Abstract
Objectives
The aim of this study was to detect levels of common lipid species in serum and synovial fluid (SF) of primary knee osteoarthritis (OA) patients and investigate their correlations with disease severity.
Materials and Methods
The study enrolled 184 OA patients receiving arthroscopic debridement or total knee arthroplasty and 180 healthy controls between April 2012 and March 2018. Total triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein A1 (ApoA1), and apolipoprotein B (ApoB) levels were analyzed in serum and SF of OA patients, and in serum of healthy individuals. The Noyes rating criteria, Kellgren-Lawrence (KL) grading system, and Western Ontario McMaster University Osteoarthritis Index (WOMAC) scores were, respectively, used to assess cartilage damage, radiographic severity, and symptomatic severity of OA.
Results
No significant differences were found in serum TG and ApoB levels between the 2 groups, while OA patients had higher TC and LDL-C levels and lower HDL-C and ApoA1 levels (P < 0.05). Pearson correlation analysis revealed SF HDL-C and ApoA1 levels were negatively correlated with cartilage damage scores, KL grades as well as WOMAC scores (P < 0.05), which were still significant after adjusting for confounding factors (P < 0.05). Receiver operating characteristic curve analysis revealed SF HDL-C (area under the curve [AUC]: 0.816) and ApoA1 (AUC: 0.793) were also good predictors of advanced-stage OA (P < 0.001).
Conclusion
SF HDL-C and ApoA1 levels were negatively correlated with cartilage damage, radiographic severity, and symptomatic severity of primary knee OA, emerging as potential biomarkers for radiographic advanced-stage OA, which may serve as predictors of disease severity.
Keywords: osteoarthritis, synovial fluid, predictor, apolipoprotein A1, lipid metabolism
Introduction
Primary knee osteoarthritis (OA) is the most prevalent degenerative joint disease in aging population, which is characterized by articular cartilage degradation, joint space narrowing, subchondral changes, and osteophyte formation. Patients with OA may suffer from pain, stiffness, swelling, and restricted motion of involved joints.1,2 It is estimated that over 30 million people suffer from OA in the United States, and this number could increase to 78 million by 2040. 3 The increased OA morbidity calls for effective methods to evaluate and predict severity of this disease.
In recent years, there have been efforts to identify biochemical markers of OA, which represent a noninvasive method to detect early changes and assess disease severity. However, it is believed that OA has multifactorial aetiology. Aging, mechanical stress, obesity, inflammation, and genetic predisposition may contribute to onset of this disease.4,5 Accumulating evidence indicates that altered lipid metabolism plays a vital role in joint degeneration, leading to a novel approach to monitor disease progression.6,7 An animal study showed that conditional knockout mice with low high-density lipoprotein cholesterol (HDL-C) and high low-density lipoprotein cholesterol (LDL-C) levels had increased ectopic bone formation and possibility of progression to knee OA. 8 There were other studies demonstrating plasma leptin level was higher in OA patients with metabolic syndrome, which might have pro-inflammatory and pro-degradative effects on joint tissues.9-11 In the joint cavity, synovial fluid (SF) can provide nutrition and lubrication to the articular cartilage, which may accumulate in a pathologic joint, reflecting the ongoing process of some disorders. 12 There was a study showing the greater SF adiponectin/leptin ratio was associated with less pain of patients with knee OA. 13
Despite previous studies have extended our knowledge about links between various lipid species and OA, there exists a paucity of research directly on relationships between lipids and lipoproteins levels with disease severity, especially their concentrations in SF. We thus investigated 6 most common species of lipid metabolism in human body, namely total triglycerides (TG), total cholesterol (TC), HDL-C, LDL-C, apolipoprotein A1 (ApoA1), and apolipoprotein B (ApoB). The goal of this study was to investigate the relationship between aforementioned parameters in serum and SF with the cartilage damage, radiographic, symptomatic severity of primary knee osteoarthritis, as well as examine potential biomarkers for predicting disease severity.
Materials and Methods
Ethics Approval
This study was reviewed and approved by the institutional review board of Nanjing First Hospital (permit number: 2012581A). A written informed consent was obtained from each participant. We also obtained consent to publication of their medical data, including medical records, photographs, and radiographic images. The study was carried out in accordance with the World Medical Association’s Declaration of Helsinki.
Study Population
A total of 184 patients with primary knee OA who underwent arthroscopic irrigation and debridement or total knee arthroplasty from April 2012 to March 2018 at the Department of Orthopaedics, Nanjing First Hospital were enrolled in the present study. These patients all met the American College of Rheumatology Clinical symptomatic and radiographic criteria for OA. 14 We excluded patients with rheumatoid arthritis or secondary osteoarthritis, previous knee injury or surgery, previous knee injection, knee pain of unknown cause, systemic inflammatory or autoimmune disorders, advanced liver or renal diseases, and known malignant disease. A total of 180 gender- and age-matched individuals receiving routine staff physical check-up arranged by their companies or factories at the Department of Physical Examination in the same hospital during the same period were selected as healthy controls. The healthy participants were excluded if they suffered from OA-related symptoms and radiographic changes, inflammatory or rheumatoid disorders, progressive liver and renal disease, hemochromatosis, cancer, and use of anti-inflammatory drugs or corticosteroids during the past 6 months.
