Abstract
Therapeutic approaches that target the metabolism of tumor cells have been a popular research topic in recent years. Previous studies have demonstrated that glycolysis inhibitors reduce the proliferation of non-small cell lung cancer (NSCLC) cells by interfering with the aerobic glycolytic pathway. However, the mitochondrial oxidative phosphorylation (OXPHOS) pathway in tumor cells has also been implicated in lung cancer metabolism. Metformin, a known inhibitor of mitochondrial OXPHOS, has been indicated to reduce NSCLC morbidity and mortality in clinical studies. The present article reviewed the therapeutic effects of metformin against NSCLC, both as a single agent and combined with other anticancer treatments, in order to provide a theoretical basis for its clinical use in adjuvant therapy for NSCLC.
Keywords: non-small cell lung cancer, metabolism, metformin, combination therapy, anticancer
1. Introduction
Lung cancer is the most common cause of cancer-related death worldwide (1). According to the latest statistical report from the American Cancer Center from 2021, lung cancer has the second-highest incidence and the highest mortality rate among all malignancies (2). There are primarily two types of lung cancer: Non-small cell lung cancer (NSCLC) and SCLC, and the former accounts for ~85% of all lung cancer cases (3). Although valuable progress has been made in the treatment of NSCLC in previous years, the high metastasis rate, post-operative recurrence rate and resistance to chemotherapeutic drugs in lung cancer have led to unsatisfactory outcomes (4,5). The rates of successful treatment and survival remain low and the 5-year survival rate is 21% (2), which may be attributed to the fact that NSCLC is usually diagnosed at an advanced stage, with no surgical options (6,7). Therefore, it is particularly important to explore new treatments and develop novel drugs.
Certain metabolic alterations, also referred to as metabolic reprogramming, are commonly observed in tumor cells and are proposed to be hallmarks of cancer (8). Given the vast differences in metabolism between healthy and tumor cells, there is hope that selective targeting of tumor metabolism may be achieved while limiting toxicity to healthy tissue. The most striking and characteristic metabolic alteration in cancer cells is anomalous glucose metabolism and cancer cells tend to utilize glycolysis to obtain energy even under aerobic conditions via a process called ‘aerobic glycolysis’ (9). The implications of this finding overshadowed the importance of mitochondria for tumor growth for a long time. However, in recent years, there has been increasing evidence that metformin exerts its anticancer effects through the inhibition of oxidative phosphorylation (OXPHOS) of tumor cell mitochondria, and metabolic pathways based on metformin targeting have only recently become the focus of intensive research. In order to establish a systematic literature review, the online search engine PubMed was used for the present study. Studies published within the last 10 years were retrieved using the key terms ‘Metformin’ and ‘Lung Cancer’. In the present review, NSCLC metabolism was discussed with a focus on the potential of metformin-based targeting of NSCLC metabolism and the associated mechanisms, and the available preclinical and clinical evidence was assessed.
2. Aerobic glycolytic pathways and targeted therapy in NSCLC
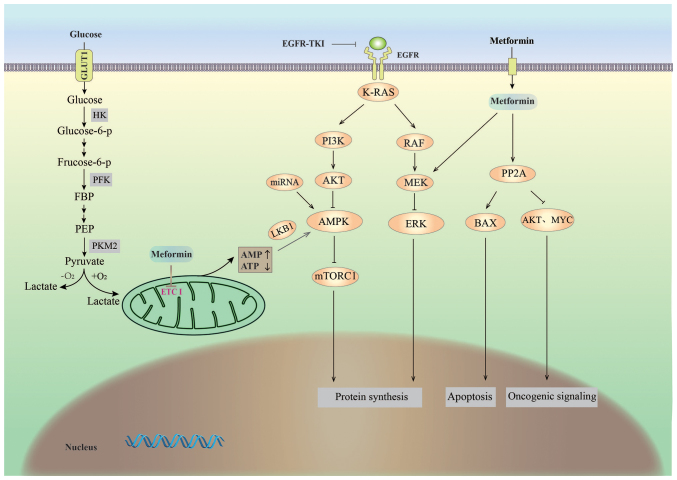
Glucose is the most abundant and important energy source in organisms and it is metabolized in cells via two major pathways (Fig. 1): Glycolysis, which takes place under anaerobic conditions, and complete oxidation, which occurs under aerobic conditions (10). In the 1920s, Otto Heinrich Warburg discovered that cancer cells, unlike normal cells, use the glycolytic pathway to obtain energy for growth even in the presence of oxygen, which is a phenomenon known as ‘aerobic glycolysis’ or the Warburg effect (9), and aerobic glycolysis is a common metabolic phenotype in NSCLC (11). In positron emission tomography (PET)/CT, the high rate of glycolysis in NSCLC is reflected by the high uptake of 18F-fluorodeoxyglucose at the corresponding tumor sites (12). It has been reported that lung cancer cells exhibit upregulated expression of all key glycolytic enzymes [hexokinase 2 (HK2), phosphofructokinase and pyruvate kinase (PK)] (13), suggesting that the essential enzymes of the aerobic glycolytic pathway have a critical role in the development of lung carcinoma. Therefore, various drugs that interfere with glycolytic glucose transport proteins and key enzymes are being studied for their potential as anticancer agents (14).
Figure 1.
Glucose metabolism and possible molecular actions of metformin in non-small cell lung cancer. GLUT1, glucose transporter 1; HK, hexokinase; PFK, phosphofructokinase; PKM2, pyruvate kinase M2; ETC I, electron transport chain complex I; K-RAS, Kirsten rat sarcoma viral oncogene homolog; PI3K, phosphoinositide 3-kinase; miRNA, microRNA; LKB1, liver kinase B1; AMPK, adenosine monophosphate-activated protein kinase; mTORC1, mechanistic target of rapamycin complex 1; RAF, Raf oncogene; MEK, mitogen-activated protein kinase kinase; ERK, extracellular signal-regulated kinase; EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitor; PP2A, protein phosphatase 2A; BAX, B-cell lymphoma 2-associated X protein; MYC, myelocytomatosis oncogene; ↑, promoting effect; T, inhibiting effect.
Glucose transporter 1 (GLUT1), which drives the intracellular transport of glucose, is the first rate-limiting factor in glycolysis (15). Lung cancer cells have a high rate of glycolysis and high GLUT1 expression (16), and research focusing on GLUT1 may be important for lung cancer treatment. WZB117 (WZB) is a synthetic small molecule that inhibits glucose transport by downregulating GLUT1 expression (17). A study suggested that WZB may enhance toxic effects on the NSCLC cell line H460 by limiting glycolysis (18). In a nude mouse tumor transplantation model of lung cancer, WZB was indicated to inhibit tumor growth by inhibiting GLUT1 and limiting the glycolytic flow (19,20). 2-Deoxy-D-glucose (2-DG), another glycolysis inhibitor, restricts tumor growth by binding to HK and preventing glucose from accessing the enzyme (5,18). In recent years, research on microRNAs (miRNAs/miRs) has expanded and their association with NSCLC has been explored. For instance, Jia et al (21) demonstrated that miR-206 levels were reduced in NSCLC cells and tissues and overexpression of miR-206 was able to inhibit glycolysis and cell proliferation by targeting the 3′-untranslated region of HK2 and downregulating HK2 expression. PKM2 is essential for tumorigenesis and An et al (22) demonstrated that small ubiquitin-related modifier 1 (SUMO1) overexpression increased glycolysis and promoted the growth of A549 cells in vitro by modifying PKM2 at Lys-336. Knockdown of SUMO1 in A549 cells resulted in a marked decrease in the protein expression of PKM2, suggesting that SUMO1-modified PKM2 may be a potential therapeutic target for NSCLC.
