Abstract
Objective
A systematic review and meta-analysis of Autologous Matrix-Induced Chondrogenesis (AMIC®) outcomes for grade III/IV chondral and osteochondral lesions of the knee treated with Chondro-Gide®.
Design
Studies with a minimum follow-up of 1 year providing clinical results of AMIC repair in the knee were included based on PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses). Methodological quality was assessed by the modified Coleman Methodology Score (mCMS). The meta-analysis was comparing pain VAS (Visual Analog Scale), Lysholm score, and IKDC score (International Knee Documentation Committee) between baseline and follow-up after 1 or 2 years and after >3 years.
Results
Twelve studies (375 patients) were included. The mCMS demonstrated a suboptimal study design (ranking between 52 and 80). The mean age was 36.2 years (14-70 years). The mean defect size was 4.24 cm2 (0.8-22 cm2). The results from the random effects model indicated a clinically significant (P < 0.05) improvement of pain VAS from baseline to follow-up at year 1 to 2 of −4.02(confidence interval −4.37; −3.67), still significant after 3 years. Lysholm score at year 1 or 2 improved significantly and remained highly significant after 3 years. IKDC score showed highly significant improvement of 32.61 between 1 and 2 years versus baseline values maintained after 3 years.
Conclusions
The AMIC procedure significantly improved the clinical status and functional scoring versus preoperative values. Evidence was obtained in a non-selected patient population, corresponding to real-life treatment of knee chondral and osteochondral defects. The evidence is sufficient to recommend AMIC in this indication.
Keywords: autologous matrix-induced chondrogenesis (AMIC®), microfracture, Chondro-Gide®, cartilage, knee
Introduction
Articular cartilage lesions caused by trauma, osteochondritis dissecans, or malalignment are a common pathology of the knee joint in young patients resulting in pain and function loss. In a retrospective analysis of 25,124 knee arthroscopy patients, chondral lesions were found in 60% of them. A total of 7% of all patients aged less than 40, and 9% under the age of 50 years, showed 1 to 3 localized grade III or IV cartilage defects according to the Outerbridge classification. 1 More than 200,000 surgical procedures for knee cartilage repair are performed annually in the United States. 2 Full-thickness articular cartilage defects only have limited regenerative potential, and spontaneous healing is unlikely. Untreated full-thickness cartilage lesions are usually associated with significant pain and arthritis, which is a major cause of disability and represents a significant socioeconomic burden. The frequent outcome for arthritis in large joints such as the knee is joint replacement, which is largely successful in older sedentary patients but less desirable for young patients.
Various surgical procedures have been developed to reduce pain and prevent/postpone the need for knee replacement while withstanding the daily activities of the patient. Surgery is primarily indicated for patients with grade III or IV defects according to Outerbridge classification. Surgical techniques include the following:
Palliative options including debridement and lavage.
Reparative options including drilling/microfracture (MFx) to stimulate migration of bone marrow cells into the cartilage defect.
Reconstructive options including osteochondral autograft transfer system (OATS®) and osteochondral allograft transplantation.
Restorative options including autologous chondrocyte implantation (ACI®).
Microfracture is a single-step procedure developed by Steadman in 1980 that can be performed arthroscopically or with a minimally invasive approach. It has gained popularity due the minimal invasive option, technical simplicity, limited surgical morbidity, low cost, and because it does not rule out other procedures although it was reported to reduce the likelihood of success with later ACI. 3 Out of 150,000 to 200,000 US Americans who undergo knee surgery for cartilage lesions every year, an estimated 60,000 are treated with this technique. Moreover, the MFx technique has been shown to be the preferred method for the treatment of articular cartilage defects in recreational and professional athletes. It is one of the world’s most frequently used cartilage repair techniques and is a currently accepted first-line treatment for full-thickness articular cartilage defects <2 to 3 cm2.
In a recent review article, Frank et al. 2 analyzed indications and failures of MFx. They summarized the existing evidence rising from several papers as follows:
Mithoefer et al. 4 : 28 studies, 3,000 patients, lesion size <4 cm2 for nonathletes and <2 cm2 for athletes. Knee function consistently improved in the first 24 months; however only 67% to 85% of patients continued to report improved outcomes between 2 and 5 years postoperatively.
Goyal et al. 5 confirmed the conclusion of Mithoefer et al. 4 and noted frequent progression to osteoarthritis in patients with lesions >4 cm2 just 5 years after the procedure.
Solheim et al. 6 reported the 12-year outcomes of MFx in 110 patients. They noted a high rate of conversion to knee arthroplasty (n = 7), with 45% of the patients presenting poor outcome (Lysholm score <64) at 14 to 15 years.
In the case series of Gobbi et al., 7 in 61 athletes there was a gradual drop in pain and functional scores over time and 11% of patients were considered as failures.
Additional information was provided by Knutsen et al. 8 who compared in a randomized multicenter trial, ACI with MFx, with a follow-up at 14 to 15 years. They reported at 15 years evaluation 17 failures in the ACI group compared with 13 in the MFx group, in opposition to the previous report of Basad et al. 9 No significant difference between the groups was found at long-term follow-up. Fifty-seven percent of the surviving patients in the ACI group and 47% of such patients in the MFx group had radiographic evidence of early osteoarthritis, the difference being nonsignificant.
Although multiple studies showed the limited successful outcomes of MFx for patients >40 with large >3 to 4 cm2 or patellar lesions, marrow stimulation remains the one technique covered by nearly all health care systems and insurance companies around the world.10,11 Seeking clarity regarding the efficacy of MFx, a recent Cochrane review of Gracitelli et al. 12 examined the evidence from randomized trials comparing MFx to other treatment options. The conclusion was that the currently available evidence is not enough to determine whether mosaicplasty, allograft transplantation, or MFx is better for treating cartilage defects in adults. The long-term results of MFx established a failure rate of about 25% at 10 years requiring an additional intervention. The Health Technology Assessment (NICE 2017) also pointed out the lack of long-term results for MFx with linearly increasing hazard (probability of failure increasing with time).
Given the limitations of MFx, efforts have focused on modifications and augmentation techniques for improving the quality of the repair tissue. Some methods improve availability of cells and growth factors, others additionally provide a scaffold on which stem cells can proliferate.
In 2003, Prof. Behrens and Prof. Steinwachs proposed to enhance MFx by covering the microfractured lesion site with a collagen I/III membrane in the knee (Chondro-Gide®, Geistlich Pharma AG). This added step extended the durability of the results as well as the size of the lesion that could be treated by MFx. The Autologous Membrane Induced Chondrogenesis (AMIC®) technique that Behrens developed involves the debridement of the cartilage lesion, the MFx procedure, and the coverage of the lesion site by the collagen membrane Chondro-Gide. This technique has been used to complete the MFx-cartilage repair process in several joints (knee, hip, talus, and metatarsal) thus far. Although 2 recent studies on the use of AMIC for the repair of articular cartilage defects have been published recently,13,14 these papers do not exclusively address the knee joint, but rather refer to all 3 major joints where AMIC is used (hip, knee, talus), and no meta-analysis of the results was performed.
The present work serves to examine outcomes from AMIC in the knee via a systematic literature review and meta-analysis. Pain and functional outcomes affect patient daily activities and are reported in all clinical series. In addition, we also evaluated the minimally clinically relevant pain score improvement 15 to evaluate the overall clinical results provided by the meta-analysis.
Material and Methods
The meta-analysis was based on the existence of several publications dealing with the results of AMIC in the treatment of chondral and osteochondral lesions (OCL) in the knee, and on the need to document the efficacy and safety of AMIC in this indication. The questions to be answered were whether or not AMIC could improve the clinical and functional status of these patients, and how stable in time the outcomes were. For this analysis, the Patient-Intervention-Comparator-Outcomes (PICO) formatted question has been:
P: adults, both sexes, all ethnicities, all nationalities
I: microfracture associated to the AMIC procedure for the treatment of isolated knee cartilage lesions
C: preoperative status, late versus early results
O: pain and functional scoring
“Among adults, compared with the preoperative status, what is the effectiveness and durability of AMIC for the relief of pain and functional disabilities related to chondral lesions of the knee joint?”
