Visual Abstract
Keywords: Acute Kidney Injury and ICU Nephrology, Acute Interstitial Nephritis, Acute Kidney Injury, Biopsy, Cohort Studies, Creatinine, Immune check point-rechallenge, Immune checkpoint inhibitors, Immune-related adverse event, Immunotherapy, Kidney Function Tests, Pneumonia, Prednisone, Proton Pump Inhibitors
Abstract
Background
The objective of this case cohort study was to describe our experience in the care of patients with immune checkpoint inhibitor–related acute interstitial nephritis (ICI-AIN) including rechallenge.
Methods
A descriptive case series of patients that received an ICI and had an AKI (defined as a ≥1.5-fold increase in serum creatinine) as an immune-related adverse event (irAE), with biopsy-proven or clinically suspected ICI-AIN from January 1, 2014 to December 1, 2018 at Mayo Clinic, Rochester. We studied details regarding diagnosis, clinical course, management, and outcomes of rechallenge of immunotherapy. Complete response (CR) was defined as return of kidney function back to baseline or <0.3 mg/dl above baseline creatinine; partial response (PR) was defined as creatinine >0.3 mg/dl from baseline, but less than twofold above the baseline by the end of steroid course.
Results
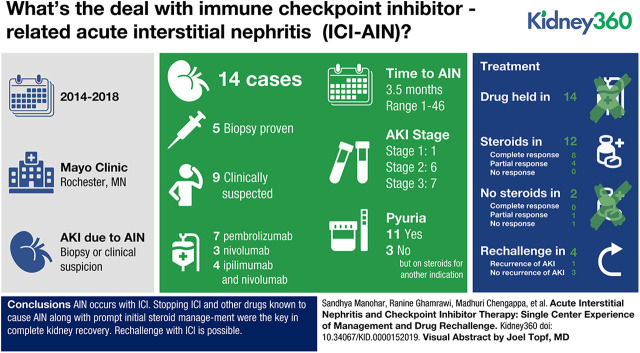
A total of 14 cases of biopsy-proven (35%) or clinically suspected (65%) ICI-AIN was identified. All patients had their ICI withheld and 12 patients received steroids. Steroid regimens were highly variable. The starting equivalent dose of prednisone was higher in those that had a CR versus a PR (median 0.77 mg/kg versus 0.66 mg/kg). Proton pump inhibitors (PPIs) were used in 11 patients and were stopped in eight (73%) patients at the time of the AKI event. A CR was seen in five (63%) of the eight patients who discontinued PPIs. Rechallenge was attempted in four of the 14 patients: three were successful with no recurrence of AKI, but one patient had recurrent AKI and fatal pneumonitis.
Conclusions
Careful review, withholding ICI and concomitant known AIN-inducing medications, along with prompt initial steroid management were the key in complete renal kidney recovery. A kidney biopsy should be strongly considered. Rechallenge of immunotherapy after a kidney irAE, although challenging, is possible and would need careful evaluation on an individual basis.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/K360/2020_01_30_KID0000152019.mp3
Introduction
Cancer therapies with immune checkpoint inhibitors (ICIs) have reached remarkable clinical response rates and have thereby substantially improved the prognosis for patients with various advanced malignancies. ICIs are monoclonal antibodies that exert inhibitory effects on surface molecules that serve as important breaks (or checkpoints) of the adaptive immune response like cytotoxic T lymphocyte–associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed death-ligand 1 (PDL-1). The untoward side effects of such an approach are immune-related adverse events (irAEs) in a variety of organs. The incidence of irAEs in patients receiving ICIs can be as high as 59%–85%, depending on the target and the use of mono- or combination therapy (1–3). The most commonly affected organs are skin, endocrine glands, gastrointestinal tract, lungs, and liver (4). Kidney toxicity is less common, but the incidence is rising as therapy with these agents continues to increase.
Although severe kidney irAEs spur concern among providers, smaller rises in creatinine may not be appreciated or may be attributed to other causes because the differential diagnosis of AKI in patients with cancer is broad. In the kidney, the most commonly reported irAE is acute interstitial nephritis (AIN) (5), although a few cases of GN have been observed (6,7). AIN is classically described as the triad of fever, rash, and eosinophilia in association with elevated serum creatinine, but this triad is present in only 5%–10% of cases (8). Histologically, AIN is characterized by the presence of inflammatory infiltrates and edema in the kidney interstitium (9). In kidney biopsy registries, AIN accounts for 1%–3% of cases and in as many as 20% of patients undergoing kidney biopsy for AKI (10,11). The incidence of AKI as an irAE has been reported in 2% and 5% of patients with AKI while on PD-1 inhibitors or combination ICI therapy, respectively, with AIN being the predominant pathologic lesion (5,12). However, this is likely an underestimation because many patients do not undergo a kidney biopsy in the setting of mild AKI, or the diagnosis is masked by steroid therapy for other irAEs.
Herein we present our experience with cases of ICI-AIN as an irAE in the setting of therapy with the CTLA-4 inhibitor, ipilimumab, and the PD-1 inhibitors, pembrolizumab and nivolumab. We reviewed the clinical and histologic characteristics, concurrent medication profiles, AKI management, and the outcomes of rechallenge of ICI in these patients at our institution.
Materials and Methods
Study Population
This is a single center, retrospective, observational study. We performed a search of our electronic medical records for all patients that received an ICI and had an AKI from January 1, 2014 to December 1, 2018 at Mayo Clinic (Rochester, Minnesota). We reviewed the following ICIs: the CTLA-4 inhibitor, ipilimumab, and the PD-1 inhibitors, pembrolizumab and nivolumab. AIN cases as a possible kidney irAE were identified either by clinical documentation by a consulting nephrologist or after mutual consensus by the authors S.M. and S.M.H. performing the retrospective chart review. Patients that did not provide research authorization were excluded. This study was approved by Mayo Clinic Institutional Review Board.
