Abstract
Objective
Cartilage damage (CD) in the temporomandibular joint (TMJ) continues being a major problem in maxillofacial field. Evidence suggests that cellular therapy may be used for repairing CD in the TMJ.
Design
A murine model of condyle CD (CCD) was generated in the TMJ to evaluate the capacity of mesenchymal stromal cells (MSCs) to induce cartilage regeneration in CCD. A large CCD was surgically created in a condyle head of the TMJ of C57BL/6 mice. Human MSC embedded into preclotted platelet-rich plasma (PRP) were placed on the surface of CCD. As controls, untreated CCD and exposed TMJ condyle (sham) were used. After 6 weeks, animals were sacrificed, and each mandibular condyle was removed and CCD healing was assessed macroscopically and histologically.
Results
Macroscopic observation of CCD treated with MSC showed the presence of cartilage-like tissue in the CCD site. Histological analysis showed a complete repair of the articular surface with the presence of cartilage-like tissue and subchondral bone filling the CCD area. Chondrocytes were observed into collagen and glycosaminoglycans extracellular matrix filling the repaired tissue. There was no evidence of subchondral bone sclerosis. Untreated CCD showed denudated osteochondral lesions without signs of cartilage repair. Histological analysis showed the absence of tissue formation over the CCD.
Conclusions
Transplantation of MSC induces regeneration of TMJ-CCD. These results provide strong evidence to use MSC as potential treatment in patients with cartilage lesions in the TMJ.
Keywords: cartilage regeneration, temporomandibular joint, mesenchymal stromal cells, MSC
Introduction
Cartilage damage (CD) in the temporomandibular joint (TMJ) is a challenging problem in maxillofacial field. Condylar CD (CCD) in the TMJ is associated with acute injury, overloading, and microtraumas in the mandibular condyle. 1 Patients suffering from CCD have decreased mobility of the TMJ, problems to perform a normal mastication and severe pain episodes. Current management of CCD in the TMJ includes conservative therapies and surgical interventions. 2 When it is left untreated, CCD can lead to a degenerative joint disease characterized by the degradation of articular cartilage and subchondral bone with the presence of fibrous or fibrocartilaginous tissue repair.3-5
It is well known that articular cartilage has a limited capacity of repairing and regeneration. It is because to the low capacity of proliferation of chondrocytes and lack of vascularization of the cartilage tissue. 6 Several clinical and surgical therapeutics strategies have been used to induce cartilage repair. 1 However, they have shown a great variety of outcomes.7,8 Cellular therapy based on autologous chondrocytes has been used as a therapeutic strategy to induce cartilage regeneration. 9 However, the dedifferentiation process that suffer these cells when they are cultured in vitro, restrict their use in cellular therapy protocols. 10 More recently, cellular therapy based on transplantation of mesenchymal stromal cells (MSCs) has been postulated as potential treatment to induce cartilage regeneration.11,12 The use of MSC is based on their multipotential capacity of differentiation to several cell lineages, including chondrocytes. 13 Likewise, there is evidence that shows that transplantation of MSC may enhance cartilage repair and regeneration. 11 There are reports that show that MSC may induce repair and regeneration of the TMJ cartilage.14,15
In this study, it is evaluated the cartilage regeneration capacity of bone marrow–derived MSC in a murine model of CCD in TMJ. Evidence of MSC transplantation as a potential treatment of CCD in TMJ is provided.
Methods
Reagents and Culture Medium
Monoclonal antibodies anti-human CD90 and CD105 were from BioLegend (USA). Alpha MEM and DMEM-F12 media were from Life Technologies (USA) and Chang medium was from Irvine Scientific (USA).
Animals
Female C57BL/6 mice (8-10 weeks old) were used in this study. Mice were obtained from the Laboratory Animal Center (Instituto Venezolano de Investigaciones Cientificas, IVIC) and maintained on a standard laboratory diet and housed in a controlled environment. All animal experimentation was performed in accordance with institutional guidelines. This project was approved by the Bioethic Committee for Animal Research (COBIANIM) of IVIC.
