Abstract
The 5-year disease-free survival (DFS) of NCI high-risk (HR) B-lymphoblastic leukemia (B-ALL) patients with end of induction (EOI) MRD≥0.1% and end of consolidation (EOC) MRD≥0.01% is 39±7%, warranting consideration of hematopoietic stem cell transplant (HSCT). However, the impact of EOC MRD in NCI standard-risk (SR) B-ALL patient using COG regimens is unknown. We found that SR patients with MRD≥0.01% at both EOI and EOC have a 4-year DFS/overall survival of 72.9%±19.0%/91.7±10.8% versus 90.7%±2.9%/95.5±2.0% (P=0.0019/0.25) for those with EOI MRD≥0.01% and EOC MRD<0.01%. These data suggest that routine use of HSCT may not be warranted in EOC MRD≥0.01%SR patients.
Introduction
The most powerful risk predictor in pediatric B-lymphoblastic leukemia (B-ALL) is end induction (EOI) minimal residual disease (MRD)1–9, which is routinely incorporated into ALL risk stratification schemes1–6,8–18. Detectable MRD at later time points is consistently associated with poor outcome3,6,10,11,15,16,18,19. NCI high risk (HR) B-ALL patients treated on Children’s Oncology Group (COG) AALL0232 with MRD ≥0.1% at EOI and ≥0.01% at end of consolidation (EOC) had a post-consolidation 5-year disease-free survival (DFS) of 39±7% compared to 79±5% for EOI MRD-positive patients with EOC MRD <0.01%20. Thus, EOC MRD-positive HR patients warrant consideration of alternative therapies, including allogeneic hematopoietic stem cell transplant (HSCT) or chimeric antigen receptor T-cell therapy (NCT03876769).
Most studies examining EOC MRD have not separately evaluated NCI standard risk (SR) patients. Importantly, the prognostic impact of EOC MRD-positivity in COG SR B-ALL trials has not been previously examined. Extrapolating data from other studies to these patients may not be appropriate because, compared to most other consortia, COG uses less intensive induction therapy for SR patients.
We hypothesized that SR patients with MRD levels ≥0.01% at both EOI and EOC would have inferior DFS compared to EOI MRD-positive who were MRD-negative at EOC, and report here outcome data to address this hypothesis.
METHODS
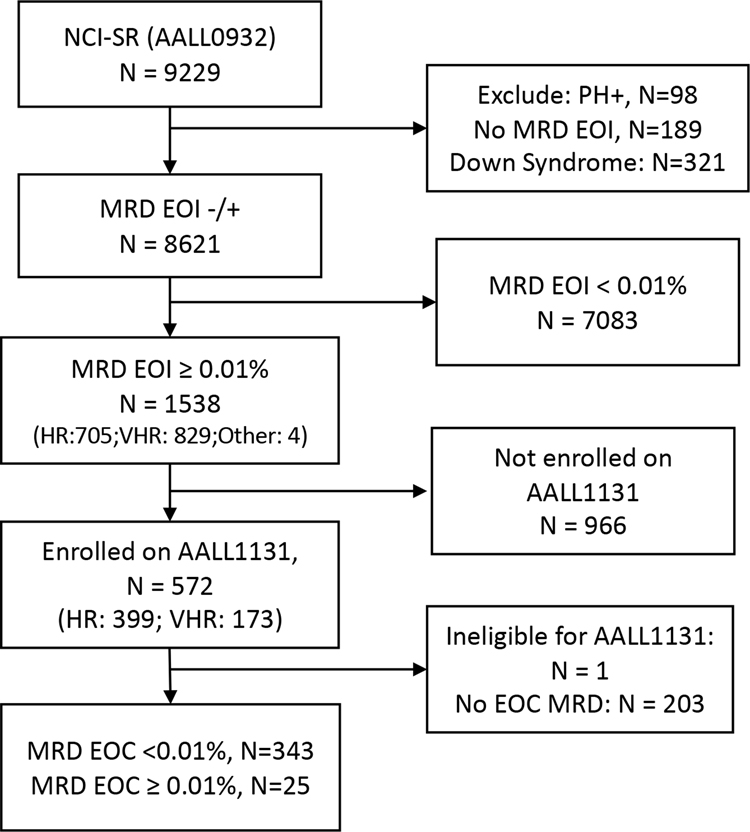
Patients
The study population included children with SR (age 1–9.99 years and initial white blood cell count <50,000/μL) B-ALL who received induction therapy on COG AALL093221 with EOI flow cytometry-determined MRD ≥0.01%. These patients were eligible to enroll and receive post-induction therapy on COG AALL113121,22. Between 08/11/2010 and 03/21/2018, AALL0932 enrolled 9,229 eligible B-ALL subjects; 8,621 non-Down syndrome Philadelphia chromosome-negative subjects had EOI MRD reported (Figure 1). Marrow EOI MRD was ≥0.01% in 1,538(17.8%); because of multiple AALL1131 temporary closures, only 572/1,538 enrolled on AALL1131 for post-induction therapy21,22 (see Supplemental Methods). EOC MRD was optional on AALL1131 and was reported for 368 EOI MRD-positive SR patients, who form the basis of this report. The median follow-up time from enrollment to last contact/death/withdrawal for these 368 patients is 1240.5 days. There were no significant differences in patient characteristics between SR EOI MRD-positive subjects who did/did not report EOC MRD (Supplemental Table S1).
