Abstract
Coronavirus disease 2019 (COVID-19) is an ongoing pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) virus. More than 5 million children have been infected in the United States. Risk factors for more severe disease progression include obesity, pulmonary disease, gastrointestinal disorders, and neurologic comorbidities. Children with COVID-19 are admitted to the pediatric intensive care unit because of severe acute COVID-19 illness or COVID-19-associated multisystem inflammatory syndrome in children. The delta surge of 2021 was responsible for an increased disease burden in children and points to the key role of vaccinating children against this sometimes-deadly disease.
Keywords: SARS-CoV-2, COVID-19, ARDS, MIS-C, Pediatric COVID, Risk factors, Epidemiology
Key points
-
•
Although children are less affected than adults with coronavirus disease 2019 (COVID-19), more than 5 million children in the United States have been infected and the overall public health implications of the pandemic on children are severe.
-
•
Certain high-risk conditions make children more prone to severe disease.
-
•
Children are admitted to the pediatric intensive care unit for severe acute COVID-19, which is severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection associated with 1 or more organ system involvement or multisystem inflammatory syndrome in children (MIS-C).
-
•
Pediatric critical care physicians should be cognizant of complications from hyperinflammation in SARS-CoV-2 infections, management of COVID-19-associated acute respiratory failure, and special precautions to be taken during aerosol-generating procedures.
-
•
Presentations of MIS-C can be like other diseases and might be especially hard to differentiate from Kawasaki disease.
-
•
Diagnosis and treatment of MIS-C using available guidelines can result in favorable outcomes in critically ill children.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has wreaked havoc across the world, with an estimated 242,688,319 human infections and 4,932,928 deaths worldwide as of November of 2021.1
Children were first thought to be immune from infection, but the truth is a much more sobering tale of infections, hospitalizations, deaths, and long-term (long-haul) symptoms. This article outlines what is known about the epidemiology of COVID-19 infection in children to date. In addition, it discusses severe COVID-19 disease in children and addresses the public health toll the pandemic has exerted on children and issues that must be addressed with lessons learned to help prepare for the next pandemic.
Epidemiology and Early Pandemic Reports
Unlike many pediatric illnesses in which knowledge of epidemiology, clinical course, and outcomes are gathered over a long time, the COVID-19 pandemic saw a shift to fast-track publication of case reports, meta-analysis, and essential updates from both the private sector and government (eg, Centers for Disease Control and Prevention [CDC]/Morbidity and Mortality Weekly Report [MMWR]). At a time of a public health emergency, rapid publication of domestic and international experience must be balanced with academic and scientific rigor.
The first reports of pediatric COVID-19 illness emerged from the Shanghai Children’s Medical Center in China in March 2020 with data from 2135 children with COVID-19 reported to the Chinese Center for Disease Control and Prevention.2 The median age of children with COVID-19 was 7 years, and 56% were male. Although 51% of the patients were said to have mild symptoms, 38% had moderate symptoms (pneumonia and wheezing), and 6% had severe or critical clinical findings such as hypoxia and respiratory failure. Lu and colleagues3 published a cohort analysis of 171 COVID-19–positive children from Wuhan Children’s Hospital in April 2020. Seventy had fever, 12 had pneumonia, 3 required mechanical ventilation, and 1 died. Although these early reports showed that children had a less severe clinical course than adults, a small but concerning percentage of children progressed to respiratory failure.
The University of Texas–San Antonio and Texas Children’s Hospital published a meta-analysis/case summary of children with COVID-19,4 with 131 studies from 26 countries and 7780 children from January to May 2020 (Table 1 ). The median age was 8.9 years, and 75.6% were exposed to an adult with COVID-19. Need for intensive care unit (ICU) care was 3.3%, and length of hospital stay was 11.6 days. Approximately 35% had underlying medical conditions, with immunodeficiency being the most common at 30.5% (Table 2 ).
Table 1.
Characteristics of children with COVID-19.
| # Studies | # Patients | N (%) | |
|---|---|---|---|
| Male gender | 113 | 4640 | 2582 (55.6) |
| Mean age (years) | 116 | 4517 | 8.9 ± 0.5 |
| Exposure from family member | 94 | 1360 | 1028 (75.6) |
| Travel to/lived-in high-risk area | 84 | 962 | 689(71.6) |
| Np/th roat SARS-CoV-2 detection | 89 | 787 | 681(86.5) |
| Positive fecal viral shedding | 31 | 321 | 67(20.9) |
| Positive urine viral shedding | 22 | 54 | 2(3.7) |
| Length of hospital stay (days) | 68 | 652 | 11.6 ± 0.3 |
| Intensive care unit admission | 88 | 3564 | 116(3.3) |
Continuous data presented as Mean ± SD. NP-nasopharyngeal.
(Hoang, A., Chorath, K., Moreira, A., Evans, M., Burmeister-Morton, F., Burmeister, F., Naqvi, R., Petershack, M., & Moreira, A. (2020). COVID-19 in 7780 pediatric patients: A systematic review. EClinicalMedicine, 24, 100433. https://doi.org/10.1016/j.eclinm.2020.100433)
Table 2.
Characteristics of children with COVID-19
| # Studies | # Patients | N (%) | |
|---|---|---|---|
| Underlying conditions | 20 | 665 | 233(35.6) |
| Immunosuppression | 71(30.5) | ||
| Respiratory | 49(21.0) | ||
| Cardiovascular | 32(13.7) | ||
| Medically complex/congenital malformations | 25(10.7) | ||
| Not reported | 17(7.3) | ||
| Hematologic | 8(3.8) | ||
| Neurologic | 8(3.4) | ||
| Obesity | 8(3.4) | ||
| Prematurity | 5(3.4) | ||
| Endocrine/metabolic | 5(2.1) | ||
| Renal | 4(1.7) | ||
| Gastrointestinal | 5(3.4) | ||
| Co-infections | 35 | 1183 | 72(5.6) |
| Bacterial | |||
| Mycoplasma pneumoniae | 42(58.3) | ||
| Enterobacter sepsis | 2(2.8) | ||
| Streptococcus pneumoniae Viral | 1(1.4) | ||
| Influenza virus A/B | 8(11.1) | ||
| Respiratory syncytial virus | 7(9.7) | ||
| Cytomegalovirus | 3(4.2) | ||
| Epstein-Barr virus | 3(4.2) | ||
| Adenovirus | 2(2.8) | ||
| Human metapneumovirus | 2(2.8) | ||
| Human parainfluenza virus | 2(2.8) |
Children and Susceptibility to Severe Acute Respiratory Syndrome Coronavirus-2
Viner and colleagues5 performed a meta-analysis on 13,926 published articles and summarized 32 studies with data from 41,460 children. Compared with data from 14 studies on adults with COVID-19, children had lower susceptibility to SARS-CoV-2 with a pooled odds ratio (OR) of 0.56 (95% confidence interval [CI], 0.37–0.85). Data regarding transmission of COVID-19 by children were inconclusive. Gaythorpe and colleagues6 reviewed 128 studies to examine COVID-19 susceptibility and transmissibility in children and showed the OR of an asymptomatic child having an infection was 21.1% (95% CI, 14.0%–28.1%), and the proportion of children with severe disease was 3.8% (95% CI, 1.5%–6.0%). The investigators were not able to determine a child’s ability to spread COVID-19.
United States experience
An analysis of 12,306 children from the United States infected with COVID-19 from April to October 2020 examined symptoms and clinical course.7 Symptoms included respiratory (16%), gastrointestinal (13.9%), rash (8.1%), and neurologic (4.8%). Eighteen percent had nonspecific findings such as fever and malaise. Five percent required hospitalization, of whom 17.6% needed mechanical ventilation. Male and female children are equally affected, and risk of hospitalization is greater among non-Hispanic black and Hispanic children compared with non-Hispanic whites. Among hospitalized children, the rate of ICU admissions is similar to adults.8
Progression and severity of disease
Graff and colleagues9 reported on which children are at most significant risk for severe complications from COVID-19 infection. At the time, there were up to 1.3 million children infected with COVID-19 in the United States. This group examined the clinical course of children with the diagnosis of COVID-19 at their institutions from March to July 2020, where 454 children tested positive for SARS-CoV-2. The most frequent risk factor for COVID-19 exposure was a family member testing positive for SARS-CoV-2. Participation in social gatherings of 10 or more was a significant risk factor as well. Forty-five percent of the children with COVID-19 were identified with at least 1 comorbid condition: pulmonary (16%), gastrointestinal (11%), and neurologic (11%). Among the comorbid conditions, asthma, diabetes, and obesity were predictors of severe COVID-19 in children. Eighty-five were hospitalized, of whom 66 were symptomatic (the remaining 19 patients were admitted for other reasons and never had COVID symptoms). Of the 66 symptomatic patients, 55% required respiratory support, and 17% required critical care (Fig. 1 ). The need for hospitalization was associated with younger age (0–3 months) and the presence of comorbidities. Requirement for respiratory support was associated with Hispanic ethnicity, age 0 to 3 months, obesity, and asthma. The need for critical care was associated with obstructive sleep apnea and increased C-reactive protein (CRP) level at the time of admission.