Serum and Synovial Fluid Analyses
All operations for OA patients were performed in unilateral knee joint by a senior surgeon (JG). Fasting venous blood samples were collected from all OA patients preoperatively and healthy controls. After centrifugation for 15 minutes (at 1500 × g), the serum samples were kept at −80°C until analysis. Due to ethical reasons, SF samples were not collected from healthy controls. For all OA patients, SF samples were taken from the affected knee joint cavity with a 10-mL sterile syringe at the beginning of the operation. The obtained SF samples were centrifuged for 10 minutes (at 2500 × g) to remove cells and joint debris and stored at −80°C until analysis.
Quantitative analysis of TG, TC, HDL-C, LDL-C, ApoA1, and ApoB from serum and SF was performed in the biochemistry laboratory of our hospital using a Synchron AU5800 Automatic Analyzer (Beckman-Coulter, Brea, CA, USA). If the SF lipid values were below detectable limits, the samples were concentrated using a Microcon YM-10 centrifuge filter device (Amicon) and the values rerun.
Cartilage Damage Severity Assessment
The cartilage damage severity of OA was assessed by 2 independent observers according to the Noyes method, 15 which evaluated the depth and area of the cartilage defect. The maximal diameter of the osteochondral defect is graded as follows: 0 point = absent; 1 point = minimal (<10 mm); 2 points = moderate (10-15 mm); 3 points = severe (>15 mm). The depth of the cartilage defect is graded as follows: 0 point = normal morphology; 1 point = minimal (less than 50% thickness); 2 points = moderate (more than 50% thickness); 3 points = severe (bone exposure). The score of cartilage damage was then calculated by summation. The knee is divided into 6 anatomical regions: medial femur, lateral femur, medial tibia, lateral tibia, patella, and femoral sulcus. The sum of scores for each region was used as the final score for cartilage damage severity.
Radiographic Severity Assessment
The standing anteroposterior radiographs of the affected knee of all OA patients were obtained before surgery. The radiographic severity of OA was assessed by the Kellgren-Lawrence (KL) grading system, 16 which consists of 5 grade scales: grade 0, no observable radiological changes; grade 1, doubtful joint space narrowing and possible osteophyte lipping; grade 2, definite osteophytes and possible joint space narrowing; grade 3, multiple moderate osteophytes, definite joint space narrowing, sclerosis with possible deformity of bone contour; grade 4, large osteophytes, significant joint space narrowing, severe sclerosis and definite deformity of bone contour. For patients with bilateral knee OA, the higher scale of the 2 knees was used for grading OA severity. The radiographic assessments were performed by 2 independent experienced radiologists who were blinded to selection of subjects.
Symptomatic Severity Assessment
The symptomatic severity of OA was assessed by the Western Ontario McMaster University Osteoarthritis Index (WOMAC), which consists of pain, stiffness, and physical function subscales. 17 The WOMAC scores are between 0 to 100, and the higher scores are corresponding to more restricted function/greater pain. The symptomatic assessments were performed by 2 independent observers who were blinded to selection of subjects.
Statistical Analysis
The power analysis was performed using Fisher’s exact test, 2-tailed with G*Power statistical software for Windows (Version 3.1.5, Franz Faul, Universität Kiel, Germany). Based on data from previous studies about the effect of hyperlipidemia on primary OA,18,19 the number of participants necessary to realize a minimum of 80% power was 162 per group.
Considering the potential effect of inflammation on serum total protein abundance, we used the Limma package of R software (Version 3.3.1, https://www.r-project.org/) to perform adjustments of comparisons of serum lipids levels between the 2 groups by a linear mixed effect model. 20 Benjamini-Hochberg method was used to adjust the P values to control the false discovery rate. 21
Statistical analysis was performed using SPSS for windows (Version 19.0, IBM Corp., Armonk, NY, USA). The continuous data were expressed as mean ± standard deviation (mean ± SD). Normality was tested using the Kolmogorov-Smirnov test. Differences between the 2 groups were analyzed using unpaired Student t test or chi-square test. Differences among KL grade subgroups were analyzed using 1-way analysis of variance (ANOVA). The correlation of serum or SF lipid levels with WOMAC scores, cartilage damage scores and KL grades were determined by Pearson correlation analysis. Possible independent relationship between variables and WOMAC scores was determined by multivariate linear regression. The independent variables entered into the model were age, gender, body mass index (BMI), and possible relevant serum or SF lipid species according to Pearson correlation analysis. Beta coefficients and 95% confidence intervals (CIs) were reported for the linear regression model. A receiver operating characteristic (ROC) curve analysis was conducted to examine prognostic ability of potential biomarkers for discriminating OA patients with advanced-stage from those with early-stage by calculating the areas under the curve (AUC). Statistical significance was accepted for P values less than 0.05.
