Abstract
Objectives
The purpose of this study was to systematically review the available evidence regarding any plausible deleterious effects of nonsteroidal anti-inflammatory drugs (NSAIDs) on chondrocytes, chondrocyte differentiation, and allograft or autograft incorporation after cartilage repair procedures.
Design
Three databases (PubMed, Science Direct, and Cochrane Library) were screened for eligible studies: investigating the effects of NSAIDs on chondrocytes, chondrogenic differentiation, or allograft/autograft incorporation. This evaluation included studies of any level of evidence, written in English, reporting clinical or preclinical results, published in peer review journals and dealing with our topic. All articles evaluating the effects of NSAIDs on either osteoarthritic (OA) chondrocyte samples or OA chondrocyte models were excluded. Moreover, articles about bone healing in which allograft or autograft incorporation was not investigated were also excluded. Methodologic quality assessment was performed for in vivo animal studies according to ARRIVE guidelines, and risk of bias of each included study was identified using the ROBINS-I tool.
Results
Eighteen studies were included in the review: 4 in vitro studies, 13 animal studies, and 1 human study. According to these studies NSAIDs have no detrimental effect on healthy mature chondrocytes; however, these drugs influence chondrocyte differentiation and graft incorporation and therefore may interfere with chondrogenesis and incorporation after transplantation of chondrocytes or osteochondral grafts.
Conclusion
The use of NSAIDs, systemic or local, after cartilage repair procedures should be avoided unless a substantial clinical benefit would otherwise be withheld from the patient. More human studies are needed to analyze the effect of NSAIDs on cartilage repair.
Keywords: cartilage, cartilage repair, anti-inflammatory agents, nonsteroidal
Introduction
Cartilage damage can lead to persistent symptoms, including swelling, pain, loss of function, and may ultimately progress to symptomatic degeneration of the joint if it is left untreated. 1 To delay or avoid a joint arthroplasty, multiple treatment options are available to restore the injured cartilage depending on patient and lesion characteristics, including marrow stimulation techniques (MST); drilling, abrasion arthroplasty, and microfracture, as well as osteochondral autograft transplantation (OATS), osteochondral allograft transplantation (OCA) and autologous chondrocyte implantation (ACI).2,3 Perioperatively nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used in orthopedics and demonstrate anti-inflammatory and analgesic effects through different mechanisms.4-9 The therapeutic effect is attributed to inhibition of cyclooxygenase (COX) activity. COX constitutes a group of enzymes responsible for the synthesis of prostaglandins and thromboxane, which are crucial mediators of pain, inflammation, and fever. 10 It is now known that at least 2 isoforms of COX exist, the inducible isoform COX-2 and the constitutive isoform COX-1. 4 NSAIDs differ based on their selectivity for COX-1 and COX-2. 5 These drugs can either inhibit the activity of both COX-1 and COX-2 with near-equal potency; preferentially inhibit the activity of COX-2 while inhibiting COX-1 with less potency; or highly selectively inhibit the COX-2 isoenzyme. 5
Use of NSAIDs in the postoperative period has been shown to reduce pain scores, improve patient satisfaction, allow earlier mobilization, and decrease opioid requirements, therefore minimizing opiate-induced adverse events. 11 However, conflicting data regarding their potential deleterious effects on new bone formation, bone remodeling, fracture healing, osseointegration, and spinal fusion have been reported.12-26 Endochondral ossification (EO), which is responsible for normal long bone formation in the growth plates, fracture healing (when bone fixation permits a small degree of movement), and ectopic generation of cartilage, is one of the two bone formation mechanisms.27,28 During EO, mesenchymal stem cells (MSCs) differentiate toward chondrocytes. Afterward, the chondrocytes become hypertrophic and direct the formation of mineralized matrix, promote angiogenesis and finally undergo apoptosis or transdifferentiate into osteoblasts.17,29,30 The remaining mineralized extracellular matrix provides a molecular scaffold for osteoblasts and osteoclasts to adhere to and remodel, setting the stage for de novo bone deposition. 31 Similar mechanisms can be identified following cartilage repair procedures. For instance, during MST procedures, small holes are created to penetrate the subchondral bone to allow the influx of blood and marrow-derived stem cells to the base of the defect with the formation of a blood clot. Subsequently, such cells differentiate toward chondrocytes and form hyaline like cartilage. However, EO often goes along in the absence of an inhibitory mechanism and results in bone formation (intralesional osteophytes).32,33 In ACI, immature tissues is implanted and it undergoes several phases to form a mature tissue: proliferative stage, in which chondrocytes are proliferating and the tissue fills the defect (up to 6 weeks); transition stage with soft, primitive repair tissue (6-12 weeks); early maturation stage, where repair begins to solidify and matrix consists mainly of type-II collagen, aggrecan, and other matrix proteins (12-26 weeks); and finally the late maturation stage, with fully matured chondrocyte and matrix (26 weeks to 3 years).34,35 Moreover, following OCA or OATS procedure, the implanted graft’s subchondral bone integrates to the host bone and undergoes remodeling, which are essential to its success.36,37 Given the fact that NSAIDs have an established detrimental effect on EO and consequently lead to an impairment of bone healing and chondrogenic differentiation and NSAIDs are commonly used perioperatively in patients undergoing cartilage repair, we were interested in whether NSAIDs have a negative effect on the outcome of cartilage repair procedures.12,13-26
The purpose of this study was to systematically review the available evidence regarding the plausible deleterious effects of NSAIDs on chondrocytes, chondrocyte differentiation and allograft or autograft incorporation which are integral parts of cartilage repair procedures. Our hypothesis was that the NSAIDs have a negative effect on chondrocyte differentiation and graft incorporation. We hope to establish the current state of the knowledge and point toward knowledge gaps that need to be studied in the future.
Methods
Literature Search Strategy
This systematic review was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). 38 A comprehensive search was performed of three medical electronic databases (PubMed, Science Direct, and The Cochrane Library) by 3 independent authors (CLL, GM, and EC) from their date of inception to August 20, 2018. To achieve the maximum sensitivity of the search strategy, we combined the terms: “NSAIDs” with “(cartilage OR chondrocytes)”; “NSAIDs” with “(chondrocyte differentiation OR chondrogenic differentiation); “NSAIDs with “(bone healing OR bone graft) OR allograft) OR autograft) OR osteochondral) as either key words or MeSH terms. The reference lists of all retrieved articles, reference lists of included papers and top hits from Google Scholar were reviewed for further identification of potentially relevant studies and assessed using the inclusion and exclusion criteria.
Study Selection
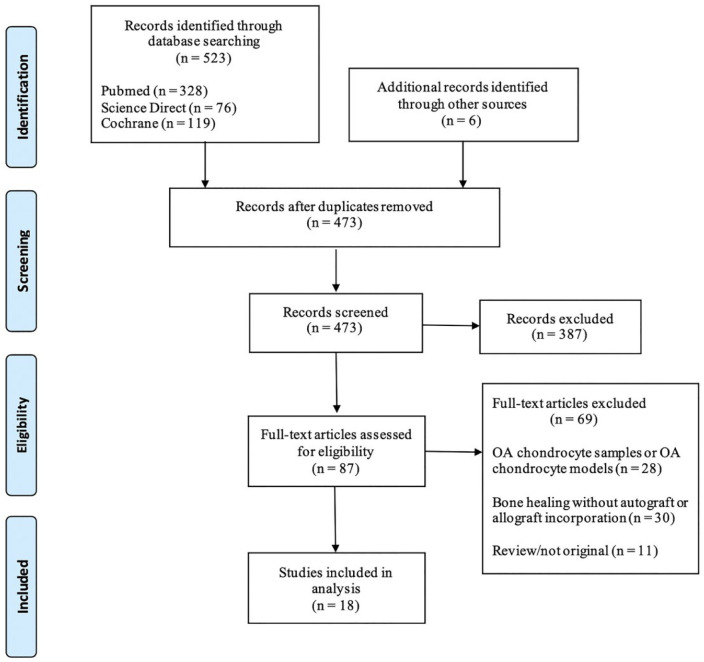
Eligible studies for the present systematic review included those dealing with the effects of NSAIDs on chondrocytes, chondrogenic differentiation and allograft or autograft incorporation. The initial titles and abstracts screening were made using the following inclusion criteria: studies of any level of evidence, written in English, reporting clinical or preclinical results, published in peer-reviewed journals and dealing with our topic. All the articles evaluating the effects of NSAIDs on either osteoarthritic (OA) chondrocyte samples or OA chondrocyte models were excluded. Moreover, articles about bone healing in which allograft or autograft incorporation was not investigated were also excluded. We also excluded all the remaining duplicates, or those without an accessible abstract ( Fig. 1 ). All publications were limited to in vivo, in vitro, animal, and human studies in the English language. Abstracts, case reports, conference presentations, reviews, editorials, and expert opinions were excluded. Studies were initially screened based on the abstracts and titles. Full texts were then obtained for all studies matching the inclusion criteria and reviewed to reconfirm the eligibility. The study selection was performed independently by 2 authors (GM and EC), and disagreement was resolved by discussion among all authors. A senior investigator (CL) was consulted in situations where disagreement persisted.
