Abstract
Objective:
To assess the cross-sectional association between serum levels of Coll2-1 and Coll2-1NO2, two cartilage degradation biomarkers; the burden of magnetic resonance imaging (MRI) features and clinical outcomes; and to evaluate the predictive value of these biomarkers on progression.
Design:
A total of 121 subjects with knee osteoarthritis (OA) were followed during 1 year with pain, function, and MRI assessment (PRODIGE study). Type II collagen-specific biomarker Coll2-1 and its nitrated form Coll2-1NO2 were directly measured in serum using immunoassays at baseline and after 3-, 6-, and 12-month follow-up.
Results:
Serum Coll2-1 and Coll2-1NO2 were correlated with several baseline knee features quantified with Whole-Organ Magnetic Resonance Imaging Score (WORMS). Coll2-1 was significantly correlated with periarticular cysts/bursitis (ρ = 0.29, P < 0.01), subarticular bone attrition (ρ = 0.25, P = 0.01), subarticular cysts (ρ = 0.24, P = 0.02), and articular cartilage integrity (ρ = 0.23, P = 0.03) WORMS subscores for the whole joint as well as with the medial femorotibial joint sum score (ρ = 0.26, P = 0.01) and medial femorotibial joint cartilage (ρ = 0.23, P = 0.02). Coll2-1NO2 correlated with WORMS total score (ρ = 0.23, P = 0.02), WORMS scores in the patellofemoral (ρ = 0.23, P = 0.02) and medial femorotibial compartments (ρ = 0.21, P = 0.03), with osteophytes scores (ρ = 0.27, P < 0.01), subarticular cysts (ρ = 0.24, P = 0.019), and intraarticular loose bodies (ρ = 0.27, P = 0.007). Baseline Coll2-1NO2 was higher in subjects with a pain worsening (426.4 pg/mL [278.04-566.95]) as compared to non-progressors (306.84 pg/mL [200.37-427.84]) over 1 year (AUC = 0.655, P = 0.015).
Conclusion:
Serum cartilage biomarkers Coll2-1 and Coll2-1NO2 are associated with several knee OA features quantified with WORMS. Our study also shows that the baseline value of Coll2-1NO2 is positively associated with pain worsening.
Keywords: osteoarthritis, diagnosis, biomarkers, diagnostics, articular cartilage, tissue, clinical trial, general, OA progression
Introduction
Osteoarthritis (OA) is a multifaceted and heterogeneous disease affecting millions of people all over the world. It has now become clear that research has to focus on a better understanding of the complexity of the disease and identify phenotypes, 1 and several projects are currently run to this end. 2 Soluble biomarkers could help at assessing burden of the disease and predicting OA evolution. 3 Among the most investigated biomarkers, Coll2-1 and Coll2-1NO2 are specific for type II collagen, the most abundant collagen of cartilage extracellular cellular matrix (ECM). 4 Coll2-1 (HRGYPGLDG) is a sequence located in the NH2-terminal part of the triple helix of type II collagen molecule, which is detectable after denaturation of the triple helix, thereby representing collagen type II degradation. 5 Data obtained before and after knee replacement suggest that serum Coll2-1 is sensitive to the structural changes occurring in a single joint, 6 therefore acting as a potential biomarker for diagnosis. 7 Importantly, a recent study showed that Coll2-1 is not affected by sampling conditions, circadian rhythm, seasonality and physical activity, indicating that Coll2-1 assay is robust for use in OA clinical trials. 8
Coll2-1NO2 (HRGY(NO2)PGLDG) is the nitrated form of Coll2-1 that reflects oxidative-related type II collagen degradation and local inflammatory reaction. 9 It was shown that unlike Coll2-1, Coll2-1NO2 was significantly correlated with C-reactive protein (CRP) levels. 9 In an interesting study conducted on a subset of patients from the OsteoArthritis Initiative cohort (OAI), Coll2-1NO2 was shown to be correlated with cartilage and meniscal features assessed with the Whole-Organ Magnetic Resonance Imaging Score (WORMS) scoring. 10
Primary aims of this study were to assess the association between serum levels of Coll2-1 and Coll2-1NO2 at baseline; magnetic resonance imaging (MRI) features of OA and clinical outcomes; and to evaluate the predictive value of these biomarkers at baseline, or their rapid change, on MRI features progression using data from the PRODIGE study. Coll2-1 is a marker of type II collagen degradation, a key and early process in cartilage degradation while its nitrated form, Coll2-1NO2, is related to oxidative stress and inflammation. Therefore, we hypothesized that these 2 biomarkers were related to different MRI and clinical features reflecting different OA phenotypes.
Materials and Methods
Participants
Patients with knee OA were recruited in the PRODIGE study, a multicenter study conducted by ARTIALIS SA (Liège, Belgium) in Belgium and France registered as NCT02070224. Written informed consent was obtained from individuals after the nature of the study was explained and understood, in accordance with International Conference on Harmonisation Good Clinical Practice (ICH GCP). The protocol was approved by the Belgian Central Ethics Committee (EC) from the University Hospital of Liege, the French Ethics Committee (Comité de Protection des Personnes EST III [CPP III]), and the French National Agency for Medicines and Health Products Safety (ANSM). The national number was B707201318719 for Belgium and ID RCB 2013-A01368-37 for France.
Males and females aged between 45 to 80 years and with a body mass index (BMI) less than or equal to 40 were recruited. Femorotibial knee OA was based on the American College of Rheumatology (ACR) criteria, 11 had to be symptomatic for more than 6 months and the Kellgren and Lawrence (KL) grade had to be of II or III. In order to increase the percentage of knee OA progressors, subjects had to show a cartilage lesion on MRI plus a phenotype generally associated with OA progression ( Table 1 ). OA was considered by the physician as polyarticular OA (polyOA) if they had previous diagnosed OA in at least 2 joints. X-rays and MRI were assessed centrally by 2 trained radiologists specialized in musculoskeletal (MSK) disorders. Subjects were followed during 1 year with visits at 3 (T3), 6 (T6), and 12 (T12) months. MRI were performed only at baseline and after 1-year follow-up. Patient flow diagram of the PRODIGE study is shown in Figure 1 .
Table 1.
