Abstract
This is a review on talus osteochondritis dissecans and talus osteochondral lesions. A majority of the osteochondral lesions are associated with trauma while the cause of pure osteochondritis dissecans is still much discussed with a possible cause being repetitive microtraumas associated with vascular disturbances causing subchondral bone necrosis and disability. Symptomatic nondisplaced osteochondral lesions can often be treated conservatively in children and adolescents while such treatment is less successful in adults. Surgical treatment is indicated when there is an unstable cartilage fragment. There are a large number of different operative technique options with no number one technique to be recommended. Most techniques have been presented in level II to IV studies with a low number of patients with short follow ups and few randomized comparisons exist. The actual situation in treating osteochondral lesions in the ankle is presented and discussed.
Keywords: repair, talus, osteochondral lesions, osteochondritis dissecans tali, cartilage
Introduction
Osteochondral lesions of the talus (OLT) are frequent after acute ankle trauma. A subset of osteochondral lesions of the talus is osteochondritis dissecans. A general problem regarding ankle OCD is that this entity is not clearly defined. In a lot of articles, the term used for the same lesion varied from osteochondral lesions (OCL), osteochondral fractures (OCF), flake fractures, or transtalar dome fractures, and so on. Other terms are such as acute or traumatic and chronic or non-traumatic OCD. In addition, there is no clear differentiation between juvenile osteochondritis dissecans (JOCD) and adult osteochondritis dissecans (AOCD).1-13
When describing treatment of OLT, OCD is also included as the treatment choices are more or less the same for both types of lesions. The majority of articles reporting on OLT subsequently include also OCD. However, the easiest is to use the term osteochondritis dissecans (OCD) when describing the most often seen nontraumatic lesions in children or adolescents and to use the term osteochondral fractures in patients with trauma, including both the cartilage and the subchondral bone most often seen in adults. 13
Epidemiology and Etiology
Incidence
Coltart 14 in 1951 was the first to report on the incidence of osteochondral talar lesions. Overlooking 25,000 fractures he found a frequency of 0.09% of osteochondral lesions, including OCD-cases. In a recent study, Kessler et al 15 reported an overall incidence of 4.6/100,000 for OCD-cases in children and adolescents between 6 and 19 years. The age dependent incidence ranged from zero for children aged 2 to 5 years, 1.1/100,000 in patients aged between 6 and 11 years. The highest incidence with 6.8/100,000 was shown in children and adolescents aged between 12 and 19 years. Furthermore, a multivariate logistic regression analysis exhibited in male patients aged between 12 and 19 years showed a 6.9 times increased risk for the development of an OCD lesion compared with females. In children and adolescents, 22.9% of the patients needed surgery. 16
The incidence of talar OLT lesions following an ankle distortion has been reported to be up to 6.5%. 17 Almost all these lesions were preceded by a trauma (sprain or fracture).1,2,17-22
Berndt and Harty 18 demonstrated the trauma cause experimentally already in 1951. Further analyses seem to be unreliable due to the differently used terms osteochondritis, osteochondral, talar dome, and transchondral fractures where sometimes it was a pure osteochondral trauma lesion and sometimes an OCD lesion.
Location
A few studies have shown that the majority of lesions (53%-58%).23-26 are located at the medial talar rim occurring in the middle third in the anterior-posterior direction. A minor percentage are seen at the lateral talar rim (total 34%-42%),23-25 occurring mostly (25.7%) in the middle third in the anterior-posterior direction. Only a small percentage is found in the central third of the talus. 25 The rate of coexisting talar and tibial lesions is up to 35%. 27
Symptomatic lesions are most often located in the mid-third of the lateral talar dome and to a lesser extent in the mid-third of the medial talar rim. 26 Only a few are found in the center of the talus. The authors speculate that these findings support the theory of a mechanical induction of OCD lesions at the talus.
Morphology
Recent morphological analyses using data obtained from high-resolution magnetic resonance imaging (MRI) 28 revealed that 2 out of 5 morphological criteria (malleolar width and length of the trochlear arc) were significantly different in idiopathic OCD and traumatic OLT-lesions when compared with healthy controls. 28 Another morphological investigation on predisposing factors for talar osteochondral lesions (OLT) using 3-dimensional computed tomography (CT) images 29 showed that the medial malleolar area and the medial malleolar volume was significantly smaller and that the anterior opening angle of the talus was significantly larger in 19 patients with medial OLT in comparison with 19 healthy controls. 29 In addition, an MRI analysis of patients who suffered from tibial shaft fractures showed in 37% concomitant occult talar lesions up to 12 months postoperatively. 30
Biomechanics
Biomechanical studies have shown that at the ankle joint forces occurring at heel rise during stance phase were found to be 3.9 to 5.0 times body weight.31,32
The average tibiotalar contact area has been estimated to be 4.4 cm2, SD 1.21. 33 Accordingly, the pressure during the stance phase can be calculated to be 650 N/cm2. During running this load may increase multiple times. 22
Furthermore, in a cadaver study mimicking either supination or pronation ankle sprains, Bruns and Rosenbach 34 were able to demonstrate that the contact areas moved from the center of the talus toward the medial rim in supination or toward the lateral rim in pronation. But this study was a static one without considering that the real load might be distinctly higher owing to the dynamics of the trauma with impulse loading.
An analysis of biomechanical characteristics of the cartilage layer in different areas in the ankle joint 35 shows that tibial cartilage is stiffer (1.19 MPa) than talar cartilage (1.06 MPa). The softest cartilage is found in the posterolateral (0.92 MPa) and in the posteromedial talus (0.92 MPa). The anterior and posterior regions of the lateral and medial sites of the tibia are found to be 18% to 37% stiffer than the corresponding sites of the talus.
Both, Simon et al. 36 and Shepherd and Seehom 37 have found that cartilage thickness correlates negatively to the congruence of the joints: The thinner the cartilage layer, the better the congruence. Loads on the articular ankle surface, for example, the talus, as well as in other joints is transferred directly into the subchondral bone (SCB).
When mimicking osteochondral lesions in a cadaver study, it was found that the peak rim stress depends on the size of the defect. 38 As an example, an osteochondral autograft transplant (OAT) procedure could restore contact biomechanics at the talar rim. 39
Clinically, bone bruises could be seen in posttraumatic MRI at the medial talar border after anterior fibulotalar and fibulocalcanear ligament tears. Thus, these findings had been discussed as a probable precursor of a talar OLT. 40
Metabolic Influence
Bruns et al. 41 have described the correlation between OCD lesions in the knee and ankle joint and vitamin D. They found in almost all patients suffering from such lesions a vitamin D insufficiency. Maier et al. 42 demonstrated a severe and significant vitamin D3 deficiency in 97.5% of their 80 patients suffering from juvenile OCD (JOCD) when compared with patients not suffering from such lesions. Fraissler et al. 43 found similar results in talar lesions in both: in patients suffering from a traumatic OLT and in those having a nontraumatic one.