Results
Baseline Clinical Characteristics
A total of 184 primary knee OA patients consisting of 76 men and 108 women with the mean age of 62.5 ± 6.3 years (range, 50-76 years) and 180 healthy individuals consisting of 75 men and 105 women with the mean age of 61.4 ± 7.1 years (range, 49-73 years) were enrolled in the present study. There were no significant differences in age, gender, and BMI between OA patients and healthy controls (P > 0.05 for all parameters).
TC and LDL-C levels in serum of OA patients were higher than those of matched controls (P = 0.026 and 0.012, respectively), while HDL-C and ApoA1 levels were markedly lower (P = 0.031 and 0.039, respectively). No significant differences were found in serum TG and ApoB levels between OA patients and healthy controls (P > 0.05 for both variables). In OA patients, TG, TC, HDL-C, LDL-C, ApoA1, and ApoB levels in SF were all significantly lower than those in paired serum samples (P < 0.05 for all variables). The baseline clinical characteristics of the study population are summarized in Table 1 .
Table 1.
Baseline Clinical Characteristics and Serum and SF Lipids Levels of OA Patients and Healthy Controls. a
| Characteristic | OA Group (n = 184) | Healthy Group (n = 180) | P b |
|---|---|---|---|
| Age (years) | 62.50 ± 6.30 | 61.4 ± 7.1 | 0.214 |
| Gender (female/male) | 108/76 | 105/75 | 0.769 |
| BMI (kg/m2) | 25.20 ± 1.90 | 24.9 ± 2.1 | 0.352 |
| TG in serum (g/L) | 0.82 ± 0.15 | 0.79 ± 0.16 | 0.307 |
| TC in serum (g/L) | 2.26 ± 0.57 | 1.99 ± 0.45 | 0.026 |
| HDL-C in serum (g/L) | 0.43 ± 0.12 | 0.52 ± 0.13 | 0.031 |
| LDL-C in serum (g/L) | 1.51 ± 0.29 | 1.14 ± 0.22 | 0.012 |
| ApoA1 in serum (g/L) | 1.67 ± 0.32 | 1.95 ± 0.43 | 0.039 |
| ApoB in serum (g/L) | 0.93 ± 0.25 | 0.99 ± 0.27 | 0.161 |
| TG in SF (g/L) | 0.19 ± 0.05 | — | — |
| TC in SF (g/L) | 0.72 ± 0.15 | — | — |
| HDL-C in SF (g/L) | 0.16 ± 0.06 | — | — |
| LDL-C in SF (g/L) | 0.45 ± 0.16 | — | — |
| ApoA1 in SF (g/L) | 0.58 ± 0.17 | — | — |
| ApoB in SF (g/L) | 0.26 ± 0.09 | — | — |
BMI, body mass index; TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SF, synovial fluid; OA, osteoarthritis; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B.
The data are presented as mean ± standard deviation (mean ± SD).
Unpaired Student t test or chi-square test. The P values shown are for intergroup comparisons. Benjamini-Hochberg adjusted P values for intergroup comparisons of TG, TC, HDL-C, LDL-C, ApoA1, and ApoB in serum. Significance was accepted for P values <0.05.
Serum and SF Lipids Levels and Cartilage Damage Severity
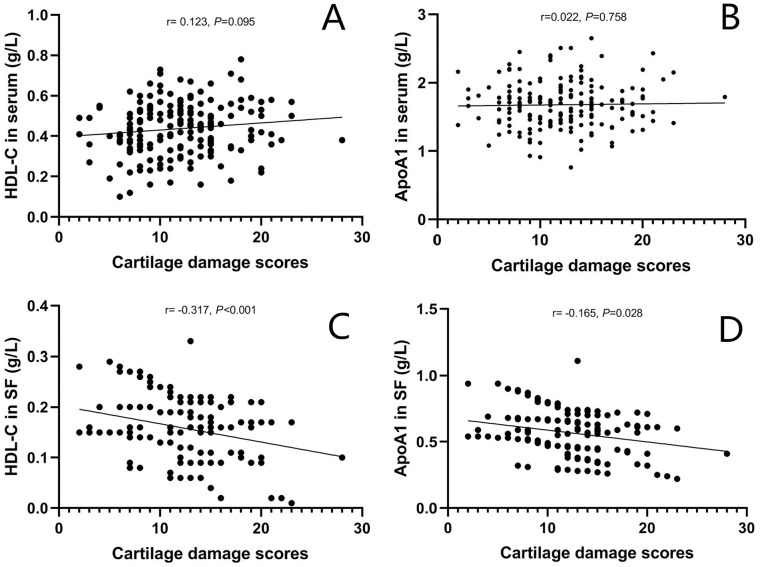
The mean cartilage damage score of OA patients was 12.4 ± 4.9. There were no significant associations between serum TG, TC, HDL-C, LDL-C, ApoA1, and ApoB as well as SF TG, TC, LDL-C, and ApoB levels and cartilage damage scores (P > 0.05 for all variables). SF HDL-C (r = −0.317, P < 0.001) and ApoA1 levels (r = −0.165, P = 0.028) were negatively correlated with cartilage damage scores. The scattergrams of correlations of HDL-C and ApoA1 levels in serum and SF with cartilage damage scores in OA patients are shown in Fig. 1.