The study of aerobic glycolytic pathways and the functions of key enzymes in tumor cells is vital for the treatment of NSCLC. Since the Warburg effect was described, there has been an increase in research focusing on aerobic glycolysis (14) and there have been several attempts to limit the growth of lung cancer by cutting off its energy supply. Although a reversal of the Warburg effect may be a broad anticancer strategy, therapeutic approaches to limit aerobic glycolysis in NSCLC have been only partially successful (23). The expression of PKM2 was previously indicated to be required for aerobic glycolysis and it was proposed that PKM2 provides a growth advantage to tumors. However, Israelsen et al (24) excised PKM exon 10 to terminate PKM2 protein synthesis while still allowing the splicing and protein expression of PKM1, which demonstrated that the loss of PKM2 accelerated tumor formation in a nude mouse xenograft tumor model. Similarly, Cortés-Cros et al (25) knocked down PKM2/M1 in established tumors and observed no significant difference in the growth of A549 lung cancer xenografts in vivo. These studies suggest the presence of other alternative metabolic pathways.
Mitochondria are the main sites of ATP release during oxidative phosphorylation and the original hypothesis of the Warburg effect was that cancer cells have a defective mitochondrial function, resulting in impaired aerobic respiration, necessitating the reliance on glycolysis for ATP supply (26). However, later studies have indicated that mitochondrial function is not impaired in most cancer cells and that mitochondria have an important role in cancer metabolism (27–29). In addition, although the ratio of glycolysis to OXPHOS increases, in absolute terms, both glycolysis and oxidative phosphorylation are more active in cancer cells than in normal cells and the two processes coexist (30). Given that mitochondria are essential for tumorigenesis and cancer cell proliferation (31–33), targeting the mitochondrial OXPHOS metabolic pathway may be a viable approach for inhibiting the growth of cancer cells (34).
3. Mechanisms underlying the effects of metformin in lung cancer treatment
Metformin has been the safest and most widely prescribed drug for type 2 diabetes (T2D) (35). It downregulates cytosolic OXPHOS by inhibiting mitochondrial electron transport chain complex I (ETC I), thereby hampering the oxidative phosphorylation required for tumor cell growth (36–38). Initial interest in the use of metformin for preventing and treating lung cancer arose from a number of clinical studies suggesting that metformin reduces the risk of lung cancer in individuals with diabetes (39–41).
Studies have gradually revealed the mechanism of action of metformin in the treatment of cancer (Fig. 1). Metformin has indirect (insulin-dependent) and direct (insulin-independent) anticancer effects (42). The indirect anticancer effect of metformin results from the attenuation of the stimulatory effect of hyperinsulinemia on lung cancer growth via an increase in insulin sensitivity and decrease in circulating insulin levels (43). By contrast, the direct effect of metformin is caused by the activation of adenosine monophosphate (AMP)-activated protein kinase (AMPK). Metformin indirectly activates AMPK by disrupting mitochondrial ETC I, leading to reduced ATP synthesis and an increased cellular AMP/ATP ratio (44). It is generally speculated that the AMPK activation-mediated anticancer activity of metformin may be dependent on liver kinase B1 (LKB1). Metformin exerts its antitumor effects mainly through the AMPK/LKB1/mammalian target of rapamycin (mTOR) complex 1 (mTORC1) signaling pathway, causing apoptosis of cancer cells (45–48). LKB1 is a classical tumor suppressor (49) and mutations in this gene are associated with Peutz-Jeghers cancer susceptibility syndrome (50,51). Genetic mutations in LKB1 are also observed in certain sporadic cancers, particularly squamous cell carcinoma and lung adenocarcinoma cells (52,53). AMPK is the direct substrate of LKB1. Metformin interferes with cellular energy metabolism by disrupting ETC I; the low energy states induce LKB1-mediated AMPK activation and indirectly inhibit mTORC1 to regulate cell growth (54–56). In addition, metformin may also have anticancer activity via AMPK activation independent of LKB1 (57–59). Guo et al (57) evaluated the effects of metformin on human NSCLC H1299 (LKB1-positive) and H460 (LKB1-deficient) cells. They indicated that metformin inhibits NSCLC proliferation in a time- and dose-dependent manner, induces cell cycle arrest in G0/G1 phase and increases apoptosis independent of LKB1 protein levels. They also observed that knockdown of LKB1 using short hairpin RNA does not affect the anti-proliferative effect of metformin on H1299 cells.
mTORC1, a serine/threonine protein kinase belonging to the PI3K-related kinase family, acts as a regulator of cell growth and metabolism (60). Activated AMPK inhibits tumor growth by inhibiting mTORC1, which blocks protein synthesis and proliferation in cancer cells (61,62). As mTORC1 is frequently mutated in cancers and functions downstream of several oncogenic pathways, various tumors, including lung cancer tumors, exhibit elevated mTORC1 activity (63). The RAS/PI3K/AKT/mTOR signaling pathway is an important cellular signaling cascade in which RAS activates PI3K and AKT and indirectly regulates mTORC1 (9,64,65). Among the three RAS genes (H-, K- and N-RAS), the highest mutation frequency was observed for the K-RAS gene in lung cancer (6.5% frequency for squamous cell carcinoma and 26% for adenocarcinoma in Western populations) (66). Metformin induces apoptosis via the downregulation of the downstream targets of K-RAS in human A549 lung adenocarcinoma cells with K-RAS mutations (67). It has also been reported that metformin inhibits mTORC1 signaling in an AMPK-independent manner and that this inhibition of mTORC1 activation and signaling may be Rag GTPase-dependent (68).
The anticancer effects of metformin are also speculated to be related to miRNAs in NSCLC. A recent study by Dong et al (69) indicated that metformin inhibits the growth, migration and invasion of A549 cells by upregulating AMPK-mediated miR-7 expression and regulating the AKT/mTOR and MAPK/ERK pathways. Recently, it has been reported that high Yes-associated protein (YAP) may induce the growth and metastasis of NSCLC. Metformin also disrupts the growth and metastasis of NSCLC by inhibiting the activity of the miR-381-YAP-Snail axis (70).
However, the gene knockout of AMPK does not completely block the effects of metformin against cancer development, suggesting the presence of alternative mechanisms (57,59). Protein phosphatase 2 (PP2A) is considered a tumor inhibitor in a variety of tumors (71) and the PP2A inhibitor α4 is usually overexpressed in tumor cells. Zhou et al (72) determined that metformin increases the apoptotic rate of A549 and H1651 lung cancer cells by disrupting the interaction of PP2A inhibitors (α4 and MID1) with the catalytic subunit and activating PP2A. This effect is associated with inhibited oncogenic activity of AKT and MYC, as well as Bax phosphorylation, suggesting that PP2A may also be a potential metformin target in lung cancer therapy.
4. Metformin monotherapy for NSCLC
Preclinical studies
Metformin has been used as a single agent in in vivo and ex vivo studies and the data obtained in preclinical studies suggested that it had certain anticancer activity (Table I). In vitro studies indicated that metformin inhibited the proliferation of lung cancer cells in a time- and concentration-dependent manner and increased phosphorylation of AMPK (73–75). Lee et al (76) exposed A549, H460, H1299, H1650 and H226 cells to 0–10 mM metformin and observed decreased cell proliferation and colony-forming capacity, and increased protein levels of p53, p21 and growth arrest and DNA damage protein 45A. In a study by Ko et al (77), treatment of H2087 cells with 0.25-4 mM metformin revealed a decrease in cell colonization and invasion, and upregulated expression of phosphorylated (p)-ERK. Wang et al (78) treated A549, H1975 and HCC827 cells with 0.2 mM metformin and observed that not only did they inhibit cell proliferation, but they also induced cell cycle arrest in the S phase and increased apoptosis.
Table I.