Inclusion and Exclusion Criteria.
| Inclusion criteria |
| Demographics • Age between 45 to 80 years • Body mass index (BMI) ≤40 Femorotibial knee OA • According to the ACR • Symptomatic for more than 6 months • KL of II or III • Cartilage lesion seen on MRI One phenotype generally associated with OA progression: • Clinical: genu varum >3°, history of meniscal surgery, known polyarthritis or joint swelling at the target knee, or • MRI-based: bone marrow edema, meniscal lesion in the central region of the medial compartment and dislocation, effusion without response to corticosteroids, presence of denuded bone/full thickness cartilage defect |
| Exclusion criteria |
| Related to OA • Isolated patellofemoral OA • Chondromatosis or villonodular synovitis of the knee • Recent trauma responsible for the painful knee • Joint pathology following articular dysplasia, aseptic osteonecrosis, acromegaly, Paget’s disease, hemophilia, hemochromatosis • Inflammatory diseases such as rheumatoid arthritis, gouty arthritis, infectious arthritis or any pathology that may interfere with the evaluation of osteoarthritis OA (radiculalgia of the lower limbs, arteritis, etc) • Joint prosthesis already programmed for the duration of the study • Genu valgum Related to previous treatments • Strontium ranelate, bisphosphonates, selective estrogen receptor modulators (SERM), and parathyroid hormone (PTH) Related to associated diseases • Patients with associated severe disease (cardiovascular, renal, hepatic or infectious disease) • Tumor of the knee MRI contraindication |
OA = osteoarthritis; ACR = American College of Rheumatology; KL = Kellgren and Lawrence; MRI = magnetic resonance imaging.
Figure 1.
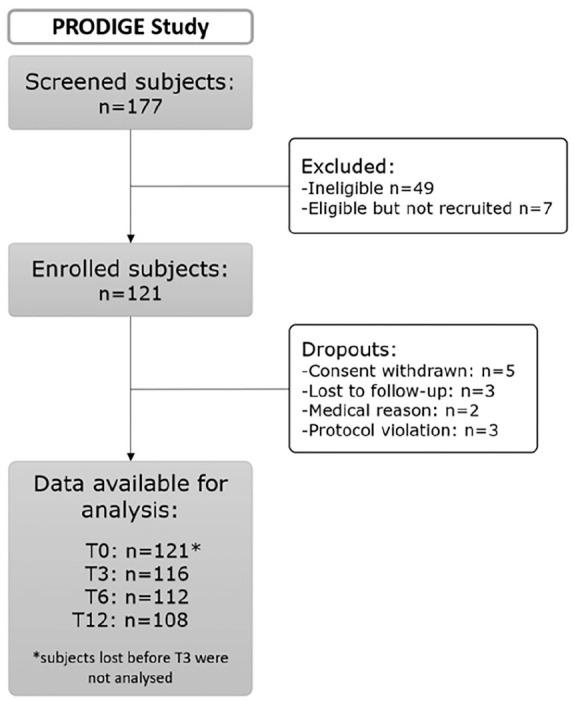
Patient flow diagram of PRODIGE study. A total of 121 subjects suffering from knee osteoarthritis were followed during one year.
Biomarkers Testing
Blood samples were collected by antecubital venepuncture with the subject in a sitting position. Samples were allowed to clot before being centrifuged at 1000g for 10 minutes. Sera were aliquoted and stored at −20°C until their analysis. Coll2-1 and Coll2-1NO2 were measured by competitive immunoassays (ARTIALIS SA, Liège, Belgium) at inclusion (baseline, T0), and after 3 (T3), 6 (T6), and 12 (T12) months. Testing was operated by the centralized biomarker platform (ARTIALIS SA) according to Good Clinical Laboratory Practice guidelines (GCLP/WHO). Samples were allocated to the different wells/plates according to a preestablished randomization list. Two controls and 2 serum samples were added to verify that interplate coefficients of variation (CV%) were below 20%. The detection and quantification limit was 67 nM for Coll2-1 and 117 pg/mL for Coll2-1NO2. Limit of detection (LOD) value was used for samples with concentration below the dynamic range of the assays.
Pain and Function Assessments
Participants completed the Knee Injury and Osteoarthritis Outcome Score (KOOS) survey in their native language. 12 Pain over the last 24 hours and Patient Global Assessment of Disease Activity (PGADA) were evaluated using a Visual Analogue Scale (VAS). Patients were classified according to the worsening of pain (“VAS progression”) or function (“KOOS progression”) at T3, T6, and T12 according to the OMERACT-OARSI responder criteria. 13 Classification was adapted because the PRODIGE study evaluates knee OA worsening instead of pain and function improvement. VAS progression was defined as a high worsening in VAS pain (pain percent change ≥50% and pain absolute change ≥20) or a high worsening in VAS PGADA (PGADA percent change ≥50% and PGADA absolute change ≥20), or a worsening in VAS pain (pain percent change ≥20% and pain absolute change ≥10) plus a worsening in VAS PGADA (PGADA percent change ≥20% and PGADA absolute change ≥10). KOOS progression was defined as a high worsening in KOOS pain (pain percent change ≥50% and pain absolute change ≥20) or a high worsening in KOOS function (KOOS function percent change ≥50% and KOOS function absolute change ≥20), or at least 2 of the 3 following: worsening of KOOS pain (KOOS pain percent change ≥20% and pain absolute change ≥10), KOOS function (KOOS function percent change ≥20% and KOOS function absolute change ≥10), and PGADA (PGADA change ≥20% and PGADA absolute change ≥10).
