Abstract
Hair is a deeply rooted component of identity and culture. Recent articles in this series have focused on scientific evidence relating to hair growth and new insights into the pathogenesis and mechanism of hair loss. This article reviews emerging evidence that has advanced our understanding of hair growth in both of these areas to provide a context for outlining current and emerging therapies. These include finasteride, minoxidil, topical prostaglandins, natural supplements, microneedling, low-level laser light, platelet-rich plasma, fractional lasers, cellular therapy, Wnt activators and SFRP1 antagonism.
Keywords: Alopecia, hair growth, androgenetic alopecia, male pattern hair loss, female pattern hair loss, antiandrogens, minoxidil, platelet rich plasma, prostaglandins, exosomes, low-level laser light, fractional lasers, micro-needling, hair cycling
Introduction
A deeply rooted component of identity and culture, the role of hair extends far beyond function, while hair disorders can significantly impact wellbeing and quality of life1. One example is in patients with breast cancer, where chemotherapy-induced anagen effluvium has been reported to be “psychologically more difficult than the loss of a breast”2–4. Another example is alopecia areata, where suicide has been described5. Androgenetic alopecia (AGA) is characterised by patterned hair loss in both men (male pattern hair loss, or MPHL) and women (female pattern hair loss, or FPHL)6–8. While the incidence of AGA varies across races, its prevalence increases with age, visibly affecting 57% of women and 73.5% of men who are at least 80 years old9.
The frequency and impact of hair loss continue to fuel a search for greater understanding of hair growth and resulting developments in therapeutics. This article aims to build on previous articles in this series that provide an excellent overview of the scientific evidence relating to hair growth10 and new insights into the pathogenesis and mechanism of hair loss11. We will also endeavour to outline current and emerging therapies to promote hair growth.
Hair cycling
The hair follicle is a “complex miniorgan” that produces hair shafts from terminally differentiated keratinocytes12. In association with the sebaceous gland, apocrine gland and arrector pili muscle (APM), the hair follicle forms the pilosebaceous unit. Eccrine glands have also recently been shown to be integrated within the pilosebaceous unit13,14. Scalp hair follicles cluster to form compound pilosebaceous units, consisting of one primary follicle and one or more secondary follicles associated with a single APM and single sebaceous gland11. All the hairs from a follicular unit emerge through a shared infundibulum11.
On average, a human has between 2 and 5 million hair follicles, of which 100,000 are on the scalp. Blonde-haired Caucasians typically have a higher hair density than dark-haired Caucasians, who in turn have a higher hair density than red-haired Caucasians, Africans and Asians15–18. The hair follicle is a complex structure that integrates tissues that arise embryologically from ectoderm, neuroectoderm and mesoderm. Epidermal and mesenchymal cells integrate, proliferate, differentiate and cycle through phases of the hair cycle called anagen, catagen and telogen. The hair fibre forms and elongates during anagen, is retained during catagen and early telogen and then is shed mid-telogen at a point in time called exogen. The late-telogen period after exogen and before the onset of the next anagen, where the follicle is empty, is called kenogen12,19–22. As the rate of linear hair growth remains relatively constant throughout life, the main determinant of hair length is anagen duration. Kenogen duration has an impact on hair density but not length.
Hair cycling involves remodelling of the lower “bulb” portion of the hair follicle during catagen. The non-cycling, upper portion of the hair follicle contains the isthmus and infundibulum, which are separated by the sebaceous gland duct. The hair bulge sits on the outer root sheath (ORS) at the lowest point of isthmus, where the APM inserts. The inner root sheath provides a mechanical barrier between the isthmus and the outside world and provides a protective microenvironment for the bulge. The bulge is a stem cell niche for hair follicle keratinocytes and melanocytes as well as arrector pili myocytes23,24. Bulge keratinocyte stem cells activate at the transition from telogen to anagen and promote regeneration of the bulb. Transient amplifying cells rapidly proliferate, migrate downwards into the dermis, reconnect with the dermal papilla (DP) to reform the hair bulb and differentiate into matrix cells. Hair bulb matrix cells are rapidly dividing cells that give rise to ORS, inner root sheath, cuticle, cortex and medulla of the hair shaft during anagen. Anagen can continue uninterrupted for many years before ending abruptly at the onset of catagen. Catagen, characterised by rapid apoptotic destruction of the entire hair bulb and partial separation from the DP, is complete in less than 2 weeks. The hair follicle remnant consists of the bulge, isthmus and infundibulum and contains a club fibre with no inner root sheath. Exogen exposes the isthmus to the environment. During telogen, the bulb and papilla remain connected by a fibrous tract. Cross-signalling between the papilla and bulge at the onset of anagen induces the new bulb to migrate down the fibrous tract into the same position as its ancestral bulb25–27.
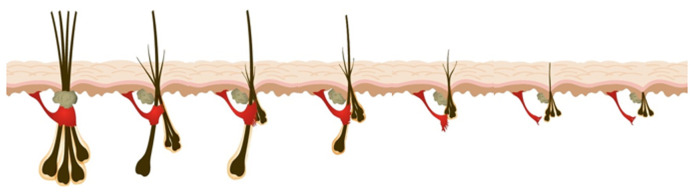
The DP also undergoes dynamic changes during hair cycling. At the onset of catagen, DP fibroblasts migrate into the dermal sheath that surrounds the ORS of the hair follicle and act as a smooth muscle. The dermal sheath, which becomes contractile during catagen, pushes the hair follicle and the surviving DP upwards, enabling its relocation to the upper reticular dermis, just below the bulge27. At the onset of anagen, dermal sheath cells migrate into the DP. Dynamic changes in hair follicle size and hair fibre diameter are a consequence of the relative influx/efflux of DP cells into the dermal sheath during hair cycling. The development of secondary sexual hair at puberty involves net recruitment of dermal sheath cells into the DP with each hair cycle. The development of AGA involves the net loss of DP cells into the dermal sheath with each hair cycle (Figure 1), leading to miniaturisation of the hair follicle (Figure 1 and Figure 2).
Figure 1. Miniaturisation of the hair follicle.
In a previous F1000 article, a model of androgenetic alopecia is presented11. Through consecutive hair cycles, progressive miniaturisation of the hair follicle unit occurs, initially affecting secondary follicles, associated with hair density reduction, before the arrector pili muscle is replaced by fat32. Ultimately, detachment of this muscle from the regenerative bulge area is associated with irreversible hair loss11,32. This figure was adapted from Sinclair et al.11 which is licensed under Creative Commons Attribution 4.0 International (CC BY 4.0) (https://creativecommons.org/licenses/by-nc/4.0/).
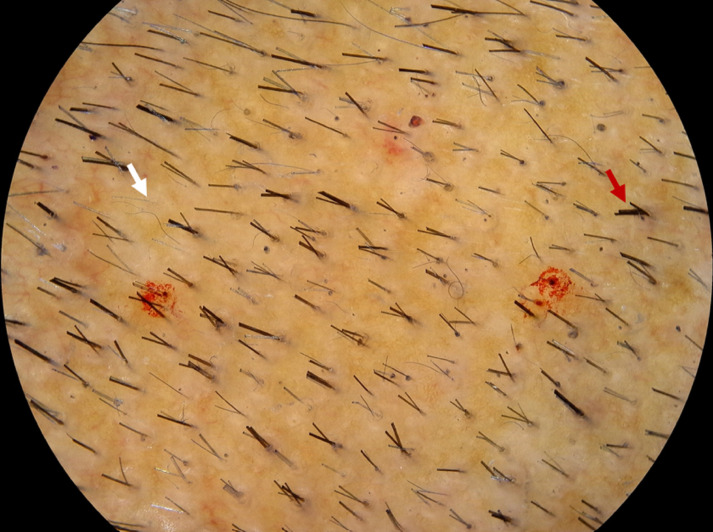
Figure 2. Androgenetic alopecia miniaturised follicles.
In androgenetic alopecia, there is a reduction in the number of hairs per follicular unit (white arrow) versus in the normal hair follicular unit (red arrow), where multiple hairs emerge from a single infundibulum. This image was kindly provided by RS from his clinic.
Regulation of the hair cycle
Regulation of the hair cycle involves multiple, incompletely understood endocrine, autocrine and paracrine signalling pathways in complex interplay. Of particular note is the Wnt family, and specific roles of members of this family are still to be elaborated. Beta-catenin is a core factor of the Wnt signalling pathway and an essential enabler of stem cell differentiation into follicular keratinocytes28. Anagen-to-telogen transition is induced by transient β-catenin signalling29,30 and is influenced by cyclical expression of bone morphogenic proteins (BMP2 and 4), produced by dermal fibroblasts and subcutaneous adipocytes31, by fibroblast growth factors (FGF 7 and 10), BMP inhibitors (transforming growth factor b2 [TGF-b2] and noggin) and Wnt7b31,33–36. Additionally, adipocyte precursor cells express platelet-derived growth factor (PDGF) alpha to activate the PDGF receptor in the DP, resulting in hair germ activation37. Perifollicular vascularisation has been shown to increase during anagen and regress during catagen and is related to ORS keratinocyte vascular endothelial growth factor (VEGF) mRNA over- and under-expression respectively38. Hepatocyte growth factor/scatter factor has been shown to stimulate hair follicle growth in vitro39,40, and insulin-like growth factor 1 (IGF-1) has been identified as a significant regulator in the hair follicle41,42.
Over the previous decade, a therapeutically relevant new method of paracrine signalling has been identified by means of nanosized extracellular phospholipid bilayer membranous vesicles (exosomes 30–120 nm and microvesicles 100–1000 nm) that can transport lipids, metabolites, nucleic acids and proteins43–45. Zhou et al. reported that DP cell-exosomes, isolated from healthy human scalp specimens, accelerated the onset of anagen and delayed catagen in mouse models, resulting in increased levels of β-catenin and Sonic hedgehog (Shh)46. A recent study identified that human dermal papillae exposed to activated human dermal fibroblasts (hDFs) produce stimulated extracellular vesicles (st-EVs) that secrete the non-Wnt ligand Norrin43. It is hypothesised that subsequent activation of the β-catenin pathway is enhanced by hDF-provided Frizzled-4 (Fzd4), the specific receptor for Norrin, resulting in the identified enhanced hair follicle growth ex vivo43.
