Abstract
Background
Emergency department (ED) workers have an increased seroprevalence of SARS-CoV-2 antibodies. However, breakthrough infections in ED workers have led to a reduced workforce within a strained healthcare system. By measuring levels of IgG antibodies to the SARS-CoV-2 nucleocapsid and spike antigens in ED workers, we determined the incidence of infection and described the course of antibody levels. We also measured the antibody response to vaccination and examined factors associated with immunogenicity.
Methods
We conducted a prospective cohort study of ED workers conducted at a single ED from September 2020–April 2021. IgG antibodies to the SARS-CoV-2 nucleocapsid antigen were measured at baseline, 3, and 6 months, and IgG antibodies to the SARS-CoV-2 spike antigen were measured at 6 months.
Results
At baseline, we found 5 out of 139 (3.6%) participants with prior infection. At 6 months, 4 of the 5 had antibody results below the test manufacturer's positivity threshold. We identified one incident case of SARS-COV-2 infection out of 130 seronegative participants (0.8%, 95% CI 0.02–4.2%). In 131 vaccinated participants (125 BNT162b2, 6 mRNA-1273), 131 tested positive for anti-spike antibodies. We identified predictors of anti-spike antibody levels: time since vaccination, prior COVID-19 infection, age, and vaccine type. Each additional week since vaccination was associated with an 11.1% decrease in anti-spike antibody levels. (95% CI 6.2–15.8%).
Conclusion
ED workers experienced a low incidence of SARS-CoV-2 infection and developed antibodies in response to vaccines and prior infection. Antibody levels decreased markedly with time since infection or vaccination.
Keywords: SARS-CoV-2, Healthcare worker, Vaccine immunogenicitiy
1. Introduction
1.1. Background and importance
Since the beginning of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, researchers have estimated the cumulative incidence of infection by measuring IgG antibodies to SARS-COV-2 [1,2]. Based on studies of anti-SARS-CoV-2 seroprevalence, frontline healthcare workers have an increased risk of infection as compared to non-healthcare workers [3,4]. Emergency department (ED) workers may be particularly vulnerable as they provide care for undifferentiated patients whose COVID-19 status is unknown and perform high-risk procedures such as nasopharyngeal swab testing, endotracheal intubation, and cardiopulmonary resuscitation. Despite initial studies suggesting high seroprevalence [5], it is unclear if ED health care workers were at increased risk for contracting COVID-19 through the pandemic [6].
In addition to their use in estimating the incidence of COVID-19, anti-SARS-CoV-2 antibody assays have also been used in the development of COVID-19 vaccines. Phase 1 trials of the BNT162b2 (Pfizer-BioNTech) vaccine used receptor-binding domain (RBD) or S1 IgG assays to measure vaccine immunogenicity [7]. In December 2020, ED healthcare workers were offered the BNT162b2 (Pfizer-BioNTech) and the mRNA-1273 (Moderna) mRNA vaccines, which were both found to have high vaccine efficacy (greater than 90%) to prevent symptomatic COVID-19 infection [8,9]. In the summer of 2021, breakthrough COVID infections, confirmed by SARS-COV-2 PCR testing, in those fully vaccinated have been increasingly reported, including among frontline healthcare workers [[10], [11], [12]]. Breakthrough infections in frontline healthcare workers have led to a reduced workforce within an already strained healthcare system. As variants (Delta and Omicron) have spread throughout the United States, concerns about reduced vaccine efficacy have arisen [13,14]. Despite initial reports of the durable protection [15], it now appears that immunity may wane.
1.2. Objectives
By measuring levels of IgG antibodies to the SARS-CoV-2 nucleocapsid and spike antigens during the COVID-19 pandemic in a cohort of ED workers, we sought to determine the cumulative incidence of infection and describe the longitudinal course of antibody levels in those who were infected. Also, as ED healthcare workers received COVID-19 vaccinations, we measured post-vaccination antibody levels to examine factors associated with immunogenicity, including time since vaccination, age, and prior COVID-19 infection. We hypothesized that time since vaccination is independently associated with reduced antibody levels.
