Abstract
In this Opinion, the antimicrobial‐resistant bacteria responsible for transmissible diseases that constitute a threat to the health of certain kept fish species have been assessed. Atlantic salmon (Salmo salar), carp (Cyprinus spp.), rainbow trout (Oncorhynchus mykiss), sea bream (Sparus aurata) and tilapia (Oreochromis spp.), selected as representative of the most important fish species and production systems that are commercially reared in fresh and saltwater farms, were the focus of this assessment. The assessment was performed following a methodology based on information collected by an extensive literature review and expert judgement. Details of the methodology used for this assessment are explained in a separate Opinion. The global state of play of antimicrobial resistance in Aeromonas hydrophila, Aeromonas salmonicida, Flavobacterium psychrophilum and Flavobacterium columnare is provided. Among these bacteria, none was identified as being among the most relevant antimicrobial‐resistant bacteria in the assessed kept fish species in the EU due to the very limited scientific evidence available.
Keywords: Animal Health Law, antimicrobial resistance, extensive literature review, fish
1. Introduction
EFSA received a mandate from the European Commission to investigate the global state of play as regards resistant animal pathogens that cause transmissible animal diseases (Term of Reference (ToR) 1), to identify the most relevant bacteria in the EU (first part of ToR 2), to summarise the current or potential animal health impact of those most relevant bacteria in the EU (second part of ToR 2) and to perform the assessment of those bacteria to be listed and categorised according to the criteria in Article 5, Appendix D according to Articles 9 and 8 within the Regulation (EU) 2016/429 on transmissible animal diseases (‘Animal Health Law’)1 (ToR 3).
This Scientific Opinion presents the global state of play for resistant animal pathogens that cause transmissible animal diseases (ToR 1) and the results of the assessment of the most relevant bacteria in the EU (first part of ToR 2) for fish following the methodology described in EFSA AHAW Panel (2021).
1.1. Background and Terms of Reference as provided by the requestor
The background and ToR as provided by the European Commission for the present document are reported in Sections 1.1 and 1.2 of the scientific Opinion on the ad hoc method to be followed for the assessment of animal diseases caused by bacteria resistant to antimicrobials within the Animal Health Law (AHL) framework (EFSA AHAW Panel, 2021).
1.2. Interpretation of the Terms of Reference
The interpretation of the ToR is as in Sections 1.3.1 and 1.3.2 of the scientific Opinion on the ad hoc method to be followed for the assessment of animal diseases caused by bacteria resistant to antimicrobials within the AHL framework (EFSA AHAW Panel, 2021).
The present document reports the results of the assessment of bacterial pathogens resistant to antimicrobials in fish.
2. Data and methodologies
The methodology applied for this Opinion is described in a dedicated document that details the ad hoc method for the assessment of animal diseases caused by bacteria resistant to antimicrobials within the AHL framework (EFSA AHAW Panel, 2021). Additional methods specific to this Opinion (data collection by an extensive literature review) are detailed below.
2.1. Extensive literature review
The process to identify the bacterial species to focus on in the extensive literature review (ELR) is described in Section 2.1.2 in the ad hoc method for the assessment of animal diseases caused by bacteria resistant to antimicrobials within the AHL (EFSA AHAW Panel, 2021). According to that methodology, the following target bacteria had been agreed upon by the EFSA Working Group: Aeromonas hydrophila, Aeromonas salmonicida, Flavobacterium columnare and Flavobacterium psychrophilum. The current assessment focused on the following kept fish species: Atlantic salmon (Salmo salar), carp (Cyprinus spp.), rainbow trout (Oncorhynchus mykiss), sea bream (Sparus aurata) and tilapia (Oreochromis spp.). These kept fish species were selected to represent the most important species and production systems for commercially rearing fish in fresh and saltwater farms.
According to FAO, the main producer countries of those selected kept fish species are reported in Figure 1. The information made available by FAO (FAO, 2020b, 2021) describing the yearly production size of the industry related to those species are summarised in the following text.
Figure 1.
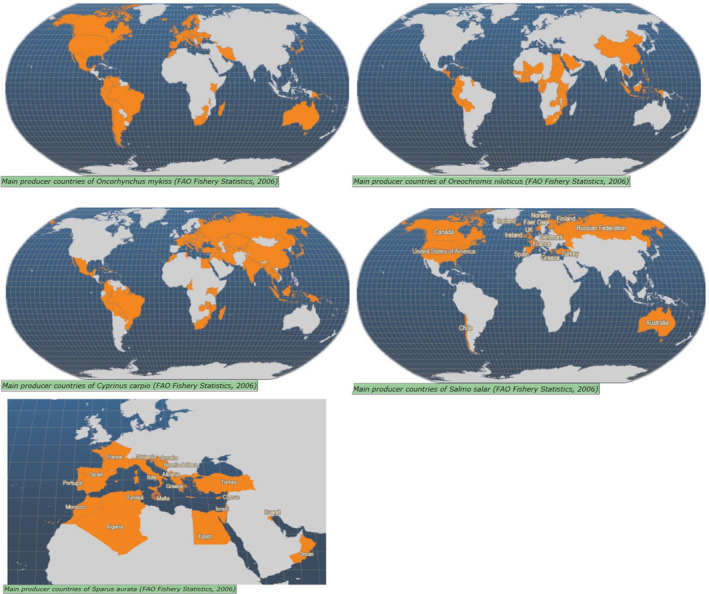
Kept common carp production represented, with more than 4,190,000 tonnes, 7.7% of the total global farmed finfish production in 2018. Common carp production increased by an average global rate of 9.5% per year between 1985 and 2002 (FAO, 2022a). A large proportion of production (e.g. 71% in 2018) takes place in China.
Tilapia (including all species) is the second most important group of kept fish after carps, and the most widely grown of any kept fish. In 2018, the production of Nile tilapia accounted with more than 4,500,000 tonnes for 8.3% of global finfish production. The biggest producer of tilapia was China, with more than 1,200,000 tonnes annual production. Indonesia reported nearly 1,200,000 tonnes of production, followed by Egypt, Brazil, Thailand and the Philippines (FAO, 2020a). Laos, Costa Rica, Ecuador, Colombia, Honduras and Taiwan are also major producers of Nile tilapia. While consumption of tilapia is very common in many parts of the world, Europeans are just discovering it and great potential exists in Europe for market expansion (FAO, 2022c).
Atlantic salmon culture began in the 19th century in the UK, and sea cage culture was first used in Norway in the 1960s to raise Atlantic salmon to marketable size. The early successes in Norway prompted the development of salmon culture in Scotland, and latterly in Ireland, the Faroe Islands, Canada, the north eastern seaboard of the USA, Chile and Australia (Tasmania). Minor production also occurs in New Zealand, France and Spain. All of the major production areas lie within latitudes 40–70° in the Northern Hemisphere, and 40–50° in the Southern Hemisphere. More than 2,400,000 tonnes of kept Atlantic salmon are produced worldwide annually, representing 4.5% of the farmed finfish production (data from 2018) (FAO, 2020b). Because of rapid increases in production over the last 10–15 years, ex‐farm prices have fallen sharply. This and the decreasing availability of suitable sites have limited further growth of production in the Northern Hemisphere. The most rapidly growing supplier now is Chile, which has lower labour and materials costs and can therefore compete effectively with traditional producing countries in distant markets. The major markets for kept Atlantic salmon are Japan, the European Union and North America (FAO, 2022d).
The production of rainbow trout was nearly 850,000 tonnes worldwide in 2018, 1.6% of total global finfish production (FAO, 2020b), with Iran being the largest producer. Other major producing countries include Turkey, Chile, Norway, Peru, China, Russia, Italy, Denmark, France, Colombia, USA, Poland, the UK and Spain (FAO, 2020a). Many other countries have reported farming production, some of them have relatively insignificant output in comparison with the larger producers (FAO, 2022b).
Global gilthead seabream production was nearly 230,000 tonnes in 2018. Most production occurs in the Mediterranean, with Turkey and Greece being the largest producers. Egypt, Tunisia, Spain and Italy are also major Mediterranean producers. In Europe, considerable production occurs also in Croatia, Cyprus, Albania and Malta (FAO, 2020a). There is also gilthead seabream production in the Red Sea, the Persian Gulf and the Arabian Sea (FAO, 2022e).
