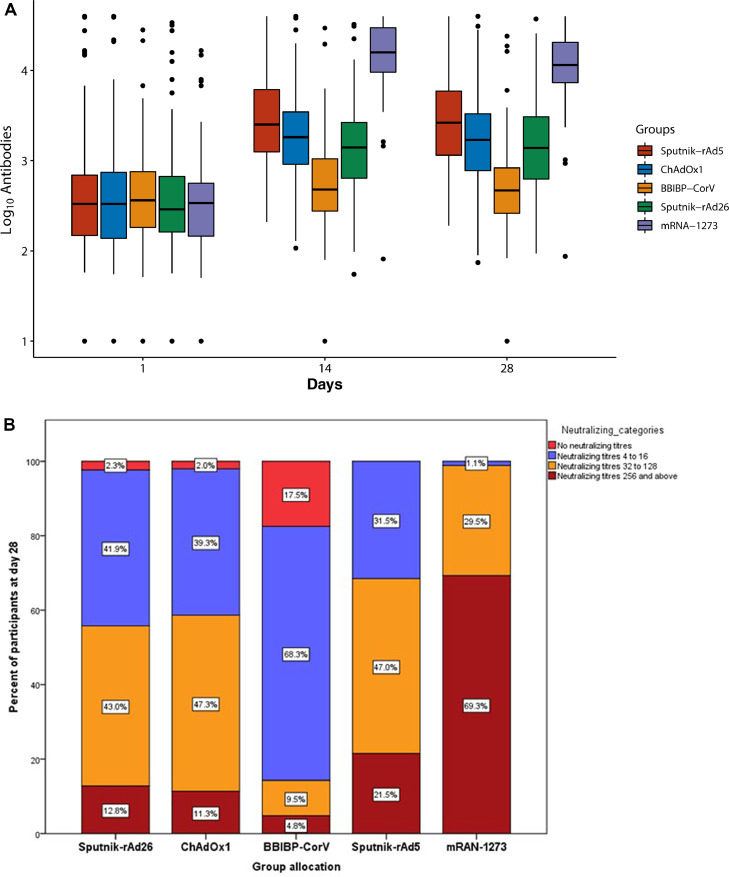
All vaccines significantly increased anti-S titers at 28 days after the second dose, except for the group randomized to BBIBP-CorV. The ratio between the geometric mean at day 28 and baseline within each treatment group was significantly increased among patients assigned to rAd26/rAd5 [5.49 (4.16–7.24)], rAd26/ChAdOx1 [4.01 (3.07–5.43)], rAd26/rAd26 [2.69 (1.76–4.11)], and rAd26/mRNA-1273 [21.98 (15.54–31.08)]. On the other hand, no significant increase in anti-S titres was observed among participants who received rAd26/BBIBP-CorV [1.22 (0.80-1.87)] (Table 2 and Figure 2A). The results were similar in all the subgroups analyzed as well as when the Architect platform was used for the assessments (data not shown).
Figure 2.
(A) Antibody titres (log10) at 0, 14 and 28 days between randomization groups; (B) Categories of neutralizing antibody titres at day 28 after second dose among the different randomization groups.
Compared to the standard rAd26/rAd5 scheme, neutralising antibody titres were significantly higher at day 28 in the group receiving rAd26/mRNA-1273 as second dose [5.83 (4.02 to 8.46)] and significantly lower in those receiving rAd26/ChAdOx1 [0.61 (0.43 to 0.86)], rAd26/rAd26 [0.56 (0.37 to 0.86)] and rAd26/BBIBP-CorV [0.11 (0.07 to 0.17)].
The highest concentration of neutralising titres (≥ 1/256) was observed in 69.3% of participants in the mRNA-1273 group and in 21.5%, 12.8%, 11.3% and 4.8% of participants in the rAd26/rAd5, rAd26/rAd26, rAd26/ChAdOx1 and rAd26/BBIBP-CorV groups, respectively. On the other hand, no neutralising antibodies titres were detected in 0% of the mRNA-1273 and rAd26/rAd5 arms; in 2.0% and 2.3% of the ChAdOx1 and rAd26/rAd26 patients, respectively; and in 17.5% of participants in the rAd26/BBIBP-CorV group at day 28 (Figure 2B).