2. Methods
2.1. Study design and setting
This was a prospective cohort study of ED healthcare workers conducted at a single medical center from September 1, 2020, to April 3, 2021. The University of California, San Francisco, is an academic medical center with EDs at separate adult and pediatric sites. These EDs are staffed by a team of ED healthcare workers including nurses, physicians, advanced practice practitioners, and patient care technicians. This study was performed in accordance with the STROBE guidelines and was approved by the local institutional review board.
2.2. Selection of participants
Participants were enrolled voluntarily as previous described [6]. Briefly, we enrolled ED healthcare workers (attending physicians, resident physicians, nurses, nurse practitioners, physician assistants, patient care technicians, and pharmacists). We excluded healthcare workers who were pregnant, immune compromised, or who were planning to move or unable to attend study visits. Participants were recruited by email and informed consent was obtained electronically.
2.3. Procedures and outcomes
The original intent of the study was to measure anti-nucleocapsid antibody levels to determine the incidence of COVID-19 infection among ED healthcare workers. Participants attended three study visits: Baseline (September 2020); three-months (December2020); and six-months – March 2021. At each study visit, participants were interviewed and underwent venipuncture. All blood specimens were tested for immunoglobulin G (IgG) antibodies to the SARS-CoV-2 nucleocapsid antigen with a chemiluminescent immunoassay (Abbott Architect SARS-CoV-2 IgG; Abbott Laboratories, Abbott Park, IL) to identify prior infection with SARS-CoV-2 [16]. At baseline and 3-months, specimens that were positive for the anti-nucleocapsid antibody were also tested with a different chemiluminescent immunoassay for anti-spike antibodies (Diasorin Liaison SARS-CoV-2 S1/S2 IgG; Diasorin Inc., Cypress, CA) to minimize false positives. We obtained both qualitative and quantitative results for all antibody assays. The manufacturer's threshold for the anti-nucleocapsid antibody test is 1.4 arbitrary units (AU); whereas the United Kingdom Medicines & Healthcare products Regulatory Agency (MHRA) published a lower threshold of 0.49 AU.
By the 6-month visit, 138/139 participants had received a COVID mRNA vaccine, and we decided to measure anti-spike antibody levels to evaluate immunogenicity of vaccines in ED healthcare workers. Vaccination with either the BNT162b2 (Pfizer-BioNTech) or MRNA-1273 (Moderna) stimulates an anti-spike antibody response but not an anti-nucleocapsid antibody response; thus testing for anti-nucleocapsid antibodies still allows identification of past infection, even in vaccinated persons [17]. To quantify antibody responses post-vaccine, we also measured IgG antibodies to the SARS-CoV-2 spike protein (Abbott AdviseDx SARS-CoV-2 IgG II). This assay for anti-spike antibodies provides a quantitative signal that correlates linearly with the antibody concentration [18,19].
2.4. Exposures of interest
The survey obtained at each visit included clinical variables about exposure to COVID-19 patients and risk factors for community-based exposure. We also surveyed participants regarding vaccination type and dates of vaccination.
2.5. Primary data analysis
Continuous participant characteristics were summarized in the demographics and results tables with means and standard deviations (SDs) or medians and interquartile ranges (IQR) as appropriate. Categorical variables were presented as frequencies and percentages.
To study the pattern of quantitative anti-nucleocapsid antibody levels over time, we identified all participants who had an anti-nucleocapsid antibody level above the MHRA threshold at any of the 3 study visits and presented their sequential levels along with additional details. We also used the cross-sectional 6-month data to compare weeks since second vaccine dose and anti-spike antibody levels. For this analysis, we used linear regression with the natural logarithm of the anti-spike antibody level as the outcome and weeks since second vaccine dose, age in decades, prior COVID-19 infection, and vaccine type as independent variables (based on a review of the prior literature) [20,21]. We excluded participants who had not yet had their second vaccine dose by their 6-month visit. We calculated the percentage change in antibody level for a 1-unit increase in the predictor as (exp(β) -1) x 100%, where β is the linear regression coefficient. All analyses were conducted with STATA MP (version 16). The sample size was initially calculated for the baseline study and was described previously [6].