Figure 1.
Search strategy according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. Eighteen studies were identified for inclusion.
Methodologic Quality Assessment and Risk of Bias
In this systematic review, quality assessment of the in vitro studies was not performed as there is no accepted grading scale for such studies. Quality assessment of all in vivo experiments selected full-text articles was performed according to the ARRIVE (Animals in Research: Reporting In Vivo Experiments) guidelines for reporting in vivo experiments in animal research. 39 The ARRIVE guidelines consist of a checklist of 20 items describing the minimum information that all scientific publications reporting research using animals should include, such as the number and specific characteristics of animals used (including species, strain, sex, and genetic background); details of housing and husbandry; and the experimental, statistical, and analytical methods (including details of methods used to reduce bias such as randomization and blinding). It was developed using the CONSORT (Consolidated Standards of Reporting Trials) statement as their foundation. 40 Quality assessment was not performed for the 1 human study. Risk of bias was assessed using the ROBINS-I (Risk Of Bias In Non-randomised Studies–of Interventions) tool for nonrandomized interventions. 41 This tool was developed by members of the Cochrane Bias Methods Group and the Cochrane Non-Randomized Studies Methods Group in 2016 and is easy to use, accessible and designed for systematic reviews. It involves assessing the risk of bias in seven domains for outcomes in a study, performed for each study outcome. Risk of bias is ranked low, moderate, serious, or critical. Sometimes a judgement could not be made as the study did not contain necessary information.
The assessments were performed by 2 authors (GM and EM) independently. Any discrepancy was discussed with the senior investigator (CL) for the final decision.
Results
Study Selection
A total of 473 studies were obtained from the databases after the removal of the duplicates. After screening of the titles and abstracts, 387 articles were filtered out because of irrelevance to our study. Finally, 18 studies with full text were included in this review after applying inclusion and exclusion criteria ( Fig. 1 ).
Methodologic Quality Assessment and Risk of Bias
Quality assessment of the included in vivo animal studies and the percentage publications in different categories per checklist item are summarized in Tables 1 and 2 , respectively. In particular, the majority of publications were associated with medium gradings when evaluating checklist items, 4 (Introduction/Objective), 5 (i.e., Methods/Ethical Statement), 7 (i.e., Methods/Experimental Procedure), 8 (i.e., Methods/Experimental Animals), 9 (Methods/Housing and Husbandry), 10 (i.e., Methods/Sample Size), 11 (Methods/Allocation Animals to Experimental Groups), 12 (i.e., Methods/Experimental Outcomes), 13 (i.e., Methods/Statistical Methods), 15 (i.e., Results/Numbers Analyzed), 17 (Results/Adverse Events), 18 (i.e., Discussion/Interpretation), and 19 (i.e., Discussion/Generalizability) corresponding to 69.2%, 92.3%, 100%, 76.9%, 92.3%, 92.3%, 100%, 76.9%, 100%, 100%, 100%, 100%, and 100% respectively. For checklist items 1 (i.e., Title), 2 (i.e., Abstract), 3 (i.e., Introduction/Background), 6 (i.e., Methods/Study Design), 14 (Results/Baseline Data), 16 (i.e., Results/Outcomes and Estimations), and 20 (Discussion) maximum gradings were assigned to a total of 100%, 76.9%, 100%, 100%, 92.3%, 100%, and 100% of the included publications, respectively. In addition, the overall risk of bias was moderate for all in vivo (animal and human) studies based on the ROBINS-I tool ( Table 3 ).
Table 1.
Quality Assessment of the Included In Vivo Studies.
| Item |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| References | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Dogan et al. | 1 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 |
| Irwin et al. | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 |
| Orak et al. | 1 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 |
| Ekici et al. | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 |
| Riggin et al. | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 |
| Ozyuvaci et al. | 1 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 |
| Shapiro et al. | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 |
| Saricaoglu et al. | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 |
| Schroeder et al. | 1 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 |
| Janssen et al. | 1 | 2 | 1 | 2 | 0 | 2 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 2 |
| O’Keefe et al. | 1 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 |
| van der Heide et al. | 1 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 |
| Antoniolli et al. | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 |
Table 2.
Percentage Publications (n = 13) in Different Categories per Checklist Item.
| Grading |
|||
|---|---|---|---|
| Item | 0 | 1 | 2 |
| 1 | 0 | 100 | |
| 2 | 0 | 23.1 | 76.9 |
| 3 | 0 | 100 | |
| 4 | 0 | 69.2 | 30.3 |
| 5 | 7.7 | 92.3 | 0 |
| 6 | 0 | 0 | 100 |
| 7 | 0 | 100 | 0 |
| 8 | 7.7 | 76.9 | 15.4 |
| 9 | 7.7 | 92.3 | 0 |
| 10 | 0 | 92.3 | 7.7 |
| 11 | 0 | 100 | 0 |
| 12 | 7.7 | 76.9 | 15.4 |
| 13 | 0 | 100 | 0 |
| 14 | 7.7 | 92.3 | |
| 15 | 0 | 100 | 0 |
| 16 | 0 | 0 | 100 |
| 17 | 0 | 100 | 0 |
| 18 | 0 | 100 | 0 |
| 19 | 0 | 100 | 0 |
| 20 | 0 | 0 | 100 |
Table 3.
Risk of Bias of the Included In Vivo Studies.
| Study | Confounding | Selection | Measurement of Intervention | Missing Data | Measurement of Outcomes | Reported Result | Overall |
|---|---|---|---|---|---|---|---|
| Dogan et al. | Moderate | Low | Low | Low | Moderate | Low | Moderate |
| Irwin et al. | Moderate | Low | Low | Low | Moderate | Low | Moderate |
| Orak et al. | Moderate | Low | Low | Low | Moderate | Low | Moderate |
| Ekici et al. | Moderate | Low | Low | Low | Moderate | Low | Moderate |
| Riggin et al. | Moderate | Low | Low | Low | Moderate | Low | Moderate |
| Ozyuvaci et al. | Moderate | Low | Low | Low | Moderate | Low | Moderate |
| Shapiro et al. | Moderate | Low | Low | Low | Moderate | Low | Moderate |
| Saricaoglu et al. | Moderate | Low | Low | Low | Moderate | Low | Moderate |
| Schroeder et al. | Moderate | Low | Low | Low | Low | Low | Moderate |
| Janssen et al. | Moderate | Low | Low | Low | Low | Low | Moderate |
| O’Keefe et al. | Moderate | Low | Low | Low | Low | Low | Moderate |
| van der Heide et al. | Moderate | Low | Low | Low | Moderate | Low | Moderate |
| Antoniolli et al. | Moderate | Low | Low | Low | Moderate | Low | Moderate |
| Soreide et al. | Moderate | Low | Low | Low | Serious | Low | Moderate |
Study Characteristics
The general characteristics of the included studies are summarized in Tables 4 and 5 .
Table 4.
Characteristics of the Included Studies I: Effects of NSAIDs on Chondrocytes.