There were no serious adverse events reported in any of the study arms during the 28-day duration of the trials, indicating all vaccines administered were safe. Reactogenesis was highest with the Moderna vaccine, followed by the non-replicating viral platforms. The inactivated virus vaccines had significantly lower reported reactions (Table 3).
Table 3.
Main adverse events reported.
| rAd26/rAd5 | rAd26/ChAdOx1 | rAd26/BBIBP-CorV | rAd26/rAd26 | rAd26/mRNA-1273 | p-value | |
|---|---|---|---|---|---|---|
| N | 150 | 150 | 65 | 87 | 88 | |
| Any adverse event at day 1 – n (%) | 103 (68·7) | 115 (76·7) | 25 (38·5) | 52 (59·8) | 77 (87·5) | <0·0001 |
| Local abscess | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Pain at the site of injection | 95 (63·4) | 105 (70·0) | 14 (21·5) | 43 (49·4) | 77 (88·5) | <0·0001 |
| Local erythema | 4 (2·7) | 6 (4·0) | 1 (1·5) | 2 (2·3) | 7 (8·0) | 0·181 |
| Injection site induration | 12 (8·0) | 13 (8·7) | 1 (1·5) | 1 (1·1) | 9 (10·2) | 0·037 |
| Mild - moderate headache | 40 (26·6) | 43 (28·6) | 11 (16·9) | 17 (19·5) | 20 (22·7) | 0·33 |
| Diarrhoea | 2 (1·3) | 3 (2·0) | 1 (1·5) | 1 (1·1) | 0 (0) | 0·776 |
| Fever | 7 (4·6) | 10 (6·7) | 0 (0) | 2 (2·3) | 9 (10·2) | 0·151 |
| Hypertension | 1 (0·7) | 0 (0) | 1 (1·5) | 0 (0) | 0 (0) | 0·412 |
| Grade II reaction (moderate) | 13 (8·7) | 14 (9·3) | 1 (1·5) | 1 (1·1) | 15 (17·2) | 0·001 |
| Any adverse event at day 5 – n (%) | 20 (13·3) | 27 (18·0) | 2 (3·1) | 11 (12·6) | 22 (25·0) | 0·003 |
| Local Abscess | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Pain at the site of injection | 17 (11·3) | 18 (12·1) | 0 (0) | 5 (5·7) | 12 (13·8) | 0·02 |
| Local erythema | 1 (0·7) | 3 (2·0) | 0 (0) | 0 (0) | 4 (4·6) | 0·062 |
| Injection site induration | 1 (0·7) | 2 (1·3) | 0 (0) | 0 (0) | 1 (1·1) | 0·724 |
| Mild - moderate headache | 5 (3·3) | 7 (4·7) | 3 (4·6) | 7 (8·0) | 9 (10·5) | 0·151 |
| Diarrhoea | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1·1) | 0·268 |
| Fever | 1 (0·7) | 0 (0) | 0 (0) | 0 (0) | 2 (2·3) | 0·112 |
| Grade II reaction (moderate) | 3 (2·0) | 1 (0·7) | 1 (1·5) | 1 (1·1) | 1 (1·1) | 0·895 |
| Any adverse event at day 10– n (%) | 4 (2·7) | 6 (4·0) | 1 (1·5) | 4 (4·6) | 7 (8·0) | 0·259 |
| Pain at the site of injection | 0 (0) | 2 (1·4) | 0 (0) | 0 (0) | 2 (2·3) | 0·217 |
| Injection site induration | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1·1) | 0·275 |
| Mild - moderate headache | 2 (1·3) | 3 (2·0) | 1 (1·5) | 4 (4·7) | 3 (3·4) | 0·415 |
| Diarrhoea | 2 (1·3) | 1 (0·7) | 0 (0) | 1 (1·1) | 1 (1·1) | 0·9 |
| Grade II reaction (moderate) | 1 (0·7) | 0 (0) | 0 (0) | 1 (1·1) | 1 (1·1) | 0·672 |
| Any adverse event at day 14– n (%) | 3 (2·0) | 3 (2·0) | 0 (0) | 0 (0) | 1 (1·1) | 0·529 |
| Pain at the site of injection | 1 (0·7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0·672 |
| Local erythema | 0 (0) | 1 (1·1) | 0 (0) | 0 (0) | 0 (0) | 0·678 |
| Mild - moderate headache | 1 (0·7) | 2 (1·4) | 1 (1·5) | 0 (0) | 1 (1·1) | 0·572 |
| Diarrhoea | 1 (0·7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0·674 |
| Grade II reaction (moderate) | 0 (0) | 0 (0) | 1 (1·5) | 0 (0) | 0 (0) | 0·406 |
For the results presented here, the control groups have been pooled across both trials, however, since some treatments are present only in one trial, many comparisons are not randomised. To check whether the pooled controls could be interfering with the results, we conducted a complementary analysis considering each trial separately and confirmed the results are unchanged when trials are analysed together or separately (Supplementary Table).