Fig. 1.
Summary of children with positive COVID-19 test
(Graff, K., Smith, C., Silveira, L., Jung, S., Curran-Hays, S., Jarjour, J., Carpenter, L., Pickard, K., Mattiucci, M., Fresia, J., McFarland, E. J., Dominguez, S. R., & Abuogi, L. (2021). Risk Factors for Severe COVID-19 in Children. Pediat)
Investigators from the CDC examined disease severity in children admitted with COVID-19 from March to October 2020 using the Premier Health Care Database and identified 20,714 children with COVID-19, 2430 of whom were hospitalized.10 Severe COVID-19 disease was associated with boys younger than 1 year, and the presence of comorbidity. There was no association between race/ethnicity and severe COVID-19.
The Delta variant surge and children
The American Academy of Pediatrics and the Children’s Hospital Association began publishing pediatric data weekly starting in the fall of 2020, indicating increasing numbers of children (<17 years old) with COVID-19 and hospitalization rates, especially during the Delta surge of 2021.11
As of October 2021, 5,899,148 children were reported to have COVID-19, representing 16.2% of US cases with an overall rate of 7838 cases per 100,000 children.
Compared with adults, the hospitalization rate for children with COVID-19 remained low until a spike in September 2021. Pediatric hospitalization rates varied between 1.3 and 3.2 per 100,000 children for ages 0 to 4 years and 0.8 to 1.4 for children 5 to 17 years.
Per Centers for Disease Control and Prevention Data
During a subsequent 6-week period after the Delta variant became predominant, COVID infection rates increased each week to 1.4 during the week ending August 14, 2021, which was 4.7 times the rate during the week ending June 26, 2021, and approached the peak hospitalization rate of 1.5 observed during the week ending January 9, 2021. Weekly rates increased among all age groups; the sharpest increase occurred among children aged 0 to 4 years, for whom the rate during the week ending August 14, 2021, (1.9) was nearly 10 times that during the week ending June 26, 2021 (0.2).
Although overall hospitalization rates remained lower in children compared with adults, 20% to 26.4% of hospitalized children required ICU care, and 9% to 12% of children required mechanical ventilation. The mortality from COVID-19 is low (for states reporting, 0%–0.26% of total COVID deaths were children.)
Effect of community vaccination on pediatric coronavirus disease 2019
However, because of areas in the United States with low vaccination rates in adults, the Delta variant emerged in 2021 as the predominant strain of COVID-19 causing infection in children. In addition, because children less than 12 years were ineligible to receive any of the emergency use approval (EUA) vaccines in early 2021, and the refusal to wear masks and adhere to social distancing recommendations, the number of children with COVID-19 infections increased, and they were 1.5 to 3 times more likely to require emergency care for COVID-19.12
Clinics Care Points
-
•
Despite lower overall hospitalization rates for COVID-19 in children, the rate of ICU admissions among hospitalized children is similar to adults.
-
•
Intensivists should be aware of underlying conditions that can put children at risk for severe COVID-19.
-
•
Hospitalization rates for children have increased since the start of the pandemic, especially during the Delta surge of 2021.
-
•
Efficacy studies for vaccines in children and vaccination recommendations for children are underway.
Pathogenesis
Transmission of SARS-CoV-2 is primarily through airborne droplets and to a lesser extent from contaminated surfaces, and rarely through body fluids. The virus can transmit over long distances, especially when indoors. Incubation period is 3 to 6 days. The entry into host cells is mediated by its spike glycoprotein (S-glycoprotein) binding to the ACE2 cellular receptor in the upper respiratory tract to begin primary replication.13 Patients can be asymptomatic carriers or have mild symptoms at this stage. Viral load is increased in the first week, followed by a progressive decline in 7 to 10 days with increase in immunoglobulin (Ig) M and IgG antibodies against viral antigens. The persistence of high viral load leads to migration of virus in the airway with entry into alveolar epithelial cells, where it replicates, causing localized inflammation and pneumonia. Cell apoptosis occurs, with increased capillary permeability and release of proinflammatory proteins. Cytokine storm can ensue with release of inflammatory markers such as interleukin (IL)-2/6/7/10, granulocyte colony-stimulating factor, interferon gamma-induced protein 10 (IP-10), macrophage chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1 (MIP-1), and tumor necrosis factor-α (TNF-α), which can cause acute respiratory distress syndrome (ARDS), septic shock, and multiorgan dysfunction.14
Coronavirus disease 2019 in critically ill children
Children with COVID-19 are admitted to the PICU because of severe acute COVID-19 illness, which is SARS-CoV-2 infection with 1 or more organ system involvement or COVID-19-associated multisystem inflammatory syndrome in children (MIS-C).
Severe acute coronavirus disease 2019
Children with severe acute COVID-19 are admitted to the PICU for respiratory problems such as pneumonia and ARDS. Cardiovascular, gastrointestinal, neurologic, hematologic, and acute kidney injury (AKI) complications can result from severe acute COVID-19. Risk factors for severe acute COVID-19 are the presence of 1 or more underlying conditions such as obesity, chronic pulmonary disease, neurologic disease, cardiovascular disease, medical complexity and technology dependence, sickle cell disease, or immunosuppresion.15, 16, 17, 18, 19 Underlying chronic respiratory diseases such as asthma and cystic fibrosis were not significantly exacerbated by SARS-CoV-2.19 Younger age, obesity, hypoxia on admission, increased white blood cell count, and bilateral infiltrates on chest radiograph are predictors of severe respiratory disease.20
Diagnosis
Laboratory Tests
Detection of SARS-CoV-2 nucleic acid using real-time reverse transcriptase-polymerase chain reaction (RT-PCR) is considered the gold-standard for the diagnosis of COVID-19.21 The virus can be detected in the upper airway (nasopharynx swab) or lower airway secretions (tracheal aspirates, bronchoalveolar lavage), blood, urine, and stool. Leukocytosis or leukopenia, lymphocytosis or lymphopenia, and increases of CRP, serum ferritin, lactate dehydrogenase (LDH), D-dimers, procalcitonin, erythrocyte sedimentation rate (ESR), serum aminotransferases, and creatine kinase-myocardial bands (CK-MB) have been observed.22 , 23 Increases of CRP, procalcitonin, pro–B-type natriuretic peptide (BNP) and platelet count are more common in children requiring PICU admission compared with other hospitalized patients.24 Organ dysfunction was associated with increased CRP, increased white blood cell count, and thrombocytopenia.25
Hyperinflammation associated with increased LDH, D-dimer, IL-6, CRP, and ferritin, and decreased lymphocyte count, platelet count, and albumin level were associated with worse outcomes in adult patients with COVID-19.26
Imaging Studies
Chest radiography is routinely performed in most children hospitalized for acute respiratory failure from COVID-19. Although chest radiographs do not have high sensitivity and specificity for the diagnosis of COVID-19, they are useful to monitor disease progression. Bilateral distribution with presence of peripheral or subpleural ground-glass opacifications and consolidation are common findings in COVID-19 pneumonia or ARDS (Fig. 2 ). Typical features of viral respiratory infections in children, such as increased perihilar markings and hyperinflation, were not reported in children with COVID-19.27 , 28
Fig. 2.
Chest radiograph of infant with bronchopulmonary dysplasia who developed COVID-19 ARDS showing bilateral ground-glass opacities.
Computed tomography (CT) scans are considered the gold standard for imaging with COVID-19 respiratory disease.29 CT scans are highly sensitive and specific and can detect infection before the appearance of clinical signs.29 , 30 Three phases of evolution have been observed in children with COVID-19 disease. These phases are the halo sign, defined as nodules or masses surrounded by ground-glass opacifications seen in the early phase of the disease; widespread ground-glass opacifications in the progressive phase; and consolidative opacities in the developed phase. Peribronchial thickening and inflammation along the bronchovascular bundle are observed more frequently in children than adults.31 Fine mesh reticulations and so-called crazy-paving sign have been reported. Pleural effusion and lymphadenopathy are rare.31 Compared with adults, children were found to have less positive CT findings, lower number of pulmonary lobes involved, and lower overall semiquantitative lung score, which measures the extent of lung involvement.31 Because of these findings and concerns for radiation exposure, transport of unstable patients to CT suites, and infection control issues, chest CT is not recommended as the initial diagnostic test in children suspected of having COVID-19. However, it may be considered to answer specific clinical questions such as presence of pulmonary embolism, for assessment of those not responding to treatment, and to track evolution of fibrotic disease. Lung ultrasonography is a useful imaging modality because semiquantitative scores in lung ultrasonography have been shown to be consistent with those in lung CT scans in adults who are critically ill with COVID-19, and should be considered in children.30 , 32
Recommendations for Diagnostic Tests in Severe Acute Coronavirus Disease 2019
Laboratory tests: SARS-CoV-2 RT-PCR, COVID-19 IgG, complete blood count (CBC), complete metabolic panel (CMP), LDH, CRP, procalcitonin, ESR, prothrombin time (PT), partial thromboplastin time (PTT), D-dimer, troponin, and BNP. Ferritin, and cytokine panel when available, provide additional information about the hyperinflammatory state.