3. Results
Our study enrolled 139 of 360 (38.6%) eligible ED healthcare workers starting September 1, 2020. All 139 participants provided baseline demographic data, survey answers, and venous blood specimens. Of the 139 participants, 90 (64.7%) were female, 88 (63.3%) white, with a median age of 36 (IQR 27–61) years. Most of the participants were nurses, attending physicians, or resident physicians. 97% of participants at baseline and 98% at 3 and 6 months reported using N95 respirators for high-risk patients, and 92–94% reported contact with at least one COVID-positive patient at each timepoint, while 50% reported contact with >10 COVID-positive patients (Table 1 ). In total, five participants (3.6%) were lost to 6-month follow-up due to the following: administrative leave (n = 1), graduating residency and leaving the area (n = 1), no longer on staff (n = 2), and no response (n = 1).
Table 1.
Characteristics of the study participants.
| Total N | 139 | ||
|---|---|---|---|
| Sex | |||
| Female | 90 | 64.7% | |
| Race | |||
| Asian | 31 | 22.3% | |
| African American | 4 | 2.9% | |
| White | 88 | 63.3% | |
| Other/Multiple | 16 | 11.5% | |
| Ethnicity | |||
| Latinx | 15 | 10.8% | |
| Age | |||
| Median (IQR) | 36 | 27–61 | |
| Provider Type | |||
| ED Nurse | 64 | 46.0% | |
| Attending Physician | 31 | 22.3% | |
| Resident Physician | 23 | 16.5% | |
| Advanced Practice Provider | 7 | 5.0% | |
| Emergency Medical Technician | 9 | 6.5% | |
| Other | 5 | 3.6% | |
| Prior History of COVID-19 infection at baseline | |||
| Yes | 5 | 3.6% | |
| Follow-Up COVID-19 infection at 3 months | 135 | ||
| Incident Cases | 1 | 0.8% | |
| Follow-up COVID-19 infection at 6 months | 134 | ||
| Incident Cases | 0 | 0% | |
| COVID-19 Vaccination Status | |||
| Received vaccination | 138 | 99.3% | |
| Vaccine manufacturer | |||
| Pfizer | 130 | 94.2% | |
| Moderna | 8 | 5.8% | |
| Time between 2nd dose and 6 mo. visit (weeks) | |||
| Median (IQR) | 9.9 | 8.7–11.3 |
3.1. Anti-nucleocapsid antibody results
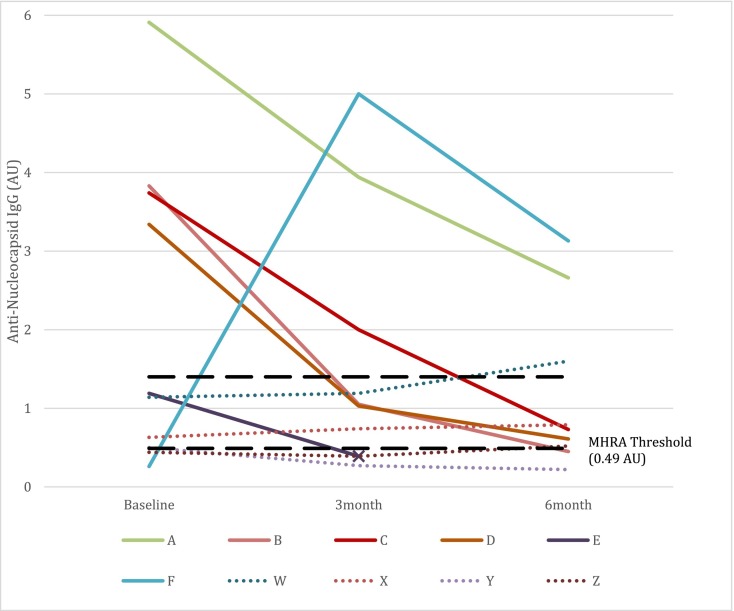
Antibody results from the first study visit were reported previously [6]. During the first study visit, we identified 4/139 (2.9%) who were positive for anti-nucleocapsid antibodies based on the manufacturer's threshold of 1.4 AU. (Table 2 , Participants A-D) One participant (Table 2, Participant E) had an elevated, albeit below-threshold, quantitative result of 1.19. This participant had a pre-study, documented positive chemiluminescent antibody assay, positive PCR for SARS-CoV-2, and clinical symptoms consistent with COVID-19 disease. Thus, we considered this participant seropositive, resulting in a first round seroprevalence of 5/139 (3.6%). Of the five who were classified as seropositive at baseline, four had 6-month results that were below the manufacturer's positivity threshold (Fig. 1 ). Of the 130 initially seronegative participants with follow-up, only one tested positive for anti-nucleocapsid antibodies at study visits 2 and 3. This incident case was an ED nurse working clinically through the winter of 2020 during which the community prevalence of acute COVID-19 in San Francisco reached its peak. This participant tested positive at the 3-month visit and had previously had a positive PCR and a consistent clinical syndrome. (Table 2, Participant F). The cumulative incidence of SARS-CoV-2 infection was therefore 1/130 (0.8%, 95% CI 0.02–4.2%) over the 6 months from 10/2020 to 3/2021.