Experimental Model of Mandibular Condyle Cartilage Damage
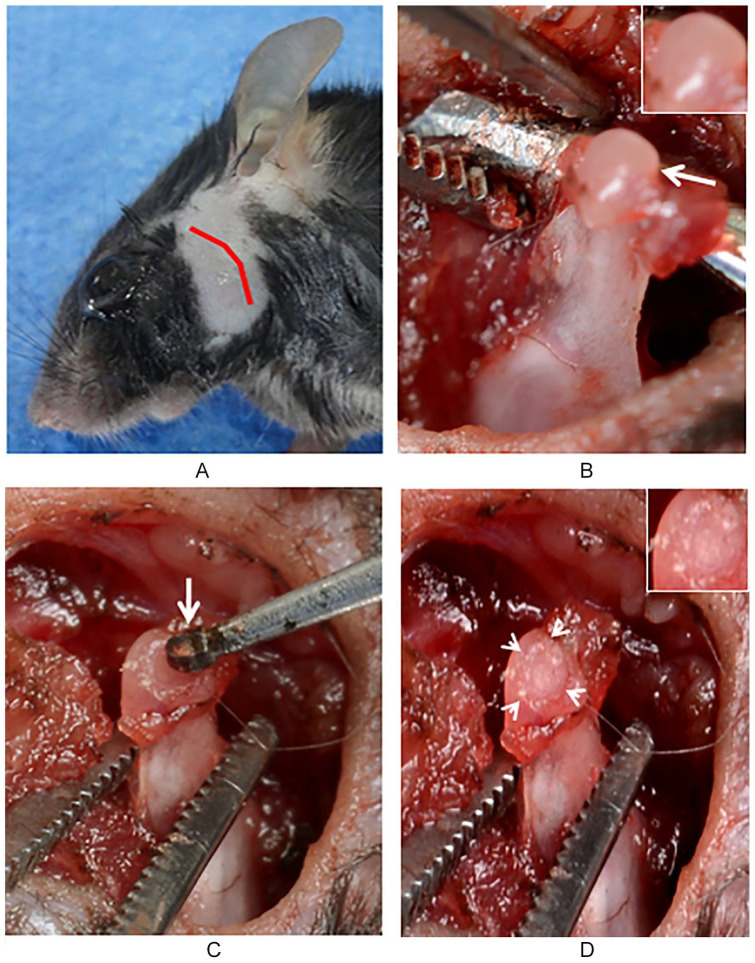
We induced cartilage damage in the mandibular condyle of mice. For this purpose, anesthetized C57BL/6 mice were placed over a surgical thermal blanket and pre-auricular hair was removed ( Fig. 1A ). An electrocautery pre-auricular skin incision was performed over the TMJ. One mandibular condyle from each mouse was exposed and disarticulated from the TMJ ( Fig. 1B ). Focal cartilage damage was performed at the mandibular condyle head by using a low-speed high torque handpiece ( Fig. 1C ). The cartilage damage was extended until subchondral bone ( Fig. 1D ). After inducing cartilage damage, the condyle head was returned to the TMJ. The articular capsule and skin were closed independently in layers. Animals were returned to the animal facility. Mandibular joint mobility was evaluated by the capacity of the mice to eat during the first 7 days, after surgery, with soft diet and later with solid diet. No detectable mandibular functional restriction was observed during the eating process. No weight loss was evident between all the experimental groups. Dipyrone was intraperitoneally administered (5 mg/kg) every 12 hours per 3 days.
Figure 1.
Surgical procedure for condylar cartilage damage CCD. Preauricular shaving and surgical pen designed skin incision (A). Mandibular condylar head and cartilage exposure (arrow) (B). Microsurgical bur (arrow) applied on the head condyle surface for inducing chondral damage (C). Focal chondral defect (head arrows) in the condylar head (D).