Preclinical studies on metformin in non-small cell lung cancer.
| A, No treatment | |||||
|---|---|---|---|---|---|
|
| |||||
| Author (year) | Cell/animal model | Metformin dose | Combination treatment | Finding/effect of treatment | (Refs.) |
| Moro et al (2018) | A549, H1299 | 50-250 mM | - | ↓ Cell proliferation | (73) |
| ↑ G1 cell cycle arrest | |||||
| ↓ MMP | |||||
| Luo et al (2019) | A549, H460 | 0-80 mM | - | ↓ Cell proliferation | (74) |
| ↑ Apoptosis | |||||
| ↓ c-FLIPL, PKA | |||||
| ↑ GSK-3β | |||||
| Riaz et al (2019) | A549, H460 | 2 µM-8 mM | - | ↓ Cell proliferation | (75) |
| ↓ Colony formation | |||||
| ↓ ERCC1 | |||||
| ↑ p-MAPK | |||||
| Lee et al (2019) | A549, H460, H1299, H1650, H226 | 0-10 mM | - | ↓ Cell proliferation | (76) |
| ↓ Colony formation | |||||
| ↓ SIRT1 | |||||
| ↑ p53, p21 | |||||
| ↑ GADD45A | |||||
| Ko et al (2020) | H2087 | 0.25-4 mM | - | ↓ Cell viability | (77) |
| ↓ Colony formation | |||||
| ↑ p-ERK | |||||
| Wang et al (2021) | A549, H1975, HCC827 | 0.2 mM | - | ↓ Cell proliferation | (78) |
| ↑ Apoptosis | |||||
| ↓ Colony formation | |||||
| ↑ Gs cell cycle arrest | |||||
| Zhou et al (2019) | A549 ×enograft | 5 mg/ml | - | ↓ Lung cancer metastases | (72) |
| Nu/J nude mice | ↓ Tumor growth | ||||
| Wang et al (2021) | A549 ×enograft male nude mice | 250 mg/d | - | ↓ Tumor growth | (78) |
| De Bruycker et al (2019) | A549 ×enograft female nude mice | 100 mg/kg | - | ↓ Tumor growth | (81) |
| ↓ Ki-67 | |||||
| Moro et al (2018) | PDXs SCID mice | 100, 800 mg/kg | - | ↓ Tumor growth | (73) |
| ↓ Ki-67 | |||||
| ↑ p-AMPK | |||||
| Borzi et al (2021) | PDXs | 400 mg/kg | - | ↑ Apoptosis | (82) |
| ↓ Tumor volume | |||||
|
| |||||
| B, Treatment | |||||
|
| |||||
| Type/author (year) | Cell/animal model | Metformin dose | Combination treatment | Finding/effect of treatment | (Refs.) |
|
| |||||
| Glycolysis inhibitor | |||||
| Hou et al (2016) | A549 | 0-10 mM | 2-dDG | ↓ Cell proliferation | (97) |
| ↑ DNA damage | |||||
| ↑ ROS level | |||||
| ↑ Apoptosis | |||||
| ↑ AMPK | |||||
| Yakisich et al (2019) | H460 | 0-10 mM | 2-DG/WZB | ↓ Cell proliferation | (18) |
| ↓ Number of colonies | |||||
| Radiation | |||||
| Wang et al (2017) | A549, H460 | 5 mM | 2 Gy | ↓ Colony formation | (104) |
| Chemotherapy | |||||
| Huang et al (2020) | A549, H838 | 3-12 mM | Cisplatin | ↓ Cell proliferation | (105) |
| ↓ Nrf2 | |||||
| ↑ Apoptosis | |||||
| EGFR-TKI | |||||
| Wang et al (2017) | A549, HCC827, H332M | 5 mM | Erlotinib | ↓ Cell proliferation | (106) |
MMP, mitochondrial membrane potential; c-FLIPL, cellular F ADD-like IL-1β-converting enzyme-inhibitory protein; PKA, protein kinase A; GSK-3β, glycogen synthase kinase 3β; ERCC1, excision repair cross complement-1; p-MAPK, phospho-mitogen-activated protein kinase; SIRT1, sirtuin 1; GADD45A, growth arrest and DNA damage protein 45A; p-ERK, phospho-ERK; PDXs, patient-derived xenografts; p-AMPK, phospho-AMP-activated protein kinase; ROS, reactive oxygen species; AMPK, AMP-activated protein kinase; Nrf2, nuclear factor erythroid 2-related factor-2; EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitor.
Progress has also been made in research on the effects of metformin in animal models of lung cancer. Nicotine-derived nitrosamides, also known as 4-(methylnitrosamino)1-(3-pyridyl)-1-butanone (NNK), have been identified as inducers of lung cancer (79). In a study by Memmott et al (80), in which A/J mice were exposed to NNK and then received intraperitoneal injections of metformin, metformin was observed to reduce tumorigenesis by 72%. This study demonstrated that metformin prevents the tobacco carcinogen-induced development of lung tumors via inhibition of Akt, upstream of mTOR, and indirect inhibition of mTOR. In three other xenogeneic models of A549 cell origin, treatment with metformin significantly reduced tumor growth and metastatic capacity in vivo, and reduced the expression of proteins such as Ki-67, proliferating cell nuclear antigen (PCNA), Akt and Myc (72,78,81). In a study by Moro et al (73) on patient-derived xenografts (PDXs), metformin (100 mg/kg/day) partially inhibited the tumor growth of PDXs with wild-type LKB1 (maximum inhibition rate, 50.5±14.8%), but had no significant inhibitory effect on LKB1-mutant PDXs, and with increasing doses of metformin, p-AMPK expression was increased and Ki67 expression was decreased, indicating that LKB1-deficient tumors have an impaired ability to adapt to metabolic stress induced by metformin treatment. More recently, in another study on wild-type LKB1 PDXs, metformin only induced apoptosis in wild-type LKB1 PDXs with high expression of miR-17, suggesting that high miR-17 expression increased sensitivity to metformin treatment (82).
Retrospective clinical studies
Preclinical studies have indicated that metformin has anticancer effects and numerous retrospective clinical studies have demonstrated that metformin significantly improved anticancer activity in patients with NSCLC compared to those not taking metformin (Table II). Several retrospective studies suggested that metformin use is associated with a decreased risk of lung cancer (41,83,84). Metformin use was also significantly associated with a favorable prognosis of patients with NSCLC (85,86). In a retrospective study assessing overall survival (OS) of patients with T2D and metastatic lung cancer, patients treated with metformin had 20% higher survival rates than those who did not take metformin (87). A comprehensive systematic evaluation and meta-analysis of 10 published retrospective studies by Cao et al (88) determined that treatment with metformin significantly improved survival, with corresponding increases in OS and progression-free survival (PFS) of 23 and 47%, respectively. In addition, analyses stratified by tissue type indicated a significant improvement in OS and PFS in NSCLC, suggesting that metformin may be an effective treatment option for patients with diabetes combined with lung cancer. However, Kim et al (89) performed a retrospective study of 336,168 individuals regarding lung cancer incidence with a median study duration of 12.86 years and observed that metformin treatment did not reduce lung cancer incidence in the diabetic population. The potential use of metformin in lung cancer prevention should be reconsidered and requires to be further validated in randomized controlled trials.
Table II.
Retrospective clinical trials on metformin in non-small cell lung cancer.
| A, Prevention | ||||
|---|---|---|---|---|
|
| ||||
| Author (year) | Stage | Number of subjects | Finding/effect of treatment | (Refs.) |
| Wang et al (2021) | NS | 295573 | ↓ Risk of lung cancer | (78) |
| Xiao et al (2020) | NS | - | ↓ Risk of lung cancer | (84) |
| Kang et al (2021) | NS | 732199 | ↓ Risk of lung cancer | (41) |
|
| ||||
| B, Treatment | ||||
|
| ||||
| Author (year) | Stage | Number of subjects | Finding/effect of treatment | (Refs.) |
|
| ||||
| Arrieta et al (2016) | IV | 1106 | ↑ OS | (85) |
| Xu et al (2018) | NS | 255 | ↑ OST | (86) |
| ↑ DFST | ||||
| Cao et al (2017) | NS | - | ↑ OS | (88) |
| ↑ PFS | ||||
NS, not specified; OS, overall survival; OST, overall survival time; DFST, disease-free survival time; PFS, progression-free survival; ↑, increase; ↓, decrease.
