Abstract
Objectives
To quantify the placebo effect of intraarticular injections for knee osteoarthritis in terms of pain, function, and objective outcomes. Factors influencing placebo effect were investigated.
Design
Meta-analysis of randomized controlled trials; Level of evidence, 2. PubMed, Web of Science, Cochrane Library, and grey literature databases were searched on January 8, 2020, using the string: (knee) AND (osteoarthritis OR OA) AND (injections OR intra-articular) AND (saline OR placebo). The following inclusion criteria were used: double-blind, randomized controlled trials on knee osteoarthritis, including a placebo arm on saline injections. The primary outcome was pain variation. Risk of bias was assessed using the RoB 2.0 tool, and quality of evidence was graded following the GRADE (Grading of Recommendations Assessment, Development and Evaluation) guidelines.
Results
Out of 2,363 records, 50 articles on 4,076 patients were included. The meta-analysis showed significant improvements up to the 6-month follow-up: Visual Analogue Scale (VAS)-pain −13.4 mean difference (MD) (95% confidence interval [CI]: −21.7/−5.1; P < 0.001), Western Ontario and McMaster Osteoarthritis Index (WOMAC)-pain −3.3 MD (95% CI: −3.9/−2.7; P < 0.001). Other significant improvements were WOMAC-stiffness −1.1 MD (95% CI: −1.6/−0.6; P < 0.001), WOMAC-function −10.1 MD (95% CI: −12.2/−8.0; P < 0.001), and Evaluator Global Assessment −21.4 MD (95% CI: −29.2/−13.6; P < 0.001). The responder rate was 52% (95% CI: 40% to 63%). Improvements were greater than the “minimal clinically important difference” for all outcomes (except 6-month VAS-pain). The level of evidence was moderate for almost all outcomes.
Conclusions
The placebo effect of knee injections is significant, with functional improvements lasting even longer than those reported for pain perception. The high, long-lasting, and heterogeneous effects on the scales commonly used in clinical trials further highlight that the impact of placebo should not be overlooked in the research on and management of knee osteoarthritis.
Keywords: osteoarthritis, knee, intraarticular injections, placebo
Introduction
Knee osteoarthritis (KOA) is the 11th cause of years lived with disability according to the World Health Organization. 1 Its chronicity leads over time to walking-related disability and increases the development of vascular diseases, ultimately causing a 1.55 higher risk ratio of death than that in the general population. 2 Intraarticular injections are a minimally invasive approach that plays a key role in the management of this condition.3,4 There is great interest in improving existing treatments and in developing new intraarticular products, testing them in randomized controlled trials (RCTs) to provide the highest level of evidence of their effectiveness. However, studies on saline suggested a large placebo effect of intraarticular injections. 5
Saline is the most common placebo administered to control groups in RCTs of injective procedures, often showing remarkable results.6-8 Indeed, the intraarticular administration of saline has been documented as the most effective placebo for KOA, 7 with pain relief being perceived by the patients as reaching the minimal clinically important difference (MCID). 9 Nevertheless, the extent of the perceived effect beyond the short-term pain modulation remains to be understood, and a more comprehensive evaluation of the results of saline injections is needed to understand if the response goes beyond a short-term pain modulating effect. 10 Understanding the overall effects of “placebo injections” would help better plan future studies using saline as a control and to quantify the real effects of injective products.
The aim of the present meta-analysis was to quantify the impact of placebo in the injective treatment of patients with KOA not only in terms of knee pain but also regarding function and objective outcomes resulting from saline injections. Moreover, the determinants of the clinical outcome of saline injections will be explored to evaluate whether different study conditions may influence the placebo effect in patients with KOA.
Materials and Methods
Data Sources
After the registration on PROSPERO (CRD42019137908), PubMed (1974-2020), Web of Science (1990-2020), Cochrane Library (no limits-2020), and gray literature databases (isrctn.org, clinicaltrials.gov, greylit.org, and opengrey.eu) were searched on January 8, 2020, with no time limitations and without any filters, using the following string: (knee) AND (osteoarthritis OR OA) AND (injections OR intra-articular) AND (saline OR placebo).
Study Selection
The following studies were included: double-blind RCTs on KOA including a placebo control arm undergoing knee intraarticular saline injections; studies assigning both knees of patients treated bilaterally to the same group; studies reporting both baseline and follow-up data (or difference); human studies; and studies published in English language. Titles and abstracts were checked to exclude articles not relevant to the study purpose. When not enough information could be obtained from the abstract, the full-text article was read to evaluate eligibility. The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were used. 11 The article selection and data extraction process was performed independently by 2 authors (GDLF, GM), with disagreement on study eligibility solved by a third author (DP). Patient/public involvement was not be feasible for this study.
Data Extraction and Outcome Measures
The following information on trial methodology was extracted: level of evidence, study design, inclusion/exclusion criteria, blinding procedure, randomization procedure, follow-up length, information on saline injection (volume, timing, injections number), and experimental treatment tested. Moreover, the following data on the study population were extracted: number of patients screened, included, and lost to follow-up; sex, age, body mass index (BMI), and OA grade; patient-reported outcome measures (PROMs); complication rate; functional test results; knee range of motion (ROM); responder rate; and radiological results. When standard deviations were not available from the full-text articles, they were estimated using established methods.12,13 When results could not be obtained from the text but were available in graphs, data were electronically extracted from the graphs following the Cochrane guidelines.14,15
The meta-analysis primary outcome was knee pain variation after saline injections, as measured with a Visual Analogue Scale (VAS). Secondary outcomes were variations in the Western Ontario and McMaster Osteoarthritis Index (WOMAC) subscores (pain, stiffness, function), Evaluator Global Assessment (EGA), and responder rate. Three different follow-up time points were analyzed: 1 month (4-6 weeks), 3 months (12-16 weeks), and 6 months (24-28 weeks). Due to the paucity of studies reporting results at 12 and 24 months, the analyses at these time points, and pooled analyses of other PROMs, were not possible. Moreover, the results in the PROMs were compared to the previously reported MCID: 13.7/100 for VAS pain score, 1.5/20 for WOMAC pain score, 0.6/8 for WOMAC stiffness subscore, and 4.6/68 for WOMAC function score.9,16 For responder rate analyses, 2 different time points were considered: 3 and 6 months. Two different analyses were performed for the responder rate: (1) including all the studies reporting it, independently from the criteria used, and (2) including only the studies using the OMERACT-OARSI criteria, the most used and accepted criteria in the field. 17
In addition, the influence of the following characteristics of the included studies and patients on the response to saline was tested: number of patients included, mean age, BMI, baseline score values, amount of saline, number of injections, answers regarding the experimental product, and symptom duration. Publication year and experimental treatment were also analyzed to evaluate possible influences on the effect of saline injection.
Risk of Bias and Quality of Evidence Assessment
The risk of bias was assessed using the revised tool for risk of bias in randomized trials (RoB 2.0), 18 and the overall quality of evidence for each outcome was graded according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines. 19
Statistical Analysis
The effect of saline injection was evaluated by comparing the results of the saline group of every trial with the absence of effects that would have been provided by no treatment (no-effect group). For continuous variables, the mean difference between the values at the different follow-ups and the baseline values were plotted against the no-effect values; the standard deviation of the saline injection group was used as a measure of variance in the no-effect group. For dichotomous variables, the effect of saline injections was expressed as the risk difference between the probability of the event in the saline injection group and that in the no-effect group. Heterogeneity was tested using Cochran’s Q statistic and the I2 metric: I2 > 25% was the cutoff of significant heterogeneity, and a fixed-effect model was used when I2 < 25%; otherwise, a random-effect model was preferred. 20
The effect size of the change in the pain-VAS and WOMAC-pain score after the injection of saline in the different studies was computed according to Cohen to assess the influence of the study and patient characteristics on the response to saline. The Kolmogorov-Smirnov test was performed to test the normality of the evaluated effect sizes. The Spearman rank correlation was used to assess the correlation between the effect sizes and continuous and rank data. The Kendall tau correlation was used to assess the correlation between the effect sizes and the number of injections. Moreover, the general linear model was used to assess the influence of the characteristics of studies and patients on the effect size corrected for study dimension (fewer than 50 patients vs. more than 50 patients). The level of statistical significance was set at P < 0.05. Analyses were performed using RevMan 5.3 and SPSS Statistics 19.0.