Isolation and Culture of Bone Marrow MSC
Bone marrow MSC used in this work was from a healthy patient treated for bone regeneration, due to pseudarthrosis secondary to a fracture, 16 who authorized the use of the cells by signing informed consent. Briefly, bone marrow aspirates were obtained from the posterior iliac crest of the patient. The mononuclear cells were isolated by using Ficoll-Hypaque (GE Healthcare, Sweden) gradient, washed and cultured in plastic culture flasks with alpha-MEM-Chang medium supplemented with 20 % autologous serum (regular medium). The MSC were isolated by its adherence to the plastic of the culture flask and expanded by culturing in regular medium. MSC were cultured, expanded, and stored at −70°C until its use. The study protocol was approved by the Bioethics Committee of Hospital Universitario de Caracas. This study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Culture and Characterization of MSC
MSC were thawed and cultured in plastic culture flasks with regular medium at 37°C and 5% CO2. When they reached almost 80% of confluency, the cells were harvested by trypsinization and examined for the expression of MSC markers (CD90 and CD105) by flow cytometry. Data collection and analysis of the fluorescent intensities were carried out using a FACSort (Becton Dickinson, San Jose, CA). Ten thousand events were acquired and analyzed using the CELLQuest software program.
Multipotent Differentiation of MSC
The multipotential capacity of MSC was examined by culturing these cells in osteogenic and chondrogenic differentiation media. Briefly, MSC monolayer were harvested by trypsinization and cultured in osteogenic and chondrogenic differentiation media (GIBCO, USA), with media change every 5 days. After 14 and 21 days (osteogenic and chondrogenic differentiation, respectively), MSC were examined by microscopic observation, photographically recorded and stained with alizarin (to stain calcium deposits) and Alcian blue (to stain glycosaminoglycans). 16
Transplant of MSC on the Surface of CCD
CCD in the TMJ were treated or untreated with MSC (n = 4 in each group). For this purpose, subconfluent MSC cultures were harvested, counted, and the viability was assessed by trypan-blue. The viability of MSC was always greater than 95%. MSCs (104 cells) were mixed with human platelet-rich plasma (PRP) from healthy donors. A solution of 5% CaCl2/thrombin was added to each vial containing MSC/PRP (final volume of 0.1 mL), and before the clot was formed it was immediately implanted onto the cartilage defect during the CCD surgery. Clot formation on the CCD was confirmed after 1 minute of MSC/PRP implantation. As control, we included a sham group without any treatment (n = 2). After 6 weeks of MSC implantation, animals were sacrificed by cervical dislocation and each condyle was macroscopically evaluated and photographed.
Histological Analysis
For histological analysis, condyles were removed and fixed in 10% buffered formalin for 24 hours, decalcified, paraffin-embedded, and sectioned at 3 µm for hematoxylin and eosin and Alcian blue staining. The degree of cartilage damage in each condyle section was determined by 3 independent observers according to Mankin scores. All condyle histological images were acquired from a Motic B1 microscope, at 10× magnification, equipped with a color video camera.
Results
Morphological, Phenotypical, and Functional Characterization of MSCs
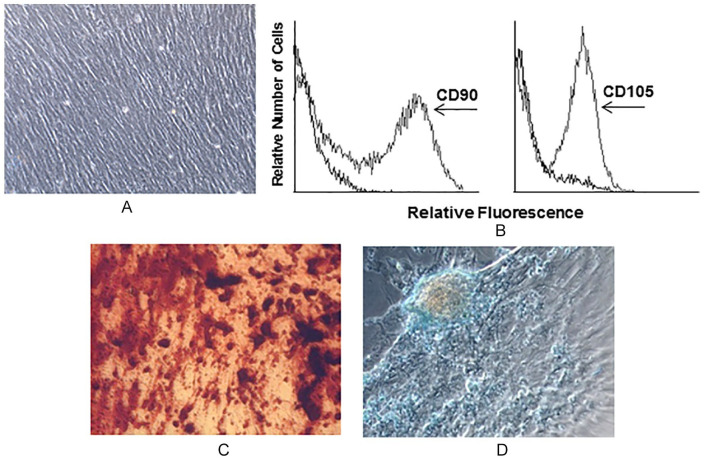
MSCs were thawed and expanded until becoming near confluent, as previously described. 16 MSC showed the typical fibroblastoid-like morphology ( Fig. 2A ) and expressed CD90 and CD105 (100% of total analyzed cells) ( Fig. 2B ). They showed osteogenic and chondrogenic capacity of differentiation ( Fig. 2C and D , respectively).