Fig 1:
CONSORT Diagram
MRD
Flow cytometric EOC MRD was determined in the COG central reference labs (University of Washington [BLW] or Johns Hopkins [MJB]) as previously described20.
Additional Methods described in Supplemental Material.
RESULTS
Among 368 SR patients with EOI MRD≥0.01%, 343 (93.2%) had EOC MRD<0.01%, whereas 25 (6.8%) remained MRD-positive at the 0.01% threshold. Comparing the characteristics of EOI MRD ≥0.01% patients by EOC MRD status, only EOI MRD level (0.01–0.1% versus ≥0.1%) was significantly different on the basis of EOC MRD (P=0.0002) (Supplemental Table S2). Logistic regression analysis showed that higher EOI MRD was strongly associated with likelihood of remaining MRD-positive at EOC, with an odds ratio of 4.2 (95% confidence limits 1.6–11.3, P <0.001) for those with EOI MRD ≥1% versus 0.1–0.99% and an odds ratio of 3.8 (95% confidence limit 1.3–11.1, P <0.001) for patients with EOI MRD 0.1–0.99% versus 0.01–0.099% (Supplemental Table S3). The EOC MRD-positive rates did not differ significantly by cytogenetic subset: 3/25 (12%) with unfavorable cytogenetics (KMT2A-rearranged, intrachromosomal amplification of chromosome 21 (iAMP21), or hypodiploidy with <44 chromosomes), 14/222 (6.3%) with favorable cytogenetics (ETV6/RUNX1 fusion or double trisomies of chromosomes 4 and 10), and 8/121 (6.6%) with neutral cytogenetics (P=0.529) (Supplemental Tables S2, S4).
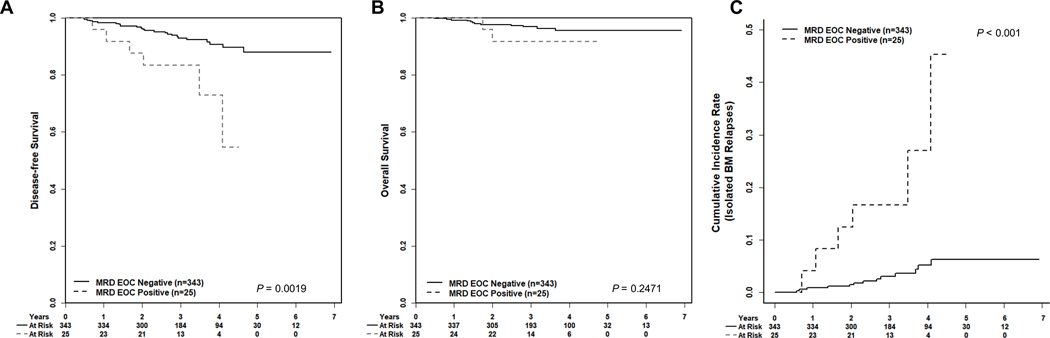
Post-induction treatment arm on AALL1131 did not impact DFS (P=0.95) for SR patients with EOI MRD ≥0.01%. The EOC MRD-positive rate was not significantly different between the various treatment regimens on the AALL1131 high risk and very high risk sub-studies (Supplemental Table S5)21,22. The SR patients with MRD≥0.01% at both EOI and EOC had a significantly worse 4-year DFS compared to EOI MRD-positive patients with EOC MRD <0.01% (72.9±19.0% versus 90.7±2.9%; P=0.0019) (Figure 2A), but 4-year overall survival (OS) was excellent and not significantly different from those with EOC MRD<0.01% (91.7±10.8% versus 95.5±2.0%, P=0.25) (Figure 2B). The 4-year cumulative incidence rate of isolated marrow relapse for EOI MRD-positive patients was 27.1±12.5% for those with EOC MRD ≥0.01% compared to 5.2±1.6% for those with EOC MRD <0.01% (P<0.001) (Figure 2C). Central nervous system (CNS) relapse was rare occurring in 0/25 EOC MRD-positive patients and 6/343 EOC MRD-negative patients, (4 isolated CNS relapse and 2 combined marrow/CNS relapse) (Supplemental Figure