Supplemental Digital Content is available in the text.
Keywords: cancer, COVID-19, Health Services Research, pandemic, treatment
Abstract
Objectives
The coronavirus disease 2019 (COVID-19) pandemic has affected the availability of healthcare resources, and adjustments to cancer care have been necessary considering the risk of morbidity by COVID-19 and of cancer progression. This study aims to quantify the impact of the COVID-19 pandemic on the care of patients with cancer by comparing a period of 4 months after the outbreak began (2 March 2020) with an equal period from 2019.
Methods
Cancer cases of the esophagus, stomach, colon and rectum, pancreas, lung, skin-melanoma, breast, cervix, prostate, non-Hodgkin lymphoma, and leukemia from the Portuguese Oncology Institute of Porto, and diagnosed between 2 March and 1 July 2019 (before COVID-19) and 2020 (after COVID-19) were identified. Those with the first treatment outside the Portuguese Oncology Institute of Porto were excluded. Sociodemographic, clinical and treatment characteristics were obtained from the cancer registry database and clinical files.
Results
The absolute number of new cancer cases decreased nearly 40% after the COVID-19 pandemic (from 1430 to 866). The largest decreases were observed for cervical (−74.3%) and prostate (−71.7%) cancers. Cases were more often diagnosed at more advanced stages in 2020 (P = 0.001), and the proportion of patients not starting any treatment until 1 July was just under 20% in 2019 and nearly 40% in 2020. The median times from symptoms onset, first medical exam and first appointment to diagnosis, and from diagnosis to first appointment, multidisciplinary tumor board meeting and first treatment were shorter after COVID-19.
Conclusions
There was a notable overall decrease in cancer diagnoses after COVID-19, with changes in the characteristics of incident cases.
Introduction
The novel coronavirus, also known as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become a worldwide threat and health concern. Since its outbreak in China in late 2019, the coronavirus disease 2019 (COVID-19) has caused over 46 million confirmed cases worldwide and over 1.2 million deaths as of 1 November 2020 (World Health Organization, 2020a). The first COVID-19 case in Portugal was confirmed on 2 March 2020 in the northern region of the country (Direção-Geral da Saúde, 2020a). On 11 March 2020, the WHO announced COVID-19 was a pandemic (World Health Organization, 2020d), and on 18 March 2020, Portugal entered a State of Emergency until 2 May 2020 (Presidente da República, 2020). Unprecedented measures were adopted worldwide to mitigate the spread and morbidity of the virus through large-scale use of isolation and quarantine, closing borders, imposing social distancing limits, and enforcing nationwide lockdowns (World Health Organization, 2020c).
Many healthcare settings implemented minimal services, with programmed activity, including routine and specialist appointments, as well as elective procedures, being canceled, postponed or replaced by telemedicine whenever possible (Direção-Geral da Saúde, 2020b, 2020c; Mann et al., 2020; Webster, 2020; World Health Organization, 2020b). Cancer screening programs were also suspended (Issaka and Somsouk, 2020; Jones et al., 2020; Richards et al., 2020; Roberts, 2020), with countries reporting decreases of over 80% (Green, 2020; Lusa, 2020), which led to declines in the number of cancer cases diagnosed (Dinmohamed et al., 2020; Green, 2020). Additionally, widespread restrictions were imposed on nonurgent medical admissions, while hospital-based outpatient care and transfers between hospitals were limited to urgent cases (Al-Shamsi et al., 2020; Driggin et al., 2020; Nepogodiev and Bhangu, 2020). These public health measures were implemented to ensure essential clinical care while reducing the risk of SARS-CoV-2 infection among patients and healthcare professionals. Concurrently, healthcare users have avoided in-person services due to fear of being infected by SARS-CoV-2 (Barómetro COVID-19, 2020; evidation, 2020). As a result, there was a decrease in the number of routine visits with healthcare professionals for follow-up of chronic diseases, such as obtaining medication refills and dose adjustments or other preventive services (evidation, 2020; Fox et al., 2020; Vaduganathan et al., 2020). There was also a drop in the access to immunization programs, particularly among children (McDonald et al., 2020; Santoli et al., 2020), bringing national and international health authorities to advise that routine immunization services should continue to function normally (Direção-Geral da Saúde, 2020d; The Regional Office for Europe of the World Health Organization, 2020).
Individuals with underlying health conditions, such as active cancer, have been found to be more vulnerable to complications from COVID-19, including increased need for ventilation or ICU admission (Liang et al., 2020; Miyashita et al., 2020; Zhang et al., 2020), as well as higher mortality (Kuderer et al., 2020; Miyashita et al., 2020; Zhang et al., 2020). Additionally, patients with cancer may have a higher risk of infection due to changes in their immune system, which may be related to cancer itself, certain types of treatment, poor nutrition, other health problems or medications unrelated to cancer (Rolston, 2017). Oncology centers introduced strict preventive strategies to reduce the risk of infection with SARS-CoV-2, namely robust infection and environmental control, additional hygiene measures, enforcing social distancing and reducing patients’ overall time spent in a hospital setting (American Society of Clinical Oncology, 2020; European Society for Medical Oncology, 2020; Ueda et al., 2020). Treatment pathways were also altered to minimize the potential exposure of patients with cancer to SARS-CoV-2 and to reduce the risk of infection (American Society of Clinical Oncology, 2020; European Society for Medical Oncology, 2020; Richards et al., 2020; Ueda et al., 2020).
The COVID-19 pandemic affected every aspect of healthcare, including the delivery of care to patients with cancer. Many oncological societies around the world, namely the European Society for Medical Oncology and the American Society of Clinical Oncology, recommend that health professionals in cancer centers adjust their routines as necessary by increasing telemedicine services, reducing clinic visits, and switching to subcutaneous or oral therapies, rather than intravenous ones, when possible (American Society of Clinical Oncology, 2020; European Society for Medical Oncology, 2020). As such, healthcare professionals involved in the diagnosis, treatment and follow-up of patients with cancer had to consider how to balance the delay in cancer diagnosis or treatment against the risk of SARS-CoV-2 infection, mitigate the risks for significant care disruptions associated with the social distancing limits in place and manage the appropriate allocation of available healthcare resources (Kutikov et al., 2020), all of which may impact the prognosis of patients with cancer (Al-Quteimat and Amer, 2020; Dinmohamed et al., 2020; Jones et al., 2020). Therefore, this study intends to quantify the impact of the COVID-19 outbreak on the care of patients treated at the Portuguese Oncology Institute of Porto (IPO-Porto) by comparing a period of four months after the beginning of the outbreak in Portugal (2 March 2020) with the same period in the previous year.