5. Metformin combined with glycolysis inhibitor
Metformin exerts toxic effects on NSCLC cells as an OXPHOS inhibitor (90). However, under standard high-glucose conditions, metformin treatment primarily causes cell cycle arrest without any signs of cell death (91). A study by Elgendy et al (92) indicated that glucose consumption and lactate production increased in a time- and dose-dependent manner after HCT116 cells were treated with metformin, indicating that the rates of glycolysis increased. Conversely, under low-glucose conditions, these cells exhibited a rapid increase in oxygen consumption and a consequent increase in OXPHOS. These findings are consistent with those of certain studies reporting that inhibition of glycolysis is associated with increased activity of OXPHOS and vice versa (93–96). Preclinical evidence suggested that, similar to other anticancer drugs, the effectiveness of metformin was limited in in vitro studies and it is feasible to combine drugs to simultaneously target multiple metabolic pathways in NSCLC to improve treatment efficacy. In a study by Hou et al (97) on a combination of metformin and the glycolysis inhibitor 2-DG in NSCLC treatment, enhanced DNA damage, DNA adduct formation, intracellular reactive oxygen species levels and mitochondrial membrane potential alteration, as well as increased apoptosis, caspase-3 activity, p-p38 and p-AMPK levels, were observed, indicating that combined treatment was more effective against NSCLC than either drug alone. Similarly, in a study by Yakisich et al (18), who studied the effect of metformin alone or in combination with 2-DG and WZB on H460 cell viability, a strong synergistic effect was discovered. The combination of metformin and glycolytic inhibitors led to a marked reduction in intracellular ATP and increased cell death by inhibiting both metabolic pathways in lung cancer.
Another strategy to influence the aerobic glycolytic pathway in cancer cells includes the inhibition of glucose concentrations in culture media in vitro and diet restrictions to lower blood glucose levels in vivo (98). Several studies have indicated that cancer cells cultured under low glucose concentrations or in sugar-free media are more susceptible to the cytotoxic effects of metformin (55,91,99). Restricted diets exhibited a strong synergistic effect on anticancer activity in preclinical models of lung adenocarcinoma. Elgendy et al (92) treated mice undergoing a 24-h feeding/fasting cycle with metformin and they observed impaired tumor growth only when the drug was administered during fasting-induced hypoglycemia. This indicated that metformin combined with fasting-induced hypoglycemia synergistically inhibited the growth of transplanted tumors in nude mice. In addition, an ongoing clinical trial aims to determine whether the combination of metformin and fasting improves PFS in patients with advanced lung adenocarcinoma compared with historical data on metformin alone (100). In another clinical trial, the investigators will assess for the first time the efficacy of combining standard-of-care platinum-based chemoimmunotherapy with metformin plus/minus a fasting-mimicking diet in patients with LKB1-inactive, advanced lung adenocarcinoma (ClinicalTrials.gov identifier no. NCT03709147).
6. Clinical progress of metformin combined with standard anticancer drugs
In recent years, under single treatment regimens [chemotherapy, immune checkpoint inhibitors (ICIs) and targeted therapies] patients have exhibited relapses due to the development of acquired drug resistance (101–103). There is growing evidence that metformin exerts its anticancer effects by inhibiting tumor metabolism and that metformin may be a potential candidate for combination therapy in NSCLC. A number of preclinical studies have reported good results of metformin acting concurrently with radiotherapy, tyrosine kinase inhibitors (TKIs) and ICIs in NSCLC (104–107), which has encouraged the use of combination therapies. In a meta-analysis of 14 clinical studies comprising 3,856 patients, the combination of metformin with standard antineoplastic drugs significantly improved OS in patients with lung cancer (108). These results suggest that metformin combined with radiotherapy may be an effective regimen for the treatment of patients with NSCLC. However, in two recent randomized clinical trials, Skinner et al (109) and Tsakiridis et al (110) reported poorer outcomes for patients with NSCLC treated with metformin in combination with radiotherapy, suggesting that the addition of metformin to radiotherapy did not improve OS in patients with NSCLC and increased toxicities, which contrasts the results of previous studies. Promising results have also been reported by two recent studies of metformin in combination with TKIs and ICIs, respectively, which suggested that metformin was able to significantly improve PFS and OS in patients with NSCLC by overcoming acquired resistance to TKIs and enhancing PD-1 blockade by anti-PD-1 antibodies, respectively (101,102). Other studies (111–114) suggested that metformin may increase tumor response to ICI through a variety of mechanisms, including upregulation of CD8+ tumor-infiltrating lymphocytes and their function, downregulation of myeloid suppressor cells with immunosuppressive effects, reduction of tumor hypoxia, anti-angiogenic effects and shifting the composition of the patient's gut flora to bacterial strains that may respond better to immunotherapy (107). Although it has been suggested that metformin treatment may exert a synergistic antitumor effect with ICIs, the study by Jacobi et al (107) did not obtain any positive association between metformin and ICIs in the treatment of patients with diabetes combined with NSCLC. More prospective studies are required to further evaluate the effect of metformin in combination with radiotherapy, TKIs and ICIs on the outcome of patients with NSCLC. A search on https://clinicaltrials.gov indicated that a number of prospective clinical trials (Table III) are currently evaluating the preventive and therapeutic effects of metformin alone or in combination with other treatment options for NSCLC. One of these is an ongoing open, single-arm, phase II clinical trial (ClinicalTrials.gov identifier no. NCT03874000) to evaluate the safety, efficacy and pharmacokinetics of the metformin-sintilimab combination in the treatment of NSCLC (115).
Table III.
Clinical trials on metformin in non-small cell lung cancer.
| Author (year) | Clinical trial number | Status | Trial phase | Stage | Metformin dose (mg/day) | Combination treatment | Primary purpose | (Refs.) |
|---|---|---|---|---|---|---|---|---|
| - | NCT03086733 | Completed | 2 | I–IIIA | 850 | - | Treatment | - |
| - | NCT01717482 | Terminated | 2 | IA-IIIA | 1700 | - | Prevention | - |
| - | NCT04931017 | Not yet recruiting | 2 | NS | - | - | Prevention | - |
| - | NCT02109549 | Completed | 2 | Advanced stage | - | - | Treatment | - |
| - | NCT04170959 | Terminated | 2 | III | 500 | - | Treatment | - |
| - | NCT02019979 | Terminated | 2 | IIIB-IV | 1000 | Restricted diet | Treatment | - |
| - | NCT02285855 | Terminated | 2 | NS | 2000 | Radiation | Treatment | - |
| Skinner et al (2021) | NCT02186847 | Active, not recruiting | 2 | III | 1000, 1500, 2000 | Chemo-radiotherapy | Treatment | (109) |
| Tsakiridis et al (2021) | NCT02115464 | Terminated | 2 | IIIA-IIIB | 2000 | Chemo-radiotherapy | Treatment | (110) |
| Kubo et al (2018) | NCT03874000 | Recruiting | 2 | IIIB, IIIC, IV | 1000 | ICIs | Treatment | (115) |
| - | NCT03048500 | Active, not recruiting | 2 | III–IV | - | ICIs | Treatment | - |
| Arrieta et al (2016) | NCT03071705 | Unknown | - | NS | 1000 | TKI | Other | (85) |
| - | NCT01864681 | Completed | 2 | IIIB-IV | 1000 | EGFR-TKI | - | - |
| - | NCT03709147 | Recruiting | 2 | IIIB-IV | 1500 | Chemo-immunotherapy + FMD | Treatment | - |
NS, not specified; ICIs, immune checkpoint inhibitors; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor; FMD, fasting-mimicking diet.
