Abstract
Background
Reports on COVID-19 skin manifestations and associated clinical outcomes are limited. Like viral diseases, cutaneous findings may be present and can help in confirmation and prognostication among those suspected or diagnosed with COVID-19.
Objective
To determine COVID-19 cutaneous manifestations and their association with disease severity and course.
Methods
This study was conducted in a designated COVID-19 referral hospital from January 1 to March 31, 2021. Skin manifestations recorded from January 1 to February 17 were retrospectively gathered. Reports from February 18 to March 31 were prospectively collected using a dermatologic checklist which was incorporated into all official medical records.
Results
A total of 507 confirmed patients with COVID-19 were included. COVID-19 skin signs were detected in 39 patients (7.7%). Morbilliform lesions were most common. Skin signs were significantly associated with severe or critical cases (odds ratio, 3.4; 95% CI, 1.3-8.7) and mortality (relative risk, 2.9; 95% CI, 2.0-4.2).
Limitations
Underestimation of prevalence of COVID-19 skin signs due to exclusion of outpatient and discharged patients and the subjective assessment in the retrospective part.
Conclusion
Cutaneous signs were significantly associated with severe/critical COVID-19 as well as death among 507 hospitalized patients in a Philippine COVID-19 referral hospital.
Key words: clinical outcome, COVID-19, cutaneous manifestations, disease severity, skin
Capsule Summary.
-
•
Worldwide, COVID-19 cutaneous signs have been documented, but reports are still limited. Association of skin signs to disease course and severity needs to be further investigated.
-
•
Documentation of COVID-19 skin signs contributes to the growing knowledge base of the various clinical features of this disease.
Introduction
In Wuhan, China, in late 2019, cases of pneumonia with unknown etiology were being reported. By the end of January 2020, cases were already reported outside China.1, 2, 3, 4, 5 In March 2020, COVID-19 was declared a pandemic.
In the Philippines, new cases were noted between January and March 2020. In response, the Department of Health in the Philippines designated institutions as official COVID-19 referral hospitals. By March 30, 2020, the University of the Philippines – Philippine General Hospital began its COVID-19 operations. Since reverse transcription polymerase chain reaction testing was still limited due to lack of kits and facilities, diagnosis was made through symptomatology, probable exposure history, other diagnostics, and imaging.
Although complaints were mainly respiratory and gastrointestinal, there were few reports on COVID-19 dermatologic manifestations. Viral infections, like COVID-19, may present with skin signs that can help confirm diagnosis. Dermatologic signs and symptoms may give an uncommon perspective and correlation to disease course and prognostication.
During protocol development, there was still scarcity of evidence on COVID-19 cutaneous manifestations. A literature search done through the PubMed database using the following search terms generated a collection of anecdotal evidence: “Coronavirus 19”, “COVID-19”, “SARS Cov-2”, “skin”, “cutaneous”, and “dermatologic” (Table I5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18).
Table I.
COVID-19 cutaneous manifestations in literature
| Skin sign morphology |
|---|
| Livedoid eruptions,7 livedo reticularis,10 livedo or necrosis14 |
| Erythematous rash,8 maculopapular eruptions,13, 14 papulosquamous rash15 |
| Urticaria,8 urticarial eruption/lesions11, 14 |
| Petechial rash,6,13 purpuric macules9 |
| Vesicles,8 vesicular eruptions,14 varicella-like/papulo-vesicular rash17 |
| Pruritic papules on bilateral heels12; acral areas of erythema, vesicles, or pustules/pseudo-chilblain14; acral ischemia16,18 |
| Unspecified rash5 |
This study aimed to document cutaneous manifestations among patients with COVID-19 and to determine associations between skin manifestations and disease severity and clinical outcomes.
Methods
This study was conducted in the University of the Philippines – Philippine General Hospital, a COVID-19 referral hospital in Metro Manila. There were 2 parts: a preliminary retrospective cross-sectional study of all COVID-19 inpatient records from January 1 to February 17, 2021, and a subsequent prospective cohort study of admitted patients with COVID-19 from February 18 to March 31, 2021. The study protocol was approved by the institution’s research ethics board.
