Abstract
Objective
To report radiographic and magnetic resonance imaging findings, patient-reported outcomes, and complications and/or reoperations following nonarthroplasty surgical intervention for focal glenohumeral cartilage defects.
Design
A literature search was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines. Patients were included if they possessed a chondral defect of the humeral head, glenoid, or both, which had been treated with a joint preserving nonarthroplasty procedure. Risk of bias assessment was performed using the Methodological Index for Non-Randomized Studies scoring system. Study demographics, surgical technique, imaging findings, patient-reported outcomes, complications, failures, and reoperations were collected.
Results
Fourteen studies with 98 patients (100 shoulders) met the inclusion criteria. Patient ages ranged from 7 to 74 years. The nonarthroplasty surgical techniques utilized included microfracture (67 shoulders), osteochondral transplantation (28 shoulders), chondrocyte transplantation (4 shoulders), and internal fixation (1 shoulder). The rates of radiographic union and progression of osteoarthritis ranged between 90% to 100% and 57% to 100%, respectively. Visual analog scores ranged from 0 to 1.9 at final follow-up. Mean postoperative ASES (American Shoulder and Elbow Surgeons) shoulder scores ranged from 75.8-100. Mean postoperative CSS (Constant Shoulder Score) scores ranged from 83.3-94. Mean postoperative SSV (Subjective Shoulder Value) ranged from 70% to 99%. Failure and reoperation rates ranged between 0% to 35% and 0% to 30%, respectively, with the most common reoperation being conversion to prosthetic arthroplasty.
Conclusions
In this systematic review, nonarthroplasty surgical techniques demonstrated acceptable rates of radiographic healing, improved patient reported outcomes, minimal complications, and low rates of failure or reoperation. Joint preserving techniques are likely viable options to prolong function of the native shoulder and provide short- to midterm pain relief in young and highly active patients.
Level of Evidence
Level IV.
Keywords: glenohumeral, cartilage, osteochondral, joint preservation
Introduction
Focal cartilage defects involving the glenohumeral joint present both a diagnostic and management challenge. The etiology of cartilage injury within the glenohumeral joint can include previous surgery, trauma, osteochondritis dissecans, infection, avascular necrosis, inflammatory arthritis, glenohumeral joint instability, rotator cuff arthropathy, osteoarthritis, and chondrolysis. 1 Patients with glenohumeral cartilage or osteochondral defects may present with a variety of symptoms, including generalized achiness exacerbated with activity, progressive pain to the point of limiting sport activities, constant deep shoulder pain, sharp pain following an acute injury, or progressive crepitation associated with increasing pain.1-6
Joint-preserving interventions are preferred in younger patients with symptomatic lesions recalcitrant to conservative management, especially in patients considered too young for prosthetic joint replacement. The incidence of symptomatic glenohumeral cartilage defects has been reported to be as high as 13% to 17% in patients with rotator cuff tears 7 and overhead throwing athletes. 8 Other authors have reported a group of patients undergoing shoulder arthroscopy for subacromial impingement to have a 29% prevalence of humeral cartilage lesions and a 15% prevalence of glenoid cartilage lesions. 9
Current surgical treatment options for cartilage lesions involving the glenohumeral joint include microfracture, osteochondral autograft transfer (OAT), 10 osteochondral allograft transplantation (OCA), autologous chondrocyte implantation (ACI), 11 bulk allograft reconstruction, and shoulder arthroplasty. 12 Although symptomatic glenohumeral arthritis can be successfully treated with shoulder arthroplasty, younger and highly active patients should be considered for joint preservation surgery due to an increasing demand on shoulder replacement and potential hardware loosening. 13 A study by Sperling et al. 14 reported only 61% survivorship at 10 years in a cohort of patients with a mean age of 46 years at time of total shoulder arthroplasty.
Therefore, the purposes of this systematic review were to report radiographic healing, patient reported outcomes, and complications or reoperations following nonarthroplasty surgical intervention for glenohumeral cartilage defects. We hypothesized that there would be high rates of radiographic union and improved patient reported outcomes among the included studies.
Methods
Literature Search
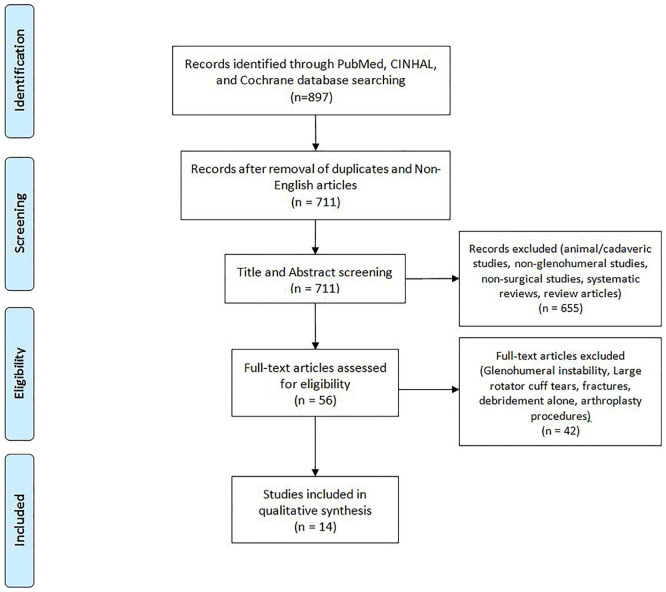
A comprehensive search of the available literature was performed on November 6, 2018 according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRIMSA) guidelines ( Fig. 1 ). 15 Searched databases included PubMed (MEDLINE), the Cumulative Index for Nursing and Allied Health Literature (CINAHL), and the Cochrane Register of Controlled Trials & Cochrane Library. The search parameters were set from January 1, 1995 to November 6, 2018 and a Boolean algebra search was employed as follows: (cartilage lesion OR cartilage lesions OR cartilage defect OR cartilage defects OR osteochondral lesions OR osteochondral lesions OR osteochondral defect OR osteochondral defects) AND (glenoid OR humerus OR glenohumeral OR glenohumeral joint OR shoulder). An initial search resulted in 820 articles from PubMed, 67 articles from CINAHL, and 10 articles from Cochrane Library. All articles were organized using Microsoft Excel (2010; Microsoft Corp, Redmond, WA). Removal of duplicates and non-English articles (186 studies) resulted in 711 articles for title and abstract screening ( Fig. 1 ).
