Abstract
Objective
Cartilage damage diagnosed by magnetic resonance imaging (MRI) is highly prevalent in the population. In this article, we explore whether such cartilage damage is associated with greater longitudinal change in 3D cartilage thickness and knee function in subjects without (risk factors of) knee osteoarthritis.
Design
Eighty-two knees of Osteoarthritis Initiative healthy reference cohort participants had baseline and 4-year follow-up MRI and knee function data. Baseline presence of semiquantitatively assessed MRI-based cartilage damage (MOAKS [MRI Osteoarthritis Knee Score] ≥ grade 1.0) was recorded by an experienced radiologist. Longitudinal femorotibial cartilage thickness change was determined after segmentation, using location-independent methodology. Knee function was evaluated by patient-reported outcomes and functional performance measures. Statistical comparisons included analysis of covariance adjusting for age, sex, and body mass index.
Results
Forty-five percent of the participants had cartilage damage in at least one femorotibial subregion; the cartilage thickness change score was 15% greater in participants with than in those without damage (1216 ± 434 vs. 1058 ± 277 µm). This difference reached borderline statistical significance with and without adjustment for age, sex, and body mass index (P = 0.05). No significant differences in the change of patient-reported outcomes of knee function (PASE [physical activity score of the elderly] and WOMAC [Western Ontario McMaster Osteoarthritis Index]) or chair stand test results were detected. Of those without femorotibial damage, 58% had cartilage damage in at least one femoropatellar subregion; these had a 9% greater femorotibial cartilage change score than those without femoropatellar or femorotibial damage (difference not statistically significant).
Conclusions
In the absence of osteoarthritis risk factors, semiquantitatively assessed MRI-based cartilage damage appears to be associated with greater longitudinal location-independent femorotibial cartilage thickness changes, but not with greater functional deteriorations.
Keywords: cartilage damage, MRI, cartilage thickness, knee function, deterioration
Introduction
Cartilage damage as diagnosed by expert radiologists’ readings on magnetic resonance imaging (MRI) is highly prevalent in the general population, even in subjects without radiographic knee osteoarthritis (OA).1,2 Such cartilage damage can be either focal or widespread. The MRI Osteoarthritis Knee Score (MOAKS) system, 3 which was used in this study, defines “focal” cartilage damage as lesions that cover less than 10% of the specific region of interest (i.e., grades 1.0 or 1.1). Widespread damage, in contrast, is defined as extending over areas greater than 10% of the region of interest (i.e., grades 2.0, 2.1, 2.2, 3.0, 3.1, 3.2, and 3.3). 3 Such semiquantitatively assessed cartilage damage 3 has been reported to predict the onset of pain, 4 incident radiographic knee OA in subjects without knee OA,5,6 cartilage thickness loss in subjects with knee OA, 7 and total knee replacement. 8 However, it is currently unknown to what extent such cartilage damage is associated with quantitative structural outcomes, such as longitudinal change in 3D cartilage thickness obtained from cartilage segmentation, as well as with that of functional impairments in subjects without knee OA, particularly in those without risk factors of incident OA. Exploration of this relationship in a cohort without risk factors of knee OA is critical, as these risk factors may be independently associated with both the presence of cartilage damage and with structural and functional longitudinal deterioration, and hence any relationship between cartilage damage and longitudinal deterioration may be confounded if not studied in a cohort without these (other) risk factors.
For this reason, we explored the relationship between baseline MRI-detected femorotibial cartilage damage versus longitudinal change in cartilage thickness anywhere across the femorotibial joint, and versus knee joint function in the Osteoarthritis Initiative (OAI) healthy reference cohort. The latter represents a subpopulation of the OAI without symptoms, radiographic signs, and risk factors of incident knee OA.9-11 Specifically, we determined the location-independent change in femorotibial cartilage thickness over 4 years, as this has been shown to be sensitive to change anywhere in the joint (e.g., at the site of cartilage damage, but also in the direct or indirect environment), and to be more sensitive in detecting differences between risk strata than location-dependent approaches. 12 Furthermore, we assessed the relationship between baseline cartilage damage versus functional measures, that is, 4-year change in patient-reported outcomes (PROs) and in performance measures of knee function.
Materials and Methods
Study Population
The study was based on data from the OAI, a prospective, observational cohort study (https://nda.nih.gov/oai/). The OAI enrolled 4796 participants aged 45 to 79 at 4 clinical centers, has provided clinical data, 3 Tesla MRI, and fixed-flexion radiographs of both knees,9,13 and was approved by the Committee on Human Research, the Institutional Review Board (IRB) for the University of California, San Francisco (UCSF), and the IRBs at each clinical site. Participants of the nonexposed healthy reference cohort of the OAI (Sample 0.B.1.) had
No pain, aching or stiffness in either knee in the past year.