COVID-19 cases, of any sex and age group, and disease severity types were included in the retrospective study. All patient records were retrieved using standard data collection forms with strict selection criteria. Records which did not meet at least 30% of the criteria were excluded. Information extracted were demographics; clinical data, that is, disease severity (mild/moderate/severe/critical); comorbidities; clinical outcome (good/improved/discharged or poor/deceased); and all dermatologic signs and symptoms. Case definitions were adapted from the Philippine Society for Microbiology and Infectious Diseases Interim Guidance.19
The prospective part of the study included all admitted COVID-19-confirmed patients and excluded probable and suspect cases. A dermatologic checklist was developed based on current literature of the COVID-19 cutaneous presentations (Table I). These presentations were categorized according to morphology: morbilliform rash, pernio or chilblains, urticarial, vesicular, petechial, and livedo reticularis. Patients with preexisting dermatologic conditions or predisposing comorbidities with cutaneous signs and symptoms were categorized as “others.” The dermatologic checklist was approved by the Hospital Infection Control Unit, Department of Medicine, and COVID-19 Clearing House, and was incorporated into all official COVID-19 medical records.
Demographic, clinical, and the dermatologic checklist data were likewise extracted. Documentation of cutaneous manifestations was done by the health care workers attending in the COVID-19 wards. An infographic on the identification of dermatologic findings was provided in the wards for reference.
For patients warranting dermatologic management, the attending physicians sent official teledermatology referrals to the Department of Dermatology. The investigators secured informed consent from the patient or a legally-authorized representative (for incapacitated patients) either through face-to-face or phone call. Teledermatology followed a standard format of confidentiality and privacy protocols on photography of skin lesions and patient information. In-person dermatologic assessment and management were executed when deemed necessary and subsequently documented in the electronic medical records.
Data from the 2 study parts underwent independent analyses. Outcomes of interest for both parts were age, COVID-19 disease severity at the time of appearance of skin signs and symptoms, cutaneous manifestations (morphology), and clinical outcome.
Categorical variables (ie, age, disease severity, clinical outcome) were reported as numbers and percentages of patients.
Pearson’s χ2 test or Fisher’s exact test were used to determine associations between skin signs and age, disease severity, clinical outcome. The latter was used when more than 20% of the cells had an expected value of less than or equal to 5. Furthermore, each cutaneous morphology was statistically analyzed for association with disease severity and clinical outcome.
A bivariate analysis was done to estimate the odds of severe/critical disease and poor outcome given the presence or absence of a cutaneous sign. The crude odds ratios were presented along with their 95% confidence intervals (CIs) and P values. For the prospective cohort, relative risk and 95% CI of skin signs and poor outcome (ie, mortality) were calculated. STATA 16 (StataCorp LLC) and an online evidence-based medicine calculator (https://ebm-tools.knowledgetranslation.net/calculator/prospective/) were used for data analyses. Cases which necessitated in-person dermatologic assessment and management were presented as brief descriptive reports.
Results
A total of 507 patients (252 men and 255 women) were included in this study. The mean age was 53.0 years (SD, 17.6; range, 0-94 years).
Part 1: Retrospective chart review
Of the 152 records reviewed, 96.30% were adults and there was an almost equal proportion of mild/moderate cases and severe/critical cases. Only 27 patients (17.8%) presented with skin lesions, of which 13 (8.6%) showed the reported COVID-19 skin signs. The morbilliform rash was the most common sign (Table II).
Table II.
Frequency distribution of skin signs by morphology
| Skin sign morphology∗ | Part 1: Retrospective |
Part 2: Prospective |
||||
|---|---|---|---|---|---|---|
| COVID-19 cases (n = 27, N = 152) |
COVID-19 cases (n = 43, N = 355) |
Total (n = 70, N = 507) |
||||
| No. | % | No. | % | No. | % | |
| Morbilliform rash | 7 | 4.6 | 10 | 2.8 | 17 | 3.4 |
| Livedo reticularis | 1 | 0.7 | 6 | 1.7 | 7 | 1.4 |
| Petechial rash | 3 | 2.0 | 3 | 0.9 | 6 | 1.2 |
| Vesicular rash | 1 | 0.7 | 3 | 0.9 | 4 | 0.8 |
| Urticaria | 0 | 0.0 | 3 | 0.9 | 3 | 0.6 |
| Pernio or chilblains | 1 | 0.7 | 1 | 0.3 | 2 | 0.4 |
| Others | 14 | 9.2 | 17 | 4.8 | 31 | 6.1 |
A patient may have more than 1 skin sign reported.