Search Strategy, Search Terms
A systematic literature search was performed in the PubMed, Embase database up to May 15, 2018, by use of the following terms: “Chondro-Gide” OR “Chondrogide” OR “Autologous matrix induced chondrogenesis” OR (AMIC® AND cartilage) and knee.
Studies were included if they fulfilled all of the following criteria: (1) clinical studies including a minimum of 6 patients, with primary measures of the pain and functional outcomes; (2) studies involving cartilage defects of the knee; and (3) articles in English language.
Two reviewers independently screened the articles, where all retrieved titles and abstracts were initially screened. Case reports were excluded unless they contained safety issues or adverse events reports. The quality of the repaired tissue, if documented, was not homogeneous and was therefore only reported in the literature survey but not used in the meta-analysis. Evidence of duplicate identical patient population in different publications was analyzed and treated/justified so as to avoid repetitive use of the same cohort. Review papers were not considered in this review.
Data Extraction and Critical Appraisal
The data extracted included the level of evidence, number of patients, defect characteristics and size, treatment groups, follow-up, outcome evaluation, and main outcomes. Results of the VAS (Visual Analog Scale), Lysholm, ICRS (International Cartilage Repair Society), Modified Cincinnati, IKDC (International Knee Documentation Committee), KOOS (Knee Injury and Osteoarthritis Outcome Score), and MOCART (Magnetic Resonance Imaging Score and Classification System) scores were specified. All included articles were assessed independently by 2 reviewers, familiar with cartilage repair studies, with a modified version of the Coleman Methodology Score (mCMS), 16 modified by Ramponi et al. 17 Data were collected by one reviewer in a standardized extraction form and verified by a second reviewer. Articles included were also consolidated by comparison with already published systematic reviews on the AMIC technique performed in the knee. Excellent studies were considered those that scored 85 to 100, good studies scored 70 to 84, fair studies scored 55 to 69, and poor studies scored less than 55. 18
Statistical Methodology
Methodology for the Meta-Analysis
The meta-analysis was based on the reported Lysholm, VAS, and IKDC scores. Mean differences, P values, and confidence intervals were calculated assuming independence between time points. Under the assumption that measurements within patients are positively correlated, one would expect the true standard deviation to be less than estimated below and therefore even smaller P values. Due to the small number of studies, an assessment of the heterogeneity between studies was deemed unreliable. For this reason and because of reported difference between the subgroups in the studies, C and H random effects models were used throughout this study using the DerSimonian-Laird estimator 19 to estimate the between study variance.
Confidence interval and significance level were 95% and 5%, respectively, based on assuming a normal distribution. All analyses were performed under R version 3.5.0 and the Meta package version 4.9-2.
Results from the Data Search and Meta-Analysis
A total of 66 papers were identified utilizing the aforementioned search criteria. After removal of duplicates, articles were screened, and the following full-text articles were excluded:
Reviews
Technical notes
In vitro studies
Animal studies
Reports on autologous chondrocyte implantation, hip, talus, or anterior cruciate ligament repair
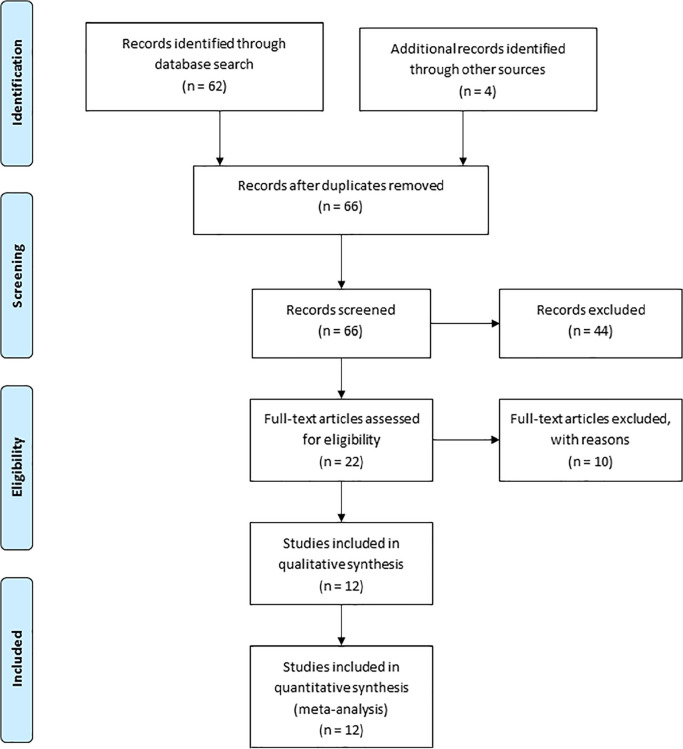
The corresponding PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) chart of the literature search results is shown in Figure 1 . Of the remaining 22 publications corresponding to the search protocol, 5 were single case reports and were not considered and 5 others were preliminary reports of another study published later by the same authors/group and therefore excluded. In these cases, only papers with the longest follow-up and the larger enrollment were considered ( Fig. 1 ).
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram. Distribution of published clinical papers on the AMIC knee outlining the application of inclusion/exclusion criteria for the meta-analysis.
None of the case reports excluded described an adverse event, but rather correspond to early clinical experience including technical tools. Finally, 12 reported clinical series were included, reporting outcomes from 375 patients corresponding to the following references as summarized in Table 1 : A, 20 B, 21 C, 22 D, 23 E, 24 F, 25 G, 26 H, 27 I, 28 J, 29 K, 30 and L. 31 Table 1 also reports the demographics and outcomes for each study. The mCMS ranking of each study is reported in Table 2 . One clinical trial was identified comparing clinical outcomes of AMIC versus MFx (study H 27 ), and none comparing AMIC versus ACI in the knee.
Table 1.
Systematic Analysis of the Reported Clinical Data for the AMIC Technique in the Knee.
| Title | Study | Authors | Number of Patients | Age | BMI | Defect Size | Follow-Up | Outcome Measures | Lysholm | VAS | IKDC | Tegner | KOOS | Cincinnati | ICRS | MRI | QoL | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | Prospective AMIC study (case series) | Gille et al. (2010) | 27 (11 females, 16 males) | 37 years (16-50) | 26 (20-32) | 4.2 cm2 (1.3-8.8) | 37 months (24-62) | Meyer, Lysholm, Tegner, ICRS, Cincinnati, MOCART | BL: 36 ± 21 1 Y: 67 ± 28 2 Y: 76 ± 24 3 Y: 62 ± 25 4 Y: 47 ± 22 |
BL: — 1 Y: 3.4 2 Y: 4.1 3 Y: 4.0 4 Y: — |
BL: 46 ± 18 1 Y: 66 ± 23 2 Y: 74 ± 23 3 Y: 62 ± 26 4 Y: 37 ± 9 |
BL: 31 ± 15 1 Y: 59 ± 24 2 Y: 68 ± 22 3 Y: 54 ± 25 4 Y: 37 ± 4 |
n = 15 Bone marrow lesions (BMLs) (n = 7), effusion (n = 8), and osseous hypertrophy underneath the repair tissue (n = 9) were found. The majority of patients (n = 10) showed a defect filling of more than 50%. |
1. Significant improvement of all scores as early as 12 months
after AMIC. Further increased values up to 24 months postop.