| Year of the Study | Study | In Vitro | In Vivo | Species | N | Follow-up, Min-Max | Outcome Measures | NSAIDs | NSAIDs Administration | Frequency of Administration | Source of Cells | Effects |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | Abrams et al. | 1 | 0 | Human chondrocytes | 16 | Duration dose equivalent to 2 half-lives plus additional 2 weeks in selected cases | Live/dead assay | Ketorolac | Chondrocytes were cultured in a bioreactor and the medication was constantly delivered | Constantly delivered—single dose injection | Human knee joint—intercondylar notch | Dose-dependent cytotoxic effect of ketorolac on human
articular chondrocytes. Specifically, 0.6% ketorolac resulted in increased cell death versus 0.3% ketorolac (P = 0.046); 0.5% meperidine was significantly less chondrotoxic versus 1.0% meperidine (P = 0.001) and 1.5% meperidine (P = 0.001). There was no significant difference in chondrotoxicity for 1.0% meperidine versus 1.5% meperidine (P = 0.665) |
| 2013 | Beitzel et al. | 1 | 0 | Human chondrocytes | 24 and 120 hours | Proliferation and viability (luminescence) assay | Keterolac | 1-hour in vitro exposure | Lonza (Hopkinton, MA) | The mean number of viable cells after 24 hours was 1488 ±
203 cells for the no-treatment 2% FBS control and 2748 ± 133 cells for the 10% FBS control (P < 0.002 compared with 2% FBS). After 120 hours, the mean viable cell number of the no-treatment 2% FBS control was 7397 ± 470 cells and 13152 ± 990 cells for the 10% FBS control treatment (P < 0.021 compared with 2% FBS). |
||
| 2004 | Dogan et al. | 0 | 1 | New Zealand male rabbits | 30 | 24 hours, 48 hours, and 10 days | Histopathological analysis | Ketorolac | Intra-articular (knee) | Single dose | Significant neutrophil and macrophage invasion and accumulation of the articular cartilage and synovial membrane, in conjunction with synovial hypertrophy, and hyperplasia following ketorolac or morphine injection into the knee. | |
| 1998 | Irwin et al. | 0 | 1 | Sprague-Dawley rats | 30 | 24 hours, 48 hours, 5 days, and 10 days | Histopathological analysis | Keterolac | Intra-articular (knee) | Single dose | At 24 hours and 5 days postinjection, the inflammation of the joint was significantly higher (P < 0.05) in ketorolac group compared with control | |
| 2015 | Orak et al. | 0 | 1 | Wistar albino rats | 100 | 48 hours, 1 week, 2 weeks, 4 weeks, and 8 weeks | Histopathological analysis | Tenoxicam and diclofenac | Intra-articular (knee) | Single dose | Increased fibroblast numbers and fibrosis levels (P < 0.05) were observed in knee joint following tenoxicam and diclofenac treatment | |
| 2004 | Ozyuvaci et al. | 0 | 1 | Sprague-Dawley rats | 50 | 24 hours, 48 hours, 7 days, 14 days, and 21 days | Histopathological analysis | Tenoxicam | Intra-articular (knee) | Single dose | Erosion of the joint surfaces and edema in the histological specimens was were observed at 24 and 48 hours after intra-articular tenoxicam injection. However, no histopathological changes were found at 7, 14, and 21-days after injection. | |
| 2014 | Ekici et al. | 0 | 1 | Sprague-Dawley rats | 40 | 1, 2, 7, 14, and 21 days | Histopathological analysis | Dexketoprofen | Intra-articular (knee) | Single dose | No significant histopathological damage up to 21 days after intraarticular administration | |
| 2014 | Riggin et al. | 0 | 1 | Sprague-Dawley rats | 64 | 2, 7, 28, and 84 days | Knee kinematics, mechanics, cartilage mechanical and histological evaluation | Ketorolac | Intra-articular (knee) | Single dose | No difference between the intervention and control groups in knee kinematics, mechanics and cartilage mechanical and histological evaluation. | |
| 2007 | Shapiro et al. | 0 | 1 | New Zealand white rabbits | 30 | 6 and 15 weeks | Histopathological analysis | Ketorolac | Intra-articular (knee) | Single dose | No significant degenerative changes were found after ketorolac administration | |
| 2008 | Saricaoglu et al. | 0 | 1 | Sprague-Dawley rats | 25 | 1, 2, 7, 14, and 21 days | Histopathological analysis | Lornoxicam | Intra-articular (knee) | Single dose | No significant differences in joint inflammation and cartilage degeneration were found, between saline and lornoxicam applied knees with histological evaluation. | |
| 2011 | Schroeder et al. | 0 | 1 | New Zealand white rabbits | 45 | 72 hours, 2 weeks, and 6 weeks | Histopathological analysis | Lornoxicam | Intra-articular (knee) | 1, 2, or 3 injections | Repeated intra-articular administration of lornoxicam was well tolerated. Mechanical irritation of the injection and to adaptive responses of the synoviocytes were demonstrated; however, no signs of toxicity to bone or chondrotoxicity was found. |
FBS = fetal bovine serum; NSAID = nonsteroidal anti-inflammatory drugs.
Table 5.
Characteristics of the Included Studies II: Effects of NSAIDs on Chondrocyte Differentiation and Allograft and Autograft Bone Incorporation.
| Year of the Study | Study | In Vitro | In Vivo | Species | N | Follow-up, Min-Max | Outcome Measures | NSAIDs | NSAIDs Administration | Frequency of Administration | Source of Cells | Effects |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2011 | Pountos et al. | 1 | 0 | Human MSCs | 10 | 21 days | Functional and quantitative assays, immunohistochemistry | Ketorolac, diclofenac, parecoxib, meloxicam | Drugs were administered into serum-free chondrogenic medium | Constantly in the medium in their stated plasma concentration | Human trabecular bone, bone marrow aspirated from superior iliac crest | No effect on MSC proliferation or its potential to
differentiate into osteogenic lineage. Use of a therapeutic concentration of diclofenac or ketorolac decreased significantly (P < 0.005) sGAG content by 45% and 55%, respectively, while parecoxib and meloxicam, inhibited sGAG to a lesser degree, 22% and 27%, respectively (P < 0.005). Pellet chondrogenesis was associated with increased COX-1 expression levels, but not COX-2, and COX-1 specific drugs suppressed MSC prostaglandin E2 (PGE-2) more than COX-2 specific inhibitors. |
| 2016 | Caron et al. | 1 | 0 | ATDC5, primary hBMSCs, ex vivo periosteal agarose cultures of New Zealand white rabbits, human mature chondrocytes | 8 | Indomethacin administration started at the onset of
differentiation (t = 0): Follow-up with
ATDC5 cells at 10 and 14 days. Follow-up with hBMSCs at 7-28
days. Follow-up with periosteal agarose culture at 21
days. Indomethacin administration beginning at varying days into differentiation onward: Follow-up at 14 days after administration of treatment. |
Bicinchoninic acid (BCA) protein assay (immunoblotting), histochemistry, quantitative reverse transcription polymerase chain reaction | Indomethacin | Drugs were added to the differentiation medium consisting of supplemented proliferation medium | N/A | Human bone marrow stem cells obtained from iliac crest bone marrow aspirate. Periosteal agarose culture harvested from the proximal tibia of skeletally mature rabbits. ATDC5 cell line obtained from mouse embryonic carcinoma cells. Human chondrocytes obtained from unaffected regions of OA cartilage from total knee arthroplasty. | Decreased gene and protein expression of chondrogenic and hypertrophic markers (measured by RT-qPCR and immunoblotting) as well as decreased glycosaminoglycan content (by Alcian blue histochemistry) was observed in all indomethacin concentration. Higher concentration of the drug resulted in more changes. When mature chondrocytes were treated with indomethacin, elevation in collagen type 2 mRNA expression was observed. Similarly, when ATDC5 cells and bone marrow stem cells were predifferentiated to obtain a chondrocyte phenotype and indomethacin was added from this time point onward, low concentrations of indomethacin also resulted in increased chondrogenic differentiation. |
| 2017 | Janssen et al. | 0 | 1 | Skeletally immature New Zealand white rabbits | 12 | 25 days | Standardized enzyme immunoassay analysis, Lane and Sandhu radiological score, micro-finite element analyses (FEA), Heiple histologic fracture score, gene expression analysis, RT-qPCR using SYBR assay | Celecoxib | Administered orally | Daily | Growth plate of the distal femur, tibia, ulna of New Zealand white rabbits | Negative effect on the initiation of the chondrogenic phase
of endochondral ossification. Celecoxib inhibited in
vivo PGE-2 synthesis by 80%. COX-2 inhibition
impaired and delayed the bone fracture healing process
(Lane-Sandhu radiologic fracture score for the control group
was 8.8 compared with a score of 5.7 for the treated group).
Mineralization front was significantly less advanced in the
celecoxib-treated group. Significant decrease in the
hypertrophic zone and total growth plate thickness in the
celecoxib-treated group, but no significant difference was
observed in the proliferative zone. Lack of cartilaginous PEO formation suggested an inhibited chondrogenic differentiation. |
| 2006 | O’Keefe et al. | 0 | 1 | C57BL/6 mice | 61 | X-ray follow-up weekly, semiquantitative histomorphometric analysis follow-up at 5 weeks postsurgery | Histological and histomorphometric analysis | Celecoxib, ketorolac | Orally for celecoxib, intramuscular injection for ketorolac | Daily | Mouse femoral bone graft | 60% decrease in bone formation of treated groups compared
with controls (P < 0.05). Differences
between bone formation in celecoxib and ketorolac were
insignificant. A lower percentage of histologic bony
bridging (55% for celecoxib, 61% for ketorolac) was observed
in both NSAID-treated groups (P < 0.05)
compared with control (82%). Stopping administration did not allow for a full recovery in bone formation; a 45% reduction in formation remained. |
| 2008 | van der Heide et al. | 0 | 1 | Dutch milk goats | 27 | 6 weeks | Histological and histomorphometric analysis: (fibrous tissue ingrowth and bone ingrowth) | Ketoprofen, meloxicam | Subcutaneously | Daily | Bone chambers (from each side in the cortical bone of the proximal medial tibia) with surrounding cortex | Normal ingrowth pattern was observed in the majority of the
bone chambers (138 specimens), with only 17 of the total
bone chambers having no ingrowth or only fatty tissue and no
fibrous tissue or bone. No significant differences in bone growth (P = 0.5) or fibrous tissue ingrowth (P = 0.6) were observed between the different medication groups. No significant differences in bone ingrowth were found with regard to whether allograft, autograft, or rinsed allograft was used. |
| 2008 | Antoniolli et al. | 0 | 1 | Wistar rats | 120 | 7, 14, and 30 days | Histological and immunohistochemistry | Diclofenac sodium, meloxicam | Intramuscular | Immediate postoperative period and for 6 consecutive days | Rat femurs | NSAIDs delayed the repair of both groups of animals,
including those with bovine devitalized matrix and
autogenous bone graft, while dexamethasone did not (null
hypothesis rejection at 5%). Inflammatory reaction was greatest in animals with bovine devitalized matrix as their bone defect filling, regardless of whether they were treated with NSAIDs or not. All groups, including those treated with NSAIDs and those not treated with NSAIDs, had a significant increase in bone neoformation at all follow-up times. Collagen formation was significantly increased at all time points. |
| 2016 | Soreide et al. | 0 | 1 | Humans | 4144 (treated with NSAIDs) | 2, 5, and 10 years postoperative | Risk of revision and survival, Knee Injury and Osteoarthritis Outcome Score (KOOS), and patient-reported quality of life (QOL) | Diclofenac (91.5% of patients), ketorolac (3.1%), celecoxib (2.4%), other (1.0%) | Not reported | Not reported | N/A | No significant difference in overall graft survival between
the groups who received NSAIDs and those who did not.