S1). Five of the 6 EOC MRD-positive patients who relapsed had EOI MRD ≥0.1%, suggesting those with higher EOI disease burden are at highest risk for relapse (Supplemental Table S6). Given the association between higher EOI MRD and likelihood of having MRD ≥0.01% at EOC, we analyzed the prognostic value of EOC MRD in patients with high (≥0.1%) EOI MRD. Among those with EOI MRD ≥0.1%, the 4-year DFS was 88.8±5.1% for those with EOC MRD <0.01% (N=141) versus 67.7±22.2% for those with EOC MRD ≥0.01% (N=20) (P=0.0247), demonstrating the prognostic import of EOC MRD even among patients with high EOI MRD (Supplemental Figure S2A).
Fig 2: Disease-free survival (DFS); Overall survival (OS); and cumulative incidence of relapse for NCI SR B-ALL EOI MRD-positive patients by MRD at EOC.
A) 4-year DFS ± SE for EOC MRD<0.01% versus EOC MRD ≥0.01%, 90.7± 2.9%, and 72.9 ± 19.0%, respectively. B) 4-year OS for EOC MRD <0.01% versus EOC MRD ≥0.01%, 95.5 ± 2.0% and 91.7 ± 10.8%, respectively. C) 4-year cumulative incidence of isolated bone marrow relapses for EOC MRD <0.01% versus EOC MRD≥0.01%, 27.1 ± 12.5% and 5.2 ±1.6%, respectively.
With recent data suggesting genotype-specific impact of EOI MRD on outcome23, we attempted to investigate the prognostic impact of EOC MRD on the basis of genetic subtype. For the entire cohort of AALL0932 SR EOI MRD-positive patients, genotype significantly impacted outcome with 5-year DFS of 89.6±10%, 84.3±8, and 70.7±19 for patients with favorable, neutral, and unfavorable cytogenetics, respectively (P=0.0004). For those with EOC MRD ≥0.01%, 4/22 with favorable or neutral cytogenetics relapsed, 2/2 patients with iAMP21 relapsed, and 1 with KMT2A rearrangement did not (Supplemental Table S6). While this suggests persistent EOC MRD may be an adverse prognostic factor in SR patients, particularly those with unfavorable cytogenetics, small patient numbers preclude definitive conclusions.
An important limitation of this study is that post-consolidation therapy was not uniform among the EOC MRD-positive patients. Eleven of the 25 EOC MRD-positive patients completed protocol therapy, 2 of those relapsed off therapy, and both remain alive. One patient remains on protocol therapy (maintenance). Thirteen came off protocol therapy for alternative treatment: 8 underwent HSCT in first CR (CR1), one of whom relapsed and died; 4 received alternative therapy without HSCT in CR1, 3 of these relapsed, and one died; and 1 has no follow-up data available (Supplemental Table S6).
