Abstract
Objective
The objective of this systematic review and meta-analysis was to report the safety of intra-articular hyaluronic acid (IAHA) in patients with symptomatic knee osteoarthritis (OA).
Methods
We identified randomized controlled trials reporting the safety of IAHA versus IA saline in adults with symptomatic knee OA. Main safety outcomes were adverse events (AEs), local AEs, serious adverse events (SAEs), study withdrawals, and AE-related study withdrawals.
Results
A total of 35 randomized controlled trials with 38 group comparisons comprising 8,078 unique patients (IAHA: 4,295, IA saline: 3,783) were included in the meta-analysis. Comparing IAHA with IA saline over a median of 6 months follow-up, there were no differences in the risk of AEs (42.4% vs. 39.7%, risk ratio [RR] = 1.01, 95% CI = 0.96-1.07, P = 0.61), SAEs (1.8% vs. 1.2%, RR = 1.44, 95% CI = 0.91-2.26, P=0.12), study withdrawals (12.3% vs. 12.7%, RR = 0.99, 95% CI = 0.87-1.12, P = 0.83), or AE-related study withdrawals (2.7% vs. 2.1%, RR = 1.37, 95% CI = 0.97-1.93, P = 0.08). Local AEs, all of which were nonserious, were more common with IAHA vs. IA saline (14.5% vs. 11.7%, RR = 1.21, 95% CI = 1.07-1.36, P = 0.003) and typically resolved within days.
Conclusion
IAHA was shown to be safe for use in patients with symptomatic knee OA. Compared with IA saline, IAHA is associated with an increased risk of nonserious, transient local reactions. There was no evidence to suggest any additional safety risks of IAHA.
Keywords: hyaluronic acid, knee, meta-analysis, osteoarthritis, systematic review
Introduction
Knee osteoarthritis (OA) is the leading cause of disability in adults1-3 and its prevalence is anticipated to rise exponentially over the next several decades. The pathogenesis of knee OA is multifactorial, but largely attributable to chronic overloading of the knee joint that promotes degradation of the articular cartilage.4,5 Hyaluronic acid (HA) is an integral component of synovial fluid. As part of its intra-articular function, it acts as a joint lubricant during shear stress and a shock absorber during compressive stress. The concentration and molecular weight of endogenous HA is often reduced in knee OA, leading to reduced viscoelastic properties of synovial fluid. Altered biodynamics with loss of other protective HA properties induce proinflammatory pathways. 6 Intra-articular hyaluronic acid (IAHA) injections are intended to alleviate knee OA symptoms by reversing the OA-induced HA deficit. 7 Numerous systematic reviews have evaluated the effectiveness of IAHA for knee OA, with varying conclusions.8-16 However, relatively less attention has been paid to the safety of IAHA. In 2012, Rutjes and colleagues 15 were among the first to report potential safety concerns with IAHA for knee OA. Since then, few studies have attempted to confirm the safety of IAHA and to further investigate the potential sources of heterogeneity among studies. The purpose of this systematic review and meta-analysis of randomized controlled trials (RCTs) was to report on the safety of IAHA in patients with symptomatic knee OA.
Methods
Data Sources and Searches
We developed and followed a review protocol that adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). 17 We searched Medline, Embase, the Cochrane Database of Systematic Reviews, and the Directory of Open Access Journals, with no language restrictions, from inception to December 31, 2018 for RCTs of IAHA for symptomatic knee OA by using a combination of study design-, diagnosis-, and treatment-specific keywords (Supplemental Table 1). Additionally, reference lists of included papers and relevant meta-analyses were manually searched. We also included results from published and unpublished sources (e.g. abstracts, Food and Drug Administration website) to reduce the risk of publication bias. 18 Manuscripts published in non-English language journals were translated to English by local-language medical translators.
Study Selection
Two independent researchers (LM, DF) reviewed titles and abstracts for possible inclusion. Primary inclusion criteria were RCTs of IAHA (IAHA group) versus IA saline (Saline group) for symptomatic knee OA; sample size at least 30 patients per group; identical treatment and follow-up conditions in each group; and at least one extractable safety outcome. The 30-patient minimum sample size criterion was included to minimize bias associated with small-study effects. 19 Duplicate publications and studies that included concomitant surgical procedures were excluded.
Data Extraction
Data were independently extracted from eligible studies by 2 researchers (LM, DF). Data extraction discrepancies between the 2 researchers were resolved by discussion and consensus. The following variables were recorded in standardized data extraction forms: general manuscript information, patient characteristics (gender, age, body mass index), study characteristics (sample size, blinding assessment, industry funding, follow-up duration), HA characteristics (trade name, molecular weight, source, cross-linking, regulatory status), procedural details (number of injections), and safety outcomes. Safety outcomes included all-cause serious adverse events (SAEs), all-cause adverse events (AEs), local AEs, patient withdrawals, and AE-related patient withdrawals. Serious AE data were extracted based on investigator determination or report of any event that led to death, serious deterioration in health, life-threatening illness/injury, permanent impairment, hospitalization or prolongation of hospitalization, or medical/surgical intervention to prevent permanent impairment. Treatment relatedness data were extracted based on investigator determination. We reviewed the article text, tables, and patient flow diagrams to determine the number of patient withdrawals in total as well as those occurring due to an AE. The Cochrane Collaboration tool was used to assess risk of bias. 20
Data Analysis
Safety outcomes in each group were compared using the risk ratio (RR) where an RR >1 indicated higher risk with IAHA and an RR <1 indicated lower risk with IAHA. When one group was compared to multiple groups within the same study, the sample size of the single group was adjusted to avoid double counting of patients. 21 We used the I2 statistic to estimate heterogeneity of outcomes among studies where a value of 0% represents no heterogeneity and larger values represent increasing heterogeneity. 22 Substantial heterogeneity was defined by a Cochran Q test P < 0.1 or I2 > 50%. When substantial heterogeneity existed, a random effects model was planned; otherwise, a fixed-effect model was planned. 23 Publication bias was visually assessed with funnel plots and quantitatively assessed with Egger’s regression test. 24 Predefined subgroup analyses were performed to explore the association of study-level factors on each safety outcome. We tested the stability of our estimates with two sensitivity analyses. The first sensitivity analysis was a one-study removed analysis in which we iteratively removed one study at a time to determine whether conclusions for each outcome were influenced by any single study. The second sensitivity analysis compared results of fixed-effect versus random effects models for each safety outcome. P-values were 2-sided with a significance level <0.05. Analyses were performed using Review Manager 5.3 (The Cochrane Collaboration, Copenhagen, Denmark).