Table 2.
Characteristics of Seropositive Participants and Indeterminate Participants
| Participant | Provider Type | Prior Diagnosis of COVID-19 | Likelihood of seropositivity | Antibody Positive at Baseline | Anti-nucleocapsid Antibody Optical Density Ratio |
Notes | ||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 Mo. Follow Up | 6 Mo. Follow Up | ||||||
| Seropositive Participants⁎ | ||||||||
| A | Resident | yes | 76–100% | Yes | 5.91 | 3.94 | 2.66 | Travelled to NYC at end of 2/2020. Believes infected at that time. |
| B | Resident | yes | 76–100% | Yes | 3.83 | 1.05 | 0.45 | Experienced fever and cough after travel to Colorado in 6/2020 |
| C | Nurse | no | 51–75% | Yes | 3.74 | 2.00 | 0.73 | Experienced fever and cough in 2/2020 prior to availability of PCR testing. |
| D | Attending | yes | 76–100% | Yes | 3.34 | 1.03 | 0.61 | Travelled to NYC at end of 2/2020. Believes infected at that time. |
| E | Nurse | yes | 76–100% | No | 1.19 | 0.39 | N/A | Experienced symptoms and positive PCR in 3/2020. Positive antibody test prior to study. |
| F | Nurse | yes | 11–25% | No | 0.26 | 5.00 | 3.13 | Symptomatic with positive PCR in 11/2020. |
| Indeterminate Participants⁎⁎ | ||||||||
| W | Attending | no | 3–5% | No | 1.14 | 1.19 | 1.60 | Denied COVID-19 symptoms or positive COVID-19 PCR testing. Banked plasma from 2019 (pre-COVID) was near threshold. Negative anti-spike antibody. Probable cross-reacting antibodies to endemic coronavirus. |
| X | Nurse | no | 26–50% | No | 0.63 | 0.74 | 0.79 | Denied COVID-19 symptoms or positive COVID-19 PCR testing. |
| Y | Resident | no | 76–100% | No | 0.5 | 0.27 | 0.22 | No prior diagnosis of COVID-19 on PCR testing. |
| Z | Nurse | no | 6–10% | No | 0.44 | 0.39 | 0.52 | Denied COVID-19 symptoms or positive COVID-19 PCR testing. |
seropositive participants had clinical syndrome, positive PCR, and AU above manufacture's threshold of 1.4 AU.
Indeterminate patients had antinucleocapsid result below manufacturer's threshold of 1.4 AU but above MHRA suggested threshold of 0.49 AU.
Fig. 1.
Quantitative anti-nucleocapsid IgG antibody levels in 10 selected participants.
Included if participants had an antinucleocapsid antibody result >0.49, the United Kingdom Medicines & Healthcare products Regulatory Agency (MHRA) threshold, at any of the 3 measurements. Immunoglobulin G (IgG) antibodies to the SARS-CoV-2 nucleocapsid antigen as determined by a chemiluminescent immunoassay (Abbott Architect SARS-CoV-2 IgG; Abbott Laboratories, Abbott Park, IL). Manufacturer's Threshold = 1.4 arbitrary units (AU).