Part 2: Prospective cohort
There were 355 patients included in this cohort, and the majority (97.46%) was adults. Mean age was 52.9 years old (SD, 17.6). Forty-three patients (12.1%) presented with skin lesions, and only 26 patients (7.3%) presented with the reported COVID-19 skin signs. Majority were severe/critical (59.3%). The morbilliform morphology was still most common, followed by livedo reticularis. Urticarial, petechial, vesicular, and pernio lesions were also documented (Table II).
Association of COVID-19 cutaneous signs to disease severity and clinical outcomes
Separate analyses for the retrospective and prospectively collected data were done. In both parts, there was no association between cutaneous manifestations and age.
Skin signs were significantly associated with more severe COVID-19. In the review of records, the odds of having a severe/critical disease among patients with COVID-19 with any cutaneous sign was almost 6 times the odds of those who did not have cutaneous signs (odds ratio, 5.9; 95% CI, 1.3-27.6). This estimate, however, was not precise (ie, wide CI). Furthermore, skin signs were not associated with poor clinical outcomes (ie, death) in this part of the study (odds ratio, 3.5; 95% CI, 1.0-11.8) (Table III).
Table III.
COVID-19 skin signs versus case severity and clinical outcomes (retrospective)
| COVID-19 skin sign | Severity |
Clinical outcome |
||||
|---|---|---|---|---|---|---|
| Severe/critical | Mild/moderate | Odds ratio (95% CI) | Poor | Good | Odds ratio (95% CI) | |
| Present | 11 | 2 | 5.9 (1.3-27.6) | 5 | 8 | 3.5 (1.0-11.8) |
| Absent | 67 | 72 | 21 | 118 | ||
| Total | 78 | 74 | 26 | 126 | ||
However, in the prospective cohort, the odds of having a severe/critical disease among those with any cutaneous sign disease was 3.4 times the odds of those who did not have cutaneous signs (odds ratio, 3.4; 95% CI, 1.3-8.7). Moreover, the risk of death was significantly higher among those with COVID-19 skin signs (relative risk, 2.9; 95% CI, 2.0-4.2) (Table IV).
Table IV.
COVID-19 skin signs versus case severity and clinical outcomes (prospective cohort)
| COVID-19 skin sign | Severity |
Clinical outcome |
||||
|---|---|---|---|---|---|---|
| Severe/critical | Mild/moderate | Odds ratio (95% CI) | Poor | Good | Relative risk (95% CI) | |
| Present | 20 | 6 | 3.4 (1.3-8.7) | 16 | 10 | 2.9 (2.0-4.2) |
| Absent | 163 | 166 | 69 | 260 | ||
| Total | 183 | 172 | 85 | 270 | ||
Due to the relatively low number of cases with COVID-19 skin signs, an association between each cutaneous lesion morphology and disease severity or outcomes was not detected.
Histopathologic examination of inpatient referrals
There were 6 inpatient referrals for in-person dermatologic assessment and management for COVID-related skin signs. Of these, 5 patients had morbilliform lesions and 1 patient had discrete dusky nodules on the abdomen and a perianal plaque (Table V).
Table V.