Beneficial significant effect on risk of revision in
patients with BPTB grafts (95% CI). Reduced risk of an Osteoarthritis Outcome Score (KOOS)–quality of life (QOL) subscale score <44 at 2-year follow-up was observed for patients who received NSAIDs and statistically significant higher mean scores for all subscales at 2-year follow-up. NSAIDs did not increase risk of revision or increase risk of worse patient-reported knee function. |
BPTB = bone–patellar tendon–bone; hBMSC = human bone marrow stem cells; MSCs = mesenchymal stem cells; N/A, not applicable; NSAIDs = nonsteroidal anti-inflammatory drugs; OA = osteoarthritis; PEO = periosteal endochondral ossification; RT-qPCR = real time quantitative polymerase chain reaction; sGAG = sulfated glycosaminoglycan.
Out of the 18 included studies, 4 in vitro, 13 animal, and 1 human study were reviewed. Among the included animal studies, varieties of animal models were investigated. Twelve studies utilized small animals (7 in rats, 4 in rabbits, and 1 in mice) and 1 study used a ruminant animal model (goat). Various type of NSAIDs were utilized in the included studies ( Tables 4 - 6 ) Ketorolac was the most commonly investigated NSAID (7 studies). Further studies evaluated the effects of diclofenac (4 studies), celecoxib (3 studies), tenoxicam (2 studies), lornoxicam (2 studies), meloxicam (2 studies), paracoxib (1 study), dexketoprofen (1 study), ketoprofen (1 study), and indomethacin (1 study).
Table 6.
Type of NSAIDs Used in the Study.
| Study | Type of NSAIDs used | Dose of NSAIDs | COX-specificity |
|---|---|---|---|
| Abrams et al. | Ketorolac | 0.3% ketorolac (15 mg area-adjusted dose); 0.6% ketorolac (30 mg area-adjusted dose) | Ketorolac more COX-1 selective |
| Beitzel et al. | Keterolac | Ketorolac tromethamine 30 mg/mL | Ketorolac more COX-1 selective |
| Dogan et al. | Ketorolac | Ketorolac: (1 mg/0.25 mL; pH 6.9-7.9) | Ketorolac more COX-1 selective |
| Irwin et al. | Keterolac | 0.25 mL of a standard preparation of tenoxicam (10 mg/mL) | Ketorolac more COX-1 selective |
| Orak et al. | Tenoxicam and diclofenac | 1 mg of tenoxicam or 0.75 mg of diclofenac-Na | Diclofenac more COX-1 selective; tenoxicam more COX-2 selective |
| Ozyuvaci et al. | Tenoxicam | 0.25 mL of a standard preparation of tenoxicam (4 mg/mL) | Tenoxicam more COX-2 selective |
| Riggin et al. | Ketorolac | 0.1 mL of ketorolac tromethamine (3 mg/0.1 mL) | Ketorolac more COX-1 selective |
| Ekici et al. | Dexketoprofen | 0.25 mL (6.25 mg) dexketoprofen trometamol | Dexketoprofen more COX-1 selective |
| Shapiro et al. | Ketorolac | 0.5 cm3 of ketorolac tromethamine (15 mg/0.5 cm3) | Ketorolac more COX-1 selective |
| Saricaoglu et al. | Lornoxicam | 0.25 mL of lornoxicam (4 mg/mL) | Lornoxicam more COX-2 selective |
| Schroeder et al. | Lornoxicam | 4 and 8 mg/mL of Lornoxicam | Lornoxicam more COX-2 selective |
| Pountos et al. | Ketorolac, diclofenac, parecoxib, meloxicam | Peak plasma concentrations: 1.1 μg/mL for ketorolac 1 μg/mL for diclofenac sodium, 1 μg/mL for parecoxib, 2 μg/mL for meloxicam | Ketorolac and diclofenac more COX-1 selective; celecoxib and ketoprofen COX-2 selective |
| Caron et al. | Indomethacin | Increasing concentrations of 2, 20, and 200 μM for the ATDC5 culture, 2 μM for the human bone marrow stem cells, 2 μM for the periosteum agarose culture, 2 μM for the human articular chondrocyte culture | Indomethacin as a nonspecific COX inhibitor |
| Janssen et al. | Celecoxib | 10 mg/kg for celecoxib | Celecoxib as COX-2 selective |
| O’Keefe et al. | Celecoxib, ketorolac | 25 mg/kg/day for celecoxib, 4 mg/kg/day for ketorolac | Celecoxib COX-2 selective, ketorolac more COX-1 selective |
| van der Heide et al. | Ketoprofen, meloxicam | 2.2 mg/kg/day for ketoprofen, 0.5 mg/kg/day for meloxicam | Ketoprofen nonselective, meloxicam COX-2 preferential |
| Antoniolli et al. | Diclofenac sodium, meloxicam | 2 mg/kg/day for diclofenac sodium, 0.5 mg/kg/day for meloxicam | Diclofenac more COX-1 selective, meloxicam COX-2 preferential |
| Soreide et al. | Diclofenac (91.5% of sample), ketorolac (3.1%), celecoxib (2.4%), other (1.0%) | Not reported | Diclofenac and ketorolac more COX-1 selective, celecoxib COX-2 selective |
COX = cyclooxygenase; NSAIDs = nonsteroidal anti-inflammatory drugs.
Outcomes
Effects of NSAIDs on Chondrocytes
One in vitro study 42 reported that ketorolac has a dose-dependent cytotoxic effect on human chondrocytes whereas another in vitro study 43 reported increased chondrocyte viability. Furthermore, 4 in vivo studies found increased inflammation in the joint with histological examinations following NSAIDs administration.44-47
Studies reporting potential negative effects of NSAIDS on chondrocytes and articular cartilage
Abrams et al. 42 investigated the effects of a single-dose ketorolac on mature healthy human chondrocytes in vitro. Chondrocytes were harvested from sixteen donors and a bioreactor was used to expose the chondrocytes to 0.3% and 0.6% ketorolac (more COX-1 selective NSAIDs). After treatment, a live/dead assay was used to assess chondrocyte viability. The study found a significantly higher (P < 0.05) chondrocyte mortality in the group receiving the highest ketorolac concentration. However, Beitzel et al. 43 in an in vitro study reported a significantly increased chondrocyte viability (with luminescence assays) at 24 and 120 hours after treatment with ketorolac (P < 0.05) alone and with platelet-rich plasma (PRP; P < 0.001), compared with a control group treated with 2% or 10% fetal bovine serum (FBS). Dogan et al. 44 performed a histological evaluation of rabbit knee joints at 24 hours, 48 hours, and 10 days after ketorolac injection. They reported significantly more histopathological changes in the treatment groups compared with the control saline injection (P < 0.05). Specifically, they demonstrated significant neutrophil and macrophage accumulation and invasion of articular cartilage and synovial membrane, as well as synovial hypertrophy and hyperplasia. In another rabbit study, Irwin et al. 45 demonstrated histologically, that at 5 and 24 days postinjection, the inflammation of the joint was significantly higher (P < 0.05) in the ketorolac group compared with control. Using a rat model, Orak et al. 46 investigated the effects of intraarticular injections with methylprednisolone, tenoxicam (COX-2 selective NSAIDs) and diclofenac (more COX-1 specific NSAIDs) respectively, following intra-articular injection. NSAIDS (tenoxicam, diclofenac), unlike methylprednisolone, increased fibroblast numbers and fibrosis levels (P < 0.05). Ozuyaci et al. 47 observed histopathological changes, including erosion of the joint surfaces and edema 24 and 48 hours following intra-articular tenoxicam injection. However, no histopathological changes were found at later time points (7, 14, and 21 days).
