Abstract
The EFSA Panel on Plant Health performed a pest categorisation of Thecodiplosis japonensis (Diptera: Cecidomyiidae) for the EU territory. This species is not included in the EU Commission Implementing Regulation 2019/2072. T. japonensis Uchida & Inouye is a well‐defined species, native to a large part of Japan, which was introduced to the Republic of Korea and eastern China: Fujian and Shandong. It attacks Pinus densiflora, P. thunbergii and P. luchuensis in Japan; P. densiflora and P. thunbergii in Korea; and P. massoniana in China, and has been observed to attack other two‐needle pine species, including species present in the EU. The pest is univoltine and the adults emerge between May and August. The adults live only for 1 day. Each female oviposits in batches on developing needles. The neonate larvae crawl to the base of the needle fascicle and create a gall in which they feed gregariously by sap sucking. The third‐instar larvae leave the galls in November, overwinter in a cocoon in the soil and pupate at the end of the winter. Degree day models have been developed to predict adult emergence. Survival of overwintering stages is poor below 15°C and above 30°C. The pest can be detected by its symptoms (stunted or dead needles, galls at the base of infected needle fascicles), and identified using morphological characters or the mitochondrial COI gene. T. japonensis is one of the major forest pests in the Republic of Korea, where 1.7 million trees were cut to control it in 2014–2015. It flies uneasily (a few hundred metres) but can be transported in galls on Pinus plants for planting, including artificially dwarfed plants, or with cut branches. Climate matching and host tree distribution suggest that T. japonensis would be able to establish and have an impact in the EU territory. T. japonensis satisfies all the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union quarantine pest.
Keywords: pine needle gall midge, Pinus spp., pest risk, plant health, plant pest, quarantine
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Background taken from Annex 2 of new mandate – to be left unchanged
The new Plant Health Regulation (EU) 2016/2031, on the protective measures against pests of plants, is applying from 14 December 2019. Conditions are laid down in this legislation in order for pests to qualify for listing as Union quarantine pests, protected zone quarantine pests or Union regulated non‐quarantine pests. The lists of the EU regulated pests together with the associated import or internal movement requirements of commodities are included in Commission Implementing Regulation (EU) 2019/2072. Additionally, as stipulated in the Commission Implementing Regulation 2018/2019, certain commodities are provisionally prohibited to enter in the EU (high risk plants, HRP). EFSA is performing the risk assessment of the dossiers submitted by exporting to the EU countries of the HRP commodities, as stipulated in Commission Implementing Regulation 2018/2018. Furthermore, EFSA has evaluated a number of requests from exporting to the EU countries for derogations from specific EU import requirements.
In line with the principles of the new plant health law, the European Commission with the Member States are discussing monthly the reports of the interceptions and the outbreaks of pests notified by the Member States. Notifications of an imminent danger from pests that may fulfil the conditions for inclusion in the list of the Union quarantine pest are included. Furthermore, EFSA has been performing horizon scanning of media and literature.
As a follow‐up of the above‐mentioned activities (reporting of interceptions and outbreaks, HRP, derogation requests and horizon scanning), a number of pests of concern have been identified. EFSA is requested to provide scientific opinions for these pests, in view of their potential inclusion by the risk manager in the lists of Commission Implementing Regulation (EU) 2019/2072 and the inclusion of specific import requirements for relevant host commodities, when deemed necessary by the risk manager.
1.1.2. Terms of Reference
ToR taken from Annex 2 of mandate – to be left unchanged
EFSA is requested, pursuant to Article 29(1) of Regulation (EC) No 178/2002, to provide scientific opinions in the field of plant health.
EFSA is requested to deliver 53 pest categorisations for the pests listed in Annex 1A, 1B, 1D and 1E (for more details see mandate M‐2021‐00027 on the Open.EFSA portal ). Additionally, EFSA is requested to perform pest categorisations for the pests so far not regulated in the EU, identified as pests potentially associated with a commodity in the commodity risk assessments of the HRP dossiers (Annex 1C; for more details see mandate M‐2021‐00027 on the Open.EFSA portal ). Such pest categorisations are needed in the case where there are not available risk assessments for the EU.
When the pests of Annex 1A are qualifying as potential Union quarantine pests, EFSA should proceed to phase 2 risk assessment. The opinions should address entry pathways, spread, establishment, impact and include a risk reduction options analysis.
Additionally, EFSA is requested to develop further the quantitative methodology currently followed for risk assessment, in order to have the possibility to deliver an express risk assessment methodology. Such methodological development should take into account the EFSA Plant Health Panel Guidance on quantitative pest risk assessment and the experience obtained during its implementation for the Union candidate priority pests and for the likelihood of pest freedom at entry for the commodity risk assessment of High Risk Plants.
1.2. Interpretation of the Terms of Reference
Thecodiplosis japonensis is one of a number of pests listed in Annex 1 to the Terms of Reference (ToR) to be subject to pest categorisation to determine whether it fulfils the criteria of a potential Union quarantine pest for the area of the EU excluding Ceuta, Melilla and the outermost regions of Member States referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores, and so inform European Commission decision‐making as to its appropriateness for potential inclusion in the lists of pests of Commission Implementing Regulation (EU) 2019/2072. If a pest fulfils the criteria to be potentially listed as a Union quarantine pest, risk reduction options will be identified.
1.3. Additional information
This pest categorisation was initiated following the commodity risk assessment of black pine (Pinus thunbergii Parl.) bonsai plants from Japan performed by EFSA (EFSA PLH Panel, 2019), in which T. japonensis was identified as a relevant non‐regulated EU pest which could potentially enter the EU on P. thunbergii.
2. Data and methodologies
2.1. Data
2.1.1. Literature search
A literature search on T. japonensis was conducted at the beginning of the categorisation in the ISI Web of Science bibliographic database, using the scientific name of the pest as search term. Papers relevant for the pest categorisation were reviewed, and further references and information were obtained from experts, as well as from citations within the references and grey literature.
2.1.2. Database search
Pest information, on host(s) and distribution, was retrieved from the European and Mediterranean Plant Protection Organization (EPPO) Global Database (EPPO, online), the CABI databases and scientific literature databases as referred above in Section 2.1.1.
Data about the import of commodity types that could potentially provide a pathway for the pest to enter the EU and about the area of hosts grown in the EU were obtained from EUROSTAT (Statistical Office of the European Communities).
The Europhyt and TRACES databases were consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network run by the Directorate General for Health and Food Safety (DG SANTÉ) of the European Commission as a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. TRACES is the European Commission's multilingual online platform for sanitary and phytosanitary certification required for the importation of animals, animal products, food and feed of non‐animal origin and plants into the European Union, and the intra‐EU trade and EU exports of animals and certain animal products. Up until May 2020, the Europhyt database managed notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the Member States and the phytosanitary measures taken to eradicate or avoid their spread. The recording of interceptions switched from Europhyt to TRACES in May 2020.
GenBank was searched to determine whether it contained any nucleotide sequences for T. japonensis which could be used as reference material for molecular diagnosis. GenBank® ( www.ncbi.nlm.nih.gov/genbank/ ) is a comprehensive publicly available database that as of August 2019 (release version 227) contained over 6.25 trillion base pairs from over 1.6 billion nucleotide sequences for 450,000 formally described species (Sayers et al., 2020).
2.2. Methodologies
The Panel performed the pest categorisation for T. japonensis following guiding principles and steps presented in the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018), the EFSA guidance on the use of the weight of evidence approach in scientific assessments (EFSA Scientific Committee, 2017) and the International Standards for Phytosanitary Measures No. 11 (FAO, 2013).