Results
Study Selection
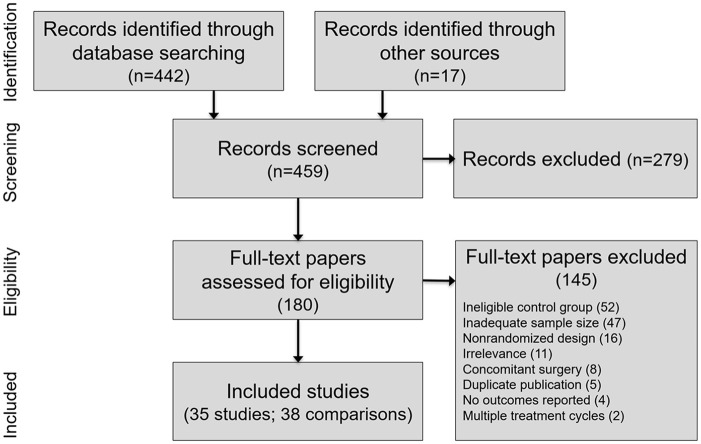
We screened 459 studies to determine eligibility for inclusion in this review. We excluded 424 studies, mostly due to a non-IA saline control group, inadequate sample size, or nonrandomized design. Ultimately, 35 RCTs with 38 group comparisons comprising 8,078 unique patients (IAHA: 4,295, Saline: 3,783) were included in this review. A flow diagram of study identification and selection is shown in Figure 1 . A listing of excluded RCTs with reasons for exclusion is provided in Supplemental Table 2.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram.
Patient and Study Characteristics
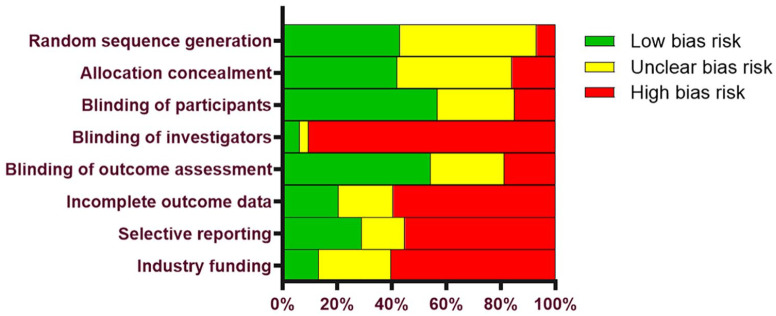
Baseline patient characteristics in the IAHA and Saline groups are reported in Table 1 . Characteristics of patients in the IAHA and Saline groups were similar, with women comprising 60% of each group, a median age of 63 years in each group, and a median body mass index of 29 kg/m2 in each group. Most studies utilized patient and outcome assessor blinding, but injector blinding was rarely specified. Most HA products were derived from avian sources. The total number of injections received in each series ranged from 1 to 5, except for one study that used 11 injections. 25 Median patient follow-up was 6 months (range: 5 weeks to 2 years) ( Table 2 ). The most common potential sources of bias were lack of investigator blinding, industry funding, and incomplete outcome data ( Figure 2 ).
Table 1.
Patient Characteristics in Randomized Controlled Trials Included in the Meta-Analysis.
| Study | No. Randomized | Age (Years) | Female Patients (%) | BMI (kg/m2) | ||||
|---|---|---|---|---|---|---|---|---|
| HA | Saline | HA | Saline | HA | Saline | HA | Saline | |
| Altman, 1998 34 | 164 | 168 | 62 | 65 | 61 | 53 | 32 | 30 |
| Altman, 2004 35 | 173 | 174 | 63 | 63 | 46 | 64 | 30 | 30 |
| Altman, 2009 36 | 293 | 295 | 63 | 61 | 63 | 63 | 32 | 33 |
| Arden, 2014 37 | 108 | 110 | 65 | 61 | 55 | 46 | 27 | 28 |
| Baltzer, 2009 38 | 135 | 107 | 57 | 60 | 55 | 64 | a | a |
| Brandt, 2001 26 | 114 | 112 | 65 | 67 | 63 | 63 | 32 | 30 |
| Chevalier, 2010 39 | 124 | 129 | 64 | 63 | 74 | 68 | 29 | 30 |
| Day, 2004 40 | 116 | 124 | 62 | 62 | 56 | 61 | 30 | 29 |
| Dickson, 2001 41 | 53 b | 57 | 65 | 64 | 57 | 56 | 29 | 29 |
| Dixon, 1988 25 | 30 | 33 | a | a | a | a | a | a |
| Dougados, 1993 42 | 55 | 55 | 67 | 69 | 76 | 65 | a | a |
| Hangody, 2017 43 | 150 | 138 | 59 | 58 | 66 | 74 | 28 | 29 |
| Henderson, 1994 44 | 45 | 46 | 69 | 64 | 67 | 72 | a | a |
| Henrotin, 2017 45 | 40 | 41 | 67 | 63 | 63 | 76 | 29 | 31 |
| Huang, 2011 46 | 100 | 100 | 66 | 64 | 74 | 78 | 26 | 25 |
| Huskisson, 1999 47 | 50 | 50 | 66 | 65 | 76 | 58 | a | a |
| Jorgensen, 2010 48 | 167 | 170 | 63 | 61 | 66 | 57 | 29 | 29 |
| Karlsson, 2002a 49 | 92 | 66 b | 72 | 71 | 67 | 61 | 28 | 28 |
| Karlsson, 2002b 49 | 88 | 66 b | 70 | 71 | 65 | 61 | 28 | 28 |
| Kosuwon, 2012 50 | 30 | 30 | 61 | 61 | 100 | 100 | 27 | 26 |
| Lohmander, 1996 27 | 120 | 120 | 59 | 58 | 56 | 56 | 28 | 27 |
| Lundsgaard, 2008 51 | 84 | 84 | 69 | 70 | 57 | 52 | 30 | 29 |
| Neustadt, 2005 52 | 128 | 124 | 58 | 59 | 45 | 50 | 29 | 29 |
| Petrella, 2002 53 | 30 | 30 | 67 | 63 | 36 | 43 | 30 | 33 |
| Petrella, 2006 54 | 53 | 53 | 62 | 64 | 43 | 46 | 30 | 31 |
| Petrella, 2008a 55 | 50 | 50 b | 69 | 71 | 54 | 60 | 27 | 27 |
| Petrella, 2008b 55 | 50 | 50 b | 71 | 71 | 58 | 60 | 27 | 27 |
| Petterson, 2018 56 | 184 | 185 | 60 | 59 | 59 | 57 | 30 | 30 |
| Pham, 2004 57 | 131 b | 85 | 65 | 65 | 71 | 69 | 29 | 29 |
| Puhl, 1993 58 | 102 | 107 | 62 | 61 | 73 | 55 | 26 | 27 |
| Rolf, 2005a 59 | 90 | 91 b | 55 | 53 | 44 | 38 | 27 | 28 |
| Rolf, 2005b 59 | 91 | 91 b | 54 | 53 | 38 | 38 | 27 | 28 |
| Saravanan, 2002 60 | 33 | 33 | a | a | a | a | a | a |
| Seikagaku, 2001 61 | 619 | 537 | 62 | 61 | 62 | 59 | 28 | 28 |
| Shichikawa, 1983 62 | 52 | 55 | a | a | a | a | a | a |
| Strand, 2012 63 | 251 | 128 | 61 | 60 | 60 | 60 | 28 | 29 |
| Wobig, 1998 64 | 52 | 54 | 60 | 64 | 56 | 74 | 27 | 28 |
| Wu, 1997 65 | 48 c | 42 c | 69 | 69 | 34 | 22 | a | a |
BMI = body mass index; HA = hyaluronic acid
Values not reported.