No radiographic findings of femorotibial OA (Osteoarthritis Research Society International [OARSI] osteophyte grade 0 and joint space narrowing grade 0) of either knee using the clinic reading of the baseline bilateral fixed flexion radiographs.
- No risk factors for the onset of knee OA, including
- ○ Obesity defined as a body weight of >170 lbs (77.1 kg) in women aged 45 to 69, >180 lbs (81.7 kg) in women aged 70 to 79, >205 lbs (93 kg) in men aged 45 to 69, and >215 lbs (97.5 kg) in men aged 70 to 79
- ○ History of knee injury, defined as having caused difficulty walking for at least a week
- ○ Knee surgery
- ○ Family history of total knee replacement in a biological parent or sibling
- ○ Heberden’s nodes, defined as self-reported bony enlargements of one or more distal interphalangeal joints in both hands
- ○ Repetitive knee bending, defined as current daily activity at work or out-side work, requiring frequent climbing, stooping, bending, lifting, squatting, or kneeling
Eighty-two right knees of 82 healthy reference cohort participants had both baseline and 4-year follow-up data by MRI as well as measures of knee function and were confirmed to be free of radiographic signs of OA in central readings performed by experts at Boston University and were confirmed to be free of radiographic signs of OA not only by the site readings (see above, but also by the central readings performed by experts at Boston University.
Evaluation of Cartilage Structure by MRI
Cartilage damage was assessed using the MOAKS, 3 at baseline by an experienced musculoskeletal radiologist with 15 years’ experience in semiquantitative standardized assessment of knee OA (FR). The coronal 2-dimensional intermediate-weighted (IW) turbo spin-echo (TSE), sagittal 3-dimensional (3D) dual-echo at steady-state (DESS), coronal and axial multiplanar reformations of the 3D DESS and sagittal IW fat-suppressed (fs) TSE sequences were used for assessment. Presence of cartilage damage in the femorotibial joint was defined as a cartilage alteration ≥grade 1.0 (i.e., a focal superficial defect) in at least 1 of 10 femorotibial subregions (i.e., the anterior, central, and posterior medial tibia; the central and posterior medial femur; the anterior, central, and posterior lateral tibia; the central and posterior lateral femur). 3 Presence of cartilage damage in the femoropatellar joint was defined as a cartilage alteration ≥grade 1.0 in at least 1 of 4 femorotibial subregions: the medial and lateral trochlea, and the medial and lateral patella. 3
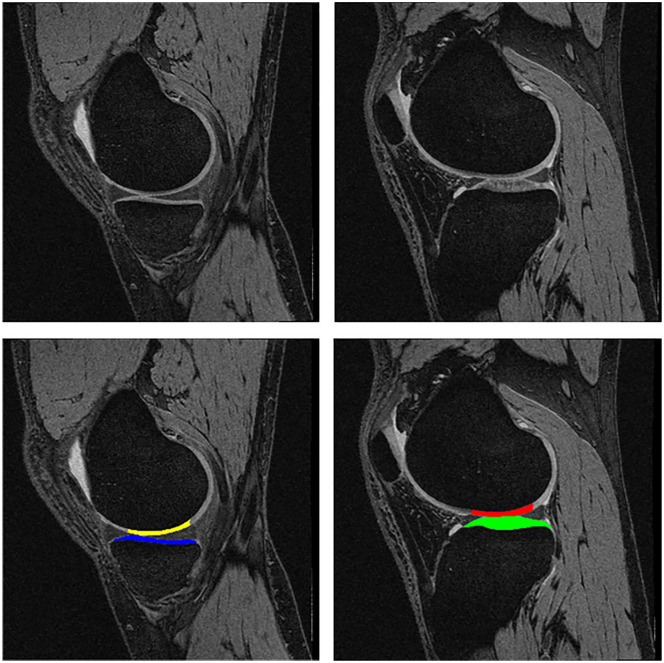
Longitudinal cartilage thickness change was determined using a sagittal DESS sequence 9 ( Fig. 1 ). Cartilage thickness was measured in the total femorotibial joint (FTJ), in the (central) medial and lateral femorotibial compartment ([c]MFTC/LFTC), and in 16 femorotibial subregions ( Fig. 2 ), using Chondrometrics Works 3.0 software (Chondrometrics GmbH, Ainring, Germany) as described previously. 14 A location-independent analysis method was then used to determine the cartilage thickness total change score, the thinning score, and the thickening score 12 : The cartilage thinning score represents the sum of all negative subregion changes, the cartilage thickening score the sum of all positive subregion changes, and the total change score the sum of all subregion changes ( Fig. 2 ), independent of direction. 12
Figure 1.