The criteria to be considered when categorising a pest as a potential Union quarantine pest (QP) are given in Regulation (EU) 2016/2031 Article 3 and Annex I, Section 1 of the Regulation. Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. In judging whether a criterion is met the Panel uses its best professional judgement (EFSA Scientific Committee, 2017) by integrating a range of evidence from a variety of sources (as presented above in Section 2.1) to reach an informed conclusion as to whether or not a criterion is satisfied.
Table 1.
Pest categorisation criteria under evaluation, as derived from Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest (article 3) |
|---|---|
| Identity of the pest (Section 3.1 ) | Is the identity of the pest clearly defined, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2 ) |
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU? Describe the pest distribution briefly |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways of entry and spread. |
| Potential for consequences in the EU territory (Section 3.5 ) | Would the pests’ introduction have an economic or environmental impact on the EU territory? |
| Available measures (Section 3.6 ) | Are there measures available to prevent pest entry, establishment, spread or impact? |
| Conclusion of pest categorisation (Section 4 ) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met. |
The Panel’s conclusions are formulated respecting its remit and particularly with regard to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, deemed to be a risk management decision, the Panel will present a summary of the observed impacts in the areas where the pest occurs, and make a judgement about potential likely impacts in the EU. Whilst the Panel may quote impacts reported from areas where the pest occurs in monetary terms, the Panel will seek to express potential EU impacts in terms of yield and quality losses and not in monetary terms, in agreement with the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018). Article 3 (d) of Regulation (EU) 2016/2031 refers to unacceptable social impact as a criterion for quarantine pest status. Assessing social impact is outside the remit of the Panel.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
Is the identity of the pest clearly defined, or has it been shown to produce consistent symptoms and/or to be transmissible?
Yes, the identity of the species is established and Thecodiplosis japonensis Uchida & Inouye is the accepted name.
The pine needle gall midge, Thecodiplosis japonensis Uchida and Inouye (1955) (Diptera: Cecidomyiidae) was described as a new species by Uchida and Inouye (1955). This species had also been described by Kim (1955 in CABI ISC, online) as Thecodiplosis pinicola Tagaki.
The EPPO code1 (Griessinger and Roy, 2015; EPPO, 2019) for this species is: THEOJA (EPPO, online).
3.1.2. Biology of the pest
The biology of the pest is summarised in Table 2, but where further detail is needed, it is presented in the text below.
Table 2.
Important features of the life‐history strategy of Thecodiplosis japonensis
| Life stage | Phenology and relation to host | Other relevant information |
|---|---|---|
| Egg | From mid‐May to late July, one or more eggs laid on developing needles. Females have ca. 140 eggs in their ovaries but do not always lay all their eggs. | Eggs hatch in 5–6 days |
| Larva | Neonate larvae crawl down the needles, and form a gall where they gregariously feed by sap sucking | One to 22 individuals per gall; three larval instars, larvae leave the galls in November, drop to the soil, spin a cocoon and overwinter. Larvae can jump over 27 cm. |
| Pupa | Pupation occurs in the soils at the end of the winter |
Conflicting information: Median cumulative adult emergence with 847.9 degree‐days and a lower larva to adult development threshold (LDT) of 5.9°C (Son et al., 2007). Median cumulative adult emergence with 626.7 degree‐days and a lower larva to adult development threshold (LDT) of 6.1°C (Nam and Choi, 2014). |
| Adult |
Emergence from mid‐May to late August; Adult life very short (1 day) Mating and oviposition on hosts nearby |
Females flew a maximum distance of 668 m in flight mills. |
The biology of T. japonensis has been reviewed by Son et al. (2007), Nam and Choi (2014), Qi et al. (2020), CABI ISC (online) and EPPO (2004). T. japonensis is a univoltine species. The third‐instar larvae leave the galls in November, overwinter in a cocoon in the soil and pupate at the end of the winter. Son et al. (2007) measured in the laboratory the temperature‐dependent larva to adult survival of the overwintering stages. At 12°C and 15°C, only 0.4 and 7% survived, respectively. Optimal survival was recorded at 21°C (49.8%), 24°C (56.8%) and 27°C (37.2%). No survival was registered at 30°C. With a calculated lower larva to adult development threshold (LDT) of 5.9°C, a predictive degree‐day model for post‐diapause larva to adult development was established from field data (1991–1995). This model, compared to field data on adult emergence in 1996, accurately predicted median cumulative emergence with 847.9 degree‐days. Nam and Choi (2014) calculated a 6.1°C LDT based on field observations, and their degree‐day model predicted median cumulative emergence with 626.7 degree‐days, correctly explaining adult emergence in 2011. The young adults emerge from mid‐May to late July. They have a short life span (ca. 1 day), and mating occurs shortly after emergence.
Oviposition takes place in the vicinity of the location of emergence. Soné (1984) counted on the average 146 eggs in the ovaries of dissected females, but the realised fecundity (number of eggs laid vs. number of eggs in the ovary) varied from 3% to 93%. One or several eggs are laid on developing needles. They hatch in 5–6 days and the neonate larvae crawl to the base of the needle fascicle and start sucking the sap. This induces the formation of a gall in which the larvae feed gregariously (Figure 1). Qi et al. (2020) report that, on P. thunbergii, the galls sheltered each 9 ± 4 larvae, with a maximum of 22 larvae; 55% of the galls contained 6–11 larvae. The infested needle fascicles have a stunted growth or die.
Figure 1.

Thecodiplosis japonensis gall and larvae at the basis of a needle fascicle of Pinus thunbergii. The long needle fascicles on the left are undamaged, a gall occupies the bases of the shorter fascicles. From Qi et al. (2020) Figure prepared by Lily Ren, no changes were made, https://creativecommons.org/licenses/by/4.0/, Available online: https://doi.org/10.1038/s41598‐020‐69231‐4
Flight has been studied in flight mills by Liu et al. (2020); the females flew a maximum distance of 668 m. Liu et al. (2020) also report that larvae are able to move by a succession of jumps; the longest jump covered 5.7 cm, and the longest distance recorded was 27 cm.
Several natural enemies play an important role in regulating the pest populations. Four hymenopteran platygastrid parasitoid species, Inostemma matsutama Yoshida, I. seoulis Ko, I. hockpari Ko and Platygaster matsutama Yoshida, are associated with T. japonensis in the Republic of Korea (Choi et al., 2017) and contributed at least partially to the control of the pest (Choi et al., 2019), with parasitism rates between 11.8% and 21.7% in 2018–2019 (Kim et al., 2020). In addition to these parasitoids, among which P. matsutama and I. seoulis were the dominant species in Japan and Korea (Duan et al., 2021), several pathogens (Beauveria bassiana, Fusarium sp., Spicaria sp., Bacillus sp., Metarhizium spp.) are also affecting the dynamics of T. japonensis populations (Duan et al., 2021).
3.1.3. Host range/Species affected
As indicated in Appendix A, T. japonensis attacks Pinus densiflora, P. thunbergii in the Republic of Korea and Japan (Honshu, Kyushu, Shikoku) (EPPO, online), P. luchuensis in Japan (Ryukyu islands: Yamauchi et al., 1982; CABI ISC online) and P. massoniana in China (EPPO, online).
CABI ISC (online) reports that ‘In Japan, the following exotic pine species were attacked by T. japonensis: P. coulteri, P. khasya, P. laricio, P. massoniana, P. mugo, P. nigra, P. radiata, P. resinosa, P. sylvestris, P. tabulaeformis, P. taiwanensis, (...) and P. thunbergii x P. massoniana’.
Among the species observed in Japan, Pinus spp. with two needles in a fascicle were attacked by T. japonensis, but no galls were observed in pine species with five needles per fascicle (Furuno, 1987 in CABI ISC online).
