Abstract
The Zeiss Airyscan microscope transforms a diffraction-limited, point-scanning confocal microscope into a super-resolution microscope using a specialized 32-channel Airyscan detector. By improving resolution two-fold and signal-to-noise ratio eight-fold relative to conventional confocal microscopes while retaining confocal functionality, the Airyscan microscope has become a very popular super-resolution imaging tool for cell biologists. In this chapter, we describe the fundamentals of Airyscan imaging, with the aim of helping the reader determine the proper acquisition settings for different types of experiments, optimize imaging conditions, and process the raw Airyscan images to obtain final images with the best quality. We also provide some tips, tricks, and best practices for Airyscan imaging. Of note, while our focus is on the Airyscan function of this microscope rather than its conventional confocal function, the Airyscan unit comes as an add-on to the conventional Zeiss laser scanning confocal microscope. This protocol is for the first generation Airyscan Zeiss 800 series microscope.
1. Introduction
The rapid development of super-resolution imaging technologies over the last decade has taken light-based imaging to new heights. These new technologies supersede the resolution limit caused by the diffraction of light as it passes through an objective lens in a traditional microscope. The limit of resolution of an optical system was first described in mathematical terms by Sir George Airy [1]. As light from a point source passes through the circular aperture of an objective lens it is diffracted. What results at the image plane is a central bright spot, termed the Airy disk, surrounded by less intense concentric rings of the diffraction pattern. In practical terms, any two fluorescent molecules located less than the radius of the Airy disk away from each will not be separable in the focused image. This distance is about half a wavelength, or about 240 nm for the blue light. The various super-resolution imaging modalities that have been developed in recent years all use a technological advance (for review see [2]) to exceed this resolution limit so that objects closer than their respective Airy disk can be resolved. These microscopes have enabled cell biologists to observe subcellular structures and their dynamics at resolutions ranging from 10 to 100 nm, compared to about 250 nm with a conventional confocal microscope. The super-resolution imaging modalities currently available offer biologists many options for optimizing resolution, speed, illumination strength, and signal-to-noise ratio to deal with their imaging challenges [3]. The Zeiss Airyscan microscope, which is based on a laser point-scanning confocal microscope, can resolve structures ~120 nm apart in the lateral dimension and ~350 nm apart in the axial dimension. Moreover, the Airyscan microscope represents one of the most gentle and sensitive live cell-compatible microscopes among all the super-resolution imaging modalities.
The key advance of the Airyscan technology over conventional confocal microscopy is the replacement of the adjustable pinhole with a circularly arrayed, 32-channel Gallium Arsenide Phosphide (GaAsP) detector. In a conventional confocal microscope, the pinhole is set ideally to the size of a central Airy disk (referred to as 1 Airy Unit) to allow passage of the signal from a point source at the focal plane while rejecting light from outside the focal plane. The pinhole thus allows optical sectioning with one Airy Unit resolution in all three dimensions. Decreasing the pinhole size to below 1 Airy Unit will improve resolution but will also result in the loss of signal strength [4]. The novel design of the Airyscan detector overcomes these limitations, allowing an enhancement of resolution without the loss of signal or an increase in noise. To accomplish this feat, the Airyscan detector uses an array detector consisting of 32 hexagonal micro-lenses arranged in a circular disk [5] (Fig. 1A), where each lens receives photons emitted from a small portion of an Airy disk and sends them through their individual optical fiber to a linear GaAsP detector array. The amount of light collected by each micro-lens is so small that each behaves as a virtual pinhole equivalent to 0.2 Airy units, while all 32 micro-lenses together cover a total area equivalent to conventional pinhole set to 1.25 Airy units. The result is a ~1.4-fold increase in resolution provided by each virtual pinhole. Along with this is an increase in detection efficiency resulting from summing the signals from all 32 PMT elements, all while minimizing the noise [5].
Figure 1.
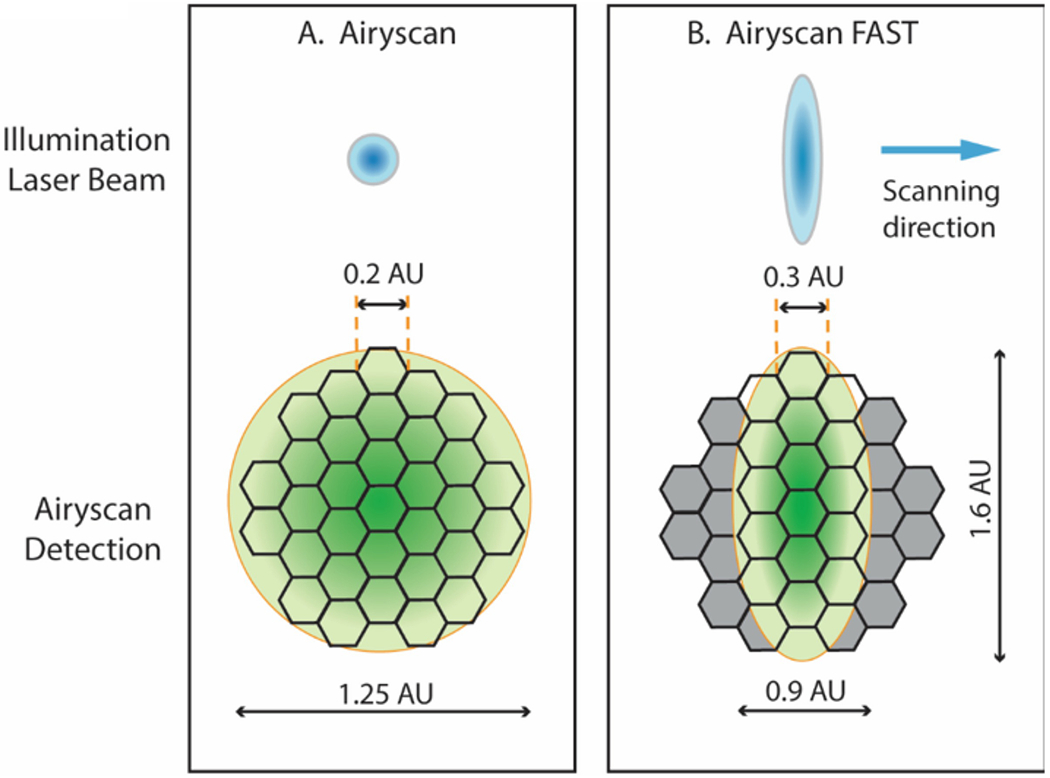
Airyscan detector in Airyscan mode (A) and Airyscan FAST mode (B). (A) The Airyscan detector consists of 32 hexagonal elements arranged in a circular disk. Each detector element acts as a pinhole the size of 0.2 Airy Units (AU). The whole detector captures the light equivalent to a pinhole of 1.25 AU. As the sample is scanned by point spot illumination from the laser, the Airy disk arising from a point emitter will be scanned by the detector. Through deconvolution and pixel reassignment, the individual images from each detector element are shifted to the center position to yield a final super-resolution image with resolutions of 120 nm in x and y and 350 nm in z. (B) In the FAST mode, the illumination is shaped like a short elliptical line. Each detector element has an equivalent pinhole size of 0.3 AU, and the central 16 elements of the detector will capture the light from 0.9 AU in x and 1.6 AU in y. Because the elliptical line scans four spots simultaneously instead of one, it scans the sample four times faster than in the regular Airyscan mode.