Diagnostics
As for OLC/OCD at the knee joint or elbow joint44,45 symptoms of OCL/OCD lesions are not specific and often vague. Most of the patients report on a diffuse ankle pain, probably in combination with swelling or blocking. There are no typical clinical signs in any joint. Thus, the diagnostic procedure should include a plain x-ray examination of the ankle using the anterior-posterior and lateral view and probably the mortice view (20° inner rotation of the leg using a course of beam) as a first examination. But, for an advanced diagnosis CT scans or MRI scans are in use to visualize the lesion 3-dimensionally. There are advantages and disadvantages for all diagnostic procedures such as radiation dose for CT, but a good visualization of the bony part of the lesion. In cases without any or with minor changes in the x-rays, MRI might be better to show bone marrow probably exhibiting a bone marrow edema as an initial stage of the lesion. Furthermore, with MRI the status of the cartilage can be visualized.46-49 Some authors are using the technetium-99m methylene diphosphonate single photon emission CT (SPECT).50,51 This procedure combines this sensitivity with the superior anatomical detail of CT, enabling better localization of pathological uptake and evaluation of associated structural changes.
There are some comparative studies: Mintz et al. 52 using MRI in 83% of the lesions a correct grading when compared with arthroscopy. A retrospective comparison of the diagnostic value of MR arthrographies versus CT arthrographies has been published by Schmid et al., 53 and they found that CT arthrography appears to be more reliable than MR arthrography for the detection of cartilage lesions in the ankle joint, for example, for the talus, tibia, and fibula.
Verhagen et al. 54 have performed a prospective analysis of the diagnostic value of MRI scans and helical CT scans compared with clinical symptoms, standard radiographs, and findings in arthroscopies. The analysis demonstrated no differences between MRI and CT and arthroscopies but significant differences to clinical symptoms and standard radiographs in 27 talar cartilage lesions. A similar study compared retrospectively CT arthrographies with MRIs and intraoperative findings was performed by Kirschke et al. 55 They found that arthro-CT improves the detection and visualization of cartilage defects.
Yasui et al. 56 also looked at the diagnostic value of MRI versus arthroscopy and found that with MRI, the size and diameter of cartilage lesions have been overestimated in the majority of cases.
Regarding the imaging techniques for follow-up examinations there are no clear recommendations. Imaging techniques such as radiographs, CT scans and MRI scans have been used inconsistently. Most often radiographs and CT scans have been used for follow-ups, and in a minor frequency MRIs have been used. 47
Treatment Options and Results
Scientifically there are almost no comparative prospectively randomized studies elaborating clear recommendations depending on the age of the patient and stage of the disease both regarding pure OCDs and osteochondral traumatic lesions. Most of those articles are level III or level IV studies. There are only a few level-I and level-II studies.57-62
Conservative Treatment
In initial stages with intact articular cartilage, it is thought that conservative treatment is indicated.1,3,6,7,9,10,22,63 Although there are no precise protocols for conservative strategies, it is generally accepted that such therapies should be the first choice, particularly in patients with JOCD.10,64,65
Conservative treatment includes10,64-66 the following:
Rest
Cast/brace immobilization
Restriction from weight bearing
Use of nonsteroidal anti-inflammatory drugs (NSAIDs)
Based on few published data, it would seem that conservative therapy in JOCD, adult OCD (AOCD), and OLT patients should be limited to a period of about 6 months with a tendency toward earlier surgical treatment in adult patients. In one study on JOCD, it was found that after 6 months only 16% of the patients’ lesions had completely healed. A further 6 percent, who still experienced some pain following immobilization, required surgery and 42% had to undergo surgery owing to unhealed lesions and pain. In contrast, 46% had no symptoms, but as could be seen in radiographs, had still persistent lesions. 10
Letts et al. 65 reported that in 23 of 25 children with JOCD initially all 26 lesions were treated conservatively with plaster-cast immobilization but 2 needed immediate surgery. Eleven out of the 24 conservatively treated children were prescribed restriction of activity, and 13 patients were treated with immobilization plaster-cast. In both groups (6/11 and 7/13) nearly half the patients still needed further surgical intervention. Thus, after unsuccessful conservative therapy in a total of 58% surgery was necessary.
However, from several studies it is obvious that clinical and radiographic results do not always correlate, meaning that even in symptomless patients, changes are still radiographically detectable.12,64-66
Surprisingly good results have been observed for so-called stage-V lesions. Such lesions exhibited subchondral cysts in 35 ankles of 34 patients. After an average of 38 months of conservative treatment, 9 JOCD patients exhibited a lower rate of excellent or good results (33%) compared with the adult patients (62%). 5 It was also observed that there was no correlation between size of the lesion and the clinical outcome and that lateral lesions did better than medial ones and that there was no significant development of osteoarthritis. Verhagen et al. 25 found in their systematic review an average success rate of 45% after conservative treatment.
Whether extracorporeal shockwave therapy (ESWT) can help in a conservative set-up is still under evaluation. 67 Others used intraoperative ESWT to improve healing after implantation of bone marrow aspirate concentrate (BMAC) or for postoperative pain relief after arthroscopic microfractures (MFX). 68 Regarding factors possibly predicting the result of conservative treatment in JOCD, Heyse et al. 69 found that higher age and stage III lesions are predictors of failure in conservative treatment.
From the 2017 International Consensus Meeting on Cartilage Repair of the Ankle, the consensus for an optimal protocol for conservative management of an acute nondisplaced osteochondral lesion of the ankle is
Immobilization for 4 to 6 weeks with touchdown weightbearing.
NSAIDs could be used in cases of significant pain and swelling.
Medium strong evidence for the use an injection of a biological product in the form of concentrated bone marrow aspirate or platelet-rich plasma if there is no improvement in symptoms after 4 to 6 weeks. 70
Surgical Therapy
Debridement, Bone Marrow Stimulations (BMS), and BMS-Augmented AMIC Repair
In the late 20th century, several articles have been published on arthroscopic treatment of OCD/OLT with shaving and debridement of the defects and/or drilling and MFX. The results have mostly been good to excellent.2,6-8,12,13,20,21 In 3 systematic reviews, comparing different surgical procedures, it was shown that the highest average success rate of nonreconstructive techniques was reached by excision, curettage, and drilling (85%, 9 85%, 11 and 86% 25 ; followed by excision and curettage (78%, 9 77%, 11 and 78% 25 ). The lowest success rate was reported for excision only (38%, 9 54%, 11 and 38%. 25 On the basis of these findings, it was summarized that conservative treatment or surgical excision alone is not to be recommended for talar OCL/OCD.9,11,25
Furthermore, owing to great diversity in the articles and variability in treatment results, no definitive conclusions could be drawn.