Results
Characteristics of the Studies and Patients
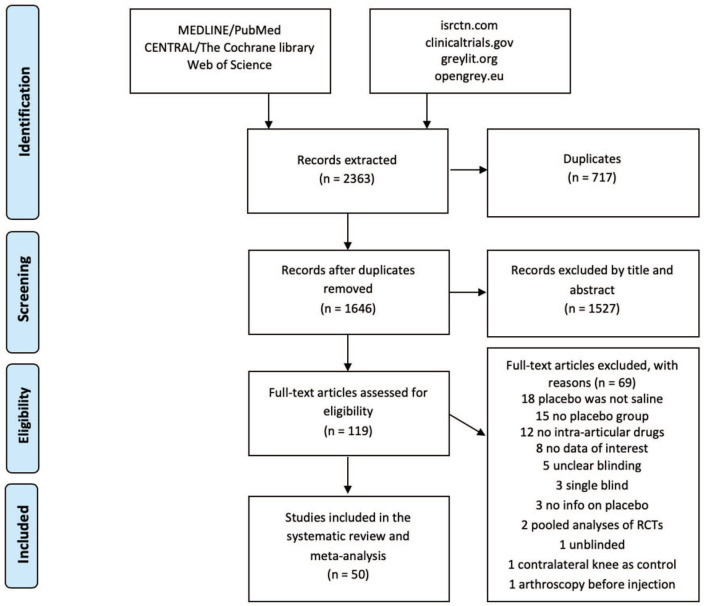
The flowchart of the article selection process is reported in Figure 1 . Of the 2,363 records retrieved with the database search, 50 articles were included in the meta-analysis. The injection protocol differed in the included studies in terms of injection number, amount of injected saline, knee aspiration, use of local anesthesia, and ultrasound guidance. The follow-up length ranged from 6 weeks to 40 months. The most common experimental drugs were hyaluronic acid (22 studies), steroids (6 studies), platelet-rich plasma (3 studies), and low-molecular-weight fraction of 5% human serum albumin (3 studies). The outcomes reported more frequently were VAS, WOMAC, EGA, and responder rate. Further details of the included studies are reported in Table 1 .
Figure 1.
PRISMA flowchart of the study selection process.
Table 1.
Characteristics of the Included Studies a .
| Study | Amount Injected | Number of Injections (Schedule) | Local Anesthesia | Aspiration Performed | US Guidance | Injection Approach | Experimental Drug | Follow-up Length | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|
| Altman, 32 2004 | 3 mL | 1 | NR | NR | NR | NR | HA | 6 m | Some concerns |
| Altman, 33 2009 | 2 mL | 3 (1/wk) | Yes | Yes | NR | Suprapatellar or infrapatellar | HA | 6 m | Some concerns |
| Arden, 34 2014 | 3 mL | 1 | NR | Yes | No | Lateral midpatellar, lateral upper-patellar, or medial | HA | 6 wk | Some concerns |
| Yang, 35 2008 | 2 mL | 6 (0, 3 d, 7 d, 10 d, 14 d, 21 d) | NR | Yes | NR | Superolateral | Anti-IL1R | 12 m | Some concerns |
| Baltzer, 36 2009 | NA | 3 (1/wk) | No | Yes | NR | Anterolateral | HA | 6 m | Some concerns |
| Bar-Or, 37 2013 | G1 4 mL; G2 10 mL | 1 | NR | NR | NR | Inferolateral | LMWF-5A | 3 m | Some concerns |
| Brandt, 38 2001 | 2 mL | 3 (1/wk) | Yes | NR | NR | NR | HA | 25 wk | Some concerns |
| Chao, 39 2016 | 1 mL | 1 | NR | NR | NR | Anterolateral | Steroids | 3 m | Some concerns |
| Chevalier, 40 2010 | 6 mL | 1 | NR | Yes | NR | Free | HA | 6 m | Low |
| Conaghan, 41 2018 | 5 mL | 1 | NR | NR | NR | NR | Steroids | 6 m | Some concerns |
| Conaghan, 42 2018 | 5 mL | 1 | Free | NR | NR | Free | Steroids | 6 m | Low |
| Day, 43 2019 | 2.5 mL | 5 (1/wk) | Yes | Yes | NR | Free | HA | 3 m | Some concerns |
| Eker, 44 2017 | 7 mL | 3 (3/wk) | No | NR | Yes | Infrapatellar | Lidocaine | 3 m | Low |
| Görmeli, 45 2017 | NR | 3 (1/wk) | NR | NR | NR | NR | PRP or HA | 6 m | Some concerns |
| Grecomoro, 46 1987 | 2 mL | 3 (1/wk) | NR | NR | NR | NR | HA | 2 m | Some concerns |
| Hangody, 47 2018 | 4 mL | 1 | NR | Yes | NR | NR | HA + steroids or HA | 6 m | Some concerns |
| Henrotin, 48 2017 | 2.2-2.5 mL | 1 | NR | NR | NR | NR | HA + mannitolo | 6 m | Some concerns |
| Huang, 49 2011 | 2 mL | 5 (1/wk) | NR | NR | NR | NR | HA | 25 wk | Some concerns |
| Huskisson, 50 1999 | 2 mL | 5 (1/wk) | NR | Yes | NR | NR | HA | 6 m | Some concerns |
| Jørgensen, 51 2010 | 2 mL | 5 (1/wk) | Yes | Yes | NR | NR | HA | 1 y | Some concerns |
| Jubb, 52 2003 | 2 mL | 3 (1/wk) | NR | NR | NR | NR | HA | 1 y | Some concerns |
| Karlsson, 53 2002 | 3 mL | 3 (1/wk) | NR | NR | NR | NR | HA | 1 y | Some concerns |
| Kim, 54 2018 | 3 mL | 1 | NR | NR | NR | NR | Allogenic-chondrocytes | 1 y | Some concerns |
| Kon, 55 2018 | 2.5 mL | 1 | NR | NR | NR | Free | PRP | 1 y | Low |
| Kotevoglu, 56 2006 | 2 mL | 3 (1/wk) | NR | NR | NR | Anterolateral | HA | 6 m | High |
| Kul-Panza, 21 2010 | 2 mL | 1 | NR | NR | NR | NR | HA | 3 m | Some concerns |
| Lee, 57 2015 | 3.5 mL | 1 | NR | Yes | NR | NR | TissueGene-c | 6 m | Some concerns |
| Lee, 58 2019 | 3 mL | 1 | NR | NR | Yes | NR | AD-MSC | 6 m | Some concerns |
| Lin, 59 2019 | 2 mL | 1 | No | NR | NR | NR | PRP or HA | 12 m | Some concerns |
| Lohmander, 60 1996 | 2.5 mL | 5 (1/wk) | Yes | Yes | NR | NR | HA | 20 wk | Some concerns |
| Lozada, 61 2017 | 4.2 mL | 3 (1/wk) | Yes | Yes | NR | Medial or lateral | Tr14/Ze14 | 12 wk | Low |
| Lundsgaard, 62 2008 | G1 2 mL; G2 20 mL | 4 (1/wk) | NR | Yes | NR | Lateral midpatellar | HA | 6 m | Low |
| McAlindon, 63 2017 | 1 mL | 8 (1/3 m) | NR | Yes | Yes | NR | Steroids | 2 y | Low |
| McAlindon, 64 2018 | 2 mL | 1 | NR | Yes | Yes | NR | Onabotulinum toxin A | 24 wk | Low |