The ~two-fold improvement in resolution provided by the final super-resolution image is achieved through a processing step involving the linear deconvolution of each of the 32 individual elements to further enhance their lateral and axial resolution, followed by pixel reassignment of each element back to the center position. The total signal is summed to yield a final image exhibiting increased resolution in all three directions, greatly increased signal relative to using the same excitation power in a conventional confocal acquisition, and reduced noise [5, 6]. The Airyscan microscope stands out among other super-resolution modalities for live cell experiments because high-quality images can be acquired with very low laser power and without frame averaging, thus reducing phototoxicity.
The Airyscan detector is an add-on module to the Zeiss LSM 800 series confocal microscope. This chapter focuses on Airyscan operation in both the regular (see Section 3.1) and the FAST modes (see Section 3.2). We cover procedures regarding detector alignment, acquisition settings, imaging condition optimization, and data processing. Typical confocal imaging tasks, such as z-stack, time series, FRAP, FRET, region scanning, tile scanning, and multi-position scanning, can be performed using the Airyscan detector in exactly the same manner as for standard confocal acquisition, and so are not covered here.
2. Materials
#1.5 coverslips or #1.5 coverslip-bottom chambers for all specimen preparations (see Note 1).
Imaging medium for live cell imaging. Two types of medium can be used. One is a complete culture medium with no phenol red that is suitable for your cell type (see Note 2 & 3). Alternatively, a, CO2-independent medium such as Leibovitz’s L15 with no phenol red and supplemented with 10% serum can be used (see Note 4).
An environmental chamber for temperature, humidity and CO2 control for live cell imaging.
An enclosure to surround the inverted microscope stand (see Note 5).
Zeiss immersion oil 30 °C for room temperature imaging.
Zeiss immersion oil 37 °C for 37 °C live-cell imaging.
Lint-free lens tissue and lens cleaning solution.
Zeiss LSM 800 series inverted confocal microscope equipped with an Airyscan detector.
3. Methods
3.1. Airyscan Imaging
Airyscan imaging involves the selection of five components: laser lines, dichroic mirrors, beam pass plate, dual filter sets, and the detector (Fig. 2). Options for each of these components must be determined before imaging to build tracks for the excitation and emission filters that match the fluorescent molecules in your sample. The configuration you set up will affect your imaging in terms of speed, resolution, and sensitivity. Keeping in mind that there are tradeoffs, careful consideration of these factors will allow you to choose the optimal combination of each element for the best imaging configuration (see Note 6 & 7)..
Figure 2.
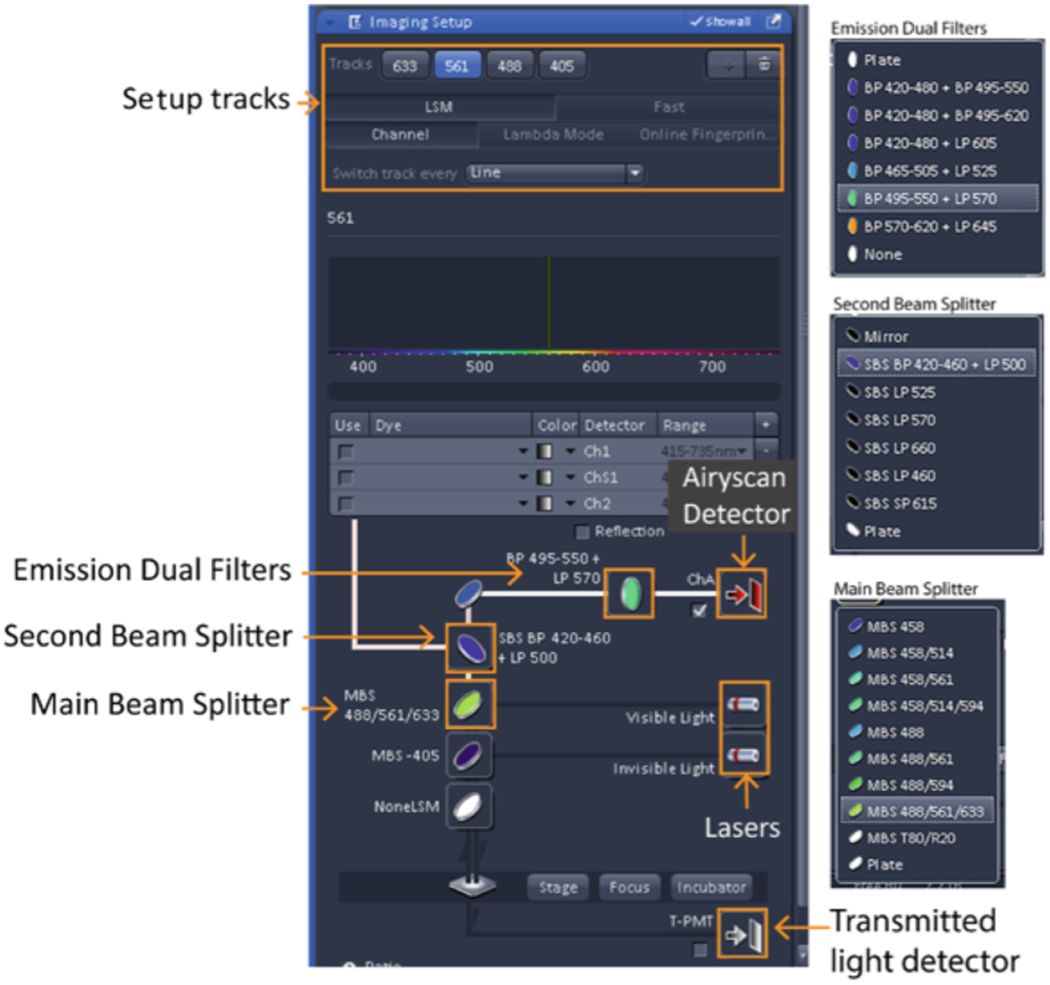
Screen shot of the imaging setup for light path configuration.