Sagittal DESS MRI sequence displaying the medial femorotibial compartment (MFTC) on the left and the lateral one (LFTC) on the right. Top row without segmentation, bottom row with segmentation of the cartilages. Yellow = weight-bearing medial femorotibial condyle (cMF); blue = medial tibia (MT); red = weight-bearing lateral femorotibial condyle (cLF); green = lateral tibia (LT).
Figure 2.
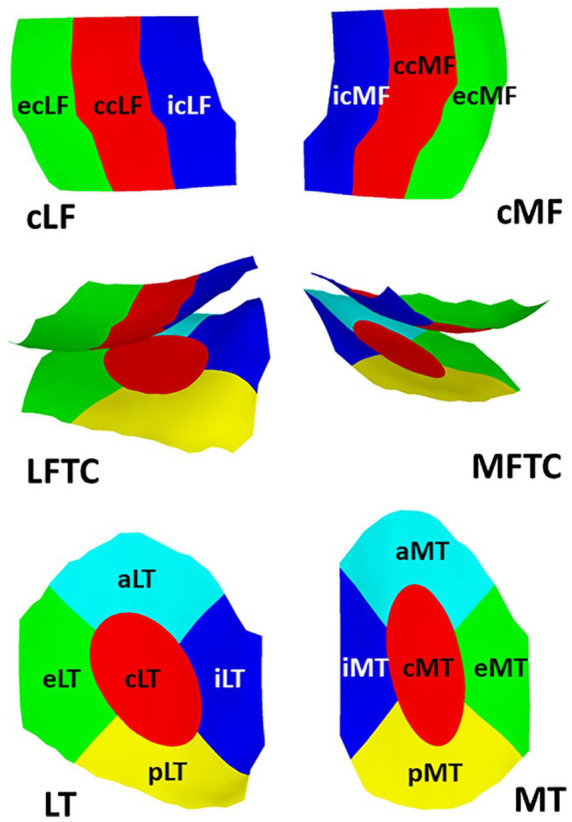
Explosion figure displaying the 16 femorotibial subregions. Top row: Inferior view of the weight-bearing femorotibial condyles, with the lateral weight-bearing (central) femorotibial condyle on the left, and the medial one on the right. Bottom row: Superior view of the tibial plateau, with the lateral tibia on the left, and the medial one on the right. The middle row shows a posterior view of the femoral condyles and tibiae together. Red = central subregions (c); green = external subregions (e); blue = internal subregions (i); yellow = posterior subregions (p); turquoise = anterior subregions (a).
Evaluation of Knee Joint Function by Patient-Reported Outcomes and Functional Performance
Knee function was evaluated by PROs, that is, the physical activity score of the elderly (PASE) and the WOMAC (Western Ontario McMaster Osteoarthritis Index) function score. Furthermore, functional performance measures were studied, that is, the chair stand test pace (CST; [stands/s]) and the 20-m walk test pace (20mWT [m/s]) as collected by the OAI (https://nda.nih.gov/oai).
Statistical Analysis
Statistical comparisons were performed using unpaired t tests, after ensuring a normal distribution of longitudinal cartilage thickness change, using the Kolmogorov-Smirnov test. This condition was tested and verified for the location-independent cartilage thickness change score as well as for medial and lateral compartment cartilage thickness change, in both those with and in those without baseline cartilage lesions. Further analyses for the cartilage thickness change score were performed using analysis of covariance (ANCOVA), adjusting for age, sex, and body mass index (BMI; IBM SPSS Statistics V24.0). In this exploratory analysis, no adjustment for multiple parallel statistical testing was performed. However, the a priori defined primary statistical focus of this analysis was the location-independent cartilage thickness change score, as this has been previously shown to be most sensitive in detecting alterations in non-OA knees. 15
Results
Demographic and Baseline Data
The healthy reference participants were 54.1 ± 7.2 years old, with a BMI of 24.2 ± 3.0 kg/m2, and with 61% being female. Thirty-seven participants (45%) had cartilage damage (MOAKS ≥ grade 1.0) in at least one femorotibial subregion of their right knee, whereas 45 did not ( Table 1 ). Of the participants with femorotibial cartilage damage, 22 (59%) were women, and of those without cartilage damage, 28 (62%) were women. The age and BMI between those with and without femorotibial cartilage damage did not differ significantly ( Table 1 ). Of the 37 knees, 11 had only medial compartment damage, 22 had only lateral compartment damage, and 4 had both medial and lateral compartment damage.
Table 1.