| Navarro-Sarabia, 65 2011 | 2.5 mL | 20 (4 × 5/wk) | NR | Yes | No | Medial/lateral/infrapatellar | HA | 40 m | High |
| Patel, 66 2013 | 8 mL | 1 | No | NR | NR | Superolateral | PRP | 6 m | Some concerns |
| Pavelka, 67 1995 | 0.5 mL | 5 (1/wk) | NR | NR | NR | NR | GAGPS | 6 m | Some concerns |
| Petterson, 22 2019 | 4 mL | 1 | NR | Yes | NR | Medial or lateral | HA | 6 m | Low |
| Ravaud, 68 1999 | 1.5 mL | 1 | NR | Yes | NR | NR | Steroids | 6 m | Some concerns |
| Raynauld, 23 2003 | 1 mL | 8 (1/3 m) | Yes | NR | NR | NR | Steroids | 2 y | Some concerns |
| Rossini, 69 2015 | 1 mL | 4 (1/wk) | NR | NR | NR | NR | Clodronate | 3 m | Some concerns |
| Salottolo, 70 2018 | 4 mL | 1 | No | NR | NR | Infero-lat | LMWF-5A | 3 m | Low |
| Scale, 71 1994 | 2 mL | NA | NR | NR | NR | NR | HA | 3 m | Some concerns |
| Schwappach, 72 2017 | 4 mL | 3 (1/2 wk) | NR | NR | NR | NR | LMWF-5A | 1 y | Low |
| Shrestha, 73 2018 | NR | 1 | NR | NR | NR | Superolateral | Steroids | 3 m | Some concerns |
| Smith, 74 2016 | 3-8 mL | 3 (1/wk) | NR | NR | NR | Lateral parapatellar | PRP | 1 y | Low |
| Soltani, 24 2019 | 10 mL | 1 | NR | NR | NR | Various | Placental MSC | 6 m | High |
| Strand, 75 2012 | 3 mL | 1 | NR | Yes | NR | NR | HA | 3 m | Low |
| van der Weegen, 25 2015 | 2 mL | 3 (1/wk) | NR | Yes | NR | NR | HA | 6 m | Some concerns |
| Wobig, 76 1998 | 2 mL | 3 (1/wk) | Optional | Yes | NR | Free | HA | 6 m | Some concerns |
NR = not reported; wk = week; m = month; y = year; US = ultrasound; HA = hyaluronic acid; LMWF-5A = low-molecular-weight fraction of 5% human serum albumin; PRP = platelet-rich plasma; AD-MSC = adipose-derived mesenchymal stem cells; GAGPS = glycosaminoglycan polysulfuric acid; MSC = mesenchymal stem cells.
A total of 4,076 patients were included in the saline control group of the analyzed studies. Overall, the mean patient age was 61.2 years (range: 46.6-71.0), the BMI was 28.6 kg/m2 (range: 25.0-34.5), and the male-to-female ratio was 0.70 (range: 0.11-1.90). Further details of the patients’ characteristics are reported in supplementary files (available online).
Results of Placebo Injections
The pain improvement in terms of 0-100 VAS was −17.7 mean difference (MD) after 1 month (95% confidence interval [CI]: −24.9/−10.5; P < 0,001), −17.9 MD after 3 months (95% CI: −24.5/−11.3; P < 0,001), and −13.4 MD after 6 months (95% CI: −21.7/−5.1; P < 0,001), and for the WOMAC-pain subscore, the improvement was −3.2 MD after 1 month (95% CI: −3.8/−2.5; P < 0,001), −3.3 MD after 3 months (95% CI: −3.8/−2.7; P < 0,001), and −3.3 MD after 6 months (95% CI: −3.9/−2.7; P < 0,001).
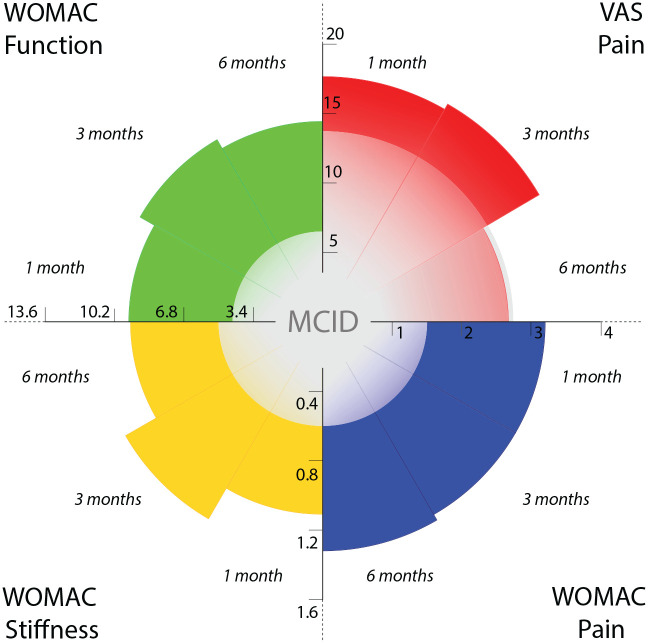
In addition to pain, a statistically significant improvement was also reported for other scores. For the WOMAC-stiffness subscore, the improvement after saline injection was −1.1 MD after 1 month (95% CI: −1.6/−0.6; P < 0,001), −1.3 MD after 3 months (95% CI: −1.5/−1.0; P < 0.001), and −1.1 MD after 6 months (95% CI: −1.5/−0.8; P < 0,001). For the WOMAC-function subscore, improvement was −9.7 MD after 1 month (95% CI: −12.5/-6.9; P < 0,001), −10.5 MD after 3 months (95% CI:−-12.6/-8.5; P < 0,001), and −10.1 MD after 6 months (95% CI: −12.2/-8.0; P < 0,001). The documented improvements were greater than the previously reported MCID for all the outcomes at all the follow-ups except for 0-100 VAS at the 6-month follow-up (summarized in Fig. 2 ; for specific forest plot analyses see supplementary material).
Figure 2.
Effect of placebo injection. The results of the meta-analysis in terms of VAS pain (0-100), WOMAC-pain (0-20), WOMAC-stiffness (0-8), and WOMAC-function (0-68) at the different follow-ups (1 month, 3 months, 6 months) are compared to the previously reported MCID. Every quadrant reports data about one of the evaluated outcomes whereas axes report the related scale: VAS pain (upper right), WOMAC-pain (bottom right), WOMAC-stiffness (bottom left), and WOMAC function (upper left). The level of the documented improvement is represented by the colored area (red for VAS pain, blue for WOMAC pain, yellow for WOMAC stiffness, green for WOMAC function), whereas the level of the central gray area represents the previously reported MCID for each outcome. All the improvements at all the follow-ups go beyond the previously reported MCID, except for VAS-pain at the 6-month follow-up.