Figure 2.
Culture, Characterization, and Differentiation of mesenchymal stromal cells (MSCs). Adherent cells from bone marrow (BM) show fibroblast-like cell morphology (A) and express CD90 and CD105 (B). MSCs differentiate into osteogenic and chondrogenic progenitors (C and D, respectively).
Macroscopic Analysis of Mandibular CCD
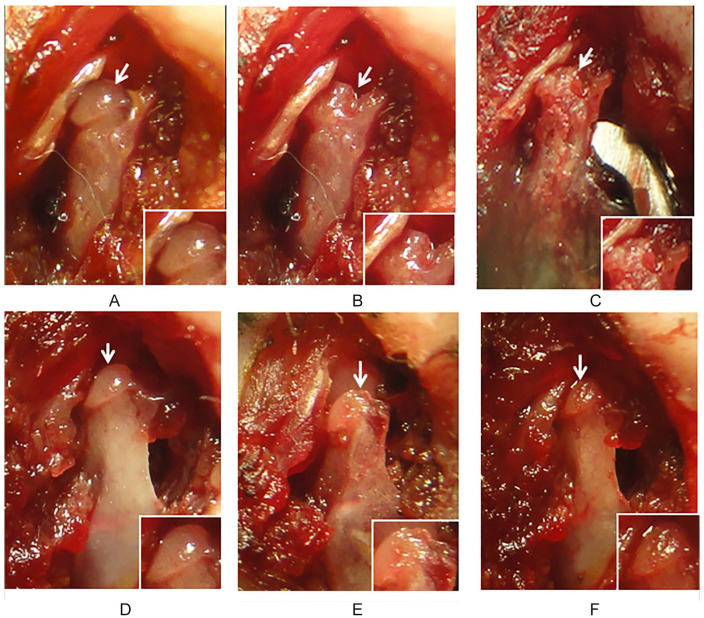
After 6 weeks of CCD surgery, there was no evidence of cartilage repair in untreated CCD (n = 4) ( Fig. 3A-C ). Macroscopic observation showed subchondral defect with no evidence of cartilage repair or new tissue filling untreated CCD ( Fig. 3C ). In some animals the subchondral lesion appeared larger than the original ones with degenerative joint characteristics ( Fig. 3C ). In contrast, macroscopic observation of CCD treated with MSCs (n = 4) showed a smooth cartilage-like tissue covering the whole condyle area ( Fig. 3F ) in which CCD was made ( Fig. 3E ). It has similar characteristics of color and brightness as normal articular cartilage ( Fig. 3D ). Neither fovea nor tissues peeling off from the articular surface were observed over the condylar head area ( Fig. 3F ). Animals have no complications after CCD surgery and the follow-up period of 6 weeks.
Figure 3.
Evaluation of condylar cartilage damage (CCD) after mesenchymal stromal cell (MSC) transplantation. Condylar head of untreated CCD (A-C). The condylar cartilage is observed before (A, arrow) and after CCD (B, arrow). Chondral defect after 6 weeks (C). The chondral defect remains without evidence of tissue repair (arrow and insert). Condylar head of MSC-treated CCD (D-F). The condylar cartilage is observed before CCD (D, arrow). Chondral defect after CCD (E, arrow), and after 6 weeks post-MSC transplantation (F, arrow). Evidence of cartilage regeneration is observed at the site of CCD were MSC were implanted (F). New cartilage-like tissue is observed filling the chondral defect (arrow and insert). Results are representative of 4 CCD (one joint in each TMJ ) in each group, all of which had similar results.
TMJ = temporo mandibular joint.