3.1.4. Intraspecific diversity
No intraspecific diversity has been reported for T. japonensis.
3.1.5. Detection and identification of the pest
Are detection and identification methods available for the pest?
Yes, visual detection is possible and identification criteria are available.
Detection – Symptoms
According to the descriptions in the literature (Son et al., 2007; Nam and Choi, 2014; Qi et al., 2020; CABI ISC, online; EPPO, 2004), the damage of T. japonensis is characteristic: the infested needle fascicles are markedly shorter than the healthy ones, and they often die. At the basis of the infested needle fascicles, a gall can be found, which shelters one to about 20 larvae feeding gregariously.
Identification
T. japonensis can be identified by morphological characters (see section Description below).
One GenBank accession is available for T. japonensis mitochondrial COI gene for cytochrome oxidase subunit I: https://www.ncbi.nlm.nih.gov/nuccore/AB105484.1
Description
A complete description is available in CABI ISC (online), based on Uchida and Inouye (1955):
Eggs are 0.1 mm long, yellow and are cylindrical. They are attached to the pair of needles. After the larvae hatch, empty egg shells remain attached to needles.
The first‐instar larva is characterized by the absence of caudal processes and by metapneustic respiration. The second‐instar larva has prominent spiracles and peripneustic respiration. The third‐instar larva is 2.8–3 mm long and is yellow. It has terminal papillae on the anal segment modified in the form of a median pair of robust sclerotized processes that are placed on a large sclerotized plate. Spatula sternalis, the sclerotized formation on the ventral side of the prothoracic segment, is well developed and has bilobed anterior part and narrow stem.
Pupa is 2.5–3 mm long, yellow‐orange, wing sheaths, legs and eyes are dark brown.
Adults are 2.5–3 mm long, body is yellow‐orange, basal parts of legs are dark brown. Wings are fine and have reduced veins. Head with holoptic eyes and three‐segmented palps. Antennae are composed of 2 + 12 flagellomeres that are sexually dimorphic. Antennae of males are long and binodose, each flagellomere consists of two globular nodes. Each node bears one whorl of circumfilar loops. Antennae of females have simple segments with relatively long necks. Females have a medium length ovipositor’.
All developmental stages have been photographed and are available in Jiao et al. (2017).
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
T. japonensis is present in eastern Asia. It is widespread in Japan and the Republic of Korea. In China, it occurs in the provinces of Fujian and Shandong (EPPO, online). See Figure 2 and Appendix B.
Figure 2.
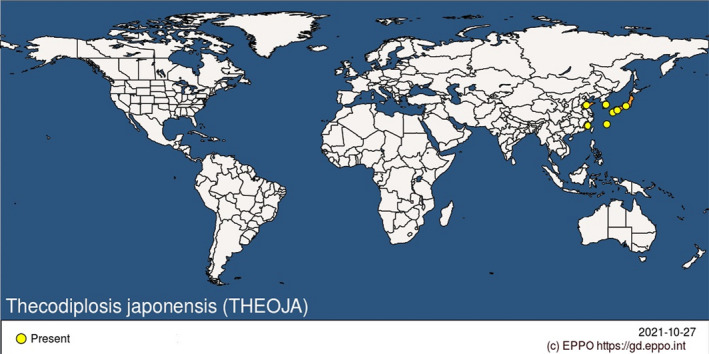
Global distribution of Thecodiplosis japonensis (Source: EPPO Global Database Accessed: 27 October 2021)
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU?
No. T. japonensis is not known to occur in the EU.
3.3. Regulatory status
3.3.1. Commission Implementing Regulation 2019/2072
T. japonensis is not listed in Annex II of Commission Implementing Regulation (EU) 2019/2072, an implementing act of Regulation (EU) 2016/2031.
3.3.2. Hosts or species affected that are prohibited from entering the Union from third countries
3.4. Entry, establishment and spread in the EU
3.4.1. Entry
Is the pest able to enter into the EU territory? If yes, identify and list the pathways
Yes, the pest is able to enter the EU territory with Pinus plants for planting, including artificially dwarfed plants, and cut branches.
Comment on plants for planting as a pathway
Pinus spp. plants are regulated, as well as the soil and growing media included in potted plants such as bonsai (see Table 3).
Table 3.
List of plants, plant products and other objects that are Thecodiplosis japonensis hosts whose introduction into the Union from certain third countries is prohibited (Source: Commission Implementing Regulation (EU) 2019/2072, Annex VI)
| List of plants, plant products and other objects whose introduction into the Union from certain third countries is prohibited | |||
|---|---|---|---|
| Description | CN code | Third country, group of third countries or specific area of third country | |
| 1. | Plants of […] Pinus L., […] other than fruit and seeds |
ex 0602 20 20 ex 0602 20 80 ex 0602 90 41 ex 0602 90 45 ex 0602 90 46 ex 0602 90 47 ex 0602 90 50 ex 0602 90 70 ex 0602 90 99 ex 0604 20 20 ex 0604 20 40 |
Third countries other than: Albania, Andorra, Armenia, Azerbaijan, Belarus, Bosnia and Herzegovina, Canary Islands, Faeroe Islands, Georgia, Iceland, Liechtenstein, Moldova, Monaco, Montenegro, North Macedonia, Norway, Russia (only the following parts: Central Federal District (Tsentralny federalny okrug), Northwestern Federal District (Severo‐Zapadny federalny okrug), Southern Federal District (Yuzhny federalny okrug), North Caucasian Federal District (Severo‐Kavkazsky federalny okrug) and Volga Federal District (Privolzhsky federalny okrug)), San Marino, Serbia, Switzerland, Turkey and Ukraine, and, until December 2023, Japan 2 |
| 19. | Soil as such consisting in part of solid organic substances |
ex 2530 90 00 ex 3824 99 93 |
Third countries other than Switzerland, and Japan until December 2023 2 |
| 20. | Growing medium as such, other than soil, […] |
ex 2530 10 00 ex 2530 90 00 ex 2703 00 00 ex 3101 00 00 ex 3824 99 93 |
Third countries other than Switzerland, and Japan until December 2023 2 |
Potential pathways for T. japonensis are presented in2 Table 4.
Table 4.
Potential pathways for Thecodiplosis japonensis into the EU 27. No special requirements in Annex VII relate to T. japonensis
| Pathways | Life stage | Relevant mitigations [e.g. prohibitions (Annex VI), special requirements (Annex VII) or phytosanitary certificates (Annex XI) within Implementing Regulation 2019/2072] |
|---|---|---|
| Pinus plants for planting | Eggs, larvae, pupae in soil or growing media | For prohibition, see Table 3 |
| Pinus cut branches | Eggs, larvae | For prohibition, see Table 3 |
| Soil or growing media | Pupae in soil or growing media | For prohibition, see Table 3 |
Pinus are prohibited as plants for planting and cut branches from third countries (see Section 3.3.2) although derogations are in place for dwarfed Pinus coming from Japan (Commission Decision 2002/887/EC) and from the Republic of Korea (Commission Decision 2002/499/EC), therefore a possible pathway exists.
Plant products, such as cut pine branches, are commonly used in floristry and in the production of Christmas decorations. Cut Pinus branches from infested hosts could carry galls and larvae therein. However, the import of cut branches of Pinus from outside of Europe is prohibited, so this pathway is closed.
Notifications of interceptions of harmful organisms began to be compiled in Europhyt in May 1994 and in TRACES in May 2020. As to 1 October 2021, there were no records of interception of T. japonensis in the Europhyt and TRACES databases.
3.4.2. Establishment
Is the pest able to become established in the EU territory?