Baseline Values in OAI Healthy Reference Cohort Participant Knees with and without MRI-Detected Femorotibial Cartilage Damage (MOAKS Scores ≥1).
| Knees without Any Femorotibial
Cartilage Damage (N = 45) |
Knees with Any Femorotibial
Cartilage Damage (n = 37) |
P Value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 53.5 | 6.3 | 54.8 | 8.3 | 0.44 |
| BMI (kg/m2) | 24.1 | 3.0 | 24.4 | 3.0 | 0.65 |
| FTJ (mm) | 7.15 | 1.00 | 7.31 | 1.09 | 0.48 |
| MFTC (mm) | 3.31 | 0.51 | 3.45 | 0.57 | 0.25 |
| LFTC (mm) | 3.91 | 0.19 | 3.94 | 0.00 | 0.32 |
| cMFTC (mm) | 4.13 | 0.69 | 4.33 | 0.77 | 0.23 |
| cLFTC (mm) | 5.19 | 0.82 | 5.25 | 0.89 | 0.73 |
| PASE | 163 | 72.3 | 180 | 85.2 | 0.33 |
| WOMAC Fct | 0.44 | 2.16 | 0.17 | 0.53 | 0.45 |
| CST Pace (stands/s) | 0.59 | 0.11 | 0.62 | 0.16 | 0.34 |
| 20m Pace (m/s) | 1.44 | 0.20 | 1.51 | 0.20 | 0.14 |
SD = standard deviation; BMI = body mass index; FTJ = total femorotibial joint cartilage thickness; MFTC = medial compartment cartilage thickness; LFTC = lateral compartment cartilage thickness; cMFTC = central MFTC; cLFTC = central LFTC; PASE = physical activity score of the elderly (range 0-793; higher values denominate higher level of activity); WOMAC Fct = Western Ontario Mac Master Function Score (range 0-68; higher values denominate higher levels of functional limitation); CST Pace = Chair Stand Tests pace in stands/s; 20m Pace = 20m walk test pace in m/s.
Of the 45 participant knees without femorotibial cartilage damage, 26 (58%) had cartilage damage in at least one femoropatellar subregion, whereas 19 did not ( Table 2 ). Of those with femoropatellar (but without femorotibial) cartilage damage, 20 (77%) were women, and of those without femoropatellar and without femorotibial cartilage damage 8 (42%) were women. Again, the age and BMI did not differ significantly between those with and without femoropatellar cartilage damage ( Table 2 ).
Table 2.
Baseline Values in OAI Healthy Reference Cohort Participant Knees with and without MRI-Detected Femoropatellar Cartilage Damage (MOAKS Scores ≥1), in the Absence of Femorotibial Cartilage Damage a .
| Knees without Any Femoropatellar
Cartilage Damage (N = 19) |
Knees with Any Femoropatellar
Cartilage Damage (N = 26) |
P Value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (year) | 52.6 | 6.7 | 54.2 | 6.0 | 0.40 |
| BMI (kg/m2) | 24.8 | 3.5 | 23.6 | 2.6 | 0.21 |
| FTJ (mm) | 7.25 | 1.29 | 7.07 | 0.73 | 0.56 |
| MFTC (mm) | 3.39 | 0.64 | 3.26 | 0.39 | 0.39 |
| LFTC (mm) | 3.87 | 0.29 | 3.94 | 0.00 | 0.20 |
| cMFTC (mm) | 4.23 | 0.85 | 4.07 | 0.56 | 0.45 |
| cLFTC (mm) | 5.21 | 1.06 | 5.17 | 0.62 | 0.89 |
| PASE | 154 | 83 | 170 | 64 | 0.48 |
| WOMAC Fct | 0.79 | 3.21 | 0.19 | 0.80 | 0.37 |
| CST Pace (stands/s) | 0.59 | 0.12 | 0.59 | 0.10 | 0.78 |
| 20m Pace (m/s) | 1.40 | 0.21 | 1.47 | 0.19 | 0.29 |
Abbreviations: See Table 1 .
No statistically significant baseline differences in cartilage thickness and knee function were observed between the above strata ( Tables 1 and 2 ).
Longitudinal (4-Year) Change of Cartilage Thickness by MRI
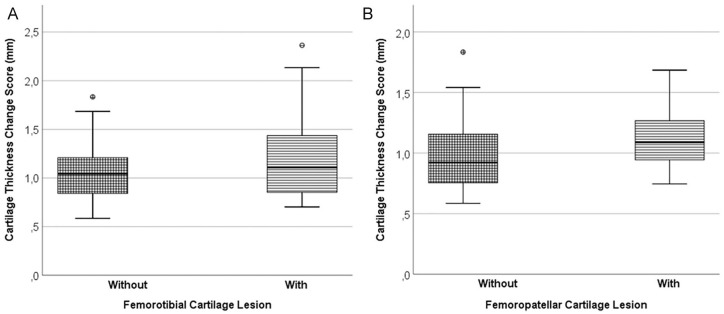
In knees with femorotibial cartilage damage, the location-independent cartilage thickness change score was 15% greater than in those without femorotibial cartilage damage (1216 ± 434 vs. 1058 ± 277 µm; Table 3 ; Fig. 3 ); this difference attained borderline statistical significance (P = 0.049). With adjustment for age, sex, and BMI (ANCOVA), the level of statistical significance for the difference was P = 0.05. The difference in the cartilage change score was mainly driven by a 28% difference in the cartilage thinning score, and to a lesser extent by a 2.4% difference in the cartilage thickening score; however, between-group differences in either of these 2 scores did not reach statistical significance ( Table 3 ). In the 28 women without femorotibial cartilage damage, the location-independent cartilage change score was 1049 ± 238 µm, and it was very similar in the 17 men without femorotibial damage (1073 ± 340 µm).