Figure 1.
Scattergrams showing correlations of HDL-C and ApoA1 levels in serum (A and B) and SF (C and D) with cartilage damage severity according to Noyes rating criteria in OA patients. HDL-C, high-density lipoprotein cholesterol; ApoA1, apolipoprotein A1; SF, synovial fluid; OA, osteoarthritis.
Serum and SF Lipids Levels and Radiographic Severity
All OA patients were classified into 3 groups according to KL grading criteria. A total of 57 patients were KL grade 2, 86 patients were KL grade 3, and 41 patients were KL grade 4. The serum and SF lipids levels in different KL grade subgroups of OA patients are summarized in Table 2 .
Table 2.
Serum and SF Lipids Levels in Different KL Grade Subgroups of OA Patients. a
| Variables (g/L) | KL Grade 2 (n = 57) | KL Grade 3 (n = 86) | KL Grade 4 (n = 41) | P b |
|---|---|---|---|---|
| TG in serum | 0.84 ± 0.16 | 0.81 ± 0.15 | 0.82 ± 0.13 | 0.396 |
| TC in serum | 2.28 ± 0.53 | 2.27 ± 0.57 | 2.25 ± 0.58 | 0.305 |
| HDL-C in serum | 0.45 ± 0.11 | 0.42 ± 0.13 | 0.44 ± 0.14 | 0.227 |
| LDL-C in serum | 1.49 ± 0.29 | 1.52 ± 0.28 | 1.54 ± 0.30 | 0.138 |
| ApoA1 in serum | 1.65 ± 0.34 | 1.61 ± 0.37 | 1.59 ± 0.31 | 0.285 |
| ApoB in serum | 0.95 ± 0.20 | 0.91 ± 0.23 | 0.94 ± 0.26 | 0.176 |
| TG in SF | 0.21 ± 0.05 | 0.19 ± 0.04 | 0.18 ± 0.04 | 0.129 |
| TC in SF | 0.69 ± 0.15 | 0.74 ± 0.13 | 0.70 ± 0.16 | 0.243 |
| HDL-C in SF | 0.24 ± 0.09 | 0.14 ± 0.07 | 0.10 ± 0.03 | 0.003 |
| LDL-C in SF | 0.46 ± 0.14 | 0.45 ± 0.18 | 0.43 ± 0.16 | 0.316 |
| ApoA1 in SF | 0.63 ± 0.17 | 0.52 ± 0.16 | 0.39 ± 0.12 | 0.014 |
| ApoB in SF | 0.24 ± 0.09 | 0.27 ± 0.09 | 0.28 ± 0.08 | 0.152 |
SF, synovial fluid; OA, osteoarthritis; KL, Kellgren-Lawrence; TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B.
The data are presented as mean ± standard deviation (mean ± SD).
One-way analysis of variance (ANOVA). The P values shown are for intergroup comparisons. Significance was accepted for P values <0.05.
There were no significant differences in serum TG, TC, HDL-C, LDL-C, ApoA1, and ApoB levels as well as in SF TG, TC, LDL-C and ApoB levels between 3 KL grade subgroups (P > 0.05 for all variables). OA patients with higher KL grade had remarkably lower SF HDL-C (F = 10.354, P = 0.009) and ApoA1 levels (F = 8.627, P = 0.014). Pearson correlation analysis revealed that SF HDL-C (r = −0.419, P < 0.001) and ApoA1 levels (r = −0.174, P = 0.022) were negatively correlated with the radiographic severity of OA.
Serum and SF Lipids Levels and Symptomatic Severity
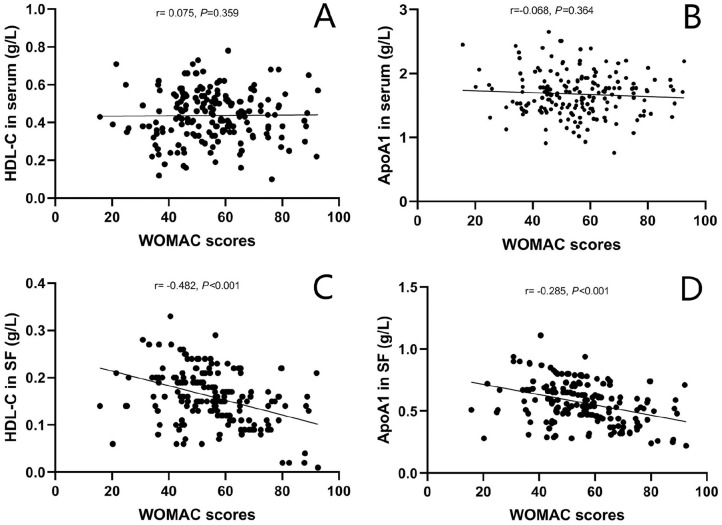
The mean WOMAC score of OA patients was 54.3 ± 15.1. No significant associations were found between serum TG, TC, HDL-C, LDL-C, ApoA1, ApoB as well as SF TG, TC, LDL-C, ApoB levels and WOMAC scores (P > 0.05 for all variables). Negative relationships were found between SF HDL-C (r = −0.482, P < 0.001) and ApoA1 levels (r = −0.285, P < 0.001) with WOMAC scores. The scattergrams of correlations of HDL-C and ApoA1 levels in serum and SF with WOMAC scores in OA patients are shown in Fig. 2 . Intra- and interobserver agreement for cartilage damage, radiographic severity, and symptomatic severity assessment outcomes are shown in Table 3 . The intraclass correlation coefficient (ICC) values for intra- and interobserver reliability of cartilage damage score, KL grade, and WOMAC score were all good.