Sample size of common comparator group within the same study adjusted to avoid double-counting in meta-analysis.
Estimated values.
Table 2.
Treatment Regimens of Randomized Controlled Trials Included in the Meta-Analysis.
| Study | Blinding | HA Characteristics | Follow-up (Days) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subjects | Injectors | Outcome Assessors | Trade Name | US Approved | Cross-Linked | Avian Origin | MW (kDa) | No. Injections | ||
| Altman, 1998 34 | Yes | No | No | HYALGAN | Yes | No | Yes | 730 | 5 | 182 |
| Altman, 2004 35 | Yes | No | Yes | DUROLANE | Yes | Yes | No | 9000 | 1 | 182 |
| Altman, 2009 36 | Yes | No | Yes | EUFLEXXA | Yes | No | No | 3600 | 3 | 182 |
| Arden, 2014 37 | Yes | No | Yes | DUROLANE | Yes | Yes | No | 9000 | 1 | 42 |
| Baltzer, 2009 38 | Yes | No | Yes | HYA-JECT | No | No | No | 1400 | 3 | 203 |
| Brandt, 2001 26 | Yes | No | Yes | ORTHOVISC | Yes | No | No | 2900 | 3 | 189 |
| Chevalier, 2010 39 | Yes | No | Yes | SYNVISC | Yes | Yes | Yes | 6000 | 1 | 182 |
| Day, 2004 40 | Yes | No | Yes | ARTZAL | Yes | No | Yes | 1170 | 5 | 126 |
| Dickson, 2001 41 | Yes | No | Yes | SYNVISC | Yes | Yes | Yes | 6000 | 3 | 84 |
| Dixon, 1988 25 | Yes | No | Yes | HYALGAN | Yes | No | Yes | 730 | 11 | 336 |
| Dougados, 1993 42 | No | No | No | HYALGAN | Yes | No | Yes | 730 | 4 | 364 |
| Hangody, 2017 43 | Yes | Yes | Yes | MONOVISC | Yes | Yes | No | 2900 | 1 | 182 |
| Henderson, 1994 44 | Yes | No | Yes | HYALGAN | Yes | No | Yes | 730 | 5 | 35 |
| Henrotin, 2017 45 | Yes | Yes | Yes | KARTILAGE CROSS | No | No | No | a | 1 | 180 |
| Huang, 2011 46 | Yes | No | Yes | HYALGAN | Yes | No | Yes | 730 | 5 | 175 |
| Huskisson, 1999 47 | Yes | No | Yes | HYALGAN | Yes | No | Yes | 730 | 5 | 182 |
| Jorgensen, 2010 48 | Yes | No | Yes | HYALGAN | Yes | No | Yes | 730 | 5 | 91 |
| Karlsson, 2002a 49 | Yes | No | Yes | ARTZAL | Yes | No | Yes | 1170 | 3 | 182 |
| Karlsson, 2002b 49 | Yes | No | Yes | SYNVISC | Yes | Yes | Yes | 6000 | 3 | 182 |
| Kosuwon, 2012 50 | Yes | No | Yes | GO-ON | No | No | No | 1500 | 5 | 182 |
| Lohmander, 1996 27 | Yes | No | Yes | ARTZAL | Yes | No | Yes | 1170 | 5 | 185 |
| Lundsgaard, 2008 51 | Yes | No | Yes | HYALGAN | Yes | No | Yes | 730 | 4 | 182 |
| Neustadt, 2005 52 | Yes | No | Yes | ORTHOVISC | Yes | No | No | 2900 | 4 | 196 |
| Petrella, 2002 53 | Yes | Yes | Yes | SUPLASYN | No | Yes | No | 1000 | 3 | 84 |
| Petrella, 2006 54 | Yes | No | Yes | SUPLASYN | No | Yes | No | 1000 | 3 | 84 |
| Petrella, 2008a 55 | Yes | No | No | HYALGAN | Yes | No | Yes | 730 | 3 | 728 |
| Petrella, 2008b 55 | Yes | No | No | SYNVISC | Yes | Yes | Yes | 6000 | 3 | 728 |
| Petterson, 2018 56 | Yes | Yes | Yes | MONOVISC | Yes | Yes | No | 2900 | 1 | 182 |
| Pham, 2004 57 | Yes | No | Yes | NRD101 | No | a | No | 1900 | 3 | 365 |
| Puhl, 1993 58 | Yes | No | Yes | ARTZAL | Yes | No | Yes | 1170 | 5 | 98 |
| Rolf, 2005a 59 | Yes | No | Yes | SYNVISC | Yes | Yes | Yes | 6000 | 3 | 364 |
| Rolf, 2005b 59 | Yes | No | Yes | ARTZAL | Yes | No | Yes | 1170 | 3 | 364 |
| Saravanan, 2002 60 | No | No | No | SYNVISC | Yes | Yes | Yes | 6000 | 3 | 42 |
| Seikagaku, 2001 61 | Yes | No | No | ARTZAL | Yes | No | Yes | 1170 | 3 | 126 |
| Shichikawa, 1983 62 | Yes | No | No | NOT REPORTED | No | a | a | a | 5 | 35 |
| Strand, 2012 63 | Yes | No | Yes | GEL-ONE | Yes | Yes | Yes | a | 1 | 91 |
| Wobig, 1998 64 | Yes | Yes | Yes | SYNVISC | Yes | Yes | Yes | 6000 | 3 | 182 |
| Wu, 1997 65 | Yes | No | No | ARTZAL | Yes | No | Yes | 1170 | 5 | 182 |
HA = hyaluronic acid; MW = molecular weight; US = United States.
Values not reported.
Figure 2.
Risk of bias assessment with the Cochrane Collaboration Tool.