The ELR was carried out by the University of Copenhagen under the contract OC/EFSA/ALPHA/2020/02 – LOT 12. On 7 June 2021, two different search strings (Appendix A) were applied in PubMed and Embase, respectively, resulting in a search result of 177 unique abstracts published since 2010. Upon import into the Rayyan software, these abstracts were screened by a senior scientist who followed the criteria described in the protocol for inclusion and exclusion of studies. When available, the full text of abstracts was downloaded into the EndNote software. In addition, the most recent national antimicrobial resistance (AMR) monitoring report from Sweden was downloaded and used in the ELR. Only the latest version of the AMR monitoring report was included in the ELR as isolates included in the report can be assumed to originate from the same sampled populations and most recent versions would therefore include the most up‐to‐date data on AMR. The previous versions of the national AMR monitoring reports, that is up to the previous 5 years, were not included in the ELR but were downloaded and analysed separately to assess changes over time when possible. Data on AMR in the full texts of national reports were evaluated for eligibility by applying the exclusion criteria as described in the ad hoc method followed for the assessment of animal diseases caused by bacteria resistant to antimicrobials within the AHL framework (EFSA AHAW Panel, 2021).
Year of bacterial isolation was neither extracted nor reported from the included studies, as in most studies, isolates had been collected over multiple years with no indication of the number of isolates per year. An exception to this rule was if only data from a certain time period within a study were extracted.
Information extracted from the eligible assessed full‐text reports/publications is described in the scientific Opinion describing the ad hoc method applied in the assessment (EFSA AHAW Panel, 2021). Information on all the full‐text studies that were assessed, including the reason for exclusion for those excluded at the full‐text screening, is presented in Appendix B. AMR was assessed for clinically relevant antimicrobials within the AMEG categories B–D. The antimicrobials deemed as clinically relevant are ampicillin, enrofloxacin, florfenicol, flumequine, oxolinic acid, sulfonamide–trimethoprim and oxytetracycline. When more than one antimicrobial from a given class was considered eligible for inclusion in the report, the following order of preference for each antimicrobial class and bacterial pathogen was considered:
For fluoroquinolone resistance data, the order of preference was enrofloxacin > ciprofloxacin
For aminopenicillin resistance data, the order of preference was ampicillin > amoxicillin
For tetracycline resistance data, the order of preference was tetracycline > oxytetracycline > doxycycline > chlortetracycline
For each study, resistance data were extracted as resistance (%R) alone and/or including the intermediate category (%R + I). The following assumptions and decisions were made when evaluating data sets:
When no information on the I category was provided in a study, it was assumed that the reported %R was equal to %R.
When the proportion of susceptibility (%S) was reported with no information on I, it was not possible to calculate %R. Instead, we calculated %R + I as 100% – %S.
When a study using epidemiological cut‐off values (ECVs) reported %R, this was considered as %R + I, as the I category is always part of the non‐wild‐type (NWT) population.
When %I was reported separately, it was extracted that along with %R and calculated %R + I.
For some drugs and presence of mecA/mecC, there is no I category for the bacterial species included; therefore for these only %R was reported, irrespective of the assumptions mentioned above.
Due to the way in which the search string was built (which required that certain fish species were mentioned in order for a study to be retrieved), the databases consulted (PubMed and Embase) and the inclusion criteria (requiring the use of a specific breakpoint for data interpretation), several studies known to the Working Group with AMR data on the pathogens and fish species of interest for this Opinion were excluded in the ELR. Because of the very limited information retrieved in the original search, relevant AMR data from these studies on phenotypic resistance to the antimicrobials and pathogens of interest were extracted and interpreted using CLSI breakpoints (CLSI, 2020b) and are mentioned in the pathogen‐specific sections. These studies ultimately considered by the Working Group also fulfilled the required minimum quality standards (e.g. use of international or national standard methods interpreted by standard criteria), but had not been retrieved through the ELR due to not being in PubMed or Embase databases, to the search string used (requiring that one of the selected fish species was used as MeSH term or included in the title/abstract), or other inclusion criteria (lack of breakpoint used for data interpretation, isolates not defined at the species level, e.g. Aeromonas hydrophila complex).
3. Assessment
3.1. ToR 1: Global state of play for resistant bacterial animal pathogens that cause transmissible animal diseases
3.1.1. General overview of studies included and excluded
3.1.1.1. Data from the extensive literature review
After screening the 177 abstracts, 24 publications were selected for evaluation according to the criteria described in the Methods sections. Of these, 18 publications were excluded with the reasons for exclusion highlighted in columns D and E of Appendix B. The reasons for exclusion of studies are listed in Table 1. The two most common reasons for exclusion were a too low number of isolates investigated (n = 5) and failure to use or report a standard for susceptibility testing (n = 4).
Table 1.
Main reasons for exclusion of studies after full‐text evaluation affecting more than one study (a study could be excluded for more than one reason)
| Reason | Code in Appendix B | Number of studies |
|---|---|---|
| Fewer than the minimum number of isolates are included in the study | 8 | 5 |
| Study does not follow a standard for antimicrobial susceptibility testing or a standard is not reported | 4 | 4 |
| Inclusion of non‐clinical isolates that cannot be distinguished from clinical isolates | 5 | 2 |
| All isolates in a study originate from the same farm | 15 | 2 |
| AMR data from several bacterial species mixed together | 17( a ) | 2 |
| AMR data reported at bacterial genus level or above | 3 | 1 |
| MIC data reported without interpretation into susceptible/resistant | 12 | 1 |
Specified in column E, Appendix B.
In total, six eligible publications from the ELR were selected for data extraction. In addition, one national report representing Sweden was selected, as it contained eligible AMR data for fish, resulting in a total of seven references finally included.
An overview of the number of eligible studies found in the ELR for each target bacterium is shown in Table 2.
Table 2.
Number of references obtained through the ELR from which AMR data were extracted
| Bacterial species | Number of eligible studies for data extraction |
|---|---|
| Aeromonas hydrophila | 5 |
| Flavobacterium psychrophilum | 2 |
| Aeromonas salmonicida | 0 |
| Flavobacterium columnare | 0 |
Figure 2 provides an overview of the seven included references found in the ELR sorted by year of publication.
Figure 2.
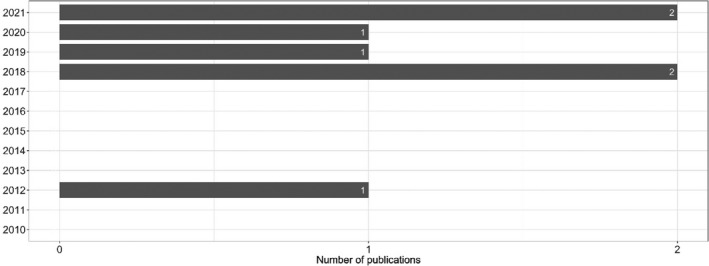
The seven included publications arranged by year of publication
Considering geographical distribution, AMR data were reported in three references from Africa (all from Egypt), two from Europe (both from Sweden) and two from Asia (one from Iraq and another from Thailand) (Figure 3).
Figure 3.
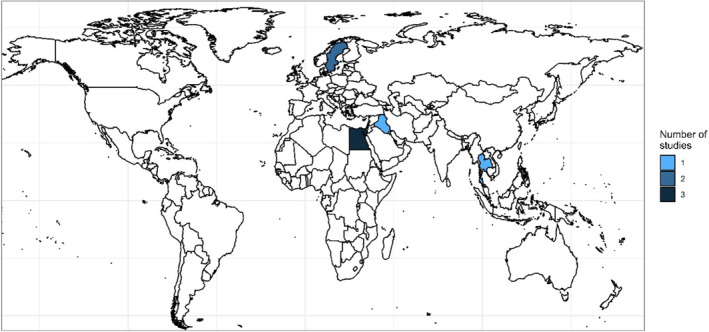
Geographical distribution of the seven included references
Based on the type of isolates analysed, references included were divided into those with isolates derived from a defined population of farmed fish, and those without – or with limited – background information on sampled fish. The latter category comprised studies with isolates from a diagnostic laboratory. Six studies had isolates obtained from samples actively collected in fish farms, whereas one study had isolates from a diagnostic laboratory, without further specification. No information on previous antimicrobial treatments was provided in the selected references.