Yes, T. japonensis would be able to establish in the EU. It could establish in the Pinus spp. areas of the EU (Figure 3).
Figure 3.
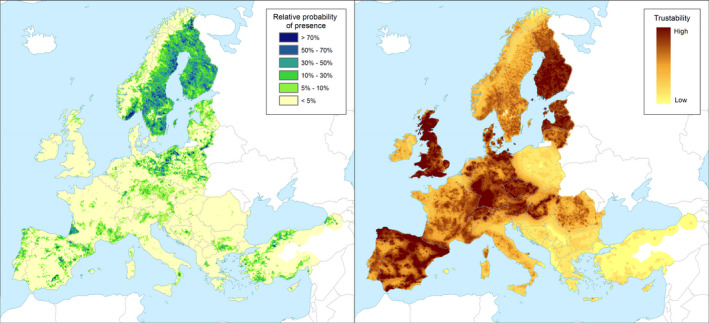
Left panel: Relative probability of the presence (RPP) of the genus Pinus in Europe, mapped at 100 km2 resolution. The underlying data are from European‐wide forest monitoring data sets and from national forestry inventories based on standard observation plots measuring in the order of hundreds m2. RPP represents the probability of finding at least one individual of the taxon in a standard plot placed randomly within the grid cell. For details, see Appendix C (courtesy of JRC, 2017). Right panel: Trustability of RPP. This metric expresses the strength of the underlying information in each grid cell and varies according to the spatial variability in forestry inventories. The colour scale of the trustability map is obtained by plotting the cumulative probabilities (0–1) of the underlying index (for details, see Appendix C)
Unless moved with plants for planting, there are uncertainties over the pests’ ability to transfer to a suitable host following arrival into the EU. Uncertainties also include its ability to find a mate and other Allee effects (effects causing reduced survival of new colonies with a small number of individuals) (Tobin et al., 2011) as well as the impact of natural enemies in the EU.
Climatic mapping is the principal method for identifying areas that could provide suitable conditions for the establishment of a pest taking key abiotic factors into account (Baker, 2002). Availability of hosts is considered in Section 3.4.2.1 and climatic factors in Section 3.4.2.2.
3.4.2.1. EU distribution of main host plants
T. japonensis attacks only Pinus spp. These are distributed throughout the EU territory (Figure 3).
3.4.2.2. Climatic conditions affecting establishment
Figure 4 indicates that climate zones in countries where T. japonensis occurs also occur in the EU.
Figure 4.
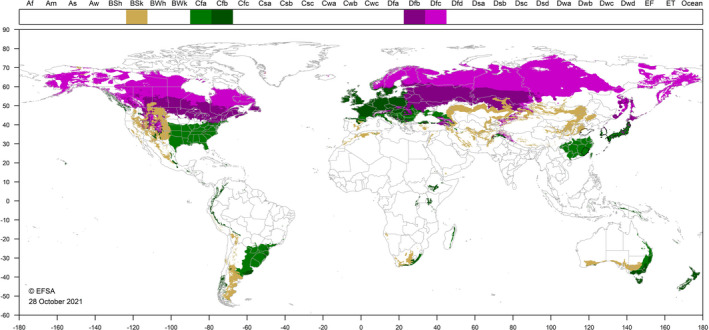
World distribution of five Köppen–Geiger climate types (BSk, Cfa, Cfb, Dfb and Dfc) that occur in the EU and which occur in countries where Thecodiplosis japonensis has been reported
3.4.3. Spread
Describe how the pest would be able to spread within the EU territory following establishment?
T. japonensis would spread naturally by flight very locally (several hundred meters only).
Comment on plants for planting as a mechanism of spread
With plants for planting the pest would spread over longer distances as compared to natural spread.
In nature, adults fly short distances (Kearby and Benjamin, 1964). Choi and Park (2012) report adult T. japonensis dispersing a maximum of 400 m whilst flight mill experiments measured adult females flying a maximum of 668 m at 26°C and 70% RH (Liu et al., 2020). Recall that adults live for 1 day.
3.5. Impacts
Would the pests’ introduction have an economic or environmental impact on the EU territory?
Yes, the pest's introduction would have an economic or environmental impact on the EU territory.
Before the arrival of the pine wood nematode, T. japonensis, an invasive species in the Republic of Korea first discovered there in 1929 on Pinus densiflora, was considered the major forest pest in this country during the 1980s and 1990s. Growth losses and tree deaths result from heavy infestations. Around 1.7 million trees were cut to control the pest in 2014–2015 (Choi et al., 2019).
P. densiflora is not grown commercially in the EU.
Because of the impacts, chemical treatments are used in nurseries (Lee et al., 1997,2000; see Table 5).
Table 5.
Selected control measures (a full list is available in EFSA PLH Panel, 2018) for pest entry/establishment/spread/impact in relation to currently unregulated hosts and pathways. Control measures are measures that have a direct effect on pest abundance
|
Control measure/Risk reduction option (Blue underline = Zenodo doc, Blue = WIP) |
RRO summary | Risk element targeted (entry/establishment/spread/impact) |
|---|---|---|
| Require pest freedom | Source hosts from pest‐free area | Entry/Spread |
| Growing plants in isolation | Screened nurseries could provide pest‐free places of production. | Entry/Spread/Impact |
| Use of resistant and tolerant plant species/varieties | Five needle Pinus species may be tolerant/resistant but this is uncertain. | Entry/Establishment/Spread/Impact |
| Roguing and pruning | Infested plants could be removed by sanitation thinning of the stands. | Spread/Impact |
| Biological control and behavioural manipulation | Several parasitoids and entomopathogenic microorganisms regulate the population dynamics of T. japonensis (see Section 3.1.2) | Impact |
| Chemical treatments on crops including reproductive material | Chemical treatments (carvacrol, neem extract, phosphamidon) have been identified for use in nurseries (Lee et al., 1997, 2000) but are not practical in forest stands. | Entry/Establishment/Impact |
| Chemical treatments on consignments or during processing | Systemic insecticides and neem extracts can be used to control T. japonensis on young/small plants (Lee et al., 1997, 2000) | Entry/Spread/Impact |
| Soil treatment | The control of soil organisms by chemical and physical methods listed below: (a) Fumigation; (b) Heating | Entry/Spread/Impact |
| Waste management | Treatment of the trees after sanitation felling (e.g. incineration, chipping, production of bio‐energy…) in authorised facilities and official restriction on the movement of waste. | Establishment/spread |
| Post‐entry quarantine and other restrictions of movement in the importing country | Artificially dwarfed plants can be kept in post entry quarantine until their needles are fully grown. Stunted, infested needle fascicles can then be identified. | Entry/Spread |
3.6. Available measures and their limitations
Are there measures available to prevent pest entry, establishment, spread or impact?
Yes, measures are available to prevent entry; the regulation of imports of plants for planting from Japan, the Republic of Korea and China is implemented in Annexes VI and VII of Implementing Regulation 2019/2072. In particular Pinus are prohibited as plants for planting and cut branches from third countries (see 3.3.2) although derogations are in place for dwarfed Pinus coming from Japan (Commission Decision 2002/887/EC) and from the Republic of Korea (Commission Decision 2002/499/EC).
3.6.1. Identification of potential additional measures
Phytosanitary measures (prohibitions) are currently applied to some host plants for planting (see Section 3.3.2).
Additional potential risk reduction options and supporting measures are shown in Sections 3.6.1.1 (Table 5) and 3.6.1.2 (Table 6).
Table 6.