Figure 1.
Flow diagram of the literature performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines. Fourteen studies were identified for inclusion. CINAHL = Cumulative Index for Nursing and Allied Health Literature.
Selection Criteria
Titles and abstracts of the 711 articles were reviewed by two independent authors (DPL and CBD), with only studies eliminated in consensus removed from the list. Any disagreements were resolved by discussion and consensus between reviewers and the senior author (CLC). Records that were excluded during title and abstract screening included animal or cadaveric studies, nonglenohumeral studies, nonsurgical studies, systematic reviews, and review articles ( Fig. 1 ). Following this process, 56 full texts remained and were manually reviewed for inclusion. Inclusion criteria were as follows: chondral defects of the humeral head, glenoid, or both, which had been treated with microfracture, fixation, OCA, OAT, or ACI. Exclusion criteria included debridement alone, shoulder arthroplasty, a clearly stated surgical indication for lesions due to glenohumeral instability, large rotator cuff tears, fractures, infection, or systemic inflammatory disease with involvement of the glenohumeral joint. If it could not be delineated which patients had procedures due to instability, the entire article was excluded. After the review, 14 studies were ultimately included in this systematic review ( Fig. 1 ).
Quality Assessment
There were not any randomized controlled trials or comparative studies found in the search. As a result, each study was assessed using the Methodological Index for Non-Randomized Studies (MINORS) scoring system. 16 MINORS is a validated tool designed for assessing the quality of non-randomized surgical studies. The maximum score is 16 points (8-item checklist scored from 0-2) for noncomparative studies and 24 points (12 item checklist scored from 0 to 2) for comparative studies, where higher scores represent a lower level of bias. Each study was scored independently by 2 reviewers (DPL and CBD) with any disagreements resolved in a consensus discussion with the senior author (CLC) when necessary. All MINORS score results are displayed in Table 1 and have been converted to percentages. Level of evidence was determined according to the Oxford Centre for Evidence-Based Medicine.
Table 1.
Demographics of Included Studies (n = 14).
| Study | MINORS Score, % | Level of Evidence | Treatment Type | Outcome Measures Reported | No. of Patients | Location | Lesion Size (Range), cm2 | Age (Range), Years | Follow-up (Range), Years |
|---|---|---|---|---|---|---|---|---|---|
| Buchmann et al. (2012) 6 | 63% | RCS, Level IV | ACT-Cs | Satisfaction Scale, VAS, Rowe, ASES, CSS and MRI | 4 | 3H, 1G | 6.0 H 2.0 G |
29.3 (21-36) | 3.4 (0.9-5.9) |
| Camp et al. (2015) 1 | 56% | RCR, Level IV | OCA | SSV, Quick DASH, ASES, X-ray and MRI | 1 | G | 1.8 | 25 | 1 |
| Frank et al. (2010) 24 | 69% | RCS, Level IV | MFX | SST, ASES, VAS | 14 (15 shoulders) | 9H, 5G, 1B | H: 5.1 (1-7.8) G: 1.7 (0.4-3.8) |
37 (18-55) | 2.3 (1-7.4) |
| Hünnebeck et al. (2017) 17 | 75% | RCS, Level IV | MFX | Subjective evaluation, ROM, CMS, SSV, and DASH, X-ray | 32 | 18H, 2G, 12B | — b | 56 (37-74) |
8.8 (5.3-12.3) |
| Johnson et al. (1997) 5 | 50% | RCR, Level IV | OCA | X-ray | 1 | H | 3.1 | 19 | 3 |
| Kircher et al. (2009)18,a | 63% | RCS, Level IV | OAT | CSS, MRI | 3 | 3H | 1.2 (1.1-1.4) | 8.5 (7.6-9.0) | 8.8 (7.6-9.8) |
| Mima et al. (2016) 19 | 56% | RCR, Level IV | Fixation with poly-l-lactic acid pins | ASES, X-ray, CT, and MRI | 1 | H | 2.0 | 29 | 4 |
| Park et al. (2006) 2 | 56% | RCR, Level IV | OAT | Arthroscopic evaluation | 1 | H | 0.6 | 13 | 2.6 |
| Pham et al. (2017) 3 | 56% | RCR, Level IV | OAT | VAS, SSV, CSS, and MRI | 1 | H | 1.5 | 14 | 2.2 |
| Provencher et al. (2010) 4 | 56% | RCR, Level IV | OCA | VAS and ROM | 1 | B | — b | 25 | 1.3 |
| Riff et al. (2017) 20 | 69% | RCS, Level IV | OCA | VAS, ASES, SST, SF-12, and X-ray | 20 | 9H, 11B | 4.9 (1.8-7.1) | 24.8 (17-49) | 5.5 |
| Siebold et al. (2003) 21 | 81% | PCS, Level IV | MFX and periosteal flap | CSS, X-ray, MRI, and arthroscopic evaluation | 5 | 5H | 3.1 (2.3-4.0) | 32 (16-56) | 2.2 (2-2.6) |
| Slabaugh et al. (2010) 22 | 50% | RCR, Level IV | MFX | VAS and ASES score | 1 | H | 6.3 | Early 40s | — b |
| Wang et al. (2018) 23 | 75% | RCS, Level IV | MFX | VAS, SST, ASES, SANE, and SF-12 scores | 13 (14 shoulders) | 8H, 5G, 1B | H: 5.2 (4.0-7.8) G: 1.5 (1.0-3.8) |
36.3 (18-55) | 10.2 (8.5-15.8) |
ACT-Cs = autologous chondrocyte transplantation–collagen membrane seeding; ASES = American Shoulder and Elbow Surgeons shoulder score; B = bipolar humeral and glenoid lesion; CMS = Constant-Murley Score; CSS = Constant Shoulder Score; DASH = Disabilities of the Arm, Shoulder and Hand Score; G = isolated glenoid lesion; H = isolated humeral head lesion; MFX = microfracture; MRI, magnetic resonance imaging; OAT = osteochondral autologous transplantation; OCA = osteochondral allograft transplantation; RCS = retrospective case series; ROM = range of motion; SANE = Single Assessment Numeric Evaluation; SF-12 = Short Form–12 (Mental and Physical); SST = Simple Shoulder Test; SSV = Subjective Shoulder Value; VAS = visual analogue score for pain.
Includes only patients in the study which met inclusion criteria as others had a history of glenohumeral instability.
Not reported or unable to calculate.