Methods
Setting
IPO-Porto is a highly differentiated organization belonging to the National Health Service, which provides specialized cancer care. IPO-Porto is the largest cancer-dedicated hospital in Portugal, admitting patients from all over the country, though mainly from the Northern region (Instituto Português de Oncologia do Porto). IPO-Porto admits over 7500 new cancer cases per year (Registo Oncológico - Instituto Português de Oncologia do Porto, 2017), corresponding to nearly half and one-fifth of all cases diagnosed in the Northern region (Registo Oncológico Regional do Norte, 2013) and the country (Ferlay et al., 2018), respectively, and provides healthcare to more than 45 000 patients covering the entire cancer continuum, from diagnosis to treatment (surgery, radiotherapy, systemic therapy and supportive care) and follow-up (Instituto Português de Oncologia do Porto, 2019). Additionally, IPO-Porto has a cancer-specific emergency service that aims to provide continuous medical care and assistance to patients with cancer. It is open 24 h a day to receive any patient with cancer registered at IPO-Porto who is in active treatment for oncological disease and whose acute situation derives from the disease itself or is induced by treatment, as well as any patient with an acute oncological pathology who has been referred (Instituto Português de Oncologia do Porto, 2020b).
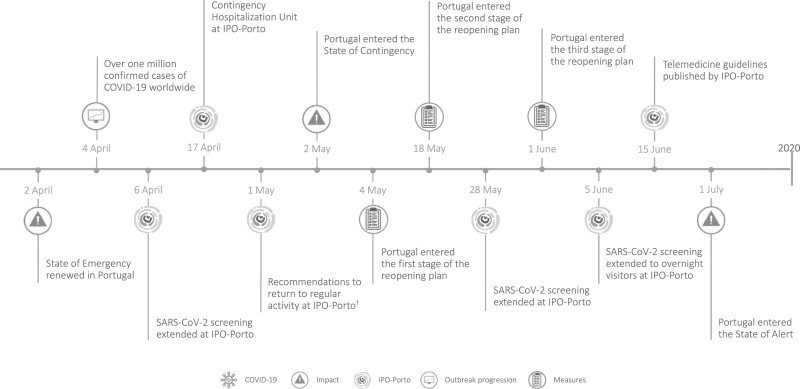
The key events and recommendations by IPO-Porto during the COVID-19 pandemic are presented in Fig. 1 and described in detail in the online Supplementary Material, Supplemental digital content 1, http://links.lww.com/EJCP/A323 (COVID-19 plan at IPO-Porto).
Fig. 1.
Timeline of selected key events and recommendations by the Portuguese Oncology Institute of Porto during the COVID-19 pandemic. *Recommendations by IPO-Porto on 16 March: surgical interventions; outpatient appointments; outpatient diagnostic tests and medical procedures; ambulatory care sessions; only patients who were referred by telephone were admitted to the cancer-specific emergency service. †Recommendations to gradually and progressively return to regular activity levels at IPO-Porto in May: surgical interventions; outpatient appointments; diagnostic tests and medical procedures; regular activity for the day hospital should resume; previously suspended clinical research activities will resume. Image credit: COVID-19 - Praewpailin (Image #30784933 at VectorStock); Impact – janjf93 (Image #2126885 at Pixabay); Outbreak progression – janjf93 (Image #2126876 at Pixabay); Measures – Kaliene (Image #4747711 at Pixabay).
COVID-19, coronavirus disease 2019; IPO-Porto, Portuguese Oncology Institute of Porto.
Study design and data collection
Cases of invasive tumors of the esophagus (code C15 of the International Statistical Classification of Diseases and Related Health Problems 10th Revision [ICD-10]) (World Health Organization, 1992), stomach (C16), colon and rectum (C18-20), pancreas (C25), lung (C34), skin-melanoma (C43), breast (C50), cervix (C53), prostate (C61), non-Hodgkin lymphoma (C82-86, C96), and leukemia (C91-95) diagnosed between 1 February 2019 and 1 July 2019, and 1 February 2020 and 1 July 2020 at IPO-Porto were identified.
Information on cases’ sociodemographic (sex, age, municipality of residence), cancer (ICD-10 code, site, laterality, histological type and stage, as applicable), presence of symptoms, referral pathway (i.e. doctor, another hospital, organized or opportunistic screening, appointment), treatment received (surgery, radiotherapy, brachytherapy, chemotherapy, chemoradiotherapy, immunotherapy, hormone therapy, targeted therapy, as applicable) and possible complications [anastomotic dehiscence, Clavien–Dindo classification (Clavien et al., 2009), oncology-specific emergency visits, hospitalizations, as applicable] were collected from the cancer registry database and clinical files, with follow-up to 1 July 2019 or 2020, as relevant. Additionally, whenever applicable, the following dates were extracted: onset of symptoms; diagnosis; first medical exam; first scheduled appointment; multidisciplinary tumor board meeting; each treatment.
Information regarding COVID-19 diagnosis from 2 March to 1 July 2020 was also obtained for all patients with cancer included in the study.
Statistical analyses
For the purposes of the current study, cancer cases who received the first cancer treatment outside IPO-Porto were excluded, except for cases with skin-melanoma. Patients’ characteristics are presented as counts and proportions for all categorical variables and were compared using the chi-square test. The numbers of cancer cases per week at IPO-Porto between 1 February and 1 July 2019 and 2020 were plotted using locally estimated scatterplot smoothing (LOESS, f = 0.3). Additionally, Joinpoint regression (Joinpoint software, Version 4.8.0.1; (Kim et al., 2000) National Cancer Institute, Bethesda, Maryland, USA) was used to evaluate changing linear trends in the difference between the numbers of cancer cases diagnosed in 2020 and 2019 across consecutive time periods (March–June) with a joinpoint occurring when the linear trend changed significantly in either direction or magnitude. For each of the identified trends, weekly percent change (WPC) estimates and corresponding 95% confidence intervals (95% CIs) were computed by fitting a regression line to the natural logarithm of the difference in the numbers of cases using calendar week as a regression variable.
The dates of manifestation of the first symptoms or routine exams, first medical exam at IPO-Porto, first scheduled appointment at IPO-Porto, multidisciplinary tumor board meeting at IPO-Porto and first treatment received at IPO-Porto were used to calculate the time between each and the date of cancer diagnosis (i.e. the date of the first histological confirmation, usually the date when the biopsy specimen was taken).
The time in days from symptoms onset, first medical exam and first appointment to diagnosis, and from diagnosis to first scheduled appointment, multidisciplinary tumor board meeting and first treatment were plotted (1-Kaplan–Meier) and compared between the two periods [before (2 March to 1 July 2019) and after (2 March to 1 July 2020) COVID-19] using the log rank test. Additionally, the median with percentiles 25 and 75 (P25-P75) time in days were quantified.
Stratified analyses were conducted by sex, age (<55, 55–64, 65–74, >75 years), residence (Porto vs. outside Porto), cancer site, cancer group (digestive, and lymphoid, hematopoietic and related tissue), stage (I, II, III, IV) and month of diagnosis (March, April, May, June).
All analyses were performed using STATA 15 (StataCorp, College Station, Texas, USA). Results were considered statistically significant for P values less than 0.05.