Selected supporting measures (a full list is available in EFSA PLH Panel, 2018) in relation to currently unregulated hosts and pathways. Supporting measures are organisational measures or procedures supporting the choice of appropriate risk reduction options that do not directly affect pest abundance
| Supporting measure | Summary | Risk element targeted (entry/establishment/spread/impact) |
|---|---|---|
| Inspection and trapping | Growing season inspections for artificially dwarfed plant | Entry/Spread |
| Sampling | Necessary as part of other RROs | |
| Phytosanitary certificate and plant passport | An official paper document or its official electronic equivalent, consistent with the model certificates of the IPPC, attesting that a consignment meets phytosanitary import requirements (ISPM 5) (a) export certificate (import) (b) plant passport (EU internal trade) |
Entry (phytosanitary certificate) Spread (plant passport) |
| Certified and approved premises | If material sourced from an approved premises e.g. in a PFA (Table 5), likelihood of commodity being infested is assumed to be reduced. | Entry/Spread |
| Delimitation of Buffer zones | ISPM 5 defines a buffer zone as ‘an area surrounding or adjacent to an area officially delimited for phytosanitary purposes in order to minimize the probability of spread of the target pest into or out of the delimited area, and subject to phytosanitary or other control measures, if appropriate’ (ISPM 5). The objectives for delimiting a buffer zone can be to prevent spread from the outbreak area and to maintain a pest‐free production place (PFPP), site (PFPS) or area (PFA). | Spread |
| Surveillance | Spread |
3.6.1.1. Additional potential risk reduction options
Potential additional control measures are listed in Table 5.
3.6.1.2. Additional supporting measures
Potential additional supporting measures are listed in Table 6.
3.6.1.3. Biological or technical factors limiting the effectiveness of measures
The cryptic nature of the galls may limit detection. However, as the infested needle fascicles are shorter than uninfested ones, visual detection is still possible.
3.7. Uncertainty
There are no key uncertainties that would cast doubt on the conclusions.
4. Conclusions
The pest satisfies the criteria which are within the remit for EFSA to assess for it to be regarded as a potential Union quarantine pest (Table 7).
Table 7.
The Panel’s conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel’s conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Key uncertainties |
|---|---|---|
|
Identity of the pest (Section 3.1 ) |
The identity of the species is clearly defined and Thecodiplosis japonensis Uchida & Inouye is the accepted name. | None |
|
Absence/ presence of the pest in the EU (Section 3.2 ) |
T. japonensis is not known to occur in the EU | None |
| Pest potential for entry, establishment and spread in the EU (Section 3.4 ) |
The pest is able to enter the EU territory with Pinus plants for planting, including artificially dwarfed plants, and cut branches. It would be able to establish in the EU, in the Pinus spp. areas of the EU. It would spread naturally by flight very locally (several hundred meters only), and with plants for planting and cut branches over longer distances. |
None |
| Potential for consequences in the EU (Section 3.5 ) | The pest's introduction would have an economic or environmental impact on the EU territory. | None |
| Available measures (Section 3.6 ) | The regulation of imports of Pinus plants for planting from Japan, the Republic of Korea and China is implemented in Annex VI of Implementing Regulation 2019/2072. | None |
| Conclusion (Section 4 ) | All criteria assessed by EFSA above for consideration as a potential quarantine pest were met. | None |
| Aspects of assessment to focus on/scenarios to address in future if appropriate | ||
Abbreviations
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- IPPC
International Plant Protection Convention
- ISPM
International Standards for Phytosanitary Measures
- MS
Member State
- PLH
EFSA Panel on Plant Health
- PZ
Protected Zone
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
Glossary
- Containment (of a pest)
Application of phytosanitary measures in and around an infested area to prevent spread of a pest (FAO, 2018)
- Control (of a pest)
Suppression, containment or eradication of a pest population (FAO, 2018)
- Entry (of a pest)
Movement of a pest into an area where it is not yet present, or present but not widely distributed and being officially controlled (FAO, 2018)
- Eradication (of a pest)
Application of phytosanitary measures to eliminate a pest from an area (FAO, 2018)
- Establishment (of a pest)
Perpetuation, for the foreseeable future, of a pest within an area after entry (FAO, 2018)
- Greenhouse
A walk‐in, static, closed place of crop production with a usually translucent outer shell, which allows controlled exchange of material and energy with the surroundings and prevents release of plant protection products (PPPs) into the environment.
- Hitchhiker
An organism sheltering or transported accidentally via inanimate pathways including with machinery, shipping containers and vehicles; such organisms are also known as contaminating pests or stowaways (Toy and Newfield, 2010).
- Impact (of a pest)
The impact of the pest on the crop output and quality and on the environment in the occupied spatial units
- Introduction (of a pest)
The entry of a pest resulting in its establishment (FAO, 2018)
- Pathway
Any means that allows the entry or spread of a pest (FAO, 2018)
- Phytosanitary measures
Any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non‐quarantine pests (FAO, 2018)
- Quarantine pest
A pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled (FAO, 2018)
- Risk reduction option (RRO)
A measure acting on pest introduction and/or pest spread and/or the magnitude of the biological impact of the pest should the pest be present. A RRO may become a phytosanitary measure, action or procedure according to the decision of the risk manager
- Spread (of a pest)
Expansion of the geographical distribution of a pest within an area (FAO, 2018)
Appendix A – Thecodiplosis japonensis host plants/species affected
Source: EPPO Global Database (online) and CABI ISC (online). In bold: the main host species.
| Host status | Host name | Plant family | Common name | Reference |
|---|---|---|---|---|
| Cultivated hosts | Pinus contorta | Pinaceae | Lodgepole pine | CABI ISC (online) |
| Pinus coulteri | Pinaceae | Big‐cone pine | CABI ISC (online) | |
| Pinus densiflora | Pinaceae | Japanese red pine | ||
| Pinus kesiya | Pinaceae | Khasya pine | CABI ISC (online) | |
| Pinus luchuensis | Pinaceae | Luchu pine | CABI ISC (online) | |
| Pinus massoniana | Pinaceae | Masson pine | CABI ISC (online) | |
| Pinus mugo | Pinaceae | Mountain pine | CABI ISC (online) | |
| Pinus nigra | Pinaceae | Black pine | CABI ISC (online) | |
| Pinus ponderosa | Pinaceae | Ponderosa pine | CABI ISC (online) | |
| Pinus radiata | Pinaceae | Radiata pine | CABI ISC (online) | |
| Pinus resinosa | Pinaceae | Red pine | CABI ISC (online) | |
| Pinus sylvestris | Pinaceae | Scots pine | CABI ISC (online) | |
| Pinus tabuliformis | Pinaceae | Chinese red pine | ||
| Pinus thunbergii | Pinaceae | Japanese black pine | ||
| Pinus taiwanensis | Pinaceae | Taiwan pine | CABI ISC (online) |
Appendix B – Distribution of Thecodiplosis japonensis
| Region | Country | Subnational (e.g. state) | Status |
|---|---|---|---|
| Asia | China | Present, restricted distribution | |
| Fujian | Present, no details | ||
| Shandong | Present, no details | ||
| Japan | Present, no details | ||
| Honshu | Present, widespread | ||
| Kyushu | Present, no details | ||
| Ryukyu Archipelago | Present, no details | ||
| Shikoku | Present, widespread | ||
| Korea Dem. People's Republic | Absent, unreliable record | ||
| Korea, Republic | Present, widespread |
Distribution records based on EPPO Global Database (EPPO, online).
Appendix C – Methodological notes on Figure 3
The relative probability of presence (RPP) reported here for Pinus spp. in Figure 3 and in the European Atlas of Forest Tree Species (de Rigo et al., 2016; San‐Miguel‐Ayanz et al., 2016) is the probability of that genus to occur in a given spatial unit (de Rigo et al., 2017). In forestry, such a probability for a single taxon is called ‘relative’. The maps of RPP are produced by means of the constrained spatial multiscale frequency analysis (C‐SMFA) (de Rigo et al., 2014, 2017) of species presence data reported in geolocated plots by different forest inventories.