Lysholm score: 15-57 (preop); 39-95 (1 year); 52-100 (2 years);
37-87 (3 years); 25-69 (4 years). Tegner score: 3.4 (1 year);
4.1 (2 years); 4.0 (3 years) 2. MRI analysis showed moderate to complete filling with a normal to incidentally hyperintense signal in most cases. 3. No clinical impact of patient’s age at the time of operation, body mass index, and number of previous operations was identified. 4. Males showed significant higher values in the ICRS score compared with their female counterparts. 5. Reoperation: arthroplasty (2/27); Complication: muscle vein thrombosis (1/27); joint effusion (1/27). |
||||
| B | Prospective AMIC study + BMA (case series) | Pascarella et al. (2010) | 19 (7 females, 12 males) | 26 years (18-50) | 3.6 cm2 (2.8-3.9) | 2 years | IKDC, Lysholm, Ikeuchi, MRI | BL: 54 (38-83) 2 Y: 98 (96-100) |
BL: 30 (24-40) 2 Y: 83 (74-94) |
Ten postoperative MRI (53%) showed a significant reduction of the defect area, both in shape, filling, interface, and subchondral edema | ||||||||
| C | Retrospective AMIC study (case series) | Kusano et al. (2012) | 40 defects (38 patients, 17 females, 23 males) | 36 years (18-50) | ocF: 26.3 ± 1.9 cP: 25.2 ± 0.9 cF: 25.7 ± 0.9 |
4.0 cm2 (2.3-4.4) | 28.7 months (13-51) | IKDC, Lysholm, Tegner, VAS, MOCART | ocF: BL: 50 ± 25 FU: 94 ± 8 cP BL: 58 ± 17 FU: 85 ± 13 cF BL: 56 ± 25 FU: 76 ± 18 |
ocF: BL: 6 ± 3 FU: 1 ± 1 cP BL: 6 ± 2 FU: 2 ± 2 cF BL: 6 ± 3 FU: 3 ± 3 |
oCF BL: 44 ± 25 FU: 88 ± 9 cP BL: 51 ± 25 FU: 74 ± 17 cF BL: 45 ± 26 FU: 68 ± 14 |
ocF BL: 2 ± 2 FU: 5 ± 2 cP BL: 3 ± 2 FU: 4 ± 1 cF BL: 4 ± 3 FU: 4 ± 1 |
— | — | — | MOCART—no overall score | Satisfaction index ocF: 98 ± 4 cP: 84 ± 24 cF: 74 ± 43 |
1. Significant improvements in clinical outcome scores were
noted. VAS: 3-9 (preop), 0-6 (1 year); Lysholm score: 25-81
(preop), 58-102 (1 year); Tegner score: 0-7 (preop), 3-7 (1
year). 2. The largest improvements were seen in the osteochondral subgroup (mean age 25.9 years), whereas patients treated for chondral defects in the patellofemoral joint and on the femoral condyles improved less. 3. Patients in all groups were generally satisfied with their results. 4. MRI evaluation showed that tissue filling was present but generally not complete or homogenous. 5. Complications: hematoma (1/38), joint stiffness (9/38). |
| D | Prognostic AMIC Registry study (case series) | Gille et al. (2013) | 57 (19 females, 38 males) | 37 years (17-61) | — | 3.4 cm2 (1-12) | 2 years | Lysholm, VAS | BL: 50.1 ± 19.6 (9-79) 1 Y: 79.9 ± 21.2 (17-100) 2 Y: 85.2 ± 18.4 (27-100) |
BL: 7.0 ± 1.8 (1-10) 1 Y: 2.7 ± 2.9 (0-9) 2 Y: 2.0 ± 2.1 (0-9) |
— | — | — | — | — | — | — | 1. Most patients were satisfied with the postoperative outcome,
reporting a significant decrease of pain. VAS: 1-10 (preop), 0-9
(1 year), 0-9 (2 years); Lysholm score: 9-79 (preop), 17-100 (1
year), 27-100 (2 years). 2. Significant improvement of the mean Lysholm score was observed at 1 year. Further increased values were noted up to 2 years postop. |
| E | Prospective AMIC + BMAC study w/o MFx (case series) | Skowronski et al. (2013) | 54 (19 females, 35 males) | 18-55 | — | 6.1 cm2 (4-12) | 5 years | KOOS, Lysholm, VAS | BL: 58.1 ± 4.6 1 Y: 93.2 ± 4.2 5 Y: 90.3 ± 2.8 |
BL: 5.6 ± 0.06 1 Y: 1.2 ± 0.1 5 Y: 0.9 ± 0.2 |
— | — | BL: 66.2 ± 2.9 1 Y: 91.1 ± 4.1 5 Y: 90.9 ± 1.9 |
— | — | |||
| F | Prospective AMIC + BMAC study w/o MFx (case series) | Gobbi et al. (2014) | 25 (9 females, 16 males) | 46.5 ± 1.71 years (32-58) | 24 ± 0.6 | 8.3 cm2 (2.5-22) | 41 months (±7) | IKDC, KOOS, Lysholm, Tegner, Marx, VAS, MRI | BL: 46.36 ± 2.25 1 Y: 82.80 ± 3.38 2 Y: 85.96 ± 3.44 FU: 86.52 ± 2.73 |
BL: 5.40 ± 0.37 1 Y: 1.16 ± 1.14 2 Y: 0.84 ± 1.02 FU: 0.48 ± 0.19 |
BL: 37.92 ± 4.52 1 Y: 74.15 ± 3.38 2 Y: 78.19 ± 3.16 FU: 81.73 ± 2.42 |
BL: 2.12 ± 0.32 1 Y: 4.44 ± 0.37 2 Y: 5.40 ± 0.35 FU: 5.64 ± 0.26 |
No overall score | — | — | The MRI analysis at final follow-up showed stable implantation and complete filling of the defect in 20 of 25 patients (80%) and incomplete filling (50% of the adjacent cartilage) in 5 of 25 patients (20%), while no signs of hypertrophy were identified. We did not identify cysts or sclerosis of the subchondral bone, while edema was identified in 6 patients. | KOOS QoL BL: 32.20 ± 4.43 1 Y: 75.52 ± 5.44 2 Y: 77.00 ± 4.44 FU: 83.04 ± 3.37 |
|
| G | Prospective AMIC study (case series) | Dhollander et al. (2014) | 10 (2 females, 8 males) | 37.2 ± 7.1 years | — | 4.2 ± 1.9 cm2 | 2 years | KOOS, Tegner, Kujala, VAS, MOCART | — | BL: 7.39 ± 2.08 6 M: 4.14 ± 2.80 1 Y: 3.60 ± 2.94 2 Y: 3.94 ± 2.88 |
— | BL: 1.5 ± 1.4 6 M: 2.1 ± 1.4 1 Y: 2.6 ± 1.4 2 Y: 2.5 ± 1.5 |
BL: 175.5 ± 83.2 6 M: 229.0 ± 82.6 1 Y: 243.2 ± 100.0 2 Y: 243.9 ± 85.9 |
— | — | MOCART 1 Y: 57.9 ± 18.2 2 Y: 54.2 ± 22.9 Hypertrophic filling of the defect was found in 10 cases (20%) at 24 months of follow-up. Bone marrow changes were observed in 4 patients (40%) at the same evaluation point. The formation of intralesional osteophytes was observed in 3 of the 10 patients (30%) |
KOOS QoL BL: 18.7 ± 16.9 6 M: 25.8 ± 11.4 1 Y: 26.4 ± 18.6 2 Y: 28.3 ± 14.8 |
1. The MOCART scoring system revealed a slight tendency to
deterioration on MRI between 1 and 2 years postop. MOCART score:
57.9 ± 18.2 (1 year), 54.2 ± 22.9 (2 years). 2. All cases showed subchondral lamina changes. A formation of intralesional osteophytes was observed in 30% of cases. 3. VAS: 5-9 (preop), 1-7 (1 year), 1-7 (2 years); Tegner score: 1.2-4.0 (1 year), 1-4 (2 years); KOOS: 93.3-259.7 (preop), 143.2-343.2 (1 year), 158-329.8 (2 years). 4. Reoperation: shaving (2/10); Complications: intralesional osteophytes (3/10), joint catching (2/10). |
| H | RCT MFx vs. AMIC (sutured and glued) study | Volz et al. (2017) | 47 (10 females, 37 males) | 37 ± 10 years (21-50) | 26.8 ± 3.9 | 3.6 ± 1.6 cm2 (2.1-6.6) | 5 years | ICRS, Cincinnati, MRI | — | Sutured BL: 5.4 ± 1.9 1 Y: 1.6 ± 1.5 2 Y: 1.8 ± 2.2 5 Y: 1.6 ± 2.2 Glued BL: 4.6 ± 2.0 1 Y: 1.5 ± 1.3 2 Y: 1.4 ± 2.5 5 Y: 1.1 ± 2.0 |
— | — | — | Sutured BL: 48 ± 15 1 Y: 82 ± 15 2 Y: 81 ± 14 5 Y: 84 ± 21 Glued BL: 45 ± 19 1 Y: 67 ± 26 2 Y: 85 ± 18 5 Y: 92 ± 6 |
— | At 1 year postoperation, 35% to 50% of the patients had a defect
filling of two thirds or more. Complete integration could be