Summary of inpatient referrals
| Patient | Demographic profile | Case severity | Cutaneous manifestation | Histologic findings | Outcome |
|---|---|---|---|---|---|
| 1 | 63-year-old woman | COVID-19 (+), severe | Morbilliform rash | Superficial and deep perivascular dermatitis, with focal area of vasculitic leukocytoclasia | Discharged |
| 2 | 58-year-old man | COVID-19 (+), severe | Morbilliform rash | Superficial perivascular dermatitis, with focal area of vessels in the papillary dermis with sparse perivascular infiltrates of neutrophils and neutrophilic nuclear dust | Discharged |
| 3 | 64-year-old woman | COVID-19 (+), severe | Morbilliform rash | Superficial perivascular dermatitis with focal leukocytoclastic vasculitis | Discharged |
| 4 | 35-year-old woman | COVID-19 (+), severe | Morbilliform rash | Interface dermatitis | Mortality |
| 5 | 8-year-old boy | COVID-19 (+), severe | (A) Discrete, deep red nodules on the abdomen (B) Well-demarcated perianal plaque |
(A) and (B) Thrombotic vasculopathy | Mortality |
| 6 | 1-year-old boy | COVID-19 (+), severe | Morbilliform rash | Superficial perivascular dermatitis, with no evidence of vasculitis | Mortality |
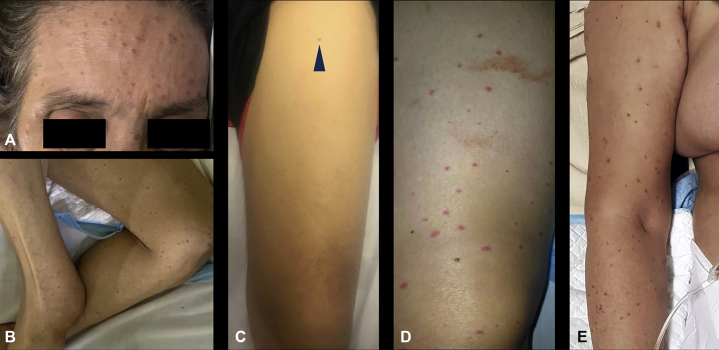
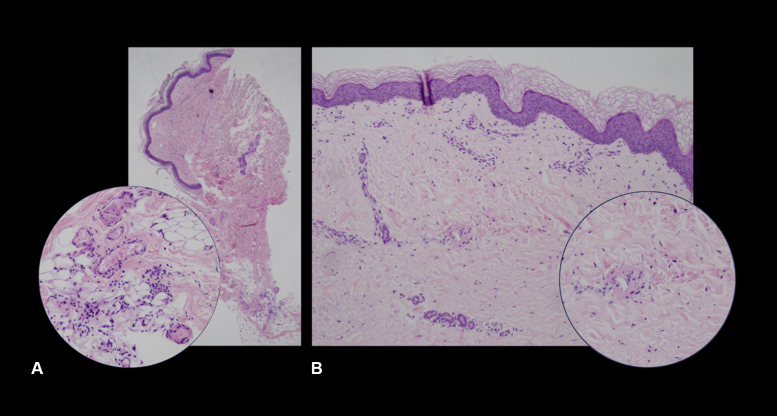
Five of the 6 patients presented with multiple erythematous macules and papules on different areas of the body (Fig 1, A-E). On histopathology, 4 cases showed superficial and superficial to deep perivascular dermatitis. Three of 4 cases had early or beginning leukocytoclastic vasculitis and focal areas of vessels in the papillary dermis with sparse perivascular infiltrates of neutrophils and neutrophilic nuclear dust (Fig 2, A and B). One case showed thrombotic vasculopathy. The last case showed interface dermatitis.
Fig 1.
Clinical cutaneous findings in patients with COVID-19. Multiple erythematous macules and papules spread over the face (A), thighs (B-D), and the upper portion of the arm (E).
Fig 2.
Histopathologic review of 4-mm punch biopsies taken from morbilliform skin eruptions in patients with COVID-19. A, Section showed superficial to deep sparse perivascular and perieccrine lymphocytic infiltrates with rare eosinophils, with inset showing a focal area of mild perivascular infiltrate of neutrophils, neutrophilic dust, and extravasated erythrocytes in the reticular dermis, with fibrin deposition involving a vessel. B, Section showed mild perivascular infiltrates of lymphocytes and occasional neutrophils in the papillary to mid dermis, with inset showing a focal area of extravasated erythrocytes surrounding a vessel with endothelial swelling and fibrin deposition.