Beyond signalling, lymphatic vessels (LVs) have been shown to localise adjacent to the bulge, with increased density and absence respectively in transgenic mice associated with prolonged anagen or accelerated entry into catagen47. Furthermore, LV-promoting VEGF-C injections have been shown to promote hair follicle growth in mice.
Therapeutics
The aim of therapeutics is to safely and effectively harness the mechanisms outlined above, to promote anagen, delay catagen and ultimately prevent or reverse AGA miniaturisation with a view to restoring or maintaining visible hair density. Finasteride and topical minoxidil are the most widely recognised agents for the treatment of AGA. Both are approved by the US Food and Drug Administration (FDA) and the European Medicines Authority48. These and other treatments which promote hair regrowth and target specific regulators involved in hair growth will be discussed below.
Finasteride
Finasteride competitively inhibits the 5-alpha-reductase type 2 enzyme, thus preventing conversion of testosterone to dihydrotestosterone (DHT)49. Finasteride is a potential teratogen and generally is contraindicated in women of childbearing potential while the efficacy of topical therapy is limited by poor adherence to treatment. Response varies: some achieve significant regrowth whereas others benefit most from slowing of additional hair loss50.
A meta-analysis of placebo-controlled randomised trials identified moderate-quality evidence supporting the use of finasteride for AGA in men51. At the end of 48 months of treatment, the mean percentage change in hair count was 24% higher in patients receiving finasteride. Most studies reporting on the benefit of finasteride have focused on the scalp vertex, but importantly a randomised trial of 326 men found it was efficacious for frontal scalp hair thinning as well52. It may occasionally adversely affect sexual function, and a systematic review of nine trials, totalling 3570 patients, identified an absolute increase of 1.5% in the risk of erectile dysfunction51. These effects usually resolve after discontinuation of the medication53,54 ; however, a small percentage have reported continued symptoms for years after stopping the medication55. In this regard, a single-centre survey study using a sexual experience scale comparing 99 non-finasteride and 663 finasteride users identified no significant difference in reporting of sexual dysfunction56.
More recently, a pharmacovigilance case–non-case study, analysing the World Health Organization’s VigiBase individual case safety report database, showed signals of suicidality and psychological adverse events associated with the use of finasteride in men under the age of 46; however, sensitivity analyses suggested that this may be influenced by “stimulated reporting”57.
The side effects associated with systemic finasteride may limit its long-term use. This has led to increased interest in topical finasteride, which is not currently FDA-approved, as an alternative therapy. The safety and efficacy of topical finasteride were first investigated over 20 years ago in a single-blind, placebo-controlled study over a 16-month period58. Patient outcomes were favourable, and 73% of patients in the treated group reported “high effectiveness”. Recently, a review exploring topical finasteride in male AGA and FPHL identified that many studies out of the 33 analysed showed positive results and a good safety profile59. Recent reviews of the use of topical finasteride in the treatment of AGA suggest that although topical finasteride is a promising therapy that may be non-inferior to systemic finasteride, there is a paucity of evidence-based research48. Studies included in the reviews showed decreased hair loss, reduction in balding areas, and increased hair diameter, follicular density, and total hair count. Combination therapy of topical finasteride with other oral and topical agents, such as minoxidil, may have synergistic effects. Although studies showed that DHT levels may be altered, no sexual side effects were noted as a result. Adverse events, such as contact dermatitis, irritation, pruritus and dryness, were due mainly to topical application but were well tolerated. Further research, however, was advised to examine the efficacy of topical finasteride in long-term regrowth, tolerability and side effect profile as well as to assess the optimum topical delivery method in monotherapy and combination therapy.
Minoxidil
Minoxidil is a 2,4-di-amino-6-piperidinopyrimidine-3-oxide and works in AGA by prolonging anagen, shortening telogen and enlarging miniaturised follicles60. Although its mechanism of action has not yet been fully elucidated, transcriptome and proteome analysis has demonstrated differential upregulation of genes in vertex versus occipital scalp of patients with AGA and has demonstrated alteration in expression following treatment with minoxidil61–64. It has been shown to increase paracrine growth factor release from and motility of adipose-derived stem cells (ADSCs), enhancing hair growth in mice65.
Minoxidil is converted to its active form, minoxidil sulphate, by sulfotransferase enzymes in the hair follicle ORS66–68. Studies from the previous decade have demonstrated the potential to predict response to minoxidil on the basis of sulfotransferase activity in plucked hair follicles66,69,70. Interestingly, low-dose aspirin has been shown to inhibit hair follicle sulfotransferase activity, possibly impacting on response to topical minoxidil71. About 30 to 40% of patients will experience visible regrowth with 6 months of twice-daily application of 5% topical minoxidil66,72,73. Early reports from one double-blind study74 suggest that increasing the concentration of topical minoxidil, which is not commercially available, to 15% can achieve a clinically significant response in 60% of non-responders to 5% topical minoxidil74,75.
The efficacy of minoxidil lotion, however, is limited by poor solubility. Solutions greater than 5% are unstable. Percutaneous absorption is saturated after twice-daily application. Crystallisation (or powdering) of minoxidil occurs on the scalp when the solvent evaporates. Powdering leads to a loss of active substance and also has an undesirable cosmetic effect on the hair. Co-solubilising agents used to prevent powdering further limit usability as they make the hair gummy, sticky or greasy. Usability is especially a problem for women with FPHL who have long hair. Whereas the hair-promoting effect of topical minoxidil generally takes 6 months to become apparent, the median duration of use is 6 weeks. Continuous use is required to maintain the hair-promoting effects of topical minoxidil, yet many who initiate treatment will discontinue use beyond 12 months.
Oral minoxidil initially was developed in the 1950s to treat hypertension. Hypertrichosis was identified as an unwanted side effect but prompted redevelopment as a treatment for AGA as Regaine/Rogaine in the 1980s. Owing to dose-related side effects, namely postural hypotension, fluid retention and hypertrichosis, it has not been used routinely in standard doses for AGA treatment76. Its efficacy and safety were evaluated in a 2015 study of 30 men with AGA given 5 mg daily for 24 weeks77. Hair growth and total hair count significantly increased in the vertex area, but hypertrichosis and pedal oedema were still common side effects. A recent study on treatment of FPHL involved therapy with 0.25 mg minoxidil combined with 25 mg spironolactone to reduce the risk of fluid retention and augment therapy with its antiandrogen effect76. This dose was well tolerated by the majority, and most noticed a reduction in hair shedding at 3 months and increased density at 6 months76. A randomised, open, 24-week study comparing oral minoxidil 1 mg with topical minoxidil 5% solution in 52 patients with FPHL identified oral minoxidil as safe and well tolerated, and there was a reduction in shedding superior to topical minoxidil78. Although there was no significant difference in hair density, there was a trend towards greater improvement in the oral group. Further data from a large multicentre study have demonstrated its efficacy and safety in AGA79.
Topical prostaglandins
Several studies have highlighted the important role of prostaglandins (PGs) in governing the hair cycle80. Prostaglandin D2 (PGD2) has hair growth–inhibitory effects and is present in increased levels in the AGA-affected scalp, whereas prostaglandin E2 (PGE2), present in reduced levels in AGA scalps, and prostaglandin F2a (PGF2a) have hair growth–stimulating effects81,82. A 2019 study in which 32 Hispanic patients with AGA were biopsied demonstrated that men with early MPHL overexpressed the enzyme prostaglandin E synthase (PTGES), which synthesises PGE2, suggesting that PGs may play differing roles, depending on the stage of AGA83.
Bimatoprost, a synthetic PGF2a analogue84, has been demonstrated to increase the thickness, length and darkness of eyelashes85 and the fullness and darkness of eyebrow hair86–90 by increasing the number of hairs and their duration in the anagen phase91–93. To determine the efficacy and safety of bimatoprost scalp solution on scalp hair growth in mild to moderate AGA, a number of clinical trials have been performed94–96 and they indicate that topical bimatoprost is not superior in efficacy to topical minoxidil. Latanoprost, a PGF2a analogue, can induce anagen and hypertrophic changes in hair follicles97,98. A randomised double-blind placebo-controlled pilot study99 assessing 0.1% topical latanoprost daily in 16 men with AGA found that, compared with placebo-treated areas and baseline, hair density increased significantly99,100.
Topical cetirizine
Cetirizine is a second-generation H1 blocker. It has anti-inflammatory properties and decreases production of PGD2, which, unlike other PGs, is thought to play a negative role in hair growth. It is safe and has a lower potential side effect profile than other topical therapies, potentially resulting in improved compliance compared with treatments such as topical minoxidil and finasteride. One case-controlled study of 60 patients with AGA showed significantly higher hair regrowth and patient satisfaction in the group that received 1% topical cetirizine (n = 30) than in the control group101. In a pilot study of 67 patients who received 1% topical cetirizine, there were increases in total and terminal hair density as well as diameter102. Currently, a number of clinical trials are looking at the use of topical cetirizine in AGA, including in females103, and comparing 1% topical cetirizine with 5% minoxidil104.
Natural ingredients
Analysis of hair has revealed a composition of iron, oxygen, hydrogen, nitrogen and sulphur. Thus, an adequate supply of blood containing these minerals is essential for hair growth during anagen. Anagen is associated with a rearrangement of skin vasculature, an increase in skin perfusion, and angiogenesis105. Various vitamins and minerals are responsible for the modulation of angiogenesis during anagen and therefore are important. In addition, multiple vitamins, minerals, and herbal drugs stimulate hair growth or prevent hair loss by various mechanisms (Table 1)106–108 and thus deficiencies in these can cause alopecia. Supplementation with these, in theory, should improve hair growth and this is particularly true for iron deficiency.