MRI Analysis
Semiquantitative scoring was assessed centrally by 2 trained radiologists specialized in MSK using the WORMS of the knee in OA. 14 They were trained for WORMS score on 10 MRI. Training was then validated on 20 imaging sets read twice by the 2 readers. This system evaluates 14 features: articular cartilage integrity, subarticular bone marrow abnormality (bone marrow lesion [BML]), subarticular cysts, subarticular bone attrition, marginal osteophytes, medial and lateral meniscal integrity, anterior and posterior cruciate ligament integrity, medial and lateral collateral ligament integrity, synovitis/effusion, intraarticular loose bodies, and periarticular cysts/bursitis. These features were evaluated in 15 different regions of the knee. 14 The baseline image was assessed first, and the 1-year-follow-up image was scored in comparison to the baseline image. All images were scored by the 2 radiologists independently. When the difference between baseline and follow-up was not in accordance between the 2 readers for any of the features, the readers were asked to find an agreement to give a final score. An increase of at least 1 grade in the summed score of WORMS cartilage, WORMS BML, WORMS effusion, or an increase of at least 2 grades in the summed total score were considered as indicative of OA progression (or worsening) over 1 year. A 1-grade increase is commonly accepted over short period of times.15-18
Analysis
Analyses were performed using the free and open-source software R Studio and the Statistical Analysis Software Statistica (Statsoft, France). Bilateral hypothesis tests were performed with a P-value <0.05 considered significant. No correction for multiple testing was made. Statistical testing was applied in order to highlight variables that have different distributions between progressors and nonprogressors. Wilcoxon-Mann-Whitney tests were applied for continuous variables and Fisher exact tests for categorical ones. In order to assess whether the joint use of variables can predict progression at T12, multivariate predictive modeling and variable selection were applied. Candidate models were generalized linear models and random forests. Their predictive performances were assessed in terms of area under the curve (AUC) within a cross-validation protocol. For these multivariate analyses, missing value imputation was applied when less than 10% values were missing. Variables were discarded otherwise. Finally, associations between variables were assessed using Spearman correlation tests for continuous variables, Kruskal tests when 1 variable was categorical, and Fisher’s exact test when the 2 variables were categorical.
Results
Subjects Demographics
Mean age was 62.7 ± 8.5 years, and mean BMI was 29.8 ± 5.1. The majority of the participants (59%) were female. A total of 45.4% of the 121 subjects had a KL grade of II and 39.7% had a KL grade of III. Mean OA duration was 8 ± 7 years, and mean pain during the 24 hours preceding the visit evaluated on a VAS was 36 ± 27 mm. Subjects’ demographics and OA history can be found in Table 2 .
Table 2.
Participants’ Characteristics.
| Characteristics | All participants |
|---|---|
| Participants’ characteristics (n = 121) | |
| Age (years), mean (SD) | 62.7 (8.5) |
| BMI (kg/m2), mean (SD) | 29.8 (5.1) |
| Gender | |
| Female | 59% |
| Male | 41% |
| OA history (n = 121) | |
| Index OA knee (% subjects) | |
| Right | 53.7 |
| Left | 46.3 |
| KL grade of the knee, n (% subjects) | |
| 0 | 0 |
| I | 3 (2.5%) |
| II | 55 (45.4%) |
| III | 48 (39.7%) |
| IV | 0 |
| Unknown | 15 (12.4%) |
| OA in other joint than index knee (%) | 74 |
| OA in collateral knee (%) | 60 |
| Concomitant hip OA (%) | 17 |
| Genu varum (%) | 25 |
| Previous meniscal surgery (%) | 26 |
| OA disease duration (years), mean (SD) | 8 (7) |
| Pain (n = 112) | |
| Pain intensity (visual scale, mm), mean (SD) | 36 (27) |
| PGADA (visual scale, mm), mean (SD) | 39 (29) |
| Function (n = 121) | |
| KOOS Pain (median [min-max]) | 58 [19-100] |
| KOOS Symptoms (median [min-max]) | 64 [17-100] |
| KOOS Activity (median [min-max]) | 66 [16-100] |
| KOOS Sports (median [min-max]) | 35 [0-100] |
| KOOS Quality of life (median [min-max]) | 44 [0-100] |
| Biomarkers | |
| Coll2-1 (nM, median [1st-3rd quartile]) (n = 111) | 483 [382-551] |
| Coll2-1NO2 (pg/mL, median [1st-3rd quartile]) (n = 116) | 326 [243-476] |
OA = osteoarthritis; BMI = body mass index; KL = Kellgren and Lawrence; PGADA = Patient Global Assessment of Disease Activity; KOOS = Knee Injury and Osteoarthritis Outcome Score.
Baseline Associations
Baseline Coll2-1 tended to be positively correlated with the total WORMS score (ρ = 0.20; P = 0.055; Table 3 ). Significant associations were found for Coll2-1 with periarticular cysts/bursitis (ρ = 0.29, P < 0.01), bone attrition (ρ = 0.25, P < 0.05), subarticular cysts (ρ = 0.24, P < 0.05), and articular cartilage integrity (ρ = 0.23, P < 0.05) WORMS subscores for the whole joint as well as with the medial femorotibial joint sum score (ρ = 0.26, P < 0.05) and medial femorotibial joint cartilage (ρ = 0.23, P < 0.05). Baseline Coll2-1NO2 was positively correlated with baseline WORMS total score (ρ = 0.23, P < 0.05), WORMS scores in the patellofemoral (ρ = 0.23, P < 0.05) and medial femorotibial compartments (ρ = 0.21, P = 0.03), with osteophytes scores (ρ = 0.27, P < 0.01), subarticular cysts (ρ = 0.24, P < 0.05), and intraarticular loose bodies (ρ = 0.27, P < 0.01). At baseline, Coll2-1 was found to be significantly associated with age (ρ = 0.32, P < 0.001) and history of meniscal surgery (P < 0.05). Coll2-1 and Coll2-1NO2 were also significantly correlated at baseline (ρ = 0.22, P < 0.05). None of the markers were associated with mean pain during the 24h preceding the visit (VAS), PGADA, or with pain and function evaluated with the KOOS.
Table 3.