Main Outcomes
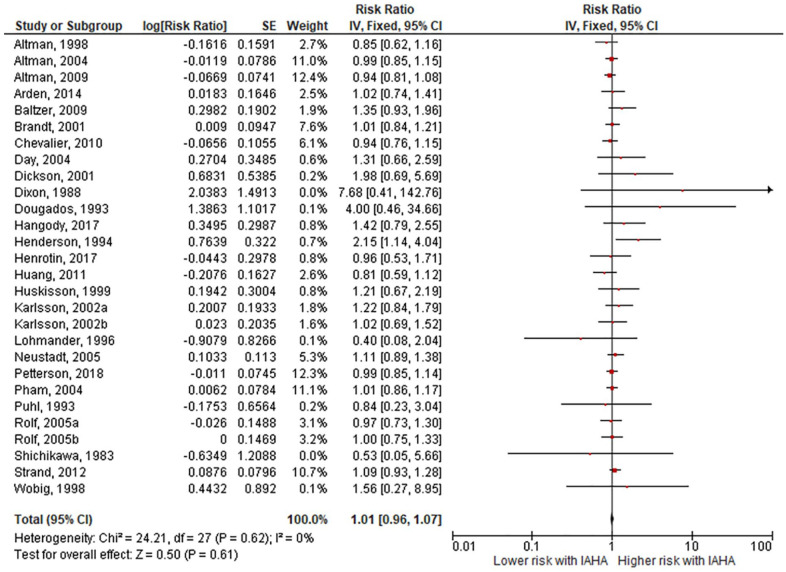
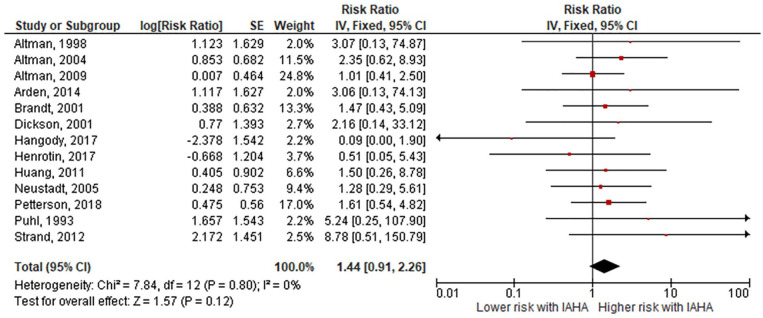
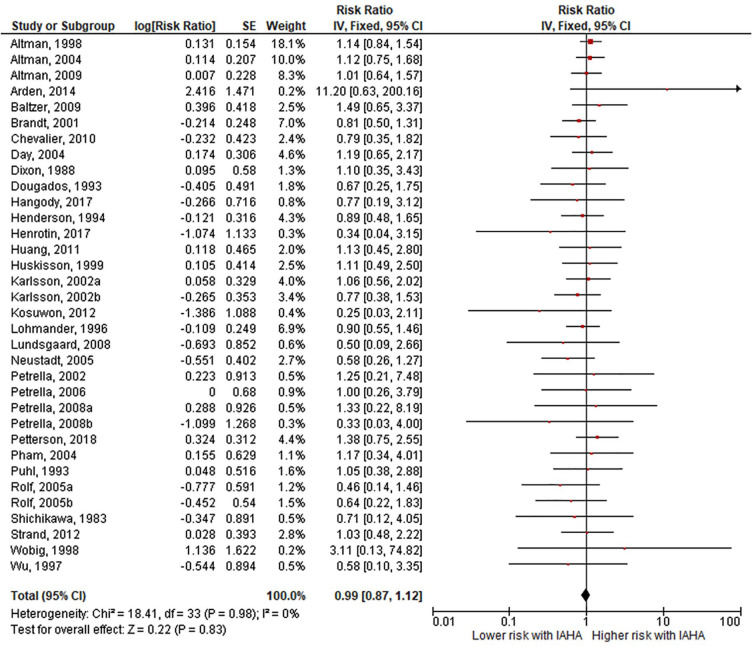
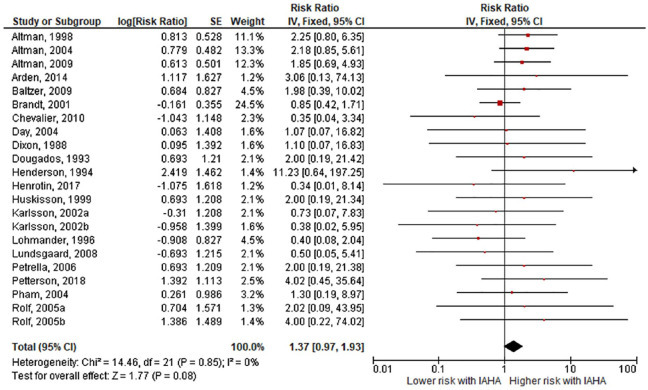
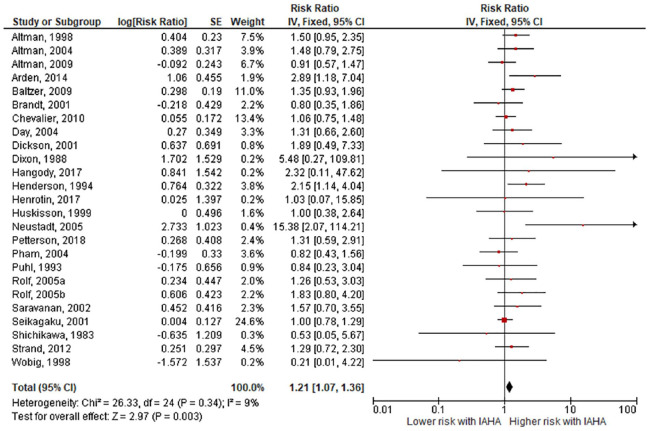
Comparing IAHA with Saline groups, there were no differences in the risk of AEs (42.4% vs. 39.7%, RR = 1.01, 95% CI = 0.96-1.07, P = 0.61; Fig. 3 ), SAEs (1.8% vs. 1.2%, RR = 1.44, 95% CI = 0.91-2.26, P = 0.12; Fig. 4 ), study withdrawals (12.3% vs. 12.7%, RR = 0.99, 95% CI = 0.87-1.12, P = 0.83; Fig. 5 ), and AE-related study withdrawals (2.7% vs. 2.1%, RR = 1.37, 95% CI = 0.97-1.93, P = 0.08; Fig. 6 ). Local AEs, none of which were classified as serious, were more common with IAHA versus Saline (14.5% vs. 11.7%, RR = 1.21, 95% CI = 1.07-1.36, P = 0.003; Fig. 7 ). Significant heterogeneity or publication bias was not observed for any outcome, with the exception of publication bias for the risk of AEs (Egger’s P = 0.04). Publication bias adjustment using the trim-and-fill method resulted in an almost identical nonsignificant difference in the risk of AEs (RR = 1.01, 95% CI = 0.96-1.06) compared with the unadjusted values (RR = 1.01, 95% CI = 0.96-1.07).
Figure 3.