observed in 15% to 30% of patients in the different
groups. At 2 years, the defect filling was more complete in the AMIC treated groups, whereas at least 60% of the patients had a defect filling of more than two thirds. In the MFx group, only 25% of the patients achieved such filling. At 5 years postoperation, the defect filling was the lowest in the MFx group, versus both AMIC treated groups ( Table 2 ). |
— | 1. After improvement for the first 2 years in all subgroups, a
progressive and significant score degradation was observed in
the microfracture group, while all functional parameters
remained stable for least 5 years in the AMIC groups. ICRS score
for pain: sutured AMIC 35-73 (preop), 1-31 (1 year); glued AMIC
26-66 (preop), 2-28 (1 year); Modified Cincinnati score: sutured
AMIC 26-64 (preop), 67-97 (1 year); glued AMIC 33-63 (preop),
41-93 (1 year). 2. At 2 and 5 years, MRI defect filling was more complete in the AMIC groups. |
| I | Prospective AMIC study (case series) | Sadlik et al. (2017) | 12 (5 females, 7 males) | 36 years (22-52) | 2.5 cm2 (0.8-4.0) | 38 months (24-70) | KOOS, IKDC, VAS, MOCART | — | BL: 7.8 (3-10) FU: 2.3 (0-6) |
BL: 37.4 (4.6-90.8) FU: 90.1 (42.5-100) |
— | BL: 50.3 (17.3-83.9) FU: 90.1 (77.4-100) |
— | — | MOCART FU: 58.3 (30-85) |
— | 1. The mean KOOS and IKDC scores increased significantly. KOOS:
17.3-83.9 (preop), 77.4-100 (mean 38 months). 2. The VAS score decreased significantly. VAS: 3-10 (preop), 0-6 (mean 38 months). 3. MOCART score: 30-85 (mean 38 months). |
|
| J | Retrospective AMIC study (case series) | Schiavone Panni et al. (2017) | 21 | No info | No info | 4.3 cm2 (2.9-8.0) | 7 years (6.5-8) | IKDC, Lysholm, MOCART | BL: 38.8 ± 12.4 7 Y: 72.6 ± 19.5 |
— | BL: 31.7 ± 8.9 7 Y: 80.6 ± 5.3 |
— | — | — | — | MOCART—no overall score On MRI evaluation, reduction both of the defect area and subchondral edema were present in 14 (66.6%) patients. |
16 (76.2%) of the patients were either satisfied or extremely satisfied with their clinical and functional outcomes. | 1. Mean IKDC and Lysholm scores improved significantly at 7
years postop. Lysholm score: 75.3-85.9 (preop), 53.1-92.1 (7
years). 2. At the last follow-up, 76.2% of patients were satisfied or extremely satisfied, while 66.6% of patients showed good quality repair tissue on MRI. |
| K | Prospective AMIC study (case series) | Schagemann et al. (2018) | 50 arthroscopic 20 (7 females, 13 males) mini-open 30 (13 females, 17 males) |
36 years (14-70) | Arthroscopic 27.0 ± 4.2 (18.7-34.7) Mini-open 23.9 ± 3.4 (18.4-28.7) |
Arthroscopic 3.1 cm2 (1.0-6.0) Mini-open 3.4 cm2 (1.5-12.0) |
24 months | KOOS, Lysholm, VAS | Arthroscopic BL: 49.1 ± 15.0 1 Y: 79.5 ± 15.5 2 Y: 81.8 ± 14.5 Mini-open BL: 45.8 ± 24.8 1 Y: 79.0 ± 20.4 2 Y: 80.5 ± 19.0 |
Arthroscopic BL: 5.18 ± 1.53 1 Y: 2.45 ± 2.04 2 Y: 1.48 ± 1.50 Mini-open BL: 6.17 ± 2.24 1 Y: 2.37 ± 2.20 2 Y: 2.07 ± 2.42 |
— | — | No overall score ( Table 2 ). | — | — | — | KOOS QoL Arthroscopic BL: 58.8 ± 15.2 1 Y: 87.3 ± 12.8 85.4 ± 13.1 Mini-open BL: 59.7 ± 21.1 88.2 ± 7.8 88.7 ± 10.4 |
|
| L | Retrospective AMIC study (case series) | Hoburg et al. (2018) | 15 (6 females, 9 males) | 26 years (17-41) | 25.6 (21-35.5) | 4.98 ± 3.02 cm2 | 49 months (36-61) | VAS, IKDC, KOOS, Lysholm, Tegner, MOCART | BL: 39.3 ± 19.5 1 Y: 73.0 ± 17.5 FU: 79.8 ± 15.1 |
BL: 7.2 ± 1.4 1 Y: 2.1 ± 1.9 FU: 2.4 ± 2.6 |
BL: 36.6 ± 20.6 1 Y: 65.4 ± 21.9 FU: 72.2 ± 18.7 |
BL: 4.3 1 Y: 4.0 FU: 4.7 |
BL: 50.0 ± 18.9 1 Y: 76.4 ± 17.0 FU: 81.7 ± 13.9 |
— | — | MOCART FU: 77.5 ± 15 |
The subjective evaluation showed 40% very satisfied, 26.6% satisfied, 20% partially satisfied, and 13.3% not satisfied with their outcome. | |
| 375 | 36.2 (14-70) | 4.24 (0.8-22) | BL: 9-83 1 Y: 17-100 2 Y: 27-100 |
BL: 1-10 1 Y: 0-9 2 Y: 0-9 3 Y: 0-6 |
BL: 4.6-90.8 2 Y: 74-94 3 Y: 42.5-100 |
AMIC = Autologous Matrix-Induced Chondrogenesis; BL = baseline; BMI = body mass index; FL = follow-up; ICRS = International Cartilage Repair Society; IKDC = International Knee Documentation Committee; KOOS = Knee Injury and Osteoarthritis Outcome Score; M = month; MFx = microfracture; MOCART = Magnetic Resonance Imaging Score and Classification System; MRI = magnetic resonance imaging; QoL = quality of life; VAS = Visual Analog Scale; Y = year.
Table 2.
Modified Coleman Methodology Score (mCMS) for the 12 Studies Considered in the Meta-Analysis a .
| A—Gille et al. (2010) 20 | B—Pascarella et al. (2010) 21 | C—Kusano et al. (2012) 22 | D—Gille et al. (2013) 23 | E—Skowronski et al. (2013) 24 | F—Gobi et al. (2014) 25 | G—Dhollanger et al. (2014) 26 | H—Volz et al. (201 7) 27 | I—Sadlik et al. (2017) 28 | J—Panni et al. (2018) 29 | K—Schagemann et al. (2018) 30 | L—Hoburg et al. (2018) 31 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Criteria Part A | ||||||||||||
| 1. Study size | 4 | 4 | 4 | 7 | 7 | 4 | 4 | 7 | 0 | 4 | 7 | 0 |
| 2. Mean duration of follow-up | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 3. Number of treatment procedures | 0 | 10 | 0 | 0 | 0 | 0 | 7 | 10 | 10 | 10 | 7 | 10 |
| 4. Type of study | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 10 | 5 | 5 | 5 | 5 |
| 5. Diagnostic certainty | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 6. Description of surgical procedure | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| 7. Description of rehabilitation procedures | 10 | 0 | 10 | 10 | 0 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Criteria Part B | ||||||||||||
| 1. Outcome criteria | 2 + 2 + 3 | 2 + 2 + 3 | 2 + 2 + 3 | 2 + 2 + 3 | 2 + 2 + 3 | 2 + 2 + 3 | 2 + 2 + 3 | 2 + 2 + 3 | 2 + 2 + 3 | 2 + 2 + 3 | 2 + 2 + 3 | 2 + 2 + 3 |
| 2. Procedure for assessing outcomes | 5 | 5 | 5 + 4 + 3 | 5 + 3 | 5 + 3 | 5 + 3 | 5 + 3 | 5 + 3 + 3 | 5 + 3 + 3 | 5 + 3 | 5 + 4 + 3 | 5 + 3 |
| 3. Subject selection process | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5+ | 5 |
| Total Part A (max 60) | 39 | 39 | 39 | 42 | 32 | 39 | 46 | 57 | 45 | 49 | 49 | 45 |
| Total Part B (max 40) | 17 | 17 | 24 | 20 | 20 | 20 | 20 | 23 | 23 | 20 | 24 | 20 |
| Total CMS | 56 | 56 | 63 | 62 | 52 | 59 | 66 | 80 | 68 | 69 | 73 | 65 |
Letters in the first row correspond to the studies identified in Table 1 with their respective references.