3.1.1. Turn on the system and launch the Zen (black) software
On the remote control box in order, press the “Main Switch”, “System/PC” and “Components” switches to the ON position.
Ensure the argon gas laser is switched to the ON position. On most systems, the laser is kept in the ON position by default. The laser is activated in the software.
Turn on the metal-halide light source for widefield epi-fluorescence imaging.
Turn on the acquisition computer and log onto the system.
-
Launch the ZEN (black) software.
Select “Start System” for image acquisition.
3.1.2. Turn on lasers
Select “Acquisition” from the main toolbar.
Click the laser dropdown bar to expand the laser window and toggle the “Power” button to ON for the Argon laser to access the 458, 488 or 514 nm laser lines. You will see the laser highlighted in red during the 5 minute warmup period. You will not be able to use the laser until it is ready, which is indicated by the disappearance of the red highlight.
Lasers other than the Argon laser typically do not need to be warmed up. Turn on the required lasers for your experiment.
3.1.3. Place the sample on the stage and locate your sample
Chose the appropriate stage holder and insert your slide your specimen chamber (see Note 8).
Select the desired objective (usually the 63X NA 1.4 oil objective) from the objective pulldown menu in the “Locate” window under the “Microscope Control” dropdown bar (see Note 9 & 10).
Apply the correct immersion oil to the objective lens (see Note 11). Use the 30 °C oil for room temperature imaging and the 37 °C oil for live-cell imaging at 37 °C.
Place the sample on the stage insert with the coverslip side down to face the objective and ensure that the sample slide or chamber is level.
Focus your sample either through the eyepiece or by laser scanning. If laser scanning is used for focusing, click the “Live” button in the “Acquisition” window after you load the correct light path configuration (see Section 3.1.4).
Use the stage joystick to position your sample in the center of the field of view.
3.1.4. Set the Airyscan light path configuration for image acquisition
Open the “Imaging setup” window (Fig. 2) and select “LSM” and “Channel” (see Notes 12 & 13).
A maximum of four tracks can be set up. By default, “Track 1” will appear in the Imaging setup window. Select “ChA” (the Airyscan detector) for this and every subsequent fluorescence track if performing multicolor imaging (see Note 14).
Select the appropriate laser line for the excitation of your chromophore.
Select the “Main Beam Splitter” (MBS) for excitation. For a single-color configuration, pick the simplest MBS dichroic that matches your laser because fewer coatings gives you better light efficiency (e.g. MBS 488 for GFP). For a multicolor configuration, pick the MBS that matches the combination of laser lines you need for multicolor excitation (e.g. MBS 516/488 for Alexa 568 and GFP). Keeping the MBS the same between different tracks in multicolor configuration is highly recommended to avoid unnecessary slow down between track changes.
Select the desired “Secondary Beam Splitter” (SBS) position to direct emission light to the Airyscan detector. The “Mirror” position blocks the light to the Airyscan detector, directing it instead to the confocal detectors and is not used. The “Plate” position allows all emission light to pass to the Airyscan detector and is the typical choice. Choice of either short pass (SP), band pass (BP) and long pass (LP) dichroic beam splitters is available to proportionally split emissions to the Airyscan and confocal detectors for experiments that utilize both types of detectors in combination (not addressed in this chapter).
Select the emission filter for your chromophores. A variety of dual bandpass filters are available. Choice is dependent upon the emission wavelengths needed for subsequent tracks. For example, if your experiment requires detection of Alexa 561 and GFP, choose BP 495-550 + LP 570 for both track 1, to detect Alexa 561, and track 2, to detect GFP (see Note 15). Using the same filter minimizes moving parts in the system and decreases scan time.
If needed, select T-PMT (the transmitted detector) for the transmitted light channel (see Note 16).
Click the “+” button to add another track if multiple colors are needed. Follow the steps above to choose the appropriate beam splitters and filters for the next chromophore. Table 1 lists examples of commonly used configurations for your convenience.
In the “Image Setup” window next to “Switch track every” choose to switch track acquisitions either between lines or between frames depending on imaging needs. If a DIC image is required, the either “Frame Fast” or “Frame” must be selected. If beam splitters or filters need to change between tracks, then “Frame” must be selected. If the experiment involves live cell imaging, especially where there is rapid movement of labeled structures, then “Line” is the optimal choice (see Notes 17 – 20).
Table 1.