Histologyc Analysis of CCD Treated with MSC
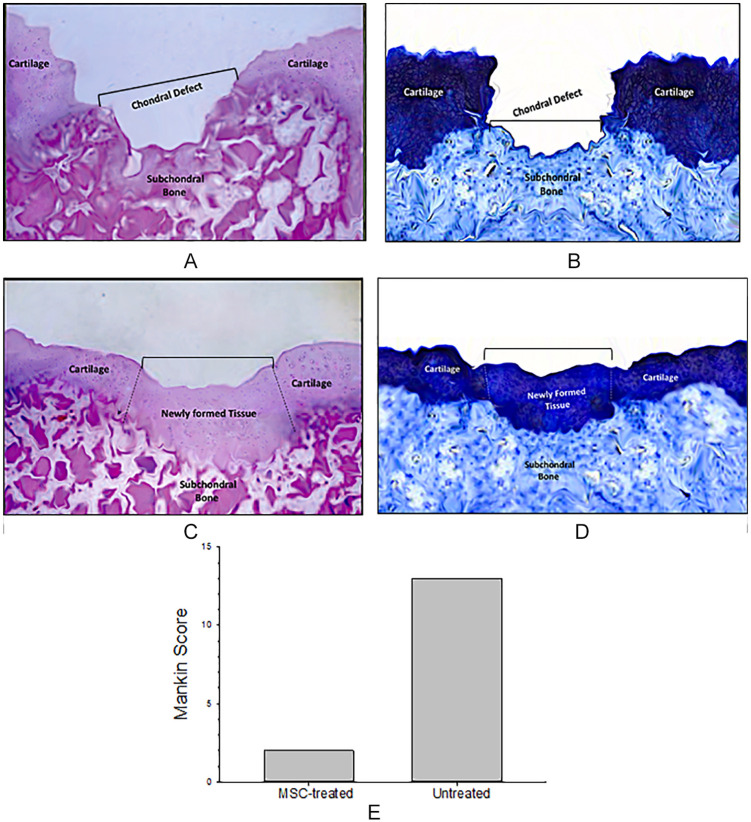
Histologic evaluation of mandibular condyles was performed in untreated or treated CCD. Untreated CCD showed the absence of tissue repair with exposed subchondral bone in the cartilage defect ( Fig. 4A ). The lack of cartilage matrix tissue repair in the CCD site was evidenced by Alcian blue staining ( Fig. 4B ). Histologic evaluation of CCD treated with MSC revealed the presence of chondral tissue integrated to the margin zones of the cartilage defect and to the subchondral bone ( Fig. 4C ). Chondrocytes within their matrix completely filled the cartilage defect ( Fig. 4C ). These cells were forming clusters but with lack of columnar distribution. A thin subchondral plate and trabeculae without evidence of subchondral bone sclerosis was observed. Alcian blue staining confirmed the presence of glycosaminoglycan in the extracellular matrix ( Fig. 4D ). Sham group showed a continuous surface of fibrocartilaginous tissue containing rounded cells (not shown). Mankin scores was lower in MSC-treated CCD group than the untreated CCD group ( Fig. 4E ). Altogether, these results are a clear evidence of neotissue formation associated with areas of chondrogenesis with cartilage extracellular matrix deposition in CCD.
Figure 4.
Histological analysis from untreated or mesenchymal stromal cell (MSC)-treated condylar cartilage damage (CCD). After 6 weeks of MSC transplantation, untreated or MSC-treated CDD lesions were processed for histological examination by hematoxylin and eosin (H&E) staining. An area of cartilage discontinuity (bucket defect) with denuded subchondral area is observed in untreated CDD lesions (A). Alcian blue staining show the absence of extracellular matrix formation in the CDD area (B). CDD transplanted with MSCs showed new a continuous cartilaginous tissue, containing chondrocytes, and ECM (C). The CCD defect was completely covered by newly cartilage and bone at the top of the condyle. A thin subchondral plate and trabeculae is evidenced at the site of CDD. The repaired cartilage defect shows a strong and homogenous Alcian blue staining, which indicates the presence of extracellular matrix with proteoglycan content (D). Representative H&E and Alcian blue staining from condyle slices are showed. Mankin scores 17 of MSC-treated CCD was lower than that of untreated CCD (E). Data are shown as the average of Mankin scores performed by 3 independent observers.