Table 3.
Longitudinal (4-Year) Change in OAI Healthy Reference Cohort Participant Knees with and without MRI-Detected Femorotibial Cartilage Damage a .
| Knees without Any Femorotibial
Cartilage Damage |
Knees with Any Femorotibial
Cartilage Damage |
P Value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Change score (µm) | 1058 | 277 | 1216 | 434 | 0.049 |
| Thinning score (µ) | −519 | 302 | −664 | 484 | 0.10 |
| Thickening score (µ) | 539 | 330 | 552 | 402 | 0.87 |
| FTJ (µm) | 20 | 141 | −11 | 197 | 0.40 |
| MFTC (µm) | 28 | 86 | −9 | 97 | 0.07 |
| LFTC (µm) | −8 | 76 | −2 | 122 | 0.80 |
| cMFTC (µm) | 20 | 130 | −8 | 150 | 0.37 |
| cLFTC (µm) | 27 | 113 | 25 | 180 | 0.95 |
| PASE | −9.7 | 67 | 5.2 | 103 | 0.43 |
| WOMAC Fct | −0.31 | 1.95 | 0.07 | 0.78 | 0.26 |
| CST Pace (stands/s) | 0.01 | 0.15 | 0.01 | 0.17 | 0.85 |
| 20m Pace (m/s) | 0.02 | 0.12 | −0.04 | 0.13 | 0.02 |
Change Score = location independent cartilage thickness change score; Thinning Score = location independent cartilage thinning score; Thickening Score = location independent cartilage thickening score. Other abbreviations: See Table 1 .
Figure 3.
Box plots showing the the location-independent cartilage thickness change score in the following: (A) Participant knees without femorotibial cartilage damage on the left (n = 45). Participant knees with femorotibial cartilage damage on the right (n = 37). (B) Participant knees without femorotibial or femoropatellar cartilage damage on the left (n = 19). Participant knees without femorotibial but with femoropatellar cartilage damage on the right (n = 26).
Location-specific cartilage thickness change across the total femorotibial joint, and in the (central) medial or lateral femorotibial compartment, did not differ significantly between those with and without any (medial or lateral) femorotibial cartilage damage ( Table 3 ). Furthermore, in the 15 knees with medial (or medial and lateral) femorotibial cartilage damage, the cartilage thickness change in the medial femorotibial compartment (MFTC) was −25.8 ± 83.3 µm, whereas in the 45 knees without any femorotibial cartilage damage it was 27.7 ± 85.6 µm; this difference did attain statistical significance (P = 0.039). In the 26 knees with lateral (or lateral and medial) femorotibial cartilage damage, the cartilage thickness change in the lateral femorotibial compartment (LFTC) was −6.5 ± 133 µm, whereas in the 45 knees without any femorotibial cartilage damage it was −7.5 ± 76 µm; this difference, in contrast, did not attain statistical significance (P = 0.97).
Among those with any femorotibial cartilage damage, 10 (of the 16) subregions displayed net cartilage thinning, and the other 6 subregions net cartilage thickening. The strongest cartilage thinning was observed in the posterior lateral tibia (−62.3 µm), the internal lateral tibia (−44.5 µm), and the anterior medial tibia (−32.9 µm), and the strongest thickening in the central lateral femur (54.6 µm), the external lateral femur (45.3 µm), and the external medial femur (22.7 µm). Among those without any femorotibial cartilage damage, 8 (of the 16) subregions displayed net cartilage thinning, and the other 8 subregions net cartilage thickening. The strongest cartilage thinning was observed in the internal lateral tibia (−62.5 µm), the external medial tibia (−28.8 µm), and the posterior lateral tibia (−26.0 µm), and the strongest thickening in the internal medial tibia (57.4 µm), the external medial femur (39.9 µm), and the central medial femur (37.4 µm).
The location-independent femorotibial cartilage thickness change score ( Fig. 3 ), thinning score, and thickening score were each 9% greater in participants with femoropatellar cartilage damage (and without femorotibial cartilage damage) than in those without femoropatellar or femorotibial cartilage damage; however, these differences did not reach statistical significance ( Table 4 ). Similarly, location-specific cartilage thickness changes across the total femorotibial joint, or in the (central) medial or lateral femorotibial compartments, were not significantly different between those with versus without femoropatellar cartilage damage ( Table 4 ).