Discussion
This study conducted a chart review and prospectively collected data over 3 months from 507 COVID-19 admitted patients in a tertiary medical facility. Significant associations with the presence of skin signs and severe/critical cases and those with poor clinical outcomes (ie, death) among patients with COVID-19 were noted.
Joob et al6 reported on how proper assessment of skin manifestations was useful in the diagnosis of COVID-19. They noted a case in Thailand who was initially diagnosed as dengue fever due to a petechial rash. Only with the presentation of respiratory symptoms was a reverse transcription polymerase chain reaction test done, which was positive for COVID-19.6 Henry et al11 reported a case of urticarial eruption prior to the onset of other classic respiratory features of COVID-19. The initial assessment by a dermatologist was urticaria, which was managed with antihistamines. However, 2 days later, the patient noted fever, chills, and chest pain and subsequently tested positive for COVID-19.11
In Lombardy, Italy, a study by Recalcati8 assessed the presence of cutaneous involvement in 88 patients with COVID-19 and showed that approximately 20% developed different types of skin manifestations, some presenting at disease onset while others after hospitalization. Kaya et al19 reviewed 57 articles and reported that cutaneous involvement in COVID-19 varies between 1.8% and 20.4%. In this present study, a total of 39 out of 507 cases (7.7%) showed cutaneous manifestations among admitted patients with COVID-19, a percentage that falls within the above-mentioned international range.
COVID-19 has been shown to affect any age, with adult predominance, as seen in this study with more than 95% of cases older than 18 years, and a mean age of around 50 years. Currently, there are scarce data on the association of skin manifestations of COVID-19 with age. This study showed no association of cutaneous signs and symptoms with age.
According to Recalcati,8 lesions varied in presentation such as erythematous rash, urticaria, and vesicles. Marzano et al17 stated that skin manifestations were similar to common viral exanthems, close to varicella-like exanthems. Suchonwanit et al20 attempted to classify the cutaneous manifestations into 2 categories, viral exanthems or systemic consequences; the former was related to an immune response to viral nucleotides while the latter was attributed to vasculopathy. There have been anecdotal reports of patients with COVID-19 presenting with transient livedoid eruptions. Manalo et al10 hypothesized that the most plausible etiology could be microthromboses, such as in 2 cases reported of transient unilateral livedo reticularis on the lower extremities with spontaneous resolution within 24 hours. Jimenez-Cauhe et al9 reported a case presenting with multiple erythemato-purpuric macules in the peri-axillary areas on the third day of hospitalization, and Estébanez et al12 presented a case of pruritic erythematous-yellowish papules on bilateral heels after approximately 2 weeks of symptoms. Other dermatologists have suggested that COVID-19 may also present with signs of acral ischemic lesions.16,18 A nationwide case collection survey through a standardized questionnaire and collection of images by Galván Casas et al14 described 5 major cutaneous clinical patterns: pseudo-chilblain, vesicular eruptions, urticarial lesions, macules and papules, and livedo or necrosis. These were noted to present at different times in the disease course and with varied duration and severity.14
To date, several reviews have been done on COVID-19 cutaneous manifestations. However, reports on association of skin manifestations with disease severity and clinical outcome are still scarce. Aside from documentation of COVID-19 skin signs, this study attempted to determine, retrospectively and prospectively, any associations with the cutaneous manifestations. There was a significant association between the presence of COVID-19 skin signs, severe/critical disease, and death. This association may be very helpful to attending physicians, not only in the diagnosis of COVID-19 itself but also in the management and prognostication of the disease.