Figure 2.
Scattergrams showing correlations of HDL-C and ApoA1 levels in serum (A and B) and SF (C and D) with symptomatic severity assessed by WOMAC scores in OA patients. HDL-C, high-density lipoprotein cholesterol; ApoA1, apolipoprotein A1; SF, synovial fluid; WOMAC, Western Ontario McMaster University Osteoarthritis Index; OA, osteoarthritis.
Table 3.
Intra- and Interobserver Agreement for Cartilage Damage, Radiographic Severity, and Symptomatic Severity Assessment Outcomes.
| Variables | ICC (95% CI) | |
|---|---|---|
| Intraobserver | Interobserver | |
| Cartilage damage score | 0.869 (0.805, 0.932) | 0.882 (0.817, 0.945) |
| KL grade | 0.847 (0.772, 0.904) | 0.915 (0.863, 0.952) |
| WOMAC score | 0.835 (0.769, 0.893) | 0.791 (0.752, 0.849) |
ICC, intraclass correlation coefficient; CI, confidence interval; KL, Kellgren-Lawrence; WOMAC, Western Ontario McMaster University Osteoarthritis Index.
We further performed the multivariate linear regression to ascertain independent associations between SF HDL-C and ApoA1 levels and WOMAC scores in OA patients. As shown in Table 4 , the correlations between SF HDL-C and ApoA1 levels with WOMAC scores were still significant after adjusting for confounding factors by age, gender, BMI, serum HDL-C, and ApoA1 (β = 0.279, 95% CI = 0.145-0.391, P < 0.001 for HDL-C; β = 0.217, 95% CI = 0.116-0.308, P = 0.002 for ApoA1).
Table 4.
Multivariate Linear Regression for Association Between Variables and WOMAC Scores.
| Variables | β (95% CI) | P a |
|---|---|---|
| Age | 0.046 (0.003, 0.128) | 0.345 |
| Gender | 0.018 (0.001, 0.057) | 0.502 |
| BMI | 0.074 (0.026, 0.114) | 0.191 |
| HDL-C in serum | 0.008 (-0.013, 0.025) | 0.783 |
| HDL-C in SF | 0.279 (0.145, 0.391) | <0.001 |
| ApoA1 in serum | 0.051 (0.009, 0.134) | 0.306 |
| ApoA1 in SF | 0.217 (0.116, 0.308) | 0.002 |
CI, confidence interval; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; SF, synovial fluid; ApoA1, apolipoprotein A1; WOMAC, Western Ontario McMaster University Osteoarthritis Index.
Significance was accepted for P values <0.05.
SF HDL-C and ApoA1 as Potential Biomarkers for OA Severity
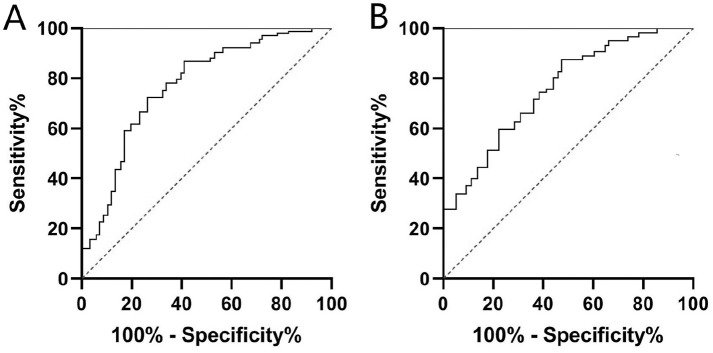
We assessed the feasibility of using SF HDL-C and ApoA1 as potential predictors for differentiating OA patients with advanced stage (KL grade 3 or 4) from early stage (KL grade 2) by ROC curve analysis. As seen in Fig. 3 , SF HDL-C (AUC, 0.816; 95% CI, 0.729-0.871; P < 0.001) and ApoA1 (AUC, 0.793; 95% CI, 0.695-0.862; P < 0.001) were also good predictors of advanced-stage OA. The optimal cutoff values were 0.18 g/L for HDL-C (sensitivity, 76.3%; specificity, 74.1%) and 0.57 g/L for ApoA1 (sensitivity, 75.9%; specificity, 72.8%) respectively.
Figure 3.