Forest plot of pooled risk ratio for adverse events with hyaluronic acid (HA) or saline injections. The risk ratio and 95% confidence interval are plotted for each study. The size of the square is proportional to the sample size of the study. The pooled risk ratio is denoted by the diamond apex and 95% confidence interval denoted by the diamond width. A pooled risk ratio of more than 1 indicates higher risk with HA. A pooled risk ratio of less than 1 indicates lower risk with HA. Heterogeneity: I2 = 0%, P = 0.62. Publication bias: Egger’s P = 0.04.
Figure 4.
Forest plot of pooled risk ratio for serious adverse events with hyaluronic acid (HA) or saline injections. The risk ratio and 95% confidence interval are plotted for each study. The size of the square is proportional to the sample size of the study. The pooled risk ratio is denoted by the diamond apex and 95% confidence interval denoted by the diamond width. A pooled risk ratio of more than 1 indicates higher risk with HA. A pooled risk ratio of less than 1 indicates lower risk with HA. Heterogeneity: I2 = 0%, P = 0.80. Publication bias: Egger’s P = 0.55.
Figure 5.
Forest plot of pooled risk ratio for study withdrawal with hyaluronic acid (HA) or saline injections. The risk ratio and 95% confidence interval are plotted for each study. The size of the square is proportional to the sample size of the study. The pooled risk ratio is denoted by the diamond apex and 95% confidence interval denoted by the diamond width. A pooled risk ratio of more than 1 indicates higher risk with HA. A pooled risk ratio of less than 1 indicates lower risk with HA. Heterogeneity: I2 = 0%, P = 0.98. Publication bias: Egger’s P = 0.13.
Figure 6.
Forest plot of pooled risk ratio for adverse event-related study withdrawal with hyaluronic acid (HA) or saline injections. The risk ratio and 95% confidence interval are plotted for each study. The size of the square is proportional to the sample size of the study. The pooled risk ratio is denoted by the diamond apex and 95% confidence interval denoted by the diamond width. A pooled risk ratio of more than 1 indicates higher risk with HA. A pooled risk ratio of less than 1 indicates lower risk with HA. Heterogeneity: I2 = 0%, P = 0.85. Publication bias: Egger’s P = 0.87.
Figure 7.
Forest plot of pooled risk ratio for local adverse events with hyaluronic acid (HA) or saline injections. The risk ratio and 95% confidence interval are plotted for each study. The size of the square is proportional to the sample size of the study. The pooled risk ratio is denoted by the diamond apex and 95% confidence interval denoted by the diamond width. A pooled risk ratio of more than 1 indicates higher risk with HA. A pooled risk ratio of less than 1 indicates lower risk with HA. Heterogeneity: I2 = 9%, P = 0.34. Publication bias: Egger’s P = 0.12.
Subgroup and Sensitivity Analyses
In subgroup analyses, no study-level factor influenced the risk of any safety outcome. The associations of these factors on the risk of local AEs are provided in Table 3 . Subgroup analysis results of other safety outcomes were not reported since no heterogeneity was observed for the other safety outcomes (all I2 = 0%). In the first sensitivity analysis, study conclusions were upheld in most scenarios when the meta-analysis was recalculated after removing one study at a time. The only safety outcome in which conclusions were influenced by individual studies was AE-related withdrawals in which one-at-a-time removal of 20 (91%) studies did not alter conclusions, but removal of Brandt et al. 26 or Lohmander et al. 27 led to the conclusion of increased risk of AE-related withdrawal with IAHA injections (Supplemental Table 3). In the second sensitivity analysis, results of each safety outcome were largely identical when comparing fixed-effect and random effects meta-analysis models owing to low heterogeneity among studies (Supplemental Table 4).
Table 3.
Subgroup Analysis of Study-Level Factors on the Risk of Local Adverse Events.
| Study-Level Factor | No. studies | RR | 95% CI | P-value |
|---|---|---|---|---|
| No. injections | 0.11 | |||
| 1-2 | 7 | 1.26 | 1.00-1.61 | |
| 3 | 10 | 1.09 | 0.92-1.28 | |
| >3 | 8 | 1.55 | 1.16-2.07 | |
| Follow-up duration | 0.12 | |||
| 1-3 mo | 7 | 1.65 | 1.20-2.28 | |
| 4-6 mo | 14 | 1.14 | 0.99-1.31 | |
| >6 mo | 4 | 1.19 | 0.77-1.84 | |
| Sample size | 0.33 | |||
| ≥100 per group | 13 | 1.17 | 1.02-1.34 | |
| <100 per group | 12 | 1.38 | 1.03-1.85 | |
| Publication year | 0.37 | |||
| Before 2000 | 7 | 1.48 | 1.07-2.04 | |
| 2000-2009 | 12 | 1.14 | 0.98-1.34 | |
| After 2009 | 6 | 1.23 | 0.95-1.60 | |
| Outcome assessor blinding | 0.41 | |||
| No | 4 | 1.12 | 0.91-1.39 | |
| Yes | 21 | 1.25 | 1.08-1.46 | |
| Molecular weight (kDa) | 0.43 | |||
| ≥6,000 | 7 | 1.28 | 1.00-1.64 | |
| >1,500 to <6,000 | 6 | 1.00 | 0.73-1.37 | |
| ≤1,500 | 9 | 1.24 | 1.05-1.46 | |
| Patient blinding | 0.52 | |||
| No | 1 | 1.57 | 0.70-3.55 | |
| Yes | 24 | 1.20 | 1.06-1.36 | |
| Cross-linked | 0.60 | |||
| Yes | 10 | 1.29 | 1.03-1.60 | |
| No | 13 | 1.20 | 1.03-1.40 | |
| Industry-funded study | 0.66 | |||
| No | 5 | 1.30 | 0.91-1.87 | |
| Yes | 20 | 1.19 | 1.05-1.36 | |
| HA origin | 0.77 | |||
| Bacterial | 10 | 1.23 | 0.99-1.53 | |
| Avian | 14 | 1.20 | 1.03-1.39 | |
| US approved HA | 0.84 | |||
| Yes | 21 | 1.21 | 1.06-1.39 | |
| No | 4 | 1.17 | 0.85-1.61 | |
| Injector blinding | 0.98 | |||
| No | 21 | 1.21 | 1.06-1.37 | |
| Yes | 4 | 1.20 | 0.58-2.46 |
HA = hyaluronic acid; RR = risk ratio; US = United States.
Discussion
IAHA injections are generally perceived as safe for use in patients with symptomatic knee OA. This conclusion was corroborated in the current meta-analysis where we confirmed that IAHA was associated with an increase in local nonserious AEs compared with saline injections (14.5% vs. 11.7%), but with no differences between treatment groups in the risk of AEs, SAEs, study withdrawals, and AE-related study withdrawals. These conclusions were robust in subgroup analyses and were not influenced by heterogeneity or publication bias.