7. Prospects and conclusions
Therapeutic methods that target the metabolic differences between tumor cells and normal cells have potential in cancer treatment and the restriction of aerobic glycolysis in tumors has been somewhat effective in inducing lung cancer cell apoptosis. There are also increasing reports confirming the important role of mitochondria in the development and growth of cancer. In recent years, there has been increasing evidence of the antitumor effects of metformin as an OXPHOS inhibitor and in a number of retrospective clinical trials, metformin has produced beneficial effects on survival outcomes in patients with NSCLC. The theory of the antitumor effects of metformin involves its action on several major signaling pathways, including indirect (insulin-dependent) and direct (activation of AMPK pathways) and corresponding targets, such as PI3K, K-RAS, mTORC, PP2A and miRNA. However, as with aerobic glycolysis inhibitors, metformin alone exhibited limitations in its effectiveness in in vitro trials. Drugs that target enzymes or metabolites of key metabolic pathways may be highly specific and effective but must be matched to responsive tumors that are likely to adapt rapidly. Preclinical evidence in recent years has demonstrated synergistic effects of metformin in combination with glycolysis inhibitors, radiotherapy, EGFR-TKIs and ICIs in NSCLC, but it is not consistent with the results of certain retrospective studies and clinical trials, and more prospective studies are required to further evaluate the influence of metformin combination effects on the outcomes for patients with NSCLC. However, metformin inhibits mitochondria in a dose-dependent manner and at high doses, although it is able to impair tumor growth, it may also lead to lactic acidosis (116). The clinical application of experimental doses of metformin may be challenging. Of note, metformin accumulates in tissues at concentrations several times higher than those in the blood and the positive charge on metformin has been indicated to promote its accumulation in the mitochondrial matrix <1,000-fold (>20 mmol/l). Hence, metformin concentrations of 1–10 mmol/l, which have been used in preclinical models, may also be effective during cancer treatment in clinical settings (117). In a recent study, Reinfeld et al (118) used PET tracers to measure glucose uptake in specific cellular subpopulations in the tumor microenvironment and determined that in a range of cancer models, myeloid cells have the greatest glucose uptake capacity within the tumor, followed by T cells and cancer cells. Furthermore, they observed that cancer cells had higher uptake of glutamine than of glucose. In the future, more in-depth basic research on target metabolic pathways in lung cancer is required to provide an improved theoretical basis for adjuvant lung cancer therapy.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
Authors' contributions
NC and JBH designed the review and edited the manuscript. NC and JBH wrote the manuscript. NC, YSZ and LCW collected and analyzed data. All authors read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wang W, Hao Y, Liu Y, Li R, Huang DB, Pan YY. Nanomedicine in lung cancer: Current states of overcoming drug resistance and improving cancer immunotherapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021;13:e1654. doi: 10.1002/wnan.1654. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 3.Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: Epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94:1623–1640. doi: 10.1016/j.mayocp.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Hai J, Zhu CQ, Bandarchi B, Wang YH, Navab R, Shepherd FA, Jurisica I, Tsao MS. L1 cell adhesion molecule promotes tumorigenicity and metastatic potential in non-small cell lung cancer. Clin Cancer Res. 2012;18:1914–1924. doi: 10.1158/1078-0432.CCR-11-2893. [DOI] [PubMed] [Google Scholar]
- 5.Meijer TWH, Peeters WJM, Dubois LJ, van Gisbergen MW, Biemans R, Venhuizen JH, Span PN, Bussink J. Targeting glucose and glutamine metabolism combined with radiation therapy in non-small cell lung cancer. Lung Cancer. 2018;126:32–40. doi: 10.1016/j.lungcan.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Yao L, Liu M, Huang Y, Wu K, Huang X, Zhao Y, He W, Zhang R. Metformin use and lung cancer risk in diabetic patients: A systematic review and meta-analysis. Dis Markers. 2019;2019:6230162. doi: 10.1155/2019/6230162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Troncone M, Cargnelli SM, Villani LA, Isfahanian N, Broadfield LA, Zychla L, Wright J, Pond G, Steinberg GR, Tsakiridis T. Targeting metabolism and AMP-activated kinase with metformin to sensitize non-small cell lung cancer (NSCLC) to cytotoxic therapy: Translational biology and rationale for current clinical trials. Oncotarget. 2017;8:57733–57754. doi: 10.18632/oncotarget.17496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv. 2016;2:e1600200. doi: 10.1126/sciadv.1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bose S, Le A. Glucose metabolism in cancer. Adv Exp Med Biol. 2018;1063:3–12. doi: 10.1007/978-3-319-77736-8_1. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, Yu X, Zhou L, Li J, Li M, Li W, Gao F. Sinomenine inhibits non-small cell lung cancer via downregulation of hexokinases II-mediated aerobic glycolysis. Onco Targets Ther. 2020;13:3209–3221. doi: 10.2147/OTT.S243212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toba H, Kawakita N, Takashima M, Matsumoto D, Takizawa H, Otsuka H, Tangoku A. Diagnosis of recurrence and follow-up using FDG-PET/CT for postoperative non-small-cell lung cancer patients. Gen Thorac Cardiovasc Surg. 2021;69:311–317. doi: 10.1007/s11748-020-01477-1. [DOI] [PubMed] [Google Scholar]
- 13.Li XB, Gu JD, Zhou QH. Review of aerobic glycolysis and its key enzymes-new targets for lung cancer therapy. Thorac Cancer. 2015;6:17–24. doi: 10.1111/1759-7714.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Outschoorn UE, Peiris-Pagés M, Pestell RG, Sotgia F, Lisanti MP. Cancer metabolism: A therapeutic perspective. Nat Rev Clin Oncol. 2017;14:11–31. doi: 10.1038/nrclinonc.2017.1. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Ran Y, Zhu Y, Zhen Q. Effect of addition of WZB117 as an inhibitor of glucose transporter 1 for venous blood glucose determination. Lab Med. 2021;52:197–201. doi: 10.1093/labmed/lmaa051. [DOI] [PubMed] [Google Scholar]
- 16.Tiemin P, Peng X, Qingfu L, Yan W, Junlin X, Zhefeng H, Ming Z, Desen L, Qinghui M. Dysregulation of the miR-148a-GLUT1 axis promotes the progression and chemoresistance of human intrahepatic cholangiocarcinoma. Oncogenesis. 2020;9:19. doi: 10.1038/s41389-020-0207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao F, Ming J, Zhou Y, Fan L. Inhibition of Glut1 by WZB117 sensitizes radioresistant breast cancer cells to irradiation. Cancer Chemother Pharmacol. 2016;77:963–972. doi: 10.1007/s00280-016-3007-9. [DOI] [PubMed] [Google Scholar]
- 18.Yakisich JS, Azad N, Kaushik V, Iyer AKV. The biguanides metformin and buformin in combination with 2-Deoxy-glucose or WZB-117 inhibit the viability of highly resistant human lung cancer cells. Stem Cells Int. 2019;2019:6254269. doi: 10.1155/2019/6254269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ojelabi OA, Lloyd KP, Simon AH, De Zutter JK, Carruthers A. WZB117 (2-Fluoro-6-(m-hydroxybenzoyloxy) Phenyl m-Hydroxybenzoate) Inhibits GLUT1-mediated sugar transport by binding reversibly at the exofacial sugar binding site. J Biol Chem. 2016;291:26762–26772. doi: 10.1074/jbc.M116.759175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Cao Y, Zhang W, Bergmeier S, Qian Y, Akbar H, Colvin R, Ding J, Tong L, Wu S, et al. A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther. 2012;11:1672–1682. doi: 10.1158/1535-7163.MCT-12-0131. [DOI] [PubMed] [Google Scholar]
- 21.Jia KG, Feng G, Tong YS, Tao GZ, Xu L. MiR-206 regulates non-small-cell lung cancer cell aerobic glycolysis by targeting hexokinase 2. J Biochem. 2020;167:365–370. doi: 10.1093/jb/mvz099. [DOI] [PubMed] [Google Scholar]