Data Collection
Clinical characteristics; details of the cancer type; comorbidities; and concurrent medications including proton pump inhibitors (PPIs), nonsteroidal anti-inflammatory drugs (NSAIDs), steroid use, and prior ICI drug use were collected. Information of prior and concurrent nonkidney irAEs as documented by care providers was collected. Baseline creatinine was defined as the last stable serum creatinine value before initiating on the ICI. ICI-related AKI cases were included if attributed directly to the ICI by the treating provider with a ≥1.5-fold increase in serum creatinine from baseline (grade 1 kidney toxicity) (13). If a kidney biopsy was done, the histologic diagnosis was used for confirmation of ICI-AIN. Data was collected on the management of AIN with details regarding the use of intravenous (IV) versus oral steroids, steroid dose, duration, and taper regimen used.
Data on kidney and overall outcomes were recorded. AKI severity was staged according to the Kidney Disease Improving Global Outcomes Work Group criteria (14). By definition, all cases were at minimum an AKI stage 1 (≥1.5-fold increase in serum creatinine). Complete response (CR) was defined as return of kidney function back to baseline or <0.3 mg/dl of baseline creatinine by the end of steroid course. Partial response (PR) was defined as creatinine >0.3 mg/dl from baseline, but less than twofold from baseline, despite the completion of steroid regimen. No response was indicated if kidney function continued to deteriorate or was unchanged with therapy.
Statistical Analysis
All continuous variables were reported as medians (minimum [min]–maximum [max]). All categoric variables were reported as counts with proportions. JMP statistical software (version 13 SAS Institute) was used to perform the analysis. No statistical comparisons were done because the event rate was low.
Results
Patient Population
A total of 1173 unique patients received ICI in our study period, with 608 patients receiving pembrolizumab, 304 patients on nivolumab, and 261 patients on ipilimumab. Of these, we found 303 patients who received ICI therapy and had an AKI (ipilimumab, 50; nivolumab, 104; pembrolizumab, 149). We excluded ten patients who did not provide research authorizations. Of the 293 patients with AKI, 14 cases with clinically suspected AIN were included in our review. The baseline characteristics are detailed in Table 1.
Table 1.
Baseline characteristics before development of AKI/acute interstitial nephritis
| Patient | Drug | Age/Gender | Cancer | Hypertension | Diabetes | CKD | Medications before AKI | Extrarenal irAE before AKI/AIN | Extrarenal irAE with AKI/AIN | On Steroids before AKI | Steroid Dose | |
| No. 1 | Nivolumab | 73/F | Lung AdenoCa | Yes | No | Yes | PPI | No | No | No | — | |
| No. 2 | Nivolumab | 79/F | Lung AdenoCa | Yes | Yes | Yes | PPI | No | No | No | — | |
| No. 3 | Nivolumab | 64/F | Melanoma | No | No | No | PPI and NSAIDs | Colitis | Colitis | Yes | Prednisone 1 mg/kg dose | |
| No. 4 | Nivolumab and ipilimumab | 54/M | Melanoma | No | No | No | NSAIDs | No | No | No | — | |
| No. 5 | Nivolumab and ipilimumab | 71/M | Melanoma | Yes | No | No | PPI and NSAIDs | Rash and uveitis | Colitis | No | — | |
| No. 6 | Nivolumab and ipilimumab | 67/F | Melanoma | Yes | No | No | PPI | Rash | No | No | — | |
| No. 7 | Nivolumab and ipilimumab | 62/M | Melanoma | No | No | No | — | Pneumonitis/thyroiditis | Hypothyroidism | No | — | |
| No. 8 | Pembrolizumab | 41/F | Melanoma | No | No | No | PPI | Colitis and iritis | No | Yes | Dexamethasone 4 mg daily | |
| No. 9 | Pembrolizumab | 74/F | Breast cancer | No | No | Yes | PPI | No | No | No | — | |
| No. 10 | Pembrolizumab | 69/M | Lung AdenoCa | No | No | No | PPI | No | No | Yes | Dexamethasone 1 mg daily | |
| No. 11 | Pembrolizumab | 80/M | Melanoma | Yes | No | No | PPI | Rash and scleroderma rash | Scleroderma rash | Yes | Prednisone 20 mg daily | |
| No. 12 | Pembrolizumab | 49/M | Melanoma | Yes | Yes | Yes | PPI | Thyroiditis | No | No | — | |
| No. 13 | Pembrolizumab | 81/F | Melanoma | Yes | No | No | PPI | Rash, pancreatitis, and arthralgias | Rash | Yes | Prednisone 30 mg daily | |
| No. 14 | Pembrolizumab | 60/M | CLL | Yes | No | Yes | PPI | No | Rash, pneumonitis | No | — | |
irAE, immune-related adverse event; AIN, acute interstitial nephritis; F, female; AdenoCa, adenocarcinoma; PPI, proton pump inhibitor; NSAID, nonsteroidal anti-inflammatory drug; M, male; CLL, chronic lymphocytic leukemia.
Our patients had a median age of 68 (min–max, 41–81) years. About 57% (eight of 14) were females. Single agent therapy with pembrolizumab was used in seven of 14 patients (50%). We found that three (21%) patients had received single agent nivolumab, whereas four (28%) patients had received a combination of ipilimumab and nivolumab before their ICI-AIN event. We found no cases of PDL-1–related AIN. The time from drug initiation to ICI-AIN had a wide range, with the median time being 3.5 (1–46) months. The predominant underlying cancer was metastatic melanoma (64%). Six (42%) of the patients had concurrent nonkidney irAEs, with rash (dermatitis) being the most common.