Results of this meta-analysis confirmed that the risk of SAEs was comparable in patients receiving IAHA or saline. This finding agrees with a previous meta-analysis in which the risk of SAEs was no different with IAHA versus saline controls (RR = 1.24, P = 0.32). 16 However, this is in contrast to the results of Rutjes et al. 15 who concluded that IAHA increased the risk of SAEs (RR = 1.41, P = 0.04). In the current study, the risk of experiencing an SAE following IAHA was low, occurring in 1.8% of patients. Furthermore, as was shown in the meta-analysis of Rutjes et al., 15 none of the SAEs were related to IAHA but instead to unrelated conditions. Overall, it appears that SAEs following IAHA are rare, occur with a similar frequency as with IA saline, and are unrelated to the treatment itself.
We determined that IAHA increased the risk of nonserious, transient local reactions compared with IA saline. The most common local reactions were injection site pain, arthralgia, joint swelling, and joint effusion, which subsided within 2 to 3 days in most instances. Despite this increased risk in local reactions, IAHA was well tolerated with the percentage of patient withdrawals overall (12.3% vs. 12.7%) and withdrawals due to an AE (2.7% vs. 2.1%) comparably low in both IAHA and IA Saline groups. It was interesting that no factors related to study design or HA product characteristics influenced safety results, but this was not particularly surprising since there was little heterogeneity among studies.
Given this evidence supporting the safety of IAHA in relation to IA saline, it would be interesting for future studies to compare the safety of IAHA to active therapies recommended for treatment of symptomatic knee OA. In a clinical practice guideline for treatment of knee OA, nonsteroidal anti-inflammatory drugs (NSAIDS) were the only nonsurgical knee OA treatment recommended by the American Academy of Orthopaedic Surgeons (AAOS). 28 Yet, knee OA pain is chronic in nature and NSAIDS are not indicated for chronic use because of safety concerns. Furthermore, selective and nonselective oral NSAIDs increase the risk of serious cardiovascular events and should be used with caution in patients with high cardiovascular risk. 29 Finally, drug fact labels specify that “NSAIDs can increase the chance of a heart attack or stroke, either of which can lead to death.” 30 Given that cardiovascular disease is highly prevalent in patients with OA, 31 there appears to be a disconnect in societal guidelines regarding the safety profile of recommended therapies for knee OA. Rigorous safety evaluations of IAHA versus oral NSAIDs would help clarify the comparative risk profile of these therapies in patients with knee OA.
Strengths of this meta-analysis were inclusion of only RCTs utilizing saline controls and robust study conclusions that were not influenced by heterogeneity among studies, publication bias, or analysis specifications. There were also several limitations of this meta-analysis that warrant additional discussion. First, patients treated with oral therapies or injection of active products were excluded from the control group. While safety comparisons to these other therapies are certainly warranted, inclusion of all potential therapies in a common control group would introduce significant biases that would complicate data interpretation. Second, in accordance with best evidence practices and following the methodology used by the AAOS in their systematic review of IAHA, 32 we excluded studies with small sample sizes. While it is possible that important safety events may be missed using this methodology, exclusion of small studies reduces bias risk since they tend to report greater treatment benefits than large trials and often suffer from lower methodological quality. 19 Finally, the effect of repeat IAHA cycles on safety outcomes could not be evaluated in this review, but has previously been reported to be safe with no reported SAEs in a previous review. 33
Conclusions
IAHA was shown to be safe for use in patients with symptomatic knee OA. Compared with IA saline, IAHA was associated with an increased risk of nonserious and transient local reactions. There was no evidence to suggest any additional safety risks of IAHA.
Supplemental Material
Supplemental material, Supplement for Safety of Intra-Articular Hyaluronic Acid for Knee Osteoarthritis: Systematic Review and Meta-Analysis of Randomized Trials Involving More than 8,000 Patients by Larry E. Miller, Samir Bhattacharyya, William R. Parrish, Michael Fredericson, Brad Bisson and Roy D. Altman in Cartilage
Footnotes
Supplementary material for this article is available on the Cartilage website at https://journals.sagepub.com/home/CAR.
Acknowledgments and Funding: We thank David Fay, PhD, for assistance with literature review and data extraction. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: DePuy Synthes provided funding for this study.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: L. Miller has an affiliation with DePuy Synthes that is related to this study and with OsteoArthritis Centers of America that is not related to this study. S. Bhattacharyya is an employee of DePuy Synthes. W. Parrish is an employee of DePuy Synthes. M. Fredericson has an affiliation with Ipsen that is not related to this study. B. Bisson is an employee of DePuy Synthes. R. Altman has affiliations with GlaxoSmithKline, Novartis, Pfizer, Sanofi, Sorrento, and Theralogix that are not related to this study.
ORCID iD: Larry E. Miller  https://orcid.org/0000-0003-1594-1885
https://orcid.org/0000-0003-1594-1885
References
- 1. Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987;30:914-8. [DOI] [PubMed] [Google Scholar]
- 2. van Saase JL, van Romunde LK, Cats A, Vandenbroucke JP, Valkenburg HA. Epidemiology of osteoarthritis: Zoetermeer survey. Comparison of radiological osteoarthritis in a Dutch population with that in 10 other populations. Ann Rheum Dis. 1989;48:271-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60:91-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arendt EA, Miller LE, Block JE. Early knee osteoarthritis management should first address mechanical joint overload. Orthop Rev (Pavia). 2014;6:5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crawford DC, Miller LE, Block JE. Conservative management of symptomatic knee osteoarthritis: a flawed strategy? Orthop Rev (Pavia). 2013;5:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dahl LB, Dahl IM, Engström-Laurent A, Granath K. Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Ann Rheum Dis. 1985;44:817-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith MM, Ghosh P. The synthesis of hyaluronic acid by human synovial fibroblasts is influenced by the nature of the hyaluronate in the extracellular environment. Rheumatol Int. 1987;7:113-22. [DOI] [PubMed] [Google Scholar]