- 22.An S, Huang L, Miao P, Shi L, Shen M, Zhao X, Liu J, Huang G. Small ubiquitin-like modifier 1 modification of pyruvate kinase M2 promotes aerobic glycolysis and cell proliferation in A549 human lung cancer cells. Onco Targets Ther. 2018;11:2097–2109. doi: 10.2147/OTT.S156918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porporato PE, Filigheddu N, Pedro JMB, Kroemer G, Galluzzi L. Mitochondrial metabolism and cancer. Cell Res. 2018;28:265–280. doi: 10.1038/cr.2017.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Israelsen WJ, Dayton TL, Davidson SM, Fiske BP, Hosios AM, Bellinger G, Li J, Yu Y, Sasaki M, Horner JW, et al. PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Cell. 2013;155:397–409. doi: 10.1016/j.cell.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortés-Cros M, Hemmerlin C, Ferretti S, Zhang J, Gounarides JS, Yin H, Muller A, Haberkorn A, Chene P, Sellers WR, Hofmann F. M2 isoform of pyruvate kinase is dispensable for tumor maintenance and growth. Proc Natl Acad Sci USA. 2013;110:489–494. doi: 10.1073/pnas.1212780110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 27.Weinhouse S. The Warburg hypothesis fifty years later. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1976;87:115–126. doi: 10.1007/BF00284370. [DOI] [PubMed] [Google Scholar]
- 28.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 29.Moreno-Sánchez R, Rodríguez-Enríquez S, Marín-Hernández A, Saavedra E. Energy metabolism in tumor cells. FEBS J. 2007;274:1393–1418. doi: 10.1111/j.1742-4658.2007.05686.x. [DOI] [PubMed] [Google Scholar]
- 30.Zacksenhaus E, Shrestha M, Liu JC, Vorobieva I, Chung PED, Ju Y, Nir U, Jiang Z. Mitochondrial OXPHOS Induced by RB1 deficiency in breast cancer: Implications for anabolic metabolism, stemness, and metastasis. Trends Cancer. 2017;3:768–779. doi: 10.1016/j.trecan.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Joshi S, Tolkunov D, Aviv H, Hakimi AA, Yao M, Hsieh JJ, Ganesan S, Chan CS, White E. The genomic landscape of renal oncocytoma identifies a metabolic barrier to tumorigenesis. Cell Rep. 2015;13:1895–1908. doi: 10.1016/j.celrep.2015.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci USA. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martínez-Reyes I, Diebold LP, Kong H, Schieber M, Huang H, Hensley CT, Mehta MM, Wang T, Santos JH, Woychik R, et al. TCA cycle and mitochondrial membrane potential are necessary for diverse biological functions. Mol Cell. 2016;61:199–209. doi: 10.1016/j.molcel.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng G, Zhang Q, Pan J, Lee Y, Ouari O, Hardy M, Zielonka M, Myers CR, Zielonka J, Weh K, et al. Targeting lonidamine to mitochondria mitigates lung tumorigenesis and brain metastasis. Nat Commun. 2019;10:2205. doi: 10.1038/s41467-019-10042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: An overview. Clin Sci (Lond) 2012;122:253–270. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez-Alvarez R, Martinez-Outschoorn UE, Lamb R, Hulit J, Howell A, Gandara R, Sartini M, Rubin E, Lisanti MP, Sotgia F. Mitochondrial dysfunction in breast cancer cells prevents tumor growth: Understanding chemoprevention with metformin. Cell Cycle. 2013;12:172–182. doi: 10.4161/cc.23058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown SL, Kolozsvary A, Isrow DM, Al Feghali K, Lapanowski K, Jenrow KA, Kim JH. A novel mechanism of high dose radiation sensitization by metformin. Front Oncol. 2019;9:247. doi: 10.3389/fonc.2019.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Q, Zhou X, Curbo S, Karlsson A. Metformin downregulates the mitochondrial carrier SLC25A10 in a glucose dependent manner. Biochem Pharmacol. 2018;156:444–450. doi: 10.1016/j.bcp.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Zhang ZJ, Bi Y, Li S, Zhang Q, Zhao G, Guo Y, Song Q. Reduced risk of lung cancer with metformin therapy in diabetic patients: A systematic review and meta-analysis. Am J Epidemiol. 2014;180:11–14. doi: 10.1093/aje/kwu124. [DOI] [PubMed] [Google Scholar]
- 40.Tsai MJ, Yang CJ, Kung YT, Sheu CC, Shen YT, Chang PY, Huang MS, Chiu HC. Metformin decreases lung cancer risk in diabetic patients in a dose-dependent manner. Lung Cancer. 2014;86:137–143. doi: 10.1016/j.lungcan.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Kang J, Jeong SM, Shin DW, Cho M, Cho JH, Kim J. The associations of aspirin, statins, and metformin with lung cancer risk and related mortality: A time-dependent analysis of population-based nationally representative data. J Thorac Oncol. 2021;16:76–88. doi: 10.1016/j.jtho.2020.08.021. [DOI] [PubMed] [Google Scholar]
- 42.Li C, Xue Y, Xi YR, Xie K. Progress in the application and mechanism of metformin in treating non-small cell lung cancer. Oncol Lett. 2017;13:2873–2880. doi: 10.3892/ol.2017.5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frasca F, Pandini G, Sciacca L, Pezzino V, Squatrito S, Belfiore A, Vigneri R. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem. 2008;114:23–37. doi: 10.1080/13813450801969715. [DOI] [PubMed] [Google Scholar]
- 44.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim KH, Song MJ, Yoo EJ, Choe SS, Park SD, Kim JB. Regulatory role of glycogen synthase kinase 3 for transcriptional activity of ADD1/SREBP1c. J Biol Chem. 2004;279:51999–52006. doi: 10.1074/jbc.M405522200. [DOI] [PubMed] [Google Scholar]
- 46.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 48.Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 49.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/S0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 50.Forcet C, Billaud M. Dialogue between LKB1 and AMPK: A hot topic at the cellular pole. Sci STKE. 2007;2007:pe51. doi: 10.1126/stke.4042007pe51. [DOI] [PubMed] [Google Scholar]
- 51.Korsse SE, Peppelenbosch MP, van Veelen W. Targeting LKB1 signaling in cancer. Biochim Biophys Acta. 2013;1835:194–210. doi: 10.1016/j.bbcan.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 52.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: Metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu B, Fan Z, Edgerton SM, Deng XS, Alimova IN, Lind SE, Thor AD. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle. 2009;8:2031–2040. doi: 10.4161/cc.8.13.8814. [DOI] [PubMed] [Google Scholar]
- 54.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, Viollet B, Thompson CB. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 56.Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- 57.Guo Q, Liu Z, Jiang L, Liu M, Ma J, Yang C, Han L, Nan K, Liang X. Metformin inhibits growth of human non-small cell lung cancer cells via liver kinase B-1-independent activation of adenosine monophosphate-activated protein kinase. Mol Med Rep. 2016;13:2590–2596. doi: 10.3892/mmr.2016.4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiao X, He Q, Lu C, Werle KD, Zhao RX, Chen J, Davis BC, Cui R, Liang J, Xu ZX. Metformin impairs the growth of liver kinase B1-intact cervical cancer cells. Gynecol Oncol. 2012;127:249–255. doi: 10.1016/j.ygyno.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Storozhuk Y, Hopmans SN, Sanli T, Barron C, Tsiani E, Cutz JC, Pond G, Wright J, Singh G, Tsakiridis T. Metformin inhibits growth and enhances radiation response of non-small cell lung cancer (NSCLC) through ATM and AMPK. Br J Cancer. 2013;108:2021–2032. doi: 10.1038/bjc.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Condon KJ, Sabatini DM. Nutrient regulation of mTORC1 at a glance. J Cell Sci. 2019;132:jcs222570. doi: 10.1242/jcs.222570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Düvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Howell JJ, Ricoult SJ, Ben-Sahra I, Manning BD. A growing role for mTOR in promoting anabolic metabolism. Biochem Soc Trans. 2013;41:906–912. doi: 10.1042/BST20130041. [DOI] [PubMed] [Google Scholar]