Common configurations for Airyscan SR imaging. Select these components in the “Imaging Setup” window. The options for each filter set are listed in Fig. 2.
| Configuration | Tracks | Laser | Main Beam Splitter (MBS) | Second Beam Splitter (SBS | Emission Dual Filters | Detector |
|---|---|---|---|---|---|---|
| 4 Color Line Switch | 1 | 633 | MBS 488/561/633; MBS 405 | Option #1 | Ch A | |
| 2 | 561 | Plate | Plate | |||
| 3 | 488 | Option #2 | ||||
| 4 | 405 | SBS BP 420-460 +LP 500 | BP495-550+ LP570 | |||
| 4 Color Frame Switch | 1 | 633 | MBS 488/561/633; MBS 405 | SBS LP 660 | BP 570-620 + LP645 | Ch A |
| 2 | 561 | SBS SP 615 | BP 570-620 + LP645 | |||
| 3 | 488 | SBS BP 420-460 +LP 500 | BP420-480 + BP495-550 | |||
| 4 | 405 | Plate | BP420-480 + BP495-550 | |||
| 2 Color (RG) Line Switch | 1 | 561 | MBS 488/561 | SBS SP 615 | BP 495-550 + LP 570 | Ch A |
| 2 | 488 | |||||
| 2 Color (RG) Frame Switch | 1 | 561 | MBS 488/561 | SBS SP 615 | BP 570-620 + LP645 | Ch A |
| 2 | 488 | BP420-480 + BP495-550 | ||||
| 3 Color (RGB)+Transmitted Light Frame Switch | 1 | 561 | MBS 488/561/633; MBS 405 | SBS SP 615 | BP 570-620 + LP645 | Ch A |
| 2 | 488 | Plate | BP420-480 + BP495-550 | |||
| 3 | 405 | Plate | BP420-480 + BP495-550 | |||
| 4 | 633 | not affect | not affect | T-PMT | ||
3.1.5. Aligning the Airyscan detector
Alignment is done with your imaging sample and with only one track (see Note 21). Choose a bright cell or region from the track with the best signal that has good structural details and contrast. Cells that have very weak and/or diffusive staining may impede the alignment process (see Note 22).
Click the “Acquisition” tab and use “Live” scan to place the cell in the image window using the joystick. The sample should be in the center position of the stage (x=0, y=0). Zoom in so that the sample fills most of the field of view. Select a small frame size such as 256 x 256 for fast scanning and adjust the signal intensity to about 50% of the total dynamic range of the PMT by increasing the main detector gain (you may increase the laser power if the detector gain is not enough to get the intensity to 50%).
Click the “Maintain” tab and expand the “Airyscan” panel. Undock the panel window so that you can monitor the alignment process. Check the “Adjust in continuous scan” box; this will allow you to activate the automatic alignment process when you click the “Continuous” scan in the next step. Check the “Weak or sparse samples” box if your signal is weak.
Return to the “Acquisition” window and click the “Live” scan button to ensure your sample is still in focus, stop and then click the “Continuous” scan button to initiate the alignment process.
To see the Airyscan detector view below the active image, click the “Airyscan” tab on the top left side of the image display, and check the “Detector view” box at the lower side of image window. If necessary, check the “Show all” box under the image display to bring up this option. During the auto-alignment process, intensities will change in the detector view and the message on the Airyscan panel opened in step 3 will go through the following indications: (i) Unknown? No beam? (Waiting), (ii) Bad, (iii) Adjusting, (iv) Good.
When “Good” is displayed in green in the Airyscan panel, your alignment is complete. Stop the continuous scan. Typically, a nice gaussian distribution pattern with a bright center and dimmer edges will appear in the detector view panel (Fig. 3A).
If the auto-alignment process does not reach the “Good” status, it is usually because the sample signal is too diffusive and/or weak. If this happens, restart the continuous scan and adjust the focus during alignment to maximize the signal. If the sample is very dim, you can select “R-S” mode for the Airyscan detector (ChA) in the “Channels” window to perform the alignment. If all this fails, you can manually adjust the detector alignment by changing the servo position in the Airyscan alignment panel so the best possible gaussian intensity distribution appears in the detector view panel. It is best to avoid this last approach, as it can alter the hardware positions. Record the servo positions before you manually change the slider if you need to return to the previous setting.
Uncheck the “Adjust in continuous scans” box to avoid triggering the auto-alignment process.
Figure 3.
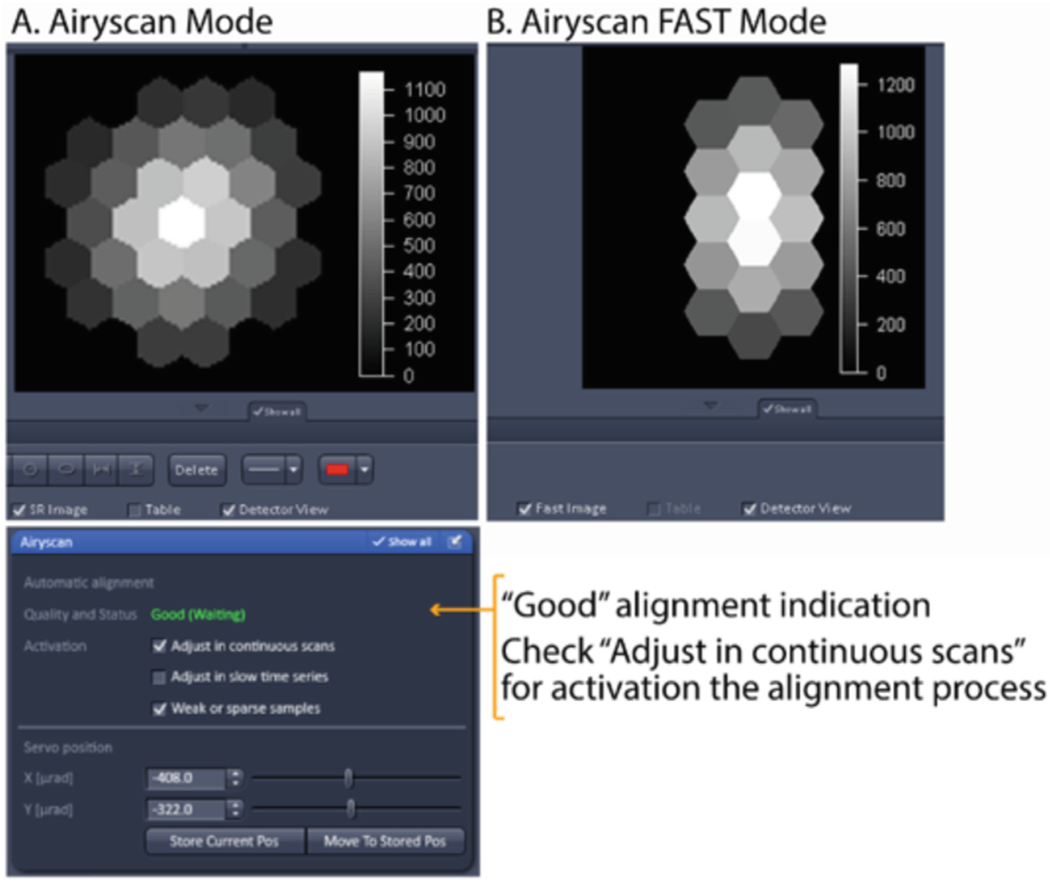
The screen shot indicating good alignment of the Airyscan detector.