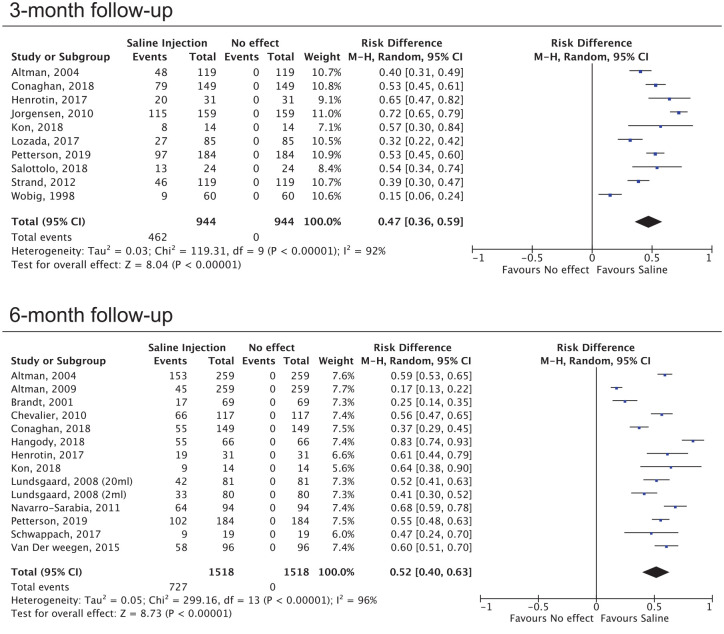
The pooled responder rate after saline injections was 47% (95%C.I.: 36%/59%) and 52% (95%C.I.: 40%/63%) after 3 and 6 months, respectively ( Fig. 3 ). When only the studies considering the OMERACT-OARSI criteria to define patients response were included the responders rate was 48% (95%C.I.: 38%/57%) and 56% (95%C.I.: 40%/72%) after 3 and 6 months, respectively. In terms of the 0-100 EGA, a statistically significant improvement was documented after 3 months (-17,5MD; 95%C.I.: -24,4/-10,7; P < 0,001) and after 6 months (-21,4MD; 95%C.I.: -29,2/-13,6; P < 0,001). Although a meta-analysis was not possible due to the heterogeneity of the data, an improvement of the knee ROM was reported in all 5 studies that documented this outcome.21-25
Figure 3.
Forest plot of the responders’ rate after 3 months and 6 months. The responders’ rate after saline injection is compared to the absence of effect and the results are reported as risk differences.
Injection-Related Complications
Data on injection-related complications were reported in 41 out of 50 studies, whereas 9 studies did not provide information about this outcome. The study with the highest complication rate was the one of Bar-Or et al., which reported a 47% complication rate, although without specifying the nature of the adverse event. In 13 out of 41 studies there were no complications after placebo injection. Adverse events manly consisted in local alterations: the most common adverse event was persistent pain after the injection, which affected 6.3% of the patients (185/2,960 patients). Other reported complications were local irritation (3.5%; 104/2,960 patients), swelling (1.0%; 29/2,960 patients), joint effusion (0.9%; 18/2,960 patients), transient stiffness (0.2%; 7/2,960 patients), and transient bleeding (0.2%; 6/2,960 patients). A summary of the injection-related complications reported in the included studies is presented in supplementary files.
Determinants of the Placebo Effect
The analysis of the characteristics that could be determinants of the effect of saline injections showed that when the experimental treatments presented better results, a higher effect was also found for saline, both in terms of 0 to 100 VAS (ρ = 0.49, P = 0.01) and WOMAC-pain (ρ = 0.77, P < 0.001). A significant negative correlation was found between the answer to saline and the duration of KOA-related symptoms (ρ = −0.70, P = 0.03). These correlations were also confirmed when the data were corrected for the number of patients included in the studies. No significant correlation with the answer to saline injection was found for the other factors studied.
Risk of Bias and Level of Evidence
The risk of bias was low in 13 studies, with some concerns in 34 studies and high in 3 studies. The main reasons for the presence of risk of bias were an unclear method for allocation concealment and the absence of an available preregistered protocol that accounted for some risk of selective reporting bias.
The level-of-evidence evaluation process for all the plotted outcomes is reported in Table 2 .
Table 2.
Summary of the Results of Saline Injection in Knee Osteoarthritis With Level of Evidence Assessment Details.
| Certainty Assessment | Summary of findings | ||||||
|---|---|---|---|---|---|---|---|
| Participants (Studies) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Quality of Evidence | Absolute Effects |
| Mean/Risk Difference With Saline | |||||||
| VAS (follow-up: range 4 weeks to 6 weeks; scale from 0 to 100) | |||||||
| 1,183 (16 RCTs) | Not serious | Serious a | Not serious | Not serious | None | ⨁⨁⨁◯ Moderate |
MD 17.69 lower (24.92 lower to 10.45 lower) |
| VAS (follow-up: range 12 weeks to 16 weeks; scale from 0 to 100) | |||||||
| 1,515 (21 RCTs) | Not serious | Serious a | Not serious | Not serious | None | ⨁⨁⨁◯ Moderate |
MD 17.89 lower (24.47 lower to 11.3 lower) |
| VAS (follow-up: range 22 weeks to 26 weeks; scale from 0 to 100) | |||||||
| 1,343 (17 RCTs) | Not serious | Serious a | Not serious | Serious b | None | ⨁⨁◯◯ Low |
MD 13.39 lower (21.68 lower to 5.11 lower) |
| Responders rate (follow-up: range 12 weeks to 16 weeks) | |||||||
| 944 (10 RCTs) | Not serious | Serious a | Not serious | Not serious | Strong association | ⨁⨁⨁⨁ High |
47 responders per 100 (from 36 to 59) |
| Responders rate (follow-up: range 22 weeks to 26 weeks) | |||||||
| 1,518 (14 RCTs) | Not serious | Serious a | Not serious | Not serious | Strong association | ⨁⨁⨁⨁ High |
52 responders per 100 (from 40 to 63) |
| WOMAC Pain (follow-up: range 4 weeks to 6 weeks; scale from 0 to 20) | |||||||
| 1,551 (17 RCTs) | Not serious | Serious a | Not serious | Not serious | None | ⨁⨁⨁◯ Moderate |
MD 3.15 lower (3.84 lower to 2.46 lower) |
| WOMAC Stiffness (follow-up: range 4 weeks to 6 weeks; scale from 0 to 8) | |||||||
| 539 (7 RCTs) | Not serious | Serious a | Not serious | Not serious | None | ⨁⨁⨁◯ Moderate |
MD 1.14 lower (1.63 lower to 0.64 lower) |
| WOMAC Function (follow-up: range 4 weeks to 6 weeks; scale from 0 to 68) | |||||||
| 1,004 (11 RCTs) | Not serious | Serious a | Not serious | Not serious | None | ⨁⨁⨁◯ Moderate |
MD 9.72 lower (12.54 lower to 6.91 lower) |
| WOMAC Pain (follow-up: range 12 weeks to 16 weeks; scale from 0 to 20) | |||||||
| 1,986 (22 RCTs) | Not serious | Serious a | Not serious | Not serious | None | ⨁⨁⨁◯ Moderate |
MD 3.26 lower (3.80 lower to 2.72 lower) |
| WOMAC Stiffness (follow-up: range 12 weeks to 16 weeks; scale from 0 to 8) | |||||||
| 1,086 (13 RCTs) | Not serious | Serious a | Not serious | Not serious | None | ⨁⨁⨁◯ Moderate |
MD 1.25 lower (1.5 lower to 0.99 lower) |
| WOMAC Function (follow-up: range 12 weeks to 16 weeks; scale from 0 to 68) | |||||||
| 1,544 (18 RCTs) | Not serious | Serious a | Not serious | Not serious | None | ⨁⨁⨁◯ Moderate |
MD 10.54 lower (12.57 lower to 8.52 lower) |
| WOMAC Pain (follow-up: range 22 weeks to 26 weeks; scale from 0 to 20) | |||||||
| 1,633 (15 RCTs) | Not serious | Serious a | Not serious | Not serious | None | ⨁⨁⨁◯ Moderate |
MD 3.31 lower (3.92 lower to 2.71 lower) |
| WOMAC Stiffness (follow-up: range 22 weeks to 26 weeks; scale from 0 to 8) | |||||||
| 1,187 (11 RCTs) | Not serious | Serious a | Not serious | Not serious | None | ⨁⨁⨁◯ Moderate |
MD 1.14 lower (1.51 lower to 0.78 lower) |
| WOMAC Function (follow-up: range 22 weeks to 26 weeks; scale from 0 to 68) | |||||||
| 1,593 (15 RCTs) | Not serious | Serious a | Not serious | Not serious | None | ⨁⨁⨁◯ Moderate |
MD 10.09 lower (12.23 lower to 7.95 lower) |
| Evaluator global assessment (follow-up: range 12 weeks to 16 weeks; scale from 0 to 100) | |||||||
| 545 (7 RCTs) | Not serious | Serious a | Not serious | Not serious | None | ⨁⨁⨁◯ Moderate |
MD 17.52 lower (24.36 lower to 10.68 lower) |
| Evaluator global assessment (follow-up: range 22 weeks to 26 weeks; scale from 0 to 100) | |||||||
| 426 (4 RCTs) | Not serious | Serious a | Not serious | Not serious | None | ⨁⨁⨁◯ Moderate |
MD 21.39 lower (29.22 lower to 13.57 lower) |
VAS = Visual Analogue Scale; WOMAC = Western Ontario and McMaster Osteoarthritis Index; RCT = randomized controlled trial; MD = Mean difference. a = Significant heterogeneity in the analysis; b = The mean difference is statistically significant but not clinically significant. GRADE level of evidence assessment has a 4-grade scale: 1 out of 4 very low (⨁◯◯◯), 2 out of 4 low (⨁⨁◯◯), 3 out of 4 moderate (⨁⨁⨁◯), 4 out of 4 high (⨁⨁⨁⨁).