The common features of the 12 clinical reports available on the AMIC procedure in the knee are the following:
Test product was Chondro-Gide, a porcine bilayer collagen type I/III membrane.
The device was placed through an arthrotomy as a cover for MFx treated defect areas. Some papers also report the use of an arthroscopic procedure.
All lesions were osteochondral or chondral type III-IV (Outerbridge classification).
MFx was performed according to the technique published by Steadman et al.32,33 Once the membrane was placed over the microfractured defect by gluing, the stable position of the membrane was checked by flexing and extending the joint 10 times.
In one study center, MFx was not always used but rather replaced or complemented by stem cell transplantation (study F, Gobbi et al. 25 ), or not used at all and only bone marrow concentrate was used (study E, Skowronski et al. 24 ).
Concurrent osteotomy was performed in cases of varus or valgus malalignment. In cases where an osteochondral defect was involved, bony tissue was removed, and the resulting defect was filled with autologous cancellous bone from the iliac crest, ipsilateral femoral or tibia metaphysis. Other associated procedures included meniscal repair, reconstruction or trochleoplasty, ligament stabilization, and patellar realignment.
Clinical evaluation was performed by the clinician before and each year after surgery.
The postoperative treatment was carried out according to the center-specific routine. Pain was treated with analgesics, and physiotherapy conducted according to the standard scheme:
Partial weight bearing for 8 weeks of 15 to 20 kg maximum.
Restricted range of motion to 0/0/60° of the femoral condyle and 0/0/30° for the patella or trochlea for the first 10 days and to 0/0/90° for 6 weeks. Weight bearing allowed after 6 weeks, but longer in cases of associated osteotomies
Mobilization exercises including continuous passive motion and proprioceptive training
Jogging allowed after 6 months and contact sports restricted for 18 months
The results of the mCMS for the included studies assessing AMIC for articular cartilage repair in the knee are reported in Table 2 . This table confirms a medium rank for almost all the studies ranging individually between 52 and 80. The mean value of the mCMS over the 12 studies was of 64.1. Only one study was classified as “poor,” 9 were considered as “fair,” and 2 were “good.” One was a level I study (scored at 80). Major areas of methodological deficiencies were study size, number of procedures (concomitant procedures are part of the standard of care), and follow-up duration.
Clinical Results: Reported Outcomes
Study Characteristics, Demographic Data, and Outcomes Reporting
Table 1 details the synthetized information from the reported literature. These studies were performed in 5 different European countries (Germany n = 5, Italy n = 3, Switzerland n = 1, Poland n = 2, and Belgium n = 1). One study was a prospective randomized trial (level I), 8 were prospective cases series, and 3 were retrospective cases series. All were published since 2010 with 5 in the last 2 years (2017-2018). Cumulatively, 375 patients were analyzed with 137 having a follow-up of more than 4 years, 102 with a follow-up of 2 to 4 years, and 136 with a follow-up of 2 years. The majority of the studies included patients aged 18 to 55 years (n = 7), and the mean age was 36.2 years (ranging from 14 to 70 years). Notably, all studies were performed in an effort to analyze the treatment of a single localized cartilage defect. The mean defect size was 4.24 cm2, ranging from 0.8 to 22 cm2. Almost all studies targeted Outerbridge grade 3 or 4 lesions for inclusion. Reported Body mass index ranged from 20 to 35 kg/m2. The most common patient-reported outcomes assessed in the included studies were the VAS (n = 9) and the Lysholm scores (n = 9), whereas Tegner and IKDC subjective knee form scores were used in 5 and 6 studies, respectively. Detailed KOOS was used only in 4 studies. All studies were based on the evaluation of at least 2 different scorings (up to 6 scorings).
Nine studies reported magnetic resonance imaging (MRI) as an endpoint and used scoring systems that could include the MOCART score.
Six studies documented the quality of life and/or patient satisfaction.
No histologic analysis of the repaired cartilage was systematically performed.
Performance Reported through the Meta-Analysis
The results of the meta-analysis of the 12 published studies are the following:
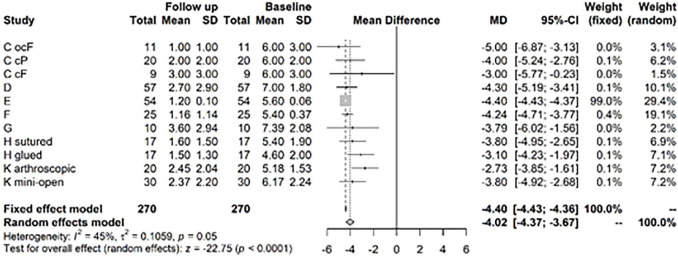
For the pain VAS, the random effects model shows a change from baseline to follow-up at years 1 to 2 of −4.02, significant at 5% level with a 95% confidence interval (CI) of (−4.37; −3.67) ( Fig. 2 ). After >3 years follow-up, there was still a significant difference in mean VAS between baseline and follow-up of −4.75, CI (−4.98; −4.53) ( Fig. 3 ).This VAS change corresponds to a clinically important difference as proposed by Tubach et al. 15 with an absolute change of −3.7 (−3.8; −3.5) of the baseline VAS score, considered as high.
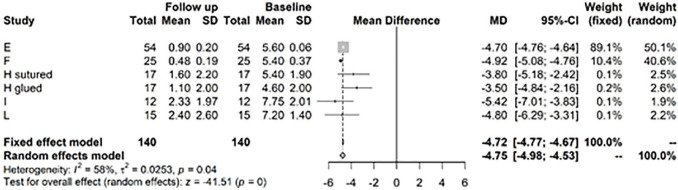
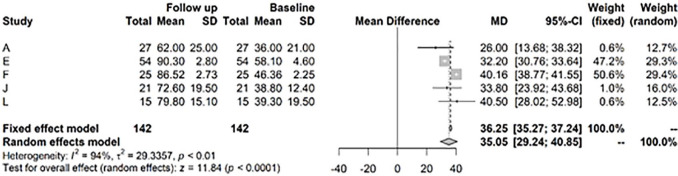
For the Lysholm score at years 1 to 2 versus baseline, there is a highly significant improvement of 34.68, CI (32.68; 36.58) ( Fig. 4 ). After >3 years, there was still a highly significant difference of the Lysholm score versus baseline of 35.1, CI (29.24; 40.85) ( Fig. 5 ).
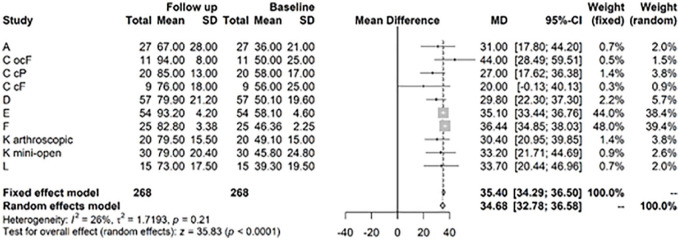
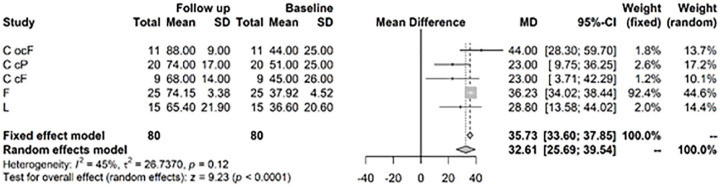
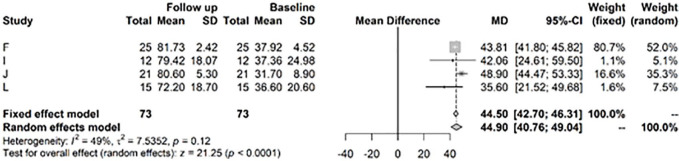
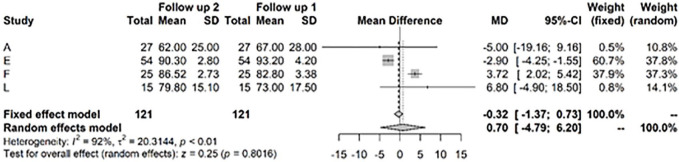
For the IKDC score, the random effects model showed a highly significant improvement at 1 to 2 years versus baseline values of 32.61, CI (25.69; 39.54) ( Fig. 6 ). After >3 years, the model showed a highly significant change in mean IKDC versus baseline of 44.9, CI (40.76; 49.04) ( Fig. 7 ).