ROC curve analysis of SF HDL-C (A) and ApoA1 (B) as diagnostic biomarkers for differentiating OA patients with advanced stage (KL grade 3 or 4) from early stage (KL grade 2). ROC, receiver operating characteristic; SF, synovial fluid; HDL-C, high-density lipoprotein cholesterol; ApoA1, apolipoprotein A1; OA, osteoarthritis; KL, Kellgren-Lawrence.
Discussion
We performed this study with the goal of recognizing potential lipid biomarkers in serum or SF related with severity of primary knee osteoarthritis. The present study demonstrated for the first time that SF HDL-C and ApoA1 levels, but not serum levels, were independently associated with cartilage damage degree, radiographic severity, as well as symptomatic severity of primary knee OA. Besides, SF HDL-C and ApoA1 both had excellent diagnostic value for predicting advanced-stage OA. These results indicated that HDL-C and ApoA1 in SF might play important roles in the pathogenesis of knee OA and serve as novel biochemical markers for assessing disease severity.
As is known to all, HDL-C is a vital constituent of the lipoprotein transport system, regulating lipid metabolism and homeostasis. It also has an anti-inflammatory effect by inhibiting production of pro-inflammatory cytokines. 22 A previous epidemiological study showed serum HDL-C level was decreased in OA patients compared with non-OA individuals. 23 Garcia-Gil et al. 24 examined serum lipid levels in 277 patients with hand OA, finding higher HDL levels was associated with lower risk of OA. ApoA1, the major constituent of HDL with ability of reversing cholesterol transport, is traditionally considered to play a protective role against cardiovascular disease. 25 The cholesterol influx genes including that encoding ApoA1 were downregulated in human osteoarthritic chondrocytes according to the gene expression research. 26 Another recent clinical study of 16 OA patients and 33 healthy individuals demonstrated that serum ApoA1 levels of OA patients markedly decreased. 19 We found serum HDL-C and ApoA1 levels of OA patients were markedly lower than matched controls, which was in concordance with previous reports.
Because the joint is bathed in SF, changes to SF composition may directly contribute to joint pathology, offering a unique opportunity to find potential effective biomarkers. A previous animal study of ovine confirmed 6 significantly altered metabolites in SF of knee joint as early OA biomarkers, namely isobutyrate, glucose, uridine, serine, asparagine, and hydroxyproline. 27 Metabolomic analysis had uncovered a variety of metabolites altered in SF of OA patients compared to healthy controls, for example, amino acid derivatives and lipoprotein lipase, which also showed promise in disease severity evaluation as predictive markers. 28 Our report may be a latest study, finding that SF HDL-C and ApoA1 levels in OA patients showed negative correlations with KL grades, WOMAC scores and cartilage damage scores. It is believed that SF is the synovial membrane–produced ultrafiltrate of blood plasma, and factors in SF accumulate by releasing from local tissues or flowing through the synovial barrier. 29 Previous studies indicated that local adipose tissues could influence cartilage metabolism through the release of cytokines, such as fatty acids, fatty acids–derived lipid mediators, and adipocytokines.30,31 Based on our findings that similar cross-associations between SF lipid levels and clinical parameters were not observed in corresponding OA blood samples, we hypothesized that HDL-C and ApoA1 in SF were possibly mostly derived from local tissues, probably the adipose tissue, and participated in the pathologic process within the articular joint. However, the accurate sources need to be further verified in future well-designed basic medical research.
It is well acknowledged that OA is a heterogeneous disease, which is recently characterized as a metabolic syndrome disorder having close connections to dyslipidemia. Previous research reported that levels of total fatty acids and arachidonic acid were significantly elevated in OA cartilage and associated with increasing histological severity. 32 The chondrocyte lipid peroxidation had also been found closely linked with cartilage matrix degradation in OA, providing more evidence for involvement of altered lipid metabolism in OA. 33 Our ROC curve analysis results demonstrated potential utility of SF HDL-C and ApoA1 as biomarkers to identify individuals who have an increased susceptibility to develop radiographic severe progressive OA, further suggesting a close relationship between dyslipidemia and OA pathogenesis.
Although the exact mechanism is still missing, a series of hypotheses have been put forward to explain the association between dyslipidemia and OA. One hypothesis is that lipid deposition in the joint may trigger OA development, on the basis of findings that substantial lipid deposits in osteoarthritic cartilage and chondrocytes at early stage of OA before apparent histological changes. 34 Some other researchers hold the opinion that biosynthesis disorders of bone marrow derived mesenchymal stem cells may be responsible for dyslipidemia in OA patients, since it will disrupt the homeostasis between adipogenesis and chondrogenesis. 35
Despite the exciting results, there were inevitably limitations to the current study. First, this study was conducted at a single center and the sample size was relatively small. And due to ethical reasons, the SF samples unfortunately lacked a control group. Without measurement of normal SF lipids levels, this study yielded no data regarding to what extent SF lipids were increased or decreased in OA patients relative to healthy individuals. Second, due to a cross-sectional research design, we could not draw a definite cause-and-effect conclusion. In addition, we could not rule out potential factors that may affect lipid levels later, such as lifestyle modifications. Therefore, further multicentral long-term prospective studies with larger sample sizes are necessary to validate any relationships. Third, we only evaluated 6 most common lipid species of human body in the present study. Further work is warranted to research the roles of some other lipid metabolism–related products in OA aetiopathogenesis.