Data Extraction and Analysis
Studies were reviewed and the extracted data included study properties (year, level of evidence, number of patients), patient demographics (age, sex, lesion size, lesion location, and follow-up), surgical details, outcomes (patient reported, functional, clinical, and imaging), complications, failures, and reoperations. In one study, only data from patients who met inclusion criteria were reported and analyzed as a subset of patients who had a history of glenohumeral instability ( Table 1 ). Patient-reported outcomes were reported only when found in 3 or more studies. Due to a lack of comparative studies, inclusion of case reports, and the resulting heterogeneity of reported outcomes, data were not pooled and was instead reported as ranges. Additionally, when ranges or standard deviations for patient-reported outcomes were available in individual studies, they were reported as a measure of dispersion. Since the outcome measures were not pooled and reported as weighted averages in a meta-analysis, subjective analysis was performed instead. All data were analyzed using JMP Pro software (2018; version 14.1.0, SAS Institute Inc., Cary, NC). Figures were created using JMP Pro.
Results
Study Characteristics
Characteristics of the 14 studies meeting all inclusion criteria can be found in Table 1 .1-6,17-24 There were 6 retrospective case series, 7 case reports, and 1 prospective case series. This resulted in a total of 100 shoulders in 98 patients. Microfracture alone was performed in 62 shoulders, OCA in 23, microfracture with periosteal flap in 5, OAT in 5, autologous chondrocyte transplantation (ACT) with collagen membrane seeding in 4, and internal fixation with poly-l-lactic acid pins in 1 patient. Lesion size ranged from 0.6 to 7.8 cm2 in patients treated with microfracture, 0.6 to 7.1 cm2 in patients treated with OAT and OCA, 2.0 to 6.0 cm2 in patients treated with ACT, and 2.0 cm2 in a patient treated with fixation. Mean patient age ranged from 13-56 years (overall age range of 7-74 years) and mean follow-up ranged from 1-10.2 years (overall range of 0.9-15.8 years).
Imaging and Second-Look Arthroscopy
Eight studies reported radiographic outcomes, 6 reported MRI outcomes, and 2 performed second-look arthroscopy ( Table 2 ). Radiographic graft incorporation and restoration of articular surfaces ranged from 90% to 100% (5 studies). Specifically, Riff et al. 20 demonstrated graft incorporation in 90% (18/20) of patients treated with OCA to the humeral head at an average of 14.8 months. Six studies reported MRI outcomes with graft integration ranging from 75% to 100%. The rate of progression of Samilson grade 25 of osteoarthritis (OA) ranged from 57% to 100% (3 studies). Hünnebeck et al 17 reported progression of OA in 57% (12/23) patients, occurring significantly more in patients with preoperative OA (P = 0.01).
Table 2.
Reported Patient Outcomes.
| Study | Outcome Measures | Results | |
|---|---|---|---|
| Buchmann et al. (2012) 6 | Satisfaction Scale, VAS, ASES, CSS, and MRI | Satisfaction Scale: 3 “Very Satisfied” and 1
“Satisfied” Mean post-op VAS: 0.3 Mean post-op Rowe (range): 91.3 (75-100) Mean post-op CSS: 83.3 (range, 69-91); Pain (14.8), ADL (16.5), ROM (31.3), Strength (15.0) |
Mean post-op ASES (range): 95.3 (83.3-100) MRI evaluation: • 75% (3) no sign of OA, 25% (1/4) sign of mild OA with a dislocated anchor • 75% (3) complete integration into border of defect |
| Camp et al. (2015) 1 | SSV, ASES, X-ray and MRI | SSV: Pre-op of 40% to post-op of 99% (59%
improvement) ASES score: Pre-op of 36 to post-op of 92 (46-point improvement) |
X-ray at 3 months: articular surface restoration. MRI at 6 months: articular surface restoration and congruity with graft incorporation |
| Frank et al. (2010) 24 | VAS, ASES, SST | Mean VAS: pre-op of 5.6 ± 1.7 vs. post-op 1.9 ± 1.4
(P < 0.001) Mean ASES: pre-op of 44.3 ± 15.3 vs. post-op of 86.3 ± 10.5 (P < 0.001) |
Mean SST: pre-op of 5.7 ± 2.1 vs. post-op of 10.3 ± 1.3 (P < 0.001) |
| Hünnebeck et al. (2017) 17 | Subjective evaluation, ROM, SSV, and X-ray | Subjective evaluation: • 48% (13/27) no pain, 44% (12) moderate pain with high activity, and 7% (2) moderate pain with normal shoulder ROM • 22% (6/27) moderate pain only at night and 11% (3/27) at rest • 59% (19/32) were “very satisfied” or “satisfied” with surgery Mean ROM: • Mean FE: pre-op 140° ± 53° to post-op 153° ± 49° (NS) • Mean ER: pre-op 45° ± 22° to post-op 49° ± 21° (NS) • Mean IR: pre-op ISJ ±32° to post-op L1 vertebral level ±11° (P = 0.03) |
Mean post-op SSV: • Operated shoulder SSV of 86 ± 13 vs. nonoperated shoulder of 88 ± 19 (P = 0.6) X-ray: • Mean Samilson OA grade pre-op of 0.8 to post-op of 1.3 • 57% (13/23) with progression of OA • Those without preoperative OA (14) had significantly less progression (P = 0.01) than those with preoperative OA (17) *Patients with preoperative OA were found to have significantly worse post-op outcome scores |