Studies reporting no or little effect of NSAIDs on chondrocytes and articular cartilage
Six studies demonstrated no pathological effects of NSAIDs on chondrocytes.43,47-52 Ekici et al. 48 used dexketoprofen (COX-1 selective NSAIDs) in a rat model. They reported no significant histopathological damage up to 21 days after intraarticular administration. Riggin et al., 49 also using a rat model, investigated the intra-articular effects of a single-dose ketorolac and found no difference between the intervention and control groups with regards to knee kinematics, mechanics and cartilage mechanical and histological evaluation at 2, 7, 28, and 84 days postinjection. Shapiro et al. 50 did not find histologically relevant degenerative changes after ketorolac administration into the rabbit knee joint at 6 and 15 weeks after intra-articular injection. Saricaogula et al., 51 using an in vivo rat model, reported no significant histological differences in joint inflammation and cartilage degeneration, between saline- and lornoxicam-treated knees. The authors found no pathological changes in both groups at 1, 2, 7, 14, and 21 days after injection. In addition to that, Shroeder et al. 52 reported that repeated administration (1, 2, or 3 times) of lornoxicam into the knee joint was well-tolerated in rabbits. Using hematoxylin and eosin staining, they reported on mechanical irritation and tract inflammation from the injection and adaptive synoviocyte responses; however, no signs of toxicity to bone or chondrotoxicity were found.
Specific Effects of NSAIDs on Chondrocyte Differentiation
For chondrocyte implantation chondrocyte differentiation is critical. We therefore report potential effects of NSAIDs on chondrocyte differentiation separately.
Two in vitro studies14,15 and 1 in vivo 19 study reported detrimental effects of NSAIDs on chondrocyte differentiation. This effect seems to be dependent on the COX-selectivity of the NSAIDs and the differentiation-stage of the chondrocytes.
Pountos et al. 14 investigated the effects of NSAIDs (ketorolac and diclofenac as being more COX-1 specific, and parecoxib and meloxicam, as more COX-2 specific drugs) on MSCs obtained from human trabecular bone and bone marrow aspirates from superior iliac crest. These are cells believed to potentially play a role in the differentiation to mature chondrocytes in cases of microfracture or microfracture-related techniques. They specifically studied proliferation and differentiation toward the osteogenic and chondrogenic linages. The effects of COX-1 and COX-2 inhibitors on MSC proliferation and osteogenic and chondrogenic differentiation were tested using Vybrant, sodium 3′-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis (4-methoxy-6-nitro) benzene sulfonic acid hydrate (XTT), functional and quantitative assays of MSC differentiation. The MSC expression of COX-1 and COX-2 and prostaglandin E2 (PGE-2) levels were evaluated serially during lineage differentiation by quantitative polymerase chain reaction (qPCR) and enzyme-linked immunosorbent assay (ELISA). In this study, treatment of MSCs with NSAIDs had no effect on cell proliferation or on their potential to differentiate into osteogenic lineage. For chondrogenic differentiation, a serum-free chondrogenic medium was used and the cells were allowed to differentiate for 21 days and form pellets. The drugs were included in the medium in their stated plasma concentration. At day 21, pellets after were assayed for their sulfated glycosaminoglycan (sGAG) content. Use of a therapeutic concentration of diclofenac or ketorolac decreased sGAG content by 45% and 55%. Parecoxib and meloxicam, inhibited sGAG to a lesser degree, 22% and 27%, respectively. Cartilage pellet immunohistochemistry confirmed the above results. Pellet chondrogenesis was associated with increased COX-1 expression levels, but not COX-2, and COX-1 specific drugs suppressed MSC PGE-2 more than COX-2 specific inhibitors.
Another in vivo study by Caron et al. 15 reported on progenitor cells differentiating in the chondrogenic lineage (ATDC5, primary human bone marrow stem cells and ex vivo periosteal agarose cultures) The cultures were treated with increasing concentrations of indomethacin (2, 20, and 200 µM). Decreased gene and protein expression of chondrogenesis and increase of hypertrophy markers (measured by real-time-qPCR and immunoblotting) as well as decreased glycosaminoglycan content (by Alcian blue histochemistry) was observed in all indomethacin treated cultures. These findings follow a linear dose response. When mature chondrocytes were treated with indomethacin, elevation in collagen type 2 mRNA expression (Col2a1) was observed. Similarly, when ATDC5 cells and bone marrow stem cells were predifferentiated to obtain a chondrocyte phenotype and indomethacin was added from this time point onward, low concentrations of indomethacin also resulted in increased chondrogenic differentiation.
Janssen et al., 19 in an in vivo rabbit model, investigated the effects of orally administered celecoxib (selective COX-2 inhibitor) or placebo (for 25 days) on endochondral ossification during fracture healing of a noncritical size defect in the ulna, femoral growth plate and ectopically induced cartilaginous tissue. Endochondral ossification was evaluated by radiography, micro-computed tomography, histology and gene expression analysis. Delayed fracture healing, alterations in growth plate development and progression of mineralization was observed. In addition, chondrogenic differentiation of ectopically induced cartilaginous tissue was severely impaired.
Effects of NSAIDs on Allograft and Autograft Bone Incorporation
Deleterious effects of NSAIDs on bone healing following allograft bone implantation were demonstrated in 2 in vivo studies.30,53 In addition, one of these studies 53 reported delayed bone healing after autograft bone implantation and NSAIDs administration. However, 1 in vivo study found no negative effects of NSAIDs on bone ingrowth for either bone graft. Furthermore, a human study 54 reported no impaired effects of these drugs on autograft bone incorporation.
O’Keefe et al., 30 in an in vivo rat model, demonstrated that ketorolac (intramuscularly) and celecoxib (orally) markedly inhibited bone repair. Mid-diaphyseal segmental femoral defect was created in this study and then repaired by frozen bone allograft. Bone healing was evaluated weekly by X-ray and by a semiquantitative histomorphometric analysis at 5 weeks postsurgery. Celecoxib and ketorolac were administered daily for 2 or 5 weeks and PGE-2 was infused locally via osmotic minipump for 4 weeks. Celecoxib or ketorolac administration for 5 weeks reduced new bone ingrowth by about 60% (P < 0.05). The percentage of bony bridging in both drug-treated groups was significantly decreased at 5 weeks. Moreover, temporal administration of celecoxib for 2 weeks significantly reduced bone formation by 45% and withdrawal of the celecoxib only led to slight recovery of bone formation at the graft side. In contrast, PGE-2 infusion stimulated bone formation and healing.
van der Heide et al. 55 investigated the effects of meloxicam (COX-2 preferential drug), ketoprofen (nonselective COX inhibitor) or no NSAIDs (utilized subcutaneously for 6 weeks) in an in vivo goat study. He used a model of a bone chamber incorporation analysis examining either autograft, rinsed allograft, or allograft that had been rinsed and irradiated. All drugs were administered subcutaneously. Histological and histomorphometric analysis revealed no significant differences in bone growth (P = 0.5) or fibrous tissue ingrowth (P = 0.6) between the different medication groups.
Antoniolli et al. 53 created a critical size defect in a rat femoral diaphysis and filled the defect with autograft or bovine bone devitalized matrix. Animals of each group were redistributed to 4 subgroups according to the intramuscular administration of diclofenac sodium, dexamethasone, meloxicam or saline solution. At 7, 14, and 30 days, specimens underwent histological evaluation consisted of quantification of inflammatory process, bone neoformation, collagen formation, and the presence of macrophages. The use of diclofenac sodium and meloxicam delayed bone healing following the use of autogenous bone graft and bovine bone devitalized matrix compared with a control group. However, significant and progressive increase of bone neoformation was observed in auto and allografts regardless of the use of NSAIDS.
Discussion
NSAIDs are widely used perioperatively in orthopedics and in patients undergoing cartilage repair.4-9 Such medications have an established effect on bone healing and chondrocyte viability and differentiation.12,13-26 Even though these mechanisms are important elements of graft incorporation and chondral repair using cell-based chondral repair procedures or auto/allograft techniques, the direct effect on cartilage repair has not been studied in randomized controlled trials (RCTs) and remain unclear. This may be in part due to the fact that it may be difficult for study investigators to maintain equipoise if there is a general perception of detrimental effects of NSAIDs on chondral repair.
The goal of this article was to systematically reviewed the available evidence regarding the plausible deleterious effects of NSAIDs on chondrocyte viability, chondrocyte differentiation, as well as osteochondral autograft and allograft incorporation.
Study Quality
Grading of the selected literature reporting on this topic revealed significant heterogeneity. The majority of publications were associated with medium gradings when evaluating checklist items 4, 5, 7, 8, 9, 10, 11, 12, 13, 15, 17, 18. For checklist items 1, 2, 3, 6, 14, 16, and 20, maximum gradings were assigned to the included publications. In addition, the overall risk of bias was moderate for all studies based on the ROBINS-I tool.