Correlation Matrix between Baseline Serum Coll2-1NO2 and Baseline Serum Coll2-1 with Participants’ Characteristics and Medical History, Pain and Function (KOOS), Pain (VAS), and Structural Features Assessed with MRI (WORMS) Scores a .
| Coll2-1NO2 |
Coll2-1 |
||||
|---|---|---|---|---|---|
| Correlation | ρ Corr. | P Value | ρ Corr. | P Value | |
| Participants characteristics and medical history | |||||
| Age | Spearman | 0.07 | 0.44 | 0.33 | <0.001 |
| BMI | Spearman | 0.02 | 0.80 | 0.03 | 0.72 |
| Sex | Kruskal | 0.09 | 0.76 | 0.33 | 0.56 |
| KL grade | Kruskal | 1.60 | 0.45 | 0.39 | 0.82 |
| OA in collateral knee | Kruskal | 0.01 | 0.92 | 1.01 | 0.31 |
| Concomitant hip OA | Kruskal | 2.95 | 0.09 | 1.14 | 0.29 |
| Previous meniscal surgery | Kruskal | 2.11 | 0.15 | 6.30 | <0.05 |
| OA in other joint than index knee | Kruskal | 0.13 | 0.72 | 0.73 | 0.39 |
| Pain and function (KOOS) | |||||
| KOOS Total | Spearman | −0.05 | 0.58 | 0.03 | 0.72 |
| KOOS Pain | Spearman | −0.07 | 0.49 | −0.05 | 0.59 |
| KOOS Knee-related quality of life | Spearman | 0.00 | 0.98 | 0.18 | 0.06 |
| KOOS Function in sport and recreation | Spearman | −0.08 | 0.38 | 0.02 | 0.80 |
| KOOS Symptoms | Spearman | −0.04 | 0.71 | 0.03 | 0.76 |
| KOOS Function in daily living | Spearman | −0.06 | 0.54 | −0.04 | 0.69 |
| Pain (VAS) | |||||
| Pain intensity | Spearman | −0.09 | 0.35 | −0.10 | 0.317 |
| PGADA | Spearman | 0.08 | 0.45 | −0.11 | 0.284 |
| WORMS | |||||
| Total | Spearman | 0.23 | <0.05 | 0.20 | 0.055 |
| LTF | Spearman | 0.11 | 0.27 | −0.02 | 0.85 |
| MTF | Spearman | 0.22 | <0.05 | 0.26 | <0.05 |
| PF | Spearman | 0.23 | <0.05 | 0.13 | 0.21 |
| WORMS articular cartilage integrity | |||||
| Total | Spearman | 0.17 | 0.10 | 0.23 | <0.05 |
| LTF | Spearman | 0.05 | 0.64 | 0.01 | 0.91 |
| MTF | Spearman | 0.11 | 0.27 | 0.23 | <0.05 |
| PF | Spearman | 0.16 | 0.12 | 0.18 | 0.08 |
| WORMS subarticular bone marrow abnormality (BML) | |||||
| Total | Spearman | 0.11 | 0.30 | 0.09 | 0.37 |
| MTF | Spearman | 0.10 | 0.32 | 0.14 | 0.17 |
| PF | Spearman | −0.06 | 0.54 | 0.09 | 0.40 |
| WORMS Other features | |||||
| Periarticular cysts/bursitis | Spearman | −0.01 | 0.94 | 0.29 | <0.01 |
| Synovitis/effusion | Spearman | 0.06 | 0.53 | 0.08 | 0.45 |
| Total lateral meniscal integrity | Spearman | −0.01 | 0.91 | −0.18 | 0.07 |
| Total medial meniscal integrity | Spearman | 0.03 | 0.78 | 0.20 | 0.05 |
| Intraarticular loose bodies | Spearman | 0.27 | <0.01 | 0.14 | 0.17 |
| Subarticular bone attrition | Spearman | 0.10 | 0.32 | 0.25 | <0.05 |
| Subarticular cysts | Spearman | 0.20 | 0.05 | 0.24 | <0.05 |
| Ligaments integrity | Spearman | 0.10 | 0.31 | 0.03 | 0.78 |
| Osteophytes | Spearman | 0.27 | <0.01 | 0.11 | 0.30 |
Corr. = partial correlation; MRI = magnetic resonance imaging; OA = osteoarthritis; BMI = body mass index; KL = Kellgren and Lawrence; KOOS = Knee Injury and Osteoarthritis Outcome Score; VAS = Visual Analogue Scale; PGADA = Patient Global Assessment of Disease Activity; WORMS = Whole-Organ Magnetic Resonance Imaging Score; LTF = lateral tibiofemoral; MTF = medial tibiofemoral; PF = patellofemoral.
Bold represents P < 0.05.
Further analysis showed that neither biomarkers’ baseline values, pain, PGADA, nor KOOS subscales differentiated polyOA subjects from mono-osteoarthritis (monoOA) subjects.
Knee OA Progressors
A total of 29.3% of the patients showed an increase in WORMS total score (Δ ≥ 2), 38.4% had an increase in subarticular BML subscore (Δ ≥ 1), 16.2% in articular cartilage integrity subscore (Δ ≥ 1), and only 7.1% in synovitis/effusion subscore (Δ ≥ 1). A total of 34.0% and 31.5% were pain (VAS) and KOOS progressors, respectively ( Table 4 ). The proportion of progressors and nonprogressors were similar among the total PRODIGE population, polyOA and monoOA subgroups for all progression criteria except for total WORMS score. The presence of polyOA was strongly associated with total WORMS progression at 1 year (P = 0.01).
Table 4.
Number and Percentages of Progressors and Nonprogressors According to Structure (WORMS), Pain and Function for the Whole Population and for “PolyOA” and “MonoOA” Subgroups a .
| Subgroup | OA Progressor | Noniprogressor | P Value (Fisher) | |
|---|---|---|---|---|
| Structural (WORMS) | ||||
| WORMS total | All population | 29 (29.3%) | 70 (70.7%) | |
| PolyOA | 27 (36.5%) | 47 (63.5%) | 0.33 | |
| MonoOA | 2 (8%) | 23 (92%) | <0.05 b | |
| Bone marrow lesion | All population | 38 (38.4%) | 61 (61.6%) | |
| PolyOA | 32 (43.2%) | 42 (56.8%) | 0.53 | |
| MonoOA | 6 (24%) | 19 (76%) | 0.10 | |
| Cartilage | All population | 16 (16.2%) | 83 (83.8%) | |
| PolyOA | 11 (14.9%) | 63 (85.1%) | 1 | |
| MonoOA | 5 (20%) | 20 (80%) | 0.54 | |
| Effusion | All population | 7 (7.1%) | 92 (92.9%) | |
| PolyOA | 6 (8.1%) | 68 (91.9%) | 1 | |
| MonoOA | 1 (4%) | 24 (96%) | 0.67 | |
| Pain and function | ||||
| Pain (VAS) | All population | 32 (34.0%) | 62 (66.0%) | |
| PolyOA | 27 (38.6%) | 43 (61.4%) | 0.62 | |
| MonoOA | 5 (20.8%) | 19 (79.2%) | 0.14 | |
| KOOS | All population | 34 (31.5%) | 74 (68.5%) | |
| PolyOA | 25 (30.5%) | 57 (69.5%) | 1 | |
| MonoOA | 9 (34.6%) | 17 (65.4%) | 0.81 | |
OA = osteoarthritis; WORMS = Whole-Organ Magnetic Resonance Imaging Score; VAS = Visual Analogue Scale.