DISCUSSION
While rare, EOC MRD-positive SR B-ALL patients present a clinical dilemma given a paucity of existing outcome data. We found that SR B-ALL patients treated on COG protocols who are EOI MRD-positive (≥0.01%) and remain MRD-positive at EOC have significantly inferior DFS but similar OS compared to those who are MRD-negative by EOC. The SR EOI MRD-positive patients who had EOC MRD <0.01% appeared to have superior outcomes to their NCI HR counterparts (4-year DFS 90.7±2.9% on this study vs. 4-year DFS 80.2±4.0% reported previously from COG AALL0232)20. Importantly, the outcomes for EOC MRD-positive SR patients (4-year DFS 72.9±19.0%) appear to be better than those of EOC MRD-positive HR B-ALL patients (4-year DFS 55.4±6.8%). Notably, the 4-year OS of 91.7±10.8% for the SR EOC MRD-positive patients treated with a variety of different approaches suggests that many of these patients can be salvaged if they relapse. Prospective analyses of larger, uniformly-treated patient cohorts with a longer duration of follow up are needed to reach definitive conclusions. However, these results suggest that while novel therapeutic approaches warrant testing in patients with EOC MRD-positive SR B-ALL, the routine use of HSCT in first CR may not be warranted.
Supplementary Material
Acknowledgements
This work was supported by grants U10 CA98543, U10 CA98413, U10 CA180886, 1U24-CA196173 and U10 CA180899 from the National Institutes of Health as well as funding from St Baldrick’s Foundation. Sanofi and Bristol-Myers Squibb were industry sponsors of the therapeutic trials described here. EAR is a KiDS of NYU Foundation Professor at NYU Langone Health. MLL is a UCSF Benioff Chair of Children’s Health and Deborah and Arthur Ablin Endowed Chair in Pediatric Molecular Oncology. SPH is the Jeffrey E. Perelman Distinguished Chair in the Department of Pediatrics at the Children’s Hospital of Philadelphia.
Conflict of Interest
RER is a consultant for Jazz and Servier. PZ-M is a paid employee of Immunogen. MJBu is a consultant and speaker for Jazz and Amgen and speaker for Servier. SPH has received consulting fees from Novartis, honoraria from Amgen, and owns common stock in Amgen. EAR serves on a DSMB for Celgene and receives research funding (institutional) from Pfizer. MLL is a consultant to MediSix Therapeutics. BLW has received honoraria from Amgen, Seattle Genetics, AbbVie, and Janssen Pharmaceuticals, has research funding (institutional) from Amgen, Seattle Genetics, Pfizer, Juno Therapeutics, BiolineRx, Biosight, and Stemline Therapeutics. MJBo is on the scientific advisory board for Amgen. All other authors have no conflicts of interest to report.
Abbreviations
- B-ALL
B lymphoblastic leukemia
- ALL
Acute lymphoblastic leukemia
- PCR
Polymerase chain reaction
- COG
Children’s Oncology Group
- HSCT
Hematopoietic stem cell transplant
- NCI
National Cancer Institute
- SR
Standard Risk
- HR
High Risk
- MRD
Minimal residual disease
- EOI
End of induction
- EOC
End of consolidation
- DFS
Disease-free survival
- OS
Overall survival
- CNS
Central nervous system
- CR
Complete remission
References
- 1.Borowitz MJ, Pullen DJ, Shuster JJ, et al. Minimal residual disease detection in childhood precursor-B-cell acute lymphoblastic leukemia: relation to other risk factors. A Children’s Oncology Group study. Leukemia. 2003;17(8):1566–1572. [DOI] [PubMed] [Google Scholar]
- 2.Borowitz MJ, Devidas M, Hunger SP, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s Oncology Group study. Blood. 2008;111(12):5477–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cave H, van der Werff ten Bosch J, Suciu S, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia. European Organization for Research and Treatment of Cancer--Childhood Leukemia Cooperative Group. N Engl J Med. 1998;339(9):591–598. [DOI] [PubMed] [Google Scholar]
- 4.Schumich A, Maurer-Granofszky M, Attarbaschi A, et al. Flow-cytometric minimal residual disease monitoring in blood predicts relapse risk in pediatric B-cell precursor acute lymphoblastic leukemia in trial AIEOP-BFM-ALL 2000. Pediatr Blood Cancer. 2019;66(5):e27590. [DOI] [PubMed] [Google Scholar]
- 5.van Dongen JJ, Seriu T, Panzer-Grumayer ER, et al. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet. 1998;352(9142):1731–1738. [DOI] [PubMed] [Google Scholar]