C.1 Geolocated plot databases
The RPP models rely on five geodatabases that provide presence/absence data for tree species and genera: four European‐wide forest monitoring data sets and a harmonised collection of records from national forest inventories (de Rigo et al., 2014, 2016, 2017). The databases report observations made inside geolocalised sample plots positioned in a forested area, but do not provide information about the plot size or consistent quantitative information about the recorded species beyond presence/absence.
The harmonisation of these data sets was performed within the research project at the origin of the European Atlas of Forest Tree Species (de Rigo et al., 2016; San‐Miguel‐Ayanz et al., 2016; San‐Miguel‐Ayanz, 2016). Given the heterogeneity of strategies of field sampling design and establishment of sampling plots in the various national forest inventories (Chirici et al., 2011a,b), and also given legal constraints, the information from the original data sources was harmonised to refer to an INSPIRE compliant geospatial grid, with a spatial resolution of 1 km² pixel size, using the ETRS89 Lambert Azimuthal Equal‐Area as geospatial projection (EPSG: 3035, https://spatialreference.org/ref/epsg/etrs89‐etrs‐laea/).
C.1.1 European National Forestry Inventories database
This data set was derived from National Forest Inventory data and provides information on the presence/absence of forest tree species in approximately 375,000 sample points with a spatial resolution of 1 km²/pixel, covering 21 European countries (de Rigo et al., 2014, 2016).
C.1.2 Forest Focus/Monitoring data set
This project is a Community scheme for harmonised long‐term monitoring of air pollution effects in European forest ecosystems, normed by EC Regulation No 2152/20033. Under this scheme, the monitoring is carried out by participating countries on the basis of a systematic network of observation points (Level I) and a network of observation plots for intensive and continuous monitoring (Level II). For managing the data, the JRC implemented a Forest Focus Monitoring Database System, from which the data used in this project were taken (Hiederer et al., 2007; Houston Durrant and Hiederer, 2009). The complete Forest Focus data set covers 30 European Countries with more than 8,600 sample points.
C.1.3 BioSoil data set
This data set was produced by one of a number of demonstration studies performed in response to the ‘Forest Focus’ Regulation (EC) No 2152/2003 mentioned above. The aim of the BioSoil project was to provide harmonised soil and forest biodiversity data. It comprised two modules: a Soil Module (Hiederer et al., 2011) and a Biodiversity Module (Houston Durrant et al., 2011). The data set used in the C‐SMFA RPP model came from the Biodiversity module, in which plant species from both the tree layer and the ground vegetation layer were recorded for more than 3,300 sample points in 19 European Countries.
C.1.4 European Information System on Forest Genetic Resources (EUFGIS)
EUFGIS (https://portal.eufgis.org) is a smaller geodatabase providing information on tree species composition in over 3,200 forest plots in 34 European countries. The plots are part of a network of forest stands managed for the genetic conservation of one or more target tree species. Hence, the plots represent the natural environment to which the target tree species are adapted.
C.1.5 Georeferenced Data on Genetic Diversity (GD2)
GD2 (https://gd2.pierroton.inra.fr) provides information about 63 species of interest for genetic conservation. The database covers 6,254 forest plots located in stands of natural populations that are traditionally analysed in genetic surveys. While this database covers fewer species than the others, it covers 66 countries in Europe, North Africa and the Middle East, making it the data set with the largest geographic extent.
C.2 Modelling methodology
For modelling, the data were harmonised in order to have the same spatial resolution (1 km²) and filtered to a study area comprising 36 countries in the European continent. The density of field observations varies greatly throughout the study area and large areas are poorly covered by the plot databases. A low density of field plots is particularly problematic in heterogeneous landscapes, such as mountainous regions and areas with many different land use and cover types, where a plot in one location is not representative of many nearby locations (de Rigo et al., 2014). To account for the spatial variation in plot density, the model used here (C‐SMFA) considers multiple spatial scales when estimating RPP. Furthermore, statistical resampling is systematically applied to mitigate the cumulated data‐driven uncertainty.
The presence or absence of a given forest tree species then refers to an idealised standard field sample of negligible size compared with the 1 km2 pixel size of the harmonised grid. The modelling methodology considered these presence/absence measures as if they were random samples of a binary quantity (the punctual presence/absence, not the pixel one). This binary quantity is a random variable having its own probability distribution which is a function of the unknown average probability of finding the given tree species within a plot of negligible area belonging to the considered 1 km2 pixel (de Rigo et al., 2014). This unknown statistic is denoted hereinafter with the name of ‘probability of presence’.
C‐SMFA performs spatial frequency analysis of the geolocated plot data to create preliminary RPP maps (de Rigo et al., 2014). For each 1 km² grid cell, the model estimates kernel densities over a range of kernel sizes to estimate the probability that a given species is present in that cell. The entire array of multiscale spatial kernels is aggregated with adaptive weights based on the local pattern of data density. Thus, in areas where plot data are scarce or inconsistent, the method tends to put weight on larger kernels. Wherever denser local data are available, they are privileged ensuring a more detailed local RPP estimation. Therefore, a smooth multiscale aggregation of the entire arrays of kernels and data sets is applied instead of selecting a local ‘best performing’ one and discarding the remaining information. This array‐based processing and the entire data harmonisation procedure are made possible thanks to the semantic modularisation which defines the Semantic Array Programming modelling paradigm (de Rigo, 2012).
The probability to find a single species (e.g. a particular coniferous tree species) in a 1‐km² grid cell cannot be higher than the probability of the presence of all the coniferous species combined. The same logical constraints applied to the case of single broadleaved species with respect to the probability of the presence of all the broadleaved species combined. Thus, to improve the accuracy of the maps, the preliminary RPP values were constrained so as not to exceed the local forest‐type cover fraction with an iterative refinement (de Rigo et al., 2014). The forest‐type cover fraction was estimated from the classes of the Corine Land Cover (CLC) maps which contain a component of forest trees (Bossard et al., 2000; Büttner et al., 2012).
The resulting probability of presence is relative to the specific tree taxon, irrespective of the potential co‐occurrence of other tree taxa with the measured plots, and should not be confused with the absolute abundance or proportion of each taxon in the plots. RPP represents the probability of finding at least one individual of the taxon in a plot placed randomly within the grid cell, assuming that the plot has negligible area compared with the cell. As a consequence, the sum of the RPP associated with different taxa in the same area is not constrained to be 100%. For example, in a forest with two co‐dominant tree species which are homogeneously mixed, the RPP of both may be 100% (see e.g. the Glossary in San‐Miguel‐Ayanz et al. (2016), https://forest.jrc.ec.europa.eu/media/atlas/Glossary.pdf).
The robustness of RPP maps depends strongly on sample plot density, as areas with few field observations are mapped with greater uncertainty. This uncertainty is shown qualitatively in maps of ‘RPP trustability’. RPP trustability is computed on the basis of the aggregated equivalent number of sample plots in each grid cell (equivalent local density of plot data). The trustability map scale is relative, ranging from 0 to 1, as it is based on the quantiles of the local plot density map obtained using all field observations for the species. Thus, trustability maps may vary among species based on the number of databases that report a particular species (de Rigo et al., 2014, 2016).
The RPP and relative trustability range from 0 to 1 and are mapped at a 1‐km spatial resolution. To improve visualisation, these maps can be aggregated to coarser scales (i.e. 10 × 10 pixels or 25 × 25 pixels, respectively, summarising the information for aggregated spatial cells of 100 and 625 km²) by averaging the values in larger grid cells.