- 64.Schneider MB, Matsuzaki H, Haorah J, Ulrich A, Standop J, Ding XZ, Adrian TE, Pour PM. Prevention of pancreatic cancer induction in hamsters by metformin. Gastroenterology. 2001;120:1263–1270. doi: 10.1053/gast.2001.23258. [DOI] [PubMed] [Google Scholar]
- 65.Bussink J, van der Kogel AJ, Kaanders JH. Activation of the PI3-K/AKT pathway and implications for radioresistance mechanisms in head and neck cancer. Lancet Oncol. 2008;9:288–296. doi: 10.1016/S1470-2045(08)70073-1. [DOI] [PubMed] [Google Scholar]
- 66.Dearden S, Stevens J, Wu YL, Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: Meta-analyses by ethnicity and histology (mutMap) Ann Oncol. 2013;24:2371–2376. doi: 10.1093/annonc/mdt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma Y, Guo FC, Wang W, Shi HS, Li D, Wang YS. K-ras gene mutation as a predictor of cancer cell responsiveness to metformin. Mol Med Rep. 2013;8:763–768. doi: 10.3892/mmr.2013.1596. [DOI] [PubMed] [Google Scholar]
- 68.Kalender A, Selvaraj A, Kim SY, Gulati P, Brûlé S, Viollet B, Kemp BE, Bardeesy N, Dennis P, Schlager JJ, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dong J, Peng H, Yang X, Wu W, Zhao Y, Chen D, Chen L, Liu J. Metformin mediated microRNA-7 upregulation inhibits growth, migration, and invasion of non-small cell lung cancer A549 cells. Anticancer Drugs. 2020;31:345–352. doi: 10.1097/CAD.0000000000000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jin D, Guo J, Wu Y, Chen W, Du J, Yang L, Wang X, Gong K, Dai J, Miao S, et al. Metformin-repressed miR-381-YAP-snail axis activity disrupts NSCLC growth and metastasis. J Exp Clin Cancer Res. 2020;39:6. doi: 10.1186/s13046-019-1503-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Fatehi Hassanabad A, MacQueen K. Molecular mechanisms underlining the role of metformin as a therapeutic agent in lung cancer. Cell Oncol (Dordr) 2021;44:1–18. doi: 10.1007/s13402-020-00570-0. [DOI] [PubMed] [Google Scholar]
- 72.Zhou X, Liu S, Lin X, Xu L, Mao X, Liu J, Zhang Z, Jiang W, Zhou H. Metformin inhibit lung cancer cell growth and invasion in vitro as well as tumor formation in vivo partially by activating PP2A. Med Sci Monit. 2019;25:836–846. doi: 10.12659/MSM.912059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moro M, Caiola E, Ganzinelli M, Zulato E, Rulli E, Marabese M, Centonze G, Busico A, Pastorino U, de Braud FG, et al. Metformin enhances cisplatin-induced apoptosis and prevents resistance to cisplatin in Co-mutated KRAS/LKB1 NSCLC. J Thorac Oncol. 2018;13:1692–1704. doi: 10.1016/j.jtho.2018.07.102. [DOI] [PubMed] [Google Scholar]
- 74.Luo Z, Zhu T, Luo W, Lv Y, Zhang L, Wang C, Li M, Wu W, Shi S. Metformin induces apoptotic cytotoxicity depending on AMPK/PKA/GSK-3β-mediated c-FLIPL degradation in non-small cell lung cancer. Cancer Manag Res. 2019;11:681–689. doi: 10.2147/CMAR.S178688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Riaz MA, Sak A, Erol YB, Groneberg M, Thomale J, Stuschke M. Metformin enhances the radiosensitizing effect of cisplatin in non-small cell lung cancer cell lines with different cisplatin sensitivities. Sci Rep. 2019;9:1282. doi: 10.1038/s41598-018-38004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee BB, Kim Y, Kim D, Cho EY, Han J, Kim HK, Shim YM, Kim DH. Metformin and tenovin-6 synergistically induces apoptosis through LKB1-independent SIRT1 down-regulation in non-small cell lung cancer cells. J Cell Mol Med. 2019;23:2872–2889. doi: 10.1111/jcmm.14194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ko E, Baek S, Kim J, Park D, Lee Y. Antitumor activity of combination therapy with metformin and trametinib in non-small cell lung cancer cells. Dev Reprod. 2020;24:113–123. doi: 10.12717/DR.2020.24.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang JL, Lan YW, Tsai YT, Chen YC, Staniczek T, Tsou YA, Yen CC, Chen CM. Additive antiproliferative and antiangiogenic effects of metformin and pemetrexed in a non-small-cell lung cancer xenograft model. Front Cell Dev Biol. 2021;9:688062. doi: 10.3389/fcell.2021.688062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Akopyan G, Bonavida B. Understanding tobacco smoke carcinogen NNK and lung tumorigenesis. Int J Oncol. 2006;29:745–752. [PubMed] [Google Scholar]
- 80.Memmott RM, Mercado JR, Maier CR, Kawabata S, Fox SD, Dennis PA. Metformin prevents tobacco carcinogen-induced lung tumorigenesis. Cancer Prev Res (Phila) 2010;3:1066–1076. doi: 10.1158/1940-6207.CAPR-10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Bruycker S, Vangestel C, Van den Wyngaert T, Pauwels P, Wyffels L, Staelens S, Stroobants S. 18F-Flortanidazole Hypoxia PET holds promise as a prognostic and predictive imaging biomarker in a lung cancer xenograft model treated with metformin and radiotherapy. J Nucl Med. 2019;60:34–40. doi: 10.2967/jnumed.118.212225. [DOI] [PubMed] [Google Scholar]
- 82.Borzi C, Ganzinelli M, Caiola E, Colombo M, Centonze G, Boeri M, Signorelli D, Caleca L, Rulli E, Busico A, et al. LKB1 down-modulation by miR-17 identifies patients with NSCLC having worse prognosis eligible for energy-stress-based treatments. J Thorac Oncol. 2021;16:1298–1311. doi: 10.1016/j.jtho.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 83.Tseng CH. Metformin and lung cancer risk in patients with type 2 diabetes mellitus. Oncotarget. 2017;8:41132–41142. doi: 10.18632/oncotarget.18239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xiao K, Liu F, Liu J, Xu J, Wu Q, Li X. The effect of metformin on lung cancer risk and survival in patients with type 2 diabetes mellitus: A meta-analysis. J Clin Pharm Ther. 2020;45:783–792. doi: 10.1111/jcpt.13167. [DOI] [PubMed] [Google Scholar]
- 85.Arrieta O, Varela-Santoyo E, Soto-Perez-de-Celis E, Sánchez-Reyes R, De la Torre-Vallejo M, Muñiz-Hernández S, Cardona AF. Metformin use and its effect on survival in diabetic patients with advanced non-small cell lung cancer. BMC Cancer. 2016;16:633. doi: 10.1186/s12885-016-2658-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu T, Li D, He Y, Zhang F, Qiao M, Chen Y. Prognostic value of metformin for non-small cell lung cancer patients with diabetes. World J Surg Oncol. 2018;16:60. doi: 10.1186/s12957-018-1362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin JJ, Gallagher EJ, Sigel K, Mhango G, Galsky MD, Smith CB, LeRoith D, Wisnivesky JP. Survival of patients with stage IV lung cancer with diabetes treated with metformin. Am J Respir Crit Care Med. 2015;191:448–454. doi: 10.1164/rccm.201407-1395OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cao X, Wen ZS, Wang XD, Li Y, Liu KY, Wang X. The clinical effect of metformin on the survival of lung cancer patients with diabetes: A comprehensive systematic review and meta-analysis of retrospective studies. J Cancer. 2017;8:2532–2541. doi: 10.7150/jca.19750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim J, Hyun HJ, Choi EA, Yoo JW, Lee S, Jeong N, Shen JJ, You HS, Kim YS, Kang HT. Diabetes, metformin, and lung cancer: Retrospective study of the Korean NHIS-HEALS Database. Clin Lung Cancer. 2020;21:e551–e559. doi: 10.1016/j.cllc.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 90.Cruz-Bermúdez A, Laza-Briviesca R, Vicente-Blanco RJ, García-Grande A, Coronado MJ, Laine-Menéndez S, Palacios-Zambrano S, Moreno-Villa MR, Ruiz-Valdepeñas AM, Lendinez C, et al. Cisplatin resistance involves a metabolic reprogramming through ROS and PGC-1α in NSCLC which can be overcome by OXPHOS inhibition. Free Radic Biol Med. 2019;135:167–181. doi: 10.1016/j.freeradbiomed.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 91.Menendez JA, Oliveras-Ferraros C, Cufí S, Corominas-Faja B, Joven J, Martin-Castillo B, Vazquez-Martin A. Metformin is synthetically lethal with glucose withdrawal in cancer cells. Cell Cycle. 2012;11:2782–2792. doi: 10.4161/cc.20948. [DOI] [PubMed] [Google Scholar]