Table 1. Vitamins, minerals and herbal drugs that stimulate hair growth or prevent hair loss by various mechanisms106–108.
| Agents improving blood supply to the scalp: |
| Niacin (vitamin B3) Vitamin B complex Ascorbic acid (vitamin C) Tocopherol (vitamin E) Grape seed Rosemary oil Sage Nettles Hibiscus rosasinensis108 |
| Cofactors for carboxylases that catalyse an essential step in intermediate metabolism: |
| Biotin |
| Antioxidants: |
| Zinc and grape seed |
| Agents that inhibit or reduce 5 alpha reductase activity: |
| Inhibitors: green tea107, ginkgo biloba and emu oil |
| Agents improving hair texture and thus preventing loss of dry brittle hairs: |
| Essential fatty acids (primrose and salmon oil) Amino acids (l-cysteine and l-methionine) |
Microneedling
Microneedling is the process of using a roller device apparatus of small fine needles to micro-puncture the stratum corneum of the epidermis. Although the procedure alone can stimulate neovascularisation, growth factor activity and Wnt protein expression109, it is often used in AGA in conjunction with hair growth stimulants: minoxidil, plasma-rich protein or topical steroids. When combined, microneedling can facilitate the percutaneous delivery of topical therapies. Lee et al. conducted a split-scalp study in 11 women with FPHL110. Microneedling was performed on half the scalp treated with growth factors and the other half treated with normal saline. At 5 weeks, the microneedling with growth factor–treated scalp had an increase in hair count (52.91 ± 10.85) compared with the microneedling with saline-treated scalp (45.91 ± 9.98) (P = 0.0001).
Low-level laser
Low-level laser therapy (LLLT) is occasionally synonymous with red light therapy, cold laser and soft laser111. It is thought to exert a biomodulation/biostimulation effect on tissue, promoting anti-inflammatory effects111–115. The exact mechanism of action in stimulating hair regrowth is not known. Improved cellular activity, reduced inflammation and improved microcirculation may be involved108. The therapeutic effects are delivered in wavelengths ranging from 500 to 1100 nm (red to near infrared) at low energy density (3 to 90 mW/cm2)111,116. A variety of LLLT devices, including in-salon hoods or overhead panels, are available to patients with hair loss. Bonnets, helmets or hand-held combs are suitable for home use. Both the HairMax® LaserComb and the head cap TOPHAT 665 have had FDA clearance for treatment in AGA.
Kim et al. conducted a 24-week, double-blind randomised controlled trial (RCT) comparing sham device to LLLT helmets (emitting wavelengths of 630-nm, 650-nm and 660-nm light-emitting diodes) in 40 patients with AGA117. The mean hair density was significantly greater in the LLLT group versus the sham group. A 26-week RCT involving 269 patients with AGA produced similar results. Patients were randomly assigned to receive different models of the HairMax® LaserComb (7-beam, 9-beam, 12-beam and 9- and 12-beam) or a sham device. The mean terminal hair count at 26 weeks from baseline was higher in the LaserComb subjects compared with the sham-treated subjects. The LaserComb patients also reported a higher percentage of overall improvement with their hair (with respect to hair thickness and fullness) compared with sham-treated subjects118. However, in an earlier case series of seven patients receiving twice-weekly LLLT, the findings were not conclusive. Although increases in terminal hairs and in hair shaft diameter were noted, the findings were not statistically significant and global image assessment did not support an improvement with LLLT119. Liu et al. conducted a system review and meta-analysis of RCTs, reviewing eight studies with 11 double-blind RCTs, and concluded that LLLT resulted in a significant increase in hair density and that low-frequency treatment can result in a better effect than high, and types of devices and course duration did not impact the effectiveness on hair growth120.
Platelet-rich plasma
Treatment with platelet-rich plasma (PRP) involves intradermal injection of the scalp with a concentrated volume of platelets, within a small volume of plasma, that has been derived from the centrifugation of a patient’s own venous blood121,122. PRP contains numerous PDGFs, including PDGF, TGF-b1 and TGF-b2, VEGF, basic fibroblast growth factor, endothelial growth factor and insulin-like growth factors121–124. It is proposed that PRP prolongs anagen, prevents catagen and shortens the period from telogen to anagen through the release of growth factors that stimulate “cell survival, proliferation, and differentiation”125–128.
A mechanistic model from Gupta and Carviel describes the stimulation of hair growth with key roles played by “growth factor mediated increased activation of wingless (Wnt)/β-catenin, extracellular signal-regulated kinase (ERK), and protein kinase B (Akt) signalling pathways”127, to improve vascularisation129 and prolong anagen127. A meta-analysis of six studies and 177 patients demonstrated increased hair number per square centimetre after PRP versus control in addition to a significantly increased hair thickness cross-section per 10−4 mm2 in the PRP group122. Earlier studies demonstrated greater improvement in hair thickness when combined with additional therapies. Combination therapy of PRP and polydeoxyribonucleotide (PDRN), delivered over 12 sessions, yielded a greater improvement in hair thickness in subjects with FPHL than treatment with PDRN therapy alone130. The largest study, involving 64 patients with AGA, is by Schiavone et al.131 Patients received leukocyte PRP plus concentrated plasmatic proteins at baseline and at 3 months. Using macrophotographs and Jaeschke rating of clinical change, two unblinded assessors noted some improvement in 62 patients. The mean changes in clinical rating were 3.2 (95% confidence interval [CI] 2.9–3.5) and 3.9 (95% CI 3.5–4.3).
In a double-blind, controlled study of 26 patients randomly assigned to receive four injections of saline or PRP, those receiving the latter were found to have significant increases in hair density, count and percentage of anagen hairs132. Interestingly, there was no correlation with either platelet counts or measured growth factors, including epidermal growth factor, VEGF or PDGF, prompting the authors to speculate whether other, unmeasured growth factors are related to the measured response to treatment. Clinical practice often involves introduction of PRP in combination with other therapies. In a retrospective review, a significant increase in hair density, but not calibre, was seen in 17 out of 24 patients 2 months after initial injections133. All 24 patients were already using 5% topical minoxidil, while 20 patients were also taking an oral antiandrogen.
A meta-analysis of 10 studies (n = 165 participants) examining PRP treatment compared with baseline calculated a statistically significant overall standardised mean difference in PRP-treated patients of 0.58134. Six of the studies (n = 99 participants) compared PRP with placebo, and the PRP group showed greater efficacy (standardised mean difference of 0.51). This led the authors to conclude that PRP treatment is beneficial in AGA. Another systematic review and network meta-analysis comparing relative efficacy of 2% minoxidil, 5% minoxidil, finasteride, PRP and LLLT indicated that LLLT had a greater increase in mean difference in hair count compared with the other treatments, which were nearly equivalent, although the quality of evidence, based on risk-of-bias assessment, was noted to be very low135. Although meta-analysis highlights evidence of increases in hair number and thickness with PRP in small studies, it is important to recognise that these studies lack comparability, highlighting an unmet need for larger RCTs122,136.
Fractional lasers
Fractional lasers are indicated for the treatment of rhytides and scarring; however, the role of lasers in treating alopecia is relatively new. Ablative (fractional 10,600-nm carbon dioxide and fractional 2940-nm erbium: yttrium aluminium garnet [YAG]) and nonablative (fractional 1550-nm erbium glass) lasers have been investigated in the context of alopecia areata and AGA124,137. Fractional lasers are unique in creating pixelated microthermal injury zones (sparing the epidermis and dermis), allowing better tolerability and fewer cutaneous side effects137.
In a study involving 28 patients with FPHL, patients received 10 treatments with 1550-nm fractional erbium glass laser at 2-week intervals138. Improvement in hair density and mean hair thickness was observed after 5 months of treatment; mean percentage changes from baseline were 57% and 77% respectively. Global photographs also confirmed improvement in 24 patients138. Furthermore, Kim et al. investigated “the effects of a 1,550 -nm fractional erbium-glass laser on the hair cycle in an alopecia areata mouse model” by laser irradiating the shaved backs of C3H/HeN mice whose hair was in telogen stage. Molecular studies revealed an increase in Wnt 5a and β-catenin signal levels, while histopathologic studies demonstrated conversion from telogen to anagen conversion. Following these results, the authors conducted a pilot study of 20 patients with MPHL treated with 1550-nm fractional erbium glass laser (five sessions at 2-weekly intervals) and found improvements in hair density and growth rates139.
Hair Stimulating Complex
Hair Stimulating Complex™ (HSC), patented by Histogen (San Diego, CA, USA)140, comes as an injectable, cell-conditioned media made up of keratinocyte growth factor, VEGF and follistatin. These growth factors are involved in stem cell proliferation and stimulate hair growth. In an RCT of 26 subjects receiving HSC, significant improvement in hair growth was noted over placebo at 12 and 52 weeks141. HSC-treated areas had increased hair shaft thickness (6.3% ± 2.5% vs. −0.63% ± 2.1%; P = 0.046), thickness density (12.8% ± 4.5% vs. −0.2% ± 2.9%; P = 0.028) and terminal hair density (20.6 ± 4.9% vs. 4.4 ± 4.9%; P = 0.029).
Cellular therapy
Autologous dermal sheath/dermal papilla/epidermal cells. Hair follicle regeneration is initiated by mesenchymal DP cell signalling to stem cells in the bulge region of the hair follicle. A viable pool of functional DP cells is necessary to maintain this process long-term. In healthy follicles, dermal sheath cup (DSC) cells may help to repopulate DP cells for each regenerative hair cycle. A gradual decline in DP numbers may account for AGA142,143. Research has shown that transplanted DP and DSC cells can promote DP formation and hair follicle induction144. Therefore, DP/DSC cells harvested from androgen-resistant areas, expanded in culture and then injected into balding scalp potentially stimulate hair growth. Several phase II trials have attempted to explore the efficacy of autologous dermal cells and/or epidermal cells injected into balding scalp to reverse miniaturisation in AGA145,146. Although study statuses are confirmed as complete, results are outstanding and not yet published.