Figure 2.
Results from random effects model comparing Visual Analog Scale (VAS) baseline versus year 1 or 2.
Figure 3.
Results from random effects model comparing Visual Analog Scale (VAS) baseline versus >3 year.
Figure 4.
Results from random effects model comparing Lysholm baseline versus year 1 or 2.
Figure 5.
Results from random effects model comparing Lysholm baseline versus >3 years.
Figure 6.
Results from random effects model comparing International Knee Documentation Committee (IKDC) baseline versus year 1 or 2.
Figure 7.
Results from random effects model comparing International Knee Documentation Committee (IKDC) baseline versus >3 years.
Comparison through the meta-analysis of the clinical outcomes between 1 and 2 years, and after at least 3 years showed the following:
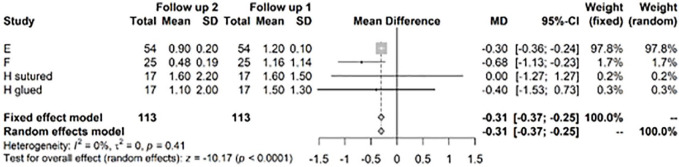
The change in mean VAS of −0.31 (CI −0.37; −0.25) was highly significant (P < 0.0001), but the absolute value was much less than the change between baseline and follow-up at years 1 to 2 ( Fig. 8 ). This VAS change corresponds to a clinically important difference as proposed by Tubach et al., 15 with an absolute change of −3.7 (−3.8; −3.5) of the baseline VAS score, considered as high.
The Lysholm score did not change significantly between follow-up of 1 to 2 years and over 3 years ( Fig. 9 ).
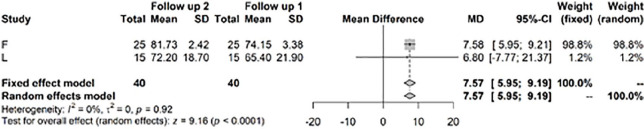
Finally, the IKDC score significantly improved by a mean difference of 7.57 (P < 0.0001) between years 1 and 2 and after year 3 ( Fig. 10 ).
Figure 8.
Results from random effects model comparing Visual Analog Scale (VAS) year 1 or 2 versus >3 years.
Figure 9.
Results from random effects model comparing Lysholm year 1 or 2 versus >3 Years.
Figure 10.
Results from random effects model comparing International Knee Documentation Committee (IKDC) year 1 or 2 versus >3 years.
Concerning the MRI data collected in this meta-analysis, they confirm the healing of the lesion as a long-lasting process. The tissue filling the defect was not complete or homogeneous even after 2 years (studies C 22 and H 27 ). MOCART scoring was reported only in 3 of the 12 studies (G, 26 I, 28 and L 31 ), but without preoperative values. Overall, MRI showed in all studies a moderate to complete filling of the defect at 2 to 3 years, with a normal to incidentally hyperintense signal in most cases. In the randomized controlled trial (RCT) (study H 27 ), at 2 and 5 years the defect filling was more complete in the AMIC group versus the MFx group. At 7 years (study J 29 ), 66% of the patients showed good-quality repair tissue on MRI.
These analyses confirm the long-term stability of the clinical outcomes after the AMIC procedure in the knee. Even more, 4 long-term studies of at least 4 years follow-up, summarized in Table 3 , show the stability of the clinical parameters at 4 to 5 years as compared to early postoperative values. No deterioration of any parameter could be identified during the 5 to 7 years’ follow-up.
Table 3.
Summary of Clinical Outcomes of Long-Term Studies of at Least 4 Years Follow-up.
| VAS Pain 1/5 Years | Lysholm 1/5 Years | IKDC 1/5 Years | Modified Cincinnati Score 1/5 Years | |
|---|---|---|---|---|
| Skowrowski et al. (2013) (study E) 24 | 1.2 ± 0.1/0.9 ± 0.2 | 93.2 ± 4.2/90.3 ± 2.8 | 91.1 ± 4.1/90.9 ± 1.9 | |
| Volz et al. (2017) (study H) 27 | 1.6 ± 1.5/1.6 ± 2.2 | 82 ± 15/84 ± 21 | ||
| Schiavone et al. (2018) (study J) 29 | No data at 1 year 72.6 ± 19.5 | No data at 1 year 80.6 ± 5.3 | ||
| Hoburg et al. (2018) (study L) 31 | 2.1 ± 1.9/2.4 ± 2.6 | 73.0 ± 17.5/79.8 ± 15.1 | 65.4 ± 72.2/72.2 ± 18.7 |
VAS, Visual Analog Scale; IKDC, International Knee Documentation Committee.
Safety
Safety Concerns among the Reported Studies
In addition to hypertrophic osteophytes reported in 3/375 patients, 9 joint stiffnesses related to complex OCL lesions were treated by passive mobilization under anesthesia; 3/375 revision surgeries were reported. According to the study reports (A 20 and H 27 ), the revisions were related to subsequent progressive osteoarthritis and were not considered as a risk after the AMIC procedure. No treatment-related adverse events were reported (see Table 4 ).
Table 4.
Safety Concerns among the Reported Studies.
| Study A 20 | Two reoperations were necessary (2/27arthroplasties for progressive osteoarthritis). Two other patients exhibited complications as muscle vein thrombosis and joint effusion without negative consequence. |
| Study B 21 | No intraoperative or postoperative complications reported. |
| Study C 22 | Hematoma was reported in 1/38 patients, and 9/38 exhibited joint stiffness needing mobilization under anesthesia but regaining full range of motion following mobilization. |
| Study D 23 | No intraoperative or postoperative complications reported. |
| Study E 24 | No intraoperative or postoperative complications reported. |
| Study F 25 | No reported complication or safety outcome. |
| Study G 26 | The technique is safe, but in 3/10 cases of patellar lesions intralesional osteophytes were observed and 2 patients developed hypertrophy of the repair tissue. The authors reported that this rate is probably lower than in an MFx (microfracture) procedure and likely related to the patellofemoral nature of all treated lesions. |
| Study H 27 | No treatment-related adverse effect reported during the 5-year follow-up. One revision arthroplasty was performed after 1 year in the glued AMIC arm among 34 patients treated with the AMIC procedure. |
| Study I 28 | No intraoperative or postoperative complications reported. |
| Study J 29 | No intraoperative or postoperative complications reported. |
| Study K 30 | No intraoperative or postoperative complications reported. |
| Study L 31 | No intraoperative or postoperative complications reported. |
Comments
The modified Coleman methodology score for studies reporting the outcomes of AMIC revealed, as already mentioned in previous systematic reviews,12,13 a suboptimal design in the majority of the recently published papers, especially regarding study size, type of study, and description of subject selection process. 13 However, this statement is not unique for AMIC but applies to almost all procedures for the treatment of chondral and osteochondral defects of the knee in adults. 34 Another factor impairing the scoring is the high number of associated procedures that is inherent to the treatment of such injuries (subchondral bone lesion, malalignment, or ligament/meniscal trauma). Although these additional procedures may have an impact on the results, they are part of the standard procedure. Treatment algorithms for cartilage lesion management dictate simultaneous correction of all the components of the pathology, particularly ligaments, meniscal ruptures, and malalignment. In addition, the presence of the bone lesion below the cartilage defect in OCL required an additional bone grafting procedure that, despite being the standard of care, impacted negatively the Coleman scoring by 10 points in 5/12 analyzed studies. These procedures represent the first line of real-life treatment for any joint. They can be avoided only in specific clinical studies that may exclude patients when an additional treatment procedure is needed (exclusion criteria), but this may introduce a patient selection bias versus the real standard of care.