- 8. Sadabad HN, Behzadifar M, Arasteh F, Behzadifar M, Dehghan HR. Efficacy of platelet-rich plasma versus hyaluronic acid for treatment of knee osteoarthritis: a systematic review and meta-analysis. Electron Physician. 2016;8:2115-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang F, He X. Intra-articular hyaluronic acid and corticosteroids in the treatment of knee osteoarthritis: a meta-analysis. Exp Ther Med. 2015;9:493-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bannuru RR, Vaysbrot EE, Sullivan MC, McAlindon TE. Relative efficacy of hyaluronic acid in comparison with NSAIDs for knee osteoarthritis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2014;43:593-9. [DOI] [PubMed] [Google Scholar]
- 11. Miller LE, Block JE. US-approved intra-articular hyaluronic acid injections are safe and effective in patients with knee osteoarthritis: systematic review and meta-analysis of randomized, saline-controlled trials. Clin Med Insights Arthritis Musculoskelet Disord. 2013;6:57-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colen S, van den Bekerom MP, Mulier M, Haverkamp D. Hyaluronic acid in the treatment of knee osteoarthritis: a systematic review and meta-analysis with emphasis on the efficacy of different products. BioDrugs. 2012;26:257-68. [DOI] [PubMed] [Google Scholar]
- 13. Bannuru RR, Natov NS, Dasi UR, Schmid CH, McAlindon TE. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis—meta-analysis. Osteoarthritis Cartilage. 2011;19:611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bannuru RR, Natov NS, Obadan IE, Price LL, Schmid CH, McAlindon TE. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum. 2009;61:1704-11. [DOI] [PubMed] [Google Scholar]
- 15. Rutjes AW, Jüni P, da Costa BR, Trelle S, Nüesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med. 2012;157:180-91. [DOI] [PubMed] [Google Scholar]
- 16. Strand V, McIntyre LF, Beach WR, Miller LE, Block JE. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65-W94. [DOI] [PubMed] [Google Scholar]
- 18. Cook DJ, Guyatt GH, Ryan G, Clifton J, Buckingham L, Willan A, et al. Should unpublished data be included in meta-analyses? Current convictions and controversies. JAMA. 1993;269:2749-53. [PubMed] [Google Scholar]
- 19. Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta-analyses of osteoarthritis trials: meta-epidemiological study. BMJ. 2010;341:c3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higgins JP, Altman DG, Gotzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. London, England: The Cochrane Collaboration; 2011. [Google Scholar]
- 22. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820-6. [DOI] [PubMed] [Google Scholar]
- 24. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dixon AS, Jacoby RK, Berry H, Hamilton EB. Clinical trial of intra-articular injection of sodium hyaluronate in patients with osteoarthritis of the knee. Curr Med Res Opin. 1988;11:205-13. [DOI] [PubMed] [Google Scholar]
- 26. Brandt KD, Block JA, Michalski JP, Moreland LW, Caldwell JR, Lavin PT. Efficacy and safety of intraarticular sodium hyaluronate in knee osteoarthritis. ORTHOVISC Study Group. Clin Orthop Relat Res. 2001;(385):130-43. [DOI] [PubMed] [Google Scholar]
- 27. Lohmander LS, Dalen N, Englund G, Hämäläinen M, Jensen EM, Karlsson K, et al. Intra-articular hyaluronan injections in the treatment of osteoarthritis of the knee: a randomised, double blind, placebo controlled multicentre trial. Hyaluronan Multicentre Trial Group. Ann Rheum Dis. 1996;55:424-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee: evidence-based guideline. Available from: https://www.aaos.org/research/guidelines/TreatmentofOsteoarthritisoftheKneeGuideline.pdf
- 29. Coxib and Traditional NSAID Trialists’ (CNT); Bhala N, Emberson J, Merhi A, Abramson S, Arber N, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382:769-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. US Food and Drug Administration. FDA Drug Safety Communication: FDA strengthens warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) can cause heart attacks or strokes. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-strengthens-warning-non-aspirin-nonsteroidal-anti-inflammatory
- 31. Hall AJ, Stubbs B, Mamas MA, Myint PK, Smith TO. Association between osteoarthritis and cardiovascular disease: systematic review and meta-analysis. Eur J Prev Cardiol. 2016;23:938-46. [DOI] [PubMed] [Google Scholar]
- 32. Jevsevar D, Donnelly P, Brown GA, Cummins DS. Viscosupplementation for osteoarthritis of the knee: a systematic review of the evidence. J Bone Joint Surg Am. 2015;97:2047-60. [DOI] [PubMed] [Google Scholar]
- 33. Altman R, Hackel J, Niazi F, Shaw P, Nicholls M. Efficacy and safety of repeated courses of hyaluronic acid injections for knee osteoarthritis: a systematic review. Semin Arthritis Rheum. 2018;48:168-75. [DOI] [PubMed] [Google Scholar]
- 34. Altman RD, Moskowitz R. Intraarticular sodium hyaluronate (Hyalgan) in the treatment of patients with osteoarthritis of the knee: a randomized clinical trial. Hyalgan Study Group. J Rheumatol. 1998;25:2203-12. [PubMed] [Google Scholar]
- 35. Altman RD, Akermark C, Beaulieu AD, Schnitzer T; Durolane International Study Group. Efficacy and safety of a single intra-articular injection of non-animal stabilized hyaluronic acid (NASHA) in patients with osteoarthritis of the knee. Osteoarthritis Cartilage. 2004;12:642-9. [DOI] [PubMed] [Google Scholar]
- 36. Altman RD, Rosen JE, Bloch DA, Hatoum HT, Korner P. A double-blind, randomized, saline-controlled study of the efficacy and safety of EUFLEXXA for treatment of painful osteoarthritis of the knee, with an open-label safety extension (the FLEXX trial). Semin Arthritis Rheum. 2009;39:1-9. [DOI] [PubMed] [Google Scholar]
- 37. Arden NK, Akermark C, Andersson M, Todman MG, Altman RD. A randomized saline-controlled trial of NASHA hyaluronic acid for knee osteoarthritis. Curr Med Res Opin. 2014;30:279-86. [DOI] [PubMed] [Google Scholar]
- 38. Baltzer AW, Moser C, Jansen SA, Krauspe R. Autologous conditioned serum (Orthokine) is an effective treatment for knee osteoarthritis. Osteoarthritis Cartilage. 2009;17:152-60. [DOI] [PubMed] [Google Scholar]
- 39. Chevalier X, Jerosch J, Goupille P, van Dijk N, Luyten FP, Scott DL, et al. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo controlled trial. Ann Rheum Dis. 2010;69:113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Day R, Brooks P, Conaghan PG, Petersen M; Multicenter Trial Group. A double blind, randomized, multicenter, parallel group study of the effectiveness and tolerance of intraarticular hyaluronan in osteoarthritis of the knee. J Rheumatol. 2004;31:775-82. [PubMed] [Google Scholar]