RepliCel (Vancouver, BC, Canada) have conducted a randomised, double-blind, placebo-controlled phase I/IIa trial to evaluate the safety and efficacy of intradermal injection of human autologous hair follicle DSC cells in 10 men and 9 women with AGA147. Stabilisation of hair loss was seen in all per-protocol participants at 24 months. Of those participants who achieved greater than a 5% hair density increase over baseline at 6 months, the top ten were noted to benefit from a sustained response (average 4.2%) at 24 months148. Over a 5-year follow-up period, no serious adverse events were reported.
Adipose-derived stem cells. Mesenchymal stem cells, rich in adipose tissue (ADSCs)149, are multipotent cells that influence surrounding cells through the generation of growth factors150,151 and have demonstrated a capacity to promote hair growth in vitro and in vivo151–154. ADSCs are available as a prepared conditioned media or extracted from adipose tissue using liposuction. This is administered to the balding scalp by serial injections. A pilot case series has suggested a possible role for autologous stem cell–enriched fat grafting for the treatment of AGA155. In an observational study of 27 patients with FPHL, improved hair density was observed in patients who received commercially available ADSC-conditioned media151. Stromal vascular fraction, containing adipose tissue–derived mesenchymal stem cells alone155 and in combination with PRP, has demonstrated significant benefit in a small number of AGA patients in one study156 and superior results compared with PRP alone in a further study157. Stem cell–conditioned media, rich in paracrine factors, has also been investigated as an alternative to cell-based therapy158,159.
Issues relating to cellular and regenerative therapies. Although advances in cellular and regenerative therapies for hair growth continue to evolve, many regulatory and ethical challenges exist. These include problems with activation of stem cells, which are dependent on pathways that may be lost in an environment altered by AGA160. As demonstrated by the use of stem cell–conditioned media as a therapy in hair loss158,159, it is becoming increasingly clear that the environment that the stem cell is exposed to is equally as important as the cell itself160. Indeed, both the cellular and acellular components of the physical environment of the stem cell are important and complicate in vitro analysis and the prediction of behaviour in a degrading in vivo state. Therefore, the ability to model the stem cell niche in vitro and predict efficacy in vivo remains a largely unmet need and is a significant challenge in the study of regenerative medicine in hair therapy.
Concerns exist that evidence generated regarding cellular and regenerative therapies can be over-hyped or unreproducible or have insufficient power to reasonably address safety concerns. Financial gain motivating the provision of unregulated services with safety concerns or little evidence must also be considered161. A report published in 2020 by the European Academies’ Science Advisory Council and the Federation of European Academies of Medicine identified significant ethical and regulatory challenges associated with regenerative medicine162. The report highlights how the ethical concerns of regenerative medicine, like those of many other fields of cellular research, typically relate to safety and efficacy, patient consent, misleading information, professional responsibility, equity and fairness as well as issues surrounding donated biological material162. Although there are strict regulatory frameworks for clinical experimental studies, there can often be premature marketing of therapies, facilitated by regulatory bodies promoting initiatives such as rapid and accelerated approval. Cossu et al. urge that regulatory procedures for regenerative medicine be transparent, robust, evidence-based, rapid and accurate and argue that much work still needs to be done, including engaging with the public, to counter misinformation163.
Wnt activators – Valproic acid
Lee et al. demonstrated hair regrowth with topical application of valproic acid to C3H mice164. Unlike minoxidil, valproic acid was found to activate the Wnt/β-catenin pathway. Other inducers of the Wnt/β-catenin pathway, including 4-phenyl butyric acid (PBA), lithium chloride and beryllium chloride, were also investigated and found to stimulate hair regrowth in vivo. Treatment with lithium chloride or beryllium chloride, in particular, triggered anagen entry. In an RCT of patients with AGA treated with topical valproic acid (VPA) or placebo for 24 weeks165, the mean change in total hair count was found to be significantly higher in the VPA group than in the placebo group (P = 0.047). Both groups experienced mild adverse effects.
Miscellaneous
Newer therapies have recently emerged. Microarray analysis led to the discovery of downregulation of secreted frizzled-related protein 1 (SFRP1), a Wnt inhibitor, by cyclosporine A166. In hair follicles, SFRP1 regulates Wnt/β-catenin activity. Using an SFRP1 antagonist (WAY-316606), the authors were able to demonstrate enhanced hair regrowth ex vivo. Sildenafil, a phosphodiesterase 5 (PDE5) inhibitor, has been shown to promote hair growth in mice. Sildenafil was found to stimulate growth factor expression (VEGF and PDGF), upregulate ERK phosphorylation and promote angiogenesis. Finally, 7-phloroeckol, a metabolite of a brown marine algae, has been shown to stimulate DP cells and ORS cells in vitro, inducing IGF-1 and promoting hair growth in human hair follicles in vitro167.
Conclusions
Treatment of AGA remains a challenge and patients with AGA often have a heterogenous response to treatment, partly because the complex aetiopathogenesis, particularly in affected women, has not yet been fully elucidated. Intricate pathways regulate hair cycling and anagen duration and determine hair growth. As we discover more about these pathways, we move into an era of a growing number of potential therapeutics for hair growth promotion. A number of ongoing clinical trials are exploring novel treatments; however, it is unlikely that one therapy alone will result in a desired, sustainable outcome. Combination therapy incorporating systemic therapy and adjuvant procedural modalities (PRP, LLLT or fractional laser) may well represent the optimal strategy to produce long-lasting results, prior to surgical considerations.
Abbreviations
ADSC, adipose-derived stem cell; AGA, androgenetic alopecia; Akt, protein kinase B; APM, arrector pili muscle; BMP, bone morphogenic protein; CI, confidence interval; DHT, dihydrotestosterone; DP, dermal papilla; DSC, dermal sheath cup; ERK, extracellular signal-regulated kinase; FDA, US Food and Drug Administration; FPHL, female pattern hair loss; hDF, (activated) human dermal fibroblast; HSC, Hair Stimulating Complex; IGF-1, insulin-like growth factor 1; LLLT, low-level laser therapy; LV, lymphatic vessel; MPHL, male pattern hair loss; ORS, outer root sheath; PDGF, platelet-derived growth factor; PDRN, polydeoxyribonucleotide; PG, prostaglandin; PGD2, prostaglandin D2; PGE2, prostaglandin E2; PGF2a, prostaglandin F2a; PRP, platelet-rich plasma; RCT, randomised controlled trial; SFRP1, secreted frizzled-related protein 1; TGF, transforming growth factor; TGF-b1, transforming growth factor b1; TGF-b2, transforming growth factor b2; VEGF, vascular endothelial growth factor; VPA, topical valproic acid
The peer reviewers who approve this article are:
Jeffrey Rapaport, Cosmetic Skin and Surgery Center, New Jersey, USA
Poonkiat Suchonwanit, Division of Dermatology, Department of Medicine, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Funding Statement
The authors declare that no grants were involved in supporting this work.
Contributor Information
Dmitri Wall, Email: dmitri.wall@gmail.com.
Nekma Meah, Email: nekma.meah@gmail.com.
References
- 1. Sherrow V: Encyclopaedia of Hair: A Cultural History. Greenwood Publishing Group; 2006. [Google Scholar]
- 2. Hunt N, McHale S: The psychological impact of alopecia. BMJ. 2005; 331(7522): 951–3. 10.1136/bmj.331.7522.951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Freedman TG: Social and cultural dimensions of hair loss in women treated for breast cancer. Cancer Nurs. 1994; 17(4): 334–341. [PubMed] [Google Scholar]
- 4. Lemieux J, Maunsell E, Provencher L: Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: A literature review. Psychooncology. 2008; 17(4): 317–28. 10.1002/pon.1245 [DOI] [PubMed] [Google Scholar]
- 5. Sinclair RD: Alopecia areata and suicide of children. Med J Aust. 2014; 200(3): 145. 10.5694/mja13.10895 [DOI] [PubMed] [Google Scholar]
- 6. Heilmann-Heimbach S, Hochfeld LM, Paus R, et al. : Hunting the genes in male-pattern alopecia: How important are they, how close are we and what will they tell us? Exp Dermatol. 2016; 25(4): 251–7. 10.1111/exd.12965 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 7. Michel L, Reygagne P, Benech P, et al. : Study of gene expression alteration in male androgenetic alopecia: Evidence of predominant molecular signalling pathways. Br J Dermatol. 2017; 177(5): 1322–36. 10.1111/bjd.15577 [DOI] [PubMed] [Google Scholar]