Ethics approval
The study was approved by the Ethics Committee of IPO-Porto (Ref. CES IPO: 164/020). The study was performed in accordance with the Declaration of Helsinki. The data contained no unique personal identifiers and were extracted from the cancer registry database and clinical files. Hence, patient informed consent was not required.
Results
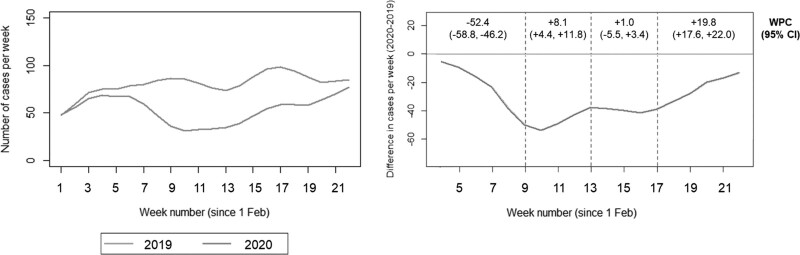
The numbers of cancer cases diagnosed per week at IPO-Porto between 1 February and 1 July of 2019 and 2020 are shown in Fig. 2. Overall, there was a significant decrease of 39% (95% CI: −44.3, −34.1) in the absolute number of cancer cases after the COVID-19 pandemic (from 1430 in 2019 to 866 in 2020), whereas there was no significant difference between years in February (−6.9%; 95% CI: −20.5 to 9.0; Supplementary Table S1, Supplemental digital content 1, http://links.lww.com/EJCP/A323). The difference in the numbers of cancer cases in 2020 in relation to 2019 fluctuated between March and July, with a 52.4% weekly variation in the difference until the ninth week, when the largest difference was observed (−71.4%; 95% CI, −81.5 to −55.8 in the absolute number of cases in 2020 in relation to 2019). Following this, there was a gradual increase in the number of cases in 2020, with the number of cases reaching that of the previous year by the end of the study period in July.
Fig. 2.
Cancer cases diagnosed between 1 February and 1 July in 2019 and 2020 by weeks (following the exclusion of cancer cases with first treatment outside the Portuguese Oncology Institute of Porto) (left), and difference in cancer cases diagnosed per week, weekly percent change and corresponding 95% confidence intervals (right). CI, confidence interval; WPC, weekly percent change.
Table 1 shows the sociodemographic, clinical and treatment characteristics of cancer cases diagnosed before (2 March and 1 July 2019) and after (2 March and 1 July 2020) the onset of COVID-19, and stratified analyses are presented in Supplementary Table S2, Supplemental digital content 1, http://links.lww.com/EJCP/A323. There was a greater decrease among males than females, which resulted in a lower proportion of cases among males after COVID-19. The largest decreases from before to after COVID-19 were observed for cervical and prostate cancers, and the lowest for non-Hodgkin lymphoma and leukemia, as well as for pancreas and lung cancers leading to a higher proportion of cases in the latter sites in 2020. After COVID-19, cases were more often diagnosed at more advanced stages and symptomatic, while less often identified through cancer screening. Just under 20% of cases diagnosed in 2019 and nearly 40% of cases from 2020 had not received any treatment until the beginning of July of the respective year of diagnosis, and there was a higher proportion of cancer-specific emergency visits among cases diagnosed in 2020. Five COVID-19 diagnoses (1 among a patient with cancer from 2019 and the remaining in patients diagnosed in 2020) were confirmed among the patients with cancer included in this study.
Table 1.
Sociodemographic, clinical and treatment characteristics of cancer cases diagnosed and treated before and after the onset of COVID-19 (2 March to 1 July 2019, and 2 March to 1 July 2020, respectively)
| Cancer diagnosis | P value | Proportional variation% (95% CI)a | ||
|---|---|---|---|---|
| Before COVID-19N (%) | After COVID-19N (%) | |||
| 1430 | 866 | −39.4 (−44.3 to −34.1) | ||
| Sex | ||||
| Men | 753 (52.7) | 418 (48.3) | −44.5 (−57.4 to −37.4) | |
| Women | 677 (47.3) | 448 (51.7) | 0.041 | −33.8 (−41.3 to −25.4) |
| Age (years) | ||||
| <55 | 349 (24.4) | 220 (25.4) | −37.0 (−46.7 to −25.4) | |
| 55–64 | 358 (25.0) | 201 (23.2) | −43.9 (−52.8 to −33.3) | |
| 65–74 | 434 (30.3) | 251 (29.0) | −42.2 (−50.5 to −32.4) | |
| >74 | 289 (20.2) | 194 (22.4) | 0.471 | −32.9 (−44.0 to −19.8) |
| Place of residenceb | ||||
| Porto Metropolitan Area | 714 (49.9) | 438 (50.6) | −38.7 (−45.5 to −31.0) | |
| Outside the Porto Metropolitan Area | 716 (50.1) | 428 (49.4) | 0.764 | −40.2 (−47.0 to −62.6) |
| Cancer sitec | ||||
| Esophagus | 27 (1.9) | 18 (2.1) | −33.3 (−62.8 to +21.0) | |
| Stomach | 147 (10.3) | 81 (9.3) | −44.9 (−58.0 to −27.7) | |
| Colon and rectum | 184 (12.9) | 115 (13.3) | −37.5 (−50.5 to −21.1) | |
| Pancreas | 41 (2.9) | 37 (4.3) | −9.8 (−42.1 to +40.7) | |
| Lungd | 209 (14.6) | 164 (18.9) | −21.5 (−36.0 to −3.8) | |
| Skin-melanoma | 81 (5.7) | 56 (6.5) | −30.9 (−50.8 to −2.8) | |
| Breastd | 370 (25.9) | 227 (26.2) | −38.6 (−48.0 to −27.6) | |
| Cervix | 35 (2.4) | 9 (1.0) | −74.3 (−87.6 to −46.6) | |
| Prostate | 233 (16.3) | 66 (7.6) | −71.7 (−78.4 to −62.8) | |
| Non-Hodgkin lymphoma | 81 (5.7) | 75 (8.7) | −7.4 (−32.4 to +26.8) | |
| Leukemia | 22 (1.5) | 18 (2.1) | <0.001 | −18.2 (−56.1 to +52.5) |
| Stagee | ||||
| I | 455 (35.5) | 216 (30.9) | −52.5 (−59.6 to −44.2) | |
| II | 282 (22.0) | 151 (21.6) | −46.5 (−56.1 to −34.7) | |
| III | 246 (19.2) | 113 (16.2) | −54.1 (−63.2 to −42.6) | |
| IV | 297 (23.2) | 219 (31.3) | 0.001 | −26.3 (−38.4 to −12.2) |
| Missing | 47 | 74 | 57.4 (+9.2 to +126.9) | |
| Symptomatic | ||||
| No | 524 (36.6) | 224 (25.9) | −57.3 (−63.4 to −50.0) | |