Conclusion
In conclusion, SF HDL-C and ApoA1 levels were negatively correlated with the cartilage damage severity, radiographic severity as well as symptomatic severity of primary knee OA, which could emerge as potential biomarkers for radiographic advanced-stage OA. This study postulated that SF HDL-C and ApoA1 could be used as predictors of disease severity. Further longitudinal investigations are warranted for enlightenment of the exact role of HDL-C and ApoA1 in fundamental processes underlying the pathogenesis of primary knee OA.
Footnotes
Author Contributions: Yiqiu Jiang and Jianchao Gui were involved with the design of the research. Kaibin Zhang wrote the manuscript and interpreted the results. Jianchao Gui performed all the surgeries. Kaibin Zhang and Yisheng Ji collected sample and performed laboratory analysis. Abdul Aleem Khan, Yiqiu Jiang, and Hanhao Dai participated in the evaluations of clinical and radiographic parameters. Yang Zhou and Ran Chen assembled the data and conducted statistical analyses. All authors read and approved the final manuscript.
Acknowledgments and Funding: We are grateful to Ran Qin and Tong Chen from Nanjing First Hospital for excellent technical assistance in sample collection. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a grant from Natural Science Foundation of Nanjing Medical University (Grant Number: 20120094).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by the Institutional Review Board of Nanjing First Hospital (Permit Number: 2012581A).
Informed Consent: A written informed consent was obtained from each participant. We obtained all patients’ consent to publication of their medical data, including medical records, photographs and images.
ORCID iD: Jianchao Gui  https://orcid.org/0000-0002-0876-5265
https://orcid.org/0000-0002-0876-5265
References
- 1. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745-59. [DOI] [PubMed] [Google Scholar]
- 2. Emery CA, Whittaker JL, Mahmoudian A, Lohmander LS, Roos EM, Bennell KL, et al. Establishing outcome measures in early knee osteoarthritis. Nat Rev Rheumatol. 2019;15:438-48. [DOI] [PubMed] [Google Scholar]
- 3. Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30(2):160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Casalone E, Tachmazidou I, Zengini E, Hatzikotoulas K, Hackinger S, Suveges D, et al. A novel variant in GLIS3 is associated with osteoarthritis. Ann Rheum Dis. 2018;77: 620-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vannini F, Spalding T, Andriolo L, Berruto M, Denti M, Espregueira-Mendes J, et al. Sport and early osteoarthritis: the role of sport in aetiology, progression and treatment of knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2016;24:1786-96. [DOI] [PubMed] [Google Scholar]
- 6. Yang CZ, Zhang YY, Zheng M. Soluble lectin-like oxidized low-density lipoprotein receptor-1 levels in synovial fluid are correlated with disease severity of knee osteoarthritis. Clin Biochem. 2011;44:1094-6. [DOI] [PubMed] [Google Scholar]
- 7. Farnaghi S, Crawford R, Xiao Y, Prasadam I. Cholesterol metabolism in pathogenesis of osteoarthritis disease. Int J Rheum Dis. 2017;20:131-40. [DOI] [PubMed] [Google Scholar]
- 8. de Munter W, van der Kraan PM, van den Berg WB, van Lent PLEM. High systemic levels of low-density lipoprotein cholesterol: fuel to the flames in inflammatory osteoarthritis? Rheumatology (Oxford). 2016;55(1_suppl):16-24. [DOI] [PubMed] [Google Scholar]
- 9. Dong N, Gao YH, Liu B, Zhao CW, Yang C, Li SQ, et al. Differential expression of adipokines in knee osteoarthritis patients with and without metabolic syndrome. Int Orthop. 2018;42(6):1283-9. [DOI] [PubMed] [Google Scholar]
- 10. Wang X, Hunter D, Xu J, Ding C. Metabolic triggered inflammation in osteoarthritis. Osteoarthritis Cartilage. 2015;23(1_suppl):22-30. [DOI] [PubMed] [Google Scholar]
- 11. Courties A, Gualillo O, Berenbaum F, Sellam J. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthritis Cartilage. 2015;23(11):1955-65. [DOI] [PubMed] [Google Scholar]
- 12. Ikeda S, Uchiyama K, Minegishi Y, Nakamura M, Takaso M. Evaluation of myeloperoxidase in synovial fluid as a biomarker for chronic periprosthetic joint infection. Int Orthop. 2020;44(10):1915-20. [DOI] [PubMed] [Google Scholar]
- 13. Gandhi R, Takahashi M, Smith H, Rizek R, Mahomed NN. The synovial fluid adiponectin-leptin ratio predicts pain with knee osteoarthritis. Clin Rheumatol. 2010;29(11):1223-8. [DOI] [PubMed] [Google Scholar]