- 8. Hamilton JB: Patterned loss of hair in man; types and incidence. Ann N Y Acad Sci. 1951; 53(3): 708–28. 10.1111/j.1749-6632.1951.tb31971.x [DOI] [PubMed] [Google Scholar]
- 9. Gan DCC, Sinclair RD: Prevalence of male and female pattern hair loss in Maryborough. J Investig Dermatol Symp Proc. 2005; 10(3): 184–9. 10.1111/j.1087-0024.2005.10102.x [DOI] [PubMed] [Google Scholar]
- 10. Bernard BA: Advances in Understanding Hair Growth [version 1; peer review: 2 approved]. F1000Res. 2016; 5: F1000 Faculty Rev-147. 10.12688/f1000research.7520.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sinclair R, Torkamani N, Jones L: Androgenetic alopecia: new insights into the pathogenesis and mechanism of hair loss [version 1; peer review: 4 approved]. F1000Res. 2015; 4(F1000 Faculty Rev): 585. 10.12688/f1000research.6401.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schneider MR, Schmidt-Ullrich R, Paus R: The hair follicle as a dynamic miniorgan. Curr Biol. 2009; 19(3): R132–42. 10.1016/j.cub.2008.12.005 [DOI] [PubMed] [Google Scholar]
- 13. Poblet E, Jiménez-Acosta F, Hardman JA, et al. : Is the eccrine gland an integral, functionally important component of the human scalp pilosebaceous unit? Exp Dermatol. 2016; 25(2): 149–50. 10.1111/exd.12889 [DOI] [PubMed] [Google Scholar]
- 14. Poblet E, Jimenez F, Escario-Travesedo E, et al. : Eccrine sweat glands associate with the human hair follicle within a defined compartment of dermal white adipose tissue. Br J Dermatol. 2018; 178(5): 1163–72. 10.1111/bjd.16436 [DOI] [PubMed] [Google Scholar]
- 15. Lai-Cheong JE, McGrath JA: Structure and function of skin, hair and nails. Medicine. 2013; 41: 317–20. 10.1016/j.mpmed.2013.04.017 [DOI] [Google Scholar]
- 16. Lieberman DE: Human locomotion and heat loss: An evolutionary perspective. Compr Physiol. 2015; 5(1): 99–117. 10.1002/cphy.c140011 [DOI] [PubMed] [Google Scholar]
- 17. Vogt A, McElwee KJ, Blume-Peytavi U: Biology of the Hair Follicle. In: Hair Growth and Disorders. Springer Berlin Heidelberg; 2003; 282: 1–22. 10.1007/978-3-540-46911-7_1 [DOI] [Google Scholar]
- 18. Szabo G: The regional anatomy of the human integument with special reference to the distribution of hair follicles, sweat glands and melanocytes. Philos Trans R Soc Lond, B Biol Sci. 1967; 252: 447–85. 10.1098/rstb.1967.0029 [DOI] [Google Scholar]
- 19. Halloy J, Bernard BA, Loussouarn G, et al. : Modeling the dynamics of human hair cycles by a follicular automaton. Proc Natl Acad Sci U S A. 2000; 97(15): 8328–33. 10.1073/pnas.97.15.8328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oh JW, Kloepper J, Langan EA, et al. : A Guide to Studying Human Hair Follicle Cycling In Vivo. J Invest Dermatol. 2016; 136(1): 34–44. 10.1038/JID.2015.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Milner Y, Sudnik J, Filippi M, et al. : Exogen, shedding phase of the hair growth cycle: Characterization of a mouse model. J Invest Dermatol. 2002; 119(3): 639–44. 10.1046/j.1523-1747.2002.01842.x [DOI] [PubMed] [Google Scholar]
- 22. Cotsarelis G: Epithelial stem cells: A folliculocentric view. J Invest Dermatol. 2006; 126(7): 1459–68. 10.1038/sj.jid.5700376 [DOI] [PubMed] [Google Scholar]
- 23. Wang AB, Jain P, Tumbar T: The Hair Follicle Stem Cell Niche: The Bulge and Its Environment. In:; 2015; 1–26. 10.1007/978-3-319-21705-5_1 [DOI] [Google Scholar]
- 24. Fuchs E, Tumbar T, Guasch G: Socializing with the neighbors: stem cells and their niche. Cell. 2004; 116(6): 769–78. 10.1016/s0092-8674(04)00255-7 [DOI] [PubMed] [Google Scholar]
- 25. Weedon D, Strutton G: Apoptosis as the mechanism of the involution of hair follicles in catagen transformation. Acta Derm Venereol. 1981; 61(4): 335–9. [PubMed] [Google Scholar]
- 26. Lindner G, Botchkarev VA, Botchkareva NV, et al. : Analysis of apoptosis during hair follicle regression (catagen). Am J Pathol. 1997; 151(6): 1601–17. [PMC free article] [PubMed] [Google Scholar]
- 27. Heitman N, Sennett R, Mok KW, et al. : Dermal sheath contraction powers stem cell niche relocation during hair cycle regression. Science. 2020; 367(6474): 161–6. 10.1126/science.aax9131 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 28. Huelsken J, Vogel R, Erdmann B, et al. : β-Catenin Controls Hair Follicle Morphogenesis and Stem Cell Differentiation in the Skin. Cell. 2001; 105(4): 533–45. 10.1016/S0092-8674(01)00336-1 [DOI] [PubMed] [Google Scholar]
- 29. Lo Celso C, Prowse DM, Watt FM: Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development. 2004; 131(8): 1787–99. 10.1242/dev.01052 [DOI] [PubMed] [Google Scholar]
- 30. van Mater D, Kolligs FT, Dlugosz AA, et al. : Transient activation of beta -catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes Dev. 2003; 17(10): 1219–24. 10.1101/gad.1076103 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 31. Plikus MV, Mayer JA, de La Cruz D, et al. : Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008; 451(7176): 340–4. 10.1038/nature06457 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 32. Torkamani N, Rufaut NW, Jones L, et al. : Destruction of the arrector pili muscle and fat infiltration in androgenic alopecia. Br J Dermatol. 2014; 170(6): 1291–8. 10.1111/bjd.12921 [DOI] [PubMed] [Google Scholar]
- 33. Greco V, Chen T, Rendl M, et al. : A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009; 4(2): 155–69. 10.1016/j.stem.2008.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 34. Oshimori N, Fuchs E: Paracrine TGF-β signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell. 2012; 10(1): 63–75. 10.1016/j.stem.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 35. Hsu YC, Li L, Fuchs E: Emerging interactions between skin stem cells and their niches. Nat Med. 2014; 20(8): 847–56. 10.1038/nm.3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kandyba E, Kobielak K: Wnt7b is an important intrinsic regulator of hair follicle stem cell homeostasis and hair follicle cycling. Stem Cells. 2014; 32(4): 886–901. 10.1002/stem.1599 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 37. Festa E, Fretz J, Berry R, et al. : Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011; 146(5): 761–71. 10.1016/j.cell.2011.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 38. Yano K, Brown LF, Detmar M: Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest. 2001; 107(4): 409–17. 10.1172/JCI11317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jindo T, Tsuboi R, Takamori K, et al. : Local injection of hepatocyte growth factor/scatter factor (HGF/SF) alters cyclic growth of murine hair follicles. J Invest Dermatol. 1998; 110(4): 338–42. 10.1046/j.1523-1747.1998.00144.x [DOI] [PubMed] [Google Scholar]
- 40. Jindo T, Tsuboi R, Imai R, et al. : The effect of hepatocyte growth factor/scatter factor on human hair follicle growth. J Dermatol Sci. 1995; 10(3): 229–32. 10.1016/0923-1811(95)00429-v [DOI] [PubMed] [Google Scholar]
- 41. Trüeb RM: Further Clinical Evidence for the Effect of IGF-1 on Hair Growth and Alopecia. Skin Appendage Disord. 2018; 4(2): 90–5. 10.1159/000479333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weger N, Schlake T: Igf-I signalling controls the hair growth cycle and the differentiation of hair shafts. J Invest Dermatol. 2005; 125(5): 873–82. 10.1111/j.0022-202X.2005.23946.x [DOI] [PubMed] [Google Scholar]
- 43. le Riche A, Aberdam E, Marchand L, et al. : Extracellular Vesicles from Activated Dermal Fibroblasts Stimulate Hair Follicle Growth Through Dermal Papilla-Secreted Norrin. Stem Cells. 2019; 37(9): 1166–75. 10.1002/stem.3043 [DOI] [PubMed] [Google Scholar]
- 44. Zaborowski MP, Balaj L, Breakefield XO, et al. : Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. BioScience. 2015; 65(8): 783–97. 10.1093/biosci/biv084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Raposo G, Stoorvogel W: Extracellular vesicles: Exosomes, microvesicles, and friends. J Cell Biol. 2013; 200(4): 373–83. 10.1083/jcb.201211138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhou L, Wang H, Jing J, et al. : Regulation of hair follicle development by exosomes derived from dermal papilla cells. Biochem Biophys Res Commun. 2018; 500(2): 325–32. 10.1016/j.bbrc.2018.04.067 [DOI] [PubMed] [Google Scholar]
- 47. Yoon SY, Dieterich LC, Karaman S, et al. : An important role of cutaneous lymphatic vessels in coordinating and promoting anagen hair follicle growth. PLoS One. 2019; 14(7): e0220341. 10.1371/journal.pone.0220341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee SW, Juhasz M, Mobasher P, et al. : A Systematic Review of Topical Finasteride in the Treatment of Androgenetic Alopecia in Men and Women. J Drugs Dermatol. 2018; 17(4): 457–63. [PMC free article] [PubMed] [Google Scholar]
- 49. Rittmaster RS: Finasteride. N Engl J Med. 1994; 330(2): 120–5. 10.1056/NEJM199401133300208 [DOI] [PubMed] [Google Scholar]
- 50. Kaufman KD, Olsen EA, Whiting D, et al. : Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998; 39(4 Pt 1): 578–89. 10.1016/s0190-9622(98)70007-6 [DOI] [PubMed] [Google Scholar]