3.1.6. Set parameters in Channels window for acquisition
Set parameters in the “Channels” window for steps 1 – 7 for each track one at a time to obtain proper signal intensities (see Fig 4A).
Ensure that SR (super-resolution) mode is checked for super-resolution imaging. This is the default mode (see Note 23).
Ensure the pinhole is set larger than 1.25 Airy units (out of the red marker on the slider) (see Note 24).
Set laser power less than 1% (see Note 25).
Using the histogram under the Image window as a reference (click the “Display” tab to view it), adjust the master gain so that the signal does not go beyond 50% of the PMT’s dynamic range. This will prevent saturation of the Airyscan detector. Saturated pixels lead to data processing artifacts (see Note 26).
If possible, leave the digital gain set to 1 and balance the signal strength for each track/channel with laser power (see Note 27). The offset is automatically adjusted by the software.
Adjust the image display by pressing the “Min/Max” button under the image window to better view the signal for samples that have a small dynamic range.
Set up the following parameters in the “Acquisition mode” window in steps 9 – 16 (see Fig. 4B):
Figure 4.
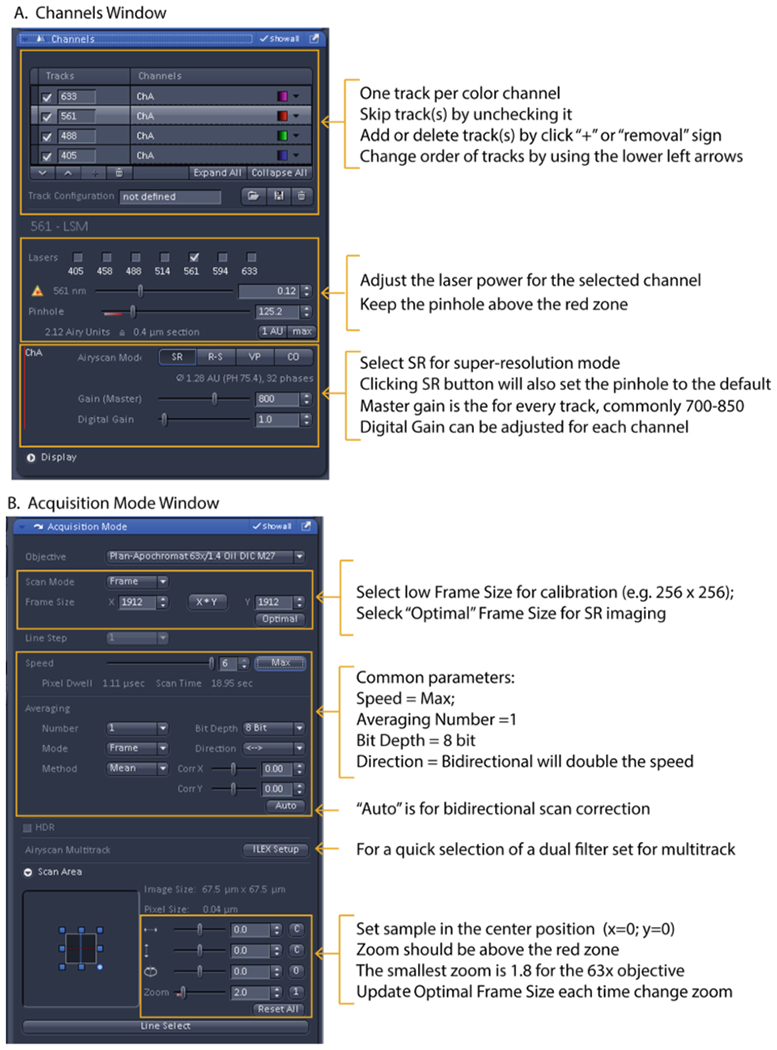
Key screen shots (left) and quick guides (right) for Airyscan Acquisition. (A) “Channel” window. (B) “Acquisition Mode” window.
Set “Zoom” higher than 1.8 for the 63X objective.
Set the “Frame Size” by pressing “Optimal” to ensure that the correct number of pixels based on Nyquist criteria are used for the size of the field of view (see Note 28).
Set “Speed” to Max; however, slower speeds may reduce the shot noise.
Set “Averaging” to 1X; however, 2X can be used with dim samples to average out background noise if time is not an issue.
Leave “Bit depth” at the default setting of 8-bit (256 grey levels) (see Note 29).
Optional: set “Direction” to bidirectional scanning to increase the image speed by 2X (see Note 30 & 31 and Table 2).
“Direction”: Bidirectional scanning will increase imaging speed.
If a 3D super-resolution images is desired, click “Optimal” in the z-stack panel to set an interval between z-planes based on Nyquist sampling (see Note 32).
Acquire single 2D images by pressing the “Snap” button or press the “Start Experiment” button for all multi-dimensional imaging modes including z-stack, time sequence, FRAP, tile, multi-positions, etc. (see Note 33).
Table 2.
Two-color, line switch Airyscan scan time at a given zoom. The scan time listed is under the following conditions: no averaging (ave 1), bi-directional scan, and at 8-bit depth.
| Zoom | Image size (um2) | Pixel size | Frame size | Max speed | Pixel dwell | Scan time |
|---|---|---|---|---|---|---|
| (um) | (pixel) | (us) | ||||
| 1.8 | 75 | 0.04 | 1764x1764 | 6 | 1.2 | 8.72 sec |
| 2.7 | 50 | 0.04 | 1176x1176 | 7 | 0.89 | 2.90 sec |
| 3.5 | 38.6 | 0.04 | 904x904 | 8 | 0.86 | 1.65 sec |
| 5 | 27 | 0.04 | 632x632 | 9 | 0.83 | 779.47 ms |
| 6.3 | 21.4 | 0.04 | 504x504 | 10 | 0.85 | 513.62 ms |
| 8.9 | 15.2 | 0.04 | 356x356 | 12 | 0.95 | 282.73 ms |
3.1.7. Saving images
Save raw images as a CZI file type for further Airyscan image processing.