- 92.Elgendy M, Cirò M, Hosseini A, Weiszmann J, Mazzarella L, Ferrari E, Cazzoli R, Curigliano G, DeCensi A, Bonanni B, et al. Combination of hypoglycemia and metformin impairs tumor metabolic plasticity and growth by modulating the PP2A-GSK3β-MCL-1 Axis. Cancer Cell. 2019;35:798–815.e5. doi: 10.1016/j.ccell.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 93.Hao W, Chang CP, Tsao CC, Xu J. Oligomycin-induced bioenergetic adaptation in cancer cells with heterogeneous bioenergetic organization. J Biol Chem. 2010;285:12647–12654. doi: 10.1074/jbc.M109.084194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jose C, Bellance N, Rossignol R. Choosing between glycolysis and oxidative phosphorylation: A tumor's dilemma? Biochim Biophys Acta. 2011;1807:552–561. doi: 10.1016/j.bbabio.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 95.Birsoy K, Possemato R, Lorbeer FK, Bayraktar EC, Thiru P, Yucel B, Wang T, Chen WW, Clish CB, Sabatini DM. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature. 2014;508:108–112. doi: 10.1038/nature13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dykens JA, Jamieson J, Marroquin L, Nadanaciva S, Billis PA, Will Y. Biguanide-induced mitochondrial dysfunction yields increased lactate production and cytotoxicity of aerobically-poised HepG2 cells and human hepatocytes in vitro. Toxicol Appl Pharmacol. 2008;233:203–210. doi: 10.1016/j.taap.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 97.Hou XB, Li TH, Ren ZP, Liu Y. Combination of 2-deoxy d-glucose and metformin for synergistic inhibition of non-small cell lung cancer: A reactive oxygen species and P-p38 mediated mechanism. Biomed Pharmacother. 2016;84:1575–1584. doi: 10.1016/j.biopha.2016.10.037. [DOI] [PubMed] [Google Scholar]
- 98.Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, Pistoia V, Wei M, Hwang S, Merlino A, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med. 2012;4:124ra27. doi: 10.1126/scitranslmed.3003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Javeshghani S, Zakikhani M, Austin S, Bazile M, Blouin MJ, Topisirovic I, St-Pierre J, Pollak MN. Carbon source and myc expression influence the antiproliferative actions of metformin. Cancer Res. 2012;72:6257–6267. doi: 10.1158/0008-5472.CAN-12-2907. [DOI] [PubMed] [Google Scholar]
- 100.Vernieri C, Signorelli D, Galli G, Ganzinelli M, Moro M, Fabbri A, Tamborini E, Marabese M, Caiola E, Broggini M, et al. Exploiting FAsting-mimicking Diet and MEtformin to improve the efficacy of platinum-pemetrexed chemotherapy in advanced LKB1-inactivated lung adenocarcinoma: The FAME Trial. Clin Lung Cancer. 2019;20:e413–e417. doi: 10.1016/j.cllc.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 101.Arrieta O, Barrón F, Padilla M, Avilés-Salas A, Ramírez-Tirado L, Arguelles Jiménez M, Vergara E, Zatarain-Barrón Z, Hernández-Pedro N, Cardona A, et al. Effect of metformin plus tyrosine kinase inhibitors compared with tyrosine kinase inhibitors alone in patients with epidermal growth factor receptor-mutated lung adenocarcinoma: A phase 2 randomized clinical trial. JAMA Oncol. 2019;5:e192553. doi: 10.1001/jamaoncol.2019.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Afzal MZ, Dragnev K, Sarwar T, Shirai K. Clinical outcomes in non-small-cell lung cancer patients receiving concurrent metformin and immune checkpoint inhibitors. Lung Cancer Manag. 2019;8:LMT11. doi: 10.2217/lmt-2018-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang J, Jiao K, Liu J, Xia Y. Metformin reverses the resistance mechanism of lung adenocarcinoma cells that knocks down the Nrf2 gene. Oncol Lett. 2018;16:6071–6080. doi: 10.3892/ol.2018.9382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang J, Wang Y, Han J, Mei H, Yu D, Ding Q, Zhang T, Wu G, Peng G, Lin Z. Metformin attenuates radiation-induced pulmonary fibrosis in a murine model. Radiat Res. 2017;188:105–113. doi: 10.1667/RR14708.1. [DOI] [PubMed] [Google Scholar]
- 105.Huang S, He T, Yang S, Sheng H, Tang X, Bao F, Wang Y, Lin X, Yu W, Cheng F, et al. Metformin reverses chemoresistance in non-small cell lung cancer via accelerating ubiquitination-mediated degradation of Nrf2. Transl Lung Cancer Res. 2020;9:2337–2355. doi: 10.21037/tlcr-20-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang X, Chen K, Yu Y, Xiang Y, Kim JH, Gong W, Huang J, Shi G, Li Q, Zhou M, et al. Metformin sensitizes lung cancer cells to treatment by the tyrosine kinase inhibitor erlotinib. Oncotarget. 2017;8:109068–109078. doi: 10.18632/oncotarget.22596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jacobi O, Landman Y, Reinhorn D, Icht O, Sternschuss M, Rotem O, Finkel I, Allen AM, Dudnik E, Goldstein DA, Zer A. The relationship of diabetes mellitus to efficacy of immune checkpoint inhibitors in patients with advanced non-small cell lung cancer. Oncology. 2021;99:555–561. doi: 10.1159/000516671. [DOI] [PubMed] [Google Scholar]
- 108.Luo X, Chen X, Wang L, Yang B, Cai S. Metformin adjunct with antineoplastic agents for the treatment of lung cancer: A meta-analysis of randomized controlled trials and observational cohort studies. Front Pharmacol. 2021;12:639016. doi: 10.3389/fphar.2021.639016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Skinner H, Hu C, Tsakiridis T, Santana-Davila R, Lu B, Erasmus JJ, Doemer AJ, Videtic GMM, Coster J, Yang AX, et al. Addition of metformin to concurrent chemoradiation in patients with locally advanced non-small cell lung cancer: The NRG-LU001 phase 2 randomized clinical trial. JAMA Oncol. 2021;7:1324–1332. doi: 10.1001/jamaoncol.2021.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tsakiridis T, Pond GR, Wright J, Ellis PM, Ahmed N, Abdulkarim B, Roa W, Robinson A, Swaminath A, Okawara G, et al. Metformin in combination with chemoradiotherapy in locally advanced non-small cell lung cancer: The OCOG-ALMERA randomized clinical trial. JAMA Oncol. 2021;7:1333–1341. doi: 10.1001/jamaoncol.2021.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Eikawa S, Nishida M, Mizukami S, Yamazaki C, Nakayama E, Udono H. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci USA. 2015;112:1809–1814. doi: 10.1073/pnas.1417636112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim SH, Li M, Trousil S, Zhang Y, Pasca di Magliano M, Swanson KD, Zheng B. Phenformin inhibits myeloid-derived suppressor cells and enhances the anti-tumor activity of PD-1 blockade in melanoma. J Invest Dermatol. 2017;137:1740–1748. doi: 10.1016/j.jid.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 113.Scharping NE, Menk AV, Whetstone RD, Zeng X, Delgoffe GM. Efficacy of PD-1 blockade is potentiated by metformin-induced reduction of tumor hypoxia. Cancer Immunol Res. 2017;5:9–16. doi: 10.1158/2326-6066.CIR-16-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang JC, Sun X, Ma Q, Fu GF, Cong LL, Zhang H, Fan DF, Feng J, Lu SY, Liu JL, et al. Metformin's antitumour and anti-angiogenic activities are mediated by skewing macrophage polarization. J Cell Mol Med. 2018;22:3825–3836. doi: 10.1111/jcmm.13655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kubo T, Ninomiya T, Hotta K, Kozuki T, Toyooka S, Okada H, Fujiwara T, Udono H, Kiura K. Study protocol: Phase-Ib trial of nivolumab combined with metformin for refractory/recurrent solid tumors. Clin Lung Cancer. 2018;19:e861–e864. doi: 10.1016/j.cllc.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 116.Granja S, Marchiq I, Le Floch R, Moura CS, Baltazar F, Pouysségur J. Disruption of BASIGIN decreases lactic acid export and sensitizes non-small cell lung cancer to biguanides independently of the LKB1 status. Oncotarget. 2015;6:6708–6721. doi: 10.18632/oncotarget.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morgillo F, Sasso FC, Della Corte CM, Festino L, Manzo A, Martinelli E, Troiani T, Capuano A, Ciardiello F. Metformin in lung cancer: Rationale for a combination therapy. Expert Opin Investig Drugs. 2013;22:1401–1409. doi: 10.1517/13543784.2013.828691. [DOI] [PubMed] [Google Scholar]
- 118.Reinfeld BI, Madden MZ, Wolf MM, Chytil A, Bader JE, Patterson AR, Sugiura A, Cohen AS, Ali A, Do BT, et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature. 2021;593:282–288. doi: 10.1038/s41586-021-03442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.