Table 4.
Longitudinal (4-Year) Change in OAI Healthy Reference Cohort Participant Knees with and without MRI-Detected Femoropatellar Cartilage Damage (in the Absence of Femorotibial Cartilage Damage) a .
| Knees without Any Femoropatellar
Cartilage Damage |
Knees with Any Femoropatellar
Cartilage Damage |
P Value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Change score (µm) | 1004 | 336 | 1098 | 224 | 0.27 |
| Thinning score (µm) | −492 | 348 | −538 | 270 | 0.62 |
| Thickening score (µm) | 511 | 388 | 559 | 286 | 0.63 |
| FTJ (µm) | 21 | 160 | 20 | 129 | 0.98 |
| MFTC (µm) | 29 | 104 | 27 | 71 | 0.93 |
| LFTC (µm) | −8 | 70 | −7 | 82 | 0.97 |
| cMFTC (µm) | 35 | 149 | 9 | 116 | 0.51 |
| cLFTC (µm) | 29 | 118 | 26 | 112 | 0.9 |
| PASE | 3.42 | 66.3 | −19.3 | 66.3 | 0.26 |
| WOMAC Fct | −0.53 | 2.87 | −0.15 | 0.83 | 0.53 |
| CST Pace (stands/s) | −0.01 | 0.01 | 0.05 | 0.03 | 0.06 |
| 20m Pace (m/s) | 0.05 | −0.04 | 0.00 | 0.00 | 0.17 |
Abbreviations: See Table 3 .
Longitudinal (4-Year) Change in Knee Function
No significant differences in the change of PROs of knee function or the CST were observed in those with and without femorotibial cartilage damage ( Table 3 ), or in those with and without femoropatellar damage ( Table 4 ). There was a significant reduction in the 20mWT pace in those with femorotibial cartilage damage ( Table 2 ); however, the difference was only 2.6% and the functional performance at follow-up was identical between both strata, whereas at baseline, those with femorotibial cartilage damage displayed slightly better performance, that is, a faster walk pace ( Table 1 ).
Discussion
Here we explore the relationship between (4-year) longitudinal cartilage thickness and knee function change versus baseline MRI-diagnosed cartilage damage, without other (general) risk factors of knee OA being present. In the absence of such potential confounding, femorotibial cartilage damage appears to be associated with greater location-independent structural change of cartilage thickness over 4 years in a nonexposed healthy reference cohort. The observed difference reached borderline statistical significance for the cartilage thickness change score, but no relevant differences in functional deterioration were noted between those with and without cartilage damage.
A limitation of the current study is the relatively small sample size; however, all participants of the OAI healthy reference cohort that had 4-year longitudinal data available were studied, and this cohort is unique in that it has actively ruled out a large number of general risk factors of knee OA. The number of those with and without femorotibial cartilage damage (and of those without femorotibial, but with and without femoropatellar cartilage damage) was relatively balanced, so that a satisfactory statistical efficiency was warranted in the small cohort. Evaluation of femorotibial and femoropatellar cartilage damage was performed by a musculoskeletal radiologist (FWR) with ample experience with MOAKS 3 and other semiquantitative scoring systems. A strength of the current study included the use of a location-independent measurement technology of cartilage thickness change 12 that has been previously shown to detect differences in “cartilage thickness perturbation” not accessible to location-specific analysis.15,16 A further strength was the use of both PROs and performance tests of knee joint function. It would have been desirable to study the participants over an even longer follow-up period than 4 years; however, the cartilage segmentation and quality control is a labor-intensive process, and running such analyses over longer periods is challenging. A further limitation of the study was that no MRI data were available on femoropatellar cartilage loss, with the OAI being focused on femorotibial OA. Another limitation of the study was that multiple measures were compared between those with and without cartilage damage, without adjusting for parallel comparisons. However, one parameter (the location-independent cartilage change score) was a priori determined as the primary analytic outcome, based on previous observations of cartilage thickness “perturbation” in anterior cruciate deficient knees without radiographic knee OA. 15
In a previous population-based cohort (Framingham), 69% of the participants without radiographic knee OA displayed MRI-based cartilage damage. The lower percentage observed here likely is due to the active exclusion of general risk factors in the OAI healthy reference cohort. As cartilage damage is considered a risk factor for future structural deterioration of the joint, the current observation of a greater cartilage change (and thinning) score in those with femorotibial (and femoropatellar) cartilage damage is plausible, with the current study suggesting that this relationship is not confounded by other general risk factors of knee OA. In contrast to these findings, a recent analysis of the relationship of cartilage damage with the longitudinal change of the cartilage transverse relaxation time (T2), a measure of cartilage composition and mechanical properties, 17 over 1 year and over 4 years, did not reveal a statistically significant relationship in the very same cohort. 18 In context of other recent observations in an early model of knee OA 19 these findings indicate that location-independent measurement methodology of cartilage thickness change may be more sensitive to change than cartilage T2, even at the earlies stages of knee OA, potentially due to the relatively large test-retest errors in the latter. 20 Although the longitudinal increase in deep cartilage T2 over 4 years was almost 50% greater in those with than in those without femorotibial cartilage damage, the difference did not reach statistical significance due to the large intersubject variability of these changes. 18
It is tempting to speculate about differences in the pattern of subregional cartilage changes between those with and those without cartilage damage: Yet these differences were subtle, with the posterior lateral tibia and internal lateral tibia being among the 3 subregions with most cartilage thinning observed both in those with and those without femorotibial cartilage damage, and the external medial femur being among the 3 subregion with the most cartilage thickening both in those with and those without femorotibial cartilage damage. Future work in larger samples and over longer observation periods should be directed at developing theories about the magnitude and directionality of changes observed at subregion level, and should relate those observations to OA risk factors as well as specific biomechanical conditions.