| Johnson et al. (1997) 5 | X-ray | No pain at rest or with overhead activities | X-ray displayed incorporation of allograft with trabeculae crossing the allograft-host junction |
| Kircher et al. (2009)18,a | CSS, MRI | Mean CSS (range): pre-op of 69 (66-71) vs. post-op of 94
(90-97) • Pain: pre-op of 7.7 (5-13) vs. post-op of 12.3 (10-14) • ADL: pre-op of 13.3 (10-16) vs. post-op of 19 (18-20) • ROM: pre-op of 36 (30-40) vs. post-op of 40 (40) • Strength (kg): pre-op of 5.6 (3.3-8.4) vs. post-op of 22.3 (21-25) |
MRI: • 66% (2/3) displayed joint surface congruency, 100% osteochondral graft integration X-ray: • Mean Samilson OA grade pre-op of 0.3 to final-follow up of 2.7 • 100% displayed progression of OA |
| Mima et al. (2016) 19 | ASES, X-ray, CT, and MRI | ASES score: pre-op of 63.3 to post-op of 89.2 (25.9-point improvement) | X-ray and CT scan: fragment union; MRI: healing of the osteochondral lesion with equal intensity as the humeral head. |
| Park et al. (2006) 2 | Arthroscopic evaluation | No symptoms with good functional results | Second-look arthroscopy: • Defects of both donor and recipient sites were healed and cartilage covered. • Biopsy specimen of recipient site demonstrated a normal pattern of hyaline cartilage. |
| Pham et al. (2017) 3 | VAS, SSV, CSS, and MRI | VAS score: pre-op of 10 to post-op of 0 SSV score: post-op of 70% CSS score: pre-op of 8 to post-op of 92 (84-point improvement) |
MRI: • Healing of the osteochondral defect with normal thickness of the grafted cartilage in comparison to the surrounding articular cartilage. |
| Provencher et al. (2010) 4 | VAS, ROM, and X-Ray | Mean ROM: • FE pre-op 120° to post-op 170° • Abduction pre-op 100° to post-op 170° • ER at the side pre-op 45° to post-op 45° • Abducted ER pre-op 50° to post-op 85° • Abducted IR pre-op 45° to post-op 65° |
VAS score: pre-op of 6 to post-op of
1 X-ray: • Complete incorporation of the humeral and glenoid allografts without resorption and demonstration of excellent articular conformity. |
| Riff et al. (2017) 20 | VAS, ASES, SST, SF-12, and X-ray | Mean VAS: pre-op of 5.8 to post-op of 1.9
(P < 0.001) Mean ASES: pre-op of 40.8 to post-op of 75.8 (P < 0.001) |
Mean SST: pre-op of 31.9 to post-op of 76.8
(P <
0.001) X-ray: • 90% (18/20) of grafts incorporated at an average of 14.8 months |
| Siebold et al. (2003) 21 | CSS, X-ray, MRI, and arthroscopic evaluation | Mean CSS: Pre-op of 43.4 to post-op of 81.8
(P = 0.002) • Pain: pre-op of 5 to post-op of 13.6 (P = 0.001) • ADL: pre-op of 13.6 to post-op of 18.6 • ROM: No significant change • Strength (kg): post-op average of 6.5 |
X-ray Samilson OA grading: pre 0.4 (0-1) post: 0.8
(0-2) • 60% (3/5) displayed progression of OA MRI: • Regeneration tissue covering the area of the chondral defect in 100% of patients. Second-look arthroscopy: • Performed in 60% (3 of 5) patients and revealed a significant reduction of the lesion. |
| Slabaugh et al. (2010) 22 | VAS and ASES score | VAS score: pre-op of 3 to post-op of 0 | ASES score: pre-op of 62 to post-op of 100 (38-point improvement) |
| Wang et al. (2018) 23 | VAS, SST, ASES, SANE, and SF-12 scores | Mean VAS: 6.1 pre-op to 1.5 post-op (P =
0.007) • Glenoid: 6 pre-op to 1.5 post-op; Humeral: 6.3 pre-op to 1.5 post-op Mean SST: 5.2 pre-op to 10.4 post-op (P = 0.009) • Glenoid: 5.7 pre-op to 9.8 post-op; Humeral: 4.3 pre-op to 11.5 post-op |
Mean ASES: 39.3 pre-op to 88.1 post-op (P =
0.014) • Glenoid: 42.5 pre-op to 87.5 post-op • Humeral: 39.0 pre-op to 92.5 post-op |
ADL = activities of daily living; ASES = American Shoulder and Elbow Surgeons shoulder score; CMS = Constant-Murley Score; CSS = Constant Shoulder Score; CT = computed tomography; DASH = Disabilities of the Arm, Shoulder and Hand Score; ER = external rotation; FE = forward elevation; IR = internal rotation; ISJ = iliosacral joint; MFX = microfracture; MRI = magnetic resonance imaging; OA = osteoarthritis; post-op = postoperative; pre-op = preoperative; ROM = range of motion; SANE = Single Assessment Numeric Evaluation; SF-12 = Short Form–12 (Mental and Physical); SST = Simple Shoulder Test; SSV = Subjective Shoulder Value; VAS = visual analogue score for pain.
Includes only patients in the study which met inclusion criteria as others had a history of glenohumeral instability.
Patient-Reported Outcomes
Patient-reported postoperative outcomes were described heterogeneously across all studies with no consistent outcome measures reported ( Table 2 ). The most commonly utilized outcomes were: visual analogue scale for pain 26 in 7 studies, American Shoulder and Elbow Surgeons (ASES) 27 questionnaire in 6 studies, Constant Shoulder Score (CSS) 28 in 4 studies, Subjective Shoulder Value (SSV) 29 in 3 studies, and Simple Shoulder Test (SST) 30 in 3 studies.