All studies were initiated by different investigators with only one exception, thus minimizing the center-induced bias. One RCT (study H) 27 was sponsored by Geistlich Pharma AG. The need for long-term data in cartilage knee repair is now established and recommended by regulatory bodies as well as by the scientific and medical communities but remains challenging in the absence of the appropriate sponsoring. Thus, a RCT sponsored by a company developing a product cannot be perceived as a bias, as suggested by Gao et al., 13 but rather as a way to generate comparative data in controlled conditions, as achieved in drug development.
The meta-analysis results in this work are based on 11 level 4 studies and one level 1 study, including a total of 375 patients with a consistent follow-up of generally at least 2 years for 136 patients, 2 to 4 years for 102 patients, and >4 years follow-up for 137 patients. For this population the amount of data generated through the meta-analysis corresponds to the updated recommendations for a long-term follow-up of repaired chondral lesions. 35
The demographic characteristics of the patients included in the meta-analysis, the studies’ duration, and the outcome reporting are almost identical to the recently published clinical trials review comparing level I and II studies, patient demographics, outcomes reporting, and methodology in cartilage repair. 36 These authors reported the following:
An age range of 18 to 50 years (mean 35.6)
A majority of VAS, IKDC, and Lysholm scoring as outcomes, MRI being performed in only 53% of the studies as a primary or secondary endpoint
A mean follow-up of 3.77 ± 3.88 years
This comparison allows to consider that the patient demographics among the 12 reported studies, the outcome measures used, and the follow-up duration are aligned with the most recent characteristics of clinical trials on cartilage repair in the knee. 36 This comparison with published outcomes used for the evaluation of knee chondral defects repair was also the background for the selection of the most frequently used outcomes to be used for the meta-analysis.
The safety of the procedure was never challenged in any of the studies and there were no reported adverse events or complications related to the procedure. The complication, or need of revision for hypertrophic osteophytes, was reported in 3 cases (3/375).
The performances are immediate with an initial clinically significant improvement of pain and functional outcomes in all AMIC studies. This improvement was stable since no study reported evidence, or a tendency to deterioration of the results with time. On the contrary, the long-term studies confirmed the stability of pain reduction and improved function score at >4 years.
Three patients in this meta-analysis required a conversion to arthroplasty after 2 to 3 years following the initial procedure. This was expected in the subject population enrolled, where several patients had undergone complex cartilage repair. No failure rate can however be derived from our literature survey since the exact number of missing patients was not systematically reported. The only exact failure value was derived from the study H, 27 in which the rate of reoperations in the AMIC group was 3%, and 14% in the MFx treatment group.
The data collected through the exhaustive literature survey and meta-analysis showed that the AMIC procedure significantly improved symptomatic knees with osteochondral or chondral lesion in the joint. The durability of the clinical results with a significant improvement of all scoring at 5 years or more after surgery versus preoperative values was also demonstrated. The present results therefore confirm the long-term clinical improvement for the treatment of grade III to IV cartilage lesions, larger than 2 cm2, with AMIC in patients of less than 55 years old.
In this long-term analysis, all efficacy data based on clinical outcomes (Lysholm scoring, VAS pain) were positive. Results showed a general and significant improvement of all parameters after 2 to 3 years following surgery and stability of the clinical results after 5 years.
The collected data address a treatment population without selection criteria. In a non-selected patient population, these data confirm the findings of long-term impairment of initial good clinical scoring after MFx, but also suggest that the AMIC procedure is able to maintain the clinical benefit for at least 5 years, potentially delaying or postponing he need for nonconservative knee surgery in this patient population between 20 and 50 years old. The therapeutic concept behind AMIC is still the bone marrow stimulation by MFx, yet AMIC extends the addressable lesion size to >2 to 3 cm2 by “protection” of the clot with the Chondro-Gide membrane. Use of Chondro-Gide maintains the cells and blood clot in the defect, an event of core importance in cartilage regeneration and the healing process. To support this view, no ACI should leave cells in the defect without protecting them from diffusing in the joint.
Finally, the follow-up period is reported as an important factor in assessing the real effectiveness and reliability of the AMIC procedure as well as the durability of the repaired cartilage. For Shaikh et al., 14 the published literature based on 13 among 16 papers on the use of Chondro-Gide suggests that AMIC in cartilage repair is a safe and effective treatment option that improves patient outcome measures and pain. Moreover, it was recognized that medium- and long-term results are necessary to evaluate cartilage repair procedures. According to this review, Chondro-Gide is by far the most used membrane for enhanced MFx procedures having also the longest and most documented safe and efficient evidence concerning the clinical follow-up. 37
In these conditions, the results from 1 RCT, comparing MFx and AMIC, as well as 11 clinical reports, analyzed through the meta-analysis, demonstrated the clinical short- and long-term benefit of the AMIC procedure in the knee. Although the indications of AMIC should not be oversized and must take into account not only the lesion size but also cofounding factors like lesion location and associated biomechanical deficiencies, the clinical outcomes of AMIC have been considered as a rationale for the introduction of AMIC procedure as a part of the treatment algorithm for chondral lesions in the knee already by the French Society for Arthroscopy 9 and more recently by the German Society of Orthopedic Surgery and Traumatology. 38 Adapted from this last recommendation, the proposed algorithm for the treatment of knee cartilage lesions can be proposed as in Figure 11 .
Figure 11.
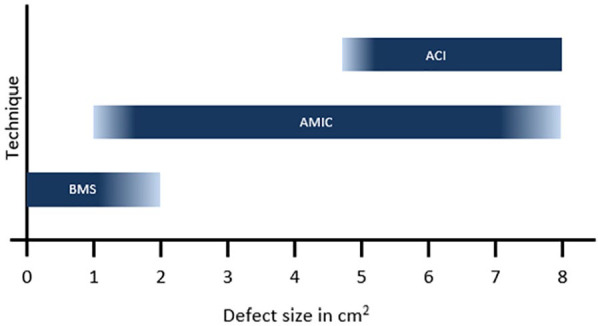
Steinwachs algorithm 2019: Proposed treatment algorithm for osteochondral lesion on the knee.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MS and RJ are board members from the ON Foundation. None of the authors has a financial interest in the subject matter discussed in the article, although they receive consultancy fees for strategic advice on future developments, by Geistlich Pharma AG.