Optional: From the “Streaming and Auto Save” window, automatically save your images in a folder after acquisition.
Optional: If your system is equipped with a secondary processing computer with an ethernet connection, stream and process large data sets directly during acquisition.
3.1.8. Tuning off the microscope
Click the laser tab to expand the laser window and toggle the “Power” button from ON to OFF for all your laser lines.
Exit the Zen and shut down the computer.
If you used the Argon laser (458, 488 or 514 nm laser lines), you will need to wait for 5 min or until the power supply unit stops cooling.
Switch off the fluorescence light source.
On the remote control box, in order, switch off “Components”, “System/PC” and “Main Switch”.
Clean your objective.
3.1.9. Airyscan image processing
Airyscan image processing applies pixel reassignment and deconvolution to the raw images to generate the final super-resolution images. Individual images can be processed after acquisition, or they can be batch-processed from the same folder at a later point. Especially beneficial for large data sets is an off-line processing computer. Data can be streamed directly to it and processed immediately during acquisition.
Choose the “Processing” tab and select “Airyscan processing” from the method list.
Click on the raw image in the image window to make it the active image.
Press the “Select” button in the “Airyscan processing” window.
Leave the processing strength on “Auto” (see Note 34).
Select “2D” or “3D” options corresponding to your image (see Note 35).
Press the “Apply” button at the top of the panel to execute image processing.
View processed images by adjusting the display using “Min/Max” for final image display in the display window.
Save your processed images as a CZI or TIFF.
To batch process raw images saved in a folder, click the “Run Batch” button on the bottom of the parameter panel. This will direct you to the file directories. Go to your image folder and highlight the image files to be processed. All files for batch processing need to be in the same format, e.g. all are 2D images, or all are 3D images. Set the processing options as above and click the “Apply” button. The processed images will be automatically saved to the same folder as your raw data with the same name followed by “out” at the end of the name.
To immediately process a streamed time series, launch Zen on the off-line processing computer. On the acquisition computer, select “Streaming” in the save tab and check the “Time” box under the “Separate Files” tab. Click the “Start Experiment” button. You will be directed to the file directory for the file destination. Enter your processing computer, give your image folder a name, and then click “OK”. Images will start streaming to your destination file folder. Open your streaming file in the processing computer as soon as you see it, select and drag the first image shown in the folder (the ones without a number in parentheses in the file name) to Zen. Click the “External Index” box in the Airyscan process window. Click “Apply”. Individual time point files (with the number in the file name) will be saved into a result subfolder inside of your raw data file folder.
3.2. Airyscan FAST Imaging
The Airyscan FAST mode is designed to increase Airyscan imaging speed four-fold with minimal sacrifice of improved resolution, field of view, and sensitivity [7]. In the Airyscan FAST mode, the excitation beam is shaped as an ellipsoid rather than the normal round spot to perform a simultaneous scan of four pixels in the y axis (see Fig. 1B). Relative to standard confocal imaging, the Airyscan FAST SR mode yields a 1.5-fold improvement in resolution and a 4-fold improvement in signal-to-noise ratio, compared to a 2- and 8-fold respective increase in the regular Airyscan mode. The significant gain in speed and gentleness in illumination, together with the improvements in confocal resolution and signal-to-noise ratio, makes the Airyscan FAST mode a valuable tool for imaging fast live-cell dynamics. In addition to the SR mode, the Airyscan FAST mode provides options to further increase the scanning speed by using a lower sampling rate (fewer pixels in a frame) for applications where resolution is not so important. Be aware the resolution improvement provided by the Airyscan FAST mode is not isometric in x and y, yielding 145 nm for x, 165 nm for y, and 450 nm for z. Operation of the Airyscan FAST mode is essentially the same as for the regular Airyscan mode with some special procedures and options described below.
3.2.1. Set the Airyscan FAST light path configuration
For single-color imaging, set the configuration the same way you do for the regular Airyscan mode line switch configuration, followed by selecting “Fast” in the “Imaging Setup” window.
For multi-color imaging, setup or load your Airyscan configurations and switch each track to the Airyscan FAST mode by clicking “Fast” in the “Imaging Setup” window for each track.
3.2.2. Aligning the Airyscan detector for the FAST mode
This is done exactly as described with the regular Airyscan mode. Notice that the detector view will be the middle 16 units instead of the entire 32 unit array (Fig. 3B).
3.2.3. Acquiring Airyscan FAST images
Set up your imaging configuration as described with the regular Airyscan mode.
Choose a sweet spot between resolution and speed for your experiment by selecting the sampling rate in terms of pixel number. The Airyscan FAST mode offers four possibilities for the sampling rates from the “Acquisition mode” window: SR −2X sampling rate (Nyquist). This offers the best resolution (145 nm and 165 nm in x and y) with 4-fold faster imaging speed than regular Airyscan SR. OPT −1X sampling rate. This offers confocal equivalent resolution with 8-fold faster imaging speed than regular Airyscan SR. FLX – 0.7X sampling rate for 11-fold faster imaging speed. FS – 0.5X sampling rate for 16-fold faster imaging speed.
In the “Acquisition Mode” panel, set the speed, averaging, bit-depth, and direction of scanning as described with the regular Airyscan mode (see Note 36).
In the “Channels” window, set the laser power and “Gain” for each track the same way as described with the regular Airyscan mode (see Note 37).
Acquire and save images as described with the regular Airyscan mode.
Acknowledgements
We wish to thank Matthew Donnelly and Zeiss specialists Alma Arnold and Sricharan Murugesan for their helpful comments and for critical reading of the chapter.
4 Notes
Sample preparation is no different from that for traditional confocal imaging of both fixed and live-cell samples in terms of choice of fluorophores, colors, and labeling methods.