Discussion
The main finding of this meta-analysis is that placebo is an important component of the effect of injective treatments for patients with KOA, with saline injections being able to provide relevant and long-lasting results not only in terms of pain relief but also with respect to stiffness resolution and function improvement. These results are both statistically and clinically significant and can be perceived by patients up to 6 months.
The present meta-analysis showed that the placebo effect exceeds what was previously reported in terms of pain perception.6,9 Pain improvement was higher than what would be considered an MCID at 6 weeks and 3 months and decreased at the MCID level after 6 months, proving to have a long-lasting effect. Similar results were found in terms of stiffness and function with not only statistical but also clinically significant improvement following saline injections that persisted even after 6 months. This finding is reflected in the responder rate documented in the different RCTs, which was approximately 50%, meaning that almost half of the patients had a significant response to the injection of a “supposed” inactive liquid. This response persisted over time, with 47% of responders after 3 months and 52% of responders after 6 months. The effect of saline has also been documented in terms of EGA, which is rated by the assessor and thus less subjective and theoretically less prone to the placebo effect. Moreover, even if a meta-analysis was not possible for this specific outcome, an improvement in knee ROM was reported in all studies documenting it.21-25 This result might challenge the concept of saline being a “mere” placebo, as suggested by previous authors. Altman and colleagues hypothesized a therapeutic effect due to the aspiration of excessive and pathologic synovial liquid before the injection and the dilution of the inflammatory molecules during the injection.6,7 However, this meta-analysis showed no influence of procedure-related variables, such as the amount of solution injected or the number of injections performed. The demonstration of an increased effect in studies in which more saline was administered would have supported the hypothesis that, beside the placebo effect, a dilution effect on the inflammatory molecules exists. No evidence was found supporting hypotheses advocating the disease-modifying role of saline injection, but the debate is far from being resolved and future scientifically robust studies comparing sham injections and saline injections are needed to shed new light on this issue. Nevertheless, even considering the documented effect of a “mere” placebo, the clinical impact of intraarticular saline is highly relevant, in terms of pain, stiffness, and functional scores. This finding is of particular importance in the field of research on KOA, since it demonstrates that a clinically significant placebo effect should always be expected for both symptoms and functional outcomes after injections and should be accounted for when planning new trials in which pain and functional scores are commonly chosen as primary outcome. 28
The clinical relevance of these findings warrants further research directed toward the underlying mechanisms and the factors influencing the placebo response. 29 To investigate possible caveats explaining these composite yet overall highly significant effects of “placebo injections,” possible predictors of a greater response to saline were evaluated: the placebo effect size was found to be correlated to the magnitude of the effect of the experimental product tested. In this light, the greater improvement documented in the experimental group could be mainly due to an increased placebo effect rather than to actual effectiveness of the tested drug. This possibility should be considered when interpreting RCT results, which should always be evaluated with respect to the entity behind the placebo effect in the specific testing condition and at the specific follow-up time. Factors such as patient perception of the treatment or the attitude of the physician could play a key role in determining the entity behind the placebo effect, although these determinants remain difficult to quantify. 30 Interestingly, the duration of symptoms before treatment was found to be inversely related to the answer to saline injections. It is likely that patients who experience KOA for a longer time have a more severe disease and are less prone to respond to placebo or, more generally, to an injective treatment. 31 On the other hand, no correlation was documented for baseline KOA characteristics, such as baseline pain. Similarly, patient-related characteristics, such as age and BMI, were not found to be related to the effect size in the saline groups, which allows us to extend the meta-analysis findings on the placebo effect to almost all patients undergoing injections for KOA.
Previous studies have focused on the importance of the placebo effect in the field of KOA. In 2008, Zhang and colleagues analyzed placebo-controlled trials (including non-RCTs) in OA and found that the placebo effect is more pronounced in studies of hand OA, when the active treatment is more effective; in studies with a higher sample size, when baseline pain is greater; and of particular interest for the present study, when placebo is administered with an injection. 5 The stronger impact of intraarticular placebo injection when compared to oral and topical placebo was confirmed in a network meta-analysis by Bannuru and colleagues and then quantified by Altman and colleagues, who documented a statistically significant effect size of 0.61 in terms of pain relief after saline injections.6,7 In 2016, Saltzman and colleagues performed a meta-analysis of 3 studies suggesting a clinically, rather than only statistically, significant effect of saline injections in decreasing pain in patients with KOA. The present meta-analysis of 48 RCTs confirms the high effect size of the placebo effect of saline injections and for the first time underlines that this effect exceeds the short-term pain modulation (significantly influencing knee stiffness and function) and EGA, with an even longer-lasting effect on more objective outcomes than on pain perception. 9
The inclusion of double-blind RCTs with a low to moderate risk of bias in this meta-analysis produced strong evidence. However, there are still some limitations. Regression to the mean is commonly observed in clinical trials and the observed symptoms improvement after saline injections could be a combination of placebo effect and of regression to the mean effect. Even though it was impossible to take into account the regression to the mean effect due to the single-group analysis design, this meta-analysis quantified the response that should be expected in clinical trials after saline injections, documenting that it goes beyond the MCID. This further underlines the need to have placebo arms in double-blind clinical trials. Some of the subanalyses, such as the one focused on the experimental products tested, were not possible due to the low number of trials. Similarly, some possible determinants of the response to saline, such as symptom duration, the use of local anesthesia, and preinjection arthrocentesis, were not reported in all trials. Site/investigator/study coordinator effects could not be analyzed as well. Finally, there was heterogeneity in the placebo administration protocols, thus hindering the possibility of performing subanalyses based on the injection protocol and introducing heterogeneity in the results. Such limitations reduced the strength of the conclusion on the determinants of the response to intraarticular saline. However, this drawback does not affect the overall conclusion on the clinical relevance of the placebo effect following injections, for which the level of evidence is moderate for almost all outcomes.