Using the MHRA alternative (lower) threshold for anti-nucleocapsid positivity, we identified 10 participants with a positive test at any of the 3 study visits. (Table 2). Five participants (A-E) were positive at baseline due to SARS-CoV-2 infection, and one (F) was the incident case identified at the 3-month visit. The remaining four participants (W-Z) are listed as “indeterminate” in Table 2. Participants with confirmed infection had steep decreases in the quantitative antibody measurement after infection. But these 4 participants showed lower but persistently elevated measurements. (Fig. 1) One of the four (Participant W) had an above-threshold anti-nucleocapsid antibody measurement at 6 months after 2 near-threshold levels at baseline and 3 months, while the anti-spike antibody results were negative. This participant was able to obtain pre-COVID plasma for testing, and it had a similarly elevated anti-nucleocapsid antibody measurement. We suspect this individual's elevated antibodies were due to prior infection with an endemic coronavirus and cross-reacting antibodies.
3.2. Anti-spike antibody results
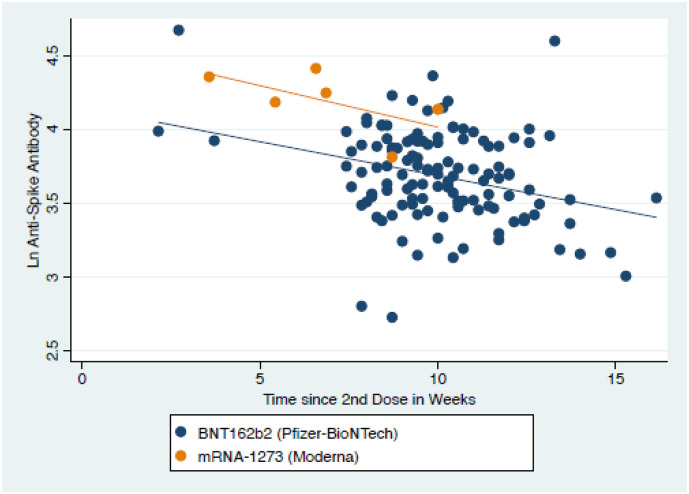
Of the 139 baseline participants, 138 were vaccinated, with 130 receiving the BNT162b2 (Pfizer-BioNTech) vaccine and 8 receiving the MRNA-1273 (Moderna) vaccine. We measured anti-spike antibody at 6 months in 134, but 3 had not yet received their second dose, leaving 131 fully vaccinated participants (125 BNT162b2, 6 MRNA-1273). 131/131 (100%, 95% CI 97.3 to 100%) vaccinated participants tested positive for anti-spike antibodies. The median anti-spike antibody level was 4938.6 AU, IQR 3098.2–8333.5 AU. (Fig. 2 ) We modelled the logarithm of the anti-spike antibody level as function of four predictors: time since vaccination, prior COVID-19 infection, age, and vaccine type. (Table 3 ) Each additional week since vaccination was associated with an 11.1% decrease in anti-spike antibody levels. (95% CI 6.2 to 15.8%) and each additional decade in age was associated with a 10.8% decrease. Prior infection was associated with 78% higher antibody levels on univariable analysis and almost 3 times the level on multivariable analysis. However, only 5 of the 131 participants included in this analysis had been infected.
Fig. 2.
Scatterplot of anti-spike antibody levels by time since second dose of vaccine.
Table 3.
Predictors of anti-spike antibody result.
| Unadjusted % Change⁎ | CI | Adjusted % Change⁎ | 95% CI | |
|---|---|---|---|---|
| Age in decades | −11.5% | −23.1% to 2.0% | −10.8% | −20.9% to 0.5% |
| Time since vaccination in weeks | −12.3% | −17.0% to −7.3% | −11.1% | −15.8% to −6.2% |
| Prior COVID-19 infection | 78.0% | 78% to −12.7% | 197.9% | 52.5% to 482.0% |
| Vaccine Type⁎⁎ | 104.6% | 14.3% to 266.2% | 91.5% | 7.6% to 240.7% |
change in anti-spike antibody level for one unit increase in predictor. (exp(β) -1) x 100%, where β is the linear regression coefficient.
Moderna vaccine vs. Pfizer.