3.1.1.2. Data from national AMR monitoring reports
Additional details/data on one of the pathogens of interest of this Opinion that are provided in previous versions of one national AMR monitoring report retrieved (up to the previous 5 years), namely Swedres‐Svarm (Sweden), were also extracted and are presented in the following section.
3.2. AMR frequency data
The following pathogen‐specific sections summarise the AMR data obtained for bacterial pathogens in fish.
In general, AMR data from different studies are extremely difficult to compare due to differences in study design, population, methods, interpretive criteria, etc. The number of antimicrobial susceptibility testing (AST) results for any given antimicrobial extracted from the seven selected references (total of 589; Appendix B) was due to results obtained in A. hydrophila (n = 361, 61.3% of all AST) and F. psychrophilum (n = 228, 38.7%). The laboratory methods applied included disk diffusion (n = 261, 44.3% of all tests), broth microdilution (n = 228, 38.7%) and agar dilution (n = 100, 17.0%) (Appendix B).
Furthermore, the definition of AMR differed across studies, as the I category defined by clinical breakpoints (CBPs) was included in the calculation of AMR frequencies in some studies, whereas it was omitted in others. Accordingly, in the figures with resistance data, we have illustrated for each study whether %R or %R + I was reported; therefore, this should be taken into account when comparing studies. It is also important to mention that no infection‐specific and host‐specific CBPs exist for pathogens in fish. This complicates the interpretation of data, as for some studies, it was unclear if the CBPs used were adapted from other bacterial or animal species, from humans, or even defined by the author. Taken together, the outcomes of the present report should be interpreted and cited with caution, as all specificities of individual studies cannot be taken into consideration. To support conclusions made from the figures or tables (e.g. a high proportion of resistance in a certain country/continent), it is strongly recommended that individual papers are consulted and checked to determine if results have been biased by previous antimicrobial treatment, sampling of animals in a certain environment, the use of certain diagnostic methods or breakpoints, or other factors.
For data included in the national AMR monitoring Swedres‐Svarm reports, details/data provided in the previous versions of the reports (up to the previous 5 years) were extracted and are presented at the end of the relevant bacterium (F. psychrophilum). Antimicrobial susceptibility tests were performed using the broth microdilution method according to CLSI guidelines (CLSI, 2020b), and test results were interpreted using the epidemiological cut‐offs (ECOFFs) proposed by Smith et al. (2016).
3.2.1. Aeromonas hydrophila
3.2.1.1. Results of the ELR by bacterium
Aeromonas hydrophila (Aeromonadales; Aeromonadaceae) is an opportunistic pathogen in freshwater fish that can cause ulcers, haemorrhages, furunculosis and haemorrhagic septicaemia, therefore compromising animal health and leading to severe economic losses. An appropriate standard testing method in this species has been available for over a decade, but ECV were only published in 2020 (VET04) (CLSI, 2020b).
In total, five studies with ≥ 10 A. hydrophila isolates and results for one or more of the relevant antimicrobials (ampicillin, enrofloxacin, florfenicol, flumequine, oxolinic acid, oxytetracycline and sulfonamide–trimethoprim) were included. Among these studies, three included isolates from Africa (all from Egypt) and two from Asia (Thailand and Iraq), respectively. All isolates were retrieved from Nile tilapia (Oreochromis niloticus) except those in the study from Iraq, which originated from carp. A. hydrophila isolates were retrieved from a mixture of organs including kidney, liver, spleen, pancreas, stomach, intestine and skin.
Figure 4 shows for each country the proportion of resistance reported in individual studies with at least 10 A. hydrophila isolates.
Figure 4.
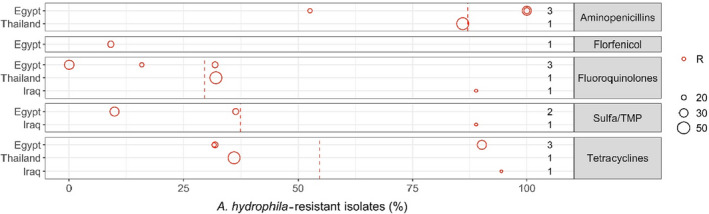
Aeromonas hydrophila resistance data for each included study sorted by country
- Each circle represents one study, and the size of each circle reflects how many isolates were included in the study. The colour of a circle illustrates resistance (red circle). The dashed lines indicate, for each antimicrobial, the weighted arithmetic mean of %R. The exact percentages that these lines represent are listed in Appendix C. Numbers written to the left of antimicrobial names reflect the number of studies for a certain drug/country combination.
Four of the five studies reporting susceptibility data for A. hydrophila were published in 2019 or later. None of these studies referred to the CLSI guidelines specific for bacteria in aquatic animals.
Out of the three papers with data on isolates from Nile tilapia from Egypt, one study (Algammal et al., 2020) evaluated the AST profiles for four antimicrobials in the categories of interest for this Opinion (amoxicillin, ciprofloxacin, sulfonamide–trimethoprim and tetracycline) of 61 isolates using the disk diffusion method. Of note, no information on the temperature used in the AST is provided, but references provided suggest it would be either 22°C or 28°C, although isolates were then classified as S, I or R according to a reference that only provides clinical breakpoints for 35°C. In the light of this limitation, results should be interpreted carefully as they may not be indicative of the true resistance of the isolates. Their results suggest very high levels of resistance to amoxicillin (100%) and tetracycline (90.1%) and low or very low levels for sulfonamides–trimethoprim (9.9%) and ciprofloxacin (0%).
Another study from Egypt on Nile tilapia using disk diffusion (El‐Bahar et al., 2019) showed very high resistance levels to aminopenicillins (in this case, ampicillin; 100%), but considerably higher levels for ciprofloxacin (31.8%) and sulfamethoxazole–trimethoprim (36.4%), and low levels of resistance to florfenicol (9.1%). Of note this study was conducted in only 22 isolates and used a standard method and human clinical breakpoints for interpretation of results.
The third study from Egypt (Zaher et al., 2021) was also based on disk diffusion but provided a reference with a non‐standard method for the medium and temperature (30°C), and then classified isolates as sensitive, intermediate and resistant according to a breakpoint designed for a different media and temperature (35°C). Hence, results should also be interpreted with care, as highlighted by the surprisingly low levels of resistance found for ampicillin among the 19 isolates tested (52.6%, with 32% of the remaining isolates classified as intermediate). The proportion of resistant isolates was also low for tetracycline (31.6%) and compared with the two previous references for ciprofloxacin (15.8%).
In the study from Thailand (Lukkana et al., 2012), AST results obtained through the agar dilution method on 50 isolates retrieved from Nile tilapia were also interpreted according to human CBP. In this case, the higher proportion of resistance was found for amoxicillin (86%), while values for enrofloxacin and tetracycline were lower (32% and 36%, respectively), with very similar values also obtained for oxytetracycline (34%).
Finally, in the study from Iraq (Taha et al., 2021), 24 A. hydrophila were tested using the disk diffusion method and results were interpreted also according to human breakpoints. In this case, very high proportions of resistance were described for three antimicrobials of the classes of interest, i.e. ciprofloxacin (89%), doxycycline (94%) and sulfonamide–trimethoprim (89%).
Only one study reported susceptibility data for florfenicol: El‐Bahar et al. (2019) found 9% of 22 Egyptian A. hydrophila isolates resistant to this antimicrobial.