Bilateral Fisher exact tests assess the difference between “all population” versus “polyOA” and “polyOA” versus “monoOA.” The percentage of progressors and nonprogressors is calculated on available data only.
Bold represents P < 0.05.
Baseline Biomarker Values Association with Knee OA Progression
In multivariate analyses, none of the 2 biochemical markers at baseline was significantly associated with a structural, pain, or functional progression of OA. However, univariate analyses showed that baseline Coll2-1NO2 was higher in VAS pain progressors (426.4 pg/mL [278.04-566.95], N = 31) as compared to nonprogressors (306.84 [200.37-427.84], N = 60) over 1 year (AUC = 0.655, P = 0.015). This was also observed in the polyOA subgroup (428.45 [276.93-598.07], N = 26 for progressors, 295.16 [200.19-408.74], N = 42 for nonprogressors, AUC = 0.690, P = 0.009) but not in the monoOA subgroup ( Fig. 2 ).
Figure 2.
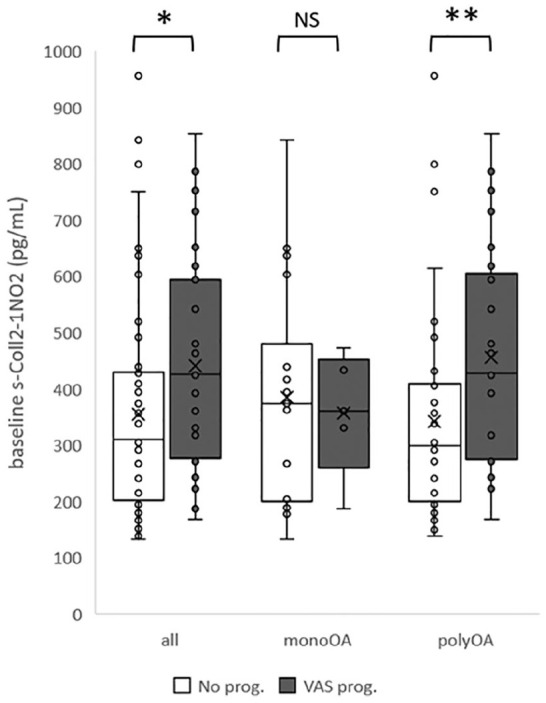
Box and whisker plots illustrate that baseline Coll2-1NO2 is higher in subjects whose VAS pain increases after 1 year (VAS prog., black) compared to subjects with no increase of VAS pain (No prog., white) in general population (all) as well as in polyosteoarthritis subgroup (polyOA) but not in monoosteoarthritis (monoOA) subgroup. prog. = progressors. *P < 0.05, **P < 0.01.
Baseline Characteristics Correlation with Knee OA Progression
Higher BMI, age, WORMS total score, BML, and cysts subscores were associated with WORMS articular cartilage integrity worsening (P < 0.05). Progression of WORMS subarticular BML was only associated with a lower score of KOOS symptoms. Worsening of WORMS total score was associated with higher VAS pain and PGADA values and lower KOOS values for symptoms, pain, and impairment in daily living subscores, indicating that patients with more symptoms at baseline show a higher progression of WORMS total score at 1 year. The only structural feature associated with a progression of WORMS total score was a higher score for subarticular cysts. In addition to higher Coll2-1NO2 concentration, VAS pain worsening was associated with less pain intensity and PGADA at baseline. KOOS worsening was associated with higher basal values of KOOS pain and KOOS knee-related quality of life, meaning that less of these symptoms were associated with a worsening in KOOS function. Gender, baseline WORMS medial menisca integrity, and synovitis/effusion were also associated with KOOS progression at 1-year follow-up ( Table 5 , all P < 0.05).
Table 5.
Median Values (Quantitative Variables) or Number of Subjects (Qualitative Variable) for Baseline Biomarkers sColl2-1, Coll2-1NO2, Demographics, Pain and Function, and Structural Knee Features According to Knee OA Progression at 1 Year Assessed with WORMS (Total, Cartilage, and BML), Pain (VAS) and Function (KOOS) a .