| Yes | 799 (55.9) | 586 (67.7) | <0.001 | −26.7 (−34. to −18.4) |
| Unknown | 107 | 56 | −47.7 (−61.1 to −27.7) | |
| Referral pathway | ||||
| Doctor | 730 (51.0) | 488 (56.3) | −33.2 (−40.4 to −25.0) | |
| Another hospital | 494 (34.6) | 258 (29.8) | −47.8 (−55.1 to −39.3) | |
| Organized screening | 142 (9.9) | 38 (4.4) | −73.2 (−81.3 to −61.7) | |
| Opportunistic screening | 31 (2.2) | 16 (1.9) | −48.4 (−71.8 to −5.6) | |
| Appointment | 30 (2.1) | 54 (6.2) | <0.001 | 80.0 (+15.2 to +181.2) |
| Missing | 3 | 12 | 300.0 (+12.9 to +1317.4) | |
| First treatmentf | ||||
| Surgery | 510 (35.7) | 219 (25.3) | −57.1 (−63.3 to −49.7) | |
| Radiotherapy | 209 (14.6) | 50 (5.8) | −76.1 (−82.4 to −67.4) | |
| Brachytherapy | 28 (2.0) | 5 (0.6) | −82.1 (−93.1 to −53.7) | |
| Systemic treatment | ||||
| Chemotherapy | 280 (19.6) | 187 (21.6) | −33.2 (−44.5 to −19.6) | |
| Chemoradiotherapy | 26 (1.8) | 3 (0.3) | −88.5 (−96.5 to −61.9) | |
| Immunotherapy | 4 (0.3) | 6 (0.7) | 50.0 (−57.7 to +431.5) | |
| Targeted therapy | 12 (0.8) | 24 (2.8) | 100.0 (0.00 to +300.0) | |
| Hormone therapy | 78 (5.4) | 40 (4.6) | −48.7 (−65.0 to −24.9) | |
| Other | 1 (0.1) | 3 (0.3) | 200.0 (−68.8 to +2784.1) | |
| None (as of 1 July)g | 282 (19.3) | 329 (38.0) | <0.001 | 16.7 (−0.5, to +36.8) |
| Complications | ||||
| Anastomotic dehiscence (yes)h | 10 (2.0) | 2 (0.9) | 0.308 | −80.0 (−95.6 to −8.7) |
| Clavien–Dindo Classificationh (Clavien et al., 2009) | ||||
| None | 449 (88.0) | 200 (91.3) | −55.5 (−62.3 to −47.4) | |
| I–II | 37 (7.2) | 12 (5.5) | −67.6 (−83.1 to −37.8) | |
| III–V | 24 (4.7) | 7 (3.2) | 0.422 | −70.8 (−87.4 to −32.3) |
| Cancer-specific emergency visits (yes)i | 173 (12.3) | 131 (15.3) | 0.038 | −24.3 (−39.6 to −5.0) |
| Hospitalizations (yes)i | 341 (24.1) | 203 (23.7) | 0.875 | −40.2 (−50.0 to −29.2) |
| COVID-19 (yes)i,j | 1 (0.1) | 4 (0.5) | – | – |
CI, confidence interval; COVID-19, coronavirus disease 2019.
aCalculated using Poisson regression.
bPorto Metropolitan Area includes the following municipalities: Arouca, Espinho, Gondomar, Maia, Matosinhos, Oliveira de Azeméis, Paredes, Porto, Póvoa de Varzim, Santa Maria da Feira, Santo Tirso, São João da Madeira, Trofa, Vale de Cambra, Valongo, Vila Nova de Gaia, Vila do Conde.
cInternational Statistical Classification of Diseases and Related Health Problems 10th Revision (World Health Organization, 1992): esophagus – C15; stomach – C16; colon and rectum – C18–20; pancreas – C25; lung – C34; skin-melanoma – C43; breast – C50; cervix – C53; prostate – C61; non-Hodgkin lymphoma – C82–86, C96; leukemia – C91–95.
dLung: 95 left, 106 right, 8 unknowns/missing and 72 left, 88 right, 4 unknowns/missing; breast: 182 left, 182 right, 6 unknowns/missing and 117 left, 106 right, 4 unknowns/missing in 2 March 2019 to 1 July 2019 and 2 March 2020 to 1 July 2020, respectively.
eSolid tumors only (P values for comparisons do not include unknowns). Esophagus: 0 I, 2 II, 7 III, 14 IV, 4 unknowns and 0 I, 1 II, 4 III, 9 IV, 4 unknowns (P value = 0.974); stomach: 42 I, 13 II, 24 III, 56 IV, 12 unknowns and 11 I, 3 II, 6 III, 37 IV, 24 unknowns (P value = 0.032); colon and rectum: 28 I, 47 II, 63 III, 37 IV, 9 unknowns and 14 I, 23 II, 39 III, 25 IV, 14 unknowns (P value = 0.776); pancreas: 4 I, 5 II, 6 III, 23 IV, 3 unknowns and 4 I, 3 II, 7 III, 19 IV, 4 unknowns (P value = 0.894); lung: 42 I, 14 II, 33 III, 115 IV, 5 unknowns and 27 I, 14 II, 18 III, 99 IV, 6 unknowns (P value = 0.380); skin-melanoma: 37 I, 18 II, 20 III, 6 IV and 28 I, 13 II, 3 III, 3 IV, 9 unknowns (P value = 0.069); breast: 263 I, 65 II, 20 III, 13 IV, 9 unknowns and 120 I, 61 II, 23 III, 14 IV, 9 unknowns (P value < 0.001); cervix: 7 I, 21 II, 4 III, 3 IV and 2 I, 6 II, 1 III, 0 IV (P value = 0.840); prostate: 32 I, 97 II, 69 III, 30 IV, 5 unknowns and 10 I, 27 II, 12 III, 13 IV, 4 unknowns (P value = 0.237) in 2019 and 2020, respectively.
fFirst treatment received until 1 July of the respective year of diagnosis. 54 and 30 cases (2019 and 2020, respectively) received chemotherapy and targeted therapy on the same day, chemotherapy was considered as first; 1 case (2020) received hormone therapy and targeted therapy on the same day, hormone therapy was considered as first; 2 cases (2019) received chemotherapy and hormone therapy on the same day, chemotherapy was considered as first; 209 and 50 cases (2019 and 2020, respectively) received hormone therapy and radiotherapy on the same day, hormone therapy was considered as first.
gReasons for no treatment: 168 and 259 cases awaiting treatment to begin; 22 and 19 cases with cancer too advanced for treatment; 24 and 24 cases cannot undergo treatment due to current physical condition; 36 and 8 cases currently under surveillance; 32 and 19 cases died before any treatment in 2019 and 2020, respectively.
hFollow-up until 1 July of the respective year of diagnosis. Among those who underwent surgery as first treatment (n = 510 and n = 219 in 2019 and 2020, respectively).
iFollow-up until 1 July of the respective year of diagnosis. Among 1412 patients with cancer (18 patients had two primary cancers) and 855 patients with cancer (11 patients had two primary cancers) in 2019 and 2020, respectively.
jCOVID-19 diagnosis between 2 March and 1 July 2020.