Inasmuch as the medical field is rapidly gaining knowledge on COVID-19, histopathologic studies of cutaneous manifestations may give a better insight of disease pathophysiology. Alramthan et al16 showed that the presence of parenchymal cell necrosis and hyaline thrombus formation in small vessels of organs could be an underlying pathology for the cutaneous lesions. Diaz-Guimaraens et al13 reported a case with a sudden appearance of erythematous macules, papules, and petechiae in the lower portion of the abdomen and bilateral peri-flexural areas which resolved after 5 days. Superficial perivascular lymphocytic infiltrate with erythrocyte extravasation, focal papillary edema, focal parakeratosis, and isolated dyskeratotic cells with no note of thrombotic vasculopathy was noted on biopsy.13 Sanchez et al15 reported a case of rapidly progressing digitate papulosquamous plaques on the trunk and thighs in an elderly patient with COVID-19, with histology revealing a foci of spongiosis with focal parakeratosis, few spongiotic vesicles with aggregates of lymphocytes and Langerhans cells, and moderate lymphohistiocytic infiltrates and papillary dermal edema. The skin sample was negative for SARS-CoV-2, and the authors noted that the cutaneous manifestations may be secondary to the immune response against the infection.15
A review of clinical and histopathologic features of COVID-19 skin lesions noted that macular and papular eruptions revealed superficial and deep perivascular dermatitis and a vasculitic pattern of lymphocytes cuffing adjacent blood vessels.21 Furthermore, thrombotic vasculopathy of small- and medium-sized vessels was seen in ischemic, livedoid, or necrotic lesions, clinically presenting as painful acral lesions, most commonly as bullae, with development into dry gangrene. Epidermal necrosis was also seen in those lesions.22
In this study, 4 skin biopsies similarly showed superficial and deep perivascular dermatitis with focal areas of vasculitic leukocytoclasia (Fig 2, A) or solely superficial perivascular dermatitis with focal leukocytoclastic vasculitis (Fig 2, B). One biopsy showed thrombotic vasculopathy. These histopathologic findings consisting of lymphocytic invasion, vasculopathy, or endothelial damage leading to the various skin signs may strengthen the possible pathophysiologic mechanisms reported and strongly hypothesized in literature. However, as Genovese et al. noted, none of these hypotheses have been substantiated by strong evidence, and they still need to be largely elucidated.23
Data in this study only involved admitted patients with COVID-19 from January 1 to March 31, 2021. One limitation is the exclusion of outpatient, discharged, or post–COVID-19 patients who may have presented with cutaneous manifestations. This exclusion may lead to underestimation of the prevalence of skin signs and association with the outcomes. Another limitation would be the subjective assessment of health care workers prior to the incorporation of the dermatologic checklist during the retrospective part, which may have led to underreporting of cutaneous involvement of COVID-19.
Conclusion and recommendations
Cutaneous signs were significantly associated with severe/critical COVID-19 as well as poor clinical outcomes among 507 hospitalized patients of the University of the Philippines – Philippine General Hospital. Skin manifestations may not be as frequent in COVID-19, but particular cutaneous signs have been strongly associated with the disease.19,20 Proper routine documentation and epidemiologic cohort studies should be done to further strengthen dermatologic association with COVID-19 among admitted or outpatient cases in the future. A significant association of the cutaneous manifestations with disease severity and clinical outcome in this study may help in diagnosis, management, and prognostication of the disease. The authors recommend that clinicians and the public gain an increased awareness and recognition of cutaneous manifestations. These signs may appear even before confirmation of COVID-19 and may lead to early avoidance of unnecessary exposure and transmission of the infection.
Conflicts of interest
None disclosed.
Acknowledgments
We thank the following individuals for their great assistance and invaluable work: Zuriel Alkian C. Tan, MD for data acquisition and organization; Al Joseph R. Molina, MD for statistical advice and analysis; Marie Len C. Balmores, MD for dermatopathology discussions; Zimri C. Tan, MD for writing suggestions and feedback; and Roy Luister C. Acos, MD, Koreen Blossom T. Chan, MD, Erickah Mary Therese R. Dy, MD, Soraya Elisse E. Escandor, MD, Doha Mae Laurisse M. Manalo, MD, Raisa Celine R. Rosete, MD, Nicole Marella G. Tan, MD and Marian Rosel D. Villaverde, MD for contributions to patient management and infographic design.
Footnotes
Funding sources: None.
IRB approval status: Reviewed and approved by the research ethics board of the University of the Philippines – Philippine General Hospital.