4. Discussion
We conducted a prospective cohort study evaluating anti-SARS-CoV-2 antibodies in ED healthcare workers from September 2020 to April 2021, spanning a total of six months. Overall, we observed a low baseline seroprevalence and identified only one incident case during our study period. Also, we observed a pattern of decline in anti-nucleocapsid antibodies in those with confirmed COVID-19 infection such that 4 out of 5 participants classified as seropositive at baseline had below-threshold results at the end of the study period.
Previous research on risk of COVID-19 infection in frontline providers has been mixed, with some studies finding substantially higher rates of infection relative to the surrounding community [3,4,6,22]. One factor that may contribute to our study's low incidence is that nearly all participants (98%) reported routinely using N95 respirators. Another factor may be the low incidence of COVID-19 in the community served. Since the beginning of the pandemic, approximately 4.1% of our community has been infected, similar to our observed seroprevalence.
Essentially all of our cohort was vaccinated during the study period and had positive anti-spike antibodies post-vaccination. However, we determined that increasing time since vaccination was significantly associated with lower antibody levels, which may have implications for immunity in healthcare workers. In a recent case-control study, reduced antibody levels were associated with breakthrough infections in those who were vaccinated [10]. Khoury et al. reported that the decay in patients' neutralizing antibody titer over the first 250 days post vaccine led to significant loss of protection against infection but still protected patients from severe disease [23]. This time frame is consistent with our regression results, which suggested a 6–16% decay in antibody levels per week post vaccination. Our results are consistent with other studies demonstrating healthcare workers with a previous COVID-19 infection had higher antibody titers compared to healthcare workers who were COVID-19 naïve [24,25], and those finding that older age is associated with a less robust antibody response to vaccination [24].
Our study is unique because we obtained both the anti-nucleocapsid antibody binary result (using manufacturer threshold) and the quantitative result. For those participants with a positive anti-nucleocapsid antibody or those vaccinated, we then obtained anti-spike antibody qualitative and quantitative results. Our dual antibody measurement strategy allowed us to differentiate prior COVID-19 infection (both positive) compared to vaccination (only anti-spike positive). We observed that, regardless of participant factors, including previous COVID-19 infection or vaccination, antibody levels decreased over time. The waning of antibody levels has important implication for population-based surveillance and research, including an understanding of the time interval required to assess whether participants have had previous infection. For example, if we only obtain binary results every 6 months, we will miss participants who were infected (e.g., Table 2, Participant E).
4.1. Limitations
Our study is subject to several limitations. We did not measure neutralizing antibodies or correlates of T-cell immunity. Instead, we used multiple commercial assays, which have been shown to predict neutralization activity against SARS-CoV-2 [19]. Also, previous research suggests that neutralizing antibody levels are highly predictive of immune protection against symptomatic infection [10,23]. In addition, few study participants had evidence of COVID infection. Our study was conducted at a single center with a low community prevalence of COVID-19, and the low sero-incidence may not be generalizable to other ED workers serving higher prevalence communities. This highlights the need for future multi-center studies in broader populations.
4.2. Conclusion
In our study, even prior to vaccination, the risk of ED healthcare workers contracting SARS-CoV-2 was relatively low. We found that antibody levels decrease markedly over the 6–9 months after infection or vaccination and that the antibody response to vaccination appears to be inversely related to age. The clinical implications of these findings are not fully understood but suggest the need for vigilant surveillance of healthcare workers for evidence of waning immunity to infection and severe disease.
Funding
This study was funded by the University of California Office of the President. The funder did not have a role in study design, analysis, or reporting.
CRediT authorship contribution statement
Ralph C. Wang: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Charles E. Murphy: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Conceptualization. Aaron E. Kornblith: Writing – original draft, Supervision, Project administration, Formal analysis, Conceptualization. Nicole A. Hohenstein: Writing – original draft. Cornelius M. Carter: Resources, Project administration. Angela H.K. Wong: Project administration, Data curation. Theodore Kurtz: Validation, Resources, Methodology, Formal analysis, Data curation. Michael A. Kohn: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of Competing Interest
All authors report no conflict of interest.