| Progression Criteria | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WORMS Total |
WORMS Cartilage |
WORMS BML |
VAS Pain |
KOOS | ||||||
| Baseline Data | Prog. | No Prog. | Prog. | No Prog. | Prog. | No Prog. | Prog. | No Prog. | Prog. | No Prog. |
| Biomarkers | ||||||||||
| Coll2-1 (nM) | 494 | 477 | 544 | 478 | 498 | 458 | 495 | 467 | 450 | 495 |
| Coll2-1NO2 (pg/mL) | 312 | 331 | 374 | 312 | 327 | 330 | 428 | 306* | 350 | 310 |
| Demographics | ||||||||||
| Age (years) | 64 | 62.5 | 69 | 62* | 62 | 63 | 63 | 63 | 64 | 63 |
| BMI (kg/m2) | 28.9 | 29.4 | 33.9 | 28.9* | 28.9 | 29.8 | 28.0 | 30.0 | 29.1 | 29.9 |
| Gender (male/female) | 8/21 | 34/36 | 7/9 | 35/48 | 13/25 | 29/32 | 14/18 | 27/35 | 8/26 | 36/38* |
| PolyOA (yes/no) | 27/2 | 47/23* | 11/5 | 63/20 | 32/6 | 42/19 | 27/5 | 43/19 | 25/9 | 57/17 |
| Pain | ||||||||||
| Pain Intensity Scale (mm) | 44 | 24* | 45 | 32 | 37 | 28 | 13 | 43* | 20 | 36 |
| Pain Impact Scale (mm) | 48 | 29* | 41 | 30 | 31 | 37 | 13 | 44* | 22 | 41 |
| Structure—X-rays | ||||||||||
| KL grade (grade II/grade III) | 8/16 | 35/27 | 4/11 | 39/32 | 14/20 | 29/23 | 11/17 | 33/20 | 19/9 | 30/35 |
| Function | ||||||||||
| KOOS Pain | 53 | 66* | 53 | 61 | 57 | 64 | 67 | 61 | 67 | 58* |
| KOOS Symptoms | 54 | 71* | 59 | 64 | 57 | 71* | 70 | 61 | 70 | 59 |
| KOOS Function in daily living | 57 | 71* | 66 | 66 | 59 | 69 | 69 | 66 | 70 | 63 |
| KOOS Function in sport and recreation | 25 | 40 | 25 | 40 | 25 | 40 | 40 | 38 | 38 | 30 |
| KOOS Knee-related quality of life | 38 | 44 | 44 | 44 | 44 | 44 | 50 | 40 | 60 | 38* |
| Structure—WORMS MRI | ||||||||||
| Total | 81 | 74 | 107 | 75* | 81 | 74 | 73 | 76 | 71 | 81 |
| Articular cartilage integrity | 32 | 32 | 39 | 32 | 32 | 32 | 32 | 32 | 27 | 35 |
| Subarticular bone marrow abnormality (BML) | 5 | 3 | 5 | 3* | 3 | 4 | 3 | 3 | 4 | 3 |
| Total lateral meniscal integrity | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Total medial meniscal integrity | 4 | 4 | 5 | 4 | 4 | 3 | 4 | 4 | 3 | 4* |
| Subarticular cysts | 4 | 2* | 4 | 3* | 3 | 3 | 3 | 2 | 3 | 3 |
| Ligaments integrity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Osteophytes | 27 | 29 | 44 | 28 | 34 | 27 | 28 | 28 | 28 | 29 |
| Periarticular cysts/bursitis | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 |
| Synovitis/effusion | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1* |
| Intraarticular Loose bodies | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subarticular bone attrition | 1 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
WORMS = Whole-Organ Magnetic Resonance Imaging Score; BML = bone marrow lesion; VAS = Visual Analogue Scale; BMI = body mass index; KL = Kellgren and Lawrence; KOOS = Knee Injury and Osteoarthritis Outcome Score; MRI = magnetic resonance imaging.
Asterisk represents P < 0.05 with Mann-Whitney test or bilateral Fisher exact test.
Bold indicate values which are different between progressors and non- progressors
Longitudinal Changes in Biomarkers Associated with Knee OA Progression
Multivariate analyses did not show significant association of Coll2-1 nor Coll2-1NO2 change over 3 or 6 months with structural, pain, or functional progression of OA. However, univariate analyses showed that Coll2-1 change at 3 and 6 months were significantly different in WORMS cartilage integrity progressors (T3/T0, 0.89 [0.82-1.10], N = 16; T6/T0, 1.00 [0.91-1.15], N = 16) as compared to nonprogressors (T3/T0, 1.07 [0.92-1.23], N = 81; T6/T0, 1.14 [1.02-1.36], N = 80) over 1 year (T3/T0, AUC = 0.68, P = 0.023; T6/T0, AUC = 0.675, P = 0.028; Fig. 3 ). Statistical significance was not observed in the polyOA and monoOA subgroups (N.S. for both ratios).
Figure 3.
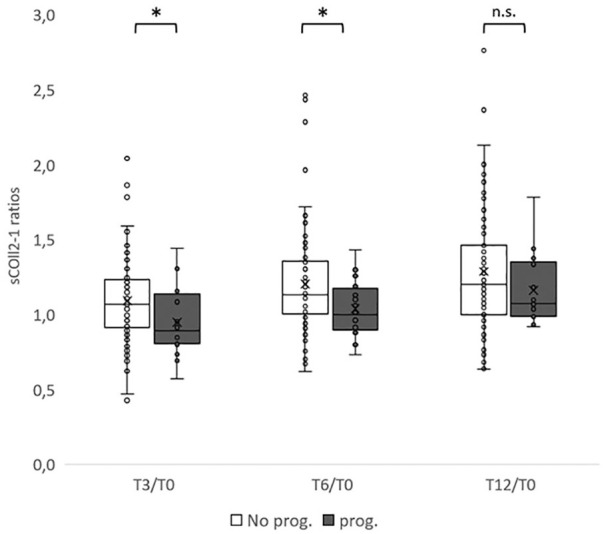
Box and whisker plots of Coll2-1 change at T3, T6, and T12 as compared to baseline values (T0) for WORMS cartilage progressors (gray) and nonprogressors (white) at 1 year. Coll2-1 ratios are lower in subjects that are progressors according to WORMS cartilage score at T3 and T6 but not at T12. prog. = progressors. *P < 0.05, n.s. (nonsignificant).
Discussion
Our study has shown significant positive correlations between the serum biomarkers Coll2-1 and Coll2-1NO2 and various MRI structural knee features. Of note, Coll2-1 correlated with WORMS cartilage lesions in the medial femorotibial compartment as well as for the whole joint, with periarticular cysts/bursitis, subarticular bone attrition, and subarticular cysts. Articular cartilage degradation over 1 year was also associated with a change of Coll2-1.
Analysis of baseline data shows that Coll2-1NO2 concentration is associated with structural damages of the knee, such as WORMS total score, WORMS scores in the patellofemoral and medial femorotibial compartments, with osteophytes and subarticular cysts WORMS subscores. These observations are consistent with a recent study of the American Foundation for the National Institutes of Health (fNIH) on the association between molecular markers and joint morphology, during which the authors found an association of serum Coll2-1NO2 with cartilage and meniscus WORMS scores in various compartments (i.e., cartilage WORMS SUM [Summation of WORMS scores across all regions], MAX [Maximum WORMS scores in any region], lateral femoral condyle, lateral tibia, patella and trochlea; meniscus WORMS MAX, lateral body and lateral posterior). 10 Other studies had also concluded that serum Coll2-1NO2 was associated with radiological findings. 7 Accordingly, serum Coll2-1NO2 marker could be of particular interest for longitudinal studies.
There are a few limitations in the interpretation for our study. The cross-sectional study design showed associations rather than causal relationship between the biomarkers and structural features in knee OA. The associations may give insight into the pathogenesis of knee OA, but the study design does not allow evaluation of pathogenic mechanisms. The sample size of the study was small and may result in limited statistical power to demonstrate correlation between soluble biomarkers and some MRI item progression. For example, the experimental population had around 30% of patients identified as progressors over 1 year in each category, except for effusion. The low number of patients showing a progression on effusion seen on MRI (7%) must be taken into account when drawing conclusions in the effusion subgroup. Semiquantitative MRI is dependent on the reader and on the quality of image acquisition. Small changes such as one grade change are commonly used in OA clinical trials over short period of time such as in the PRODIGE study.19-21 In our study, we considered 2 grades changes for WORMS total score and 1 grade change for cartilage, BML, and effusion subscores to be over the interreader coefficient of variation observed during reader’s training.
Notwithstanding these considerations, our data indicate that baseline structural characteristics are indicative of OA evolution assessed by MRI. For example, KL grade assessed on X-rays, BML, and cysts assessed on MRI were associated with subsequent cartilage degradation worsening. Similar findings have been observed in the MOST study, with radiographic OA, cartilage damage, and meniscal lesions being associated with cartilage thickness loss. 22 Consistent with a systematic review on patients characteristics’ predictive value on knee OA, 23 we found that age, BMI, and the presence of polyOA seem to be predictors of WORMS cartilage or WORMS total scores evolution.
Our data do not indicate any association between baseline structural characteristics and the evolution of pain; on the contrary, we found that higher pain and higher symptoms are correlated with a structural worsening of knee OA defined with the WORMS total score evolution. Associations of pain and structure are conflicting among published work with several studies reporting some predictive value of pain on structure, of structure on pain, as well as the contrary.24,25
Pain and PGADA at baseline seem to be good predictors of pain evolution according to our data. This can be explained by the fact that subjects with low pain are the more susceptible to show an increase in pain according to our definition while subjects with a high pain at baseline are less keen to reach the thresholds of pain increase. The same is observed with function assessed with KOOS questionnaire and can be, at least in part, explained in the same way. However, our study also shows that the baseline value of biochemical marker Coll2-1NO2 is positively associated with subsequent progression of VAS pain. Subjects that are classified after 1-year follow-up as pain progressors had greater baseline values of Coll2-1NO2 than subjects without pain change. It is interesting to see that the baseline value of Coll2-1NO2 is also correlated with subsequent progression of VAS pain in the subgroup of polyOA subjects. These new findings could be of special interest for targeting the right patient to treat. Furthermore, in contrast to VAS evaluation, Coll2-1NO2 is an objective biomarker that could be used to select pain progressors in clinical trials.
In conclusion, cartilage biomarkers Coll2-1 and Coll2-1NO2 are correlated with several knee features quantified with WORMS scoring system on MRI. Therefore, they could be indicative of the burden of OA disease. Furthermore, they are correlated with different knee MRI features suggesting that they could investigate different OA phenotypes. This should be confirmed in the future in larger cohort designed to investigate a panel of OA phenotypes. Basal serum values of Coll2-1NO2 were also correlated with a worsening of target knee pain over 1 year. It would be interesting to research on a larger population the association between COll2-1NO2 and synovitis features. Results show that Coll2-1 and Coll2-1NO2, in association with other structural features, pain and function, could help at identifying OA phenotypes and patients at risk of OA worsening.
Footnotes
Author Contributions: ACH and YH conceived and designed the study. MM, DL, TC, and YM were investigators of the study and collected patients’ data; FP analyzed the imaging data. CT and ACH made the statistical analyses. ACH and YH wrote the manuscript. All authors revised the article critically for important intellectual content. All authors have given final approval of the version to be published. All authors agreed to be accountable for all aspects of the work.
Acknowledgments and Funding: The authors thank the patients for their engagement in the PRODIGE study. The authors thank Dr. Jean-Marc Lemaire for MRI scoring. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: PRODIGE study (NCT02070224) was performed in the framework of a convention between the Walloon region and ARTIALIS SA (convention no. 6905).
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: YH is the founder and the President of ARTIALIS SA, a spin-off company of the University of Liege. He has also received fees from Bepharbel, GSK, KiOmed Pharma SA (formerly Synolyne Pharma SA), Nestle, Genequine, Flexion Therapeutics, IBSA, BioIberica, Laboratoires Expanscience, Royal Canin, MagPharm, LABRHA, Pfizer, Thuasne, and Tilman SA. ACH is employee of ARTIALIS S.A. The other authors have no conflicts of interest to declare.
Ethical Approval: The study protocol was approved by the Belgian Central Ethics Committee (EC) from the University Hospital of Liege, the French Ethics Committee (Comité de Protection des Personnes EST III [CPP III]), and the French National Agency for Medicines and Health Products Safety (ANSM). The National Number was B707201318719 for Belgium and ID RCB 2013-A01368-37 for France.
Informed Consent: Written informed consent was obtained from individuals after the nature of the study was explained and understood, in accordance with International Conference on Harmonisation Good Clinical Practice (ICH GCP).
ORCID iD: Anne-Christine Hick  https://orcid.org/0000-0001-5931-052X
https://orcid.org/0000-0001-5931-052X
References
- 1. Van Spil WE, Kubassova O, Boesen M, Bay-Jensen AC, Mobasheri A. Osteoarthritis phenotypes and novel therapeutic targets. Biochem Pharmacol. 2019;165:41-8. [DOI] [PubMed] [Google Scholar]
- 2. APPROACH Project. Accessed May 18, 2021. www.approachproject.eu
- 3. Mobasheri A, van Spil WE, Budd E, Uzieliene I, Bernotiene E, Bay-Jensen AC, et al. Molecular taxonomy of osteoarthritis for patient stratification, disease management and drug development: biochemical markers associated with emerging clinical phenotypes and molecular endotypes. Curr Opin Rheumatol. 2019;31(1_suppl):80-9. [DOI] [PubMed] [Google Scholar]
- 4. Cancedda R. Cartilage and bone extracellular matrix. Curr Pharm Des. 2009;15(12):1334-48. [DOI] [PubMed] [Google Scholar]
- 5. Mobasheri A, Lambert C, Henrotin Y. Coll2-1 and Coll2-1NO2 as exemplars of collagen extracellular matrix turnover–biomarkers to facilitate the treatment of osteoarthritis? Exp Rev Mol Diagn. 2019;19(9):803-12. [DOI] [PubMed] [Google Scholar]
- 6. Deberg M, Dubuc JE, Labasse A, Sanchez C, Quettier E, Bosseloir A, et al. One-year follow-up of Coll2-1, Coll2-1NO2 and myeloperoxidase serum levels in osteoarthritis patients after hip or knee replacement. Ann Rheum Dis. 2008;67(2):168-74. doi: 10.1136/ard.2007.073452 [DOI] [PubMed] [Google Scholar]
- 7. Henrotin Y, Chevalier X, Deberg M, Balblanc JC, Richette P, Mulleman D, et al. Early decrease of serum biomarkers of type II collagen degradation (Coll2-1) and joint inflammation (Coll2-1 NO2) by hyaluronic acid intra-articular injections in patients with knee osteoarthritis: a research study part of the Biovisco study. J Orthop Res. 2013;31(6):901-7. [DOI] [PubMed] [Google Scholar]
- 8. Hick AC, Fonck M, Costes B, Cobraiville E, Pirson S, Garcia L, et al. Serum Levels of Coll2-1, a specific biomarker of cartilage degradation, are not affected by sampling conditions, circadian rhythm, and seasonality. Cartilage. doi: 10.1177/1947603519878489. Epub Oct 20, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deberg M, Labasse A, Christgau S, Cloos P, Henriksen DB, Chapelle JP, et al. New serum biochemical markers (Coll 2-1 and Coll 2-1 NO2) for studying oxidative-related type II collagen network degradation in patients with osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2005;13(3):258-65. [DOI] [PubMed] [Google Scholar]
- 10. Joseph GB, Nevitt MC, McCulloch CE, Neumann J, Lynch JA, Heilmeier U, et al. Associations between molecular biomarkers and MR-based cartilage composition and knee joint morphology: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2018;26(8):1070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29(8):1039-49. [DOI] [PubMed] [Google Scholar]
- 12. Roos EM, Lohmander LS. Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pham T, Van Der Heijde D, Lassere M, Altman RD, Anderson JJ, Bellamy N, et al. Outcome variables for osteoarthritis clinical trials: the OMERACT-OARSI set of responder criteria. J Rheumatol. 2003;30(7):1648-54. [PubMed] [Google Scholar]
- 14. Peterfy CG, Guermazi A, Zaim S, Tirman PFJ, Miaux Y, White D, et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177-90. [DOI] [PubMed] [Google Scholar]
- 15. Culvenor AG, Segal NA, Guermazi A, Roemer F, Felson DT, Nevitt MC, et al. The sex-specific influence of quadriceps weakness on worsening patellofemoral and tibiofemoral cartilage damage: the MOST study. Arthritis Care Res (Hoboken). 2019;71(10):1360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Teng HL, MacLeod TD, Link TM, Majumdar S, Souza RB. Higher knee flexion moment during the second half of the stance phase of gait is associated with the progression of osteoarthritis of the patellofemoral joint on magnetic resonance imaging. J Orthop Sports Phys Ther. 2015;45(9):656-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee B, Parvizi J, Bramlet D, Romness DW, Gumazi A, Noh M, et al. Results of a phase II randomized study to determine the efficacy and safety of genetically engineered allogeneic human chondrocytes expressing TGF-β1. J Knee Surg. 2020;33:167-72. [DOI] [PubMed] [Google Scholar]
- 18. Goldman LH, Tang K, Facchetti L, Heilmeier U, Joseph GB, Nevitt MC, et al. Role of thigh muscle cross-sectional area and strength in progression of knee cartilage degeneration over 48 months—data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2016;24(12):2082-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roemer FW, Nevitt MC, Felson DT, Niu J, Lynch JA, Crema MD, et al. Predictive validity of within-grade scoring of longitudinal changes of MRI-based cartilage morphology and bone marrow lesion assessment in the tibio-femoral joint–the MOST study. Osteoarthritis Cartilage. 2012;20(11):1391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roemer FW, Guermazi A, Javaid MK, Lynch JA, Niu J, Zhang Y, et al. Change in MRI-detected subchondral bone marrow lesions is associated with cartilage loss: the MOST study. A longitudinal multicentre study of knee osteoarthritis. Ann Rheum Dis. 2009;68(9):1461-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roemer FW, Zhang Y, Niu J, Lynch JA, Crema MD, Marra MD, et al. Tibiofemoral joint osteoarthritis: risk factors for MR-depicted fast cartilage loss over a 30-month period in the multicenter osteoarthritis study. Radiology. 2009;252(3):772-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guermazi A, Eckstein F, Hayashi D, Roemer FW, Wirth W, Yang T, et al. Baseline radiographic osteoarthritis and semi-quantitatively assessed meniscal damage and extrusion and cartilage damage on MRI is related to quantitatively defined cartilage thickness loss in knee osteoarthritis: the Multicenter Osteoarthritis Study. Osteoarthritis Cartilage. 2015;23(12):2191-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chapple CM, Nicholson H, Baxter GD, Abbott JH. Patient characteristics that predict progression of knee osteoarthritis: a systematic review of prognostic studies. Arthritis Care Res (Hoboken). 2011;63(8):1115-25. [DOI] [PubMed] [Google Scholar]
- 24. Joseph GB, Hou SW, Nardo L, Heilmeier U, Nevitt MC, McCulloch CE, et al. MRI findings associated with development of incident knee pain over 48 months: data from the osteoarthritis initiative. Skeletal Radiol. 2016;45(5):653-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wegrzyn J, D’Apuzzo MR, Amrami KK, Larson DR, Kaufman KR. Could Whole-Organ Magnetic Resonance Imaging Score (WORMS) components predict pain in knee osteoarthritis? Motion Analysis Laboratory; 2012. [Google Scholar]