- 51. Mella JM, Perret MC, Manzotti M, et al. : Efficacy and safety of finasteride therapy for androgenetic alopecia: A systematic review. Arch Dermatol. 2010; 146(10): 1141–50. 10.1001/archdermatol.2010.256 [DOI] [PubMed] [Google Scholar]
- 52. Leyden J, Dunlap F, Miller B, et al. : Finasteride in the treatment of men with frontal male pattern hair loss. J Am Acad Dermatol. 1999; 40(6 Pt 1): 930–7. 10.1016/s0190-9622(99)70081-2 [DOI] [PubMed] [Google Scholar]
- 53. Gillespie JDN: Long-term (5-year) multinational experience with finasteride 1mg in the treatment of men with androgenetic alopecia. Hair Transplant Forum International. 2002; 12(3): 129. 10.33589/12.3.0129 [DOI] [PubMed] [Google Scholar]
- 54. Rossi A, Cantisani C, Scarnò M, et al. : Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10-year follow-up. Dermatol Ther. 2011; 24(4): 455–61. 10.1111/j.1529-8019.2011.01441.x [DOI] [PubMed] [Google Scholar]
- 55. Price VH, Menefee E, Sanchez M, et al. : Changes in hair weight and hair count in men with androgenetic alopecia after treatment with finasteride, 1 mg, daily. J Am Acad Dermatol. 2002; 46(4): 517–23. 10.1067/mjd.2002.120537 [DOI] [PubMed] [Google Scholar]
- 56. Haber RS, Gupta AK, Epstein E, et al. : Finasteride for androgenetic alopecia is not associated with sexual dysfunction: A survey-based, single-centre, controlled study. J Eur Acad Dermatol Venereol. 2019; 33(7): 1393–7. 10.1111/jdv.15548 [DOI] [PubMed] [Google Scholar]
- 57. Nguyen DD, Cone EB, Trinh QD: Association of Hair Loss With Suicidality and Psychological Adverse Events vs Finasteride Use-Reply. JAMA Dermatol. 2021; 157(6): 738. 10.1001/jamadermatol.2021.0377 [DOI] [PubMed] [Google Scholar]
- 58. Mazzarella GF, Loconsole GF, Cammisa GA, et al. : Topical finasteride in the treatment of androgenic alopecia. Preliminary evaluations after a 16-month therapy course. J Dermatol Treat. 1997; 8(3): 189–92. 10.3109/09546639709160517 [DOI] [Google Scholar]
- 59. Suchonwanit P, Iamsumang W, Leerunyakul K: Topical finasteride for the treatment of male androgenetic alopecia and female pattern hair loss: A review of the current literature. J Dermatolog Treat. 2020; 1–6. 10.1080/09546634.2020.1782324 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 60. Messenger AG, Rundegren J: Minoxidil: Mechanisms of action on hair growth. Br J Dermatol. 2004; 150(2): 186–94. 10.1111/j.1365-2133.2004.05785.x [DOI] [PubMed] [Google Scholar]
- 61. Moon PG, Kwack MH, Lee JE, et al. : Proteomic analysis of balding and non-balding mesenchyme-derived dermal papilla cells from androgenetic alopecia patients using on-line two-dimensional reversed phase-reversed phase LC-MS/MS. J Proteomics. 2013; 85: 174–91. 10.1016/j.jprot.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 62. Mirmirani P, Consolo M, Oyetakin-White P, et al. : Similar response patterns to topical minoxidil foam 5% in frontal and vertex scalp of men with androgenetic alopecia: A microarray analysis. Br J Dermatol. 2015; 172(6): 1555–61. 10.1111/bjd.13399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lai VWY: Systemic Treatments for Alopecia Areata: The Efficacy of Cyclosporin Thesis: Bachelor of Medical Science ( Honours ). 2018. Reference Source [Google Scholar]
- 64. Martinez-Jacobo LA, Ancer-Arellano CI, Villarreal-Villarreal CD, et al. : Global expression profile and global genome methylation signatures in male patients with androgenetic alopecia. J Eur Acad Dermatol Venereol. 2020; 34(5): e216–e218. 10.1111/jdv.16169 [DOI] [PubMed] [Google Scholar]
- 65. Choi N, Shin S, Song SU, et al. : Minoxidil Promotes Hair Growth through Stimulation of Growth Factor Release from Adipose-Derived Stem Cells. Int J Mol Sci. 2018; 19(3): 691. 10.3390/ijms19030691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Goren A, Shapiro J, Roberts J, et al. : Clinical utility and validity of minoxidil response testing in androgenetic alopecia. Dermatol Ther. 2015; 28(1): 13–6. 10.1111/dth.12164 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 67. Baker CA, Uno H, Johnson GA: Minoxidil sulfation in the hair follicle. Skin Pharmacol. 1994; 7(6): 335–9. 10.1159/000211315 [DOI] [PubMed] [Google Scholar]
- 68. Anderson RJ, Kudlacek PE, Clemens DL: Sulfation of minoxidil by multiple human cytosolic sulfotransferases. Chem Biol Interact. 1998; 109(1–3): 53–67. 10.1016/s0009-2797(97)00120-8 [DOI] [PubMed] [Google Scholar]
- 69. Goren A, Castano JA, McCoy J, et al. : Novel enzymatic assay predicts minoxidil response in the treatment of androgenetic alopecia. Dermatol Ther. 2014; 27(3): 171–3. 10.1111/dth.12111 [DOI] [PubMed] [Google Scholar]
- 70. Roberts J, Desai N, McCoy J, et al. : Sulfotransferase activity in plucked hair follicles predicts response to topical minoxidil in the treatment of female androgenetic alopecia. Dermatol Ther. 2014; 27(4): 252–4. 10.1111/dth.12130 [DOI] [PubMed] [Google Scholar]
- 71. Goren A, Sharma A, Dhurat R, et al. : Low-dose daily aspirin reduces topical minoxidil efficacy in androgenetic alopecia patients. Dermatol Ther. 2018; 31(6): e12741. 10.1111/dth.12741 [DOI] [PubMed] [Google Scholar]
- 72. Olsen EA, Dunlap FE, Funicella T, et al. : A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol. 2002; 47(3): 377–85. 10.1067/mjd.2002.124088 [DOI] [PubMed] [Google Scholar]
- 73. Olsen EA, Whiting D, Bergfeld W, et al. : A multicenter, randomized, placebo-controlled, double-blind clinical trial of a novel formulation of 5% minoxidil topical foam versus placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol. 2007; 57(5): 767–74. 10.1016/j.jaad.2007.04.012 [DOI] [PubMed] [Google Scholar]
- 74. McCoy J, Goren A, Kovacevic M, et al. : Minoxidil dose response study in female pattern hair loss patients determined to be non-responders to 5% topical minoxidil. J Biol Regul Homeost Agents. 2016; 30(4): 1153–5. [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 75. Goren A, Naccarato T: Minoxidil in the treatment of androgenetic alopecia. Dermatol Ther. 2018; 31(5): e12686. 10.1111/dth.12686 [DOI] [PubMed] [Google Scholar]
- 76. Sinclair RD: Female pattern hair loss: A pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018; 57(1): 104–9. 10.1111/ijd.13838 [DOI] [PubMed] [Google Scholar]
- 77. Lueangarun S, Panchaprateep R, Tempark T, et al. : Efficacy and safety of oral minoxidil 5 mg daily during 24-week treatment in male androgenetic alopecia. J Am Acad Dermatol. 2015; 72(5): AB113. 10.1016/j.jaad.2015.02.466 [DOI] [Google Scholar]
- 78. Ramos PM, Sinclair RD, Kasprzak M, et al. : Minoxidil 1 mg oral versus minoxidil 5% topical solution for the treatment of female-pattern hair loss: A randomized clinical trial. J Am Acad Dermatol. 2020; 82(1): 252–3. 10.1016/j.jaad.2019.08.060 [DOI] [PubMed] [Google Scholar]
- 79. Vañó-Galván S, Pirmez R, Hermosa-Gelbard A, et al. : Safety of low-dose oral minoxidil for hair loss: A multicenter study of 1404 patients. J Am Acad Dermatol. 2021; 84(6): 1644–51. 10.1016/j.jaad.2021.02.054 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 80. Khidhir KG, Woodward DF, Farjo NP, et al. : The prostamide-related glaucoma therapy, bimatoprost, offers a novel approach for treating scalp alopecias. FASEB J. 2013; 27(2): 557–67. 10.1096/fj.12-218156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Johnstone MA, Albert DM: Prostaglandin-Induced Hair Growth. Surv Ophthalmol. 2002; 47 Suppl 1: S185–S202. 10.1016/s0039-6257(02)00307-7 [DOI] [PubMed] [Google Scholar]
- 82. Garza LA, Liu Y, Yang Z, et al. : Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci Transl Med. 2012; 4(126): 126ra34. 10.1126/scitranslmed.3003122 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 83. Villarreal-Villarreal CD, Sinclair RD, Martínez-Jacobo L, et al. : Prostaglandins in androgenetic alopecia in 12 men and four female. J Eur Acad Dermatol Venereol. 2019; 33(5): e214–e215. 10.1111/jdv.15479 [DOI] [PubMed] [Google Scholar]
- 84. Barrón-Hernández YL, Tosti A: Bimatoprost for the treatment of eyelash, eyebrow and scalp alopecia. Expert Opin Investig Drugs. 2017; 26(4): 515–22. 10.1080/13543784.2017.1303480 [DOI] [PubMed] [Google Scholar]
- 85. Yoelin S, Walt JG, Earl M: Safety, effectiveness, and subjective experience with topical bimatoprost 0.03% for eyelash growth. Dermatol Surg. 2010; 36(5): 638–49. 10.1111/j.1524-4725.2010.01519.x [DOI] [PubMed] [Google Scholar]
- 86. Beer KR, Julius H, Dunn M, Wilson F, et al. : Treatment of eyebrow hypotrichosis using bimatoprost: A randomized, double-blind, vehicle-controlled pilot study. Dermatol Surg. 2013; 39(7): 1079–87. 10.1111/dsu.12199 [DOI] [PubMed] [Google Scholar]
- 87. Carruthers J, Beer K, Carruthers A, et al. : Bimatoprost 0.03% for the Treatment of Eyebrow Hypotrichosis. Dermatol Surg. 2016; 42(5): 608–17. 10.1097/DSS.0000000000000755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Schweiger ES, Pinchover L, Bernstein RM: Topical bimatoprost for the treatment of eyebrow hypotrichosis. J Drugs Dermatol. 2012; 11(1): 106–8. [PubMed] [Google Scholar]