Cardiac evaluation: baseline electrocardiogram (ECG) should be obtained in all patients, and those with abnormal troponin should undergo echocardiography.
Imaging studies: chest radiograph in all patients, and CT scan if pulmonary embolism is suspected.
Clinics Care Points
-
•
Severe acute COVID-19, which is SARS-CoV-2 infection with 1 or more organ system involvement, requires PICU admission.
-
•
Pediatric intensivists should be familiar with MIS-C and its complications.
-
•
The gold standard for diagnosis of COVID-19 is detection of SARS-CoV-2 nucleic acid using RT-PCR.
-
•
Hyperinflammation plays a major role in pathogenesis of SARS-CoV-2 and complications of severe acute COVID-19.
-
•
Intensivists should be familiar with chest radiograph changes in severe acute COVID-19, and chest CT should be considered only in those patients in whom pulmonary embolism is a concern.
Severe acute coronavirus disease 2019 complications
Acute Respiratory Failure
Clinical features: SARS-CoV-2 pneumonia can cause acute respiratory failure and progress to ARDS. Diagnostic criteria for COVID-19 ARDS are the same as for pediatric ARDS (PARDS) from other causes. Patients typically have worsening respiratory symptoms 1 week after disease onset, new opacities on chest imaging that are not caused by cardiac failure or volume overload, partial pressure of oxygen (Pao 2) to fraction of inspired oxygen (Fio 2) ratio less than or equal to 300 mm Hg or oxygen saturation by pulse oximetry/Fio 2 less than or equal to 264 during noninvasive ventilation, oxygenation index (OI) greater than or equal to 4, or oxygen saturation index (OSI) greater than or equal to 5 during invasive mechanical ventilation. Mild, moderate, and severe PARDS are defined as OI/OSI of 4 to 8 or 5 to 7.5, 8 to 16 or 7.5 to 12.3, and greater than 16 or greater than 12.3 respectively.33
Pathologic changes in these patients are like PARDS from other causes with initial diffuse alveolar damage and fibrosis with disease progression. Differences have been noted in adults between ARDS from COVID-19 compared with ARDS from other causes, including phenotypic subtypes such as type L, characterized by low elastance with preserved compliance, and type H, characterized by high elastance with low compliance, and increased association with thrombosis.34 Studies in children have not shown significant differences in compliance between PARDS from COVID-19 and other causes.
Management
General principles of management
Management of COVID-19–associated acute respiratory failure is outlined in Fig. 3 . The principles of management and end goals of respiratory therapy are the same as for other causes of acute respiratory failure in children.33 , 35 , 36 Patients who have Spo 2 less than 90% need supplemental oxygen, noninvasive ventilation, or intubation and mechanical ventilation based on severity. Intubation protocols with special precautions for patients with COVID-19 should be developed based on resources available.37 , 38 Ventilator strategies as outlined in Fig. 3 help in the management of COVID-19 PARDS, and ARDSNet protocols for positive end-expiratory pressure (PEEP)/Fio 2 may be followed. In a retrospective study in children before the COVID-19 pandemic, use of lower PEEP relative to Fio 2 than what is recommended by the ARDSNet model resulted in higher mortality.38, 39, 40 In addition to recommendations in Fig. 3, intravascular volume expansion should be avoided in patients without hypotension. Adequate mean arterial pressure should be maintained, and inotropic support provided as needed, and nutritional support must be adequate.38 , 41 Patients who have refractory hypoxemia may need treatment such as inhaled nitric oxide, high-frequency oscillatory ventilation, or extracorporeal membrane oxygenation (ECMO) as recommended in the management of PARDS from other causes.
Fig. 3.
Management of acute respiratory failure in severe COVID-19. BiPAP, bilevel positive airway pressure; CPAP, continuous positive airway pressure; ETT, endotracheal tube; HEPA, high-efficiency particulate air filter; HFNC, high-flow nasal cannula; LMA, laryngeal mask airway; NIV, noninvasive ventilation; PAPR, powered air purifying respirator; PEEP, positive end-expiratory pressure; POCUS, point-of-care ultrasonography; PPE, personal protective equipment; Pplat, plateau pressure; RSI, rapid sequence intubation; Spo2, oxygen saturation by pulse oximetry.
COVID-19–specific management
-
1.
Rapid spread of infection from SARS-CoV-2 can occur during various aerosol-generating procedures (AGPs). Appropriate personal protection equipment (PPE) should be used by all staff and visitors. Special precautions should be taken to minimize spread during AGP, such as coughing and sneezing, use of noninvasive ventilation including high-flow nasal cannula, bag-mask ventilation, intubation, tracheal suction, planned or accidental extubation, chest physiotherapy, cardiopulmonary resuscitation, and use of nebulized medications outside of a closed circuit.38
-
2.
Antiviral therapy: remdesivir is an antiviral medication that is an inhibitor of the SARS-CoV-2 RNA-dependent RNA polymerase (RdRp), which is essential for viral replication. Remdesivir is approved by the US Food and Drug Administration (FDA) for treatment of patients greater than or equal to 12 year old hospitalized with COVID-19 who weigh greater than or equal to 40 kg, and is FDA authorized via EUA for use in hospitalized patients less than 12 years of age or weighing from 3.5 to less than 40 kg.42 , 43 In neonates less than 3.5 kg, use should be directed by recommendations from infectious disease consultants on weighing the risks versus benefits. Intravenous remdesivir is most beneficial if used early in the course of illness (<10 days) and has been shown to reduce symptom duration in adults but does not seem to reduce mortality. There are few studies in children, but remdesivir seems to be well tolerated.44 , 45 Lyophilized powder formulation should be used in children less than 40 kg because it contains half the amount of sulfobutylether-β-cyclodextrin sodium salt, an excipient in remdesivir that is cleared through the kidneys and can accumulate in patients with decreased renal function. Children weighing greater than or equal to 3.5 kg and less than 40 kg should receive a loading dose of 5 mg/kg on day 1 followed by 2.5 mg/kg/dose once daily. For those greater than or equal to 40 kg, a loading dose of 200 mg is recommended on day 1 followed by 100 mg daily. Duration of therapy is 5 days or until hospital discharge, whichever is earlier, and 10 days for those who require mechanical ventilation or ECMO. Laboratory monitoring during remdesivir therapy should include CBC, CMP, PT/International Normalized Ratio (INR) at baseline, day 5 of therapy, and more often if there is concern for toxicity. Common adverse reactions to remdesivir include reversible increases of transaminase levels and hypersensitivity reactions. Bradycardia and hypotension have been reported in adults but may have been related to concomitant use of other medications.46 Contraindications to its use are hypersensitivity to remdesivir or any component of the formulation. Remdesivir is not recommended in children older than 28 days with estimated glomerular filtration rate less than 30 mL/min, and in full-term neonates with serum creatinine level 1 mg/dL or greater and should be used with caution in those with baseline alanine transaminase (ALT) levels more than 5 times the upper limit of normal. Transaminase levels might be increased because of COVID-19 and, if remdesivir is used, it should be discontinued if ALT levels increase to more than 10 times the upper limit of normal or if ALT increase is accompanied by signs or symptoms of liver inflammation. Dose adjustments are needed for those on ECMO or renal replacement therapy (RRT) because of interactions between remdesivir and the circuits, which can cause significant changes in the pharmacokinetics of the drug.
-
3.
Antiinflammatory therapy: dexamethasone is recommended for hospitalized children with COVID-19 who require high-flow oxygen, noninvasive ventilation, invasive mechanical ventilation, or ECMO.47 The dexamethasone dosing regimen for children is 0.15 mg/kg/dose (maximum dose, 6 mg) once daily for up to 10 days. Steroids should be used with caution because there are reports of increased mortality and decreased viral clearance with certain viral infections and development of neuropathy and myopathy in critically ill patients. However, dexamethasone significantly reduces mortality in adult patients with COVID-19 who require mechanical ventilation and is recommended for treatment of children with severe acute COVID-19 disease.48, 49, 50 Patients with exacerbation of asthma with COVID-19 should receive methylprednisolone and those with adrenal insufficiency and catecholamine-resistant refractory shock should receive hydrocortisone in doses recommended for such conditions.
-
4.