Effect of NSAIDS on Articular Cartilage and Chondrocytes
There is controversial literature regarding the overall effect of NSAIDs on chondrocytes.42,43 A recent in vitro study by Abrams et al. 42 reported a significantly higher (P < 0.05) human chondrocyte mortality in the highest ketorolac (0.6%) concentration compared with isotonic saline. However, Beitzel et al., 43 in another in vitro model, reported a significantly increased chondrocyte viability after treatment with ketorolac (P < 0.05) alone and with PRP (P < 0.001). The discrepancies between the 2 studies may be due to differences in the duration of treatment as well as the model used to study chondrocyte death. In particular, Abrams et al. 42 used qPCR to analyze the differentiation status of human chondrocytes and a bioreactor for continuous medication delivery, consistent with a single-dose injection. By contrast, Beitzel et al. 43 exposed chondrocytes for only 1 hour to ketorolac and used only human chondrocytes obtained from Lonza (Hopkinton, MA) as culture without evaluating the status of chondrocyte differentiation. Consequently, intra-articular injection of ketorolac following ACI is not suggested, given the fact that ACI graft is susceptible until the late maturation stage (26 weeks to 3 years)34,35 and this drug might affect the chondrocyte survival. Moreover, chondrocyte viability in the OCA graft is believed to be a major determinant of graft performance in vivo, and any drug which can result in a lower viability should be avoided.56,57
Dogan et al. 44 and Irwin et al. 45 both found that intra-articular injection of ketorolac in an animal model caused a moderate to high-grade inflammation in the knee joint, compared with saline injection (P < 0.05). Another in vivo study by Orak et al., 46 involving the use of diclofenac and tenoxicam, found increased fibroblast numbers and fibrosis levels. Furthermore, Ozuyaci et al. 47 observed early (24-48 hours) inflammatory changes following intra-articular tenoxicam injection in the knee joint, which changes dissolved by 7 days. However, Riggin et al. 49 and Shapiro et al. 50 found no histologically relevant degenerative changes following ketorolac administration into the rat and rabbit knee, respectively. Other animal studies further confirmed the safeness of the use of dexketoprofen 48 and lornoxicam.51,52
It appears that studies looking at the early time points within hours or days after injury report a detrimental effect of NSAIDS on chondrocyte proliferation, and histological parameters of chondral repair. However, the effect seems to be significantly muted or absent at later time points. This could be rationalized by assuming that a general healing response requires an initial level of inflammation during the early phases of tissue repair but not in the remodeling or repair phases. 58 There are certainly some concerns based up the findings that ketorolac may in fact induce an increase in inflammation, which would be unintended.
Effects of NSAIDs on Chondrocyte Differentiation
Pountos et al., 14 in an in vitro study, indicated that ketorolac, diclofenac, parecoxib, and meloxicam inhibited the chondrogenic potential of MSCs. Moreover, pellet chondrogenesis was associated with increased COX-1 expression levels but not COX-2, and COX-1 specific drugs suppressed MSC PGE-2 more than COX-2 specific inhibitors. Therefore, it is possible that early administration of NSAIDs inhibits bone healing 59 with decreased chondrogenic differentiation of MSCs, as supported by the findings of Caron et al. 15 in the study of indomethacin (nonspecific COX inhibitor) administration at the early start of differentiation. However, exposure of (pre)differentiated chondrocytes to indomethacin did not negatively influence a chondrogenic phenotype, and instead stimulated it. This implies that indomethacin’s effects on chondrogenic differentiation depend on the cell’s differentiation stage. Janssen et al., 19 in an in vivo study, found that COX-2 selective inhibition caused impaired chondrogenic differentiation during EO in his use of orally administered (25 days) celecoxib or placebo on cartilaginous phase of 3 different endochondral ossification scenarios. In addition, chondrogenic differentiation of ectopically induced cartilaginous tissue was severely impaired, indicating that a cell’s differentiation status and sensitivity to NSAIDs is influenced by differentiation stage-dependent COX-1 and COX-2 expression patterns. 15 COX-2 expression during chondrogenic differentiation is biphasic.17,18 The first phase takes place during chondrogenic differentiation of progenitor cells and later during chondrocyte hypertrophy. Taken together, specific inhibition of the COX-1 enzyme results in overall inhibition of chondrogenic differentiation, whereas COX-2 inhibition specifically negatively affects the first phase of chondrogenic differentiation and also the level of chondrocyte hypertrophy.14,16-19 The use of anti-inflammatory agents might therefore contribute to poor cartilage formation following MST procedures and cell-based chondral repairs as chondrocyte differentiation is an component of such procedures. However, in a later phase NSAIDs might also help the chondrocytes to avoid hypertrophy and obtain an optimal phenotype and therefore avoid intralesional osteophyte formation a known complication of MST procedures.32,33
Effect of NSAIDs on Bone Healing and Graft Incorporation
We found that detrimental effects of NSAIDs on fracture healing and osseointegration have been reported.12,13,19-26 In particular, we were concerned about the effects of NSAIDs on allograft and autograft incorporation as described by O’Keefe et al., 30 indicating that the utilization of ketorolac and celecoxib inhibits bone repair in an in vivo allograft-healing model and delayed allograft incorporation. There may be a difference based up the selectiveness of the COX-inhibitor, however, as van der Heide et al. 55 showed no differences in bone ingrowth using either ketoprofen or meloxicam in titanium bone chambers loaded with fresh autograft, rinsed allograft, or irradiated rinsed allograft. This observation is contradictory to the earlier mentioned article by OKeefe et al., 30 which may be due to the use of a COX-2 preferential drug (meloxicam) instead of a COX-2 selective drug (celecoxib).12,13,19,20 However, Antoniolli et al., 53 in another in vivo model, reported that the use of diclofenac sodium and meloxicam delayed bone healing following the use of autogenous bone graft and bovine bone devitalized matrix indicating that the data remain controversial.
Taken together, the effects of NSAIDs on autograft and allograft incorporation is controversial and has a wide range of effects. COX-2 inhibition seems to have more deleterious effects on incorporation, as was reported in the case of fracture healing.12,13,19,20 Since allo- and autografts rely on osteoinductive and angiogenic activity to incorporate, we theorized that they may potentially be sensitive to NSAIDs.20,30,60 Incorporation of OCA and OATS plugs is a crucial factor for effective repair and its impairment is a major source of graft failure.25,61,62 Therefore, further studies are needed to investigate the effect of NSAIDs on autograft and allograft incorporation.
Summarizing the effect of NSAIDs on chondrocytes, it appears that there is a likely effect on articular cartilage and chondrocyte differentiation in the early phases of chondral repair. This effect has not been proven in the human situation yet. Future RCTs investigating this effect could answer this question; however, based on the basic science concerns it is unlikely for such an RCT to be performed as the risk/benefit profile appears prohibitive. On the other hand, it is well established that NSAIDs interfere with the early phases of bone healing and this is corroborated by the available literature on graft incorporation that we reviewed. Even though the effect and mechanism are not entirely clear there is certainly enough clinical concern to avoid NSAID use during the time of graft incorporation.
It may be prudent to avoid NSAIDs after cartilage repair; however, there may be situations in which the use of NSAIDs is necessary to allow for progression during rehabilitation that may affect the outcome of the procedure more than a theoretical negative effect on chondrocyte proliferation (i.e., impending knee stiffness). In those case a careful risk benefit analysis should include the potential gain of the use of NSAIDs and the time from surgery. Use of NSAIDs within the first 2 to 3 months after chondral repair should likely be avoided; at later time points, the risk may be lower based on the basic science studies reviewed.
While the demand for long-term randomized control studies to investigate the effects of NSAIDs on cartilage repair appears necessary, it may be difficult to rationalize the use of NSAIDs at early time points. It may be more promising to investigate other anti-inflammatory avenues such as emerging orthobiologics (BMAC, PRP).
Limitations
A major limitation of this systematic review is the variability of the included studies. Given the fact that out of the 18 included studies 4 in vitro, 13 animal and 1 human study were reviewed, the accurate comparison of the studies was difficult. Another limitation is that no quality assessment was performed in the in vitro and human studies. However, there is no accepted grading scale for the in vitro studies and utilization of other quality assessment tool for only 1 human study would not have yield valuable additional information. Moreover, the considerable heterogeneity of the included studies, such as the differences in NSAID type, dose, duration, and outcome assessment, and the only one human study on the topic makes the interstudy comparison difficult and hinders drawing additional convincing conclusions.
Although studies included in this review are variable in terms of NSAID type, dose, and duration, and human studies are lacking, useful information can still be extracted from the existing literature.
Conclusion
The present systematic review demonstrates no detrimental effect of NSAIDs use on healthy mature chondrocytes; however, there are possible deleterious effects of NSAIDs on cartilage that is in the process of repair or cartilage repair technology relying on chondrocyte biology. In addition, these drugs seem to have an influence on graft incorporation and osteoconduction. We believe that at this point the use of NSAIDs systemic or local immediately after cartilage repair procedures should be avoided unless a substantial clinical benefit would otherwise be withheld from the patient. More human studies are needed to analyze the effect of NSAIDs on cartilage repair techniques.