Discussion
Repair and regeneration of CD in the TMJ is a challenging problem in the maxillofacial field. An optimal treatment for CD should not only repair the cartilage defect but also restore the structure and functionality of the TMJ. Although several therapeutic strategies have been used to induce cartilage regeneration,8,14,15 there is no single optimal treatment for TMJ disorders associated with CD. Recently, experimental and clinical protocols based on cellular therapy have been used to induce repair and regeneration of CD in the TMJ.8,14,15
MSC are the most used cells in cellular therapy for tissue repair and regeneration.11,12 There are experimental and clinical studies using MSC transplantation for articular cartilage repair in orthopedic and maxillofacial trials.8,14,15,18 It is known that scaffolds should be used for MSC transplantation as support and vehicle of these cells.19-21 An optimal scaffold for MSC should support not only the survival but also maintains the biological functions of these cells. Numerous scaffolds have been used as support of MSC, including protein-based scaffolds (i.e., fibrin).19-21 We have previously showed that PRP clot constitutes an excellent scaffold because it provides a tridimensional framework to MSC, in which these cells can survive, proliferate, differentiate, and migrate. 22 Additionally, PRP can also provide growth signals derived from activated platelets. 23 Recently, we have used MSCs incorporated into PRP clots for inducing bone regeneration in patients with pseudoarthrosis. 16
Here, the capacity of MSC to induce cartilage regeneration in CCD of the TMJ was investigated. For this purpose, a large cartilage defect involving subchondral bone was made in the condyle head of the TMJ. In this CCD model, mice did not show evidence of secondary effects and weigh loss after surgery. Although, there is evidence that shows that articular cartilage of the TMJ can heal and regenerate,24-26 our results indicate that the size of the lesion made on the condylar cartilage of the TMJ (untreated CCD) does not allow spontaneous repairing and regeneration of the CCD. In fact, untreated CCD was not only not repaired but also macroscopic and histological observation showed a larger lesion than the one observed after surgery.
In the present work, human MSCs were implanted embedded in a PRP clot in the CCD site. In contrast with untreated-CCD, transplantation of MSC induced cartilage and bone tissue regeneration in CCD. After 6 weeks of MSC implantation, macroscopic evaluation of MSC-treated CCD showed a significant difference in terms of filling, color, and smoothness, as compared with untreated CCD. Thus, the surface of the mandibular CCD treated with MSC showed a smoother and brighter surface. Histological studies confirmed a complete repair and regeneration of the whole cartilage defect. Cartilage repair was associated with fibrocartilage formation, chondrocytes within extracellular matrix formed by proteoglycans and subchondral bone repair. The fibrocartilage tissue formed on CCD was associated to the underlying subchondral bone plate. Altogether, these results constitute strong evidence that MSC may induce new cartilaginous tissue characterized by the presence of areas of chondrogenesis with cartilage extracellular matrix deposition. Based on previous evidence suggesting that paracrine signals from MSC may exert effects on other cells,27-30 we propose that growth factors produced by donor MSC induce migration of host MSC, which may differentiate in chondrocytes, resulting in healing of the CCD. However, there is evidence suggesting that MSC paracrine effects may also result from the release of exosomes by these cells.31-34 It has been reported that MSC-derived exosomes may promote restoration of cartilage and subchondral bone, and also enhance extracellular matrix deposition (type II collagen and sulfated glycosaminoglycan). 35 Finally, based on the low immunogenicity of human MSC, the possibility of rejection of these cells by murine cells is very low. 18 In fact, we did not observe any evidence of rejection in CCD treated with human MSC.
Our results support previous evidence that transplantation of MSC can induce articular repair.11,27-31 It has been reported that injection of differentiated MSC stimulates cartilage regeneration in an experimental model of TMJ osteoarthritis. 29 However, unlike the degenerative environment associated with this osteoarthritis model, our CCD model is associated with an acute damage of the condylar cartilage, which involves a great surface of the cartilage and subchondral bone. To our knowledge, our results constitute the first evidence showing that MSCs induce regeneration of cartilage and subchondral bone in CCD of the TMJ.
In conclusion, our work shows that transplantation of MSC induces repair and regeneration of cartilage and subchondral bone in CCD of the TMJ. Our results provide new evidence for using allogeneic MSC transplantation as a potential treatment in patients with cartilage defects of the TMJ.