Immunomodulator therapy: IL-1 receptor antagonist such as anakinra should be considered in children for whom corticosteroids is contraindicated, who are refractory to corticosteroids, or who have severe acute COVID-19 causing ARDS, shock, or signs of significant hyperinflammation.50 Dosing guidelines as mentioned in relation to MIS-C treatment may be followed.
-
5.
Other specific treatments, such as monoclonal antibodies, convalescent plasma, and IL-6 inhibitors, are not recommended in critically ill children.50
Sepsis and Septic Shock
Manifestations are like those resulting from other infections, and recommendations of the 2020 Surviving Sepsis Campaign should be followed.51
Acute Kidney Injury
Presentation and management are the same as for any critically ill patient developing AKI. The hypercoagulable state in COVID-19 can cause clotting of filters used in RRT and can be prevented with the addition of prefilter heparin and/or citrate.52
Neurologic Complications
Meningitis, encephalitis, acute disseminated encephalomyelitis, Guillain-Barré syndrome, myositis, acute necrotizing hemorrhagic encephalopathy, seizures, and cerebrovascular disease from hypercoagulable state have all been reported in severe acute COVID-19.53 Diagnosis and management are the same as when these complications arise from other causes.
Hypercoagulable State
COVID-19 induces a prothrombotic state from hyperactivation of the inflammatory and hemostatic pathways.54 Thrombotic complications in adults with COVID-19 is well recognized but are rare in children with COVID-19 and, when they occur, are usually in the lungs.55 Serum D-dimer levels are used to assess for hypercoagulation, and a daily screen of D-dimer, PT, and platelet count is recommended.38 When not contraindicated, pharmacologic thromboprophylaxis combined with mechanical thromboprophylaxis with sequential compression devices is recommended. Anticoagulant thromboprophylaxis with low-molecular-weight heparin is recommended in patients who have increased D-dimer levels or clinical risk factors for venous thromboembolism. Children who are at high risk for venous thromboembolism include those who are critically ill, with a history of thromboembolism, or those who have increased inflammatory markers (CRP>150 mg/L, D-dimer>1500 ng/mL, IL-6>100pg/mL, ferritin>500 ng/mL), and should be treated with subcutaneous low-molecular weight-heparin (<2 months, 1.5 mg/kg/dose every 12 hours; ≥2 months, 1 mg/kg/dose every 12 hours) to achieve anti-Xa factor levels of 0.5 to 1 IU/mL.56 Children who are clinically unstable or have severe renal impairment should receive continuous intravenous infusion of unfractionated heparin as anticoagulant thromboprophylaxis using pediatric heparin nomogram to guide therapy.57 , 58
Myocarditis
Patients with MIS-C commonly have myocarditis, and, occasionally, in severe acute COVID-19. Presentation and management are the same as for myocarditis from other infections.
Clinics Care Points
-
•
Pathophysiology and diagnosis of PARDS from COVID-19 is the same as for PARDS from other causes.
-
•
Intensivists must be familiar with additional precautions to be taken during intubation and AGPs.
-
•
Intensivists should know ventilator strategies and therapies used specifically in COVID-19 acute respiratory failure, including antiviral, antiinflammatory, and immunomodulator therapies.
-
•
Multiorgan dysfunction and failure from severe acute COVID-19 should be recognized and treated.
-
•
COVID-19 induces a prothrombotic state, and thrombotic complications in severe acute COVID-19 should be diagnosed and treated and thromboprophylaxis instituted in children at high risk for venous thromboembolism.
-
•
A multidisciplinary approach should be instituted to minimize spread of the virus within critical care units while still providing excellent patient care.
Coronavirus Disease 2019-associated Multisystem Inflammatory Syndrome in Children
The diagnosis of MIS-C is usually made weeks after a child is infected with SARS-CoV-2, and almost all patients are positive for SARS-CoV-2 either by RT-PCR, SARS-CoV-2 antibody testing, or both, whereas the rest have a history of contact with someone with COVID-19.59, 60, 61 The CDC, World Health Organization, and Royal College of Pediatrics and Child Health provided definitions of MIS-C from SARS-CoV-2 infection.62, 63, 64 All 3 definitions have many similarities but the CDC definition is the most widely used in North America.
Definition
Centers for Disease Control and Prevention Definition of Multisystem Inflammatory Syndrome in Children
-
•
An individual less than 21 years old presenting with fever, laboratory evidence of inflammation, and evidence of clinically severe illness requiring hospitalization, with multisystem (≥2) organ involvement (cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic, or neurologic); and
-
•
No alternative plausible diagnoses; and
-
•
Positive for current or recent SARS-CoV-2 infection by RT-PCR, serology, or antigen test; or exposure to a suspected or confirmed COVID-19 case within the 4 weeks before the onset of symptoms.
Fever here is defined as greater than or equal to 38.0°C for greater than or equal to 24 hours, or report of subjective fever lasting greater than or equal to 24 hours. Evidence of inflammation includes, but is not limited to, 1 or more of the following: increased CRP, fibrinogen, procalcitonin, D-dimer, ferritin, LDH, IL-6, or neutrophil levels; increased ESR; reduced lymphocyte levels; and low albumin levels.
The CDC suggests that some individuals may fulfill full or partial criteria for Kawasaki disease (KD) but should be reported if they meet the case definition for MIS-C, and to consider MIS-C in any child who dies with evidence of SARS-CoV-2 infection.
Clinical features
Patients with MIS-C usually present with persistent fever, cardiorespiratory and gastrointestinal symptoms, mucocutaneous lesions, and, in severe cases, hypotension, and shock. Cardiac, cardiorespiratory, and gastrointestinal complications are the most common reasons for PICU admission. Belay and colleagues60 reported illness involving at least 4 organ systems in almost 90% of cases in a cohort of 1733 patients. Children with MIS-C have required intensive care more than those with severe acute COVID-19, and intensivists should be cognizant of the similarities and differences between MIS-C and severe acute COVID-19.20 , 60 , 61 Children with MIS-C are often male and previously healthy, whereas severe acute COVID-19 is more common in children with existing risk factors with no gender predilection. Large studies from United States and United Kingdom have shown that MIS-C and severe acute COVID-19 are both more common in African American, Hispanic, and Asian children compared with white children. Mortality in hospitalized children is less than 2%.60 , 61 , 65 , 66 Differences have been observed between the occurrence of MIS-C and severe acute COVID-19 among various age groups. Severe acute COVID-19 rates are higher in children 0 to 5 years and 13 to 20 years of age, whereas MIS-C is higher in the 6- year to 12-year age group.61 MIS-C has been associated with more severe outcomes in children older than 5 years, whereas severe acute COVID-19 is associated with worse outcomes in children less than 1 year of age.67 , 68 Higher values of D-dimer, CRP, and ferritin, and lower platelet and absolute lymphocyte count have been shown to be predictive of severe MIS-C. Higher neutrophil to lymphocyte ratio, higher CRP, and lower platelet count have been observed in MIS-C compared with COVID-19.61 Mucocutaneous signs and symptoms on presentation are seen in almost two-thirds of patients with MIS-C, but only in 10% of patients with COVID-19.61
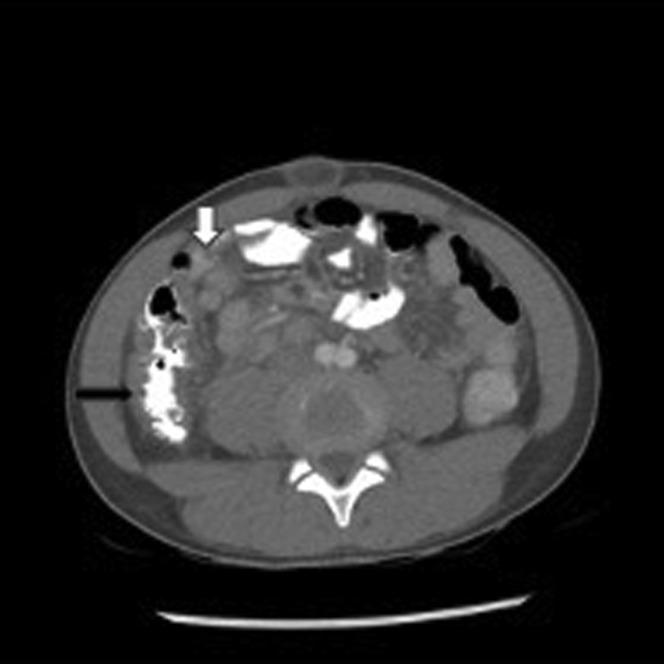
Abdominal pain and vomiting can occur in 60% of patients with MIS-C and can be of such severity as to be mistaken for acute appendicitis.60 The possibility of MIS-C coexisting with acute appendicitis should be considered.69 , 70 Patients with severe acute COVID-19 can present with gastrointestinal symptoms but these are usually not as severe as those seen in patients with MIS-C. Feldstein and colleagues61 reported gastrointestinal symptoms on presentation in 90% of patients with MIS-C compared with 58% of patients with severe acute COVID-19. Abdominal imaging in patients with MIS-C have demonstrated inflammation, including mesenteric adenopathy, mesenteric edema, ascites, bowel wall thickening, and gallbladder wall thickening (Fig. 4 ).