References
- 1.Sood N., Simon P., Ebner P., et al. Seroprevalence of SARS-CoV-2-specific antibodies among adults in Los Angeles County, California, on April 10-11, 2020. Jama. 2020;323(23):2425–2427. doi: 10.1001/jama.2020.8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deeks J.J., Dinnes J., Takwoingi Y., et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev. 2020;6(6) doi: 10.1002/14651858.CD013652. Cd013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett ES, Horton DB, Roy J, et al. Prevalence of SARS-CoV-2 infection in previously undiagnosed health care workers in New Jersey, at the onset of the U.S. COVID-19 pandemic. BMC Infect Dis 2020;20(1):853. [DOI] [PMC free article] [PubMed]
- 4.Self W.H., Tenforde M.W., Stubblefield W.B., et al. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network - 13 academic medical centers, April-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(35):1221–1226. doi: 10.15585/mmwr.mm6935e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madsen T., Levin N., Niehus K., et al. Prevalence of IgG antibodies to SARS-CoV-2 among emergency department employees. Am J Emerg Med. 2020;38(12):2752. doi: 10.1016/j.ajem.2020.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang R.C., CET Murphy, Kornblith A.E., Kurtz T., Kohn M.A. Prevalence of SARS-Cov-2 antibodies in emergency medicine healthcare workers. Ann Emerg Med. 2021;77(5):556–557. doi: 10.1016/j.annemergmed.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh E.E., Frenck R.W., Jr., Falsey A.R., et al. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergwerk M., Gonen T., Lustig Y., et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouton T.C., Lodi S., Turcinovic J., et al. COVID-19 vaccine impact on rates of SARS-CoV-2 cases and post vaccination strain sequences among healthcare workers at an urban academic medical center: a prospective cohort study. medRxiv. 2021 doi: 10.1101/2021.03.30.21254655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hacisuleyman E., Hale C., Saito Y., et al. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med. 2021;384(23):2212–2218. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez Bernal J., Andrews N., Gower C., et al. Effectiveness of covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Rio C., Malani P.N., Omer S.B. Confronting the delta variant of SARS-CoV-2, Summer 2021. Jama. 2021;326(11):1001–1002. doi: 10.1001/jama.2021.14811. [DOI] [PubMed] [Google Scholar]
- 15.Widge A.T., Rouphael N.G., Jackson L.A., et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bryan A., Pepper G., Wener M.H., et al. Performance characteristics of the Abbott architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58(8) doi: 10.1128/JCM.00941-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suhandynata R.T., Bevins N.J., Tran J.T., et al. SARS-CoV-2 serology status detected by commercialized platforms distinguishes previous infection and vaccination adaptive immune responses. medRxiv. 2021 doi: 10.1101/2021.03.10.21253299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradley B.T., Bryan A., Fink S.L., et al. Anti-SARS-CoV-2 antibody levels measured by the AdviseDx SARS-CoV-2 assay are concordant with previously available serologic assays but are not fully predictive of sterilizing immunity. J Clin Microbiol. 2021;59(9) doi: 10.1128/JCM.00989-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suhandynata R.T., Hoffman M.A., Huang D., et al. Commercial serology assays predict neutralization activity against SARS-CoV-2. Clin Chem. 2021;67(2):404–414. doi: 10.1093/clinchem/hvaa262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibarrondo F.J., Fulcher J.A., Goodman-Meza D., et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild covid-19. N Engl J Med. 2020;383(11):1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lustig Y., Sapir E., Regev-Yochay G., et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. 2021;9:999–1009. doi: 10.1016/S2213-2600(21)00220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He Z., Ren L., Yang J., et al. Seroprevalence and humoral immune durability of anti-SARS-CoV-2 antibodies in Wuhan, China: a longitudinal, population-level, cross-sectional study. Lancet. 2021;397(10279):1075–1084. doi: 10.1016/S0140-6736(21)00238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khoury D.S., Cromer D., Reynaldi A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 24.Abu Jabal K., Ben-Amram H., Beiruti K., et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021;26(6) doi: 10.2807/1560-7917.ES.2021.26.6.2100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saadat S., Rikhtegaran Tehrani Z., Logue J., et al. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. Jama. 2021;325(14):1467–1469. doi: 10.1001/jama.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]