This meta-analysis documented and quantified the statistically and clinically significant improvement after saline injections in terms of pain relief, stiffness resolution, functional impairment, and EGA improvement. While the mechanism and the determinants of these effects remain uncertain, the placebo effects are high, persistent, and even stronger when saline is used as a control for more effective treatments. Moreover, while the effect on pain decreased over time, it was stable for up to 6 months with respect to functional scores, which are commonly used as the primary outcome in RCTs on new injective treatments. This meta-analysis documented high, long-lasting, and heterogeneous effects, urging not to overlook the impact of placebo in the research on and management of KOA.
Supplemental Material
Supplemental material, Supplements_Update for The Long-Lasting Effects of “Placebo Injections” in Knee Osteoarthritis: A Meta-Analysis by Davide Previtali, Giulia Merli, Giorgio Di Laura Frattura, Christian Candrian, Stefano Zaffagnini and Giuseppe Filardo in CARTILAGE
Footnotes
Supplementary material for this article is available on the Cartilage website at https://journals.sagepub.com/home/CAR.
Authors’ Note: The study was performed at the Ospedale Regionale di Lugano, EOC, Lugano, Switzerland, and the IRCCS Istituto Ortopedico Rizzoli, Bologna, Italy.
Acknowledgments and Funding: The authors would like to thank Elettra Pignotti for her help with the statistical analysis. The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest Statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors declare that no support was reveived from any organization for the submitted work. CC has received institutional support outside the present work from Medacta International SA, Johnson & Johnson, Lima Corporate, Zimmer Biomet, and Oped AG. SZ received personal fees from I+ SRL, research grants outside the submitted work from Fidia Farmaceutici SPA, CartiHeal Ltd, IGEA Clinical Biophysics, BIOMET, and Kensey Nash, and has a patent with Springer, with royalties paid. GF received institutional support outside the submitted work from Finceramica Faenza SPA, Fidia Farmaceutici SPA, CartiHeal (2009) Ltd, EON medical SRL, IGEA Clinical Biophysics, BIOMET, and Kensey Nash.
Ethical Approval: Ethical approval was not sought for the present study because it is a meta-analysis.
Informed Consent: Informed consent was not sought for the present study because it is a meta-analysis.
ORCID iDs: Davide Previtali  https://orcid.org/0000-0002-0284-4368
https://orcid.org/0000-0002-0284-4368
Giulia Merli  https://orcid.org/0000-0001-7895-5606
https://orcid.org/0000-0001-7895-5606
References
- 1. Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nüesch E, Dieppe P, Reichenbach S, Williams S, Iff S, Jüni P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ. 2011;342:d1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1_suppl):46-54. [DOI] [PubMed] [Google Scholar]
- 4. Bruyère O, Cooper C, Pelletier JP, Branco J, Brandi ML, Guillemin F, et al. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum. 2014;44(3):253-63. [DOI] [PubMed] [Google Scholar]
- 5. Zhang W, Robertson J, Jones A, Dieppe P, Doherty M. The placebo effect and its determinants in osteoarthritis: meta-analysis of randomised controlled trials. Ann Rheum Dis. 2008;67(12):1716-23. [DOI] [PubMed] [Google Scholar]
- 6. Altman RD, Devji T, Bhandari M, Fierlinger A, Niazi F, Christensen R. Clinical benefit of intra-articular saline as a comparator in clinical trials of knee osteoarthritis treatments: a systematic review and meta-analysis of randomized trials. Semin Arthritis Rheum. 2016;46:151-9. [DOI] [PubMed] [Google Scholar]
- 7. Bannuru RR, McAlindon TE, Sullivan MC, Wong JB, Kent DM, Schmid CH. Effectiveness and implications of alternative placebo treatments: a systematic review and network meta-analysis of osteoarthritis trials. Ann Intern Med. 2015;163(5):365-72. [DOI] [PubMed] [Google Scholar]
- 8. Reiter-Niesert S, Boers M, Detert J. Short-term placebo response in trials of patients with symptomatic osteoarthritis: differences between hip and knee. Osteoarthritis Cartilage. 2016;24(6):1007-11. [DOI] [PubMed] [Google Scholar]
- 9. Saltzman BM, Leroux T, Meyer MA, Basques BA, Chahal J, Bach BR, Jr, et al. The therapeutic effect of intra-articular normal saline injections for knee osteoarthritis: a meta-analysis of evidence level 1 studies. Am J Sports Med. 2017;45(11):2647-53. [DOI] [PubMed] [Google Scholar]
- 10. Kaptchuk TJ, Miller FG. Placebo effects in medicine. N Engl J Med. 2015;373(1_suppl):8-9. [DOI] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-9. [DOI] [PubMed] [Google Scholar]
- 12. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higgins J, Deeks J, Altman D. Special topics in statistics. In: Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Version 5.1. 0. Available from: https://training.cochrane.org/handbook/archive/v5.1/
- 14. Kadic AJ, Vucic K, Dosenovic S, Sapunar D, Puljak L. Extracting data from figures with software was faster, with higher interrater reliability than manual extraction. J Clin Epidemiol. 2016;74:119-23. [DOI] [PubMed] [Google Scholar]
- 15. Vucic K, Kadic AJ, Puljak L. Survey of Cochrane protocols found methods for data extraction from figures not mentioned or unclear. J Clin Epidemiol. 2015;68(10):1161-4. [DOI] [PubMed] [Google Scholar]
- 16. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45(4):384-91. [DOI] [PubMed] [Google Scholar]
- 17. Pham T, van der Heijde D, Altman RD, Anderson J, Bellamy N, Hochberg M, et al. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage. 2004;12(5):389-99. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JP, Sterne JA, Savovic J, Page MJ, Hróbjartsson A, Boutron I, et al. A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst Rev. 2016;10(Suppl 1):29-31. [Google Scholar]
- 19. Ryan R, Hill S. How to GRADE the quality of the evidence. Available from: https://colorectal.cochrane.org/sites/colorectal.cochrane.org/files/public/uploads/how_to_grade.pdf
- 20. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kul-Panza E, Berker N. Is hyaluronate sodium effective in the management of knee osteoarthritis? A placebo-controlled double-blind study. Minerva Med. 2010;101(2):63-72. [PubMed] [Google Scholar]
- 22. Petterson SC, Plancher KD. Single intra-articular injection of lightly cross-linked hyaluronic acid reduces knee pain in symptomatic knee osteoarthritis: a multicenter, double-blind, randomized, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2019;27(6):1992-2002. [DOI] [PubMed] [Google Scholar]
- 23. Raynauld JP, Buckland-Wright C, Ward R, Choquette D, Haraoui B, Martel-Pelletier J, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48(2):370-7. [DOI] [PubMed] [Google Scholar]
- 24. Soltani SK, Forogh B, Ahmadbeigi N, Kharazi HH, Fallahzadeh K, Kashani L, et al. Safety and efficacy of allogenic placental mesenchymal stem cells for treating knee osteoarthritis: a pilot study. Cytotherapy. 2019;21(1_suppl):54-63. [DOI] [PubMed] [Google Scholar]