Discussion
Vaccine shortages are a global health problem. The perpetuation of this shortage threatens the right to health and increases the likelihood of new viral mutations that could prolong the pandemic. The main finding of the trials presented here is that neither non-replicative viral platforms nor those based on inactivated viruses are methodologically - at least in canonical terms - valid alternatives to substitute or interchange schemes for those who received a Sputnik V first dose. On the other hand, the Moderna vaccine results in higher titers of antibodies than any of the other alternatives. Additionally, the same conclusions are reached when examining the behavior of neutralizing antibodies, which appear to be highly predictive of immune protection from symptomatic SARS-CoV-2 infection.12
The reasons for these findings are still speculative. A lower response with inactivated virus vaccines is not surprising. Results from clinical trials,13 observational studies14 and pharmacological considerations15 support a lower response. In the case of non-replicative virus platforms, as the Astra Zeneca vaccine, it should be considered that the vaccine contains lower viral load compared to the Sputnik V.7,16 Despite this, its use was associated with a significant increase in the level of antibodies 28 days after the second dose, which - although lower than that conferred by the traditional schedule and by mRNAs - is significantly higher than the baseline antibody level. Whether this finding should authorize vaccine interchangeability in a context of shortage is controversial. From a strictly methodological point of view and following the guidelines of WHO recommendations,17 an inferior vaccine should not be approved or recommended as an alternative and, in fact, its approval could configure a new standard of lower quality with which new alternatives would be compared in the future, progressively lowering the reference standard.18 On the other hand, in the context of a pandemic with a high case fatality rate and marked inequity in access to vaccines, the recommendations might not conform to these standards.19
The shortage of vaccine puts millions of people around the world in a context of uncertainty about the emergence of new and more virulent variants of the virus. The solution to this problem has so far not been formally explored and, as far as we know, this is the first trial aimed at getting the first clues to the answers.
The problems surrounding the Sputnik V vaccine go far beyond a question of efficacy and availability. The publication of phase I/II and III trials in the Lancet early in 20211 promoted enormous visibility of the Russian vaccine, however, since then, there has been an overwhelming absence of data.
Our work has several limitations that should be acknowledged. First, antibody measurement does not necessarily correlate with clinical events. Although there is evidence that antibody levels can be used as correlates of protection,20 the results are still far from conclusive and the determination of what levels relate to reasonable clinical protection is still unknown.21 Second, the mechanisms underlying immunity cannot be reduced to the level of anti-S antibodies and neutralizing antibodies. Our work did not measure cell-mediated immunity and we cannot rule out that the different platforms may have different effects on this unmeasured aspect. The population studied was young and without major comorbidities, so our results are not representative of the general population but to a fraction of it. The results reported are the combination of two separate clinical trials. Therefore some comparisons are non-randomised. The separate results of each trial are reported in the supplementary table and the results are materially identical to those reported with the combination of the two trials.