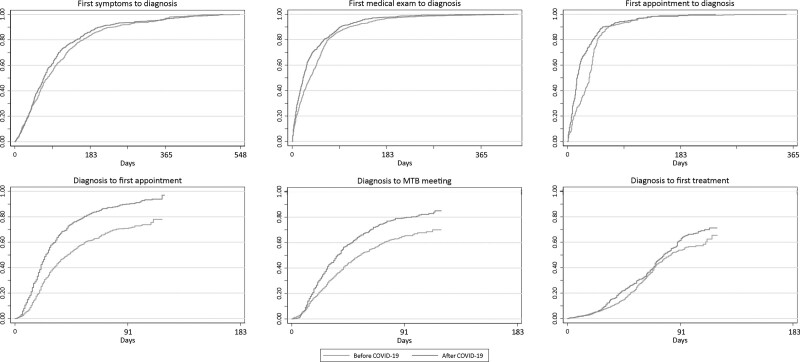
Figure 3 depicts the cumulative probability of diagnosis following first symptoms, first medical exam and first appointment, and of first appointment, multidisciplinary tumor board meeting and first treatment following diagnosis; the median time between each is presented in Table 2, and stratified analyses are presented in Supplementary Table S3, Supplemental digital content 1, http://links.lww.com/EJCP/A323. The median time between the onset of symptoms and diagnosis decreased from 84 days before COVID-19 to 71 after; likewise, the median time from first medical exam to diagnosis declined from 39 days before to 21 days after COVID-19. Regardless of when the first appointment at IPO-Porto occurred, a shorter median time was observed after COVID-19 (16 days for appointments before diagnosis and 22 days for appointments after diagnosis) than before (36 and 34 days, respectively). The median time from diagnosis to multidisciplinary tumor board meeting decreased from 46 days before COVID-19 to 35 after, and there was also a decline in the median time between diagnosis and first treatment from 84 days before COVID-19 to 80 days in 2020.
Fig. 3.
Observed cumulative proportion (calculated using the 1-Kaplan–Meier estimator) of diagnosis following first symptoms, first medical exam and first appointment, and of first appointment, multidisciplinary tumor board meeting and first treatment following diagnosis according to the period of diagnosis (2 March to 1 July 2019 vs. 2 March to 1 July 2020 with follow-up to 1 July 2019 or 2020, respectively). COVID-19, coronavirus disease 2019; MTB, multidisciplinary tumor board meeting.
Table 2.
Median time in days from onset of symptoms, first medical exam and first appointment to diagnosis, and from diagnosis to first appointment, multidisciplinary tumor board meeting and first treatment before and after the onset of COVID-19 (2 March to 1 July 2019 and 2 March to 1 July 2020 with follow-up to 1 July 2019 or 2020, respectively)
| Before COVID-19 | After COVID-19 | ||||||
|---|---|---|---|---|---|---|---|
| Total (N) | Event [N (%)] | Median time in days (P25-P75) | Total (N) | Event [N (%)] | Median time in days (P25–P75) | P value | |
| Onset of symptoms and diagnosis | 567 | – | 84 (41–150) | 448 | – | 71 (39–129) | 0.020 |
| First medical exam and diagnosis | 1254 | – | 39 (14–64) | 788 | – | 21 (8–53) | <0.001 |
| First appointment and diagnosis | 364 | – | 36 (15–46) | 243 | – | 16 (8–36) | <0.001 |
| Diagnosis and first appointment | 1066 | 613 (57.5) | 34 (20–70) | 623 | 443 (71.1) | 22 (14–38) | <0.001 |
| Diagnosis and multidisciplinary tumor board meetinga | 1398 | 692 (49.5) | 46 (24–98) | 846 | 501 (59.2) | 35 (18–63) | <0.001 |
| Diagnosis and first treatment | 1430 | 1148 (80.3) | 84 (57–..) | 866 | 537 (62.0) | 80 (51–..) | <0.001 |
.., not yet available.
COVID-19, coronavirus disease 2019; P25–P75, percentiles 25 and 75.
a32 and 20 cancer cases had a multidisciplinary tumor board meeting before diagnosis in 2019 and 2020, respectively.
Discussion
The current study shows a notable decrease in the absolute number of cancer cases after the COVID-19 outbreak compared to the same period in the previous year. This was particularly evident during the month of March with a WPC in the difference in the number of cases of −52.4%, which was followed by a gradual increase until the end of the study period. Significant differences in the characteristics of incident cases, namely regarding sex, cancer site and stage at diagnosis, presence of symptoms, referral pathway, first treatment received, and cancer-specific emergency visits, were observed. Moreover, although fewer cancer cases received the first treatment in 2020, IPO-Porto provided timely care to patients as the median times from symptoms onset, first medical exam and first appointment to diagnosis, and from diagnosis to the first appointment, multidisciplinary tumor board meeting and first treatment were shorter after compared to before COVID-19.
The substantial impact of the COVID-19 pandemic on the number of cancer diagnoses at IPO-Porto, with a nearly 40% decrease compared to the same period in 2019, is in accordance with the observed in other countries. A study from the United States of America found that the weekly number of new cancer cases, considering breast, colon and rectum, lung, pancreas, stomach, and esophagus, decreased by over 45% during the pandemic (Kaufman et al., 2020). Likewise, in the Netherlands, data from the nationwide cancer registry between 24 February and 12 April 2020 showed a decrease in the number of diagnoses compared to the period before the outbreak (Dinmohamed et al., 2020).
The current study found a lower proportion of cases among males after COVID-19, which was also observed when comparing February 2019 and 2020. Although the number of cancer cases diagnosed in February was low, and the latter difference was not statistically significant, the sex proportions were similar to those before and after COVID-19. In the United States of America, Kaufman et al. (2020) also did not find differences in the overall sex distribution when comparing patients diagnosed from 6 January 2019 to 29 February 2020 to those from 1 March to 18 April 2020.
Among the cancer sites considered in the present study, the largest decreases were observed for cervical and prostate cancers leading to a lower proportion of cases in these sites following COVID-19. In the United States of America, the largest differences in the weekly number of new cancer cases were observed for breast cancer, followed by colorectal cancer (Kaufman et al., 2020). While, in the Netherlands, the decrease in cancer cases was most pronounced for skin cancers (excluding basal cell carcinoma), followed by breast and hematological cancers (Dinmohamed et al., 2020).