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Gonthier P, Jaques Miret JA, Justesen AF, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Grégoire J‐C, Malumphy C, Kertesz V, Maiorano A and MacLeod A, 2022. Scientific Opinion on the pest categorisation of Thecodiplosis japonensis . EFSA Journal 2022;20(2):7088, 24 pp. 10.2903/j.efsa.2022.7088
Requestor: European Commission
Question number: EFSA‐Q‐2021‐00638
Panel members: Claude Bragard, Paula Baptista, Elisavet Chatzivassiliou, Francesco Di Serio, Paolo Gonthier, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe L Reignault, Emilio Stefani, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent Civera, Jonathan Yuen and Lucia Zappalà.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: EFSA wishes to acknowledge the contribution of Caterina Campese and Oresteia Sfyra to this opinion.
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder: Figure 1: Qi et al., 2020 Figure prepared by Lily Ren; Figure 2: © EPPO
Adopted: 16 December 2021
Notes
An EPPO code, formerly known as a Bayer code, is a unique identifier linked to the name of a plant or plant pest important in agriculture and plant protection. Codes are based on genus and species names. However, if a scientific name is changed, the EPPO code remains the same. This provides a harmonised system to facilitate the management of plant and pest names in computerised databases, as well as data exchange between IT systems (Griessinger and Roy, 2015; EPPO, 2019).
The import of black pine (Pinus thunbergii Parl.) bonsai (rooted bonsai plants 3–30 years old and potted in disinfected growing media) from Japan was subjected in March 2019 by EFSA to a Commodity risk assessment . (EFSA PLH Panel, 2018). An Expert Knowledge Elicitation (EKE) was performed for T. japonensis. Plants of black pines can be imported to the EU from Japan until December 2023, following specific conditions to ensure pest freedom. https://eur‐lex.europa.eu/legal‐content/EN/TXT/PDF/?uri=CELEX:32020R1217&from=EN
Council of the European Union, 2003. Regulation (EC) No 2152/2003 of the European Parliament and of the Council of 17 November 2003 concerning monitoring of forests and environmental interactions in the Community (Forest Focus). Official Journal of the European Union 46 (L 324), 1–8.
References
- Baker RHA, 2002. Predicting the limits to the potential distribution of alien crop pests. In: Hallman GJ and Schwalbe CP (eds.), Invasive Arthropods in Agriculture: Problems and Solutions. Science Publishers Inc, Enfield, USA. pp. 207–241. [Google Scholar]
- Bossard M, Feranec J and Otahel J, 2000. CORINE land cover technical guide ‐ Addendum 2000. Technical Report 40, European Environment Agency. Available online: https://www.eea.europa.eu/ds_resolveuid/032TFUPGVR
- Büttner G, Kosztra B, Maucha G and Pataki R, 2012. Implementation and achievements of CLC2006, Technical report, European Environment Agency. Available online: https://www.eea.europa.eu/ds_resolveuid/GQ4JECM8TB [Google Scholar]
- CABI Invasive Species Compendium Datasheet report , online. Thecodiplosis japonensis (pine needle gall midge). Available online: https://www.cabi.org/isc/datasheet/53511 [Accessed: 8 October 2021].
- Chirici G, Bertini R, Travaglini D, Puletti N and Chiavetta U, 2011a. The common NFI database. In: Chirici G, Winter S and McRoberts RE (eds). National Forest Inventories: Contributions to Forest Biodiversity Assessments. Springer, Berlin. pp. 99–119. [Google Scholar]
- Chirici G, McRoberts RE, Winter S, Barbati A, Brändli U-B, Abegg M, Beranova J, Rondeux J, Bertini R, Alberdi Asensio I and Condes S, 2011b. Harmonization tests. In: Chirici G, Winter S and McRoberts RE (eds). National Forest Inventories: Contributions to Forest Biodiversity Assessments. Springer, Berlin. pp. 121–190. [Google Scholar]
- Choi WI and Park YS, 2012. Dispersal patterns of exotic forest pests in South Korea. Insect Science, 19, 535–548. [Google Scholar]
- Choi WI, Jeon MJ and Park YS, 2017. Structural dynamics in the host‐parasitoid system of the pine needle gall midge (Thecodiplosis japonensis) during invasion. PeerJ, 5, e3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WI, Nam Y, Lee CY, Choi BK, Shin YJ, Lim J‐H, Koh S‐H and Park Y‐S, 2019. Changes in major insect pests of pine forests in Korea over the last 50 years. Forests, 10, 692. [Google Scholar]
- Duan C, Jiao J, Ren L and Wu H, 2021. Discovery of Thecodiplopsis japonensis Uchida & Inouye (Diptera: Cecidomyiidae) Infesting Pine Trees in the Huangdo Area of Shandong Province, China. Entomological News, 129, 546–552. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gregoire J‐C, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van Der Werf W, West J, Winter S, Hart A, Schans J, Schrader G, Suffert M, Kertesz V, Kozelska S, Mannino MR, Mosbach‐Schulz O, Pautasso M, Stancanelli G, Tramontini S, Vos S and Gilioli G, 2018. Guidance on quantitative pest risk assessment. EFSA Journal 2018;16(8):5350, 86 pp. 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Panel on Plant Health (EFSA PLH Panel) , Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Fejer Justesen A, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Reignault PL, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Vettraino AM, Leuschner R, Mosbach‐Schulz O, Rosace MC and Potting R, 2019. Commodity risk assessment of black pine (Pinus thunbergii Parl.) bonsai from Japan. EFSA Journal 2019;17(5):5667, 184 pp. 10.2903/j.efsa.2019.5667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Benfenati E, Chaudhry QM, Craig P, Frampton G, Greiner M, Hart A, Hogstrand C, Lambre C, Luttik R, Makowski D, Siani A, Wahlstroem H, Aguilera J, Dorne J‐L, Fernandez Dumont A, Hempen M, Valtueña Martínez S, Martino L, Smeraldi C, Terron A, Georgiadis N and Younes M, 2017. Scientific Opinion on the guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal 2017;15(8):4971, 69 pp. 10.2903/j.efsa.2017.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization) , 2004. Mini data sheet on Thecodiplosis japonensis. Available online: https://gd.eppo.int/download/doc/1097_minids_THEOJA.pdf
- EPPO (European and Mediterranean Plant Protection Organization) , 2019. EPPO codes. Available online: https://www.eppo.int/resources/eppo_databases/eppo_codes
- EPPO (European and Mediterranean Plant Protection Organization) , online. EPPO Global Database. Available online: https://gd.eppo.int [Accessed: 26 November 2021].