- 25. van der Weegen W, Wullems JA, Bos E, Noten H, van Drumpt RA. No difference between intra-articular injection of hyaluronic acid and placebo for mild to moderate knee osteoarthritis: a randomized, controlled, double-blind trial. J Arthroplasty. 2015;30(5):754-7. [DOI] [PubMed] [Google Scholar]
- 26. Trouvin AP, Marty M, Goupille P, Perrot S. Determinants of daily pain trajectories and relationship with pain acceptability in hip and knee osteoarthritis. A national prospective cohort study on 886 patients. Joint Bone Spine. 2019;86(2):245-50. [DOI] [PubMed] [Google Scholar]
- 27. Gooberman-Hill R, Woolhead G, Mackichan F, Ayis S, Williams S, Dieppe P. Assessing chronic joint pain: lessons from a focus group study. Arthritis Rhuem. 2007;57(4):666-71. [DOI] [PubMed] [Google Scholar]
- 28. Enck P, Bingel U, Schedlowski M, Rief W. The placebo response in medicine: minimize, maximize or personalize? Nat Rev Drug Discov. 2013;12(3):191-204. [DOI] [PubMed] [Google Scholar]
- 29. Kamper SJ, Williams CM. The placebo effect: powerful, powerless or redundant? Br J Sports Med. 2013;47(1_suppl):6-9. [DOI] [PubMed] [Google Scholar]
- 30. Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci. 2015;16(7):403-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Filardo G, Kon E, Longo UG, Madry H, Marchettini P, Marmotti A, et al. Non-surgical treatments for the management of early osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1775-85. [DOI] [PubMed] [Google Scholar]
- 32. Altman RD, Åkermark C, Beaulieu AD, Schnitzer T; Durolane International Study Group. Efficacy and safety of a single intra-articular injection of non-animal stabilized hyaluronic acid (NASHA) in patients with osteoarthritis of the knee. Osteoarthritis Cartilage. 2004;12(8):642-9. [DOI] [PubMed] [Google Scholar]
- 33. Altman RD, Rosen JE, Bloch DA, Hatoum HT, Korner P. A double-blind, randomized, saline-controlled study of the efficacy and safety of EUFLEXXA® for treatment of painful osteoarthritis of the knee, with an open-label safety extension (The FLEXX Trial). Semin Arthritis Rheum. 2009;39(1_suppl):1-9. [DOI] [PubMed] [Google Scholar]
- 34. Arden NK, Åkermark C, Andersson M, Todman MG, Altman RD. A randomized saline-controlled trial of NASHA hyaluronic acid for knee osteoarthritis. Curr Med Res Opin. 2014;30(2):279-86. [DOI] [PubMed] [Google Scholar]
- 35. Yang KA, Raijmakers N, Van Arkel E, Caron J, Rijk P, Willems W, et al. Autologous interleukin-1 receptor antagonist improves function and symptoms in osteoarthritis when compared to placebo in a prospective randomized controlled trial. Osteoarthritis Cartilage. 2008;16(4):498-505. [DOI] [PubMed] [Google Scholar]
- 36. Baltzer A, Moser C, Jansen S, Krauspe R. Autologous conditioned serum (Orthokine) is an effective treatment for knee osteoarthritis. Osteoarthritis Cartilage. 2009;17(2):152-60. [DOI] [PubMed] [Google Scholar]
- 37. Bar-Or D, Salottolo KM, Loose H, Phillips MJ, McGrath B, Wei N, et al. A randomized clinical trial to evaluate two doses of an intra-articular injection of LMWF-5A in adults with pain due to osteoarthritis of the knee. PLoS One. 2014;9(2):e87910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brandt KD, Block JA, Michalski JP, Moreland LW, Caldwell JR, Lavin PT. Efficacy and safety of intraarticular sodium hyaluronate in knee osteoarthritis. Clin Orthop Relat Res. 2001;(385):130-43. [DOI] [PubMed] [Google Scholar]
- 39. Chao J, Wu C, Sun B, Hose MK, Quan A, Hughes TH, et al. Inflammatory characteristics on ultrasound predict poorer long-term response to intraarticular corticosteroid injections in knee osteoarthritis. J Rheumatol. 2010;37(3):650-5. [DOI] [PubMed] [Google Scholar]
- 40. Chevalier X, Jerosch J, Goupille P, van Dijk N, Luyten FP, Scott DL, et al. Single, intra-articular treatment with 6 ml hylan GF 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo controlled trial. Ann Rheum Dis. 2010;69(1_suppl):113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Conaghan PG, Cohen SB, Berenbaum F, Lufkin J, Johnson JR, Bodick N. Brief Report: A Phase II b Trial of a Novel Extended-Release Microsphere Formulation of Triamcinolone Acetonide for Intraarticular Injection in Knee Osteoarthritis. Arthritis & Rheumatology, 2018;70(2), 204-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Conaghan PG, Hunter DJ, Cohen SB, Kraus VB, Berenbaum F, Lieberman JR, et al. Effects of a single intra-articular injection of a microsphere formulation of triamcinolone acetonide on knee osteoarthritis pain: a double-blinded, randomized, placebo-controlled, multinational study. J Bone Joint Surg Am. 2018;100(8):666-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Day R, Brooks P, Conaghan PG, Petersen M; Multicenter Trial Group. A double blind, randomized, multicenter, parallel group study of the effectiveness and tolerance of intraarticular hyaluronan in osteoarthritis of the knee. J Rheumatol. 2004;31(4):775-82. [PubMed] [Google Scholar]
- 44. Eker HE, Cok OY, Aribogan A, Arslan G. The efficacy of intra-articular lidocaine administration in chronic knee pain due to osteoarthritis: a randomized, double-blind, controlled study. Anaesth Crit Care Pain Med. 2017;36(2):109-14. [DOI] [PubMed] [Google Scholar]
- 45. Görmeli G, Görmeli CA, Ataoglu B, Çolak C, Aslantürk O, Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25(3):958-65. [DOI] [PubMed] [Google Scholar]
- 46. Grecomoro G, Martorana U, Di CM. Intra-articular treatment with sodium hyaluronate in gonarthrosis: a controlled clinical trial versus placebo. Pharmatherapeutica. 1987;5(2):137-41. [PubMed] [Google Scholar]
- 47. Hangody L, Szody R, Lukasik P, Zgadzaj W, Lénárt E, Dokoupilova E, et al. Intraarticular injection of a cross-linked sodium hyaluronate combined with triamcinolone hexacetonide (Cingal) to provide symptomatic relief of osteoarthritis of the knee: a randomized, double-blind, placebo-controlled multicenter clinical trial. Cartilage. 2018;9(3):276-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Henrotin Y, Berenbaum F, Chevalier X, Marty M, Richette P, Rannou F. Reduction of the serum levels of a specific biomarker of cartilage degradation (Coll2-1) by hyaluronic acid (KARTILAGE® CROSS) compared to placebo in painful knee osteoarthritis patients: the EPIKART study, a pilot prospective comparative randomized double blind trial. BMC Musculoskelet Disord. 2017;18(1_suppl):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huang TL, Chang CC, Lee CH, Chen SC, Lai CH, Tsai CL. Intra-articular injections of sodium hyaluronate (Hyalgan®) in osteoarthritis of the knee. A randomized, controlled, double-blind, multicenter trial in the Asian population. BMC Musculoskelet Disord. 2011;12:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huskisson E, Donnelly S. Hyaluronic acid in the treatment of osteoarthritis of the knee. Rheumatology (Oxford). 1999;38(7):602-7. [DOI] [PubMed] [Google Scholar]