Neither the PROs (WOMAC and PASE) nor the performance tests (chair stand and 20m walk) of knee function displayed relevant longitudinal differences between those with and without femorotibial or femoropatellar cartilage damage. This observation most likely explains that cartilage damage is not associated with knee pain or other reasons of decreased function at such in an early stage of structural joint damage.
In conclusion, the current study shows that—in the absence of general risk factors of knee OA—MRI-based cartilage damage appears to be associated with greater longitudinal location-independent cartilage thickness change. Yet cartilage damage does not appear to be a strong predictor of structural and functional deterioration in knees without osteoarthritis.
Footnotes
Authors’ Note: This study was conducted at the Institute of Anatomy & Cell Biology, Paracelsus Medical University, Salzburg, Austria.
Author Contributions: AW, AL, FR, and FE were responsible for the conception and design of the study. AW, AL, SM, and WW were responsible for the acquisition of data. All authors were responsible for the analysis and interpretation of data. AW and AL wrote the first draft of the manuscript with guidance for FE. All authors critically revised the manuscript for important intellectual content. All authors approved the final manuscript to be submitted
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The MOAKS reading analysis was funded by the Bundesministerium für Bildung und Forschung (BMBF—01EC1408D; OVERLOAD-PREVOP). These readings and the cartilage thickness segmentations were performed using publicly available data from the Osteoarthritis Initiative (OAI): The OAI is a public-private partnership comprised of 5 contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health. Funding partners include Merck Research Laboratories, Novartis Pharmaceuticals Corporation, GlaxoSmithKline, and Pfizer, Inc. Private sector funding for the Consortium and OAI is managed by the FNIH. The authors would further like to thank the readers of the fixed flexion radiographs at Boston University for the central KL grading, the OAI investigators, clinic staff and OAI participants at each of the OAI clinical centers for their contributions in acquiring the publicly available clinical and imaging data, and the team at the OAI coordinating center. The statistical analysis and writing of this article were independent from and not contingent upon approval from the study sponsors.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AW, DF, SM, WW, and FE are employees of Chondrometrics GmbH. SM, WW, and FE are co-owners of Chondrometrics GmbH. FR was a part-time employee of Chondrometrics GmbH during the time at which the MOAKS readings were performed; he is shareholder, CMO, and Director of Research of Boston Imaging Core Lab (BICL), LLC. GND has no conflicts to declare. FE has provided consulting services to Merck KGaA, Bioclinica, Samumed, Kolon-Tissuegene, Servier, Galapagos, Roche, Novartis, ICM, and Health Link.
Ethical Approval: The OAI is a publicly accessible image repository and database. Ethical approval was obtained at the 4 clinical centers.
Informed Consent: Written informed consent was obtained from all subjects before the study.