Footnotes
Author Contributions: Marcos Gomez and Olga Wittig designed research, analyzed data, and wrote the paper; D. Diaz-Solano performed experiments and analyzed data; J. E. Cardier designed research, analyzed data, and wrote the paper.
Acknowledgments and Funding: The authors wish to thank the support of Esal. The authors also wish to thank G. Cardier for his assistance in the preparation of this manuscript. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by funds from Mppeuct (Ministerio del Poder Popular para la Educación Universitaria, Ciencia y Tecnología), PEI Project No. 284.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This project was approved by the Bioethic Committee for Animal Research (COBIANIM) of Instituto Venezolano de Investigaciones Científicas (IVIC).
Animal Welfare: Institutional guidelines for the care and use of animals were followed in this work. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (COBIANIM, IVIC)
ORCID iD: José E. Cardier  https://orcid.org/0000-0002-5597-5505
https://orcid.org/0000-0002-5597-5505
References
- 1. LeResche L. Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit Rev Oral Biol Med. 1997;8(3):291-305. [DOI] [PubMed] [Google Scholar]
- 2. Tanaka E, Detamore MS, Mercuri LG. Degenerative disorders of the temporomandibular joint: etiology, diagnosis, and treatment. J Dent Res. 2008;87(4):296-307. [DOI] [PubMed] [Google Scholar]
- 3. Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg. 1982;64(3):460-6. [PubMed] [Google Scholar]
- 4. Mitchell N, Shepard N. The resurfacing of adult rabbit articular cartilage by multiple perforations through the subchondral bone. J Bone Joint Surg. 1976;58(2):230-3. [PubMed] [Google Scholar]
- 5. Salter RB, Simmonds DF, Malcolm BW, Rumble EJ, Macmichael D, Clements ND. The biological effect of continuous passive motion on the healing of full-thickness defects in articular cartilage: an experimental investigation in the rabbit. J Bone Joint Surg Am. 1980;62(8):1232-51. [PubMed] [Google Scholar]
- 6. Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432-63. [DOI] [PubMed] [Google Scholar]
- 7. De Riu G, Vaira LA, Carta E, Meloni SM, Sembronio S, Robiony M. Bone marrow nucleated cell concentrate autograft in temporomandibular joint degenerative disorders: 1-year results of a randomized clinical trial. J Craniomaxillofac Surg. 2019;47(11):1728-38. [DOI] [PubMed] [Google Scholar]
- 8. Zhang S, Yap AU, Toh WS. Stem cells for temporomandibular joint repair and regeneration. Stem Cell Rev Rep. 2015;11(5):728-42. [DOI] [PubMed] [Google Scholar]
- 9. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889-95. [DOI] [PubMed] [Google Scholar]
- 10. Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982:30(1_suppl):215-24. [DOI] [PubMed] [Google Scholar]
- 11. Nejadnik H, Hui JH, Choong EPF, Tai BC, Lee EH. Autologous bone marrow–derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38(6):1110-6. [DOI] [PubMed] [Google Scholar]
- 12. Toh WS, Foldager CB, Pei M, Hui JH. Advances in mesenchymal stem cell–based strategies for cartilage repair and regeneration. Stem Cell Rev Rep. 2014;10(5):686-96. [DOI] [PubMed] [Google Scholar]
- 13. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-7. [DOI] [PubMed] [Google Scholar]
- 14. Wu Y, Gong Z, Li J, Meng Q, Fang W, Long X. The pilot study of fibrin with temporomandibular joint derived synovial stem cells in repairing TMJ disc perforation. Biomed Res Int. 2014;2014:454021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahtiainen K, Mauno J, Ellä V, Hagström J, Lindqvist C, Miettinen S, et al. Autologous adipose stem cells and polylactide discs in the replacement of the rabbit temporomandibular joint disc. J R Soc Interface. 2013;10(85):20130287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wittig O, Romano E, González C, Díaz-Solano D, Márquez ME, Tovar P, et al. A method of treatment for nonunion after fractures using mesenchymal stromal cells loaded on collagen microspheres and incorporated into platelet-rich plasma clots. Int Orthop. 2016;40(5):1033-8. [DOI] [PubMed] [Google Scholar]