- 89. Suwanchatchai W, Tanglertsampan C, Pengsalae N, et al. : Efficacy and safety of bimatoprost 0.03% versus minoxidil 3% in enhancement of eyebrows: A randomized, double-blind, split-face comparative study. J Dermatol. 2012; 39(10): 865–6. 10.1111/j.1346-8138.2012.01579.x [DOI] [PubMed] [Google Scholar]
- 90. Elias MJ, Weiss J, Weiss E: Bimatoprost ophthalmic solution 0.03% for eyebrow growth. Dermatol Surg. 2011; 37(7): 1057–9. 10.1111/j.1524-4725.2011.01908.x [DOI] [PubMed] [Google Scholar]
- 91. Tauchi M, Fuchs TA, Kellenberger AJ, et al. : Characterization of an in vivo model for the study of eyelash biology and trichomegaly: Mouse eyelash morphology, development, growth cycle, and anagen prolongation by bimatoprost. Br J Dermatol. 2010; 162(6): 1186–97. 10.1111/j.1365-2133.2010.09685.x [DOI] [PubMed] [Google Scholar]
- 92. Cohen JL: Enhancing the growth of natural eyelashes: The mechanism of bimatoprost-induced eyelash growth. Dermatol Surg. 2010; 36(9): 1361–71. 10.1111/j.1524-4725.2010.01522.x [DOI] [PubMed] [Google Scholar]
- 93. Law SK: Bimatoprost in the treatment of eyelash hypotrichosis. Clin Ophthalmol. 2010; 4: 349–58. 10.2147/opth.s6480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Allergan: A Safety and Efficacy Study of Bimatoprost in Men With Androgenic Alopecia (AGA). In: ClinicalTrials.gov. [Cited 2021, May 24]. Reference Source [Google Scholar]
- 95. Allergan: Safety and Efficacy Study of Bimatoprost in the Treatment of Men with Androgenic Alopecia. In ClinicalTrials.gov. [Cited 2021, May 24]. Reference Source [Google Scholar]
- 96. Allergen: Safety and Efficacy Study of Bimatoprost in the Treatment of Women with Female Pattern Hair Loss. In: Clinicaltrials.gov. [Cited 2021, May 29]. Reference Source [Google Scholar]
- 97. Toris CB, Gulati V: The biology, pathology and therapeutic use of prostaglandins in the eye. Clin Lipidol. 2011; 6(5): 577–91. Reference Source [Google Scholar]
- 98. Xu XG, Chen HD: Prostanoids and Hair Follicles: Implications for Therapy of Hair Disorders. Acta Derm Venereol. 2018; 98(3): 318–23. 10.2340/00015555-2843 [DOI] [PubMed] [Google Scholar]
- 99. Blume-Peytavi U, Lönnfors S, Hillmann K, et al. : A randomized double-blind placebo-controlled pilot study to assess the efficacy of a 24-week topical treatment by latanoprost 0.1% on hair growth and pigmentation in healthy volunteers with androgenetic alopecia. J Am Acad Dermatol. 2012; 66(5): 794–800. 10.1016/j.jaad.2011.05.026 [DOI] [PubMed] [Google Scholar]
- 100. Choi YM, Diehl J, Levins PC: Promising alternative clinical uses of prostaglandin F2α analogs: Beyond the eyelashes. J Am Acad Dermatol. 2015; 72(4): 712–6. 10.1016/j.jaad.2014.10.012 [DOI] [PubMed] [Google Scholar]
- 101. Zaky MS, Abo Khodeir H, Ahmed HA, et al. : Therapeutic implications of topical cetirizine 1% in treatment of male androgenetic alopecia: A case-controlled study. J Cosmet Dermatol. 2021; 20(4): 1154–9. 10.1111/jocd.13940 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 102. Rossi A, Campo D, Fortuna MC, et al. : A preliminary study on topical cetirizine in the therapeutic management of androgenetic alopecia. J Dermatolog Treat. 2018; 29(2): 149–51. 10.1080/09546634.2017.1341610 [DOI] [PubMed] [Google Scholar]
- 103. Farid SF: Topical Cetirizine in Androgenetic Alopecia in Females. In: ClinicalTrials.gov [Cited 2021, August 16]. Reference Source [Google Scholar]
- 104. Ibrahim RA: Topical Cetirizine 1% vs Minoxidil 5% Gel in Treatment of Androgenetic Alopecia. ClinicalTrials.gov [Cited 2021, August 16]. Reference Source [Google Scholar]
- 105. Mecklenburg L, Tobin DJ, Müller-Röver S, et al. : Active hair growth (anagen) is associated with angiogenesis. J Invest Dermatol. 2000; 114(5): 909–16. 10.1046/j.1523-1747.2000.00954.x [DOI] [PubMed] [Google Scholar]
- 106. Semalty M, Semalty A, Joshi GP, et al. : Hair growth and rejuvenation: An overview. J Dermatolog Treat. 2011; 22(3): 123–32. 10.3109/09546630903578574 [DOI] [PubMed] [Google Scholar]
- 107. Kwon OS, Han JH, Yoo HG, et al. : Human hair growth enhancement in vitro by green tea epigallocatechin-3-gallate (EGCG). Phytomedicine. 2007; 14(7–8): 551–5. 10.1016/j.phymed.2006.09.009 [DOI] [PubMed] [Google Scholar]
- 108. Adhirajan N, Ravi Kumar T, Shanmugasundaram N, et al. : In vivo and in vitro evaluation of hair growth potential of Hibiscus rosa-sinensis Linn. J Ethnopharmacol. 2003; 88(2–3): 235–9. 10.1016/s0378-8741(03)00231-9 [DOI] [PubMed] [Google Scholar]
- 109. Fertig RM, Gamret AC, Cervantes J, et al. : Microneedling for the treatment of hair loss? J Eur Acad Dermatol Venereol. 2018; 32(4): 564–9. 10.1111/jdv.14722 [DOI] [PubMed] [Google Scholar]
- 110. Lee YB, Eun YS, Lee JH, et al. : Effects of topical application of growth factors followed by microneedle therapy in women with female pattern hair loss: A pilot study. J Dermatol. 2013; 40(1): 81–3. 10.1111/j.1346-8138.2012.01680.x [DOI] [PubMed] [Google Scholar]
- 111. Zarei M, Wikramanayake TC, Falto-Aizpurua L, et al. : Low level laser therapy and hair regrowth: An evidence-based review. Lasers Med Sci. 2016; 31(2): 363–71. 10.1007/s10103-015-1818-2 [DOI] [PubMed] [Google Scholar]
- 112. Chung PS, Kim YC, Chung MS, et al. : The effect of low-power laser on the murine hair growth. J Korean Soc Plast. 2004; (i): 1–8. Reference Source [Google Scholar]
- 113. Vlachos SP, Kontoes PP: Development of terminal hair following skin lesion treatments with an intense pulsed light source. Aesthetic Plast Surg. 2002; 26(4): 303–7. 10.1007/s00266-002-2002-1 [DOI] [PubMed] [Google Scholar]
- 114. Satino JL, Markou M: Hair Regrowth and Increased Hair Tensile Strength Using the HairMax LaserComb for Low-Level Laser Therapy. International Journal of Cosmetic Surgery and Aesthetic Dermatology. 2003; 5(2): 113–7. 10.1089/153082003769591209 [DOI] [Google Scholar]
- 115. Conlan MJ, Rapley JW, Cobb CM: Biostimulation of wound healing by low-energy laser irradiation. A review. J Clin Periodontol. 1996; 23(5): 492–6. 10.1111/j.1600-051x.1996.tb00580.x [DOI] [PubMed] [Google Scholar]
- 116. Sadick NS: New-Generation Therapies for the Treatment of Hair Loss in Men. Dermatol Clin. 2018; 36(1): 63–7. 10.1016/j.det.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 117. Kim H, Choi JW, Kim JY, et al. : Low-level light therapy for androgenetic alopecia: A 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013; 39(8): 1177–83. 10.1111/dsu.12200 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 118. Jimenez JJ, Wikramanayake TC, Bergfeld W, et al. : Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: A multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol. 2014; 15(2): 115–27. 10.1007/s40257-013-0060-6 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 119. Avram MR, Rogers NE: The use of low-level light for hair growth: Part I. J Cosmet Laser Ther. 2009; 11(2): 110–7. 10.1080/14764170902842531 [DOI] [PubMed] [Google Scholar]
- 120. Liu KH, Liu D, Chen YT, et al. : Comparative effectiveness of low-level laser therapy for adult androgenic alopecia: A system review and meta-analysis of randomized controlled trials. Lasers Med Sci. 2019; 34(6): 1063–9. 10.1007/s10103-019-02723-6 [DOI] [PubMed] [Google Scholar]
- 121. Marx RE: Platelet-rich plasma: Evidence to support its use. J Oral Maxillofac Surg. 2004; 62(4): 489–96. 10.1016/j.joms.2003.12.003 [DOI] [PubMed] [Google Scholar]
- 122. Giordano S, Romeo M, Lankinen P: Platelet-rich plasma for androgenetic alopecia: Does it work? Evidence from meta analysis. J Cosmet Dermatol. 2017; 16(3): 374–81. 10.1111/jocd.12331 [DOI] [PubMed] [Google Scholar]
- 123. Eppley BL, Woodell JE, Higgins J: Platelet quantification and growth factor analysis from platelet-rich plasma: Implications for wound healing. Plast Reconstr Surg. 2004; 114(6): 1502–8. 10.1097/01.prs.0000138251.07040.51 [DOI] [PubMed] [Google Scholar]
- 124. York K, Meah N, Bhoyrul B, et al. : A review of the treatment of male pattern hair loss. Expert Opin Pharmacother. 2020; 21(5): 603–12. 10.1080/14656566.2020.1721463 [DOI] [PubMed] [Google Scholar]
- 125. Stevens J, Khetarpal S: Platelet-rich plasma for androgenetic alopecia: A review of the literature and proposed treatment protocol. Int J Womens Dermatol. 2018; 5(1): 46–51. 10.1016/j.ijwd.2018.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Alves R, Grimalt R: Randomized Placebo-Controlled, Double-Blind, Half-Head Study to Assess the Efficacy of Platelet-Rich Plasma on the Treatment of Androgenetic Alopecia. Dermatol Surg. 2016; 42(4): 491–7. 10.1097/DSS.0000000000000665 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 127. Gupta AK, Carviel J: A Mechanistic Model of Platelet-Rich Plasma Treatment for Androgenetic Alopecia. Dermatol Surg. 2016; 42(12): 1335–9. 10.1097/DSS.0000000000000901 [DOI] [PubMed] [Google Scholar]