Clinical Presentation
At the time of presentation, the median serum creatinine was 2.75 (min–max, 1.4–6.5) mg/dl, with a peak serum creatinine of 3.25 (1.5–7.31) mg/dl. We found that 13 of the 14 (92%) patients had at least a doubling of creatinine. Seven patients (50%) had AKI stage 3 and six (43%) had AKI stage 2. Only one of the patients (patient 13) had AKI stage 1, creatinine increased from 0.8 to 1.5 mg/dl while she was on prednisone 30 mg daily for a skin rash at the time of the kidney event. The median random urine protein/osmolality ratio was 0.69 (0.6–12.3) mg/mOsm. We found that 71% of patients had sterile pyuria on the urinalysis at the time of the AKI event, but the remaining three patients had no white cells on urine microscopy and these were patients already on steroids for a nonkidney irAE.
We found that only five (35%) of the 14 patients underwent kidney biopsy. The kidney biopsies showed diffuse interstitial inflammatory infiltrate with interstitial edema and tubulitis. The infiltrating cells were mononuclear cells only (n=1), mononuclear cells plus eosinophils (n=2), mononuclear and plasma cells (n=1), and all three types of cells (n=1). The reasons for not doing a kidney biopsy in the remaining nine patients were high bleeding risk due to concurrent drugs (n=2), already on steroids with improving kidney function at the time of nephrology evaluation (n=2), biopsy was considered to not change plans for management (n=3), and no documentation of reason (n=2). One of the patients (patient 6), who presented with AKI stage 3, had a positron emission tomography–computed tomography scan that was suggestive of AIN. In the two patients that had no initial documentation of biopsy, the diagnosis of AIN was made retrospectively when a concurrent irAE was being treated with subsequent improvement in kidney function. Patient 5 also had concurrent enterocolitis and the initial AKI was thought to be from acute tubular injury. The patient was started on dialysis for a couple of days and after aggressive steroid therapy, initially with IV methylprednisolone, he had improvement in his kidney function with CR. Patient 12 had underlying diabetic nephropathy with CKD stage 4 at baseline and the worsening kidney function was initially believed to be from natural progression of diabetic kidney disease, but the patient’s melanoma progressed on pembrolizumab and, when the therapy was discontinued, he had a some improvement and stabilization of kidney function.
Patient 14 had AKI, fever, significant rash, and pneumonitis. He required dialysis for 2 days and improved with IV steroids. Additional details are outlined in Table 2.
Table 2.
Clinical characteristics at the time of AKI/acute interstitial nephritis
| Patient | Drug | Baseline Creatinine (mg/dl) | Drug Duration before AKI (mo) | Creatinine at AKI (mg/dl) | Peak Creatinine at AKI (mg/dl) | Urine WBC/hpf at AKI | Proteinuria/Osmolality Ratio (mg/mOsm) | Kidney Biopsy | Initiation of Dialysis |
| No. 1 | Nivolumab | 1 | 4 | 4.2 | 5.6 | 1–3 | 0.64 | Yes, AIN | No |
| No. 2 | Nivolumab | 1.1 | 4 | 2.3 | 2.3 | 21–30 | 1.8 | No | No |
| No. 3 | Nivolumab | 0.8 | 12 | 3.27 | 3.4 | No | 0.76 | Yes, AIN | No |
| No. 4 | Nivolumab and ipilimumab | 0.9 | 1 | 6.5 | 6.5 | No | 0.54 | No | No |
| No. 5 | Nivolumab and ipilimumab | 0.9 | 1 | 5.3 | 6.8 | 1–3 | 4.78 | No | Yes |
| No. 6 | Nivolumab and ipilimumab | 1 | 3 | 5.2 | 5.9 | 31–40 | 1.16 | No | No |
| No. 7 | Nivolumab and ipilimumab | 0.6 | 3 | 1.7 | 1.7 | 1–3 | 0.56 | No | No |
| No. 8 | Pembrolizumab | 0.8 | 46 | 2.4 | 2.5 | 11–20 | 0.57 | Yes, AIN | No |
| No. 9 | Pembrolizumab | 1.3 | 1 | 2.7 | 3.1 | 21–30 | 0.75 | Yes, AIN | No |
| No. 10 | Pembrolizumab | 0.9 | 3 | 1.89 | 1.89 | 4–10 | 0.37 | No | No |
| No. 11 | Pembrolizumab | 0.8 | 6 | 1.6 | 1.7 | No | 0.86 | No | No |
| No. 12 | Pembrolizumab | 3 | 13 | 6 | 7.31 | 11–20 | 12.3 | No | No |
| No. 13 | Pembrolizumab | 0.8 | 4 | 1.4 | 1.5 | 1–3 | 0.16 | Yes, AIN | No |
| No. 14 | Pembrolizumab | 1.7 | 1 | 2.8 | 6.8 | NA | 0.64 | No | Yes |
WBC, white blood cell; hpf, high power field; AIN, acute interstitial nephritis; NA, not available.
Management of Patients with Suspected and/or Confirmed AIN
All of the 14 patients were closely followed and the details of the management of their kidney irAEs are noted in Table 3.
Table 3.