The imaging medium should not contain phenol red as it increases fluorescence background.
Complete culture medium is recommended for sensitive cell types and for long-term time-lapse imaging of all cell types. Such imaging requires an environmental chamber system to maintain a supply of humidified 5% CO2 at 37 °C during imaging.
While CO2 is not required during imaging using a HEPES-based medium, an environmental chamber is needed if imaging needs to be done at 37 °C.
An enclosure for the inverted stand is strongly recommended by Zeiss to avoid vibration from airflow and ensure environmental stability. The enclosure is required even for room temperature experiments.
An easy way for routine configuration setup is to modify an existing four-channel configuration. Simply load the four-channel configuration, and then remove the channels you don’t need by dragging them to the dust bin, or by unselecting the channel in the channel window.
For a quick two-channel line scan setup, you can pick your dual emission filter from the “Acquisition Mode” window. Click the “ILEX Setup” button to access the pulldown menu for the dual filter sets.
If your system is equipped with a piezo stage, turn off the power at its control box when you change stage holder to avoid damaging the sensitive piezo mechanism.
The 63X NA 1.4 oil special super-resolution objective is the best objective lens for Airyscan imaging, as it ensures the best resolution and brightness. However, other objectives will also yield improved image quality in Airyscan mode compared to confocal mode. For example, a 20X objective in Airyscan mode will behave like a 60X objective in standard confocal mode.
While a DIC slider is not compatible with Airyscan imaging, you are able to image in brightfield without the DIC slider.
An oil immersion objective lens will have “Oil” printed on the barrel of the lens. Do not use immersion oil on air lenses or lenses that use different immersion media such as water, glycerol or silicon.
The Zeiss Zen Black software that runs the 880 series system does not have the “Smart Setup” wizard to assist users to set up tracks in the Airyscan mode.
Section 3.1.4 describes how to manually set up an imaging configuration. Once a configuration is made, it can be saved and recalled as premade setup from the dropdown in the Experimental Manager window. Pay attention to whether the configuration is set for frame or line switch mode for your needs. Alternatively a configuration can be reused from a previously saved image. This is a simple shortcut and is especially useful when you want to repeat your exact imaging conditions. One tip is to save an image from an experiment as a configuration template. The “Reuse” button is found under the image window. From a saved or reused configuration, tracks can be modified or deleted as necessary.
Understanding imaging “Tracks”: One imaging track in Airyscan mode corresponds to one color channel because there is only one Airyscan detector in the system. For single-color imaging, you only need to select the correct laser line and filter set that match the excitation and emission wavelengths of your one chromophore. For multi-color imaging, each color must be imaged sequentially using separate tracks. You can image up to four colors because there are only four available tracks. How the tracks are switched can affect your image speed and image quality.
After multiple tracks have been created, track order can be changed using the up and down arrow buttons under the tracks in the “Channels” window. Typically, imaging from red to blue wavelengths helps best to lower the phototoxicity and protect a sensitive chromophore from bleaching.
To include a transmitted light image, you need to set up a separate track using the “Frame” switch-based configuration, not “Line” switch configuration. While any laser line can be used for the transmitted channel, the laser that will not affect your fluorescence signal will be the best one to use. If you need a transmitted light image, you will be limited to three fluorescent tracks.
Understanding “Line” switch: Here the different tracks (i.e. colors) are switched for every line scan. This requires that the hardware filters remain the same for all colors. The colors can be separated by the dual color emission filters together with the dichroic filters and specific excitation lasers. This option will give you almost simultaneous scanning for each line of different colors because no change in hardware is required. It is, therefore, the fastest approach for multi-color imaging. However, it is subject to color bleed-through artifacts, especially if one color is much brighter than the others. Having said that, Zeiss provides good emission dual filter sets for two-color imaging. It usually works well, and the bleed-through artifact is minimal when the signal intensities of the different colors are comparable. In combination with the excitation lasers and the dichroic beam splitters, three-color and even four-color line switch imaging is possible. Therefore, the “Line” switch is the first choice if you need to do fast time series for live-cell specimens. If one color is much brighter than the others, the bleed-through problem is more noticeable. Note that a variety of specialized dual filters are available from the manufacturer to suit experimental needs.
Understanding “Frame” switch: Here the different tracks (colors) are switched for every frame scan. With this option you can pick different filter sets and beam splitters that are more specific for each color as regards both excitation and emission. This will help prevent bleed-through. However, the time required for imaging is considerably longer than using the line switch option because the hardware must be changed for every frame scan.
Understanding “Z-Stack” switch: Here the different tracks (colors) are switched every full z-stack. This 3D imaging option is only available when “Z-Stack” is selected in the “Acquisition” window. It performs an entire z-stack per color so that you can use the same filter sets you selected for the frame switch mode. It is faster to image a z-stack per color than to image multiple colors per z-section, especially when you use a z-piezo motor (by selecting “Use Piezo” in the “Z-Stack” setup window). However, for live-cell imaging you need to be aware that there will be a time interval between tracks at the same z position, and that this interval could be large if the z-stack consists of many planes. This could cause color overlay problems. If this turns out to be a problem for you, the line switch or frame switch options at every z plane will be better choices.
Understanding “Frame-Fast” switch: Here the different tracks (colors) switch every frame just as in the frame switch setup, but the hardware components are kept constant for each track (as in the line switch setup). Although this setup does not provide faster speeds than the line switch option for multi-color fluorescence tracks, it does provide an advantage by adding a transmitted light track together with the fluorescence tracks (this cannot be done using the line switch option).
The Airyscan detector needs to be aligned with the optical axis. The alignment is an automated process and normally takes about a minute and need only be done with one track (all other tracks are co-aligned). Zeiss recommends performing it at the beginning of your imaging session and each time you change the objective lens. You don’t have to do an alignment when you change samples unless your next sample is a different type (e.g. going from a single cell layer to a tissue sample). Alignment is required in this case because the differences in the refractive index affect the alignment.
The process of optimizing the alignment may bleach your sample. Use an area of your specimen for the alignment that is not critical for your subsequent imaging session.