Fig. 4.
Abdominal CT of 11-year-old African-American male with MIS-C who presented with fever, mucocutaneous symptoms, severe abdominal pain, vomiting, and CoV-2 antibody (Ab) IgG+. Bowel wall thickening of ascending colon (black arrow) with several enlarged lymph nodes (white arrow).
Cardiorespiratory involvement and the need for vasoactive agents were observed in 56%, 67%, and 45% respectively in patients with MIS-C compared with 9%, 12%, and 9% respectively in patients with severe acute COVID-19 in a case series of 1116 patients studied by Feldstein and colleagues.61 Belay and colleagues60 reported hypotension (51%), shock (37%), cardiac dysfunction (31%), and myocarditis (17%) in the largest cohort of patients with MIS-C reported thus far. Mucocutaneous lesions and conjunctival injection and laboratory markers of BNP and IL-6 were associated with coronary artery abnormalities.61 , 67 The incidence of coronary artery dilatation and aneurysms (CAA) in MIS-C is 4% to 24%.60 , 61 , 71, 72, 73 In patients with KD, the risk of coronary artery thrombosis is directly related to size of CAA and increases exponentially at z-scores of more than 10.74 , 75 Depressed left ventricular (LV) function has been noted in a third of patients.60 , 61 Similar to patients with other causes of poor cardiac function, children with MIS-C or severe acute COVID-19 with LV dysfunction are at risk for intracardiac thrombosis.76 Knowledge of duration of persistence of abnormalities in inflammatory markers, troponin, D-dimer, LV dysfunction, and CAA is limited because of lack of consistent follow-up protocols and patient compliance. In the small number of children seen in follow-up so far, most of the abnormalities return to normal.61 , 77
Respiratory complications in MIS-C can be like those seen in severe acute COVID-19 with some differences. Lower respiratory infection was reported in 17% of patients with MIS-C compared with 36% of patients with severe acute COVID-19. Severe respiratory disease without cardiovascular involvement was observed in 24% of MIS-C compared with 71% of patients with severe acute COVID-19 in the study by Feldstein and colleagues.61 However, patients with MIS-C had a greater need for noninvasive and invasive ventilation (36% and 18%) compared with those with severe acute COVID-19 (33% and 15%). This finding may be related to higher prevalence of cardiorespiratory complications in patients with MIS-C. Radiographic abnormalities in MIS-C with cardiorespiratory complications include pleural effusions and bilateral pulmonary consolidation with lower zone predominance (Figs. 5 and 6 ). Pleural effusions are rarely reported in patients with severe acute COVID-19.78 Depressed myocardial function, shock, need for aggressive intravascular volume expansion, severe systemic inflammation, and hypoalbuminemia are seen more often in patients with MIS-C compared with those with severe acute COVID-19, likely contributing to third spacing and pleural effusion in patients with MIS-C.
Fig. 5.
Chest radiographs of a 5-year-old Hispanic male with MIS-C who presented with fever; cardiorespiratory, mucocutaneous, and abdominal symptoms; hypoalbuminemia; positive for SARS-CoV-2 RT-PCR and CoV-2 IgG Ab. (A) On presentation, when he had moderately decreased LV systolic function and required BiPAP. (B) Three days after presentation, with normal biventricular systolic function and resolution of respiratory symptoms, hypoalbuminemia, and fever.
Fig. 6.
Chest CT of an 18-year-old African-American male with MIS-C who presented with fever, shock with LV dysfunction requiring inotropic/vasoactive medication, pneumonia, mucocutaneous symptoms, hypoalbuminemia, CoV-2 Ab IgG+, requiring BiPAP with pleural effusion (black arrow) and bilateral lower lobe consolidation (white arrow).
Diagnosis
The diagnostic pathway for MIS-C recommended by the American College of Rheumatology is a clinically useful tool.50 The tier 1 and tier 2 evaluations shown in Fig. 7 are a comprehensive list of tests for evaluation of MIS-C. Recommendations for laboratory studies for patients in the ICU include daily CBC, basic metabolic panel, and D-dimer; troponin every 6 hours; and BNP every 48 hours, and are adjusted in frequency based on clinical condition. Recommendations for monitoring of cardiac complications in MIS-C in addition to those listed in tier 2 include the following: (1) ECG every 48 hours in hospitalized patients or more frequently for those with conduction abnormalities and again at follow-up. (2) Echocardiogram repeated at 1 to 2 weeks and 4 to 6 weeks after initial presentation. Patients with LV dysfunction and coronary artery aneurysm require more frequent echocardiography. (3) Cardiac MRI 2 to 6 months after the acute illness to assess for myocardial fibrosis and scarring.
Fig. 7.
Diagnostic pathway for MIS-C. Moderate to high consensus was reached by the task force in the development of this diagnostic pathway for MIS-C associated with SARS-CoV-2. aAn epidemiologic link to SARS-CoV-2 infection is defined as a child with any of the following criteria: positive for SARS-CoV-2 by polymerase chain reaction (PCR), positive for SARS-CoV-2 by serology, preceding illness resembling COVID-19, or close contact with an individual with confirmed or suspected COVID-19 in the past 4 weeks. bSuggestive clinical features include rash (polymorphic, maculopapular, or petechial, but not vesicular), gastrointestinal symptoms (diarrhea, abdominal pain, or vomiting), oral mucosal changes (red and/or cracked lips, strawberry tongue, or erythema of the oropharyngeal mucosa), conjunctivitis (bilateral conjunctival infection without exudate), and neurologic symptoms (altered mental status, encephalopathy, focal neurologic deficits, meningismus, or papilledema). cThe CMP includes measurement of sodium, potassium, carbon dioxide, chloride, blood urea nitrogen, creatinine, glucose, calcium, albumin, total protein, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and bilirubin. dProcalcitonin, cytokine panel, and blood smear test results should be sent, if available. eSerologic test results should be sent if not sent in tier 1 evaluation, and if possible, SARS-CoV-2 IgG, IgM, and IgA test results should be sent. ALC, absolute lymphocyte count; u/a, urinalysis.
(With permission from Henderson LA, et al. Arthritis & Rheumatology, Volume: 73, Issue: 4, Pages: e13-e29, First published: 05 December 2020, DOI: (10.1002/art.41616).
Patients who do not meet all the criteria for diagnosis of MIS-C should be evaluated for diseases with similar presentations, such as KD, toxic shock syndrome, or hemophagocytic lymphohistiocytosis.
Management
Treatment should be directed at supportive care of multiorgan dysfunction and mitigation of the underlying inflammatory process. The treatment of MIS-C as recommended by the American College of Rheumatology is outlined in Fig. 8 .50
Fig. 8.
Algorithm for initial immunomodulatory treatment of MIS-C. Moderate to high consensus was reached by the task force in the development of this treatment algorithm for MIS-C associated with SARS-CoV-2. aIntravenous immunoglobulin (IVIG) dosing is 2 g/kg based on ideal body weight. Cardiac function and fluid status should be assessed before IVIG is given. In some patients with cardiac dysfunction, IVIG may be given in divided doses (1 g/kg daily over 2 days). bMethylprednisolone or another steroid at equivalent dosing may be used. cRefractory disease is defined as persistent fevers and/or ongoing and significant end-organ involvement. dLow-dose to moderate-dose glucocorticoids (methylprednisolone 1–2 mg/kg/d) may be considered for first-line therapy in some MIS-C patients with concerning features (ill appearance, highly increased B-type natriuretic peptide levels, unexplained tachycardia) who have not yet developed shock or organ-threatening disease. eIf the patient was given low-dose to moderate-dose glucocorticoids as first-line therapy, methylprednisolone IV dosing should be 10 to 30 mg/kg/d for intensification treatment.
(With permission from Henderson LA, et al. Arthritis & Rheumatology, Volume: 73, Issue: 4, Pages: e13-e29, First published: 05 December 2020, DOI: (10.1002/art.41616).
Additional treatment guidelines:
-
1.
Initial treatment with intravenous immunoglobulin (IVIG) and glucocorticoids is associated with lower risk of LV dysfunction and shock, and decreased need for adjunctive therapy compared with IVIG alone.79
-
2.
Anakinra 1 to 2 mg/kg/d should be considered in patients in whom corticosteroids are contraindicated.
-
3.
High-dose anakinra, greater than 4 mg/kg/d, is recommended for those refractory to treatment with IVIG with or without steroids. In some cases, anakinra as high as 10 mg/kg/d (maximum 100 mg/dose) through subcutaneous or intravenous routes divided every 6 to 12 hours may be needed. If the patient does not show improvement with this regimen, the diagnosis of MIS-C should be reconsidered.