- 14. Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039-49. [DOI] [PubMed] [Google Scholar]
- 15. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17:505-13. [DOI] [PubMed] [Google Scholar]
- 16. Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833-40. [PubMed] [Google Scholar]
- 18. Oliviero F, Lo Nigro A, Bernardi D, Giunco S, Baldo G, Scanu A, et al. A comparative study of serum and synovial fluid lipoprotein levels in patients with various arthritides. Clin Chim Acta. 2012;413:303-7. [DOI] [PubMed] [Google Scholar]
- 19. Oliviero F, Sfriso P, Baldo G, Dayer JM, Giunco S, Scanu A, et al. Apolipoprotein A-I and cholesterol in synovial fluid of patients with rheumatoid arthritis, psoriatic arthritis and osteoarthritis. Clin Exp Rheumatol. 2009;27(1_suppl):79-83. [PubMed] [Google Scholar]
- 20. Listgarten J, Emili A. Statistical and computational methods for comparative proteomic profiling using liquid chromatography-tandem mass spectrometry. Mol Cell Proteomics. 2005;4(4):419-34. [DOI] [PubMed] [Google Scholar]
- 21. Wu G, Wan X, Xu B. A new estimation of protein-level false discovery rate. BMC Genomics. 2018;19(Suppl 6):567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rye KA, Barter PJ. Regulation of high-density lipoprotein metabolism. Circ Res. 2014;114(1_suppl):143-56. [DOI] [PubMed] [Google Scholar]
- 23. Karvonen-Gutierrez CA, Sowers MR, Heeringa SG. Sex dimorphism in the association of cardiometabolic characteristics and osteophytes-defined radiographic knee osteoarthritis among obese and non-obese adults: NHANES III. Osteoarthritis Cartilage. 2012;20(7):614-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garcia-Gil M, Reyes C, Ramos R, Sanchez-Santos MT, Prieto-Alhambra D, Spector TD, et al. Serum lipid levels and risk of hand osteoarthritis: the Chingford prospective cohort study. Sci Rep. 2017;7(1_suppl):3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arciello A, Piccoli R, Monti DM. Apolipoprotein A-I: the dual face of a protein. FEBS Lett. 2016;590:4171-9. [DOI] [PubMed] [Google Scholar]
- 26. Tsezou A, Iliopoulos D, Malizos KN, Simopoulou T. Impaired expression of genes regulating cholesterol efflux in human osteoarthritic chondrocytes. J Orthop Res. 2010;28(8):1033-9. [DOI] [PubMed] [Google Scholar]
- 27. Mickiewicz B, Heard BJ, Chau JK, Chung M, Hart DA, Shrive NG, et al. Metabolic profiling of synovial fluid in a unilateral ovine model of anterior cruciate ligament reconstruction of the knee suggests biomarkers for early osteoarthritis. J Orthop Res. 2015;33(1_suppl):71-7. [DOI] [PubMed] [Google Scholar]
- 28. Mickiewicz B, Kelly JJ, Ludwig TE, Weljie AM, Wiley JP, Schmidt TA, et al. Metabolic analysis of knee synovial fluid as a potential diagnostic approach for osteoarthritis. J Orthop Res. 2015;33(11):1631-8. [DOI] [PubMed] [Google Scholar]
- 29. de Boer TN, van Spil WE, Huisman AM, Polak AA, Bijlsma JW, Lafeber FP, et al. Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthritis Cartilage. 2012;20(8):846-53. [DOI] [PubMed] [Google Scholar]
- 30. de Jong AJ, Klein-Wieringa IR, Kwekkeboom JC, Toes REM, Kloppenburg M, Ioan-Facsinay A. Inflammatory features of infrapatellar fat pad in rheumatoid arthritis versus osteoarthritis reveal mostly qualitative differences. Ann Rheum Dis. 2018;77(7):1088-90. [DOI] [PubMed] [Google Scholar]
- 31. Frommer KW, Schäffler A, Rehart S, Lehr A, Müller-Ladner U, Neumann E. Free fatty acids: potential proinflammatory mediators in rheumatic diseases. Ann Rheum Dis. 2015;74: 303-10. [DOI] [PubMed] [Google Scholar]
- 32. Van de Vyver A, Clockaerts S, van de Lest CHA, Wei W, Verhaar J, Van Osch GJVM, et al. Synovial fluid fatty acid profiles differ between osteoarthritis and healthy patients. Cartilage. 2020;11(4):473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morquette B, Shi Q, Lavigne P, Ranger P, Fernandes JC, Benderdour M. Production of lipid peroxidation products in osteoarthritic tissues: new evidence linking 4-hydroxynonenal to cartilage degradation. Arthritis Rheum. 2006;54(1_suppl):271-81. [DOI] [PubMed] [Google Scholar]
- 34. Lee SW, Rho JH, Lee SY, Chung WT, Oh YJ, Kim JH, et al. Dietary fat-associated osteoarthritic chondrocytes gain resistance to lipotoxicity through PKCK2/STAMP2/FSP27. Bone Res. 2018;6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aspden RM, Scheven BA, Hutchison JD. Osteoarthritis as a systemic disorder including stromal cell differentiation and lipid metabolism. Lancet. 2001;357:1118-20. [DOI] [PubMed] [Google Scholar]