- 41. Dickson DJ, Hosie G, English JR. A double-blind, placebo-controlled comparison of hylan G-F 20 against diclofenac in knee osteoarthritis. J Clin Res. 2001;4:41-52. [Google Scholar]
- 42. Dougados M, Nguyen M, Listrat V, Amor B. High molecular weight sodium hyaluronate (hyalectin) in osteoarthritis of the knee: a 1 year placebo-controlled trial. Osteoarthritis Cartilage. 1993;1:97-103. [DOI] [PubMed] [Google Scholar]
- 43. Hangody L, Szody R, Lukasik P, Zgadzaj W, Lenart E, Dokoupilova E, et al. Intraarticular injection of a cross-linked sodium hyaluronate combined with triamcinolone hexacetonide (Cingal) to provide symptomatic relief of osteoarthritis of the knee: a randomized, double-blind, placebo-controlled multicenter clinical trial. Cartilage. 2018;9:276-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Henderson EB, Smith EC, Pegley F, Blake DR. Intra-articular injections of 750 kD hyaluronan in the treatment of osteoarthritis: a randomised single centre double-blind placebo-controlled trial of 91 patients demonstrating lack of efficacy. Ann Rheum Dis. 1994;53:529-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Henrotin Y, Berenbaum F, Chevalier X, Marty M, Richette P, Rannou F. Reduction of the serum levels of a specific biomarker of cartilage degradation (Coll2-1) by hyaluronic acid (KARTILAGE® CROSS) compared to placebo in painful knee osteoarthritis patients: the EPIKART study, a pilot prospective comparative randomized double blind trial. BMC Musculoskelet Disord. 2017;18:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang TL, Chang CC, Lee CH, Chen SC, Lai CH, Tsai CL. Intra-articular injections of sodium hyaluronate (Hyalgan®) in osteoarthritis of the knee. a randomized, controlled, double-blind, multicenter trial in the Asian population. BMC Musculoskelet Disord. 2011;12:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huskisson EC, Donnelly S. Hyaluronic acid in the treatment of osteoarthritis of the knee. Rheumatology (Oxford). 1999;38:602-7. [DOI] [PubMed] [Google Scholar]
- 48. Jørgensen A, Stengaard-Pedersen K, Simonsen O, Pfeiffer-Jensen M, Eriksen C, Bliddal H, et al. Intra-articular hyaluronan is without clinical effect in knee osteoarthritis: a multicentre, randomised, placebo-controlled, double-blind study of 337 patients followed for 1 year. Ann Rheum Dis. 2010;69:1097-102. [DOI] [PubMed] [Google Scholar]
- 49. Karlsson J, Sjögren LS, Lohmander LS. Comparison of two hyaluronan drugs and placebo in patients with knee osteoarthritis. A controlled, randomized, double-blind, parallel-design multicentre study. Rheumatology (Oxford). 2002;41:1240-8. [DOI] [PubMed] [Google Scholar]
- 50. Kosuwon W, Sirichatiwapee W, Visanuyotin T, Jeeravipoolvarn P, Laupattarakasem W. Determination of cartilage volume using MRI in patients with knee osteoarthritis: efficacy study of 25 milligrams of sodium hyaluronate (2.5 ml) versus placebo. J Clin Exp Pharmacol. 2012;2:112. [Google Scholar]
- 51. Lundsgaard C, Dufour N, Fallentin E, Winkel P, Gluud C. Intra-articular sodium hyaluronate 2 mL versus physiological saline 20 mL versus physiological saline 2 mL for painful knee osteoarthritis: a randomized clinical trial. Scand J Rheumatol. 2008;37:142-50. [DOI] [PubMed] [Google Scholar]
- 52. Neustadt D, Caldwell J, Bell M, Wade J, Gimbel J. Clinical effects of intraarticular injection of high molecular weight hyaluronan (Orthovisc) in osteoarthritis of the knee: a randomized, controlled, multicenter trial. J Rheumatol. 2005;32:1928-36. [PubMed] [Google Scholar]
- 53. Petrella RJ, DiSilvestro MD, Hildebrand C. Effects of hyaluronate sodium on pain and physical functioning in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled clinical trial. Arch Intern Med. 2002;162:292-8. [DOI] [PubMed] [Google Scholar]
- 54. Petrella RJ, Petrella M. A prospective, randomized, double-blind, placebo controlled study to evaluate the efficacy of intraarticular hyaluronic acid for osteoarthritis of the knee. J Rheumatol. 2006;33:951-6. [PubMed] [Google Scholar]
- 55. Petrella RJ, Cogliano A, Decaria J. Combining two hyaluronic acids in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Clin Rheumatol. 2008;27:975-81. [DOI] [PubMed] [Google Scholar]
- 56. Petterson SC, Plancher KD. Single intra-articular injection of lightly cross-linked hyaluronic acid reduces knee pain in symptomatic knee osteoarthritis: a multicenter, double-blind, randomized, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2019;27:1992-2002. [DOI] [PubMed] [Google Scholar]
- 57. Pham T, Le Henanff A, Ravaud P, Dieppe P, Paolozzi L, Dougados M. Evaluation of the symptomatic and structural efficacy of a new hyaluronic acid compound, NRD101, in comparison with diacerein and placebo in a 1 year randomised controlled study in symptomatic knee osteoarthritis. Ann Rheum Dis. 2004;63:1611-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Puhl W, Bernau A, Greiling H, Köpcke W, Pforringer W, Steck KJ, et al. Intra-articular sodium hyaluronate in osteoarthritis of the knee: a multicenter, double-blind study. Osteoarthritis Cartilage. 1993;1:233-41. [DOI] [PubMed] [Google Scholar]
- 59. Rolf CG, Engstrom B, Ohrvik J, Valentin A, Lilja B, Levine DW. A comparative study of the efficacy and safety of hyaluronan viscosupplements and placebo in patients with symptomatic and arthroscopy-verified cartilage pathology. J Clin Res. 2005;8:15-32. [Google Scholar]
- 60. Saravanan V, Morgan T, Stobbs D, Daymond TJ. Inflammatory effusion after viscosupplementation with Hylan G-F 20. Rheumatology (Oxford). 2002;41:121.11886956 [Google Scholar]
- 61. US Food and Drug Administration. Summary of safety and effectiveness data: SUPARTZ 2001. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf8/P080020b.pdf
- 62. Shichikawa K, Igarashi M, Sugawara SIwasaka Y. Clinical evaluation of high molecular weight sodium hyaluronate (SPH) on osteoarthritis of the knee—multi-center well controlled comparative study [in Japanese]. Jpn J Clin Pharmacol Ther. 1983;4:545-58. [Google Scholar]
- 63. Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2012;20:350-6. [DOI] [PubMed] [Google Scholar]
- 64. Wobig M, Dickhut A, Maier R, Vetter G. Viscosupplementation with hylan G-F 20: a 26-week controlled trial of efficacy and safety in the osteoarthritic knee. Clin Ther. 1998;20:410-23. [DOI] [PubMed] [Google Scholar]
- 65. Wu JJ, Shih LY, Hsu HC, Chen TH. The double-blind test of sodium hyaluronate (ARTZ) on osteoarthritis knee. Zhonghua Yi Xue Za Zhi (Taipei). 1997;59:99-106. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplement for Safety of Intra-Articular Hyaluronic Acid for Knee Osteoarthritis: Systematic Review and Meta-Analysis of Randomized Trials Involving More than 8,000 Patients by Larry E. Miller, Samir Bhattacharyya, William R. Parrish, Michael Fredericson, Brad Bisson and Roy D. Altman in Cartilage