Management of patients with AKI from suspected/confirmed immune checkpoint–related acute interstitial nephritis
| Patient | Drug | ICI Withheld | PPI Withheld | Steroids Initiated | IV Pulse Steroids | Total IV Steroids (g) | Prednisone Starting Dose (mg) | Prednisone Starting Dose (mg/kg) | Approximate Weekly Steroid Taper Regimen (mg) | Renal Response | Nadir Creatinine (mg/dl) | Cancer Outcome | Patient Outcome | |
| No. 1 | Nivolumab | Yes | Yes | Yes | Yes | 0.75 | 60 | 1.0 | 60–60–40–20–10–5–X | PR | 1.6 | Progression | Hospice | |
| No. 2 | Nivolumab | Yes | Yes | Yes | No | — | 60 | 0.9 | 60–80–40–20–20–20–20–20–20–20–hospice | CR | 1.2 | Unknown | Deceased | |
| No. 3 | Nivolumab | Yes | Yes | Yes | No | — | 40 | 0.5 | 40–30–20–15–7.5–5-4–3-2–1–X | PR | 1.22 | Complete response | Alive | |
| No. 4 | Nivolumab and ipilimumab | Yes | NA | Yes | Yes | 1 | 60 | 0.7 | 60–60–60–60–40–40–40–40–20–10–5–4–3–2–1–X | CR | 1.3 | Progression | Deceased | |
| No. 5 | Nivolumab and ipilimumab | Yes | No | Yes | Yes | 9.75 | 100 | 1.1 | 100–80–60–40–30–20–10–7.5–5–2.5–X | CR | 1.1 | Complete response | Alive | |
| No. 6 | Nivolumab and ipilimumab | Yes | Yes | Yes | Yes | 3 | 80 | 0.79 | 80–60–40–30–20–10–20–40–30–20–10–5–X | PR | 1.5 | Complete Response | Alive | |
| No. 7 | Nivolumab and ipilimumab | Yes | NA | No | No | — | — | — | — | PR | 1 | Progression | Alive on therapy | |
| No. 8 | Pembrolizumab | Yes | Yes | Yes | Yes | 0.25 | 40 | 0.77 | 40–30–30–30–30–20–15–15–10–7.5–7.5–5–2.5–X | CR | 1 | Progression | Deceased | |
| No. 9 | Pembrolizumab | Yes | Yes | Yes | No | — | 60 | 0.78 | 60–50–40–30–40–40–35–30–25–20–15–10–5–X | CR | 1.5 | Complete response | Alive | |
| No. 10 | Pembrolizumab | Yes | Yes | Yes | No | — | 60 | 0.66 | 60–40–20–20–15–10–> | CR | 1.1 | Progression | Deceased | |
| No. 11 | Pembrolizumab | Yes | No | Yes | No | — | 30 | 0.38 | 30–20–10–5-65–60–50–40–30–20–10–7.5–5–2.5–X | PR | 1.5 | Progression | Deceased | |
| No. 12 | Pembrolizumab | Yes | No | No | No | — | — | — | — | NR | 6.4 | Progression | Deceased | |
| No. 13 | Pembrolizumab | Yes | Yes | Yes | Yes | 2 | 60 | 1.0 | 60–40–30–30–20–10–5–X | CR | 1.1 | Complete response | Alive | |
| No. 14 | Pembrolizumab | Yes | NA | Yes | Yes | 5 | 60 | 0.68 | 60–40–20–10–5–2.5–X | CR | 1.1 | Stable | Alive on therapy | |
ICI, immune checkpoint inhibitor; PPI, proton pump inhibitor; IV, intravenous; X, stopped; >, dose continued at the time of last follow up; PR, partial response (creatinine improved from peak but did not return to within 0.3 mg/dl of baseline); CR, complete response (creatinine improved to a value within 0.3 mg/dl of baseline creatinine); NA, not applicable; NR, no response (creatinine unchanged or worsened).
Possible Offending Medications.
All 14 patients had their ICI withheld. We looked at the medication lists and identified any drugs associated with AIN. PPIs were the most commonly used drugs in our series. Out of 14 patients, 11 were on a PPI at the time of the AKI. Of these 11 patients, eight (73%) stopped their PPI at the time of kidney injury event. Of those that stopped the PPI, five of eight (63%) had a CR and the other three (38%) had a PR. Only three patients (patients 3, 4, and 5) were intermittently taking NSAIDs prior the AKI, two of them were on PPIs as well. Only one patient (patient 11) was on sulfa for infection prophylaxis in the setting of steroid use for a skin rash irAE and this patient was also on a PPI at the time of the AKI.
Steroid Regimen.
Two of the 14 patients did not receive any steroids at the time of AKI. The first (patient 7) had a PR with drug holiday alone; whereas in the second patient (patient 12), the diagnosis of ICI-AIN was not considered initially and AKI was presumed to be a progression of primary diabetic kidney disease. Steroids were later not initiated due to uncontrolled diabetes and creatinine improved slightly and stabilized with stage 5 CKD.
For the remaining 12 patients, the steroid regimen used to treat ICI-AIN was highly variable. IV pulse steroids were used in seven of the 12 patients (58%), the median peak serum creatinine for those patients was 5.9 (min–max, 2.5–6.8) mg/dl. Five of the seven patients who received IV pulse steroids had a complete recovery of kidney function. For the five patients that did not receive IV pulse steroids, the median peak serum creatinine was 2.3 (1.79–3.25) mg/dl.