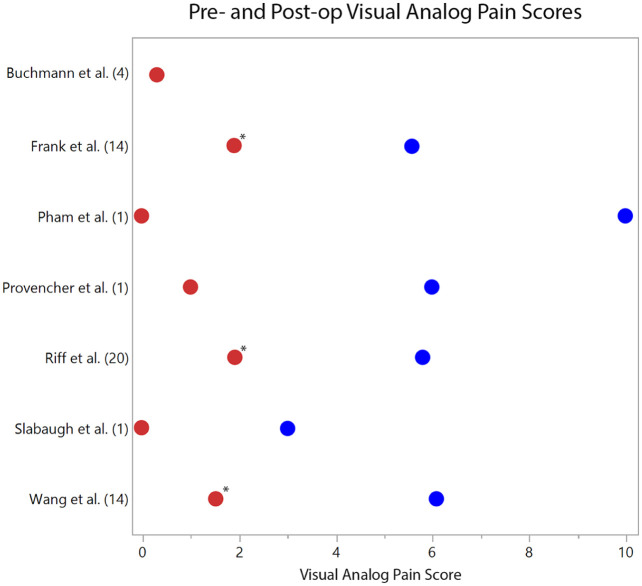
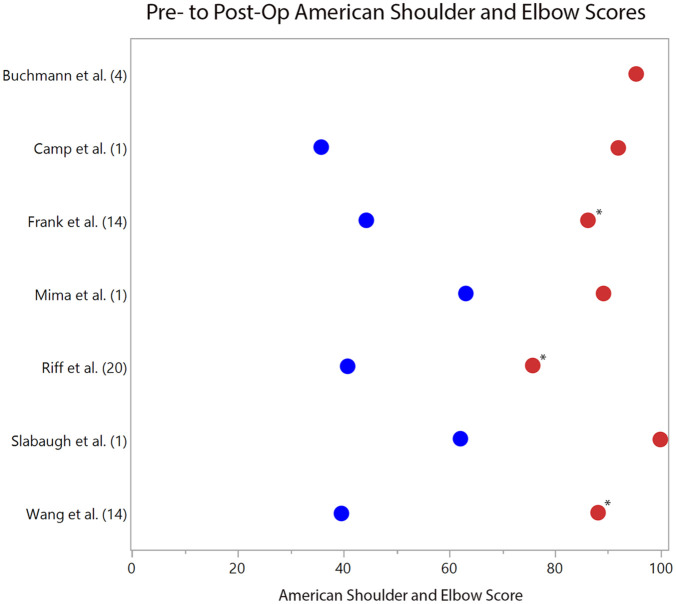
Mean postoperative VAS scores ranged from 0 to 1.9 across 7 studies, with all 3 retrospective case series reporting statistically significant pre- to postoperative improvement (P < 0.05).20,23,24 ( Fig. 2 ). Of 7 studies reporting ASES scores, the mean postoperative range was 75.8 to 100 ( Fig. 3 ). In the 6 studies reporting preoperative scores, the mean improvement ranged from 25.9 to 48.8 with 3 series reporting statistically significant improvement at final follow-up (P < 0.05).20,23,24 Mean CSS scores were reported in 4 studies and ranged from 81.8 to 94. Mean postoperative SSV scores were available in 3 studies and ranged from 70% to 99%.
Figure 2.
Pre- (blue dots) and postoperative (red dots) visual analogue scale (VAS) pain scores on a scale of 0 to 10, with 0 being no pain and 10 being the most. The respective studies are listed with their cohort size (n). *Statistically significant improvement in VAS scores (P < 0.05) is denoted when provided by the study.
Figure 3.
Pre- (blue dots) and postoperative (red dots) American Shoulder and Elbow Surgeons (ASES) questionnaire scores. The respective studies are listed with their cohort size (n). *Statistically significant improvement in ASES scores (P < 0.05) is denoted when provided by the study.
The largest study meeting inclusion reported additional subjective patient outcomes on 32 patients. Hünnebeck et al. 17 reported no difference for overall Constant Murley Scores 28 (CMS) between the operative and nonoperative shoulder, with mean scores of 74 ± 26 versus 75 ± 28 (P = 0.5), respectively. Fifty-nine percent (19/32) were “satisfied” or “very satisfied” with their surgical outcomes, with 48% (13/27) reporting no pain, and 44% (12/27) reporting moderate pain only while performing high-level activity.
Complications, Reoperations, and Failures
Similar to the outcomes reported among included studies, complications, reoperations, and failures were reported heterogeneously ( Table 3 ). Complications were rare, as only 1 patient experienced a transient postoperative brachial plexopathy with return of normal strength and function at 6-month follow-up. 1 The other 13 studies reported no complications in the perioperative period.2-6,17-24 The overall rate of failure and/or reoperation ranged from 0% to 35% and 0% to 30%, respectively6,17,20,23,24 ( Table 3 ). Of note, patients who required reoperation and patients who would choose not to undergo the initial procedure again were considered failures. The most common reoperation was conversion to prosthetic arthroplasty, occurring at a mean follow-up of 47 months, 17 25 months, 20 and 2.5 months. 23
Table 3.
Surgical Details, Complications, and Failures.
| Study | Surgical Details | Concomitant Procedures | Complications | Reoperations and Failures | Additional Notes |
|---|---|---|---|---|---|
| Buchmann et al. (2012) 6 | ACT-Cs | 3 (75%) patients: LBE, BTD, and anchor extraction, MFX |
None | 25% (1/4) failure with progressive OA and a new cartilage defect adjacent to the ACT-Cs | None |
| Camp et al. (2015) 1 | OCA | BTD | Transient brachial plexopathy | None | None |
| Frank et al. (2010) 24 | MFX | 10 (71%) patients: SAD, BTD, CR, LBE |
None | 20% (3/15) failure and reoperation: 1 hemiarthroplasty, 1 biological resurfacing, 1 debridement and CR | None |
| Hünnebeck et al. (2017) 17 | MFX | 27 (84%) patients: SAD, biceps long head tenotomy, AC joint resection, SLAP, BTD |
None | 16% (5/32) failure and reoperation: 3 hemiarthroplasty and 2 TSA at a mean of 47 months (range, 5-79 months) | 97% (31/32) grade IV lesions, 1 grade III lesion. 53% (17) identified to have preoperative OA 16% (5) with previous operations |
| Johnson et al. (1997) 5 | OCA | None | None | None | None |
| Kircher et al. (2009)18,a | OAT (ipsilateral knee) | 1 (33%) patient: labral augmentation |
None | None | 33% (1/3) preoperative grade I OA |
| Mima et al. (2016) 19 | Poly-l-lactic acid pin fixation | None | None | None | None |
| Park et al. (2006) 2 | OAT (lateral femoral condyle) | None | None | None | New osteochondral lesion developed on the posterolateral humeral head with follow-up MRI |
| Pham et al. (2017) 3 | OAT (femoral trochlea) | None | None | None | No donor site complications |
| Provencher et al. (2010) 4 | OCA | None | None | None | Bipolar Outerbridge grade IV lesion. Four prior surgeries: MFX, debridement, anchor removal, and CR with chondroplasty. Patient “extremely satisfied” with outcome. |
| Riff et al. (2017) 20 | OCA | 11 (55%) patients: MFX, meniscal allograft glenoid resurfacing |
None | 35% (7/20) failure: 22% (4) required TSA at an average of 25
months, 17% (3) were dissatisfied and would not undergo the
procedure again. 30% (6/20) reoperation: 4 TSA, 2 postoperative CR. |
Those with concomitant meniscal allograft glenoid resurfacing demonstrated worse subjective scores and worse ASES scores (66 vs. 83, P = 0.049) |
| Siebold et al. (2003) 21 | MFX and periosteal flap | 4 (80%) patients: capsular shift, Anchor removal |
None | None | None |
| Slabaugh et al. (2010) 22 | MFX | Labral debridement and subacromial bursectomy | None | None | None |
| Wang et al. (2018) 23 | MFX | 9 (64%) patients: SAD, CR, BTD, LBE |
None | 28% (4/14) failure and reoperation: 1 hemiarthroplasty at 2.5 months, 1 distal tibia allograft at 1.4 years, and 1 TSA at 9.6 years, 1 debridement, synovectomy, SAD, and DCR at 2.7 years | Survivorship of 93.8% at 1 year, 87.5% at 3 years, and 76.6% at 9.6 years. |
ACT-Cs = autologous chondrocyte transplantation–collagen membrane seeding; BTD = biceps tenodesis; CR = capsular release; DCR = distal clavicle resection; ER = external rotation; FE = forward elevation; IR = internal rotation; LBE = loose body excision; MFX = microfracture; MRI = magnetic resonance imaging; OA = osteoarthritis; OAT = osteochondral autologous transplantation; OCA = osteochondral allograft transplantation; SAD = subacromial decompression; SLAP = superior labrum anterior and posterior repair; TSA = total shoulder arthroplasty.
Includes only patients in the study which met inclusion criteria as others had a history of glenohumeral instability.
Discussion
Osteochondral defects of the glenohumeral joint occur secondary to a number of etiologies and present a management challenge in young and highly active patients. Prosthetic arthroplasty has been well described as a treatment option for advanced cartilage defects involving the glenohumeral joint for older patients; however, for younger and more active patients, literature is limited regarding outcomes following non-arthroplasty surgical management. The current systematic review demonstrates rates of radiographic union and progression of OA to range between 90% to 100% and 57%-100%, respectively, across 8 studies. Postoperative VAS improved to a range of 0 to 1.9 (7 studies), mean ASES scores improved to 75.8 to 100 (7 studies), mean CSS scores improved to 83.3 to 94 (3 studies), and mean SSV improved to 70% to 99% (3 studies).
Overall, this systematic review found high rates of radiographic graft incorporation and restoration of articular surfaces; ranging from 90% to 100% (5 studies). Six studies reporting MRI outcomes revealed graft integration ranging from 75% to 100%.
In spite of high healing rates, progression of Samilson osteoarthritis grade ranging from 57% to 100% (3 studies). One study revealed no sign of osteoarthritis in 75% of patients with progression of osteoarthritis in the remaining 25%. Radiographic and MRI evaluation in this series demonstrates high rates of healing without significantly preventing progression and development of glenohumeral osteoarthritis. To our knowledge, no studies have evaluated outcomes of non-arthroplasty management with respect to radiographic healing, graft incorporation, and progression to OA.
This review also demonstrated improved patient reported outcomes (VAS, ASES, CSS, and SSV scores) following nonarthroplasty surgical management of glenohumeral cartilage lesions. With several studies reporting statistically significant improvements, these results suggest patients experience improved pain and function. It has been established that patients of younger age undergoing shoulder arthroplasty have higher rates of prosthetic loosening and failure, thus suggesting the need for joint preserving options. 14 Several studies in current literature have also provided similar short-term results of pain relief and improved function; however, results are generally temporary and patients with features of advanced disease experience lesser postoperative improvement and high probability for conversion to shoulder arthroplasty.31-35 Van Thiel et al. 34 published a retrospective series of 71 patients undergoing arthroscopic debridement in patients with glenohumeral OA. Patients reported subjective pain relief in 77% (55/71) at a mean of 2.25 years postoperatively. Patients in this cohort with ongoing pain and progressing to shoulder arthroplasty exhibited higher grade of preoperative arthritis scores, joint space narrowing, and large osteophyte formation.
In this systematic review, the rate of complications was extremely low, occurring in only one patient who was treated with OAT. The complication was a brachial plexopathy and it ultimately resolved without further treatment. Reoperations ranged from 0% to 30% and failures ranged from 0% to 35%. A prospective study of 9,410 patients undergoing shoulder arthroscopy also reported a very low complication rate of 0.99%. 36 Similar to studies evaluating nonarthroplasty management of glenohumeral cartilage lesions, the most common reoperation remains conversion to prosthetic shoulder arthroplasty, occurring at a mean of 2.5 to 47 months (4 studies) in this review. Mitchell et al. 37 reported a series of 49 shoulders with a mean age of 52 years undergoing arthroscopic management of glenohumeral cartilage loss. In that study, 26% of patients progressed to total shoulder arthroplasty at a mean of 2.6 years. Skelly et al. 38 reviewed 33 patients, with an average age of 55.2 years, who underwent arthroscopic debridement and capsular release. Fourteen patients (42.4%) progressed to shoulder arthroplasty at a mean of 8.8 months. While also considering the patient reported outcomes following nonarthroplasty surgical management of glenohumeral cartilage lesions, the literature suggests joint preserving techniques to be viable options to prolong the function of the native shoulder and provide short- to midterm pain relief without significantly decreasing the progression of osteoarthritis. Despite evidence of healing and attempted joint preservation, many patients experience progression of OA to the point of needing reoperation, most often in the form of conversion to prosthetic arthroplasty. Additional studies are needed to further investigate nonarthroplasty surgical treatment options to delineate which techniques provide the most beneficial long-term outcomes in young and active patients.
Limitations
The results of this systematic review should be interpreted in the context of several limitations. First, there is no consensus as to the best management of glenohumeral cartilage lesions in young and highly active patients. Therefore, the majority of studies included were level IV case reports or retrospective case series with relatively small patient cohorts. Second, the included studies also generally lack randomization, blinding, and comparative control groups. Third, the outcome measures were inconsistent across the 14 included studies, allowing for a source of bias while making it difficult to draw meaningful conclusions. 39 When attempting to explore the causes of heterogeneity of included studies, no significant correlations were found between the type of surgical intervention pursued, glenohumeral cartilage defect size or location, level of evidence, patient age, or follow up timelines. Fourth, the exclusion criteria may have eliminated pertinent data that could have altered conclusions, specifically studies evaluating glenohumeral cartilage defects as a result of joint instability. Fifth, various surgical treatment options were employed to address lesions and resulting follow-up timelines varied. Several of the nonarthroplasty treatment options utilized in this review resulted in small cohorts of patients. There is certainly surgeon selection bias in terms of management strategies, which further contributes to the heterogeneity of the data. Finally, this review only includes published data with reported outcomes and may therefore have publication bias.
Conclusions
There are numerous joint-preserving surgical options for the treatment of osteochondral lesions involving the glenohumeral joint in young and highly active patients without a clear consensus as to which approach provides the best outcomes. In this systematic review, nonarthroplasty surgical techniques demonstrated acceptable rates of radiographic healing, improved patient-reported outcomes, minimal complications, and low rates of failure or reoperation. The rate of arthritis progression, however, was high. Joint preserving techniques are likely viable options to prolong function of the native shoulder and provide short- to midterm pain relief.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Aaron J. Krych discloses the following: Aesculap/B. Braun: research support; American Journal of Sports Medicine: editorial or governing board; Arthrex, Inc.: IP royalties; paid consultant; Arthritis Foundation: research support; Ceterix: research support; Histogenics: research support; International Cartilage Repair Society: board or committee member; International Society of Arthroscopy, Knee Surgery, and Orthopaedic Sports Medicine: board or committee member; Minnesota Orthopedic Society: board or committee member; Musculoskeletal Transplantation Foundation: board or committee member; Vericel: paid consultant; Open Payments Database: general payments from Arthrex Inc., Musculoskeletal Transplant Foundation, Ceterix; Orthopedics, Inc., Gemini Medical LLC, and Gemini Mountain Medical. Jonathan D. Barlow discloses the following: Stryker: paid consultant; CDC Medical: education fees; Arthrex Inc.: travel and lodging, food and beverage. Diane L. Dahm discloses the following: American Orthopaedic Society for Sports Medicine: board or committee member; Arthrex Inc.: research support; GE Healthcare: travel and Lodging; NBA/GE Strategic Advisory Board: board or committee member; spouse owns stock in Tenex Health, Inc. and Sonex Health, LLC: stock or stock options; spouse receives royalties from Tenex Health, Inc. and Sonex Health, LLC: IP royalties. Christopher L. Camp discloses the following: Arthrex Inc.: travel and lodging. The other authors have nothing to disclose.
ORCID iDs: Devin P. Leland  https://orcid.org/0000-0003-0695-5441
https://orcid.org/0000-0003-0695-5441
Aaron J. Krych  https://orcid.org/0000-0003-3248-8007
https://orcid.org/0000-0003-3248-8007
Christopher L. Camp  https://orcid.org/0000-0003-3058-7327
https://orcid.org/0000-0003-3058-7327
References
- 1. Camp CL, Barlow JD, Krych AJ. Transplantation of a tibial osteochondral allograft to restore a large glenoid osteochondral defect. Orthopedics. 2015;38(2):e147-52. [DOI] [PubMed] [Google Scholar]
- 2. Park TS, Kim TS, Cho JH. Arthroscopic osteochondral autograft transfer in the treatment of an osteochondral defect of the humeral head: report of one case. J Shoulder Elbow Surg. 2006;15(6):e31-6. [DOI] [PubMed] [Google Scholar]
- 3. Pham TT, Kany J, Lakhal W, Sales de Gauzy J, Accadbled F. Arthroscopic osteochondral autograft transfer for juvenile osteochondritis dissecans of the humeral head: a case report. JBJS Case Connect. 2017;7(3):e63. [DOI] [PubMed] [Google Scholar]
- 4. Provencher MT, LeClere LE, Ghodadra N, Solomon DJ. Postsurgical glenohumeral anchor arthropathy treated with a fresh distal tibia allograft to the glenoid and a fresh allograft to the humeral head. J Shoulder Elbow Surg. 2010;19(6):e6-11. [DOI] [PubMed] [Google Scholar]
- 5. Johnson DL, Warner JJ. Osteochondritis dissecans of the humeral head: treatment with a matched osteochondral allograft. J Shoulder Elbow Surg. 1997;6(2):160-3. [DOI] [PubMed] [Google Scholar]
- 6. Buchmann S, Salzmann GM, Glanzmann MC, Wortler K, Vogt S, Imhoff AB. Early clinical and structural results after autologous chondrocyte transplantation at the glenohumeral joint. J Shoulder Elbow Surg. 2012;21(9):1213-21. [DOI] [PubMed] [Google Scholar]
- 7. Gartsman GM, Taverna E. The incidence of glenohumeral joint abnormalities associated with full-thickness, reparable rotator cuff tears. Arthroscopy. 1997;13(4):450-5. [DOI] [PubMed] [Google Scholar]
- 8. Paley KJ, Jobe FW, Pink MM, Kvitne RS, ElAttrache NS. Arthroscopic findings in the overhand throwing athlete: evidence for posterior internal impingement of the rotator cuff. Arthroscopy. 2000;16(1_suppl):35-40. [DOI] [PubMed] [Google Scholar]
- 9. Guntern DV, Pfirrmann CW, Schmid MR, Zanetti M, Binkert CA, Schneeberger AGet al. Articular cartilage lesions of the glenohumeral joint: diagnostic effectiveness of MR arthrography and prevalence in patients with subacromial impingement syndrome. Radiology. 2003;226(1_suppl):165-70. [DOI] [PubMed] [Google Scholar]
- 10. Mitchell JJ, Vap AR, Sanchez G, Liechti DJ, Chahla J, Moatshe Get al. Concomitant reverse hill-sachs lesion and posterior humeral avulsion of the glenohumeral ligament: treatment with fresh talus osteochondral allograft and arthroscopic posterior humeral avulsion of the glenohumeral ligament and labrum repair. Arthrosc Tech. 2017;6(4):e987-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Flatow EL, Altchek DW, Gartsman GM, Iannotti JP, Miniaci A, Pollock RGet al. The rotator cuff. Commentary. Orthop Clin North Am. 1997;28(2):277-94. [DOI] [PubMed] [Google Scholar]
- 12. Elser F, Braun S, Dewing CB, Millett PJ. Glenohumeral joint preservation: current options for managing articular cartilage lesions in young, active patients. Arthroscopy. 2010;26(5):685-96. [DOI] [PubMed] [Google Scholar]
- 13. Denard PJ, Wirth MA, Orfaly RM. Management of glenohumeral arthritis in the young adult. J Bone Joint Surg Am. 2011;93(9):885-92. [DOI] [PubMed] [Google Scholar]
- 14. Sperling JW, Antuna SA, Sanchez-Sotelo J, Schleck C, Cofield RH. Shoulder arthroplasty for arthritis after instability surgery. J Bone Joint Surg Am. 2002;84-A(10):1775-81. [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew Met al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712-6. [DOI] [PubMed] [Google Scholar]
- 17. Hünnebeck SM, Magosch P, Habermeyer P, Loew M, Lichtenberg S. Chondral defects of the glenohumeral joint: Long-term outcome after microfracturing of the shoulder. Obere Extrem. 2017;12(3):165-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kircher J, Patzer T, Magosch P, Lichtenberg S, Habermeyer P. Osteochondral autologous transplantation for the treatment of full-thickness cartilage defects of the shoulder: results at nine years. J Bone Joint Surg Br. 2009;91(4):499-503. [DOI] [PubMed] [Google Scholar]
- 19. Mima Y, Matsumura N, Ogawa K, Iwamoto T, Ochi K, Sato Ket al. Osteochondritis dissecans on the medial aspect of the humeral head. Int J Shoulder Surg. 2016;10(2):89-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Riff AJ, Yanke AB, Shin JJ, Romeo AA, Cole BJ. Midterm results of osteochondral allograft transplantation to the humeral head. J Shoulder Elbow Surg. 2017;26(7):e207-15. [DOI] [PubMed] [Google Scholar]
- 21. Siebold R, Lichtenberg S, Habermeyer P. Combination of microfracture and periostal-flap for the treatment of focal full thickness articular cartilage lesions of the shoulder: a prospective study. Knee Surg Sports Traumatol Arthrosc. 2003;11(3):183-9. [DOI] [PubMed] [Google Scholar]
- 22. Slabaugh MA, Frank RM, Cole BJ. Resurfacing of isolated articular cartilage defects in the glenohumeral joint with microfracture: a surgical technique & case report. Am J Orthop (Belle Mead NJ). 2010;39(7):326-32. [PubMed] [Google Scholar]
- 23. Wang KC, Frank RM, Cotter EJ, Davey A, Meyer MA, Hannon CPet al. Long-term clinical outcomes after microfracture of the glenohumeral joint: average 10-year follow-up. Am J Sports Med. 2018;46(4):786-94. [DOI] [PubMed] [Google Scholar]
- 24. Frank RM, Van Thiel GS, Slabaugh MA, Romeo AA, Cole BJ, Verma NN. Clinical outcomes after microfracture of the glenohumeral joint. Am J Sports Med. 2010;38(4):772-81. [DOI] [PubMed] [Google Scholar]
- 25. Samilson RL, Prieto V. Dislocation arthropathy of the shoulder. J Bone Joint Surg Am. 1983;65(4):456-60. [PubMed] [Google Scholar]
- 26. Spiegler PA, Vassalle M. Role of voltage oscillations in the automaticity of sheep cardiac Purkinje fibers. Can J Physiol Pharmacol. 1995;73(8):1165-80. [DOI] [PubMed] [Google Scholar]
- 27. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-94. [DOI] [PubMed] [Google Scholar]
- 28. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-4. [PubMed] [Google Scholar]
- 29. Gilbart MK, Gerber C. Comparison of the subjective shoulder value and the Constant score. J Shoulder Elbow Surg. 2007;16(6):717-21. [DOI] [PubMed] [Google Scholar]
- 30. Angst F, Schwyzer HK, Aeschlimann A, Simmen BR, Goldhahn J. Measures of adult shoulder function: Disabilities of the Arm, Shoulder, and Hand Questionnaire (DASH) and its short version (QuickDASH), Shoulder Pain and Disability Index (SPADI), American Shoulder and Elbow Surgeons (ASES) Society standardized shoulder assessment form, Constant (Murley) Score (CS), Simple Shoulder Test (SST), Oxford Shoulder Score (OSS), Shoulder Disability Questionnaire (SDQ), and Western Ontario Shoulder Instability Index (WOSI). Arthritis Care Res (Hoboken). 2011;63(Suppl 11):S174-88. [DOI] [PubMed] [Google Scholar]
- 31. Mitchell JJ, Warner BT, Horan MP, Raynor MB, Menge TJ, Greenspoon JAet al. Comprehensive arthroscopic management of glenohumeral osteoarthritis: preoperative factors predictive of treatment failure. Am J Sports Med. 2017;45(4):794-802. [DOI] [PubMed] [Google Scholar]
- 32. Spiegl UJ, Faucett SC, Horan MP, Warth RJ, Millett PJ. The role of arthroscopy in the management of glenohumeral osteoarthritis: a Markov decision model. Arthroscopy. 2014;30(11):1392-9. [DOI] [PubMed] [Google Scholar]
- 33. Tashjian RZ, Deloach J, Green A, Porucznik CA, Powell AP. Minimal clinically important differences in ASES and simple shoulder test scores after nonoperative treatment of rotator cuff disease. J Bone Joint Surg Am. 2010;92(2):296-303. [DOI] [PubMed] [Google Scholar]
- 34. Van Thiel GS, Sheehan S, Frank RM, Slabaugh M, Cole BJ, Nicholson GPet al. Retrospective analysis of arthroscopic management of glenohumeral degenerative disease. Arthroscopy. 2010;26(11):1451-5. [DOI] [PubMed] [Google Scholar]
- 35. Weinstein DM, Bucchieri JS, Pollock RG, Flatow EL, Bigliani LU. Arthroscopic debridement of the shoulder for osteoarthritis. Arthroscopy. 2000;16(5):471-6. [DOI] [PubMed] [Google Scholar]
- 36. Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-5.e1661. [DOI] [PubMed] [Google Scholar]
- 37. Mitchell JJ, Horan MP, Greenspoon JA, Menge TJ, Tahal DS, Millett PJ. Survivorship and patient-reported outcomes after comprehensive arthroscopic management of glenohumeral osteoarthritis: minimum 5-year follow-up. Am J Sports Med. 2016;44(12):3206-13. [DOI] [PubMed] [Google Scholar]
- 38. Skelley NW, Namdari S, Chamberlain AM, Keener JD, Galatz LM, Yamaguchi K. Arthroscopic debridement and capsular release for the treatment of shoulder osteoarthritis. Arthroscopy. 2015;31(3):494-500. [DOI] [PubMed] [Google Scholar]
- 39. Cote MP, Lubowitz JH, Rossi MJ, Brand JC. Reviews pooling heterogeneous, low-evidence, high-bias data result in incorrect conclusions: but heterogeneity is an opportunity to explore. Arthroscopy. 2018;34(12):3126-8. [DOI] [PubMed] [Google Scholar]