- European Information System on Forest Genetic Resources . EUFGIS database. Available online: https://portal.eufgis.org
- FAO (Food and Agriculture Organization of the United Nations) , 2013. ISPM (International Standards for Phytosanitary Measures) 11—Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. Available online: https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014‐04‐30_201405121523‐494.65%20KB.pdf
- FAO (Food and Agriculture Organization of the United Nations) , 2018. International Standards for Phytosanitary Measures. ISPM 5 Glossary of phytosanitary terms. Revised version adopted CPM 13, April 2018. FAO, Rome. Available online: https://www.ippc.int/en/publications/621/
- Furuno T, 1987. Studies on the insect damage to exotic pine species introduced in Japan. (No. 8) Further report on Japanese pine needle gall midge, Thecodiplosis japonensis Uchida et Inouye. Bulletin of the Kyoto University Forests, no, 59, 16–30. [Google Scholar]
- Griessinger D and Roy A‐S, 2015. EPPO codes: a brief description. Available online: https://www.eppo.int/media/uploaded_images/RESOURCES/eppo_databases/A4_EPPO_Codes_2018.pdf
- Hiederer R, Houston Durrant T, Granke O, Lambotte M, Lorenz M, Mignon B and Mues V, 2007. Forest focus monitoring database system ‐ Validation methodology. Vol. EUR 23020 EN of EUR – Scientific and Technical Research. Office for Official Publications of the European Communities. 10.2788/51364 [DOI]
- Hiederer R, Houston Durrant T and Micheli E, 2011. Evaluation of BioSoil demonstration project ‐ Soil data analysis. Vol. 24729 of EUR ‐ Scientific and Technical Research. Publications Office of the European Union. 10.2788/56105 [DOI]
- Houston Durrant T and Hiederer R, 2009. Applying quality assurance procedures to environmental monitoring data: a case study. Journal of Environmental Monitoring, 11, 774–781. 10.1039/b818274b [DOI] [PubMed] [Google Scholar]
- Houston Durrant T, San‐Miguel‐Ayanz J, Schulte E and Suarez Meyer A, 2011. Evaluation of BioSoil demonstration project: forest biodiversity ‐ Analysis of biodiversity module. Vol. 24777 of EUR – Scientific and Technical Research. Publications Office of the European Union. 10.2788/84823 [DOI]
- INRA, Biogeco, EvolTree . GD² database. Available online: https://gd2.pierroton.inra.fr
- Jiao J, Wu H, Ren L, Chen R and Luo Y, 2017. Reports on the discovery and preliminary studies of the invasive species Thecodiplosis japonensis (Uchida & Inouye) in Huangdao area of Shandong province. Chinese Journal of Applied Entomology, 54, 915–923. [Google Scholar]
- Kearby WH and Benjamin DM, 1964. The biology and ecology of the red‐pine needle midge and its role in fall browning of red pine foliage 1. The Canadian Entomologist, 96, 1313–1322. [Google Scholar]
- Kim CW, 1955. Thecodiplosis pinicola n.sp. Bulletin of the Collage Literature Science, Korea University, 1, 1–12. [Google Scholar]
- Kim J, Ha M, Lee S, Kim H and Lee C, 2020. Difference of gall formation rates and parasitic rates of Thecodiplosis japonensis (Diptera: Ceidomyiidae) Larvae in Pine Forests around Urban and Mountain Villages. Journal of Forest and Environmental Science, 36, 290–297. [Google Scholar]
- Lee SG, Kim SI, Ahn YJ, Kim JB and Lee BY, 1997. Effectiveness of carvacrol derived from Thujopsis dolabrata var. hondai sawdust against Thecodiplosis japonensis (Diptera: Cecidomyiidae). Pesticide Science, 49, 119–124. [Google Scholar]
- Lee SG, Park JD and Ahn YJ, 2000. Effectiveness of neem extracts and carvacrol against Thecodiplosis japonensis and Matsucoccus thunbergianae under field conditions. Pest Management Science: Formerly Pesticide Science, 56, 706–710. [Google Scholar]
- Liu H, Duan C, Qi Y, Ren L and Wu H, 2020. Movement behavior of the Pine Needle Gall Midge (Diptera: Cecidomyiidae). Journal of Insect Science, 20, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam Y and Choi WI, 2014. An empirical predictive model for the spring emergence of Thecodiplosis japonensis (Diptera: Cecidomyiidae): model construction and validation on the basis of 25 years of field observation data. Journal of Economic Entomology, 107, 1136–1141. [DOI] [PubMed] [Google Scholar]
- Qi Y, Duan C, Ren L and Wu H, 2020. Growth dynamics of galls and chemical defence response of Pinus thunbergii Parl. to the pine needle gall midge. Thecodiplosis Japonensis Uchida & Inouye (Diptera: Cecidomyiidae). Scientific Reports, 10, 12289. 10.1038/s41598-020-69231-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rigo D, 2012. International Environmental Modelling and Software Society (iEMSs). In: Seppelt R, Voinov AA, Lange S and Bankamp D (eds.), International Congress on Environmental Modelling and Software ‐ Managing Resources of a Limited Planet: Pathways and Visions under Uncertainty, Sixth Biennial Meeting. pp. 1167–1176. [Google Scholar]
- de Rigo D, Caudullo G, Busetto L and San‐Miguel‐Ayanz J, 2014. Supporting EFSA assessment of the EU environmental suitability for exotic forestry pests: final report. EFSA Supporting Publications, 11, EN‐434+. 10.2903/sp.efsa.2014.EN-434 [DOI] [Google Scholar]
- de Rigo D, Caudullo G, Houston Durrant T and San‐Miguel‐Ayanz J, 2016. The European Atlas of Forest Tree Species: modelling, data and information on forest tree species. In: San‐Miguel‐Ayanz J, de Rigo D, Caudullo G, Houston Durrant T and Mauri A (eds.), European Atlas of Forest Tree Species. Publ. Off. EU, Luxembourg, pp. e01aa69+ Available online: https://w3id.org/mtv/FISE‐Comm/v01/e01aa69 [Google Scholar]
- de Rigo D, Caudullo G, San‐Miguel‐Ayanz J and Barredo JI, 2017. Robust modelling of the impacts of climate change on the habitat suitability of forest tree species. Publication Office of the European Union, 58 pp. ISBN: 978‐92‐79‐66704‐6. 10.2760/296501 [DOI] [Google Scholar]
- San‐Miguel‐Ayanz J, 2016. The European Union Forest Strategy and the Forest Information System for Europe. In: San‐Miguel‐Ayanz J, de Rigo D, Caudullo G, Houston Durrant T and Mauri A (eds.). European Atlas of Forest Tree Species, Publ. Off. EU, Luxembourg, pp. e012228. [Google Scholar]
- San‐Miguel‐Ayanz J, de Rigo D, Caudullo G, Houston Durrant T and Mauri A (eds.), 2016. European Atlas of Forest Tree Species, Publication Office of the European Union, Luxembourg. Available online: https://w3id.org/mtv/FISE‐Comm/v01. ISBN: 978‐92‐79‐36740‐3. [Google Scholar]
- Sayers EW, Cavanaugh M, Clark K, Ostell J, Pruitt KD and Karsch‐Mizrachi I, 2020. Genbank. Nucleic Acids Research, 48, Database issue, doi: 10.1093/nar/gkz956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son Y, Lee JH and Chung YJ, 2007. Temperature‐dependent post‐diapause development and prediction of spring emergence of the pine needle gall midge (Dipt., Cecidomyiidae). Journal of Applied Entomology, 131, 674–683. [Google Scholar]
- Sone K, 1984. Realized fecundity of the pine needle gall midge, Thecodiplosis japonensis Uchida & Inouye: Diptera: Cecidomyiidae. Applied Entomology and Zoology, 19, 534–536. [Google Scholar]
- Tobin PC, Berec L and Liebhold AM, 2011. Exploiting Allee effects for managing biological invasions. Ecology Letters, 14, 615–624. [DOI] [PubMed] [Google Scholar]
- Toy SJ and Newfield MJ, 2010. The accidental introduction of invasive animals as hitchhikers through inanimate pathways: a New Zealand perspective. Revue Scientifique Et Technique (International Office of Epizootics), 29, 123–133. [DOI] [PubMed] [Google Scholar]
- Uchida T and Inouye M, 1955. Eine neue Thecodiplosis‐Art (Diptera: Itonididae). Insecta Matsumurana, 19, 44–45. [Google Scholar]
- Yamauchi S, Ikenaga H and Yukawa J, 1982. Midge galls collected from the south‐east islands of Japan. Satsuma, 31, 1–23. (in CABI ISC online). [Google Scholar]