It is important to note here the difficulty in trying to differentiate among diverse Aeromonas species on the basis of phenotypical traits or even 16S rRNA gene sequencing (Beaz‐Hidalgo et al., 2010) and, therefore, in some of these articles, assignation of isolates to an Aeromonas species or complex must be taken with caution. In addition to these papers, another study (Scarano et al., 2018) included information that was considered useful by the Working Group although it had not be included in the results of the ELR since it did not fulfil the inclusion criteria. Scarano et al. (2018) used the broth microdilution method to determine the MIC of 15 antimicrobials against 104 Aeromonas spp. isolated from Sparus aurata in Italy, 21 of which were assigned to A. hydrophila or the A. hydrophila complex using phenotypical and 16S rRNA sequence characterisation. MIC50, MIC90 and ECVs values and the distribution among the different categories were calculated for the whole Aeromonas collection. Resistance (%R + I) to aminopenicillins ranged from 59.60% for ampicillin to 57.7% for amoxicillin, whereas much lower values were obtained for oxolinic acid (37.5%), flumequine (22.1%), florfenicol (2.9%) and oxytetracycline (19.2%).
As described above, three references (Lukkana et al., 2012; Scarano et al., 2018; Zaher et al., 2021) reported different proportions of susceptible isolates to amoxicillin and/or ampicillin in their bacterial panels, even though mesophilic Aeromonads are considered intrinsically resistant to aminopenicillins (CLSI, 2016), thus highlighting the difficulties associated with the evaluation of the resistance phenotype in A. hydrophila isolates (and in bacteria from aquatic animals in general), a process in which small variations may lead to significant variations in the results.
3.2.2. Flavobacterium psychrophilum
3.2.2.1. Results of the ELR by bacterium
Flavobacterium psychrophilum (Flavobacteriales; Flavobacteriaceae) is the causative agent of Bacterial Cold Water Disease (BCWD) as well as rainbow Trout Fry Syndrome (RTFS). These infections can be associated with high mortality in young fish in particular.
Standard methods with appropriate quality controls have been published by CLSI (VET03) (CLSI, 2020a) for determining MIC values of F. psychrophilum. Some ECVs applicable to the data generated by these tests have been published in VET04 (CLSI, 2020b).
Two Swedish studies with ≥ 10 F. psychrophilum isolates and results for one or more of the relevant antimicrobials were included. Both studies included mainly isolates from rainbow trout. From one study, 20 isolates – mostly of kidney origin – collected between 2014 and 2016 were included (Soderlund et al., 2018). The other study comprised 94 isolates collected between 2015 and 2018 from non‐specified organ sites (Swedres‐Svarm, 2019). Although not stated, it cannot be ruled out that some overlap exists in these isolate collections.
None of the studies found resistance to florfenicol, whereas 75% (Soderlund et al., 2018) and 55% (Swedres‐Svarm, 2019) of isolates were resistant to oxolinic acid. Slightly higher proportions of resistance were detected to oxytetracycline (80% and 76%) in the two studies that, for all drugs, used the tentative ECOFFs proposed by Smith et al. (2016) for interpretation of susceptibility data.
Six studies known to the Working Group with AMR data on F. psychrophilum in fish species of interest for this Opinion were left out of the ELR performed by the contractor. Four of these studies, three including isolates from Europe (Smith et al., 2016; Ngo et al., 2018; Saticioglu et al., 2019) and one from North America (USA) (Van Vliet et al., 2017), used a standard CLSI microdilution method. These studies provided their raw MIC data and, therefore, these could be interpreted using the ECVs in VET04 (CLSI, 2020b). The frequency of NWT (%R + I) found in these studies is presented in Table 3 for all antimicrobials except florfenicol, which was tested in all isolates but for which only wild‐type strains were found. This susceptibility to florfenicol is in agreement with the evidence shown in the two studies from Sweden retrieved in the literature review (Soderlund et al., 2018; Swedres‐Svarm, 2019).
Table 3.
Proportion of non‐wild‐type (% NWT) isolates according to the ECV proposed by CLSI (VET04) found in F. psychrophilum isolates for which MIC raw data were available
| Reference | Country | Date of isolation | Number of isolates | Antimicrobial | % NWT |
|---|---|---|---|---|---|
| Smith et al. (2016) | Denmark | 1988–2012 | 31 | Oxolinic acid | 75 |
| Oxytetracycline | 25 | ||||
| UK | 2006–2013 | 22 | Oxolinic acid | 70 | |
| Oxytetracycline | 65 | ||||
| Ngo et al. (2018) | UK | 2005–2015 | 118 | Oxolinic acid | 70 |
| Enrofloxacin | 80 | ||||
| Oxytetracycline | 51 | ||||
| Van Vliet et al. (2017) | US | 2008–2013 | 50 | Oxolinic acid | 0 |
| Enrofloxacin | 0 | ||||
| Oxytetracycline | 24 | ||||
| Saticioglu et al. (2019) | Turkey | 2014–2017 | 25 | Oxolinic acid | 88 |
| Enrofloxacin | 88 | ||||
| Oxytetracycline | 51 |
Of note, the three European studies suggested that the proportion of NWT phenotypes to quinolones was high (70–88%) while in the study from the USA only wild‐type phenotypes were reported for oxolinic acid or enrofloxacin. This could reflect differences in antimicrobial use in aquaculture between the two regions, as several members of this antimicrobial class (flumequine, oxolinic acid and enrofloxacin) have received authorisation for use in certain European countries, while their use is not allowed in the USA.
Miranda et al. (2016) determined the susceptibility to antimicrobials commonly used in Chilean fish farming. In total, 125 Chilean isolates of F. psychrophilum isolates from reared salmonids were analysed using an agar dilution MIC method. They used normalised resistance interpretation (NRI) analysis to calculate an ECV (COWT) of ≤ 0.125, ≤ 2 and ≤ 0.5 mg/mL for amoxicillin, florfenicol and oxytetracycline, respectively. For the quinolones, the COWT were ≤ 1, ≤ 0.5 and ≤ 0.125 mg/mL for oxolinic acid, flumequine and enrofloxacin, respectively. These COWT values are, in general, two dilutions lower than the consensus ECVs set by VET04 for MIC data generated under the same incubation conditions by the standard microdilution method. This would suggest that the agar dilution and microdilution methods generate different data and, therefore, it would not be valid to reinterpret the data of Miranda et al. (2016) by applying the consensus ECVs. The frequencies of NWT phenotypes calculated by Miranda et al. (2016) by applying the COWT that were calculated from their own data (Table 4) were very similar to those for the European studies as shown in Table 3.
Table 4.
Epidemiological cut‐offs (COwt) and proportion of NWT isolates among a collection of 125 F. psychrophilum isolates retrieved from reared salmonids in Chile as described in Miranda et al. (2016)
| Antimicrobial | MIC data | |
|---|---|---|
| COwt (µg mL–1) | NWT (%) | |
| Amoxicillin | ≤ 0.125 | 24 |
| Florfenicol | ≤ 2 | 2 |
| Oxytetracycline | ≤ 0.5 | 70 |
| Oxolinic acid | ≤ 1 | 45 |
| Flumequine | ≤ 0.5 | 39 |
| Enrofloxacin | ≤ 0.125 | 38 |
Hesami et al. (2010) in Ontario (Canada) tested 72 isolates from salmonids (rainbow trout, Atlantic salmon and speckled trout Salvelinus fontinalis) using a broth microdilution AST method that differed from the standard method given in VET03. Therefore, there were no acceptable ranges for reference strains that could be applied in this study. Some concern must be expressed about the data presented in this paper. Particularly disturbing is that few if any of the distribution of MIC values for any agent showed a low‐end peak that would be expected from wild‐type isolates. Equally the very different distributions for the very similar agent oxolinic acid and flumequine were inconsistent with the data from other studies. This made it very difficult to determine suitable cut‐off values or to make any reliable estimate of any frequencies of NWT phenotypes.
3.2.2.2. Results from the national AMR monitoring reports
Information on AMR in fish F. psychrophilum originating from Swedish farmed salmonid fish was included in the last annual reports for two antimicrobials of interest in this Opinion (florfenicol and oxolinic acid). Data are reported for multiple years together, preventing the extraction of yearly results, but historic results for the 2004–2018 period were provided in the last report (Swedres‐Svarm, 2019) and indicated that there was an increase in the proportion of isolates resistant to quinolones (nalidixic acid/oxolinic acid) from levels below 40% before 2010 to more than 50% after the present year, with the proportion of resistant isolates in the 2014–2018 period ranging between approximately 40 and 75%.