- 6.Paganin M, Fabbri G, Conter V, et al. Postinduction minimal residual disease monitoring by polymerase chain reaction in children with acute lymphoblastic leukemia. J Clin Oncol. 2014;32(31):3553–3558. [DOI] [PubMed] [Google Scholar]
- 7.Stow P, Key L, Chen X, et al. Clinical significance of low levels of minimal residual disease at the end of remission induction therapy in childhood acute lymphoblastic leukemia. Blood. 2010;115(23):4657–4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vrooman LM, Blonquist TM, Harris MH, et al. Refining risk classification in childhood B acute lymphoblastic leukemia: results of DFCI ALL Consortium Protocol 05–001. Blood Adv. 2018;2(12):1449–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou J, Goldwasser MA, Li A, et al. Quantitative analysis of minimal residual disease predicts relapse in children with B-lineage acute lymphoblastic leukemia in DFCI ALL Consortium Protocol 95–01. Blood. 2007;110(5):1607–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conter V, Bartram CR, Valsecchi MG, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115(16):3206–3214. [DOI] [PubMed] [Google Scholar]
- 11.Escherich G, Horstmann MA, Zimmermann M, Janka-Schaub GE, group Cs. Cooperative study group for childhood acute lymphoblastic leukaemia (COALL): long-term results of trials 82,85,89,92 and 97. Leukemia. 2010;24(2):298–308. [DOI] [PubMed] [Google Scholar]
- 12.Schmiegelow K, Forestier E, Hellebostad M, et al. Long-term results of NOPHO ALL-92 and ALL-2000 studies of childhood acute lymphoblastic leukemia. Leukemia. 2010;24(2):345–354. [DOI] [PubMed] [Google Scholar]
- 13.Vora A, Goulden N, Mitchell C, et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2014;15(8):809–818. [DOI] [PubMed] [Google Scholar]
- 14.Vora A, Goulden N, Wade R, et al. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2013;14(3):199–209. [DOI] [PubMed] [Google Scholar]
- 15.Yamaji K, Okamoto T, Yokota S, et al. Minimal residual disease-based augmented therapy in childhood acute lymphoblastic leukemia: a report from the Japanese Childhood Cancer and Leukemia Study Group. Pediatr Blood Cancer. 2010;55(7):1287–1295. [DOI] [PubMed] [Google Scholar]
- 16.Yeoh AE, Ariffin H, Chai EL, et al. Minimal residual disease-guided treatment deintensification for children with acute lymphoblastic leukemia: results from the Malaysia-Singapore acute lymphoblastic leukemia 2003 study. J Clin Oncol. 2012;30(19):2384–2392. [DOI] [PubMed] [Google Scholar]
- 17.Campana D, Pui CH. Minimal residual disease-guided therapy in childhood acute lymphoblastic leukemia. Blood. 2017;129(14):1913–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pui CH, Pei D, Coustan-Smith E, et al. Clinical utility of sequential minimal residual disease measurements in the context of risk-based therapy in childhood acute lymphoblastic leukaemia: a prospective study. Lancet Oncol. 2015;16(4):465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flohr T, Schrauder A, Cazzaniga G, et al. Minimal residual disease-directed risk stratification using real-time quantitative PCR analysis of immunoglobulin and T-cell receptor gene rearrangements in the international multicenter trial AIEOP-BFM ALL 2000 for childhood acute lymphoblastic leukemia. Leukemia. 2008;22(4):771–782. [DOI] [PubMed] [Google Scholar]
- 20.Borowitz MJ, Wood BL, Devidas M, et al. Prognostic significance of minimal residual disease in high risk B-ALL: a report from Children’s Oncology Group study AALL0232. Blood. 2015;126(8):964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burke MJ, Salzer WL, Devidas M, et al. Replacing cyclophosphamide/cytarabine/mercaptopurine with cyclophosphamide/etoposide during consolidation/delayed intensification does not improve outcome for pediatric B-cell acute lymphoblastic leukemia: a report from the COG. Haematologica. 2019;104(5):986–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salzer WL, Burke MJ, Devidas M, et al. Toxicity associated with intensive postinduction therapy incorporating clofarabine in the very high-risk stratum of patients with newly diagnosed high-risk B-lymphoblastic leukemia: A report from the Children’s Oncology Group study AALL1131. Cancer. 2018;124(6):1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Connor D, Enshaei A, Bartram J, et al. Genotype-Specific Minimal Residual Disease Interpretation Improves Stratification in Pediatric Acute Lymphoblastic Leukemia. J Clin Oncol. 2018;36(1):34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.