References
- 1.2019 novel coronavirus (2019-nCoV), Wuhan, China. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/novel-coronavirus-2019.html
- 2.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi H., Han X., Jiang N., et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Pneumonia of unknown cause in China. January 5, 2020. https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/
- 5.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joob B., Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82(5):e177. doi: 10.1016/j.jaad.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otto A. Skin manifestations are emerging in the Coronavirus pandemic. MDedge: Internal Medicine News. April 3, 2020. http://www.mdedge.com/internalmedicine/article/220183/coronavirus-updates/skin-manifestations-are-emerging-coronavirus?channel=63993
- 8.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34(5):e212–e213. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 9.Jimenez-Cauhe J., Ortega-Quijano D., Prieto-Barrios M., Moreno-Arrones O.M., Fernandez-Nieto D. Reply to “COVID-19 can present with a rash and be mistaken for Dengue”: petechial rash in a patient with COVID-19 infection. J Am Acad Dermatol. 2020;83(2):e141–e142. doi: 10.1016/j.jaad.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manalo I.F., Smith M.K., Cheeley J., Jacobs R. A dermatologic manifestation of COVID-19: transient livedo reticularis. J Am Acad Dermatol. 2020;83(2):700. doi: 10.1016/j.jaad.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry D., Ackerman M., Sancelme E., Finon A., Esteve E. Urticarial eruption in COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34(6):e244–e245. doi: 10.1111/jdv.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estébanez A., Pérez-Santiago L., Silva E., Guillen-Climent S., García-Vázquez A., Ramón M.D. Cutaneous manifestations in COVID-19: a new contribution. J Eur Acad Dermatol Venereol. 2020;34(6):e250–e251. doi: 10.1111/jdv.16474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz-Guimaraens B., Dominguez-Santas M., Suarez-Valle A., et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156(7):820–822. doi: 10.1001/jamadermatol.2020.1741. [DOI] [PubMed] [Google Scholar]
- 14.Galván Casas C., Català A., Carretero Hernández G., et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183(1):71–77. doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez A., Sohier P., Benghanem S., et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156(7):819–820. doi: 10.1001/jamadermatol.2020.1704. [DOI] [PubMed] [Google Scholar]
- 16.Alramthan A., Aldaraji W. Two cases of COVID-19 presenting with a clinical picture resembling chilblains: first report from the Middle East. Clin Exp Dermatol. 2020;45(6):746–748. doi: 10.1111/ced.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marzano A.V., Genovese G., Fabbrocini G., et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol. 2020;83(1):280–285. doi: 10.1016/j.jaad.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y., Cao W., Xiao M., et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Article in Chinese. Zhonghua Xue Ye Xue Za Zhi. 2020;41(0):E006. doi: 10.3760/cma.j.issn.0253-2727.2020.0006. [DOI] [PubMed] [Google Scholar]
- 19.Kaya G., Kaya A., Saurat J.H. Clinical and histopathological features and potential pathological mechanisms of skin lesions in COVID-19: review of the literature. Dermatopathol. 2020;7(1):3–16. doi: 10.3390/dermatopathology7010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suchonwanit P., Leerunyakul K., Kositkuljorn C. Cutaneous manifestations in COVID-19: lessons learned from current evidence. J Am Acad Dermatol. 2020;83(1):e57–e60. doi: 10.1016/j.jaad.2020.04.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Philippine Society for Microbiology and Infectious Diseases Interim guidance on the clinical management of adult patients with suspected or confirmed COVID-19 infection, Version 3.1, as of July 20, 2020. https://www.psmid.org/wpcontent/uploads/2020/07/Final-PCP-PSMID-PCCP-COVID-19-Guidelines-20July2020b.pdf
- 22.Rongioletti F., Ferreli C., Sena P., Caputo V., Atzori L. Clinicopathologic correlations of COVID-19–related cutaneous manifestations with special emphasis on histopathologic patterns. Clin Dermatol. 2021;39(1):149–162. doi: 10.1016/j.clindermatol.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Genovese G., Moltrasio C., Berti E., Marzano A.V. Skin manifestations associated with COVID-19: current knowledge and future perspectives. Dermatology. 2021;237(1):1–12. doi: 10.1159/000512932. [DOI] [PMC free article] [PubMed] [Google Scholar]