- 17. Lee YJ, Park JA, Yang SH, Kim KY, Kim BK, Lee EY, et al. Evaluation of osteoarthritis induced by treadmill-running exercise using the modified Mankin and the new OARSI assessment system. Rheumatol Int. 2011;31(12):1571-6. [DOI] [PubMed] [Google Scholar]
- 18. Caliari-Oliveira C, Yaochite JN, Ramalho LN, Palma PV, Carlos D, Cunha F, et al. Xenogeneic mesenchymal stromal cells improve wound healing and modulate the immune response in an extensive burn model. Cell Transplant. 2016;25(2):201-15. [DOI] [PubMed] [Google Scholar]
- 19. Li YY, Cheng HW, Cheung KM, Chan D, Chan BP. Mesenchymal stem cell-collagen microspheres for articular cartilage repair: cell density and differentiation status. Acta Biomater. 2014;10(5):1919-29. [DOI] [PubMed] [Google Scholar]
- 20. Kim TG, Shin H, Lim DW. Biomimetic scaffolds for tissue engineering. Adv Funct Mater. 2012;22(12):2446-68. [Google Scholar]
- 21. Wang H, Li Y, Zuo Y, Li J, Ma S, Cheng L. Biocompatibility and osteogenésis of biomimetic nano-hydroxyapatite/polyamide composta scaffolds for bone tissue engineering. Biomaterials. 2007;28(22):3338-48. [DOI] [PubMed] [Google Scholar]
- 22. Wittig O, Diaz-Solano D, Cardier J. Viability and functionality of mesenchymal stromal cells loaded on collagen microspheres and incorporated into plasma clots for orthopaedic application: effect of storage conditions. Injury. 2018;49(6):1052-7. [DOI] [PubMed] [Google Scholar]
- 23. Alsousou J, Thompson M, Hulley P, Noble A, Willet K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br. 2009;91(8):987-96. [DOI] [PubMed] [Google Scholar]
- 24. Robinson PD. Articular cartilage of the temporomandibular joint: can it regenerate? Ann R Coll Surg Engl. 1993;75(4):231-6. [PMC free article] [PubMed] [Google Scholar]
- 25. Robinson PD. Histological study of articular cartilage repair in the marmoset condyle. J Oral Maxillofac Surg. 1993;51(10):1088-94. [DOI] [PubMed] [Google Scholar]
- 26. Blackwood HJ. Cellular remodelling in articular tissue. J Dent Res. 1966;45:480-9. [Google Scholar]
- 27. Sordi V. Mesenchymal stem cell homing capacity. Transplantation. 2009;87(9 Suppl.):S42-S45. [DOI] [PubMed] [Google Scholar]
- 28. Sato M, Uchida K, Nakajima H, Miyazaki T, Guerrero AR, Watanabe S, et al. Direct transplantation of mesenchymal stem cells into the knee joints of Hartley strain guinea pigs with spontaneous osteoarthritis. Arthritis Res Ther. 2012;14(1_suppl):R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen K, Man C, Zhang B, Hu J, Zhu SS. Effect of in vitro chondrogenic differentiation of autologous mesenchymal stem cells on cartilage and subchondral cancellous bone repair in osteoarthritis of temporomandibular joint. Int J Oral Maxillofac Surg. 2013;42(2):240-8. [DOI] [PubMed] [Google Scholar]
- 30. Da Silva ML, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20(5-6):419-27. [DOI] [PubMed] [Google Scholar]
- 31. Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16-27. [DOI] [PubMed] [Google Scholar]
- 32. Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7(1_suppl):16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu Y, Zou R, Wang Z, Wen C, Zhang F, Lin F. Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem J. 2018;475(22):3629-38. [DOI] [PubMed] [Google Scholar]
- 34. Toh WS, Lai RC, Hui JHP, Lim SK. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin Cell Dev Biol. 2017;67:56-64. [DOI] [PubMed] [Google Scholar]
- 35. Zhang S, Chu WC, Lai RC, Lim SK, Hui JHP, Toh WS. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24(12):2135-40. [DOI] [PubMed] [Google Scholar]