The Airyscan detector can be used in several alternative non-super-resolution modes. Note that all images acquired in these modes must be processed as images acquired in the SR mode. By changing the way the signals are reassigned and summed over the array detectors, these modes offer advantages to dealing with weak signals. “R-S” mode (Resolution vs Sensitivity): This mode is useful for capturing very dim and/or fast dynamic processes. The detector receives the complete Airydisk pattern of 2 Airy units instead of 1.25. This mode maximizes the signal-to-noise ratio but sacrifices resolution and confocality. It provides resolution improvements of 1.6 to 1.9-fold over standard confocal imaging depending on the pixel size you choose. “CO” mode (Confocal): This mode offers standard resolution and sensitivity and is useful for weak signals without sacrificing confocality. It acts like a standard GaAsp detector. “VP” mode (Virtual Pinhole): In this mode, the detector mimics the different pinhole sizes as apparent pinhole sizes, thereby allowing confocal sectioning of the sample after image capture. It is useful for examining optical sectioning effects post acquisition.
Because the Airyscan operates without a pinhole, the pinhole should be open to ensure that sufficient light is received by the detector. By clicking the SR button, the pinhole will go to the default setting. Closing down the pinhole to some degree will help eliminate background/stray light, but never close the pinhole down to below the red marker in the pinhole setting.
To minimize bleaching and photon toxicity, the laser power should be kept low, which usually means less than 1% for most applications.
The master gain is commonly set between 700 to 850, and its value will apply to every track/channel.
In line switch imaging mode, the same gain setting for ChA must be used by all tracks to take advantage of faster acquisition times. If increasing laser power is not acceptable, either because of phototoxicity or photobleaching, then raising the digital gain for a dim track/channel can help correct unbalanced signal intensities by bringing them to similar levels in line switch imaging.
Properly sampled images are essential for obtaining high-quality super-resolution Airyscan images.
The bit depth does not affect the spatial resolution; however, more resolution in intensity variations is obtained if one selects 16-bit (65,536 grey levels). Tradeoffs, are a longer scan time, because the read-out time is doubled for a 16-bit image, and larger data size per image (keep in mind that a raw Airyscan image is composed of 32 individual images from the detector array). If 256 gray levels are good enough for your image, then the 8-bit is the better choice.
Bidirectional scanning sometimes produces a skewed image caused by scan head positional errors due to changes in scan direction. The Zen software automatically corrects such errors by having the “Online scanner calibration” option checked, which is found on the “Maintain” tab, on the “Hardware” tab in the “Systems Options” window. Errors can be also corrected manually with the Corr x and Corr y sliders or by clicking “Auto” in the “Acquisition Mode” window (using either of these latter options requires the “Online scanner calibration” option to be unchecked).
Considerations regarding image size and scan speed: As for all point-scanning microscopes, the acquisition time of the Airyscan is proportional to the observation volume. The largest field of view is 75 um2 (which corresponds to the smallest zoom factor of 1.8). To help in estimating scan time, Table 2 lists common two-color line scan times in relation to image size at the maximum scan speed with no averaging (average of 1), using a bi-directional scan at 8-bit depth. Doubling your bit-depth or using one-directional scans will reduce your scan speed two-fold. For 3D imaging, multiplying the number of z-stacks by the 2D scan time will give you your total time for 3D volume imaging.
The z interval is about half of the longest wavelength you select for imaging. Usually it ranges from 0.19-0.3 µm depending on the wavelength. If z-resolution is not important for your experiment, you can choose any z-stack interval. If so, you will get a z-stack of 2D super-resolution images. In this case you should apply 2D data processing for your z-stack accordingly in the processing step (see Section 3.1.9).
Do not press the “Airyscan” tab on the left side of the image window during a time lapse acquisition. Although it will update the super-resolution image next to the raw image, it may overwhelm the computer, causing a stall or software crash.
Considerations regarding data processing strength. The processing strength is set as an arbitrary number from 1 to 14. Higher strength numbers give better resolution but are subject to more processing artifacts, and vice versa. The Zeiss “Auto” process setting usually works very well as it takes into account the contributions from objective NA, wavelengths, sample quality, signal-to-noise ratio, etc. for its deconvolution computation. Nevertheless, you can still set the processing strength manually to refine your results. Use an “Auto” processed image as a reference point to observe the strength number used by the software algorithm by clicking the “Info” tab on the left side of the image window. We recommend not exceeding +/− 1 units from the “Auto” strength number.
Note that if your 3D data set is not done with the optimal Z interval, you should select “2D” processing instead of “3D” processing both because you lack Z information needed for SR processing and because you can save processing time using 2D stacks.
Bidirectional scanning is the preferred selection in the Airyscan FAST mode, which has a built-in correction factor for bidirectional scanning.
The laser power in the Airyscan FAST mode displays two values: the first shows the effective laser power after the beam is reshaped in Airyscan FAST, the second value represents the equivalent laser power in regular Airyscan mode.
References
- [1].Airy GB (1835) On the Diffraction of an Object-glass with Circular Aperture. Transactions of the Cambridge Philosophical Society 5: 283–291. [Google Scholar]
- [2].Schermelleh L, Ferrand A, Huser T, Eggeling C, Sauer M, Biehlmaier O, Drummen GPC (2019) Super-resolution microscopy demystified. Nat Cell Biol 21(1): 72–84. [DOI] [PubMed] [Google Scholar]
- [3].Lambert TJ, Waters JC (2017) Navigating challenges in the application of superresolution microscopy. J Cell Biol 216(1): 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sheppard CJ (1988) Super-resolution in confocal imaging Optik 80(2): 53–54. [Google Scholar]
- [5].Huff J (2015) The Airyscan Detector from Zeiss: confocal Imaging with Improved Signal-to-Noise Ratio and Surperresolution. Nature methods 12: 1205. [Google Scholar]
- [6].Weisshart K (2014) The Basic Principle of Airyscanning. Zeiss Technology Note. [Google Scholar]
- [7].Huff J (2016) The Fast mode for Zeiss LSM 880 with Airyscan: high-speed confocal imaging with super-resolution and improved signal-to-noise ratio. Nature Meodthods 13: 958 [Google Scholar]