ORCID iD: Matthias R. Steinwachs  https://orcid.org/0000-0002-2287-1654
https://orcid.org/0000-0002-2287-1654
References
- 1. Negrin L, Kutscha-Lissberg F, Gartlehner G, Vecsei V. Clinical outcome after microfracture of the knee: a meta-analysis of before/after-data of controlled studies. Int Orthop. 2012;36(1_suppl):43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frank RM, Cotter EJ, Nassar I, Cole B. Failure of bone marrow stimulation techniques. Sports Med Arthrosc. 2017;25(1_suppl):2-9. [DOI] [PubMed] [Google Scholar]
- 3. Bert JM. Abandoning microfracture of the knee: has the time come? Arthroscopy. 2015;31(3):501-5. [DOI] [PubMed] [Google Scholar]
- 4. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053-63. [DOI] [PubMed] [Google Scholar]
- 5. Goyal D, Keyhani S, Lee EH, Hui JH. Evidence-based status of microfracture technique: a systematic review of level I and II studies. Arthroscopy. 2013;29(9):1579-88. [DOI] [PubMed] [Google Scholar]
- 6. Solheim E, Hegna J, Inderhaug E, Oyen J, Harlem T, Strand T. Results at 10-14 years after microfracture treatment of articular cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24(5):1587-93. [DOI] [PubMed] [Google Scholar]
- 7. Gobbi A, Karnatzikos G, Kumar A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):1986-96. [DOI] [PubMed] [Google Scholar]
- 8. Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Ludvigsen TC, Løken S, et al. A randomized multicenter trial comparing autologous chondrocyte implantation with microfracture: long-term follow-up at 14 to 15 years. J Bone Joint Surg Am. 2016;98(16):1332-9. [DOI] [PubMed] [Google Scholar]
- 9. Basad E, Ishaque B, Bachmann G, Sturz H, Steinmeyer J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):519-27. [DOI] [PubMed] [Google Scholar]
- 10. Farr J, Gracitelli GC, Shah N, Chang EY, Gomoll AH. High failure rate of a decellularized osteochondral allograft for the treatment of cartilage lesions. Am J Sports Med. 2016;44(8):2015-22. [DOI] [PubMed] [Google Scholar]
- 11. Behery O, Siston RA, Harris JD, Flanigan DC. Treatment of cartilage defects of the knee: expanding on the existing algorithm. Clin J Sport Med. 2014;24(1_suppl):21-30. [DOI] [PubMed] [Google Scholar]
- 12. Gracitelli GC, Moraes VY, Franciozi CE, Luzo MV, Belloti JC. Surgical interventions (microfracture, drilling, mosaicplasty, and allograft transplantation) for treating isolated cartilage defects of the knee in adults. Cochrane Database Syst Rev. 2016;(9):CD010675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gao L, Orth P, Cucchiarini M, Madry H. Autologous matrix-induced chondrogenesis: a systematic review of the clinical evidence. Am J Sports Med. 2019;47(1_suppl):222-31. [DOI] [PubMed] [Google Scholar]
- 14. Shaikh N, Seah MKT, Khan WS. Systematic review on the use of autologous matrix-induced chondrogenesis for the repair of articular cartilage defects in patients. World J Orthop. 2017;8:588-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tubach F, Wells GA, Ravaud P, Dougados M. Minimal clinically important difference, low disease activity state, and patient acceptable symptom state: methodological issues. J Rheumatol. 2005;32(10):2025-9. [PubMed] [Google Scholar]
- 16. Coleman BD, Khan KM, Maffulli N, Cook JL, Wark JD. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10(1_suppl):2-11. [DOI] [PubMed] [Google Scholar]
- 17. Ramponi L, Yasui Y, Murawski CD, Ferkel RD, DiGiovanni CW, Kerkhoffs GMMJ, et al. Lesion size is a predictor of clinical outcomes after bone marrow stimulation for osteochondral lesions of the talus: a systematic review. Am J Sports Med. 2017;45(7):1698-705. [DOI] [PubMed] [Google Scholar]
- 18. Jakobsen RB, Engebretsen L, Slauterbeck JR. An analysis of the quality of cartilage repair studies. J Bone Joint Surg Am. 2005;87(10):2232-9. [DOI] [PubMed] [Google Scholar]
- 19. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
- 20. Gille J, Schuseil E, Wimmer J, Gellissen J, Schulz AP, Behrens P. Mid-term results of autologous matrix-induced chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1456-64. [DOI] [PubMed] [Google Scholar]
- 21. Pascarella A, Ciatti R, Pascarella F, Latte C, Di Salvatore MG, Liguori L, et al. Treatment of articular cartilage lesions of the knee joint using a modified AMIC technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):509-13. [DOI] [PubMed] [Google Scholar]
- 22. Kusano T, Jakob RP, Gautier E, Magnussen RA, Hoogewoud H, Jacobi M. Treatment of isolated chondral and osteochondral defects in the knee by autologous matrix-induced chondrogenesis (AMIC). Knee Surg Sports Traumatol Arthrosc. 2012;20(10):2109-15. [DOI] [PubMed] [Google Scholar]
- 23. Gille J, Behrens P, Volpi P, de Girolamo L, Reiss E, Zoch W, et al. Outcome of autologous matrix induced chondrogenesis (AMIC) in cartilage knee surgery: data of the AMIC registry. Arch Orthop Trauma Surg. 2013;133(1_suppl):87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Skowronski J, Skowronski R, Rutka M. Large cartilage lesions of the knee treated with bone marrow concentrate and collagen membrane—results. Ortop Traumatol Rehabil. 2013;15(1_suppl):69-76. [DOI] [PubMed] [Google Scholar]
- 25. Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42(3):648-57. [DOI] [PubMed] [Google Scholar]
- 26. Dhollander A, Moens K, Van der Maas J, Verdonk P, Almqvist KF, Victor J. Treatment of patellofemoral cartilage defects in the knee by autologous matrix-induced chondrogenesis (AMIC). Acta Orthop Belg. 2014;80(2):251-9. [PubMed] [Google Scholar]
- 27. Volz M, Schaumburger J, Frick H, Grifka J, Anders S. A randomized controlled trial demonstrating sustained benefit of autologous matrix-induced chondrogenesis over microfracture at five years. Int Orthop. 2017;41(4):797-804. [DOI] [PubMed] [Google Scholar]
- 28. Sadlik B, Puszkarz M, Kosmalska L, Wiewiorski M. All-arthroscopic autologous matrix-induced chondrogenesis-aided repair of a patellar cartilage defect using dry arthroscopy and a retraction system. J Knee Surg. 2017;30(9):925-9. [DOI] [PubMed] [Google Scholar]
- 29. Panni AS, Del Regno C, Mazzitelli G, D’Apolito R, Corona K, Vasso M. Good clinical results with autologous matrix-induced chondrogenesis (Amic) technique in large knee chondral defects. Knee Surg Sports Traumatol Arthrosc. 2018;26(4):1130-6. [DOI] [PubMed] [Google Scholar]
- 30. Schagemann J, Behrens P, Paech A, Riepenhof H, Kienast B, Mittelstadt H, et al. Mid-term outcome of arthroscopic AMIC for the treatment of articular cartilage defects in the knee joint is equivalent to mini-open procedures. Arch Orthop Trauma Surg. 2018;138(6):819-25. [DOI] [PubMed] [Google Scholar]
- 31. Hoburg A, Leitsch JM, Diederichs G, Lehnigk R, Perka C, Becker R, et al. Treatment of osteochondral defects with a combination of bone grafting and AMIC technique. Arch Orthop Trauma Surg. 2018;138(8):1117-26. [DOI] [PubMed] [Google Scholar]
- 32. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001(391 Suppl):S362-S369. [DOI] [PubMed] [Google Scholar]
- 33. Steadman JR, Rodkey WG, Briggs KK, Rodrigo JJ. The microfracture technic in the management of complete cartilage defects in the knee joint [in German]. Orthopade. 1999;28(1_suppl):26-32. [DOI] [PubMed] [Google Scholar]
- 34. Loveday D, Clifton R, Robinson A. Interventions for treating osteochondral defects of the talus in adults. Cochrane Database Syst Rev. 2010;(8):CD008104. [DOI] [PubMed] [Google Scholar]
- 35. Marquez-Lara A, Mannava S, Howse EA, Stone AV, Stubbs AJ. Arthroscopic management of hip chondral defects: a systematic review of the literature. Arthroscopy. 2016;32(7):1435-43. [DOI] [PubMed] [Google Scholar]
- 36. Saltzman BM, Redondo ML, Beer A, Cotter EJ, Frank RM, Yanke AB, et al. Wide variation in methodology in level I and II studies on cartilage repair: a systematic review of available clinical trials comparing patient demographics, treatment means, and outcomes reporting [October 31, 2018]. Cartilage. doi: 10.1177/1947603518809398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shive MS, Stanish WD, McCormack R, Forriol F, Mohtadi N, Pelet S, et al. BST-CarGel(R) treatment maintains cartilage repair superiority over microfracture at 5 years in a multicenter randomized controlled trial. Cartilage. 2015;6(2):62-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Niemeyer P, Becher C, Brucker PU, Buhs M, Fickert S, Gelse K, et al. Significance of matrix-augmented bone marrow stimulation for treatment of cartilage defects of the knee: a consensus statement of the DGOU Working Group on Tissue Regeneration [in German]. Zeitschrift fur Orthopadie und Unfallchirurgie. 2018;156(5):513-32. [DOI] [PubMed] [Google Scholar]