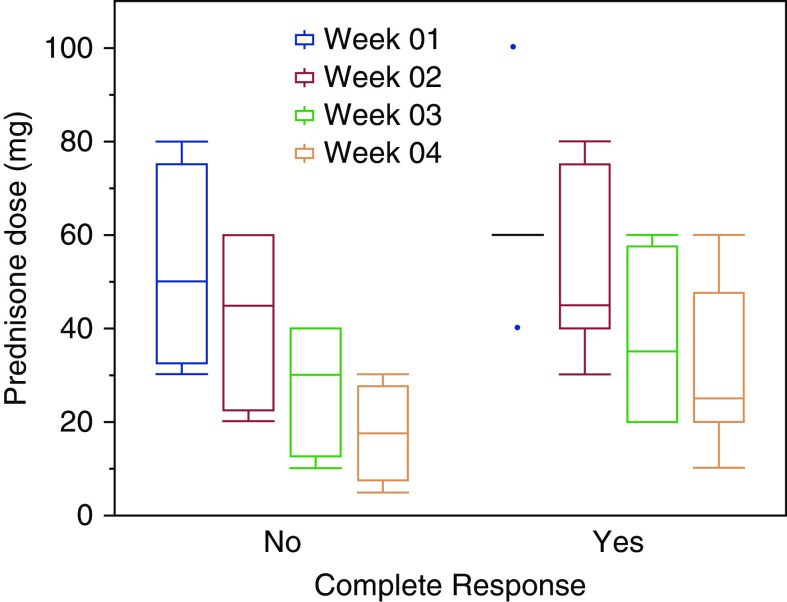
Overall, the starting dose of prednisone was higher in those that had a CR compared to those that had a PR: median 0.77 (0.66–1.11) mg/kg versus 0.66 (0.37–1.07) mg/kg. Because the steroid taper regimens were very heterogeneous as well, we used the total prednisone dose received (in milligram per kilogram) per month for analyses. At the end of the first month, patients who had a CR received higher steroid doses than those that had only a PR (median 2.79 [1.45–3.2] mg/kg per month versus 1.74 [0.8–3.2] mg/kg per month). Head-to-head comparisons of taper regimens were difficult, but there appeared to be a trend to suggest that higher initial steroid doses had improved recovery of kidney function. See Figure 1 for a representative first month on steroid doses stratified by their kidney response.
Figure 1.
Trend of prednisone dose used in the first 4 weeks stratified according to complete response at the end of 3 months.
Rechallenge of Immunotherapy After Suspected/Confirmed AIN
Four of the 14 patients had to be rechallenged with the same immunotherapy (Table 4). Median time of rechallenge from the initial AKI event was 95 days (range 53–511). Two of the patients (patients 4 and 7) had developed AIN on combination therapy with ipilimumab and nivolumab. These two patients were rechallenged after return of kidney function close to baseline with nivolumab alone without recurrence of AKI (one patient remained on low-dose steroids). The two patients with AIN on pembrolizumab were rechallenged while still on prednisone of 10 mg daily (patient 10) or 20 mg daily (patient 11). Patient 10 was on a PPI at the time of rechallange. Patient 11 had recurrent AKI, also in part secondary to obstructive uropathy initially and pneumonitis within one month of rechallenge, subsequently patient did deteriorate clinically despite steroids and eventually transitioned to comfort care.
Table 4.
Rechallenge of immunotherapy after a confirmed/suspected acute interstitial nephritis
| Patient | Drug | Previous irAE | Rechallenged | Creatinine at Rechallenge (mg/dl) | Days from AKI | ICI Used at Rechallenge | PPI at Rechallenge | AKI Recurrence | Outcome |
| No. 4 | Nivolumab and ipilimumab | AIN alone | Yes on 4 mg prednisone | 0.8 | 58 | Nivolumab | Yes | No | Died a month later from disease progression |
| No. 7 | Nivolumab and ipilimumab | Thyroiditis and AIN | Yes on no steroids | 0.9 | 132 | Nivolumab | No | No | Alive with normal kidney function |
| No. 10 | Pembrolizumab | AIN alone | Yes on 10 mg prednisone | 1.2 | 53 | Pembrolizumab | Yes | No | Alive with normal kidney function |
| No. 11 | Pembrolizumab | Scleroderma, pneumonitis, AIN | Yes on 20 mg prednisone | 2.5 | 511 | Pembrolizumab | No | Yes | Pneumonitis and AKI progressed and died about 1 mo later |
irAE, immune-related adverse event; ICI, immune checkpoint inhibitor; PPI, proton pump inhibitor; AIN, acute interstitial nephritis.
Discussion
Among 293 patients with AKI while on ICI therapy at our institution, we report 14 cases (5%) of ICI-AIN. We have described our experience in clinical features, diagnostic challenges, management, and response to treatment. We have also described our experience with rechallenging the ICI in four of the 14 patients. Although the incidence of AIN is low with ICI (12), patients who do develop AIN as an irAE are in a unique predicament, especially if their cancer has been responding appropriately to the ICI. We had previously described that medications were the etiology of AIN in 70% of the patients (15), with antibiotics being the most common followed by PPIs. However, as compared with the AIN from “routine” medications, ICI-AIN has other aspects to be considered including mechanistic differences, time of onset, and duration between other drugs to name a few. ICI-AIN must prompt us to think differently about this disorder and we must manage it unlike a “regular” AIN because this may affect the cancer-related prognosis of the patient.
Our findings are consistent with other prior studies showing AIN as the most common cause of ICI-AKI, although AIN is not the sole etiology of AKI associated with ICIs, different kidney irAEs have been described in recent reports. Recently, Izzedine et al. (16) reported the occurrence of acute tubular injury/necrosis (ATI/ATN) in approximately 40% of their patients, and AIN in 30%, followed by two cases of minimal change disease. One can argue that the patients reported by Izzedine et al. were biopsied early because the median serum creatinine was 1.1 (min–max, 0.8–1.6) mg/dl. These patients could have had AKI related to hemodynamics or concurrent chemotherapies. In our series, we only included patients with at least a 50% increase of creatinine from baseline and those in which the authors were clinically suspicious of AKI related to ICIs. This may have led to the predominant renal pathology identified to be AIN.
Glomerular diseases associated with ICIs have been described (6,7,17). MD Anderson recently published a case series of 16 patients that had undergone a kidney biopsy while on ICIs. Interestingly, only five of their patients had AIN alone, whereas the remaining nine patients had simultaneous presentation of AIN with a glomerulopathy (pauci-immune vasculitis, membranous nephropathy, IgA nephropathy, C3 glomerulopathy, FSGS, and AA amyloidosis) (7). Furthermore, tubular damage by ICIs may also cause electrolyte abnormalities and some ICIs have been reported in association with renal tubular acidosis in the setting of AIN (18,19). Mechanistically, this is believed to be an autoimmune process causing an alteration of H+-ATPase or Cl-/HCO3- in in the type A intercalated cells (19).