- 128. Li ZJ, Choi HI, Choi DK, et al. : Autologous platelet-rich plasma: A potential therapeutic tool for promoting hair growth. Dermatol Surg. 2012; 38(7 Pt 1): 1040–6. 10.1111/j.1524-4725.2012.02394.x [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 129. Gentile P, Garcovich S, Bielli A, et al. : The Effect of Platelet-Rich Plasma in Hair Regrowth: A Randomized Placebo-Controlled Trial. Stem Cells Transl Med. 2015; 4(11): 1317–23. 10.5966/sctm.2015-0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Lee SH, Zheng Z, Kang JS, et al. : Therapeutic efficacy of autologous platelet-rich plasma and polydeoxyribonucleotide on female pattern hair loss. Wound Repair Regen. 2015; 23(1): 30–6. 10.1111/wrr.12250 [DOI] [PubMed] [Google Scholar]
- 131. Schiavone G, Raskovic D, Greco J, et al. : Platelet-rich plasma for androgenetic alopecia: A pilot study. Dermatol Surg. 2014; 40(9): 1010–9. 10.1097/01.DSS.0000452629.76339.2b [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 132. Rodrigues BL, Montalvão SAL, Cancela RBB, et al. : Treatment of male pattern alopecia with platelet-rich plasma: A double-blind controlled study with analysis of platelet number and growth factor levels. J Am Acad Dermatol. 2019; 80(3): 694–700. 10.1016/j.jaad.2018.09.033 [DOI] [PubMed] [Google Scholar]
- 133. Ho A, Sukhdeo K, Lo Sicco K, et al. : Trichologic response of platelet-rich plasma in androgenetic alopecia is maintained during combination therapy. J Am Acad Dermatol. 2020; 82(2): 478–9. 10.1016/j.jaad.2018.03.022 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 134. Gupta AK, Cole J, Deutsch DP, et al. : Platelet-Rich Plasma as a Treatment for Androgenetic Alopecia. Dermatol Surg. 2019; 45(10): 1262–73. 10.1097/DSS.0000000000001894 [DOI] [PubMed] [Google Scholar]
- 135. Gupta AK, Mays RR, Dotzert MS, et al. : Efficacy of non-surgical treatments for androgenetic alopecia: A systematic review and network meta-analysis. J Eur Acad Dermatol Venereol. 2018; 32(12): 2112–25. 10.1111/jdv.15081 [DOI] [PubMed] [Google Scholar]
- 136. Alves R, Grimalt R: A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Skin Appendage Disord. 2018; 4(1): 18–24. 10.1159/000477353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Perper M, Aldahan AS, Fayne RA, et al. : Efficacy of fractional lasers in treating alopecia: A literature review. Lasers Med Sci. 2017; 32(8): 1919–25. 10.1007/s10103-017-2306-7 [DOI] [PubMed] [Google Scholar]
- 138. Lee GY, Lee SJ, Kim WS: The effect of a 1550 nm fractional erbium-glass laser in female pattern hair loss. J Eur Acad Dermatol Venereol. 2011; 25(12): 1450–4. 10.1111/j.1468-3083.2011.04183.x [DOI] [PubMed] [Google Scholar]
- 139. Kim WS, Lee HI, Lee JW, et al. : Fractional photothermolysis laser treatment of male pattern hair loss. Dermatol Surg. 2011; 37(1): 41–51. 10.1111/j.1524-4725.2010.01833.x [DOI] [PubMed] [Google Scholar]
- 140. Histogen: Hair Stimulating Complex. Reference Source [Google Scholar]
- 141. Zimber MP, Ziering C, Zeigler F, et al. : Hair regrowth following a Wnt- and follistatin containing treatment: Safety and efficacy in a first-in-man phase 1 clinical trial. J Drugs Dermatol. 2011; 10(11): 1308–12. [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 142. Rahmani W, Abbasi S, Hagner A, et al. : Hair follicle dermal stem cells regenerate the dermal sheath, repopulate the dermal papilla, and modulate hair type. Dev Cell. 2014; 31(5): 543–58. 10.1016/j.devcel.2014.10.022 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 143. Randall VA: Androgens and hair growth. Dermatol Ther. 2008; 21(5): 314–28. 10.1111/j.1529-8019.2008.00214.x [DOI] [PubMed] [Google Scholar]
- 144. McElwee KJ, Kissling S, Wenzel E, et al. : Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J Invest Dermatol. 2003; 121(6): 1267–75. 10.1111/j.1523-1747.2003.12568.x [DOI] [PubMed] [Google Scholar]
- 145. Aderans Research Institute: A Study to Evaluate and Compare Injections of Autologous Dermal and Epidermal Cells into the Balding Scalp of Subjects with Hair Loss (CA-0002013). In ClinicalTrials.gov. [Cited 2021 May 26]. Reference Source [Google Scholar]
- 146. Aderans Research Institute: A Study to Evaluate and Compare Injections of Autologous Mixed Population of Dermal Cells into the Balding Scalp of Subjects with Hair Loss (CA-0004542). In ClinicalTrials.gov. [Cited 2021 May 26]. Reference Source [Google Scholar]
- 147. TrichoScience Innovations: Safety and Efficacy Study of Human Autologous Hair Follicle Cells to Treat Androgenetic Alopecia. In ClinicalTrials.gov. [Cited in 2021 May 26]. Reference Source [Google Scholar]
- 148. McElwee K, Lortkipanidze N, Panich D, et al. : 281 Phase I/IIa clinical trial for the treatment of androgenetic alopecia using intradermal injections of cultured autologous dermal sheath cup cells. J Investig Dermatol. 2017; 137(10): S241. 10.1016/j.jid.2017.07.279 [DOI] [Google Scholar]
- 149. Kern S, Eichler H, Stoeve J, et al. : Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006; 24(5): 1294–301. 10.1634/stemcells.2005-0342 [DOI] [PubMed] [Google Scholar]
- 150. Kinnaird T, Stabile E, Burnett MS, et al. : Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004; 94(5): 678–85. 10.1161/01.RES.0000118601.37875.AC [DOI] [PubMed] [Google Scholar]
- 151. Shin H, Ryu HH, Kwon O, et al. : Clinical use of conditioned media of adipose tissue-derived stem cells in female pattern hair loss: A retrospective case series study. Int J Dermatol. 2015; 54(6): 730–5. 10.1111/ijd.12650 [DOI] [PubMed] [Google Scholar]
- 152. Park BS, Kim WS, Choi JS, et al. : Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: Evidence of increased growth factor secretion. Biomed Res. 2010; 31(1): 27–34. 10.2220/biomedres.31.27 [DOI] [PubMed] [Google Scholar]
- 153. Won CH, Yoo HG, Park KY, et al. : Hair growth-promoting effects of adiponectin in vitro. J Invest Dermatol. 2012; 132(12): 2849–51. 10.1038/jid.2012.217 [DOI] [PubMed] [Google Scholar]
- 154. Lockhart AR, Hakakian SC, Birnbaum EZ, et al. : Adipose derived stem cell based therapies or male/female pattern hair loss. J Stem Cell Res Med. 2016; 1(2): 59–63. 10.15761/JSCRM.1000109 [DOI] [Google Scholar]
- 155. Perez-Meza D, Ziering C, Sforza M, et al. : Hair follicle growth by stromal vascular fraction-enhanced adipose transplantation in baldness. Stem Cells Cloning. 2017; 10: 1–10. 10.2147/SCCAA.S131431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Stevens HP, Donners S, de Bruijn J: Introducing Platelet-Rich Stroma: Platelet-Rich Plasma (PRP) and Stromal Vascular Fraction (SVF) Combined for the Treatment of Androgenetic Alopecia. Aesthet Surg J. 2018; 38(8): 811–22. 10.1093/asj/sjy029 [DOI] [PubMed] [Google Scholar]
- 157. Butt G, Hussain I, Ahmad FJ, et al. : Stromal vascular fraction-enriched platelet-rich plasma therapy reverses the effects of androgenetic alopecia. J Cosmet Dermatol. 2020; 19(5): 1078–85. 10.1111/jocd.13149 [DOI] [PubMed] [Google Scholar]
- 158. Osugi M, Katagiri W, Yoshimi R, et al. : Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng Part A. 2012; 18(13–14): 1479–89. 10.1089/ten.TEA.2011.0325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Gunawardena TNA, Masoudian Z, Rahman MT: Dental derived stem cell conditioned media for hair growth stimulation. PLoS One. 2019; 14(5): e0216003. 10.1371/journal.pone.0216003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Dimmeler S, Ding S, Rando TA, et al. : Translational strategies and challenges in regenerative medicine. Nat Med. 2014; 20(8): 814–21. 10.1038/nm.3627 [DOI] [PubMed] [Google Scholar]
- 161. Fu W, Smith C, Turner L, et al. : Characteristics and Scope of Training of Clinicians Participating in the US Direct-to-Consumer Marketplace for Unproven Stem Cell Interventions. JAMA. 2019; 321(24): 2463–4. 10.1001/jama.2019.5837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. EASAC, FEAM: Challenges and potential in regenerative medicine: a joint report from EASAC and FEAM. German National Academy of Sciences Leopoldina, Halle (Saale). 2020; Accessed 16th August 2021. Reference Source [Google Scholar]
- 163. Cossu G, Fears R, Griffin G, et al. : Regenerative medicine: Challenges and opportunities. Lancet. 2020; 395(10239): 1746–7. 10.1016/S0140-6736(20)31250-2 [DOI] [PubMed] [Google Scholar]
- 164. Lee SH, Yoon J, Shin SH, et al. : Valproic acid induces hair regeneration in murine model and activates alkaline phosphatase activity in human dermal papilla cells. PLoS One. 2012; 7(4): e34152. 10.1371/journal.pone.0034152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Jo SJ, Shin H, Park YW, et al. : Topical valproic acid increases the hair count in male patients with androgenetic alopecia: A randomized, comparative, clinical feasibility study using phototrichogram analysis. J Dermatol. 2014; 41(4): 285–91. 10.1111/1346-8138.12422 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 166. Hawkshaw NJ, Hardman JA, Haslam IS, et al. : Identifying novel strategies for treating human hair loss disorders: Cyclosporine A suppresses the Wnt inhibitor, SFRP1, in the dermal papilla of human scalp hair follicles. PLoS Biol. 2018; 16(5): e2003705. 10.1371/journal.pbio.2003705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Bak SS, Sung YK, Kim SK: 7-Phloroeckol promotes hair growth on human follicles in vitro. Naunyn Schmiedebergs Arch Pharmacol. 2014; 387(8): 789–93. 10.1007/s00210-014-0986-0 [DOI] [PubMed] [Google Scholar]