To relieve the demand on the healthcare system due to COVID-19, national and regional organized screening programs for breast, colorectal and cervical cancer were temporarily suspended (Green, 2020; Issaka and Somsouk, 2020; Jones et al., 2020). In the United States of America, screening appointments for cervical, colon and breast cancer in March 2020 decreased by over 85% compared to mean volumes between 1 January 2017 and 19 January 2020. Also, an estimated 420 000 cancer screenings were deferred between 15 March and 16 June 2020 as a result of the COVID-19 pandemic (Mast and Rio, 2020). Likewise, data from Cancer Research UK revealed that over two million people were waiting for cancer screening, tests and treatments due to the 10-week lockdown in the country (Roberts, 2020). In Portugal, imaging centers reported to be conducting only 10% of their usual volume of mammograms, while colorectal screening by endoscopy came to a halt during the State of Emergency (Lusa, 2020). In fact, we observed a smaller number of cancer cases being referred to IPO-Porto from organized screening during the COVID-19 pandemic, and there were fewer breast, cervical and colorectal cancer cases in 2020, which are included in Portugal’s organized cancer screening programs (Direção-Geral da Saúde, 2017). On the other hand, we found that cancer cases were more often diagnosed at more advanced stages, and there was a higher proportion of symptomatic cases during the COVID-19 pandemic. More importantly, the effect of cancer screening suspensions may be more pronounced in the future as early diagnoses from screening will be delayed leading to symptoms-based diagnosis at more advanced stages (Hamilton et al., 2016).
The lower number of cancer diagnoses during the COVID-19 pandemic, as well as changes in the distribution of cancer sites, occurred as a result of patient, doctor and healthcare factors. Healthcare institutions, including oncology centers, implemented several measures to ensure necessary services, while reducing the risk of infection by COVID-19 among patients, as well as healthcare professionals (Direção-Geral da Saúde, 2020b; c; Mann et al., 2020; Webster, 2020; World Health Organization, 2020b). At IPO-Porto specifically, a Contingency Plan was first published in mid-February, which was revised a month later with additional recommendations (Instituto Português de Oncologia do Porto, 2020a). These measures, along with the State of Emergency declared in Portugal on 18 March (República Portuguesa, 2020), likely contributed to the significant WPC decrease in the difference of the number of cancer cases diagnosed during the month of March. Additionally, beginning in May, IPO-Porto recommended that activity levels should gradually and progressively return to normal (Instituto Português de Oncologia do Porto, 2020a), and Portugal entered the State of Contingency (República Portuguesa, 2020), which probably led to the observed WPC increase in the difference of the number of cancer cases diagnosed in May and June.
Furthermore, in many healthcare settings, routine and specialist medical appointments, including opportunistic cancer screening, were canceled, postponed or replaced by telehealth (Núcleo Regional do Norte, 2020), which also likely contributed to fewer cancer diagnoses or referrals. Patients may have also delayed routine health procedures or the assessment of mild symptoms due to fear of visiting healthcare providers that were also handling suspected COVID-19 cases (Rosenbaum, 2020). Nevertheless, we may expect that patients with well-recognized symptoms, including a new mass or rectal bleeding, will present to healthcare services, while vague cancer symptoms, namely fatigue or weight loss, may be dismissed by patients (Jones et al., 2020). A report from a Slovenian cancer-dedicated institute showed a significant decrease in first referrals for cancer services, first appointments, imaging studies and cancer notifications in April 2020 (Zadnik et al., 2020). In the United States of America, all outpatient visits dropped by nearly 60% in April 2020, which recovered until mid-June to nearly reaching prepandemic levels by the end of July. Nevertheless, the cumulative number of missed ambulatory care visits since mid-March remained important and continued to grow (Mehrotra et al., 2020). In Portugal, the National Health Service recorded a decrease of nearly half a million primary healthcare and hospital appointments, while there were 9000 fewer surgeries and emergency room visits since the COVID-19 pandemic began in March (Oliveira, 2020). Indeed, we found fewer referrals from other hospitals and from opportunistic screening, while there were more referrals from doctors and from other appointments at IPO-Porto, as well as more symptomatic cases after COVID-19.
Although IPO-Porto made several changes to their regular operations between mid-March and early May, we found that the institute was generally able to continue providing services with shorter median wait times being observed in the post-COVID-19 period. This may be due to the fact that a lower number of cancer cases was diagnosed overall during the COVID-19 pandemic and that over one-third of patients with cancer had not received the first treatment by the end of the study period in 2020 in comparison with 2019, which may have facilitated the continuum of care of the patients undergoing treatment. Treatment disruptions or modifications as a result of the pandemic were described elsewhere. The Portuguese Oncology Institute of Lisbon reported a decrease in the number of appointments during the COVID-19 period compared to the same period in the previous year, and despite delays and postponements, more chemotherapy sessions, radiotherapy and immunotherapy treatments were performed in 2020 (Simões, 2020). In Canada, thoracic oncologists at a university health center found that over half of patients receiving treatment for lung cancer between March and May 2020 underwent at least one change in their cancer treatment plan (Elkrief et al., 2020). In particular, there were delays in palliative and adjuvant chemotherapy, while some treatments stopped, and there were also changes in the dosing and scheduling of chemotherapy. Likewise, a survey conducted among patients with breast cancer in the United States of America found that nearly half encountered treatment delays, particularly younger patients (Papautsky and Hamlish, 2020). Finally, two leading cancer centers in Pakistan, which usually receive 850 new patients with cancer per month for treatment, admitted no patients in the first weeks of April 2020 (Yusuf, 2020). The authors also found that the number of patients beginning chemotherapy and radiotherapy decreased by two-thirds and by half, respectively, and less than 5% of usual elective surgical procedures were performed.
Strengths and limitations
We used two interrupted time series of 5 months for a standardized comparison of incident cancer cases diagnosed before COVID-19 and after, which allowed us to evaluate the impact of the COVID-19 pandemic at the start in March 2020 as well as the return to nearly normal levels by the end of the study period. The possibility of assessing trends since the beginning of the pandemic, and to compare them with a similar period from the previous year, as well as the comparison of data from the month of February, support the validity of our findings.
Despite a usual lag between the incidence of cancer cases and their registration, which improves data completeness and quality but prevents the timely availability of data, to overcome the potential delay in the registration of cases, particularly in 2020, we opted to exclude patients who received the first treatment outside IPO-Porto from our analyses, to ensure the comparability between the two periods. Supplementary Figure S1, Supplemental digital content 1, http://links.lww.com/EJCP/A323, shows the number of cancer cases diagnosed per week at IPO-Porto between 1 February and 1 July of 2019 and 2020 without excluding those with the first treatment outside IPO-Porto, and as expected, there was a higher number of patients with cancer diagnosed throughout the entire study period in 2019. This is because cancer cases diagnosed in 2019 and treated elsewhere are more likely to have potentially received additional care at IPO-Porto until 2020 than those diagnosed in 2020, and as such, were included in the cancer registry.
Detailed patient-level data were obtained, which allowed us to understand the impact of the COVID-19 pandemic on cancer diagnoses and treatment beyond quantifying differences in the distribution of cancer sites or the activity of the institution in terms of the number of surgeries, treatments or appointments. In fact, we were also able to compare median times to and from diagnosis considering symptoms, appointments and first treatment, among others, as well as describe differences in complications among patients diagnosed with cancer before and after COVID-19.