Clinically, the classic drug-induced AIN symptoms of low-grade fever, rash, fatigue, and peripheral eosinophilia are rarely seen and we did not see them as a feature in our cases either. In our study, we found sterile pyuria in many of the ICI-AIN cases but it was not a consistent presenting feature. Cortazar et al. (5) reported on 12 cases with similar findings of pyuria and subnephrotic range proteinuria as the most common manifestations, whereas Shirali et al. (20) found no common clinical presentation in their six cases of ICI-induced AIN. Extrarenal irAE was not present in almost 60% of our patients at the time of the AKI, therefore absence of irAE does not exclude ICI-AIN.
The AIN infiltrate is predominantly composed of T lymphocytes, macrophages, and eosinophils as well as neutrophils on occasion. Spanou et al. (21) very eloquently demonstrated that patients with drug-induced AIN harbor drug-specific T cells. The possibility that ICIs may lead to a loss of tolerance in drug-specific T cells and thereby result in AIN has been speculated upon (5,20). In our study, although we noted that many of the patients had been on PPIs, there was no clear pattern to suggest this was the offending drug, but it certainly could be a potential trigger. With polypharmacy being common among patients with cancer who are immunosuppressed, their medications have to be reviewed.
It is possible that some of the patients in our series that did not undergo a kidney biopsy could have had another cause for AKI such as ATI/ATN, but our clinical suspicion was high for AIN as the etiology of AKI. The absence of other etiologies of AKI, in conjunction with the time line from the time of initiation of ICIs, the rapidity of renal function decline, presence of concurrent nonrenal irAEs, and the response to empirical steroids led to our strong clinical suspicion for ICI-AIN.
If we do try to apply the lessons learned from drug-induced AIN to the management of ICI-related AIN, then understanding the role of steroids is also important. Early steroid initiation in addition to drug withholding is recommended because delays are associated with poorer kidney outcomes (22,23). Therefore, we should strongly consider kidney biopsy when in doubt because delaying diagnosis may affect kidney recovery. Furthermore, it would help to differentiate AIN or glomerular diseases from other causes such as ATI/ATN, which would influence the decision on whether or not to continue with ICI therapy. Our experience has been to start patients on steroids fairly quickly, but the initial dose and duration of high-dose steroid, as well as the subsequent tapering rate, are quite heterogeneous. Interestingly, we found that the patients who had complete recovery of kidney function had received higher doses of steroids in the first 1–2 months (2.79 mg/kg per month; min–max, 1.45–3.2) compared with those that had a partial recovery (1.74 mg/kg per month; 0.8–3.2). Five of seven patients who received IV pulse steroids initially had complete recovery of kidney function. Although this pattern is suggestive, the patient’s clinical status can limit one’s ability to give high doses of steroids for a prolonged period of time.
The American Society of Clinical Oncology (ASCO) guidelines recommend starting steroids for a grade 2 kidney irAE (creatinine of two to three times above baseline) and, once it improves to grade 1 or less, to start a taper over 4–6 weeks (4,24). Our experience has been that a rapid taper over 4 weeks has led to rebound AKI and a slow taper over the course of at least 8–12 weeks has been better tolerated. The key is to closely monitor creatinine levels during the taper. We also noted that, in our cohort, five of the 14 patients (patients 3, 8, 10, 11, and 13) were already on some type of steroids for either nonrenal irAE or another reason. The patients that had an increase in the prescribed steroid dose at the time of the AKI had a CR, whereas those that were left on the same dose or continued tapering only had a PR to steroids. This cautions the oncologists who, per ASCO guidelines, may start patients on steroids for a nonrenal irAE: they must be watchful for a concurrent kidney (or other organ) injury that may need additional immunosuppression.
Rechallenge is another uncharted territory that requires close attention. Additional AKI events can result in further loss of kidney function and may influence the overall prognosis (25). Rechallenge after a severe irAE has been attempted with mixed experience in the literature (26,27), but we had an overall favorable outcome except in one patient (patient 11) that rapidly deteriorated due to recurrent pneumonitis. His case was challenging because he had extensive metastatic disease that had failed multiple lines of treatment and the only drug to which he had shown a reasonable response was pembrolizumab. Although ASCO guidelines (24) would not recommend a rechallenge in his case, the decision to try pembrolizumab was a mutual one between the patient and his oncologist because the alternative was a referral to hospice. Simonaggio et al. (28) described, in their French cohort of 93 patients that had recently had recovered from a grade 2 or higher irAE, that a rechallenge led to recurrence of a second adverse event in 55% of patients within a median follow-up time of 14 months. Out of those 55% patients, 13% had a different irAE than their initial event. There are two patients with renal failure in the cohort, but details regarding their rechallenge experience were not described in their paper. Overall the authors believed that the severity of the second event was not greater than the first (28). A similar study by Abu-Sbeih et al. (29) looked at recurrence of immune-related diarrhea and colitis after rechallenge of ICI and found that this was less severe in PD-1 drugs as compared to CTLA-4 inhibitors.
We chose to challenge a few of our patients while on low-dose steroids. Although there is concern that being on a higher baseline dose of steroids at the time of ICI therapy may affect the response to ICI, there has not been any change in progression-free survival or overall survival when the prednisone dose has been kept <10 mg/d (30). When we review the risk/benefit ratio of baseline steroids we recommend, based on our small experience, that the patient recovering from a ICI-AIN be rechallenged while on low-dose prednisone (10 mg/d). There is a need for collaboration of nephrologists and oncologists to guide the community on managing this unique clinical conundrum.
Our study has several limitations. First, it is a retrospective, single-center study. Second, we may not have captured all of the AKI/AIN events that have occurred. Our aim was to capture all of the clinically significant cases of AKI by restricting our inclusion criteria to a 50% increase in serum creatinine from baseline, therefore we may have missed milder AKI events. Third, we limited our search to patients with biopsy-proven AIN or a high clinical level of suspicion for ICI-AIN. Hence, we may have not captured all of the patients who had AKI by the search strategies we used. We attempted to minimize this potential confounder by having two investigators independently search and procure cases. Fourth, we had many cases that were treated for AIN without a kidney biopsy and, based on their response to steroids, we presumed a diagnosis of ICI-related AIN and one could argue that these patients had acute tubular injury and the response to steroids was merely coincidental. Fifth, we had few events to report and this limited our ability to perform statistical analysis for associations or correlations. Lastly, because the AIN and other irAEs were managed by providers of varied specialties, the treatment was not consistent, stressing the importance of dedicated onco-nephrology teams in the care of these patients. Our study highlights the need for future prospective studies to accurately identify and characterize kidney irAEs and response to immune suppression, in addition to obtaining an early nephrology consult and kidney biopsy when indicated.
In conclusion, we provide a summary of our experience with ICI-AIN. An attempt must be made to have a prompt diagnosis of the cause of AKI, including a strong consideration of a kidney biopsy, in addition to stopping concurrent medications that have AIN as a known adverse event. An increase in serum creatinine, evidence of abnormalities of urine sediment, or even persistent electrolyte abnormalities should be recognized and prompt early referral to nephrology providers. We believe that having a baseline serum creatinine, electrolyte, and urinalysis evaluation before initiation and during each ICI treatment is paramount to identify the occurrence of kidney irAE early on, but also that a kidney biopsy is necessary to understand the lesion being treated. Use of high doses of steroids (at least 0.8 mg/kg) in the initial period for patients with AKI stage 1/2 and consideration of the use of IV steroids for patients with more severe kidney dysfunction (AKI stage 3) with a slow taper is recommended, if there are no other contraindications. Rechallenge of immunotherapy after recovery from a kidney irAE can be attempted in the right setting and would need to take into account the severity of prior irAEs. In our small experience, rechallenge while on low-dose steroids may help reduce the risk of recurrence of kidney irAE. We need more experience to know if this is a consistent benefit and the effect on overall patient outcome.
Disclosures
L. Kottschade reports personal fees from Array Biopharma and grants from Bristol Myers Squibb outside the submitted work. M. Chengappa, R. Dronca, H. Finnes, Dr. Ghamrawi, B. Goksu, J. Herrmann, S. Herrmann, K. Leventakos, and S. Manohar have nothing to disclose.
Funding
Dr. S.M. Herrmann is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (grant K08 DK118120) and by the Mary Kathryn and Michael B. Panitch Career Development Award. Dr. J. Herrmann is supported by the National Heart, Lung, and Blood Institute (K08 HL116952) and the National Cancer Institute (R01 CA233610).
Author Contributions
Concept and design: all authors. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: S. Manohar, R. Ghamrawi, and S. Herrmann. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: S. Manohar, R. Ghamrawi, and S. Herrmann. Preparation of figures and tables: S. Manohar, R. Ghamrawi, and S. Herrmann. All authors approved the final version of the manuscript, and all are accountable for all aspects of the submitted work.
References
- 1.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD: Combined nivolumab and ipilimumab or monotherapy in untreated melanoma [published correction appears in N Engl J Med 379: 2185, 2018]. N Engl J Med 373: 23–34, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, Smylie M, Dummer R, Hill A, Hogg D, Haanen J, Carlino MS, Bechter O, Maio M, Marquez-Rodas I, Guidoboni M, McArthur G, Lebbé C, Ascierto PA, Long GV, Cebon J, Sosman J, Postow MA, Callahan MK, Walker D, Rollin L, Bhore R, Hodi FS, Larkin J: Overall survival with combined nivolumab and ipilimumab in advanced melanoma [published correction appears in N Engl J Med 377: 1345–1356, 2017]. N Engl J Med 377: 1345–1356, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K; ESMO Guidelines Committee : Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up [published correction appears in Ann Oncol 29(suppl 4): iv264–iv266, 2018]. Ann Oncol 28[Suppl 4]: iv119-iv142, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Postow MA, Sidlow R, Hellmann MD: Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378: 158–168, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, Le DT, Lipson EJ, Glezerman IG, Wolchok J, Cornell LD, Feldman P, Stokes MB, Zapata SA, Hodi FS, Ott PA, Yamashita M, Leaf DE: Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 90: 638–647, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fadel F, El Karoui K, Knebelmann B: Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med 361: 211–212, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Mamlouk O, Selamet U, Machado S, Abdelrahim M, Glass WF, Tchakarov A, Gaber L, Lahoti A, Workeneh B, Chen S, Lin J, Abdel-Wahab N, Tayar J, Lu H, Suarez-Almazor M, Tannir N, Yee C, Diab A, Abudayyeh A: Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: Single-center experience. J Immunother Cancer 7: 2, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perazella MA, Markowitz GS: Drug-induced acute interstitial nephritis. Nat Rev Nephrol 6: 461–470, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Cameron JS: Allergic interstitial nephritis: Clinical features and pathogenesis. Q J Med 66: 97–115, 1988 [PubMed] [Google Scholar]
- 10.Praga M, Sevillano A, Auñón P, González E: Changes in the aetiology, clinical presentation and management of acute interstitial nephritis, an increasingly common cause of acute kidney injury. Nephrol Dial Transplant 30: 1472–1479, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Raghavan R, Eknoyan G: Acute interstitial nephritis - a reappraisal and update. Clin Nephrol 82: 149–162, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manohar S, Kompotiatis P, Thongprayoon C, Cheungpasitporn W, Herrmann J, Herrmann SM: Programmed cell death protein 1 inhibitor treatment is associated with acute kidney injury and hypocalcemia: Meta-analysis. Nephrol Dial Transplant 34: 108–117, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, Budde LE, Costa L, Davies M, Dunnington D, Ernstoff MS, Frigault M, Hoffner B, Hoimes CJ, Lacouture M, Locke F, Lunning M, Mohindra NA, Naidoo J, Olszanski AJ, Oluwole O, Patel SP, Reddy S, Ryder M, Santomasso B, Shofer S, Sosman JA, Wahidi M, Wang Y, Johnson-Chilla A, Scavone JL: Management of immunotherapy-related toxicities, version 1.2019. J Natl Compr Canc Netw 17: 255–289, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, Herzog CA, Joannidis M, Kribben A, Levey AS, MacLeod AM, Mehta RL, Murray PT, Naicker S, Opal SM, Schaefer F, Schetz M, Uchino S: Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 15.Muriithi AK, Leung N, Valeri AM, Cornell LD, Sethi S, Fidler ME, Nasr SH: Biopsy-proven acute interstitial nephritis, 1993-2011: A case series. Am J Kidney Dis 64: 558–566, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Izzedine H, Mathian A, Champiat S, Picard C, Mateus C, Routier E, Varga A, Malka D, Leary A, Michels J, Michot JM, Marabelle A, Lambotte O, Amoura Z, Soria JC, Kaaki S, Quellard N, Goujon JM, Brocheriou I: Renal toxicities associated with pembrolizumab. Clin Kidney J 12: 81–88, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitchlu A, Fingrut W, Avila-Casado C, Chan CT, Crump M, Hogg D, Reich HN: Nephrotic syndrome with cancer immunotherapies: A report of 2 cases. Am J Kidney Dis 70: 581–585, 2017 [DOI] [PubMed] [Google Scholar]
- 18.El Bitar S, Weerasinghe C, El-Charabaty E, Odaimi M: Renal tubular acidosis an adverse effect of PD-1 inhibitor immunotherapy. Case Rep Oncol Med 2018: 8408015, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charmetant X, Teuma C, Lake J, Dijoud F, Frochot V, Deeb A: A new expression of immune checkpoint inhibitors’ renal toxicity: When distal tubular acidosis precedes creatinine elevation. Clin Kidney J, 2019. Available at: 10.1093/ckj/sfz051. Accessed 13 November, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirali AC, Perazella MA, Gettinger S: Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis 68: 287–291, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Spanou Z, Keller M, Britschgi M, Yawalkar N, Fehr T, Neuweiler J, Gugger M, Mohaupt M, Pichler WJ: Involvement of drug-specific T cells in acute drug-induced interstitial nephritis. J Am Soc Nephrol 17: 2919–2927, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Juarez G, Perez JV, Caravaca-Fontán F, Quintana L, Shabaka A, Rodriguez E, Gadola L, de Lorenzo A, Cobo MA, Oliet A, Sierra M, Cobelo C, Iglesias E, Blasco M, Galeano C, Cordon A, Oliva J, Praga M; Spanish Group for the Study of Glomerular Diseases (GLOSEN) : Duration of treatment with corticosteroids and recovery of kidney function in acute interstitial nephritis. Clin J Am Soc Nephrol 13: 1851–1858, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.González E, Gutiérrez E, Galeano C, Chevia C, de Sequera P, Bernis C, Parra EG, Delgado R, Sanz M, Ortiz M, Goicoechea M, Quereda C, Olea T, Bouarich H, Hernández Y, Segovia B, Praga M; Grupo Madrileño De Nefritis Intersticiales : Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int 73: 940–946, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA; National Comprehensive Cancer Network : Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol 36: 1714–1768, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pannu N, James M, Hemmelgarn B, Klarenbach S; Alberta Kidney Disease Network : Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol 8: 194–202, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdel-Wahab N, Shah M, Suarez-Almazor ME: Adverse events associated with immune checkpoint blockade in patients with cancer: A systematic review of case reports. PLoS One 11: e0160221, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delyon J, Brunet-Possenti F, Leonard-Louis S, Arangalage D, Baudet M, Baroudjian B, Lebbe C, Hervier B; PATIO Group : Immune checkpoint inhibitor rechallenge in patients with immune-related myositis. Ann Rheum Dis 78: e129, 2019 [DOI] [PubMed] [Google Scholar]
- 28.Simonaggio A, Michot JM, Voisin AL, Le Pavec J, Collins M, Lallart A, Cengizalp G, Vozy A, Laparra A, Varga A, Hollebecque A, Champiat S, Marabelle A, Massard C, Lambotte O: Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer [published online ahead of print June 6, 2019]. JAMA Oncol doi: 10.1001/jamaoncol.2019.1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abu-Sbeih H, Ali FS, Naqash AR, Owen DH, Patel S, Otterson GA, Kendra K, Ricciuti B, Chiari R, De Giglio A, Sleiman J, Funchain P, Wills B, Zhang J, Naidoo J, Philpott J, Gao J, Subudhi SK, Wang Y: Resumption of immune checkpoint inhibitor therapy after immune-mediated colitis. J Clin Oncol 37: 2738–2745, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ricciuti B, Dahlberg SE, Adeni A, Sholl LM, Nishino M, Awad MM: Immune checkpoint inhibitor outcomes for patients with non-small-cell lung cancer receiving baseline corticosteroids for palliative versus nonpalliative indications. J Clin Oncol 37: 1927–1934, 2019 [DOI] [PubMed] [Google Scholar]