ORCID iDs: Frank Roemer  https://orcid.org/0000-0001-9238-7350
https://orcid.org/0000-0001-9238-7350
Felix Eckstein  https://orcid.org/0000-0002-2014-8278
https://orcid.org/0000-0002-2014-8278
References
- 1. Guermazi A, Niu J, Hayashi D, Roemer FW, Englund M, Neogi T, et al. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study). BMJ. 2012;345:e5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laberge MA, Baum T, Virayavanich W, Nardo L, Nevitt MC, Lynch J, et al. Obesity increases the prevalence and severity of focal knee abnormalities diagnosed using 3T MRI in middle-aged subjects—data from the Osteoarthritis Initiative. Skeletal Radiol. 2011;41(6):633-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthritis Cartilage. 2011;19(8):990-1002. doi: 10.1016/j.joca.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Joseph GB, Hou SW, Nardo L, Heilmeier U, Nevitt MC, McCulloch CE, et al. MRI findings associated with development of incident knee pain over 48 months: data from the osteoarthritis initiative. Skeletal Radiol. 2016;45(5):653-60. doi: 10.1007/s00256-016-2343-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roemer FW, Kwoh CK, Hannon MJ, Hunter DJ, Eckstein F, Fujii T, et al. What comes first? Multi-tissue involvement leading to radiographic osteoarthritis: MRI-based trajectory analysis over 4 years in the Osteoarthritis Initiative. Arthritis Rheumatol. 2015;67(8):2085-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharma L, Hochberg M, Nevitt M, Guermazi A, Roemer FW, Crema MD, et al. Knee tissue lesions and prediction of incident knee osteoarthritis over 7 years in a cohort of persons at higher risk. Osteoarthritis Cartilage. 2017;25(7):1068-75. doi: 10.1016/j.joca.2017.02.788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guermazi A, Eckstein F, Hayashi D, Roemer FW, Wirth W, Yang T, et al. Baseline radiographic osteoarthritis and semi-quantitatively assessed meniscal damage and extrusion and cartilage damage on MRI is related to quantitatively defined cartilage thickness loss in knee osteoarthritis: the Multicenter Osteoarthritis Study. Osteoarthritis Cartilage. 2015;23(12):2191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roemer FW, Kwoh CK, Hannon MJ, Hunter DJ, Eckstein F, Wang Z, et al. Can structural joint damage measured with MR imaging be used to predict knee replacement in the following year? Radiology. 2015;274(3):810-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eckstein F, Wirth W, Nevitt MC. Recent advances in osteoarthritis imaging—the Osteoarthritis Initiative. Nat Rev Rheumatol. 2012;8(10):622-30. doi: 10.1038/nrrheum.2012.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eckstein F, Kwoh CK, Link TM. Imaging research results from the Osteoarthritis Initiative (OAI): a review and lessons learned 10 years after start of enrolment. Ann Rheum Dis. 2014;73(7):1289-300. doi: 10.1136/annrheumdis-2014-205310 [DOI] [PubMed] [Google Scholar]
- 11. Eckstein F, Yang M, Guermazi A, Roemer FW, Hudelmaier M, Picha K, et al. Reference values and Z-scores for subregional femorotibial cartilage thickness—results from a large population-based sample (Framingham) and comparison with the non-exposed Osteoarthritis Initiative reference cohort. Osteoarthritis Cartilage. 2010;18(10):1275-83. doi: 10.1016/j.joca.2010.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eckstein F, Buck R, Wirth W. Location-independent analysis of structural progression of osteoarthritis—taking it all apart, and putting the puzzle back together makes the difference. Semin Arthritis Rheum. 2017;46(4):404-10. doi: 10.1016/j.semarthrit.2016.08.016 [DOI] [PubMed] [Google Scholar]
- 13. Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16(12):1433-41. doi: 10.1016/j.joca.2008.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wirth W, Eckstein F. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging. 2008;27(6):737-44. [DOI] [PubMed] [Google Scholar]
- 15. Eckstein F, Wirth W, Lohmander LS, Hudelmaier MI, Frobell RB. Five-year followup of knee joint cartilage thickness changes after acute rupture of the anterior cruciate ligament. Arthritis Rheumatol. 2015;67(1_suppl):152-61. [DOI] [PubMed] [Google Scholar]
- 16. Eckstein F, Maschek S, Roemer FW, Duda GN, Sharma L, Wirth W. Cartilage loss in radiographically normal knees depends on radiographic status of the contralateral knee—data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2019;27(2):273-7. doi: 10.1016/j.joca.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8(4):355-68. [DOI] [PubMed] [Google Scholar]
- 18. Roemer FW, Eckstein F, Duda GN, Guermazi A, Maschek S, Wirth W. Baseline structural tissue pathology is not strongly associated with longitudinal change in transverse relaxation time (T2) in knees without osteoarthritis. Eur J Radiol. 2019;118:161-8. [DOI] [PubMed] [Google Scholar]
- 19. Wirth W, Maschek S, Roemer FW, Sharma L, Duda GN, Eckstein F. Radiographically normal knees with contralateral joint space narrowing display greater change in cartilage transverse relaxation time than those with normal contralateral knees: a model of early OA? Data from the Osteoarthritis Initiative (OAI). Osteoarthritis Cartilage. 2019;27(11):1663-8. doi: 10.1016/j.joca.2019.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mosher TJ, Zhang Z, Reddy R, Boudhar S, Milestone BN, Morrison WB, et al. Knee articular cartilage damage in osteoarthritis: analysis of MR image biomarker reproducibility in ACRIN-PA 4001 multicenter trial. Radiology. 2011;258(3):832-42. doi: 10.1148/radiol.10101174 [DOI] [PMC free article] [PubMed] [Google Scholar]