3.2.3. Aeromonas salmonicida
A. salmonicida (Aeromonadales; Aeromonadaceae) can cause ulcerative and haemorrhagic skin ulcers in fish under stress, and is the causative agent of several relevant fish diseases (furunculosis, goldfish ulcer disease and carp erythrodermatitis).
Although no references fulfilling the inclusion criteria used in the ELR were found, several studies including results on clinical A. salmonicida strains from fish were known to the Working Group members and are described here.
Early studies to determine possible ECVs for A. salmonicida were made by two groups (Table 5). Miller and Reimschuessel (2006) assessed the resistance phenotype from four antimicrobials in 217 isolates from fish retrieved in eight countries in North America and Europe (~half of the isolates originated from the USA) (Miller and Reimschuessel, 2006). Applying the ECVs in VET04, all the isolates studied had WT phenotypes for florfenicol, while the proportion of NWT isolates for the other antimicrobials were 9% (ormetoprim–sulfadimethoxine), 13% (oxolinic acid) and 25% (oxytetracycline). Interestingly, the oxolinic acid MIC obtained in isolates coming from the USA were consistently lower than those found in isolates from other countries (with all US strains falling in the wild‐type population for this antimicrobial), indicating again a possible effect of the different antimicrobial usage in aquaculture depending on the country. Smith et al. (2007) used disc diffusion to study the susceptibility of 106 isolates from five European countries. Applying the ECVs in VET04 to the data generated in these studies, all the isolates studied had wild‐type phenotypes for florfenicol, while the proportions of NWT isolates for the other antimicrobials were 25% (trimethoprim–sulfamethoxazole), 56% (oxolinic acid) and 48% (oxytetracycline).
Table 5.
Frequencies of NWT phenotypes in studies on A. salmonicida
| Miller and Reimschuessel (2006) | Smith and colleagues( a ) | Baron et al. (2021) | ANSES( b ) | CEFAS( b ) | |
|---|---|---|---|---|---|
| Method | MIC | Disc | MIC | MIC | MIC |
| Number of isolates | 217 | 106 | 74 | 50 | 154 |
| Florfenicol | 0 | 0 | 25 | 18 | 2 |
| Oxytetracycline | 25 | 48 | – | 51 | 16 |
| Oxolinic acid | 13 | 56 | 95 | 91 | 30 |
| Trimethoprim–sulfamethoxazole | – | 25 | – | – | – |
| Ormetoprim–sulfadimethoxine | 9 | – | – | – | – |
A recent study reporting the agreement between AST results obtained by disc diffusion and broth microdilution at 22°C included AMR information for three antimicrobials of interest on 74 A. salmonicida isolates from diseased fish in France (Baron et al., 2021). When results were interpreted according to ECVs in VET04, the proportion of NWT strains was highest for oxolinic acid (95–96%), medium for tetracycline (59–66%) and lowest for florfenicol (24–27%).
Two yet unpublished MIC studies providing data on the susceptibility of A. salmonicida were known to the Working Group. Both used the standard CLSI testing method (VET03) and interpreted their data with the ECVs in VET04. CEFAS studied 50 UK isolates and demonstrated that the frequencies of NWT were 2% for florfenicol, 30% for oxolinic acid and 16% for oxytetracycline. ANSES studied 154 French isolates and demonstrated that the frequencies of NWT were 18% for florfenicol, 91% for oxolinic acid and 51% for oxytetracycline.
Duman et al. (2020) studied 102 motile Aeromonas isolates recovered from multiple fish species, some of which had clinical signs, and water in Turkey. They categorised 18% of their isolate as A. salmonicida. As A. salmonicida is a non‐motile species, this is somewhat confusing. They reported that A. salmonicida was the species with the highest proportion of isolates carrying florfenicol, sulfonamide and tetracycline resistance genes. However, as they also recorded that resistance genes were very common in isolates that were phenotypically wild type, it is difficult to know what value to give these genetic studies.
Most phenotypic resistance profiles in Duman et al. (2020) are provided combined for all 102 isolates, and therefore, it was not possible to differentiate A. salmonicida‐specific results. Tetracycline was the only agent for which MIC data specifically on the 18 A. salmonicida isolates were presented. Unfortunately, CLSI have not published quality control requirements or ECVs for data generated for this agent using the testing method adopted in this work. There were too few putative wild‐type isolates to allow an NRI analysis of these A. salmonicida data for tetracycline; however, a visual examination would suggest that approximately half of the isolates would be categorised as NWT. This figure would be in agreement with other European studies.
3.2.4. Flavobacterium columnare
Flavobacterium columnare (Flavobacteriales; Flavobacteriaceae) is a Gram‐negative gliding bacterium ubiquitous in freshwater environments. The bacterium causes columnaris disease in a large variety of wild and cultured freshwater fish throughout the world.
No eligible papers were found by conducting the ELR on F. columnare. However, two studies that included results on clinical F. columnare strains from fish were known to the Working Group members and are described here.
Declercq et al. (2013) assessed the in vitro antimicrobial susceptibility of 97 F. columnare isolates collected worldwide between 1987 and 2011 from 17 fish species using the broth microdilution technique. None of the isolates displayed acquired resistance to florfenicol, gentamicin, ormetoprim–sulfadimethoxine and trimethoprim‐sulfamethoxazole. Acquired resistance to chloramphenicol was detected in 1%, to nitrofuran in 5%, to oxytetracycline in 11% and to enrofloxacin, flumequine and oxolinic acid in 10%, 16% and 16%, respectively, of the isolates. The isolates displaying acquired resistance originated from species other than the ones of interest in this Opinion (ornamental fish species or Vietnamese catfish, except for two isolates coming from wild channel catfish in which acquired resistance was encountered towards oxytetracycline only).
Gieseker et al. (2016) using the optimised protocol of the broth microdilution method analysed the susceptibility of 120 F. columnare isolates against antimicrobials commonly used in aquaculture, including ampicillin, erythromycin, florfenicol, oxytetracycline, enrofloxacin, flumequine, oxolinic acid, gentamicin, ormetoprim/sulfadimethoxine and trimethoprim–sulfamethoxazole. Isolates were obtained from five different countries and 20 fish species, including different salmonids, carps and catfish among others. Frequencies of NWT isolates [inferred according to the interpretative criteria for F. columnare included in the CLSI VET04 document] were 3.3% for ampicillin and florfenicol, 5.8% for erythromycin, 5% for oxytetracycline and enrofloxacin, 7.5% for flumequine and 6.6% for oxolinic acid.
3.3. ToR 2: identifying the most relevant bacteria in the EU
Following the methodology presented in the scientific Opinion on the ad hoc method for the assessment of animal diseases caused by bacteria resistant to antimicrobials within the AHL framework (EFSA AHAW Panel, 2021), the evidence available was assessed individually by Working Group members who had expertise in aquaculture and who provided individual judgements on the perceived relevance to certain kept fish species of the antimicrobial‐resistant bacteria included in the list.
After discussion of the individual judgements for each bacterium (relevant/non‐relevant/cannot be assessed based on available evidence), it was agreed that based on the available evidence, it was not possible to identify with sufficient certainty (i.e. > 66%) any of the pathogens assessed among the most relevant antimicrobial‐resistant bacteria in certain kept fish species in the EU (Figure 5).
Figure 5.