- 51. Jørgensen A, Stengaard-Pedersen K, Simonsen O, Pfeiffer-Jensen M, Eriksen C, Bliddal H, et al. Intra-articular hyaluronan is without clinical effect in knee osteoarthritis: a multicentre, randomised, placebo-controlled, double-blind study of 337 patients followed for 1 year. Ann Rheum Dis. 2010;69(6):1097-102. [DOI] [PubMed] [Google Scholar]
- 52. Jubb R, Piva S, Beinat L, Dacre J, Gishen P. A one-year, randomised, placebo (saline) controlled clinical trial of 500-730 kDa sodium hyaluronate (Hyalgan) on the radiological change in osteoarthritis of the knee. Int J Clin Pract. 2003;57(6):467-74. [PubMed] [Google Scholar]
- 53. Karlsson J, Sjogren L, Lohmander L. Comparison of two hyaluronan drugs and placebo in patients with knee osteoarthritis. A controlled, randomized, double-blind, parallel-design multicentre study. Rheumatology (Oxford). 2002;41(11):1240-8. [DOI] [PubMed] [Google Scholar]
- 54. Kim MK, Ha CW, In Y, Cho SD, Choi ES, Ha JK, et al. A multicenter, double-blind, phase III clinical trial to evaluate the efficacy and safety of a cell and gene therapy in knee osteoarthritis patients. Hum Gene Ther Clin Dev. 2018;29(1_suppl):48-59. [DOI] [PubMed] [Google Scholar]
- 55. Kon E, Engebretsen L, Verdonk P, Nehrer S, Filardo G. Clinical outcomes of knee osteoarthritis treated with an autologous protein solution injection: a 1-year pilot double-blinded randomized controlled trial. Am J Sports Med. 2018;46(1_suppl):171-80. [DOI] [PubMed] [Google Scholar]
- 56. Kotevoglu N, Iyıbozkurt PC, Hız O, Toktas H, Kuran B. A prospective randomised controlled clinical trial comparing the efficacy of different molecular weight hyaluronan solutions in the treatment of knee osteoarthritis. Rheumatol Int. 2006;26(4):325-30. [DOI] [PubMed] [Google Scholar]
- 57. Lee MC, Ha C, Elmallah R, Cherian J, Cho J, Kim TW, et al. A placebo-controlled randomised trial to assess the effect of TGF-β1-expressing chondrocytes in patients with arthritis of the knee. Bone Joint J. 2015;97(7):924-32. [DOI] [PubMed] [Google Scholar]
- 58. Lee WS, Kim HJ, Kim KI, Kim GB, Jin W. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl Med. 2019;8(6):504-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lin KY, Yang CC, Hsu CJ, Yeh ML, Renn JH. Intra-articular injection of platelet-rich plasma is superior to hyaluronic acid or saline solution in the treatment of mild to moderate knee osteoarthritis: a randomized, double-blind, triple-parallel, placebo-controlled clinical trial. Arthroscopy. 2019;35(1_suppl):106-17. [DOI] [PubMed] [Google Scholar]
- 60. Lohmander LS, Dalen N, Englund G, Hämäläinen M, Jensen EM, Karlsson K, et al. Intra-articular hyaluronan injections in the treatment of osteoarthritis of the knee: a randomised, double blind, placebo controlled multicentre trial. Hyaluronan Multicentre Trial Group. Ann Rheum Dis. 1996;55(7):424-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lozada CJ, del Rio E, Reitberg DP, Smith RA, Kahn CB, Moskowitz RW. A double-blind, randomized, saline-controlled study of the efficacy and safety of co-administered intra-articular injections of Tr14 and Ze14 for treatment of painful osteoarthritis of the knee: the MOZArT trial. Eur J Integr Med. 2017;13:54-63. [Google Scholar]
- 62. Lundsgaard C, Dufour N, Fallentin E, Winkel P, Gluud C. Intra-articular sodium hyaluronate 2 mL versus physiological saline 20 mL versus physiological saline 2 mL for painful knee osteoarthritis: a randomized clinical trial. Scand J Rheumatol. 2008;37(2):142-50. [DOI] [PubMed] [Google Scholar]
- 63. McAlindon TE, LaValley MP, Harvey WF, Price LL, Driban JB, Zhang M, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317(19):1967-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. McAlindon TE, Schmidt U, Bugarin D, Abrams S, Geib T, DeGryse R, et al. Efficacy and safety of single-dose onabotulinumtoxinA in the treatment of symptoms of osteoarthritis of the knee: results of a placebo-controlled, double-blind study. Osteoarthritis Cartilage. 2018;26(10):1291-9. [DOI] [PubMed] [Google Scholar]
- 65. Navarro-Sarabia F, Coronel P, Collantes E, Navarro F, de la Serna AR, Naranjo A, et al. A 40-month multicentre, randomised placebo-controlled study to assess the efficacy and carry-over effect of repeated intra-articular injections of hyaluronic acid in knee osteoarthritis: the AMELIA project. Ann Rheum Dis. 2011;70(11):1957-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356-64. [DOI] [PubMed] [Google Scholar]
- 67. Pavelka K, Jr, Sedláčková M, Gatterová J, Bečvář R, Pavelka K, Sr. Glycosaminoglycan polysulfuric acid (GAGPS) in osteoarthritis of the knee. Osteoarthritis Cartilage. 1995;3(1_suppl):15-23. [DOI] [PubMed] [Google Scholar]
- 68. Ravaud P, Moulinier L, Giraudeau B, Ayral X, Guerin C, Noel E, et al. Effects of joint lavage and steroid injection in patients with osteoarthritis of the knee: results of a multicenter, randomized, controlled trial. Arthritis Rheum. 1999;42(3):475-82. [DOI] [PubMed] [Google Scholar]
- 69. Rossini M, Adami S, Fracassi E, Viapiana O, Orsolini G, Povino MR, et al. Effects of intra-articular clodronate in the treatment of knee osteoarthritis: results of a double-blind, randomized placebo-controlled trial. Rheumatol Int. 2015;35(2):255-63. [DOI] [PubMed] [Google Scholar]
- 70. Salottolo K, Cole B, Bar-Or D. Intra-articular injection of the anti-inflammatory compound LMWF-5A in adults with severe osteoarthritis: a double-blind prospective randomized controlled multi-center safety and efficacy trial. Patient Saf Surg. 2018;12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Scale D, Wobig M, Wolpert W. Viscosupplementation of osteoarthritic knees with hylan: a treatment schedule study. Curr Ther. 1994;55(3):220-232. [Google Scholar]
- 72. Schwappach J, Dryden SM, Salottolo KM. Preliminary trial of intra-articular LMWF-5A for osteoarthritis of the knee. Orthopedics. 2017;40(1_suppl):e49-e53. [DOI] [PubMed] [Google Scholar]
- 73. Shrestha R, Shrestha R, Thapa S, Khadka SK, Shrestha D. Clinical outcome following intra-articular triamcinolone injection in osteoarthritic knee at the community: a randomized double blind placebo controlled trial. Kathmandu Univ Med J (KUMJ). 2018;16(62):175-80. [PubMed] [Google Scholar]
- 74. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44(4):884-91. [DOI] [PubMed] [Google Scholar]
- 75. Strand V, Baraf HS, Lavin P, Lim S, Hosokawa H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2012;20(5):350-6. [DOI] [PubMed] [Google Scholar]
- 76. Wobig M, Dickhut A, Maier R, Vetter G. Viscosupplementation with hylan GF 20: a 26-week controlled trial of efficacy and safety in the osteoarthritic knee. Clin Ther. 1998;20(3):410-23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplements_Update for The Long-Lasting Effects of “Placebo Injections” in Knee Osteoarthritis: A Meta-Analysis by Davide Previtali, Giulia Merli, Giorgio Di Laura Frattura, Christian Candrian, Stefano Zaffagnini and Giuseppe Filardo in CARTILAGE