This study includes data from a single-center located in Northern Portugal, which may impair the generalizability of our results to other settings. Nonetheless, IPO-Porto is the largest cancer-dedicated hospitals in Portugal, receiving patients from any part of the country with different sociodemographic backgrounds, and our study includes patients presenting a large spectrum of cancer sites, including all stages and histological types.
Conclusion
The results presented here describe the impact of the COVID-19 outbreak on the care of patients with cancer at IPO-Porto, while identifying needs to be considered in planning for the coming months. As the pandemic progresses, it will be important to determine how delays in diagnosis and treatment will impact clinical outcomes among these patients, which may subsequently lead to an increase in cancer morbidity and mortality. Furthermore, when normal service resumes at a population and health-service level, there will likely be a backlog of patients with potential cancer symptoms needing assessment along with the expected volume of new cancer cases under normal circumstances that may overload oncology cancer services. Therefore, healthcare services should plan accordingly to ensure high-quality cancer care for all.
Acknowledgements
The authors gratefully acknowledge the work of Filipa Gonçalves, Roxanne Garcia, Tatiana Dominguez, Anabela Sousa and Vânia Teixeira.
This study was funded by the Foundation for Science and Technology – FCT (Portuguese Ministry of Science, Technology and Higher Education) in collaboration with the Agency for Clinical Research and Biomedical Innovation (AICIB), under the scope of the project “Impacto da pandemia COVID-19 nos cuidados prestados a doentes oncológicos” (Research 4 COVID 174_596850546), and national funding from FCT, under the Unidade de Investigação em Epidemiologia – Instituto de Saúde Pública da Universidade do Porto (EPIUnit; UIDB/04750/2020). SM was funded under the scope of the project “NEON-PC - Neuro-oncological complications of prostate cancer: longitudinal study of cognitive decline” (POCI-01-0145-FEDER-032358; ref. PTDC/SAU-EPI/32358/2017), which is funded by the European Regional Development Fund through the Operational Programme Competitiveness and Internationalization, and national funding from FCT. The funding sources had no involvement in the conduct of the research or preparation of the article.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (www.eurjcancerprev.com).
References
- Al-Quteimat OM, Amer AM. (The impact of the COVID-19 pandemic on cancer patients. Am J Clin Oncol (2020). 43:452–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, Coomes EA, Chemaly RF, Almuhanna M, et al. (A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist (2020). 25:e936–e945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Society of Clinical Oncology (ASCO Special Report: a guide to cancer delivery during the COVID-19 pandemic. (2020). Alexandria, VA: American Society of Clinical Oncology. [Google Scholar]
- Barómetro COVID-19 (Utilização dos serviços de saúde em tempos de COVID-19 [Use of healthcare services in COVID-19 times]. (2020). Lisbon, Portugal: Escola Nacional de Saúde Pública & Universidade Nova de Lisboa. [Google Scholar]
- Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. (The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg (2009). 250:187–196. [DOI] [PubMed] [Google Scholar]
- Dinmohamed AG, Visser O, Verhoeven RHA, Louwman MWJ, van Nederveen FH, Willems SM, et al. (Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol (2020). 21:750–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Direção-Geral da Saúde (Programa nacional para as doenças oncológicas [National program for oncological disease]. (2017). Lisbon, Portugal: Direção-Geral da Saúde. [Google Scholar]
- Direção-Geral da Saúde (Novo Coronavírus COVID-19 - Relatório de Situação [New Coronavirus COVID-19 - Situation Report]. (2020a). Lisbon, Portugal: Direção-Geral da Saúde. [Google Scholar]
- Direção-Geral da Saúde (Orientações: Conheça as principais Orientações e Informações da DGS [Guidelines: Know the main DGS Guidelines and Information]. (2020b). Lisbon, Portugal: Direção-Geral da Saúde. [Google Scholar]
- Direção-Geral da Saúde (Normas: Consulte as normas publicadas [Norms: See the published norms]. (2020c). Lisbon, Portugal: Direção-Geral da Saúde. [Google Scholar]
- Direção-Geral da Saúde (Diretora-Geral da Saúde apela à vacinação [Director-General of Health calls for vaccination]. (2020d). Lisbon, Portugal: Direção-Geral da Saúde. [Google Scholar]
- Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, et al. (Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol (2020). 75:2352–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkrief A, Kazandjian S, Bouganim N. (Changes in lung cancer treatment as a result of the coronavirus disease 2019 pandemic. JAMA Oncol (2020). 6:1805–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Society for Medical Oncology (Cancer patient management during the COVID-19 pandemic. (2020). Lugano, Switzerland: European Society for Medical Oncology. [Google Scholar]
- evidation (COVID-19 Pulse: delivering regular insights on the pandemic from a 150,000+ person connected cohort. (2020). San Mateo, CA: evidation. [Google Scholar]
- Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. (Global cancer observatory: cancer today. (2018). Lyon, France: International Agency for Research on Cancer. Available from: https://gco.iarc.fr/today. [Accessed 2 November 2020] [Google Scholar]
- Fox ER, Stolbach AI, Mazer-Amirshahi M. (The landscape of prescription drug shortages during the COVID-19 pandemic. J Med Toxicol (2020). 16:311–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A. (Routine cancer screenings plunge during COVID-19. Cancer Health. (2020). Available from: https://www.cancerhealth.com/article/routine-cancer-screenings-plunge-covid19. [Accessed 2 November 2020]
- Hamilton W, Walter FM, Rubin G, Neal RD. (Improving early diagnosis of symptomatic cancer. Nat Rev Clin Oncol (2016). 13:740–749. [DOI] [PubMed] [Google Scholar]
- Instituto Português de Oncologia do Porto. Institucional [Institutional]. Porto, Portugal: Instituto Português de Oncologia do Porto. [Google Scholar]
- Instituto Português de Oncologia do Porto (Relatório e contas 2018 IPO-Porto [Annual Report 2018 IPO-Porto]. (2019). Porto, Portugal: Instituto Português de Oncologia do Porto. [Google Scholar]
- Instituto Português de Oncologia do Porto (Medidas COVID-19 [COVID-19 Measures]. (2020a). Porto, Portugal: Instituto Português de Oncologia do Porto. [Google Scholar]
- Instituto Português de Oncologia do Porto (SANP – Serviço de atendimento não programado [Cancer-specify emergency service]. (2020b). Porto, Portugal: Instituto Português de Oncologia do Porto. [Google Scholar]
- Issaka R, Somsouk M. (Colorectal cancer screening and prevention in the COVID-19 era. JAMA Health Forum. (2020). [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D, Neal RD, Duffy SRG, Scott SE, Whitaker KL, Brain K. (Impact of the COVID-19 pandemic on the symptomatic diagnosis of cancer: the view from primary care. Lancet Oncol (2020). 21:748–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman HW, Chen Z, Niles J, Fesko Y. (Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw Open (2020). 3:e2017267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Fay MP, Feuer EJ, Midthune DN. (Permutation tests for joinpoint regression with applications to cancer rates. Stat Med (2000). 19:335–351. [DOI] [PubMed] [Google Scholar]
- Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, et al.; COVID-19 and Cancer Consortium. (Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet (2020). 395:1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutikov A, Weinberg DS, Edelman MJ, Horwitz EM, Uzzo RG, Fisher RI. (A war on two fronts: cancer care in the time of COVID-19. Ann Intern Med (2020). 172:756–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. (Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol (2020). 21:335–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusa (COVID-19: rastreios do cancro colorretal feitos por endoscopia desapareceram - Ordem [COVID-19: colorectal cancer screening by endoscopy disappeared - Order of Physicians]. (2020). Lisbon, Portugal: Saúde Mais TV. [Google Scholar]
- Mann DM, Chen J, Chunara R, Testa PA, Nov O. (COVID-19 transforms health care through telemedicine: evidence from the field. J Am Med Inform Assoc (2020). 27:1132–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast C, Rio AMd. (Delayed cancer screenings – a second look. (2020). Verona, WI: Epic Health Research Network. [Google Scholar]
- McDonald HI, Tessier E, White JM, Woodruff M, Knowles C, Bates C, et al. (Early impact of the coronavirus disease (COVID-19) pandemic and physical distancing measures on routine childhood vaccinations in England, January to April 2020. Euro Surveill (2020). 25:2000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra A, Chernew M, Linetsky D, Hatch H, Cutler D, Schneider EC. (The impact of the COVID-19 pandemic on outpatient visits: changing patterns of care in the newest COVID-19 hot spots. (2020). New York: The Commonwealth Fund. [Google Scholar]
- Miyashita H, Mikami T, Chopra N, Yamada T, Chernyavsky S, Rizk D, et al. (Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol (2020). 31:1088–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepogodiev D, Bhangu A. (Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. Br J Surg (2020). 107:1440–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núcleo Regional do Norte (Plano de Contingência COVID-19 no Núcleo Regional do Norte [COVID-19 Contingency Plan at the Northern Regional Nucleus]. (2020). Porto, Portugal. [Google Scholar]
- Oliveira DC. (Governo e DGS dizem aos portugueses: não temam idas aos hospitais [Government and the Directorate-General for Health tell the Portuguese: do not fear visiting hospitals]. (2020). Lisbon, Portugal: Público. [Google Scholar]
- Papautsky EL, Hamlish T. (Patient-reported treatment delays in breast cancer care during the COVID-19 pandemic. Breast Cancer Res Treat (2020). 184:249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presidente da República (Mensagem do Presidente da República ao País sobre a declaração do Estado de Emergência [Message of the President of the Republic to the Country on the declaration of a State of Emergency]. (2020). Lisbon, Portugal. [Google Scholar]
- Registo Oncológico - Instituto Português de Oncologia do Porto (Registo oncológico 2017 [Cancer registry 2017]. (2017). Porto, Portugal: Registo Oncológico - Instituto Português de Oncologia do Porto. [Google Scholar]
- Registo Oncológico Regional do Norte (Projeções de Incidência de Cancro Região Norte - 2013, 2015 e 2020 [North Region Cancer Incidence Projections - 2013, 2015 and 2020]. (2013). Porto, Portugal: Registo Oncológico Regional do Norte. [Google Scholar]
- República Portuguesa (Não paramos - Estamos on: Resposta de Portugal à COVID-19 [We don’t stop - We are on: Portugal’s response to COVID-19]. (2020). Lisbon, Portugal: República Portuguesa. [Google Scholar]
- Richards M, Anderson M, Carter P, Ebert BL, Mossialos E. (The impact of the COVID-19 pandemic on cancer care. Nat Cancer (2020). 1:565–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts K. (Over 2 million people waiting for cancer screening, tests and treatments. (2020). London, United Kingdom: Cancer Research UK. [Google Scholar]
- Rolston KVI. (Infections in cancer patients with solid tumors: a review. Infect Dis Ther (2017). 6:69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum L. (The untold toll - the pandemic’s effects on patients without Covid-19. N Engl J Med (2020). 382:2368–2371. [DOI] [PubMed] [Google Scholar]
- Santoli JM, Lindley MC, DeSilva MB, Kharbanda EO, Daley MF, Galloway L, et al. (Effects of the COVID-19 pandemic on routine pediatric vaccine ordering and administration - United States, 2020. MMWR Morb Mortal Wkly Rep (2020). 69:591–593. [DOI] [PubMed] [Google Scholar]
- Simões S. (Quimioterapia atrasada, radioterapia adiada e um transplante por marcar. O impacto preocupante nos doentes com cancro [Delayed chemotherapy, postponed radiation therapy and an unscheduled transplant. The worrying impact on cancer patients]. (2020). Lisbon, Portugal: Observador. [Google Scholar]
- The Regional Office for Europe of the World Health Organization (Guidance on routine immunization services during COVID-19 pandemic in the WHO European Region. (2020). Copenhagen, Denmark: The Regional Office for Europe of the World Health Organization. [Google Scholar]
- Ueda M, Martins R, Hendrie PC, McDonnell T, Crews JR, Wong TL, et al. (Managing cancer care during the COVID-19 pandemic: agility and collaboration toward a common goal. J Natl Compr Canc Netw (2020). 20:1–4. [DOI] [PubMed] [Google Scholar]
- Vaduganathan M, van Meijgaard J, Mehra MR, Joseph J, O’Donnell CJ, Warraich HJ. (Prescription fill patterns for commonly used drugs during the COVID-19 pandemic in the United States. JAMA (2020). 323:2524–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster P. (Virtual health care in the era of COVID-19. Lancet (2020). 395:1180–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (International Statistical Classification of Diseases and Related Health Problems 10th Revision. (1992). Geneva, Switzerland: World Health Organization. [Google Scholar]
- World Health Organization (Coronavirus disease (COVID-19) weekly epidemiological update and weekly operational update. (2020a). Geneva, Switzerland: World Health Organization. [Google Scholar]
- World Health Organization (Maintaining essential health services: operational guidance for the COVID-19 context (Interim guidance). (2020b). Geneva, Switzerland: World Health Organization. [Google Scholar]
- World Health Organization (2019 novel Coronavirus (2019-nCov): strategic preparedness and response plan. (2020c). Geneva, Switzerland: World Health Organization. [Google Scholar]
- World Health Organization (WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. (2020d). Geneva, Switzerland: World Health Organization. [Google Scholar]
- Yusuf A. (Cancer care in the time of COVID-19-a perspective from Pakistan. Ecancermedicalscience (2020). 14:1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadnik V, Mihor A, Tomsic S, Zagar T, Bric N, Lokar K, Oblak I. (Impact of COVID-19 on cancer diagnosis and management in Slovenia - preliminary results. Radiol Oncol (2020). 54:329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, et al. (Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol (2020). 31:894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.