-
4.
If used, immunomodulation therapy should be tapered over 2 to 3 weeks or longer to avoid rebound inflammation.
-
5.
Antiplatelet and antithrombotic therapy with low-dose aspirin (3–5 mg/kg/d up to 81 mg/d) is recommended in all patients with MIS-C if they do not have uncontrolled bleeding or risk for bleeding. Aspirin therapy should be continued until normalization of platelet count and normal coronary arteries are confirmed at greater than or equal to 4 weeks after diagnosis.
-
6.
Anticoagulation with enoxaparin to achieve anti–factor Xa level of 0.5 to 1 or warfarin with INR level of 2 to 3 is recommended in patients with coronary artery Z-score greater than 10 and in those with moderate or severe LV dysfunction with ejection fraction less than 35%.
-
7.
Empiric antibiotics should be used in all patients with severe MIS-C until cultures are negative for 48 hours or as directed by infectious disease consultants. Ceftriaxone may be used alone or in combination with metronidazole for possible appendicitis or vancomycin/clindamycin for those with possible toxic shock syndrome.
-
8.
Stress ulcer prophylaxis is recommended in patients receiving aspirin and or steroids.
-
9.
Consultation with infectious disease, immunology, and cardiology subspecialists is recommended for all patients. Hematologists and endocrinologists may also be needed to guide anticoagulation and steroid management.
Multisystem inflammatory syndrome in children and Kawasaki disease
MIS-C may be especially difficult to differentiate from KD despite well-established diagnostic criteria.62 , 80 The following are differences between MIS-C and KD:
-
1.
MIS-C is common among black and Hispanic children, whereas incidence of KD is highest in children of Asian descent.
-
2.
MIS-C is reported in children aged from 3 months to 20 years, with those older than 5 years more severely affected, whereas KD is usually seen in children less than 5 years of age.
-
3.
Patients with MIS-C frequently need PICU admission, whereas patients with KD rarely do.
-
4.
Increased serum ferritin, leukopenia, lymphopenia, and thrombocytopenia are common in MIS-C. Thrombocytosis is a characteristic feature of KD.
-
5.
Myocarditis, LV cardiac dysfunction, shock, the need for intravascular fluid expansion, and vasopressor/inotropic support is more common in MIS-C.
-
6.
Coronary artery dilatations and aneurysms are reported in 4% to 24% of children with MIS-C. The progression and long-term sequelae are not known at this time. In the pre-IVIG era, CAA occurred in 20% to 25% of children with KD.81 With IVIG therapy, persistent CAAs are much less common, but are still noted in 4% to 6% of patients, with approximately 1% developing giant CAA despite treatment.82 , 83
-
7.
Respiratory and cardiorespiratory complications requiring noninvasive or invasive ventilation are more common in children with MIS-C.
-
8.
Gastrointestinal and neurologic complications and coagulopathy are more common in MIS-C.
-
9.
IVIG and moderate-dose to high-dose aspirin are the established recommended treatment of KD.84 In addition to IVIG and aspirin, steroids and biologic drugs are frequently used in patients with MIS-C.50
-
10.
Most children with KD have a good prognosis, whereas the long-term clinical outcomes of MIS-C are not clear.
Clinics Care Points
-
•
Intensivists should be familiar with the CDC definition of MIS-C and various clinical presentations of MIS-C.
-
•
Intensivists should know the differences in clinical manifestations of severe acute COVID-19 and MIS-C.
-
•
Algorithms for diagnosis and management of MIS-C should be followed.
-
•
Intensivists should be aware of the cardiorespiratory, cardiac, and gastrointestinal complications of MIS-C, their presentation, and management.
-
•
Intensivists should be familiar with the differential diagnosis for MIS-C and especially its differentiation from KD.
Unique pediatric intensive care unit care issues related to the pandemic
The SARS-CoV-2 pandemic has demanded unprecedented and rapid adaptation of all personnel involved in the care of critically ill children. Surges of this pandemic, which caused acute shortage of ICU beds worldwide, resulted in many PICU teams providing care for adults with COVID-19 in addition to children with severe acute COVID-19.85 , 86 Hospitals must develop protocols for implementation in their critical care units based on their needs and resources, with emphasis on minimizing the spread of virus while still providing excellent patient care.
The following recommendations can help PICUs during the current pandemic and future infection outbreaks:87
-
1.
Monitoring: monitoring patients from outside the room while having a direct line of sight might require installation of windows or glass doors.
-
2.
Nursing care: moving intravenous pumps outside of patient rooms while paying attention to the possibilities of inadvertent dislodgement of catheters, increased risk of central line–associated bloodstream infection, and inability of nurses to hear pump alarms when they are inside the patient rooms with PPE. Reduction, or grouping, of blood sampling as much as possible.
-
3.
Respiratory care: coordination of team members to minimize entry into rooms, address measures to decrease aerosol generation, set appropriate ventilator alarm limits, change ventilator circuits or filters as needed rather than by protocol, and use of metered-dose inhalers instead of nebulizers when possible. Consider vibrating mesh nebulizer rather than in-line gas-driven nebulizer when nebulized medication must be given. Prone positioning teams and protocols should be in place to safely place patients in the prone position while addressing possible dislodgement of tubes and catheters and development of pressure ulcers.
-
4.
Pharmacy: critical care pharmacists can help with development of specific management guidelines as treatments evolve during the pandemic and help with measures to reduce the number of times nurses must enter patient rooms for medication administration.
-
5.
Structure related: zones and protocols should be developed for donning and doffing PPE. A protocol should be developed for room cleaning and disinfection with approved disinfectants while ensuring safety of environmental service workers.
-
6.
Patient communication: social workers, child-life specialists, patient representatives, and pastoral care providers can be enlisted, along with the use of audio or video communication to help facilitate communication with family members during pandemic-induced restricted visiting.
-
7.
Mental health issues of all team members should be addressed. Posttraumatic stress (PTS) has been noted to be high among pediatric critical care physicians in association with various COVID-19 patient care experiences. These observations, along with association of PTS with thoughts of quitting the profession because of the pandemic, could have implications for the workforce in the future.88
Public health concerns for children
-
1.
Mental health: the mental health crisis facing children was substantial even before the pandemic. The significant pressures on families, schools, and communities resulting from the pandemic have made the situation worse. Children’s hospitals are feeling the considerable burden of this crisis every day. Emergency rooms are filled to capacity, and staff are at the breaking point. According to the Kaiser Family Foundation, there have been marked increases in suicidal ideations, anxiety disorders, obsessive-compulsive disorder diagnosis, and substance abuse in children.89 Significant efforts and resources are needed to address the mental health crisis in children.
-
2.
The fragility of the medical home and health system: children are best served in a coordinated, fully staffed medical home. Care is coordinated, and the most medically fragile children receive timely and coordinated care. However, the pandemic has had a negative impact on America’s pediatric practices. In a recent survey by the American Academy of Pediatrics, two-thirds of practices have experienced a significant decrease in visits.90 This decrease has both public health impacts (delays in vaccines, late diagnosis) and a negative fiscal impact on the long-term survival of the medical home.
Tags for SEO: severe acute COVID-19, MIS-C, hyperinflammation, KD.
Clinics care points
• Mental health issues of critical care teams and children affected during this pandemic should be addressed.
Acknowledgment
The authors thank Dr. Tej Phatak, MD, MBA, Chief of Pediatric Radiology at Children’s Hospital of New Jersey at Newark Beth Israel Medical Center, for providing us with the radiology images used in this review article.
Disclosure
The authors have nothing to disclose.
References
- 1.Centers for Disease Control and Prevention . 2021. Laboratory-confirmed COVID-19-associated hospitalizations.https://gis.cdc.gov/grasp/COVIDNet/COVID19_5.html#virusTypeDiv [Google Scholar]
- 2.Dong Y., Mo X., Hu Y., et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6):e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 3.Lu X., Zhang L., Du H., et al. SARS-CoV-2 Infection in Children. N Engl J Med. 2020;382(17):1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoang A., Chorath K., Moreira A., et al. COVID-19 in 7780 pediatric patients: a systematic review. EClinicalMedicine. 2020;24:100433. doi: 10.1016/j.eclinm.2020.100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viner R.M., Ward J.L., Hudson L.D., et al. Systematic review of reviews of symptoms and signs of COVID-19 in children and adolescents. Arch Dis Child. 2020 doi: 10.1136/archdischild-2020-320972. [DOI] [PubMed] [Google Scholar]
- 6.Gaythorpe K.A.M., Bhatia S., Mangal T., et al. Children's role in the COVID-19 pandemic: a systematic review of early surveillance data on susceptibility, severity, and transmissibility. Sci Rep. 2021;11(1):13903. doi: 10.1038/s41598-021-92500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parcha V., Booker K.S., Kalra R., et al. A retrospective cohort study of 12,306 pediatric COVID-19 patients in the United States. Sci Rep. 2021;11(1):10231. doi: 10.1038/s41598-021-89553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delahoy M.J., Ujamaa D., Whitaker M., et al. Hospitalizations associated with COVID-10 among children and adolescents-COVID-NET, 14 states, March 1, 2020-August 14, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1255–1260. doi: 10.15585/mmwr.mm7036e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graff K., Smith C., Silveira L., et al. Risk factors for severe cOVID-19 in children. Pediatr Infect Dis J. 2021;40(4):e137–e145. doi: 10.1097/INF.0000000000003043. [DOI] [PubMed] [Google Scholar]
- 10.Preston L.E., Chevinsky J.R., Kompaniyets L., et al. Characteristics and disease severity of US children and adolescents diagnosed With COVID-19. JAMA Netw Open. 2021;4(4):e215298. doi: 10.1001/jamanetworkopen.2021.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Children’s Hospital Association and the American Academy of Pediatrics Children and COVID-19: state-level data report. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report Available at.