Clinical Relevance
NSAIDs are widely used perioperatively before and after orthopedic procedures. Repair of damaged cartilage is necessary because if it is left untreated it progresses to osteoarthritis. However, to this date it is unclear what effects NSAIDs have on the outcome of cartilage repair procedures. The results of the cartilage repair can be influenced by multiple factors and this review gives an insight into the possible deleterious effect of NSAIDs on its success.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Gergo Merkely  https://orcid.org/0000-0001-6660-6994
https://orcid.org/0000-0001-6660-6994
Emanuele Chisari  https://orcid.org/0000-0003-0933-6806
https://orcid.org/0000-0003-0933-6806
References
- 1. Batty L, Dance S, Bajaj S, Cole BJ. Autologous chondro-cyte implantation: an overview of technique and outcomes. ANZ J Surg. 2011;81(1-2):18-25. doi: 10.1111/j.1445-2197.2010.05495.x [DOI] [PubMed] [Google Scholar]
- 2. Rosenberger RE, Gomoll AH, Bryant T, Minas T. Repair of large chondral defects of the knee with autologous chondrocyte implantation in patients 45 years or older. Am J Sports Med. 2008;36(12):2336-44. doi: 10.1177/0363546508322888 [DOI] [PubMed] [Google Scholar]
- 3. Farr J, Cole B, Dhawan A, Kercher J, Sherman S. Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res. 2011;469(10):2696-705. doi: 10.1007/s11999-010-1764-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coxib and Traditional NSAID Trialists’ (CNT) Collaboration; Bhala N, Emberson J, Merhi A, Abramson S, Arber N, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382:769-79. doi: 10.1016/S0140-6736(13)60900-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brooks P, Emery P, Evans JF, Fenner H, Hawkey CJ, Patrono C, et al. Interpreting the clinical significance of the differential inhibition of cyclooxygenase-1 and cyclooxygenase-2. Rheumatology (Oxford). 1999;38:779-88. doi: 10.1093/rheumatology/38.8.779 [DOI] [PubMed] [Google Scholar]
- 6. Park KD, Kim TK, Bae BW, Ahn JK, Lee WY, Park Y. Ultrasound guided intra-articular ketorolac versus corticosteroid injection in osteoarthritis of the hip: a retrospective comparative study. Skeletal Radiol. 2015;44:1333-40. doi: 10.1007/s00256-015-2174-9 [DOI] [PubMed] [Google Scholar]
- 7. Min KS, St, Pierre P, Ryan PM, Marchant BG, Wilson CJ, Arrington ED. A double-blind randomized controlled trial comparing the effects of subacromial injection with corticosteroid versus NSAID in patients with shoulder impingement syndrome. J Shoulder Elbow Surg. 2013;22:595-601. doi: 10.1016/j.jse.2012.08.026 [DOI] [PubMed] [Google Scholar]
- 8. Reuben SS, Connelly NR. Postoperative analgesia for outpatient arthroscopic knee surgery with intraarticular bupivacaine and ketorolac. Anesth Analg. 1995;80:1154-7. doi: 10.1097/00000539-199506000-00015 [DOI] [PubMed] [Google Scholar]
- 9. Unlu Z, Ay K, Tuzun C. Comparison of intra-articular tenoxicam and oral tenoxicam for pain and physical functioning in osteoarthritis of the knee. Clin Rheumatol. 2006;25:54-61. doi: 10.1007/s10067-005-1136-3 [DOI] [PubMed] [Google Scholar]
- 10. Smith WL, Meade EA, DeWitt DL. Interactions of PGH synthase isozymes-1 and -2 with NSAIDs. Ann N Y Acad Sci. 1994;177:50-7. doi: 10.1111/j.1749-6632.1994.tb52723.x [DOI] [PubMed] [Google Scholar]
- 11. Gupta A, Bah M. NSAIDs in the treatment of postoperative pain. Curr Pain Headache Rep. 2016;20:62. doi: 10.1007/s11916-016-0591-7 [DOI] [PubMed] [Google Scholar]
- 12. Simon AM, Manigrasso MB, O’Connor JP. Cyclo-oxygenase 2 function is essential for bone fracture healing. J Bone Miner Res. 2002;17:963-76. doi: 10.1359/jbmr.2002.17.6.963 [DOI] [PubMed] [Google Scholar]
- 13. O’Connor JP, Capo JT, Tan V, Cottrell JA, Manigrasso MB, Bontempo N, et al. A comparison of the effects of ibuprofen and rofecoxib on rabbit fibula osteotomy healing. Acta Orthop. 2009;80(5):597-605. doi: 10.3109/17453670903316769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pountos I, Giannoudis PV, Jones E, English A, Churchman S, Field S, et al. NSAIDS inhibit in vitro MSC chondrogenesis but not osteogenesis: implications for mechanism of bone formation inhibition in man. J Cell Mol Med. 2011;15(3):525-34. doi: 10.1111/j.1582-4934.2010.01006.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caron MMJ, Emans PJ, Cremers A, Surtel DAM, van Rhijn LW, Welting TJM. Indomethacin induces differential effects on in vitro endochondral ossification depending on the chondrocyte’s differentiation stage. J Orthop Res. 2017;35(4):847-57. doi: 10.1002/jor.23324 [DOI] [PubMed] [Google Scholar]
- 16. Caron MMJ, Emans PJ, Sanen K, Surtel DA, Cremers A, Ophelders D, et al. The role of prostaglandins and COX-enzymes in chondrogenic differentiation of ATDC5 progenitor cells. PLoS One. 2016;11(4):e0153162. doi: 10.1371/journal.pone.0153162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caron MMJ, Emans PJ, Surtel DAM, Cremers A, Voncken JW, Welting TJ, et al. Activation of NF-κB/p65 facilitates early chondrogenic differentiation during endochondral ossification. PLoS One. 2012;7(3):e33467. doi: 10.1371/journal.pone.0033467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Welting TJM, Caron MMJ, Emans PJ, Janssen MP, Sanen K, Coolsen MM, et al. Inhibition of cyclooxygenase-2 impacts chondrocyte hypertrophic differentiation during endochondral ossification. Eur Cell Mater. 2011;22:420-7. [DOI] [PubMed] [Google Scholar]
- 19. Janssen MPF, Caron MMJ, van Rietbergen B, Surtel DA, van Rhijn LW, Welting TJ, et al. Impairment of the chondrogenic phase of endochondral ossification in vivo by inhibition of cyclooxygenase-2. Eur Cell Mater. 2017;34:202-16. doi: 10.22203/eCM.v034a13 [DOI] [PubMed] [Google Scholar]
- 20. Goodman S, Ma T, Trindade M, Ikenoue T, Matsuura I, Wong N, et al. COX-2 selective NSAID decreases bone ingrowth in vivo. J Orthop Res. 2002;20(6):1164-9. doi: 10.1016/S0736-0266(02)00079-7 [DOI] [PubMed] [Google Scholar]
- 21. Wurnig C, Schwameis E, Bitzan P, Kainberger F. Six-year results of a cementless stem with prophylaxis against heterotopic bone. Clin Orthop Relat Res. 1999;(361)150-8. doi: 10.1097/00003086-199904000-00020 [DOI] [PubMed] [Google Scholar]
- 22. Cook SD, Barrack RL, Dalton JE, Thomas KA, Brown TD. Effects of indomethacin on biologic fixation of porous-coated titanium implants. J Arthroplasty. 1995;10(3):351-8. doi: 10.1016/S0883-5403(05)80184-6 [DOI] [PubMed] [Google Scholar]
- 23. Goodman SB, Ma T, Mitsunaga L, Miyanishi K, Genovese MC, Smith RL. Temporal effects of a COX-2-selective NSAID on bone ingrowth. J Biomed Mater Res A. 2005;72(3):279-87. doi: 10.1002/jbm.a.30231 [DOI] [PubMed] [Google Scholar]
- 24. Dodwell ER, Latorre JG, Parisini E, Zwettler E, Chandra D, Mulpuri K, et al. NSAID exposure and risk of nonunion: a meta-analysis of case-control and cohort studies. Calcif Tissue Int. 2010;87(3):193-202. doi: 10.1007/s00223-010-9379-7 [DOI] [PubMed] [Google Scholar]
- 25. Oladeji LO, Stannard JP, Cook CR, Kfuri M, Crist BD, Smith MJ, et al. Effects of autogenous bone marrow aspirate concentrate on radiographic integration of femoral condylar osteochondral allografts. Am J Sports Med. 2017;45(12):2797-803. doi: 10.1177/0363546517715725 [DOI] [PubMed] [Google Scholar]
- 26. Krischak GD, Augat P, Sorg T, Blakytny R, Kinzl L, Claes L, et al. Effects of diclofenac on periosteal callus maturation in osteotomy healing in an animal model. Arch Orthop Trauma Surg. 2007;127(1_suppl):3-9. doi: 10.1007/s00402-006-0202-x [DOI] [PubMed] [Google Scholar]
- 27. Park J, Gebhardt M, Golovchenko S, Perez-Branguli F, Hattori T, Hartmann C, et al. Dual pathways to endochondral osteoblasts: a novel chondrocyte-derived osteoprogenitor cell identified in hypertrophic cartilage. Biol Open. 2015;4(5):608-21. doi: 10.1242/bio.201411031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332-6. doi: 10.1038/nature01657 [DOI] [PubMed] [Google Scholar]