In conclusion, the findings confirm and extend what has been reported in relation to the interchangeability efficacy of adenoviral vaccines with those based on RNA technology and not with other vaccines of the same platform or those based on inactivated viruses. These results emphasize the importance of the availability of vaccines to complete a full scientifically-proven effective vaccination scheme in the population.
Contributors
Analysis was done by AM and DF. The manuscript was drafted by AM, DF and FBQ, with all other authors editing the manuscript for intellectual content. AM and DF provided supervision and were responsible for the decision to submit the manuscript. AM and DF had full access to the data and verified the data. All authors always had access to the data whenever requested.
Data sharing statement
Individual patient data may be shared anonymously and upon reasonable request.
Declaration of interests
None of the participating authors declared any conflict of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lana.2022.100196.
Appendix. Supplementary materials
References
- 1.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nogrady B. Mounting evidence suggests Sputnik COVID vaccine is safe and effective. Nature. 2021;595:339–340. doi: 10.1038/d41586-021-01813-2. [DOI] [PubMed] [Google Scholar]
- 3.Macchia A., Ferrante D., Angeleri P., et al. Evaluation of a COVID-19 vaccine campaign and SARS-CoV-2 infection and mortality Among Adults Older than 60 years in a middle income country using Sputnik V, AstraZeneca and SinoPharm vaccines: an observational study. JAMA Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.30800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Covid: stalled Russian vaccines cause global anger. available at https://www.bbc.com/news/world-europe-58003893. Accessed 9 May 2021
- 5.Borobia A.M., Carcas A.J., Pérez-Olmeda M., et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398:121–130. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X., Shaw R.H., Stuart A.S.V., et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398:856–869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voysey M., Clemens S.A.C., Madhi S.A., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al Kaabi N., Zhang Y., Xia S., et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021;326:35–45. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ojeda D.S., Gonzalez Lopez Ledesma M.M., et al. Emergency response for evaluating SARS-CoV-2 immune status, seroprevalence and convalescent plasma in Argentina. PLOS Pathogens. 2001;17 doi: 10.1371/journal.ppat.1009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.English E., Cook L.E., Piec I., Dervisevic S., Fraser W.D., John W.G. Performance of the abbott SARS-CoV-2 IgG II quantitative antibody assay including the new variants of concern, VOC 202012/V1 (United Kingdom) and VOC 202012/V2 (South Africa), and first steps towards global harmonization of COVID-19 antibody methods. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.00288-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khoury D.S., Cromer D., Reynaldi A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 13.Wu Z., Hu Y., Xu M., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:803–812. doi: 10.1016/S1473-3099(20)30987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sauré D., O'Ryan M., Torres J.P., Zuniga M., Santelices E., Basso L.J. Dynamic IgG seropositivity after rollout of CoronaVac and BNT162b2 COVID-19 vaccines in Chile: a sentinel surveillance study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burrell C.J., Howard C.R., Murphy F.A. Vaccines and vaccination. Fenner White's Med Virol. 2017:155–167. doi: 10.1016/b978-0-12-375156-0.00011-4. [DOI] [Google Scholar]
- 16.van Doremalen N., Lambe T., Spencer A., et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586:578–582. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO. Guidelines on clinical evaluation of vaccines: regulatory expectations. 2016. https://www.who.int/biologicals/BS2287_Clinical_guidelines_final_LINE_NOs_20_July_2016.pdf. Accessed 17 September 2021.
- 18.Krause P.R., Fleming T.R., Longini I.M., et al. SARS-CoV-2 variants and vaccines. N Engl J Med. 2021;385:179–186. doi: 10.1056/NEJMsr2105280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodgson S.H., Mansatta K., Mallett G., Harris V., Emary K.R.W., Pollard A.J. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis. 2021;21:e26–e35. doi: 10.1016/S1473-3099(20)30773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Earle K.A., Ambrosino D.M., Fiore-Gartland A., et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39:4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krammer F. A correlate of protection for SARS-CoV-2 vaccines is urgently needed. Nat Med. 2021;27:1147–1148. doi: 10.1038/s41591-021-01432-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.