- 12.Siegel D.A., Reses H.E., Cool A.J., et al. Trends in COVID-19 cases, emergency department visits, and hospital admissions among children and adolescents aged 0-17 years - United States, August 2020-August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(36):1249–1254. doi: 10.15585/mmwr.mm7036e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cevik M., Kuppalli K., Kindrachuk J., et al. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ. 2020;371:m3862. doi: 10.1136/bmj.m3862. [DOI] [PubMed] [Google Scholar]
- 14.Mangalmurti N., Hunter C.A. Cytokine storms: understanding COVID-19. Immunity. 2020;53(1):19–25. doi: 10.1016/j.immuni.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bixler D., Miller A.D., Mattison C.P., et al. SARS-CoV-2-associated deaths among persons aged <21 years – United States, February 12-July 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1324. doi: 10.15585/mmwr.mm6937e4. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Dambrauskas S., Vasquez-Hoyos P., Camporesi A., et al. Pediatric critical care and COVID-19. Pediatrics. 2020;146(3):e20201766. doi: 10.1542/peds.2020-1766. [DOI] [PubMed] [Google Scholar]
- 17.Shekerdemian L.S., Mahmood N.R., Wolfe K.K., et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174(9):868–873. doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalyanaraman M., McQueen D., Morparia K., et al. ARDS in an ex-premature infant with bronchopulmonary dysplasia and COVID-19. Pediatr Pulmonology. 2020;55(10):2506–2507. doi: 10.1002/ppul.24989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moeller A., Thanikkel L., Duijts L., et al. COVID-19 in children with underlying chronic respiratory diseases: survey results from 174 centres. ERJ Open Res. 2020;6(4):00409–02020. doi: 10.1183/23120541.00409-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandes D.M., Oliveira C.R., Guerguis S., et al. Severe acute respiratory syndrome Coronavirus 2 clinical syndromes and predictors of disease severity in hospitalized children and youth. J Pediatr. 2021;230:23–31. doi: 10.1016/j.jpeds.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corman V.M., Landt O., Kaiser M., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z., Fu J., Shu O., et al. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr. 2020;16(3):240–246. doi: 10.1007/s12519-020-00345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu X., Zhang L., Hui D., et al. SARS-CoV-2 infection in children. N Engl J Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chao J.Y., Derespina K.R., Herold B.C., et al. Clinical characteristics and outcomes of hospitalized and critically ill children and adolescents with coronavirus disease 2019 (COVID-19) at a tertiary care medical center in New York City. J Pediatr. 2020;223:14–19.e2. doi: 10.1016/j.jpeds.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisler G., Izard S.M., Shah S., et al. Characteristics and risk factors associated with critical illness in pediatric COVID-19. Annals Intensive Care. 2020;10(1):171. doi: 10.1186/s13613-020-00790-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hariyanto T.I., Japar K.V., Kwenandar F., et al. Inflammatory and hematologic markers as predictors of severe outcomes in COVID-19 infection: a systematic review and meta-analysis. Am J Emerg Med. 2021;41:110–119. doi: 10.1016/j.ajem.2020.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foust A.M., Phillips G.S., Chu W.C., et al. International expert consensus statement on chest imaging in pediatric COVID-19 patient management: imaging findings, imaging study reporting, and imaging study recommendations. Radiol Cardiothorac Imaging. 2020;2(2):e200214. doi: 10.1148/ryct.2020200214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nino G., Zember J., Sanchez-Jacob R., et al. Pediatric lung imaging features of COIVD-19: a systematic review and meta-analysis. Pediatr Pulmonol. 2021;56(1):252–263. doi: 10.1002/ppul.25070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung M., Bernheim A., Mei X. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar J., Meena J., Yadav A., et al. Radiological findings of COVID-19 in children: a systematic review and meta-analysis. J Trop Pediatr. 2021;67(3):fmaa045. doi: 10.1093/tropej/fmaa045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen A., Huang J., Liao Y., et al. Differences in clinical and imaging presentation of pediatric patients with COVID-19 in comparison with adults. Radiol Cardiothorac Imaging. 2020;2(2):e200117. doi: 10.1148/ryct.2020200117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denina M., Scolfaro C., Silvestro E., et al. Lung ultrasound in children with COVID-19. Pediatrics. 2020;146(1):e20201157. doi: 10.1542/peds.2020-1157. [DOI] [PubMed] [Google Scholar]
- 33.Pediatric acute respiratory distress syndrome: consensus recommendations from the pediatric acute lung injury consensus conference. Pediatr Crit Care Med. 2015;16(5):428–439. doi: 10.1097/PCC.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gattinoni L., Chiumello D., Caironi P., et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kneyber M.C.J., de Luca D., Calderini E., et al. Section respiratory failure of the European Society for Paediatric and Neonatal Intensive Care. Recommendations for mechanical ventilation of critically ill children from the paediatric mechanical ventilation consensus conference (PEMVECC) Intensive Care Med. 2017;43:1764–1780. doi: 10.1007/s00134-017-4920-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rimensberger P.C., Cheifetz I.M., Pediatric Acute Lung Injury Consensus Conference Group Ventilatory support in children with pediatric acute respiratory syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16:S51–S60. doi: 10.1097/PCC.0000000000000433. [DOI] [PubMed] [Google Scholar]
- 37.Matava C.T., Kovatsis P.G., Lee J.K., et al. Pediatric airway management in COVID-19 patients: consensus guidelines from the Society for Pediatric Anesthesia’s Pediatric Difficult Intubation Collaborative and the Canadian Pediatric Anesthesia Society. Anesth Analg. 2020;131(1):61–73. doi: 10.1213/ANE.0000000000004872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rimensberger P.C., Kneyber M.C.J., Deep A., et al. Caring for critically ill children with suspected or proven coronavirus disease 2019 infection: recommendations by the scientific sections’ collaborative of the European Society of Pediatric and Neonatal Intensive Care. Pediatr Crit Care Med. 2021;22(1):56–67. doi: 10.1097/PCC.0000000000002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brower R.G., Matthay M.A., Morris A., et al. Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 40.Khemani R.G., Parvathaneni K., Yehya N., et al. Positive end-expiratory pressure lower than the ARDS network protocol is associated with higher pediatric acute respiratory distress syndrome mortality. Am J Respir Crit Care Med. 2018;198(1):77–89. doi: 10.1164/rccm.201707-1404OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kache S., Chisti M.J., Gumbo F., et al. COVID-19 PICU guidelines: for high- and limited-resource settings. Pediatr Res. 2020;88(5):705–716. doi: 10.1038/s41390-020-1053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/214787Orig1s000lbl.pdf
- 43.Available at: https://www.fda.gov/media/137564/download
- 44.Goldman D.L., Aldrich M.L., Hagmann S.H.F., et al. Compassionate use of remdesivir in children with severe acute COVID-19. Pediatrics. 2021;147(5) doi: 10.1542/peds.2020-047803. e2020047803. [DOI] [PubMed] [Google Scholar]
- 45.Chiotos K., Hayes M., Kimberlin D.W., et al. Multicenter interim guidance on use of antivirals for children with coronavirus disease 2019/severe acute respiratory syndrome coronavirus 2. J Pediatr Infect Dis Soc. 2021;10(1):34–48. doi: 10.1093/jpids/piaa115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacinto J.P., Patel M., Goh J., et al. Remdesivir-induced symptomatic bradycardia in the treatment of COVID-19 disease. Heart Rhythm Case Rep. 2021;7(8):514–517. doi: 10.1016/j.hrcr.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/ Available at. Accessed. [PubMed]
- 48.Horby P., Lim W.S., Emberson J., et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sterne J.A., Murthy S., Diaz J.V., et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henderson L.A., Canna S.W., Friedman K.G., et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 2. Arthritis Rheumatol. 2021;73(4):e13–e29. doi: 10.1002/art.41616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiss S.L., Peters M.J., Alhazzani W., et al. Surviving Sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med. 2020;21(2):e52–e106. doi: 10.1097/PCC.0000000000002198. [DOI] [PubMed] [Google Scholar]