- 29. Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42(6):551-5. doi: 10.1016/j.injury.2011.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O’Keefe RJ, Tiyapatanaputi P, Xie C, Li TF, Clark C, Zuscik MJ, et al. COX-2 has a critical role during incorporation of structural bone allografts. Ann N Y Acad Sci. 2006;1068:532-42. doi: 10.1196/annals.1346.012 [DOI] [PubMed] [Google Scholar]
- 31. Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol. 2008;40(1_suppl):46-62. doi: 10.1016/j.biocel.2007.06.009 [DOI] [PubMed] [Google Scholar]
- 32. Frisbie DD, Oxford JT, Southwood L, Trotter GW, Rodkey WG, Steadman JR, et al. Early events in cartilage repair after subchondral bone microfracture. Clin Orthop Relat Res. 2003;(407):215-27. doi: 10.1097/00003086-200302000-00031 [DOI] [PubMed] [Google Scholar]
- 33. Demange MK, Minas T, von Keudell A, Sodha S, Bryant T, Gomoll AH. Intralesional osteophyte regrowth following autologous chondrocyte implantation after previous treatment with marrow stimulation technique. Cartilage. 2017;8(2):131-8. doi: 10.1177/1947603516653208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krill M, Early N, Everhart JS, Flanigan DC. Autologous chondrocyte implantation (ACI) for knee cartilage defects. JBJS Rev. 2018;6(2):e5. doi: 10.2106/jbjs.rvw.17.00078 [DOI] [PubMed] [Google Scholar]
- 35. Breinan HA, Minas T, Hsu HP, Nehrer S, Sledge CB, Spector M. Effect of cultured autologous chondrocytes on repair of chondral defects in a canine model. J Bone Joint Surg Am. 1997;79(10):1439-51. [DOI] [PubMed] [Google Scholar]
- 36. Henderson IJP, Tuy B, Connell D, Oakes B, Hettwer WH. Prospective clinical study of autologous chondrocyte implantation and correlation with MRI at three and 12 months. J Bone Joint Surg Br. 2003;85(7):1060-6. doi: 10.1302/0301-620X.85B7.13782 [DOI] [PubMed] [Google Scholar]
- 37. Murphy RT, Pennock AT, Bugbee WD. Osteochondral allograft transplantation of the knee in the pediatric and adolescent population. Am J Sports Med. 2014;42(3):635-40. doi: 10.1177/0363546513516747 [DOI] [PubMed] [Google Scholar]
- 38. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Animals. 2013;4(1_suppl):35-44. doi: 10.3390/ani4010035 [DOI] [PubMed] [Google Scholar]
- 40. Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abrams GD, Chang W, Dragoo JL. In vitro chondrotoxicity of nonsteroidal anti-inflammatory drugs and opioid medications. Am J Sports Med. 2017;45(14):3345-50. doi: 10.1177/0363546517724423 [DOI] [PubMed] [Google Scholar]
- 43. Beitzel K, McCarthy MB, Cote MP, Apostolakos J, Russell RP, Bradley J, et al. The effect of ketorolac tromethamine, methylprednisolone, and platelet-rich plasma on human chondrocyte and tenocyte viability. Arthroscopy. 2013;29(7):1164-74. doi: 10.1016/j.arthro.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 44. Dogan N, Erdem AF, Gundogdu C, Kursad H, Kizilkaya M. The effects of ketorolac and morphine on articular cartilage and synovium in the rabbit knee joint. Can J Physiol Pharmacol. 2004;82(7):502-5. doi: 10.1139/y04-066 [DOI] [PubMed] [Google Scholar]
- 45. Irwin MG, Cheung KMC, Nicholls JM, Thompson N. Intra-articular injection of ketorolac in the rat knee joint: Effect on articular cartilage and synovium. Br J Anaesth. 1998;80(6):837-9. doi: 10.1093/bja/80.6.837 [DOI] [PubMed] [Google Scholar]
- 46. Orak MM, Ak D, Midi A, Laçin B, Purisa S, Bulut G. Comparison of the effects of chronic intra-articular administration of tenoxicam, diclofenac, and methylprednisolone in healthy rats. Acta Orthop Traumatol Turc. 2015;49(4):438-46. [DOI] [PubMed] [Google Scholar]
- 47. Ozyuvaci H, Bilgic B, Ozyuvaci E, Altan A, Altug T, Karaca C. Intra-articular injection of tenoxicam in rats: assessment of the local effects on the articular cartilage and synovium. J Int Med Res. 2004;32(3):312-6. doi: 10.1177/147323000403200311 [DOI] [PubMed] [Google Scholar]
- 48. Ekici AG, Akyol O, Ekici M, Sitilci T, Topacoglu H, Ozyuvaci E. Intra-articular injection of dexketoprofen in rat knee joint: Histopathologic assessment of cartilage & synovium. Indian J Med Res. 2014;140(2):227-30. [PMC free article] [PubMed] [Google Scholar]
- 49. Riggin CN, Tucker JJ, Soslowsky LJ, Kuntz AF. Intra-articular tibiofemoral injection of a nonsteroidal anti-inflammatory drug has no detrimental effects on joint mechanics in a rat model. J Orthop Res. 2014;32(11):1512-9. doi: 10.1002/jor.22674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shapiro PS, Rohde RS, Froimson MI, Lash RH, Postak P, Greenwald AS. The effect of local corticosteroid or ketorolac exposure on histologic and biomechanical properties of rabbit tendon and cartilage. Hand (N Y). 2007;2(4):165-72. doi: 10.1007/s11552-007-9042-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Saricaoglu F, Dal D, Atilla P, Iskit AB, Tarhan O, Aşan E, et al. Effect of intraarticular injection of lornoxicam on the articular cartilage & synovium in rat. Indian J Med Res. 2008;127(4):362-5. [PubMed] [Google Scholar]
- 52. Schroeder S, Heuser A, Tellmann A, Goebel KJ, Woehrmann T. Local tolerance of intraarticular administration of lornoxicam into the rabbit knee joint. Rheumatol Int. 2012;32(9):2661-7. doi: 10.1007/s00296-011-2012-x [DOI] [PubMed] [Google Scholar]
- 53. Silva RA, Fagundes DJ, Silva ACA, Sisti KE, Brochado de, Carvalho TM, Silva DN. Effect of anti-inflammatory agents on the integration of autogenous bone graft and bovine bone devitalized matrix in rats. Acta Cir Bras. 2008;23(2):140-8. doi: 10.1590/S0102-86502008000200006 [DOI] [PubMed] [Google Scholar]
- 54. Soreide E, Granan LP, Hjorthaug GA, Espehaug B, Dimmen S, Nordsletten L. The effect of limited perioperative nonsteroidal anti-inflammatory drugs on patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(12):3111-8. doi: 10.1177/0363546516657539 [DOI] [PubMed] [Google Scholar]
- 55. van der Heide HJL, Hannink G, Buma P, Schreurs BW. No effect of ketoprofen and meloxicam on bone graft ingrowth: a bone chamber study in goats. Acta Orthop. 2008;79(4):548-54. doi: 10.1080/17453670710015562 [DOI] [PubMed] [Google Scholar]
- 56. Mickevicius T, Pockevicius A, Kucinskas A, Gudas R, Maciulaitis J, Noreikaite A, et al. Impact of storage conditions on electromechanical, histological and histochemical properties of osteochondral allografts. BMC Musculoskelet Disord. 2015;16(1_suppl):314. doi: 10.1186/s12891-015-0776-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pallante AL, Bae WC, Chen AC, Görtz S, Bugbee WD, Sah RL. Chondrocyte viability is higher after prolonged storage at 37°C than at 4°C for osteochondral grafts. Am J Sports Med. 2009;37(Suppl 1):24S-32S. doi: 10.1177/0363546509351496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Anderson DD, Chubinskaya S, Guilak F, Martin JA, Oegema TR, Olson SA, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res. 2011;29(6):802-9. doi: 10.1002/jor.21359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Boursinos LA, Karachalios T, Poultsides L, Malizos KN. Do steroids, conventional non-steroidal anti-inflammatory drugs and selective Cox-2 inhibitors adversely affect fracture healing? J Musculoskelet Neuronal Interact. 2009;9(1_suppl):44-52. [PubMed] [Google Scholar]
- 60. Khan SN, Cammisa FP, Jr, Sandhu HS, Diwan AD, Girardi FP, Lane JM. The biology of bone grafting. J Am Acad Orthop Surg. 2005;13(1_suppl):77-86. doi: 10.5435/00124635-200501000-00010 [DOI] [PubMed] [Google Scholar]
- 61. von Rechenberg B, Akens MK, Nadler D, Bittmann P, Zlinszky K, Kutter A, et al. Changes in subchondral bone in cartilage resurfacing—an experimental study in sheep using different types of osteochondral grafts. Osteoarthritis Cartilage. 2003;11(4):265-77. doi: 10.1016/S1063-4584(03)00006-2 [DOI] [PubMed] [Google Scholar]
- 62. Pallante-Kichura AL, Cory E, Bugbee WD, Sah RL. Bone cysts after osteochondral allograft repair of cartilage defects in goats suggest abnormal interaction between subchondral bone and overlying synovial joint tissues. Bone. 2013;57:259-68. doi: 10.1016/j.bone.2013.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]