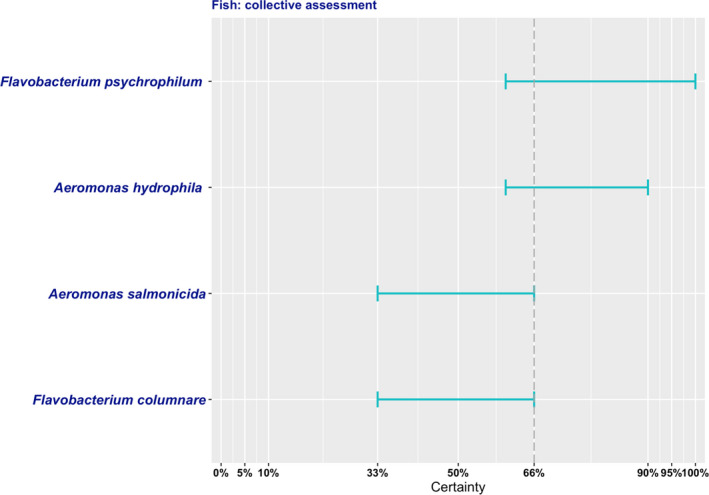
Level of certainty for the inclusion of the selected antimicrobial‐resistant pathogens of certain kept fish species among the most relevant in the EU
Aeromonas hydrophila, Aeromonas salmonicida, Flavobacterium columnare and Flavobacterium psychrophilum are highly relevant pathogens in the EU in relation to the fish species considered here, due to their wide distribution, and the frequent usage of antimicrobials required for their treatment with the possible exception of A. salmonicida, for which effective vaccines are available. Given the very limited available therapeutic options that are licensed to treat infected fish, resistance to any of those licensed antimicrobials has a major importance. Evidence of widespread resistance to some of these antimicrobials (e.g. tetracyclines and quinolones) has been found for the four pathogens considered here, further limiting the alternatives for treatment. Decreased levels of susceptibility (i.e. increase in MIC values) to antimicrobials commonly used in aquaculture such as tetracycline, oxytetracycline and trimethoprim were already reported in Aeromonas in 1991 (Inglis et al., 1991). The reported widespread circulation of strains with reduced susceptibility to tetracyclines in particular (> 30–40%) has been attributed to the generalised use of this antimicrobial class in aquaculture for the treatment of infections due to pathogenic Aeromonas species (Duman et al., 2020). Nevertheless, many uncertainties were identified in the assessment, preventing the inclusion of any of the four pathogens among the most relevant AMR bacteria in the fish species evaluated here, including the lack of clinical breakpoints (and the availability of standardised ECVs implemented only very recently), and the possible geographic variations in the relative importance of phenotypically resistant strains belonging to one or more of the species assessed (which cannot be taken into account given the very limited data from only a few countries). However, F. psychrophilum and A. hydrophila were the two highest ranked bacterial species due to the high prevalence of infection in the kept fish species considered here and the limited options for preventing/limiting its impact beyond antimicrobial treatment. For A. salmonicida, the availability of effective vaccines resulted in a lower ranking, although difficulties associated with vaccination (e.g. cost, need for individually handling each fish) may prevent its use in certain circumstances, therefore resulting in the use of antimicrobials. Finally, F. columnare was also ranked lower due to the availability of other preventive measures related to improved husbandry that may also help to limit its impact without necessarily relying only on antimicrobial treatment. However, it should be noted that most countries within the EU have licensed only a very limited number of antimicrobials (3–5) for use in kept fish. Consequently, resistance to any of these agents can have a proportionately greater impact on the therapeutic options available for treatment and, therefore, a potentially larger impact on animal welfare.
4. Conclusions
In this Opinion, EFSA presents the results of the assessment conducted to answer ToR 1 (global state of play of antimicrobial‐resistant animal bacteria) and the first part of ToR 2 (identifying the most relevant resistant bacteria in the EU) according to the ad hoc methodology (EFSA AHAW Panel, 2021). The second part of ToR 2 and ToR 3, namely the animal health impact of the selected species on fish in the EU, and their eligibility for being listed and categorised in the framework of the AHL, will not be assessed in later steps of this EFSA mandate given that none of the pathogens in the scope of this Opinion was included among the most relevant antimicrobial‐resistant pathogens in the selected kept fish species for the EU. EFSA summarised the global state of play on AMR in certain kept fish species (Atlantic salmon, carp, rainbow trout, sea bream and tilapia) for the following bacteria: Aeromonas hydrophila, Aeromonas salmonicida, Flavobacterium columnare and Flavobacterium psychrophilum.
Based on the evidence available and expert Opinion, none of the evaluated bacterial species was included among the most relevant antimicrobial‐resistant pathogens in certain kept fish species in the EU (lower uncertainty ranges for all pathogens were lower than 66%). However, F. psychrophilum and A. hydrophila were ranked higher than the other pathogens based on the limited evidence retrieved, which suggested that resistance to certain clinically relevant antimicrobials may not be uncommon and the limited options for their prevention and control beyond antimicrobial treatment.
The scientific assessment of the global state of play of the resistant bacterial pathogens of certain kept fish species included in this Opinion and of their EU relevance is hampered by several important sources of uncertainty mainly due to a lack of data and to the methodology followed in this assessment, as mentioned in Section 2.4 of EFSA AHAW Panel (2021) and in the preceding sections of this Opinion:
Due to the scope of the ELR, only studies published in the last 10 years and in English were considered eligible, therefore introducing a possible selection bias.
Information on the rationale and study design for the references retrieved in the ELR was limited and very heterogeneous, making the detailed assessment of the representativeness of the isolates included in each study very difficult. For example, it was not always possible to differentiate AMR data from clinical vs. environmental strains. Moreover, isolates included in the studies reviewed often originated from animals subjected to previous antimicrobial treatments, which may have led to higher levels of resistance in tested isolates. In fact, strain collections available at veterinary diagnostic laboratories, in which several of the studies reviewed were based, may in a high proportion of cases have originated from disease outbreaks that were not effectively controlled by antimicrobial treatment at first and were investigated in more detail. Furthermore, strains with abnormal susceptibilities may be more often kept at historical strain collections in diagnostic laboratories, therefore contributing to a bias towards an overrepresentation of isolates with reduced susceptibility to antimicrobials.
Even though only studies that exceeded a minimum quality threshold were included in the assessment (e.g. use of international or national standards), the methodology used was also diverse (e.g. use of disk diffusion or microdilution methods, CBP or ECOFFs, consideration or not of the intermediate category, etc.). Therefore, results provided here should be considered carefully as they may not be representative of the true underlying situation, particularly for cases in which the sample size was small.
The impact of the uncertainties derived from these data gaps on the scientific assessment was incorporated into the results through expert opinion.
5. Recommendation
Data on AMR in bacterial pathogens are necessary to enhance animal health, promote the rational use of antimicrobials and identify specific therapeutic challenges attributable to AMR.
There is an urgent need for good quality data on susceptibility for bacteria isolated from aquatic animals. Data of suitable quality can only be generated by the use of standardised methods and internationally harmonised interpretive criteria.
Standardised methods for many of the most relevant bacterial species are now available (Smith, 2019). The current urgent need is to expand the number of species‐specific interpretive criteria for data generated by those methods.
Abbreviations
- 3GC
Third‐generation cephalosporin
- AHAW
Animal Health and Welfare
- AHL
Animal health law
- AMR
Antimicrobial resistance
- AST
Antimicrobial susceptibility testing
- BCWD
Bacterial cold water disease
- CLSI
Clinical and Laboratory Standards Institute
- ECOFF
Epidemiological cut‐off
- ECV
Epidemiological cut‐off values
- ELR
Extensive literature review
- ESBL
Extended‐spectrum beta‐lactamase
- ESC
Extended‐spectrum cephalosporinase
- EUCAST
European Committee on Antimicrobial Susceptibility Testing
- I
Intermediate
- MIC
Minimum inhibitory concentration
- MR
Methicillin resistance
- MRSA
Methicillin‐resistant Staphylococcus aureus
- MRSP
Methicillin‐resistant Staphylococcus pseudintermedius
- NRI
Normalised resistance interpretation
- NWT
Non‐wild‐type
- PCR
Polymerase chain reaction
- R
Resistant
- RTFS
Rainbow trout fry syndrome
- S
Susceptible
- ToR
Term of Reference
- UTI
Urinary tract infection
Appendix A – Search strings applied
-
1
PubMed:
Common search string “Antimicrobials”
((“antibiotic”Title/Abstract] OR “antibiotics”[Title/Abstract] OR “antimicrobial” [Title/Abstract] OR “antimicrobials”[Title/Abstract] OR “Anti‐Bacterial Agents”[MeSH Terms:noexp]) AND (“resistan*”[Title/Abstract] OR “susceptib*”[Title/Abstract])) OR (“Microbial Sensitivity Tests”[MeSH Terms] OR “drug resistance, microbial”[MeSH Terms])
Host‐based strings
((“Tilapia”[Title/Abstract] OR “Carp”[Title/Abstract] OR “Carps”[Title/Abstract] OR “Oncorhynchus mykiss”[Title/Abstract] OR “Sea Bream”[Title/Abstract] OR (“Tilapia”[MeSH Terms] OR “Carps”[MeSH Terms] OR “Oncorhynchus mykiss”[MeSH Terms] OR “Sea Bream”[MeSH Terms]))
“Bacterial species”
“Aeromonas hydrophila”[Title/Abstract] OR “Flavobacterium columnare”[Title/Abstract] OR “Aeromonas salmonicida”[Title/Abstract] OR “Flavobacterium psychrophilum”[Title/Abstract] OR “Aeromonas hydrophila”[MeSH Terms] OR “Flavobacterium columnare”[Supplementary Concept] OR “Aeromonas salmonicida”[MeSH Terms] OR “Flavobacterium psychrophilum”[Supplementary Concept]
-
2
Embase:
Common search string “Antimicrobials”
antibiotic resistance/or exp antibiotic sensitivity/or exp drug resistance/
susceptib*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
resistan*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
2 or 3
antibiotic.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
antibiotics.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
antimicrobial.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
antimicrobials.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
5 or 6 or 7 or 8
antibiotic agent/
10 or 9
11 and 4
12 or 1
Host‐based string
Tilapia/
carp/
oncorhynchus mykiss/
sea bream/
tilapia.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
carp.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
sea bream.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
rainbow trout.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
oncorhynchus mykiss.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
sea bream.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
1 or 2 or 3 or 4
5 or 6 or 7 or 8 or 9 or 10
11 or 12
“Bacterial species”
aeromonas hydrophila/
columnaris disease/
Aeromonas salmonicida/
Flavobacterium psychrophilum/
Aeromonas hydrophila.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
aeromonas salmonicida.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
flavobacterium psychrophilum.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
flavobacterium columnare.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
1 or 2 or 3 or 4
5 or 6 or 7 or 8
9 or 10
Appendix B – Excel file with all the data extracted
Information on all the full‐text studies that were assessed, including the reason for exclusion for those that were excluded at the full‐text screening and the data extracted from the included studies, can be consulted at https://doi.org/10.5281/zenodo.5575786
Appendix C – Exact percentages of weighted arithmetic means of %R and %R + I, respectively, displayed as dashed lines in figures
| Antibiotic | How resistance is reported (%R or %R + I) | Weighted arithmetic mean proportion of resistance (%) | Maximum resistance % observed | Minimum resistance % observed | Standard deviation (SD) | Bacterial species/genus |
|---|---|---|---|---|---|---|
| Aminopenicillins | R | 87.1 | 100 | 52.6 | 16.1 | A. hydrophila |
| Fluoroquinolones | R | 29.6 | 88.9 | 0 | 26.1 | A. hydrophila |
| Sulfa/TMP | R | 37.4 | 88.9 | 9.9 | 31.8 | A. hydrophila |
| Tetracyclines | R | 54.7 | 94.4 | 31.6 | 27.8 | A. hydrophila |
| Florfenicol | R + I | 0 | 0 | 0 | 0 | F. psychrophilum |
| Oxolinic acid | R + I | 58.5 | 75 | 55 | 7.6 | F. psychrophilum |
Suggested citation: EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortazar Schmidt C, Herskin M, Michel V, Miranda Chueca MA, Padalino B, Pasquali P, Roberts HC, Spoolder H, Stahl K, Velarde A, Viltrop A, Winckler C, Dewulf J, Guardabassi L, Hilbert F, Mader R, Romalde JL, Smith P, Baldinelli F, Kohnle L and Alvarez J, 2022. Scientific Opinion on the assessment of animal diseases caused by bacteria resistant to antimicrobials: kept fish species. EFSA Journal 2022;20(2):7076, 23 pp. 10.2903/j.efsa.2022.7076
Requestor: European Commission
Question number: EFSA‐Q‐2021‐00666
Panel members: Søren Saxmose Nielsen, Julio Alvarez, Dominique Joseph Bicout, Paolo Calistri, Elisabetta Canali, Julian Ashley Drewe, Bruno Garin‐Bastuji, Jose Luis Gonzales Rojas, Christian Gortazar Schmidt, Mette Herskin, Virginie Michel, Miguel Angel Miranda Chueca, Barbara Padalino, Paolo Pasquali, Helen Clare Roberts, Hans Spoolder, Karl Stahl, Antonio Velarde, Arvo Viltrop and Christoph Winckler.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch
Acknowledgements: The AHAW Panel wishes to thank Peter Damborg, Carmen Espinosa‐Gongora, Steffen Lynge Jørgensen from the University of Copenhagen for conducting the extensive literature review under the contract OC/EFSA/ALPHA/2020/02 – LOT 1, Verena Oswaldi from EFSA for the support provided for this scientific output.
Reproduction of the image listed below is prohibited and permission must be sought directly from the copyright holder: Figure 1: © FAO
Adopted: 15 December 2021
Footnotes
References
- Algammal AM, Mohamed MF, Tawfiek BA, Hozzein WN, Kazzaz WME and Mabrok M, 2020. Molecular typing, antibiogram and PCR‐RFLP based detection of Aeromonas hydrophila complex isolated from Oreochromis niloticus. Pathogens, 9, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron S, Larvor E, Jouy E, Kempf I, Le Bouquin S, Chauvin C, Boitard PM, Jamin M, Le Breton A and Thuillier B, 2021. Agreement between the categorization of isolates of Aeromonas salmonicida and Yersinia ruckeri by disc diffusion and MIC tests performed at 22°C. Journal of Fish Diseases, 44, 979–985. [DOI] [PubMed] [Google Scholar]
- Beaz‐Hidalgo R, Alperi A, Buján N, Romalde JL and Figueras MJ, 2010. Comparison of phenotypical and genetic identification of Aeromonas strains isolated from diseased fish. Systematic and Applied Microbiology, 33, 149–153. [DOI] [PubMed] [Google Scholar]
- CLSI (Clinical and Laboratory Standards Institute) , 2016. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria. CLSI guideline M45, 3rd Edition. Place Clinical and Laboratory Standards Institute. [Google Scholar]
- CLSI (Clinical and Laboratory Standards Institute) , 2020a. Methods for Antimicrobial Broth Dilution and Disk Diffusion Susceptibility Testing of Bacteria Isolated From Aquatic Animals. CLSI guideline VET03, 2nd Edition. Place Clinical and Laboratory Standards Institute. [Google Scholar]
- CLSI (Clinical and Laboratory Standards Institute) , 2020b. Performance Standards for Antimicrobial Susceptibility Testing of Bacteria Isolated From Aquatic Animals. CLSI supplement VET04, 3rd Edition. Place Clinical and Laboratory Standards Institute. [Google Scholar]
- Declercq A, Boyen F, Van Den Broeck W, Bossier P, Karsi A, Haesebrouck F and Decostere A, 2013. Antimicrobial susceptibility pattern of F lavobacterium columnare isolates collected worldwide from 17 fish species. Journal of Fish Diseases, 36, 45–55. [DOI] [PubMed] [Google Scholar]
- Douglas I, Ruane NM, Geary M, Carroll C, Fleming GT, McMurray J and Smith P, 2007. The advantages of the use of discs containing single agents in disc diffusion testing of the susceptibility of Aeromonas salmonicida to potentiated sulphonamides. Aquaculture, 272, 118–125. [Google Scholar]
- Duman M, Saticioglu IB and Altun S, 2020. The determination of antimicrobial susceptibility by MIC and epidemiological cut‐off values and the detection of resistance genes in Aeromonas species isolated from cultured fish. Letters in Applied Microbiology, 71, 531–541. [DOI] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare (AHAW) , Nielsen SS, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortazar Schmidt C, Herskin M, Michel V, Miranda Chueca MA, Padalino B, Pasquali P, Roberts HC, Sihvonen LH, Spoolder H, Stahl K, Velarde A, Viltrop A, Winckler C, Dewulf J, Guardabassi L, Hilbert F, Mader R, Smith P, Aznar I, Baldinelli F and Alvarez J, 2021. Ad hoc method for the assessment of animal diseases caused by bacteria resistant to antimicrobials. EFSA Journal 2021;19. 10.2903/j.efsa.2021.6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Bahar HM, Ali NG, Aboyadak IM, Khalil SAES and Ibrahim MS, 2019. Virulence genes contributing to Aeromonas hydrophila pathogenicity in Oreochromis niloticus. International Microbiology: The Official Journal of the Spanish Society for Microbiology, 22, 479–490. [DOI] [PubMed] [Google Scholar]
- FAO , 2020a. FAO Yearbook. Fishery and Aquaculture Statistics 2018/FAO annuaire. Statistiques des pêches et de l'aquaculture 2018/FAO anuario. Estadísticas de pesca y acuicultura 2018. Rome, FAO. Available online: https://www.fao.org/documents/card/en/c/cb1213t
- FAO , 2020b. The State of World Fisheries and Aquaculture 2020. Sustainability in action. Rome, Italy, FAO. 10.4060/ca9229en [DOI]
- FAO , 2021. Cultured Aquatic Species. Available online: https://www.fao.org/fishery/en/culturedspecies/search [Google Scholar]
- FAO , 2022a. Cyprinus carpio. Cultured Aquatic Species Information Programme. Text by Peteri, A. Fisheries and Aquaculture Division. Rome. Available online: https://www.fao.org/fishery/en/culturedspecies/cyprinus_carpio/en [Google Scholar]
- FAO , 2022b. Oncorhynchus mykiss. Cultured Aquatic Species Information Programme. Text by Cowx, I. G. Fisheries and Aquaculture Division. Rome. Available online: https://www.fao.org/fishery/en/culturedspecies/oncorhynchus_mykiss/en [Google Scholar]
- FAO , 2022c. Oreochromis niloticus, Cultured Aquatic Species Information Programme. Text by Rakocy, J. E. Fisheries and Aquaculture Division. Rome. Available online: https://www.fao.org/fishery/en/culturedspecies/oreochromis_niloticus/en [Google Scholar]
- FAO , 2022d. Salmo salar. Cultured Aquatic Species Information Programme. Text by Jones, M. Fisheries and Aquaculture Division. Rome. Available online: https://www.fao.org/fishery/en/culturedspecies/salmo_salar/en [Google Scholar]
- FAO , 2022e. Sparus aurata. Cultured Aquatic Species Information Programme. Text by Colloca, F.; Cerasi, S. Fisheries and Aquaculture Division. Rome. Available online: https://www.fao.org/fishery/en/culturedspecies/sparus_aurata/en [Google Scholar]
- Gieseker CM, Crosby TC, Mayer TD, Bodeis SM and Stine CB, 2016. Development of similar broth microdilution methods to determine the antimicrobial susceptibility of Flavobacterium columnare and F. psychrophilum. Journal of Aquatic Animal Health, 28, 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesami S, Parkman J, MacInnes JI, Gray JT, Gyles CL and Lumsden JS, 2010. Antimicrobial susceptibility of Flavobacterium psychrophilum isolates from Ontario. Journal of Aquatic Animal Health, 22, 39–49. [DOI] [PubMed] [Google Scholar]
- Inglis V, Frerichs G, Millar S and Richards R, 1991. Antibiotic resistance of Aeromonas salmonicida isolated from Atlantic salmon, Salmo salar L., in Scotland. Journal of Fish Diseases, 14, 353–358. [Google Scholar]
- Lukkana M, Wongtavatchai J and Chuanchuen R, 2012. Class 1 integrons in Aeromonas hydrophila isolates from farmed Nile tilapia (Oreochromis nilotica). Journal of Veterinary Medical Science, 74, 435–440. [DOI] [PubMed] [Google Scholar]
- Miller RA and Reimschuessel R, 2006. Epidemiologic cutoff values for antimicrobial agents against Aeromonas salmonicida isolates determined by frequency distributions of minimal inhibitory concentration and diameter of zone of inhibition data. American Journal of Veterinary Research, 67, 1837–1843. [DOI] [PubMed] [Google Scholar]
- Miranda CD, Smith P, Rojas R, Contreras‐Lynch S and Vega J, 2016. Antimicrobial susceptibility of Flavobacterium psychrophilum from Chilean salmon farms and their epidemiological cut‐off values using agar dilution and disk diffusion methods. Frontiers in Microbiology, 7, 1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo TP, Smith P, Bartie K, Thompson KD, Verner‐Jeffreys DW, Hoare R and Adams A, 2018. Antimicrobial susceptibility of Flavobacterium psychrophilum isolates from the United Kingdom. Journal of Fish Diseases, 41, 309–320. [DOI] [PubMed] [Google Scholar]
- Ruane NM, Douglas I, Geary M, Carroll C, Fleming GT and Smith P, 2007. Application of normalised resistance interpretation to disc diffusion data on the susceptibility of Aeromonas salmonicida to three quinolone agents. Aquaculture, 272, 156–167. [Google Scholar]
- Saticioglu IB, Duman M, Smith P, Wiklund T and Altun S, 2019. Antimicrobial resistance and resistance genes in Flavobacterium psychrophilum isolates from Turkey. Aquaculture, 512, 734293. [Google Scholar]
- Scarano C, Piras F, Virdis S, Ziino G, Nuvoloni R, Dalmasso A, De Santis E and Spanu C, 2018. Antibiotic resistance of Aeromonas ssp. strains isolated from Sparus aurata reared in Italian mariculture farms. International Journal of Food Microbiology, 284, 91–97. [DOI] [PubMed] [Google Scholar]
- Smith P, 2019. The performance of antimicrobial susceptibility testing programmes relevant to aquaculture and aquaculture products. FAO Fisheries and Aquaculture Circular No. 1191.
- Smith P, Endris R, Kronvall G, Thomas V, Verner‐Jeffreys D, Wilhelm C and Dalsgaard I, 2016. Epidemiological cut‐off values for Flavobacterium psychrophilum MIC data generated by a standard test protocol. Journal of Fish Diseases, 39, 143–154. [DOI] [PubMed] [Google Scholar]
- Smith P, Ruane NM, Douglas I, Carroll C, Kronvall G and Fleming GT, 2007. Impact of inter‐lab variation on the estimation of epidemiological cut‐off values for disc diffusion susceptibility test data for Aeromonas salmonicida. Aquaculture, 272, 168–179. [Google Scholar]
- Soderlund R, Hakhverdyan M, Aspan A and Jansson E, 2018. Genome analysis provides insights into the epidemiology of infection with flavobacterium psychrophilum among farmed salmonid fish in Sweden. Microbial Genomics, 4, 000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedres‐Svarm , 2019. Sales of antibiotics and occurrence of resistance in Sweden. 1650–6332 Solna/Uppsala Available online: https://www.sva.se/media/0hihej1c/swedres‐svarm‐2019.pdf [Google Scholar]
- Taha ZM, Sadiq ST, Khalil WA, Ali KY, Yosif HS and Shamil HN, 2021. Investigation of gcat gene and antibiotic resistance pattern of aeromonas hydrophila isolated from hemorrhagic septicemia's cases in fish farms. Iraqi Journal of Veterinary Sciences, 35, 375–380. [Google Scholar]
- Van Vliet D, Loch TP, Smith P and Faisal M, 2017. Antimicrobial susceptibilities of Flavobacterium psychrophilum isolates from the Great Lakes Basin, Michigan. Microbial Drug Resistance, 23, 791–798. [DOI] [PubMed] [Google Scholar]
- Zaher HA, Nofal MI, Hendam BM, Elshaer MM, Alothaim AS and Eraqi MM, 2021. Prevalence and Antibiogram of Vibrio parahaemolyticus and Aeromonas hydrophila in the Flesh of Nile Tilapia, with Special Reference to Their Virulence Genes Detected Using Multiplex PCR Technique. Antibiotics (Basel), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]