- 52.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 53.Siracusa L., Cascio A., Giordano S., et al. Neurological complications in pediatric patients with SARS-CoV-2 infection: a systematic review of the literature. Ital J Pediatr. 2021;47:123. doi: 10.1186/s13052-021-01066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levi M., Thachil J., Iba T., et al. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zaffanello M., Piacentini G., Nosetti L., et al. Thrombotic risk in children with COVID-19 infection: a systematic review of the literature. Thromb Res. 2021;205:92–98. doi: 10.1016/j.thromres.2021.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loi M., Branchford B., Kim J., et al. COVID-19 anticoagulation recommendations in children. Pediatr Blood Cancer. 2020;67(9):e28485. doi: 10.1002/pbc.28485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldenberg N.A., Sochet A., Albisetti M., et al. Consensus-based clinical recommendations and research priorities for anticoagulant thromboprophylaxis in children hospitalized for COVID-19-related illness. J Thromb Haemost. 2020;18(11):3099–3105. doi: 10.1111/jth.15073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monagle P., Chan A., Goldenberg N., et al. Antithrombotic therapy in neonates and children: antithrombotic therapy and prevention of thrombosis 9th edition: American College of Chest Physicians evidence based clinical practice guidelines. Chest. 2012;141(2 Suppl):e737S–e801S. doi: 10.1378/chest.11-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hospitalizations associated with COVID-19 among children and adolescents — COVID-NET, 14 states, March 1, 2020–August 14, 2021. Weekly/September 10, 2021/70(36);1255- 1260. https://www.cdc.gov/mmwr/volumes/70/wr/mm7036e2.htm [DOI] [PMC free article] [PubMed]
- 60.Belay E.D., Abrams J., Oster M.E., et al. Trends in geographic and temporal distribution of US children with multisystem inflammatory syndrome during the COVID-19 pandemic. JAMA Pediatr. 2021;175(8):837–845. doi: 10.1001/jamapediatrics.2021.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feldstein L.R., Tenforde M.W., Friedman K.G., et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;323(11):1074–1087. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Information for healthcare providers about multisystem inflammatory syndrome in children (MIS-C) https://www.cdc.gov/mis/mis-c/hcp/index.html Available at:
- 63.Royal College of Paediatrics and Child Health . 2020. Guidance: paediatric multisystem inflammatory syndrome temporally associated with COVID-19.https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims Available at. [Google Scholar]
- 64.World Health Organization . 2020. Multisystem inflammatory syndrome in children and adolescents with COVID-19.https://www.who.int/publications/i/item/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 Available at. [Google Scholar]
- 65.Saatci D., Ranger T.A., Garriga C., et al. Association between race and COVID-19 outcomes among 2.6 million children in England. JAMA Pediatr. 2021;175(9):928–938. doi: 10.1001/jamapediatrics.2021.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Payne A.B., Gilani Z., Godfred-Cato S., et al. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Netw Open. 2021;4(6):e2116420. doi: 10.1001/jamanetworkopen.2021.16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abrams J.Y., Oster M.E., Godfred-Cato S.E., et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: a retrospective surveillance study. Lancet Child Adolesc Health. 2021;5(5):323–331. doi: 10.1016/S2352-4642(21)00050-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bellino S., Punzo O., Rota M.C., et al. COVID-19 disease severity risk factors for pediatric patients in Italy. Pediatrics. 2020;146(4) doi: 10.1542/peds.2020-009399. e2020009399. [DOI] [PubMed] [Google Scholar]
- 69.Anderson J.E., Campbell J.A., Durowoju L., et al. COVID-19-associated multisystem inflammatory syndrome in children (MIS-C) presenting as appendicitis with shock. J Pediatr Surg Case Rep. 2021;71:101913. doi: 10.1016/j.epsc.2021.101913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meyer J.S., Robinson G., Moonah S., et al. Acute appendicitis in four children with SARS-CoV-2 infection. J Pediatr Surg Case Rep. 2021;64:101734. doi: 10.1016/j.epsc.2020.101734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dufort E.M., Koumans E.H., Chow E.J., et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kavurt A.V., Bağrul D., Gül A.E.K., et al. Echocardiographic findings and correlation with laboratory values in multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19. Pediatr Cardiol. 2021:1–13. doi: 10.1007/s00246-021-02738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Valverde I., Singh Y., Sanchez-de-Toledo J., et al. Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID-19 infection in Europe. Circulation. 2021;143(1):21–32. doi: 10.1161/CIRCULATIONAHA.120.050065. [DOI] [PubMed] [Google Scholar]
- 74.McCrindle B.W., Rowley A.H., Newburger J.W., et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association [review] Circulation. 2017;135(17):e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 75.Tsuda E., Tsujii N., Hayama Y. Stenotic lesions and the maximum diameter of coronary artery aneurysms in Kawasaki disease. J Pediatr. 2018;194:165–170. doi: 10.1016/j.jpeds.2017.09.077. [DOI] [PubMed] [Google Scholar]
- 76.Giglia T.M., Massicotte M.P., Tweddell J.S., et al. Prevention and treatment of thrombosis in pediatric and congenital heart disease: a scientific statement from the American Heart Association. Circulation. 2013;128(24):2622–2703. doi: 10.1161/01.cir.0000436140.77832.7a. [DOI] [PubMed] [Google Scholar]
- 77.Davies P., du Pré P., Lillie J., et al. One-year outcomes of critical care patients post-COVID-19 multisystem inflammatory syndrome in children. JAMA Pediatr. 2021;30:e212993. doi: 10.1001/jamapediatrics.2021.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rostad B.S., Shah J.H., Rostad C.A., et al. Chest radiograph features of multisystem inflammatory syndrome in children (MIS-C) compared to pediatric COVID-19. Pediatr Radiol. 2021;51(2):231–238. doi: 10.1007/s00247-020-04921-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Son M.B.F., Murray N., Friedman K., et al. Multisystem inflammatory syndrome in children - Initial therapy and outcomes. N Engl J Med. 2021;385(1):23–34. doi: 10.1056/NEJMoa2102605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Q.Y., Xu B.W., Du J.B. Similarities and differences between multiple inflammatory syndrome in children associated with COVID-19 and Kawasaki disease: clinical presentations, diagnosis, and treatment. World J Pediatr. 2021;17(4):335–340. doi: 10.1007/s12519-021-00435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kato H., Sugimura T., Akagi T., et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 1996;94(6):1379–1385. doi: 10.1161/01.cir.94.6.1379. [DOI] [PubMed] [Google Scholar]
- 82.Newburger J.W. Treatment of Kawasaki disease. Lancet. 1996;347(9009):1128. doi: 10.1016/s0140-6736(96)90600-5. [DOI] [PubMed] [Google Scholar]
- 83.Ogata S., Tremoulet A.H., Sato Y., et al. Coronary artery outcomes among children with Kawasaki disease in the United States and Japan. Int J Cardiol. 2013;168(4):3825–3828. doi: 10.1016/j.ijcard.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Newburger J.W., Takahashi M., Burns J.C., et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315(6):341–347. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 85.Yager P.H., Whalen K.A., Cummings B.M. Repurposing a pediatric ICU for adults. N Engl J Med. 2020;382(22):e80. doi: 10.1056/NEJMc2014819. [DOI] [PubMed] [Google Scholar]
- 86.Wasserman E., Toal M., Nellis M., et al. Rapid transition of a PICU space and staff to adult coronavirus disease 2019 ICU care. Pediatr Crit Care Med. 2021;22(1):50–55. doi: 10.1097/PCC.0000000000002597. [DOI] [PubMed] [Google Scholar]
- 87.Halpern N.A., Kaplan L.J., Rausen M., et al. Configuring ICUs in the COVID-19 era. 2020. https://www.sccm.org/COVID19RapidResources/Resources/Configuring-ICUs-in-the-COVID-19-Era-A-Collection
- 88.Kalyanaraman M., Sankar A., Timpo E., et al. Posttraumatic stress among pediatric critical care physicians in the United States in association with coronavirus disease 2019 patient care experiences. J Intensive Care Med. 2021 doi: 10.1177/08850666211059385. [DOI] [PubMed] [Google Scholar]
- 89.Kaiser Family Foundation. www.kff.org/coronavirus-covid-19/issue-brief/mental-health-and-substance-use-considerations-among-children-during-the-covid-19-pandemic/ Available at:
- 90.American Academy of Pediatrics: PLACES Survey. 2020. https://publications.aap.org/aapnews/news/14172/Survey-Pediatricians-reeling-from-pandemic-s?searchresult=1 Available at: