Abstract
Objective
The effect of lumbar traction on low back pain (LBP) patients is controversial. Our study aims to assess changes in the intervertebral disc water content after lumbar traction using T2 mapping and explore the correlation between changes in the T2 value and Oswestry Disability Index (ODI)/visual analogue scale (VAS) score.
Design
Lumbar spine magnetic resonance imaging was performed, and the ODI/VAS scores were recorded in all 48 patients. Midsagittal T2-weighted imaging and T2 mapping were performed to determine the Pfirrmann grade and T2 value. Then, the T2 values were compared between pre- and posttraction, and the correlation between changes in the T2 value and ODI/VAS scores were examined.
Results
In the traction group, the changes in the nucleus pulposus (NP) T2 values for Pfirrmann grades II-IV and the annulus fibrosus (AF) T2 values for Pfirrmann grade II were statistically significant after traction (P < 0.05). Changes in the mean NP T2 value of 5 discs in each patient and in the ODI/VAS score showed a strong correlation (r = 0.822, r = 0.793).
Conclusion
T2 mapping can be used to evaluate changes in the intervertebral disc water content. Ten sessions of traction resulted in a significant increase in quantitative T2 measurements of the NP in discs for Pfirrmann grade II-IV degeneration and remission of the patients’ clinical symptoms in the following 6 months. Changes in the mean NP T2 value of 5 discs in each patient were strongly correlated with changes in the ODI/VAS score.
Keywords: T2 mapping, low back pain, intervertebral disc, lumbar traction, ODI/VAS score
Introduction
Low back pain (LBP) causes great inconvenience, reduces the quality of life, and leads to a high rate of disability. Intervertebral disc degeneration (IVDD) has been considered 1 factor of LBP among a variety of etiologies. 1 With disc degeneration, several factors, including the lack of a blood supply, decreased proteoglycan and water levels, an inflammatory environment, and immune imbalance, lead to the emergence of pain. 2 Patients who have no or only slight disc bulging or disc herniation are more likely to choose conservative treatment, especially lumbar traction. 3 Traction may reduce intradiscal pressure, increasing the level of oxygen, nutrient uptake, and water through reverse osmosis. 4 The duration, symptoms (with or without leg pain and/or sciatica), and pathogenic process (acute, subacute, or chronic LBP) of LBP, and the type and parameters of traction have been inconsistent among previous, so the results have varied.5,6 Several studies4,6,7 have failed to support the efficacy of lumbar traction in relieving LBP and have proposed that this treatment should not receive high priority. However, many clinicians still firmly insist that an undefined subset of patients truly benefit from traction. 8 Therefore, a noninvasive and quantitative method is needed to detect changes in disc composition after lumbar traction and explore the correlation between these changes and clinical improvement.
Magnetic resonance imaging (MRI) has a high resolution for soft tissue and can clearly show disc herniation or bulging, annular tears, and nerve root compression. MRI can also show disc dehydration on T2-weighted imaging but cannot be used to quantify the water content. Recently, quantitative MRI has been widely used to detect the chemical composition of tissues and organs; for example, T2 mapping can reflect information regarding the interaction between water molecules and collagen in discs and can thus be used to detect differences in the disc water content between pre- and posttraction. 9 Guehring et al. 10 and Chow et al. 11 showed that continuous spinal distraction could increase the disc height, reorganize the disc structure, and improve the disc water content in animal trials and in humans, respectively. To date, most scholars have focused on changes in the morphology of intervertebral discs, clinical symptoms, disability scale scores, and neurological deficits rather than changes in the underlying microstructure of discs in patients after traction. 12
Therefore, this study aimed to quantitatively evaluate changes in the T2 value of the nucleus pulposus (NP) and annulus fibrosus (AF) separately after lumbar traction using T2 mapping and assess the clinical effect to provide an objective basis for clinicians to evaluate the therapeutic efficacy in patients with LBP.
Materials and Methods
Subjects
Forty-eight patients (28 females and 20 males) from the Orthopedic Clinic or Rehabilitation Clinic of our hospital participated in our study between June 2017 and July 2019. All subjects complained of LBP without leg pain or sciatica lasting for at least 6 months and underwent MRI examination and scheduled follow-up observation. They were randomly divided into the control group and the traction group, with 24 subjects in each group. All patients underwent lumbar spine MRI and were diagnosed with no or only slight disc bulging or disc herniation before traction. In the traction group (15 females and 9 males), the patient age ranged from 22 to 51 years (females, 34-51 years; males, 22-48 years). The mean age was 38.5 years (females, 39 years; males, 34.5 years). The mean body mass index (BMI) was 23.5 kg/m2 (females, 22.7 kg/m2; males, 24.3 kg/m2). In the control group (13 females and 11 males), the patient age ranged from 21 to 53 years (females, 30-53 years; males, 21-50 years). The mean age was 37.5 years (females, 40 years; males, 34 years). The mean BMI was 23.9 kg/m2 (females, 23.1 kg/m2; males, 24.7 kg/m2). The exclusion criteria were as follows: scoliosis, stenosis, intrauterine device or other artifacts in the pelvic cavity, lumbar surgical treatment, severe lumbar trauma or infection, lumbar tumor, lumbar instability or spondylolisthesis, claustrophobia, lack of cooperation due to LBP, and failure to follow-up examinations on time after traction. None of the subjects used nonsteroidal anti-inflammatory drugs (NSAIDs) or any other type of analgesic. Our study was approved by the institutional review board, and informed consent was obtained from all participants.
A total of 120 discs (Pfirrmann grade II, 58; Pfirrmann grade III, 33; Pfirrmann grade IV, 24; and Pfirrmann grade V, 5) were enrolled in the traction group. Meanwhile, 120 discs (Pfirrmann grade II, 58; Pfirrmann grade III, 38; Pfirrmann grade IV, 22; and Pfirrmann grade V, 2) were enrolled in the control group.
Intervention Strategy
All 48 patients underwent lumbar spine MRI, and 24 patients who were randomly divided into the traction group began traction treatment within 3 days. The patients underwent lumbar spine MRI again within 3 days of finishing all traction sessions. In addition, 24 patients in the control group were instructed not to bend, not to stand or sit for long periods of time, not to lift heavy items, and to lie down as much as possible for 1 month; these patients then underwent lumbar spine MRI again within 3 days of the end of this period.
The traction device we used was a motorized traction bed (OG GIKEN, Ortho Trac OL-2000). The patient assumed a supine position with a holder under the knee maintaining knee and hip flexion and preventing lumbar fatigue; a pelvic belt and armpit retainer were used to maintain the position. The traction force was selected as 40% of the patient’s body weight and could be slightly adjusted according to the patient’s tolerance. All patients were treated with intermittent motorized traction (hold: rest time = 99:33 seconds) for 30 minutes per session in 2 to 3 sessions per week, for a total of 10 sessions.
Magnetic Resonance Imaging
This study was performed using an MRI scanner (Philips Achieva/Intera 1.5 T, Best, Netherlands) with a dedicated 5-channel SENSE spine coil. MRI examinations were carried out before and after the patient completed all intervention sessions. The scanning protocols were as follows:
Sagittal turbo spin echo T1-weighted/T2-weighted sequences: repetition time (TR)/echo time (TE) = 500/8 ms/2602/120 ms; field of view (FOV) = 304 × 160 × 39 mm3; slice gap = 0.4 mm; slice thickness = 4 mm; slices = 9; scanning time = 2.04 minutes/2.15 minutes.
Axial turbo spin echo T2-weighted sequences: TR/TE = 2032/100 ms; FOV = 200 × 200 × 13 mm3; slice gap = 0 mm; slice thickness = 4 mm; slices = 12; scanning time = 2.14 minutes.
Sagittal turbo spin echo T2-mapping sequences: TR/TE = 2000/20-130 (12 echo time) ms; flip angle = 90°; FOV = 200 × 117 × 32 mm3; slice gap = 0 mm; slice thickness = 4 mm; slices = 6; scanning time = 7.52 minutes.
Image Analysis
All lumbar spine MRI sequences, including sagittal T1-weighted imaging (T1WI), sagittal T2-weighted imaging (T2WI), axial T2WI, and sagittal T2-mapping sequences, were performed. Two musculoskeletal radiologists with 8 years and 7 years of experience who were blinded to the clinical data assessed the grade of the L1/L2-L5/S1 intervertebral discs using the MRI data before the intervention according to the Pfirrmann grading system. 13 One of the radiologists also reviewed the disc grades again 2 months later.
The T2 values of the NP and AF (as the mean value of the anterior and posterior AF) for each intervertebral disc were measured on midsagittal T2 mapping. 14 Each area was measured 3 times, and the mean value was selected. The regions of interest were approximately 50 to 70 mm3 (NP) and 15 to 30 mm3 (AF). After the 48 patients completed the second lumbar spine MRI examination, all pre- and postintervention T2-mapping images were measured and evaluated by the 2 radiologists who were blinded to the clinical and MRI data.
Clinical Data
The Oswestry Disability Index (ODI) questionnaire and visual analogue scale (VAS) score have been widely used in patients with LBP undergoing physical therapy or nonoperative treatment. 15 The ODI and VAS scores were evaluated within 3 days before the intervention, after all sessions (traction group) or a month later (control group) and 6 months later (both groups). They were recorded with the assistance of a physiatrist with 5 years of experience who was blinded to the intervention and baseline ODI score and VAS score. The ODI questionnaire consisted of 10 items related to different functional aspects, such as pain intensity, lifting, walking, and traveling, and each item was rated from 0 to 5 points. The ODI score was calculated as follows: ODI% = total score/(number of items×5)×100%. The total ODI score ranged from 0 to 100, with higher scores representing more serious dysfunction. 16 The VAS score range from 0 to 10, and the higher scores representing the more severe pain.
Minimum clinical important difference (MCID) was defined as having posttraction improved score of ODI > 12 and VAS > 3.17,18
Statistical Analysis
All data were analyzed using SPSS 22.0. Differences in the T2 value of the NP and AF of discs between pre-and postintervention for each Pfirrmann grade were analyzed using paired t tests. In addition, the correlation between changes in the T2 value and ODI score or VAS score were analyzed by Pearson correlation separately. The reliability of the NP and AF T2 values (independently determined by 2 radiologists) was evaluated with the intraclass correlation coefficient (ICC), as was the reliability of the Pfirrmann grade of the discs. A P-value of less than 0.05 was considered to be significant.
Results
Subjects and Discs
There were no significant differences in the mean age and the BMI between the traction group (24 patients) and the control group (24 patients).
Statistical analysis of Pfirrmann grade V discs (only 5) in the traction group and the control group (only 2) was not included to avoid large deviation.
Image Analysis
In qualitative determination of the Pfirrmann grade, the interobserver agreement was high (ICC = 0.969, 95% CI = 0.952-0.974); the intraobserver agreement was also high (ICC = 0.990, 95% CI = 0.985-0.994).
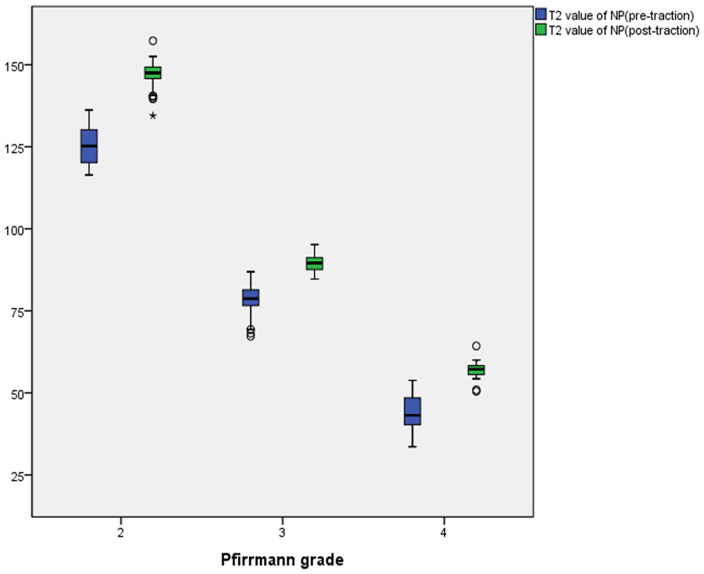
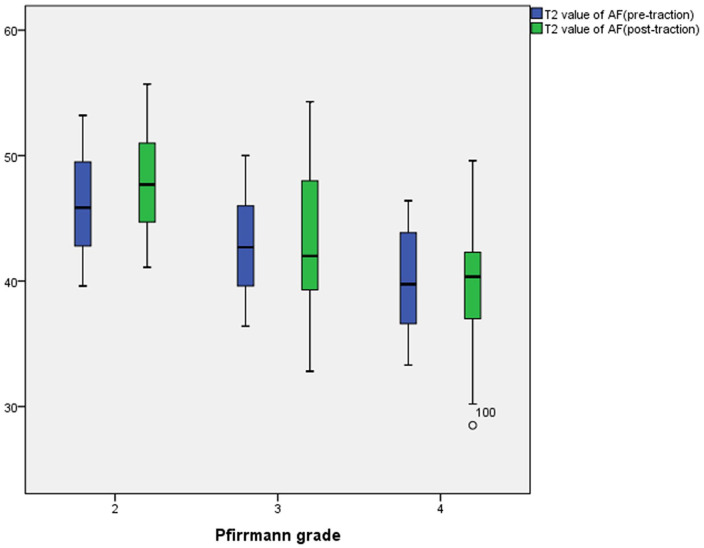
In the traction group, the NP T2 values for Pfirrmann grades II to IV were 125.22 ± 5.27, 78.28 ± 5.43, and 44.32 ± 5.41 before traction and 146.93 ± 3.77, 89.65 ± 2.53, and 56.95 ± 2.84 after traction, respectively. Meanwhile, the AF T2 values for Pfirrmann grades II to IV were 46.19 ± 3.69, 42.96 ± 3.84, and 40.10 ± 4.07 before traction and 48.05 ± 3.61, 42.74 ± 5.86, and 39.38 ± 5.21 after traction, respectively. The NP T2 values for Pfirrmann grades II and III increased after traction, and that for Pfirrmann grade IV slightly increased after traction. Otherwise, the AF T2 values for Pfirrmann grades II to IV did not obviously change after traction ( Figs. 1 - 5 ).
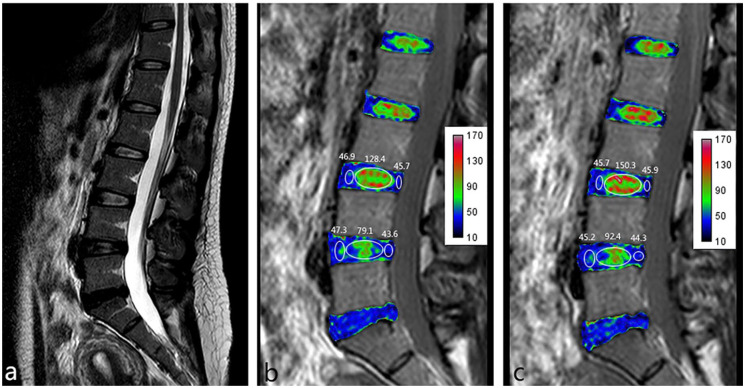
Figure 1.
Female, 35 years old, complaining of low back pain lasting for 8 months. (a) Sagittal T2 weighted image (T2WI) showing that the L3/L4 disc is classified as Pfirrmann grade II and the L4/L5 disc is classified as Pfirrmann grade III. (b) T2 mapping pretraction. (c) T2 mapping posttraction. Images b and c show that the nucleus pulposus (NP) T2 value increased visually after traction (from 128.4 to 150.3 in the L3/L4 disc and from 79.1 to 92.4 in the L4/L5 disc) but that the annulus fibrosus (AF) T2 value did not obviously change (from 46.3 to 45.8 in the L3/L4 disc and from 45.5 to 44.8 in the L4/L5 disc).
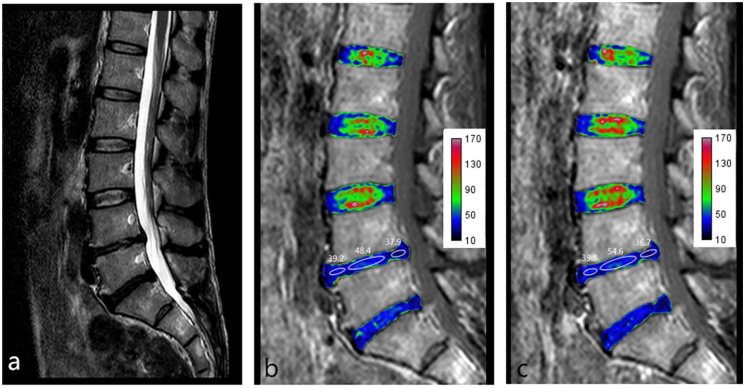
Figure 2.
Male, 47 years old, complaining of low back pain lasting for 1.5 years. (a) Sagittal T2 weighted image (T2WI) showing that the L4/L5 disc is classified as Pfirrmann grade IV. (b) T2 mapping pretraction. (c) T2 mapping posttraction. Images b and c show that the nucleus pulposus (NP) T2 value increased after traction (from 48.4 to 54.6) but that the annulus fibrosus (AF) T2 value showed no obvious change (from 38.6 to 38.3).
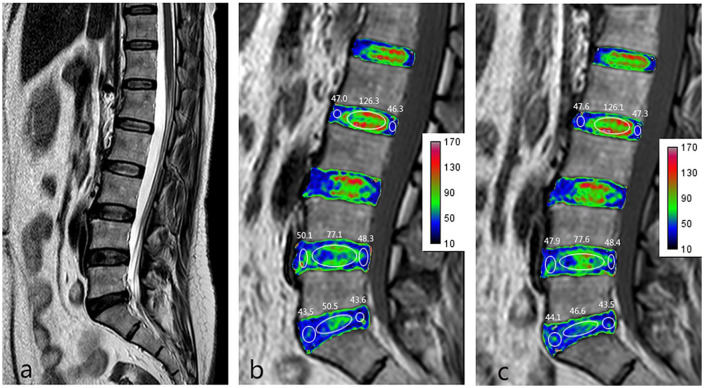
Figure 3.
Female, 39 years old, complaining of low back pain lasting for 3 years. (a) Sagittal T2 weighted image (T2WI) showing that the L2/L3 disc is classified as Pfirrmann grade II, the L4/L5 disc as Pfirrmann grade III, and the L5/S1 disc as Pfirrmann grade IV. (b) T2 mapping preintervention. (c) T2 mapping postintervention. Images b and c show no obvious changes in the nucleus pulposus (NP) T2 value for the Pfirrmann grade II to IV discs (from 126.3 to 126.1 for Pfirrmann grade II, from 77.1 to 77.6 for Pfirrmann grade III, and from 50.5 to 46.6 for Pfirrmann grade IV) or the annulus fibrosus (AF) T2 value for Pfirrmann grades II to IV (from 46.7 to 47.5 for Pfirrmann grade II, from 49.2 to 48.2 for Pfirrmann grade III, and from 43.6 to 43.8 for Pfirrmann grade IV).
Figure 4.
In the traction group, the mean nucleus pulposus (NP) T2 value for Pfirrmann grades II to IV significantly increased from pre- to posttraction (P < 0.05).
Figure 5.
In the traction group, the mean anulus fibrosus (AF) T2 value for Pfirrmann grade II significantly increased from pre- to posttraction (P < 0.05). Otherwise, changes in the mean AF T2 value for Pfirrmann grades III and IV from pre- to posttraction were not statistically significant (P > 0.05).
In the control group, the NP T2 values for Pfirrmann grades II to IV were 126.57 ± 7.83, 74.22 ± 5.83, and 46.33 ± 6.01 before intervention and 126.89 ± 8.05, 74.88 ± 6.10, and 46.30 ± 6.07 after intervention, respectively. Meanwhile, the AF T2 values for Pfirrmann grades II to IV were 49.39 ± 1.69, 44.69 ± 1.82, and 42.26 ± 1.97 before intervention and 49.12 ± 2.04, 44.83 ± 1.70, and 41.75 ± 1.75 after intervention, respectively. The NP and AF T2 values for Pfirrmann grades II to IV did not increase after 1 month.
T2 Value Analysis
In quantitative measurement of the NP and AF T2 values by T2 mapping, the interobserver agreement was high (ICC = 0.954, 95% CI = 0.943-0.966); the intraobserver agreement was also high (ICC = 0.982, 95% CI = 0.971-0.991).
In the traction group, the mean NP and AF T2 values and 95% CIs for each Pfirrmann grade pre- and posttraction are summarized in Table 1 . After traction, the changes in the NP T2 values for Pfirrmann grades II to IV and the AF T2 values for Pfirrmann grade II were statistically significant (P < 0.05).
Table 1.
The Mean T2 Value of Nucleus Pulposus (NP) and Annulus Fibrosus (AF) in Each Pfirrmann Grade Pre- and Posttraction in the Traction Group.
| NP |
AF |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mean T2 Value (95% CI) in Pretraction | Mean T2 Value (95% CI) in Posttraction | Change (Post − Pre) | P | Mean T2 Value (95% CI) in Pretraction | Mean T2 Value (95% CI) in Posttraction | Change (Post − Pre) | P | |
| Pfirrmann grade II | 125.22 (123.90-126.47) | 146.93 (145.96-147.92) | 21.71 | 0.001 | 46.19 (45.22-47.06) | 48.05 (47.16-48.92) | 1.86 | 0.001 |
| Pfirrmann grade III | 78.28 (76.46-80.02) | 89.65 (88.80-90.45) | 11.37 | 0.001 | 42.96 (41.60-44.20) | 42.74 (40.75-44.72) | −0.22 | 0.730 |
| Pfirrmann grade IV | 44.32 (42.20-46.31) | 56.95 (55.85-58.07) | 12.63 | 0.001 | 40.10 (38.48-41.68) | 39.38 (37.28-41.28) | −0.72 | 0.453 |
In the control group, the mean NP and AF T2 values and 95% CIs for each Pfirrmann grade pre- and postintervention are summarized in Table 2 . After the intervention, the changes in the NP and AF T2 values for Pfirrmann grades II to IV were not statistically significant (P > 0.05).
Table 2.
The Mean T2 value of Nucleus Pulposus (NP) and Annulus Fibrosus (AF) in Each Pfirrmann Grade Pre- and Postintervention in the Control Group.
| NP |
AF |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mean T2 Value (95% CI) in Preintervention | Mean T2 Value (95% CI) in Postintervention | Change (Post − Pre) | P | Mean T2 Value (95% CI) in Preintervention | Mean T2 Value (95% CI) in Postintervention | Change (Post − Pre) | P | |
| Pfirrmann grade II | 126.57 (124.56-128.45) | 126.89 (124.82-128.97) | 0.32 | 0.615 | 49.39 (48.95-49.85) | 49.12 (48.58-49.63) | −0.27 | 0.259 |
| Pfirrmann grade III | 74.22 (72.44-76.09) | 74.88 (73.01-76.87) | 0.66 | 0.107 | 44.69 (44.15-45.25) | 44.83 (44.27-45.37) | 0.14 | 0.680 |
| Pfirrmann grade IV | 46.33 (43.90-48.93) | 46.30 (43.91-48.90) | −0.03 | 0.956 | 42.26 (41.43-43.08) | 41.75 (41.07-42.47) | −0.51 | 0.464 |
Correlation between the T2 Value and ODI Score or VAS Score
The overall achievement of MCID for ODI score and VAS score were 58.3% (14/24) and 29.2% (7/24), separately.
In the traction group, the mean ODI score before and after traction was 43.08 ± 11.90 and 31.00 ± 14.20 (P < 0.05) and 31.12 ± 15.02 after 6 months, which has no statistical significance when compared with that after traction (P = 0.229). However, in the control group, the mean ODI score before and after the intervention was 42.83 ± 12.51 and 42.17 ± 13.46 (P = 0.267), and 43.15 ± 11.77 after 6 months, which has no statistical significance when compared with that before and after traction (P = 0.310, P = 0.278).
In the traction group, the mean VAS score before and after traction was 5.43 ± 1.67 and 3.11 ± 1.47 (P < 0.05) and 3.18±1.63 after 6 months, which has no statistical significance when compared with that after traction (P = 0.573). However, in the control group, the mean VAS score before and after the intervention was 5.46 ± 1.26 and 5.43 ± 1.35 (P = 0.769) and 5.36 ± 1.34 after 6 months, which has no statistical significance when compared with that before and after traction (P = 0.375, P = 0.646).
In the traction group, changes in the mean NP T2 value of 5 discs in each patient (16.11 ± 3.72) and in the ODI score (12.08 ± 4.59) or VAS score (2.32 ± 0.91) showed a strong correlation (r = 0.822, r = 0.793). Meanwhile, changes in the NP T2 value of the most severely affected disc at baseline in each patient (11.17 ± 7.64) and in the ODI score (12.08 ± 4.59) or VAS score (2.32 ± 0.91) showed a moderate correlation (r = 0.634, r = 0.594). Changes in the NP T2 value of the disc whose T2 value increased the most after traction in each patient (24.22 ± 4.39) and in the ODI score (12.08 ± 4.59) or VAS score (2.32 ± 0.91) also showed a moderate correlation (r = 0.477, r = 0.489).
Discussion
MRI is widely used to diagnose IVDD, and it can demonstrate changes in disc morphology. 9 This view is also supported by this study. In one study, T2 mapping was performed using a multiecho fast spin echo (FSE) sequence followed by exponential fitting, 19 and the T2 value of the disc could be obtained. To date, we have found no studies evaluating changes in the T2 value of the disc between pre- and posttraction using T2 mapping, which can indicate the water content, compared with T2WI. Our study found that T2 mapping could be used to evaluate changes in the T2 value of the lumbar disc between pre- and posttraction according to the water content of the disc; this result is consistent with those of previous studies.9,14 The results of the present study demonstrate that short-term motorized lumbar traction can increase the T2 value of the disc, especially in the NP, the changes in the mean NP T2 value of 5 discs in each patient are strong correlated with changes in the ODI score and the VAS score, and no significant progress in clinical symptoms was observed in the following 6 months.
Many fibrils (including collagen type 1 and type 2 fibrils) and proteoglycan are essential components of discs and are closely related to the water content, especially proteoglycan. The inner NP is rich in proteoglycan and water, while the outer AF is rich in collagen. 10 Several researchers have reported that the water and proteoglycan levels gradually decrease with disc degeneration.20,21 The ability to absorb water will diminish because the diffusion of material in the intervertebral disc decreases along with disc degeneration, and then cells become oxygen deficient and decline in number; furthermore, the synthesis of matrix macromolecules that have the ability to maintain the water content will be destroyed, 22 resulting in reduced signals on T2WI. The majority of studies have reported pathological or biochemical changes in discs after traction in animal experiments, suggesting that decompression or traction would increase the disc nutritional supply, promote cell proliferation, and increase extracellular matrix gene expression, which could stimulate the production of collagen and some kinds of proteoglycan, thereby improving the capacity of the disc to bind water and ability of water to flow into the disc.10,23 Studies have also shown that some discs with early or moderate degeneration could recover in terms of histological signs after decompression.10,23 Until now, only morphological changes (e.g., enlargement of the intervertebral disc space, relief of the nuclear protrusion of the disc, and disc recovery on a biological and biomechanical level) after decompression in vivo have been reported.24-26 However, changes in the T2 value of intervertebral discs after traction have not been reported. In our study, T2 mapping served as a feasible method for detecting changes in intervertebral disc components (water content) after traction in the case of no or only slight changes in morphology.
In this study, the NP T2 value increased for Pfirrmann grades II to IV after lumbar traction, as did the AF T2 value for Pfirrmann grade II, demonstrating that motorized traction could increase the T2 value of the intervertebral disc (especially in the NP). Therefore, we supposed that lumbar traction might increase the water content in the disc, and this viewpoint is consistent with that in a previous study. 27 In our study, the NP T2 value increased more after all sessions of traction for Pfirrmann grade II than those for Pfirrmann grades III and IV, so we speculated that the compensatory ability of the water molecules recovery would be better due to the early stage in which less water would lost.20-23,27 Although the exact mechanisms of lumbar traction are still controversial, it has been confirmed that this intervention could enlarge the intervertebral disc space, decrease mechanical stress on the intervertebral disc, improve circulation, and loosen facet joint adhesions.25,28 Then, water presumably diffuses from the adjacent cartilage endplate or capillaries of the vertebral body into the disc because of negative intradiscal pressure.29,30 Therefore, nutrient transportation, inflammation-mediated resorption, and metabolism in the intervertebral disc are improved. 29 In the present study, there was no significant difference in the AF T2 value between pre- and posttraction for Pfirrmann grade III or IV; one possible explanation might be the small number of subjects, and another might be that there is little water in the AF in Pfirrmann grades III and IV due to the more severe degeneration than in Pfirrmann grade II. Alternatively, 10 sessions of motorized traction could be insufficient to ensure disc recovery.
Several clinical trials have reported that traction had no effect or showed no difference when compared with sham, placebo, no treatment, or other therapies.4,6,31 Some researchers have speculated that the unsatisfactory effect of traction may be related to the multiple traction parameters, different control groups, and lack of biomechanical confirmation of the mechanism in vivo.32,33 In our study, we applied motorized traction as the traction type, which has been applied by the majority of researchers, and we set the traction force as 40% of the patient’s body weight, which not only standardized the treatment but was also accepted by the patients as an individualized plan. 24
It has been reported that LBP patients experience approximately 63% relief after continuous lumbar traction 25 ; in addition, decompression therapy and segmental traction therapy could obviously decrease the pain intensity, improve the lumbar range of motion and lead to an approximately 40% reduction in the VAS score.29,30,34 Nevertheless, Aybala Koçak 35 reported that the ODI score of patients with LBP decreased after traction but showed no significant difference from the baseline value. This inconsistency might be explained by the interval between the increased water content and decreased inflammatory mediator levels. 36 In our study, overall, the patients’ symptoms improved after 10 sessions of traction, and the mean ODI score and VAS score reduction were 12.08 and 2.32, respectively. Furthermore, the ODI score of more than half of the subjects achieved MCID and the VAS score more than a quarter. Meanwhile, in this study, we found that changes in the mean NP T2 value of 5 discs in each patient were highly correlated with changes in the ODI score and the VAS score, with a correlation coefficient of 0.822 and 0.793, respectively. Therefore, we speculate that the patients’ LBP, in some way, might be caused by the combined effect of all lumbar intervertebral discs and that the improvement in clinical symptoms is correlated with microstructural changes in all discs.
The limitations of this study are as the following. First is the small number of subjects (especially subjects with Pfirrmann grade V discs). Second is the lack of MRI examination in the following 6 months and the long-term follow-up data. In future work, more patients, more traction sessions and measurement of the T2 values of discs at several fixed time points are needed to explore changes in the disc water content under long-term lumbar traction therapy.
In conclusion, T2 mapping can be used to evaluate changes in the intervertebral disc water content after lumbar traction in patients with chronic LBP. Short-term traction resulted in a significant increase in the NP T2 value for Pfirrmann grades II to IV and a remission of the patients’ clinical symptoms in the following 6 months. Changes in the mean NP T2 value of 5 discs in each patient were strongly correlated with changes in the ODI score and the VAS score
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the National Natural Science Foundation of China (No. 81801757), the Natural Science Foundation of Guangdong Province (No. 2018A030310322), Guangdong Basic and Applied Basic Research Foundation (No. 2019A1515012051), and the Guangdong Medical Research Foundation (No. A2018106).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by the Institutional Research Ethics Committee of the Third Affiliated Hospital of Sun Yat-Sen University (nos. [2018]02 and [2018]03).
Informed Consent: Written informed consent was obtained from all study participants.
Trial Registration: Not applicable.
ORCID iD: Zhen-zhen Liu  https://orcid.org/0000-0002-8018-7260
https://orcid.org/0000-0002-8018-7260
References
- 1. Wenger HC, Cifu AS. Treatment of low back pain. JAMA. 2017;318:743-4. [DOI] [PubMed] [Google Scholar]
- 2. Niu T, Lv C, Yi G, Tang H, Gong C, Niu S. Therapeutic effect of medical ozone on lumbar disc herniation. Med Sci Monit. 2018;24:1962-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Madson TJ, Hollman JH. Lumbar traction for managing low back pain: a survey of physical therapists in the United States. J Orthop Sports Phys Ther. 2015;45:586-95. [DOI] [PubMed] [Google Scholar]
- 4. Macario A, Pergolizzi JV. Systematic literature review of spinal decompression via motorized traction for chronic discogenic low back pain. Pain Pract. 2006;6:171-8. [DOI] [PubMed] [Google Scholar]
- 5. Wegner I, Widyahening IS, van Tulder MW, Blomberg SE, de Vet HC, Brønfort G, et al. Traction for low-back pain with or without sciatica. Cochrane Database Syst Rev. 2013;2013(8):CD003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beurskens AJ, de Vet HC, Koke AJ, Lindeman E, Regtop W, van der Heijden GJ, et al. Efficacy of traction for non-specific low back pain: a randomised clinical trial. Lancet. 1995;346:1596-600. [DOI] [PubMed] [Google Scholar]
- 7. Fritz JM, Thackeray A, Childs JD, Brennan GP. A randomized clinical trial of the effectiveness of mechanical traction for sub-groups of patients with low back pain: study methods and rationale. BMC Musculoskelet Disord. 2010;11:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng Y, Hsu C, Lin Y. The effect of mechanical traction on low back pain in patients with herniated intervertebral disks: a systemic review and meta-analysis. Clin Rehabil. 2020;34:13-22. [DOI] [PubMed] [Google Scholar]
- 9. Yoon MA, Hong SJ, Kang CH, Ahn KS, Kim BH. T1rho and T2 mapping of lumbar intervertebral disc: correlation with degeneration and morphologic changes in different disc regions. Magn Reson Imaging. 2016;34:932-9. [DOI] [PubMed] [Google Scholar]
- 10. Guehring T, Omlor GW, Lorenz H, Engelleiter K, Richter W, Carstens C, et al. Disc distraction shows evidence of regenerative potential in degenerated intervertebral discs as evaluated by protein expression, magnetic resonance imaging, and messenger ribonucleic acid expression analysis. Spine (Phila Pa 1976). 2006;31:1658-65. [DOI] [PubMed] [Google Scholar]
- 11. Chow D, Yuen E, Xiao L, Leung M. Mechanical effects of traction on lumbar intervertebral discs: a magnetic resonance imaging study. Musculoskelet Sci Pract. 2017;29:78-83. [DOI] [PubMed] [Google Scholar]
- 12. Cleland JA, Whitman JM, Fritz JM, Palmer JA. Manual physical therapy, cervical traction, and strengthening exercises in patients with cervical radiculopathy: a case series. J Orthop Sports Phys Ther. 2005;35:802-11. [DOI] [PubMed] [Google Scholar]
- 13. Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976). 2001;26:1873-8. [DOI] [PubMed] [Google Scholar]
- 14. Menezes-Reis R, Salmon CEG, Carvalho CS, Bonugli GP, Chung CB, Nogueira-Barbosa MH. T1ρ and T2 mapping of the intervertebral disk: comparison of different methods of segmentation. Am J Neuroradiol. 2015;36:606-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vianin M. Psychometric properties and clinical usefulness of the Oswestry Disability Index. J Chiropr Med. 2008;7:161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murphy HA, Warnick E, McEntee R, Nicholson K, Hollern DA, Stawicki C, et al. Which domains of the ODI best predict change in physical function in patients after surgery for degenerative lumbar spondylolisthesis? Spine (Phila Pa 1976). 2018;43:805-12. [DOI] [PubMed] [Google Scholar]
- 17. Van Der Roer N, Ostelo RWJG, Bekkering GE, Van Tulder MW, De Vet HCW. Minimal clinically important change for pain intensity, functional status, and general health status in patients with nonspecific low back pain. Spine (Phila Pa 1976). 2006;31(5):578-82. [DOI] [PubMed] [Google Scholar]
- 18. Seuk J, Bae J, Shin S, Lee S. Long-term minimum clinically important difference in health-related quality of life scores after instrumented lumbar interbody fusion for low-grade isthmic spondylolisthesis. World Neurosurg. 2018;117:e493-e499. [DOI] [PubMed] [Google Scholar]
- 19. Bojorquez JZ, Bricq S, Acquitter C, Brunotte F, Walker PM, Lalande A. What are normal relaxation times of tissues at 3 T? Magn Reson Imaging. 2017;35:69-80. [DOI] [PubMed] [Google Scholar]
- 20. Stelzeneder D, Welsch GH, Kovács BK, Goed S, Paternostro-Sluga T, Vlychou M, et al. Quantitative T2 evaluation at 3.0T compared to morphological grading of the lumbar intervertebral disc: a standardized evaluation approach in patients with low back pain. Eur J Radiol. 2012;81:324-30. [DOI] [PubMed] [Google Scholar]
- 21. Hwang D, Kim S, Abeydeera NA, Statum S, Masuda K, Chung CB, et al. Quantitative magnetic resonance imaging of the lumbar intervertebral discs. Quant Imaging Med Surg. 2016;6:744-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mitchell UH, Beattie PF, Bowden J, Larson R, Wang H. Age-related differences in the response of the L5-S1 intervertebral disc to spinal traction. Musculoskel Sci Prac. 2017; 31:1-8. [DOI] [PubMed] [Google Scholar]
- 23. Kuo Y, Hsu Y, Chuang I, Chao PG, Wang J. Spinal traction promotes molecular transportation in a simulated degenerative intervertebral disc model. Spine (Phila Pa 1976). 2014;39:E550-E556. [DOI] [PubMed] [Google Scholar]
- 24. Chung TS, Yang HE, Ahn SJ, Park JH. Herniated lumbar disks: real-time MR imaging evaluation during continuous traction. Radiology. 2015;275:755-62. [DOI] [PubMed] [Google Scholar]
- 25. Ozturk B, Gunduz OH, Ozoran K, Bostanoglu S. Effect of continuous lumbar traction on the size of herniated disc material in lumbar disc herniation. Rheumatol Int. 2006;26:622-6. [DOI] [PubMed] [Google Scholar]
- 26. Chung TS, Lee YJ, Kang SW, Park CJ, Kang WS, Shim YW. Reducibility of cervical disk herniation: evaluation at MR imaging during cervical traction with a nonmagnetic traction device. Radiology. 2002;225:895-900. [DOI] [PubMed] [Google Scholar]
- 27. Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine (Phila Pa 1976). 2004;29:2700-9. [DOI] [PubMed] [Google Scholar]
- 28. Chung CT, Tsai SW, Chen CJ, Wu TC, Wang D, Lan HCH, et al. Comparison of the intervertebral disc spaces between axial and anterior lean cervical traction. Eur Spine J. 2009;18:1669-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Choi J, Lee S, Hwangbo G. Influences of spinal decompression therapy and general traction therapy on the pain, disability, and straight leg raising of patients with intervertebral disc herniation. J Phys Ther Sci. 2015;27:481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Isner-Horobeti ME, Dufour SP, Schaeffer M, Sauleau E, Vautravers P, Lecocq J, et al. High-force versus low-force lumbar traction in acute lumbar sciatica due to disc herniation: a preliminary randomized trial. J Manipulative Physiol Ther. 2016;39:645-54. [DOI] [PubMed] [Google Scholar]
- 31. Thackeray A, Fritz JM, Childs JD, Brennan GP. The effectiveness of mechanical traction among subgroups of patients with low back pain and leg pain: a randomized trial. J Orthop Sports Phys Ther. 2016;3:144-54. [DOI] [PubMed] [Google Scholar]
- 32. Tadano S, Tanabe H, Arai S, Fujino K, Doi T, Akai M. Lumbar mechanical traction: a biomechanical assessment of change at the lumbar spine. BMC Musculoskel Dis. 2019;20(1_suppl):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alrwaily M, Almutiri M, Schneider M. Assessment of variability in traction interventions for patients with low back pain: a systematic review. Chiropr Man Therap. 2018;26:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karimi N, Akbarov P, Rahnama L. Effects of segmental traction therapy on lumbar disc herniation in patients with acute low back pain measured by magnetic resonance imaging: a single arm clinical trial. J Back Musculoskelet Rehabil. 2017;30:247-53. [DOI] [PubMed] [Google Scholar]
- 35. Aybala Koçak F. Comparison of the short-term effects of the conventional motorized traction with non-surgical spinal decompression performed with a DRX9000TM device on pain, functionality, depression, and quality of life in patients with low back pain associated with lumbar disc herniation: a single-blind randomized-controlled trial. Turk J Phys Med Rehab. 2017;64:17-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Modic MT, Obuchowski NA, Ross JS, Brant-Zawadzki MN, Grooff PN, Mazanec DJ, et al. Acute low back pain and radiculopathy: MR imaging findings and their prognostic role and effect on outcome. Radiology. 2005;237:597-604. [DOI] [PubMed] [Google Scholar]