Transchondral drilling is not without complications; anterograde trans-osseous drilling can initiate iatrogenic bone cysts and produces drill holes to the cartilage. 71 In the authors’ experience, the use of a hand-driven awl used in a retrograde manner can avoid transchondral drilling, drill heat and thus does not negatively affect the osseous tissue. Similar recommendations were made in a review article analyzing 29 studies about a combined therapy including arthroscopic debridement and MFX. 72 Results from 295 patients who had undergone this treatment were monitored and, in 80%, the results were either good or excellent. However, all 29 publications are level-IV studies describing several techniques, mostly applied arthroscopically, without differentiation of OCL from talar dome fractures or OCD. Goh et al. 73 reported “favorable” results after arthroscopic chondroplasty, removal of loose bodies, and microfracture for OCD lesions at the talus after 12 months, but 41% of their patients showed poor and fair results.
The main problem with drilling or any other perforation procedures of the subchondral bone (SCB) is that synovial fluid may penetrate into the SCB resulting in destruction and/or development of subchondral bone cysts owing to its osteolytic potential.22,74 This is in accordance with findings described for the etiopathology of SCB cysts.74-77
Furthermore, degradation of the SCB following MFX has been reported. 78 With an MRI-follow-up using a new SCB health score evaluating different criteria such as the amount of edema, subchondral cyst diameter, and qualitative and thickness changes in the SCB, Shimozono et al. 77 found in 42 patients a significant decrease of the SCB healing score over time from preoperatively to the fourth MRI-follow-up 6 years postoperatively. The conclusion was that such damage to the SCB during MFX may irreversibly change the joint-loading support of the ankle with bone degradation over time. 77 Those results correlated with the clinical postoperative FAOS-scores at mid-term follow-up and are in accordance with results from an animal study reported by Chen et al. 78
Seow et al. 79 presented recently a systematic review on 520 chondral lesion reported in 17 preclinical animal studies. Assessing the SCB with micro-CT (for bone density) and histology (for microarchitecture), they found that the SCB after BMS/MFX did not recover. Recovery was inferior in the deep SCB and superior in the superficial SCB and cartilage. In addition, this study demonstrated that biological adjuvants appeared to improve the morphology of the SCB compared with BMS alone. In this regard, an experimental cadaveric study showed that smaller hole sizes (1 or 1.25 mm) reduced the damage to the SCB compared with larger hole sizes (2 mm). 80 Lambers et al. 81 reported on their arthroscopic lift, drill, fill, and fix (LDFF) technique. If a fragment is viable, the surgeons lift the fragment up, debride, drill, and refixate the fragment. The LDFF technique was used for primary fixable lesions in 25 patients. In all cases they found postoperatively a significant reduction of pain score, a significant improvement in the FAOS and quality of life (Short Form–36 [SF-36]) score after a mean follow-up of 27 months and in CT scans a fusion was seen in 92%. 81
A comparison of the arthroscopic LDFF procedure with arthroscopic BMS exhibited after one year no clinical differences. However, the authors found that the SCB plate was restored significantly superior after arthroscopic LDFF compared with arthroscopic BMS and suggested that LDFF leads to a lower rate of ankle osteoarthritis and thus a better long-term outcome. In contrast, it is well-known that BMS will results in a rate of ankle osteoarthritis in 33% to 34%. 82 However, many lesions are not able to be treated by LDFF procedures as the osteochondral fragments often are necrotic at time of surgery.
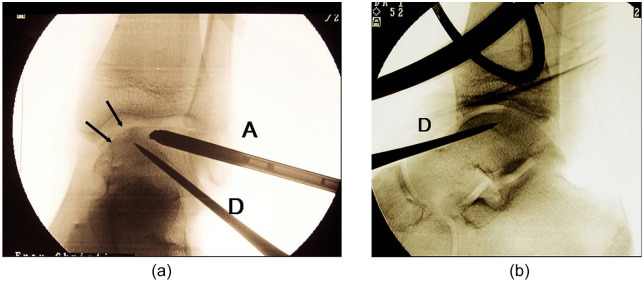
Zengerink et al. 11 summarized results of different surgical procedures and separated them into reconstructive and nonreconstructive techniques. They found that retrograde drilling (example in Fig. 1a and b ) and refixation of loose fragments had a success rate of almost 90%. 11
Figure 1.
(a) Intraoperative fluoroscopy during arthroscopy of the ankle joint and simultaneous retrograde drilling of a talar osteochondritic lesions (anteroposterior view). Arrow = talar lesion. (b) Intraoperative fluoroscopy during arthroscopy of the ankle joint and simultaneous retrograde drilling of a talar osteochondritic lesions (anteroposterior view). A = arthroscope; D = drill.
Evidence for Bone Marrow Stimulations
Zwingmann et al., 17 who looked through almost all the literature on OCD lesions at the talus excluding OLT which were treated surgically, did not find a single article fulfilling the criteria of level I to III. Reviewing 54 studies on 1,105 patients, the authors found similar results for drilling procedures in most of the cases. The reported overall success rate was 79%. Differentiation into the I to IV Berndt and Harty stages 18 revealed that the highest rate of success was found in stage I to III lesions (82%-86%), the lowest in stage IV lesions needing more complex reconstruction.
In another study, the best overall success rate, independent of the stages but in correlation to the different surgical techniques was found to be 84% for transplantation procedures, 82% for fragment refixation, 76% for BMS/MFX, and 71% for only debridement. 11
In a study including 130 patients with OLT treated with arthroscopic debridement and drilling/MFX, Cuttica et al. 83 summarized their results as predominantly good. But the follow-up period was rather short (mean follow-up 37.2 weeks). The mean lesion size was >1.5 cm2 in 20 patients, <1.5 cm2 in 110 patients, contained in 113 patients, and uncontained in 17 patients. Predictors for the clinical outcome were size of the lesion and its containment: larger lesions (>1.5 cm2) and uncontained lesions did worse. However, there is great confusion owing to the inconsistent use of definitions, surgical procedures, and grouping in JOCD or AOCD lesions. In addition, there were a lot of combined treatment procedures used such as excision and curettage, or these two combined with MFX and/or one of the different ways of drilling the bony lesion in an anterograde, retrograde, or trans-malleolar manner.
Ramponi et al. 84 recommended that based on results of their systematic results BMS may be best for OLT sizes less than 107.4 mm2 in area and/or 10.2 mm in diameter.
Another systematic review BMS/MFX demonstrated once more the rather disappointing scientific situation. Yasui et al. 85 analyzed 22 articles, but 21 of 22 had a low level of evidence of III and IV, just one was level II. In this level II study comparing chondroplasty versus MFX and versus OAT, they did not find any differences using different scores. Only 1 article had an excellent methodological quality of evidence, but 14 studies exhibited a fair quality and 7 showed a poor quality. Becher et al. 86 analyzed whether arthroscopic autologous matrix-induced chondrogenesis (AMIC) provides better results than simple MFX procedure (lesions size AMIC group: defect size 111 ± 52 mm2 [range 37-189 mm2]; MFX-group 106 ± 47 mm2 [range 52-198 mm2]) and found in a comparative study (level III) that both procedures resulted in similar good results and thus stated that “it is not worthwhile adding a collagen I/III matrix to microfractures.” Weigelt et al. 87 used a medial malleolar osteotomy to approach the lesion for treatment with AMIC in 5 patients without any other procedure and AMIC combined with bone drilling and bone grafting talus in 28 patients. Complete filling was seen in 88% of the lesions, but in 52% hypertrophy of the cartilage layer was detected and in 58 % of the patients a reoperation mostly due to symptoms from the hardware had to be performed after a mean follow-up of almost 5 years. Baums et al. 88 observed failures in up to 40% of the patients owing to an incomplete filling of the defect and development of SCB cysts and/or osteophytes. Such cystic development may explain why results may deteriorate with time.89,90
In a most recent second systematic review on BMS, Toale et al. 91 summarized results of 15 studies comprising 853 patients (858 ankles) with a mean follow-up of 71.9 months. First, they also claimed a low level of evidence (15 studies: 11 level IV, 3 levels III, 1 level II). Regarding the quality of methodology only 1 each exhibited an excellent and good quality, 9 studies had a fair quality, and 4 ha a poor quality of methodology. Good clinical outcomes after BMS were seen at midterm follow-up for primary OLTs, but radiological examinations showed damages to the surface of the repair tissue in the majority of patients. Thus, they suggested that this may be a harbinger for long-term problems, which has also been confirmed by several studies that have shown deterioration of results in the long term after BMS. In addition, Gao et al. 92 compared, in a systematic review, the clinical evidence of 28 publication on the AMIC procedure performed in the knee, hip, and ankle joint. The authors found a rather low Coleman methodology score for all 3 joints (knee, 57.8; ankle, 55.3; hip, 57.7) and no available clinical trial comparing AMIC versus MFX or ACI. 92
Based on this medium strong evidence and high international consensus, BMS can be considered for the surgical treatment of full-thickness chondral or osteochondral lesions that have failed conservative treatment in lesions <10 mm in diameter and <5 mm in depth. 93
Few reports on the time for return to sports exist but in 1 article an average return to play length of 15 ± 4 weeks in athletes treated with BMS has been reported. 94
More Complex OLT Repair Methods
Advanced cases such as stage IV or cystic lesions and those with severe damage to the cartilage are treated with more advanced surgical techniques such as osteoarticular transplants (OATS) ( Fig. 2a-d )/mosaicplasty or autologous chondrocyte implantation (ACI). In general, related to the 2017 consensus meeting, 93 defects larger than 10 mm in diameter and with a depth >5 mm are not suitable for BMS. Such lesions need a more direct filling at the time of surgery. The alternatives presented augment, in different ways, either as BMS with a porous scaffold inducing the repair with chondrogenic cells or a fill of the defect with an osteochondral full tissue. There is no evidence that there are different indications for those treatment alternatives but very large loss of bone needs osteochondral allografting.
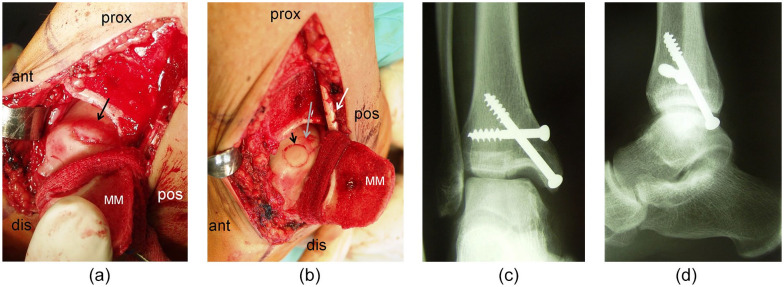
Figure 2.
(a) Intraoperative findings of a medial osteochondritis dissecans (OCD) lesion of the talus after medial malleolar osteotomy (arrow = lesion, ant = anterior, prox = proximal, pos = posterior, dis = distal, MM = medial malleolus after osteotomy). (b) Intraoperative findings of a medial OCD lesion of the talus after medial malleolar osteotomy (arrowhead = osteochondral autograft transplant in the anterior part of the lesion, gray arrow: remaining posterior part of the lesion to be transplanted; ant = anterior, prox = proximal, pos = posterior, dis = distal, MM = medial, malleolus after osteotomy, white arrow: retromalleolar tendons). (c) Postoperative x-ray (anteroposterior view) showing the ankle joint after refixation of the medial malleolus with 2 screws. (d) Postoperative x-ray (lateral view) showing the ankle joint after refixation of the medial malleolus with 2 screws.
Autologous Chondrocyte Implantation (ACI)
There are several papers reporting good and excellent results for different generations of ACI in the treatment of OCL/OCD lesion.95-102 In all these level IV studies, there was a distinct and significant postoperative improvement detectable.
Recently, Erickson et al. 103 presented a review of 19 articles including 343 patients that had been treated by ACI. Six studies used arthroscopic ACI with membrane assisted AC; MACI, 8 studied open MACI, and 5 studies presented open periosteal ACI (PACI). All studies were level IV evidence.103,104 No differences were found between of open or arthroscopic MACI and PACI in the management of lesions less than 2.5 cm2. In another study, a comparison of ACI with BMAC (both applied arthroscopically on a hyaluronic acid membrane) for treatment of talar OLT in 40 patients each (with similar characteristics regarding different criteria) revealed similar results in both groups in terms of clinical scores and MRI analysis (MOCART, Magnetic Resonance Observation of Cartilage Repair Tissue), but patients who received BMAC had a higher presence of hyaline like value, a lower evidence of fibrocartilage and slightly better return to sports activities. 105
With the third- and fourth-generation ACI, no lesion containment is needed. From the International Consensus Meeting in 2017, a high consensus and evidence was found that ACI can be considered in primary or revision procedures for large lesions greater than 1 cm2, with or without cysts, including shoulder lesions. 106 Normally, return to sports after ACI procedures in the knee is depending on type of sport and similar is for ACI for osteochondral ankle lesions with a mean return around 12 months postsurgery.
Osteo(chondral) Transplantation
Osteochondral Autograft Transplantation System (OATS) and Mosaicplasty
Several publications are reporting on OATS and/or mosaicplasty for the treatment of OLT or OCD in the talus. In some articles, both terms OATS and mosaicplasty are used synonymously. 107
However, there is an important technical difference between them: While with OATS using a technique with graft implantation in an overlapping manner filling up the defect completely instead in mosaicplasties, the osteochondral grafts are implanted side by side leaving clefts between them. These clefts allow the synovial fluid to penetrate into the SCB until fibrous tissue will have filled up the clefts with scar tissue ingrowing from the SCB. Thus, the osteolytic character of the synovial fluid may be harmful to the SCB including the transplanted grafts.22,75-77
Hangody et al. 108 treated 36 patients with medial or lateral OCD talar lesions with mosaicplasty and observed 34 excellent or good and 2 moderate results after a mean follow-up of 4.2 years. Comparison of results after mosaicplasty either as a first-line therapy or a second-line treatment revealed no differences with favorable results for both groups 3 years postoperatively. 109 Similar results were found by others.110,111 In contrast, Valderrabano et al. 112 experienced only moderate clinical results and no significant increase of sports activities in 12 out of 21 follow-up patients (57%) who had been treated with mosaicplasty for talar OLT. Postoperative MRI exhibited SCB cysts in 66% of the patients and the SCB plate was either disrupted or absent in almost all patients. In contrast, comparison of patients exhibiting subchondral talar cysts (n = 13) with those without any cysts (n = 15) revealed successful results at follow-up 2 years postoperatively.110,113 One of the first reports on a quite large group of 39 patients with talar OLT (27 OCD lesions) revealed a distinct postoperative improvement in almost all patients after OAT. 114
Baltzer and Arnold 115 achieved good and excellent results in almost all 43 patients including 22 patients with OCD lesions. Their results were depending on the size of the graft—the smaller the graft the better. Woelfle et al. 116 found that advanced age (>40 years) is associated with higher donor-site morbidity, indication for OAT in older patients should be carefully considered but did not find any correlation of the outcome in dependency to obesity, pre-existing osteoarthritis, size of the lesion, necessity of malleolar osteotomy, localization of the lesions and number of previous surgeries. 116
Two of the most important biomechanical criteria for osteochondral reconstructive procedures seems to be restoration of the contact area and pressure distribution. Studies have shown that both mosaicplasty 108 and OATS could fulfill such criteria. 39
Park et al. 117 examined whether there is a difference using the OATS as a primary or secondary repair method and could not find any significant difference after a mean follow-up time of 6 years.
A systematic review of 11 studies on 500 ankles with OCL treated with OAT 118 exhibited, at a mean 62.8 months’ follow-up, an excellent or good result in 87.4% of the patients. Thirty-one patients (6.2%) underwent reoperations and 5 ankles (1.0%) were regarded as failed. Donor site morbidity was observed in 18 patients (3.6%) at final follow-up. 118
A meta-analysis on donor site morbidity being a specific problem after OAT and mosaicplasty exhibited a donor site morbidity ranging from 6.7% to 10.8%. In larger groups (n > 30) the rate was estimated to be <5% in contrast to smaller groups (n < 30). 118 Thus, donor site morbidity still remains a problem.118,119
The use of autologous transplants taken from the injured talus itself has, to our knowledge, been first reported in 2002. 120 The authors used transplants from the talus itself, thus avoiding the more invasive technique of harvesting grafts from the intact knee joint. A significant postoperative improvement was seen after 2 years in all 12 patients. This was confirmed by Kreuz et al.121,122 using the talar facet as donor site combined with a tibial wedge-osteotomy as one of several approaches to the lesion. Four years postoperatively it was clear that best results had been achieved in patients where no osteotomy was necessary. The general problem seems to be that the talar donor site is located at the vertical talar articular surface. Most of the lesions are located just above this donor site at the edge of the medial or lateral talar rim and it might be that the transplants are compromised regarding the press-fit situation. For this reason, this technique may only be applicable for chondral and not for OCL/OCD lesions.
Regarding OAT/mosaicplasty, in rare cases where a lesion is located in the anterior third of the talus and an osteotomy is not necessary, it can be done arthroscopically. 123 However, this is technically demanding since the transplants have to be placed vertically and flush with the healthy surrounding cartilage.
The combination of autologous osteochondral transplants taken from the ipsilateral knee joint with cancellous allografts has been used in 15 patients suffering from OCL/OCD accompanied by SCB cysts >15 mm). 124 After 12 months, the VAS (visual analogue scale) score exhibited a distinct pain reduction and an increased AOFAS (American Orthopaedic Foot and Ankle Society) score. The radiolucent area of the cysts disappeared on the plain radiographs in all cases. 124
The return to sports has been found to be rather high. Nguyen et al. 125 analyzed a total of 38 athletes, including 11 professionals with rather large OCL (249 mm2). After a mean follow-up of 45 months, mean lesion size was 249 mm2 and 33 patients could return to sport at their previous level, 4 returned at a lower level compared with preinjury, and 1 did not return to sport (mean return to play, 8.2 months). 125 Similar results have been described by Fraser et al. 126 They found that 19 of 21 (90%) professional athletes were still competing or had unlimited activities. In recreational athletes 13 of 15 (87%) regained their preinjury activity levels, 2 (13%) reached only a reduced intensity or with restrictions. These rates of return to sports were similar to that found in a systematic review on 2347 cases published by Steman et al. 127 in 2019. However, the authors criticized the poor methodological quality of the articles reviewed. Return to sport rates decreased when considering patients’ return to preinjury levels versus return at any level. 127
Bone Grafts Alone Without Cartilage Layer
A transplantation procedure using only cortical-cancellous bone grafts without any cartilage layer resulted in 46% failures (6 of 13 patients). Five of the 7 remaining patients were still suffering from mild to moderate pain at a mean follow-up of 52 months. 128 Struckmann et al. 59 compared, in a preliminary prospective randomized trial, autologous vascularized bone grafts taken from the medial femoral condyles with nonvascularized cancellous bone graft for the treatment of OCL Berndt and Harty stages II and III. Vascularized grafts resulted in better clinical outcomes than nonvascularized cancellous grafts. MRI analyses exhibited 9 or 10 partial malperfusions and 1 hypervascularized cancellous graft whereas vascularized grafts resulted in 8 of 9 well-vascularized grafts with a good incorporation and in 1 case a partial malperfusion.
There exist acceptable evidence and high consensus that cancellous bone grafting is the preferred method of treatment in cases of subchondral bone marrow lesions of the ankle. There is no evidence for or against bone void substitutes. 129 Bone grafting can be utilized in lesions with large subchondral cysts, as well as in cases with large and/or deep lesions with or without subchondral cysts ( Fig. 3 ). For cystic lesions, bulk bone transplantation (e.g., osteochondral autograft/allograft) should be considered. 127 The rate of return to preinjury level of sports was 79% for patients after bone marrow stimulation, 72% for patients after autograft transplantation, and 69% for patients after autologous chondrocyte implantation.
Figure 3.
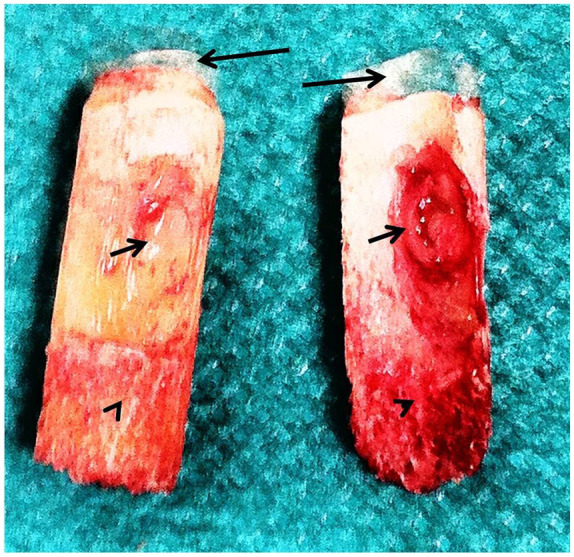
Osteochondral explants of a cystic osteochondritis dissecans (OCD) lesion of the talus (long black arrows = damaged cartilage on the top of the explants; short black arrows = the cystic subchondral lesion, arrowheads = subchondral normal bone).
Osteochondral Allografts
Osteochondral allografts for repair of OCL/OCD have been used since many years.130-132 But, as for as other repair techniques the level of evidence and the methodological quality is low.131,132 Almost all reports are level IV or even level V. In principle, the problem is immunogenicity. 132 While chondrocytes embedded in extracellular matrix were believed to be immunologically privileged the bony part is even more at risk, particularly without any immunological matching and without immunosuppression.132,133
There are different kinds of allografts, such as
bipolar allografts
bulk transplants
small osteochondral allografts.
There is no clear definition of bulk/structural transplants versus small allografts and their size, whereas bipolar allografts seem to be not appropriate for treatment of OCL/OCD-lesions. Mulholland and Gross 129 were the first to present a paper on osteochondral allografts used for talar osteochondral lesions. A longer follow-up was presented in 2001 by Gross et al. 130 on fresh osteochondral allografts for repair of talar osteochondral lesions in 9 patients with a mean graft survival of 11 years (4-19 years. 131 In 2011, Berlet et al. 133 summarized their results achieved from 12 patients with a minimum follow-up of 2 years. All 12 allografts remained intact and no revision had to be performed up to the most recent follow-up. This report included a heterogenous group of patients who received either block allografts (n = 6) or smaller allogenic osteochondral plugs (n = 6). All patients exhibited a distinct improvement in terms of AOFAS score and pain reduction but without improvement of the physical and mental health components using the SF-12. In this article, the authors summarized also data from at that time already published articles on allografts with a minimum of 1 and a maximum of 15 patients. The graft survival rate ranged between 0% (1/1) 134 to 100% (13/13), 135 (15/15), 135 between 1.6 and 12 years, postoperatively.131,136
In a retrospective study, El-Rashidy et al. 137 published their results on fresh osteochondral allografts in a rather large cohort of 42 patients with symptomatic, refractory osteochondral talar lesions. Complete postoperative follow-up after a mean of 37.7 months was achieved in 38 patients with an average age of 44.2 years. Clinical evaluation was performed with use of the AOFAS ankle-hind foot score and a visual analog pain scale. Graft failure occurred in 4 patients (10.5%). The mean VAS pain score improved from 8.2 to 3.3 points and the mean AOFAS ankle-hind foot score improved from 52 to 79 points. Patients’ satisfaction was rated as excellent, very good, or good by 28 of 38 (73%) and as fair or poor by 10 patients. Of 15 MRI scans, most analyses showed minimal graft subsidence, reasonable graft stability, and persistent articular congruence.
Adams et al. 138 reported in their prospective level IV analysis on structural allografts a success in 12 of 14 patients (86%) after a mean follow-up of more than 4 years although 5 patients needed additional surgery (36%). In talar lesions located at the medial or lateral talar shoulders are of particular interest because most of these lesions are located there. Fourteen patients with an average age of 40 years (range, 18-69 years) and a mean follow-up of 55 months (range, 24-97 months) underwent structural fresh osteochondral allograft transplantation to reconstruct the talar shoulder. For this demanding situation the authors reported a significant (P < 0.05) improvement in regard to VAS pain scale, AOFAS scale, SF-36, and Short Musculoskeletal Function Assessment scores. Five (36%) of the patients required additional surgery for pain and stiffness and two patients had cartilage delamination and were considered treatment failures. Therefore, the success rate was 86% (12 of 14). 138
In contrast to above statements, Orr et al. 139 studying 8 structural allograft transfers in a highly active group (soldiers) had already, after a mean follow-up of 28.5 months, observed rather modest results in terms of AOFOS score and pain (VAS) with 3 patients requiring ankle arthrodesis. Similar outcomes in athletes have recently been reported. 140 Excellent and good occupational results were seen in 61% but in 12 of 31 lesions (39%), the patients were unable to continue their previous active occupation. 140
Van Tienderen et al. 141 summarized the results of their systematic review “that this kind of treatment can effectively prevent or delay the eventual for ankle arthrodesis or replacement.” But the question is whether a clinical failure rate of 13%, need for reoperation in 25% and a rate of revision surgery in 8.8% is acceptable.
Comparison of allografts with OAT has been reported twice: In a prospective randomized level II study on 20 patients who had received OAT were compared with 20 patients treated with allografts. 142 At final follow-up, no patient who had received OAT developed ankle degenerative changes or knee complications while one of the allograft patients developed symptoms of asymptomatic arthritis. The mean Foot and Ankle Ability Measure score of the 16 patients (exclusion of 4 patients) who had received osteochondral allograft plugs could be followed up increased from 55.2 preoperatively to 80.7 at the time of final follow-up. The postoperative score was lower than after OAT, but statistically not significant (P = 0.25). The mean VAS pain score decreased from 7.8 of 10 preoperatively to 2.7 of 10 at final follow-up. The postoperative pain score was higher than after OAT, and also statistically not significant (P = 0.15). Three patients (18.8%) who received allografts, 2 for recurrent OLTs sized less than 1.5 cm2 and 1 for a primary OLT of 2.2 cm2 in size developed a symptomatic nonunion at the entire graft (18.8%). In 2 of these 3 patients, the allograft had to be converted to OAT-plugs harvested from the ipsilateral femoral condyle and achieved full graft incorporation. Regarding pain relieve and clinical scoring autografts resulted postoperatively in slightly better results than patients with allograft implantation. Another comparative but nonrandomized retrospective level III analysis of patients treated with autografts or allografts for OLT 143 has recently been published: 25 patients with autografts and 16 with allografts with a mean follow-up of 26 months in the autograft group and 22 months in the allograft group could be analyzed. In this small cohort study, OAT provided better clinical and MRI outcomes than allografts. The rate of chondral wear detected on MRI was higher in allografts than in autografts. In addition, allograft-treated patients had a higher rate of clinical failures. 143
Consensus and some evidence exist that bulk osteochondral allograft transplant can be considered in uncontained/shoulder lesions or lesions that cannot be addressed with an osteochondral autograft. There is strong evidence and consensus for the use of fresh, nonfrozen allografts in the ankle. No special reports on the return to sport after ankle osteochondral allografts have so far been published. 144
Extracellular Matrix Allografts and Particulated Juvenile Cartilage Allografts
Extracellular matrix cartilage allografts (ECMA) and particulate juvenile cartilage allografts (PJCA) are relatively new biologics that may improve the quality of cartilage repair. ECMA are developed from allogenic cartilage and contain extracellular matrix that is native to articular cartilage, including key components such as type II collagen, proteoglycans, and cartilaginous growth factors. 145
PJCA consist of pieces of fresh juvenile cartilage allografts containing vital cells within their native extracellular matrix. Fibrin glue is used to secure PJCA inside the lesion. Several experimental studies on PJCA have already been published but only a couple reporting on its clinical application. 146
ECMA
In contrast to PJCA, there exist several preclinical studies on ECMA147-151) but, to our knowledge, only 1 clinical report 152 exists. This prospective study evaluated the intermediate-term results of operatively treated primary OCL with a size of 1.5 cm2 or smaller with arthroscopic excision, MFX combined with ECM in a level III study. 151 After a maximal follow-up of 19 months, ECMA resulted in a high rate of improvement in ankle function and pain.
PJCA
Saltzman et al. 153 gave in 2017 a systematic review on PJA studies that at that time were published combined with their own experiences on PJCA transplants. Owing to the small number of patients and the heterogeneity of these studies the authors decided to give a predominantly qualitative, descriptive report on a total of 33 ankles (32 patients) already published in level IV and level V articles in addition with 6 own cases. Their preliminary data suggested that treatment of large, traumatic or atraumatic, symptomatic osteochondral talar defects with particulated juvenile cartilage transplantation may improve patient subjective complaints of pain and function.
Up to now there are at least 5 additional articles on this particular allogenic transplantation procedure and 1 further retrospective study.153-158
A comparative study on PJCA in combination with BMAC versus patients treated with MFX only has been performed by Karnovsky et al. 154 Both groups resulted in improved functional outcomes. The majority of patients improved, but functionally the outcome and quality of repair tissue were still not normal and appeared on MRI as fibrocartilage.
Dekker et al. 155 reported on the use of PJCA as a salvage procedure after failed previous repair techniques such as debridement or MFX. In 15 patients, they found a failure rate of 40%. after a mean follow-up of 34.6 months (minimum 15 months). Age, stage, body mass index and other factors did not influence the results but lesion size. However, a lesion larger than 125 mm2 resulted in worse outcome.
In another study, 46 patients with critical-size OCLs (≥6 mm widest diameter) were arthroscopically treated with a mixture of PJCA and BMAC. 156 Factors associated with outcomes were lesion size and hypertrophy. An increasing lesion size was associated with a decreased clinical score and hypertrophy was accompanied with a higher postoperative pain score. In twenty-two patients who received a postoperative MRI, the reparative tissue still exhibited only fibrocartilage. This is in accordance with results reported by Karnovsky et al. 154
Another analysis on PJCA has most recently been given by Heida et al. 157 They used PJCA without any adjunct in thirty-three consecutive cases and found a distinct and significant pain reduction in about half of the patients (51%) at a mean follow-up of 3.5 years and a clinical improvement in 40%. Interestingly, they stated that the presence of at least one or more behavioral health diagnoses was a risk factor for decreased pain relief, while moderate to severe preoperative pain predicted improved postoperative pain relief. Age, body mass index, tobacco use, and morphology of the lesion did not influence the outcome.
A second systematic review on PJCA has been published by Aldawsari et al. 158 The authors summarized their results on the repair of OCL with PJCA reviewing 10 studies involving 132 patients. The AOFAS and FAOS average scores increased significantly postoperatively after a mean follow-up of 25.5 months while the MOCART for MRI evaluation exhibited rather disappointing results 158 in terms of infill hypertrophy, incomplete border zone integration, deep surface disruption, structure inhomogeneity, patches of hyperintense signals, damage of subchondral lamina and SCB, adhesions, and absence of effusion. The authors concluded that the “heterogeneous picture of regenerate cartilaginous tissue and lack of repair in SCB and subchondral lamina are not in favor with claims of full restoration of lost normal hyaline articular cartilage.” Ryan et al. 146 investigated the use of DeNovo NT natural tissue graft used either open or trans-arthroscopic and found no significant differences in outcome at 2 years regardless of whether the graft was inserted with an arthroscopic or open technique. Both groups demonstrated improvement from baseline.
Biologics: Scaffolds, Platelet-Rich Plasma (PRP), and Bone Marrow Aspirate Concentrate (BMAC)
Osteochondral scaffolds
Apart from the aforementioned AMIC procedure, there are many scaffolds with different biochemical characteristics in use. In 2017, Shimozono et al. 159 gave an interesting review on already used scaffold for treatment OLT. The authors sampled 897 cases/patients reviewing data from 30 different treatment groups/articles: (13 matrix-induced autologous chondrocyte transplantation (MACI), 9 bone marrow–derived cell transplantation (BMDCT/BMAC), 4 autologous matrix-induced chondrogenesis (AMIC), and 4 studies of other techniques. Within this review, they found only 1 study of level II, 160 3 studies of level III,105,161,162 and 24 studies of level IV. In addition, the authors 159 criticized the low level of evidence and quality. Furthermore, 13 different scoring systems had been used for the clinical evaluation, mostly AOFAS and VAS. Thus, scientifically the comparability assumed to be very low. Nevertheless, the weighted mean AOFAS score at final follow-up was 86.7 in MACI, 88.2 in BMAC, and 82.3 in AMIC. 159 Thus, they stated that scaffold-based therapy for OLT “may produce favorable clinical outcomes,” but low levels of evidence, poor qualities of evidence and a great variability of the data have confounded the effectiveness of this treatment. They summarized with the statement that further well-designed studies are necessary to determine the effectiveness of scaffold-based therapy for OLT, especially when compared with the available traditional treatments.
In another systematic review on the clinical evidence of articles reporting on the AMIC procedure in different joints Gao et al. 92 found a rather poor score for talar lesions (knee, 57.8; ankle, 55.3; hip, 57.7). Furthermore, they could not find a single clinical trial comparing AMIC versus MFX or ACI in the ankle joint.
The use of biomimetic collagen scaffolds has been reported in 2 articles: Christensen et al. 163 used these scaffolds to treat lesions in the knee (n = 6) or in the talus (n = 4). Two patients had to be reoperated and were excluded. In the remaining patients, this scaffold resulted in incomplete cartilage repair and poor subchondral bone repair at 1- and 2.5-year follow-up. Albano et al. 164 had used the same scaffold and found that among 16 patients, 6 (37%) were not satisfied at the end of follow-up, 6 (37%) were moderately satisfied, and 4 (25%) were highly satisfied. The treatment was considered failed in 5 out of 16 patients (31%). Among them, 4 (25%) required reinterventions with implantation of ankle prostheses, whereas one patient was treated with a further AMIC technique combined with autologous bone graft and platelet-rich plasma.
McGoldrick et al. 165 have critically summarized the recent situation with their statement “A number of exciting new techniques have been developed which show promise. Robust randomized control trials are required to identify the optimal surgical strategy.”
The addition of a scaffold to a bone marrow stimulation (e.g., matrix-augmented bone marrow stimulation) can be considered in the following cases:
Primary and revision cases with lesions >1 cm2
Cases in which a 1-step procedure is to be used
In cases where an additional bone grafting may be needed. 106
Platelet-rich plasma
PRP is an autologous product of concentrated blood plasma that contains at least twice of the normal platelet concentration in blood and contains of several growth factors and cytokines166-169 and is produced with a bedside procedure concentrating PRP from autologous blood samples for individual use. Thus, the concentration is differing from individual to individual. PRP contains different growth-factors such as transforming growth factor-β1 (TGF-β1), fibroblast growth factor (FGF), epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF).166-170 These factors are capable for the proliferation of chondrocytes in a porcine model 171 and human PRP stimulates migration and chondrogenic proliferation of subchondral progenitor cells. 172
In addition, PRP has also been used in orthopedic surgery with the purpose to improving the healing process in different tissues such as tendons, bone, and cartilage or musculoskeletal disorders either as a single agent or as an adjunct in combination with BMAC.173-180
Regarding the role of PRP in cartilage repair of talar OCL the diversity of clinical studies is confusing and similar to other reports on talar cartilage repair: Most of these studies are level IV studies with a limited scientific value: In 2015, Guney et al., 60 in a level II study, compared arthroscopic MFX alone (n = 16) versus a combination of MFX with PRP (n = 19) for treatment of talar OCL and found significantly better clinical results with PRP as an adjunct. With another study, Guney et al. 61 compared MFX alone and MFX + PRP with mosaicplasty. Results after mosaicplasty were better with regard to pain relief than both MFX groups. 61 Görmeli et al., 57 in a level I study, compared debridement and MFX in combination with PRP versus debridement, MFX and intra-articular hyaluronic acid (HA) versus a control group without any adjunct. Using the AOFAS score and the VAS, all groups exhibited a postoperative improvement. PRP-adjunct exhibited the largest improvement which was significantly better in comparison to the remaining groups. In addition, also the HA group showed significant better result than controls.
For treatment of larger subchondral cystic OCL/OCD lesions (so-called Hepple-V-lesions), Gu et al. 181 used a combination of cancellous bone with PRP scaffolds. In their evaluation using different clinical scores as well as radiographs and MRI, they could reexamine 13 of 14 patients with a mean follow-up of 18 months: All patients demonstrated a distinct clinical improvement and MRI exhibited a complete restoration of the bone and cartilage in all.
In a recent systematic review, Yausep et al. 58 summarized the results of already published article on the treatment of OCL with PRP. Unfortunately, the results were sparse: Only 4 papers satisfied their inclusion criteria. But these 4 publications exhibited a rather high level of evidence (2 level I and 2 level II studies). These reports were accompanied with a high grade of methodological quality of evidence (1 excellent, 3 good quality). Furthermore, the authors stated that a comparison resulted in “that patients that received surgery along with PRP injections improved more than those that received PRP only.”
The International Consensus Group did not make any analysis on the use of PRP for different repairs of ankle osteochondral lesions and there is no consensus or strong evidence that an augmentation of PRP to different repair procedures will speed up return to play. 106
BMAC
Friedenstein et al. 182 in 1966 were the first to report on the capability of mesenchymal stem cells (MSCs) to promote osteogenesis. BMAC is an autologous concentrated centrifuged blood product of a bone marrow aspirate that contains several cytokines and MSCs. Meanwhile, experimental trials using different animal models and several clinical studies have corroborated this capability and have shown the potential of BMAC to repair different tissues.183-185
BMAC has been proven for cartilage repair in different combinations with scaffolds and/or PRP.
In 2009, Giannini et al. 185 reported the first time on the use of arthroscopically applied bone marrow–derived cells for the treatment of talar OCL. These cells technique have been applied in different ways: on collagen powder mixed with platelet gel, or on HA membranes also mixed with an addition of platelet gel. Forty-eight patients received these mixtures and were followed up 6, 12, 18, and 24 months postoperatively clinically and with MRI. All patients exhibited a distinct and significant postoperative improvement in either group (collagen powder and HA membrane. MRIs at 24 months exhibited newly formed tissue at the lesion site in all patients, but in 2 patients hypertrophy of the tissue was observed at 12 months.
Four-year clinical results had been reported from the same institution in 2013. 186 Forty-nine patients exhibited a significant improvement 24 months postoperatively followed by a significant decrease in the AOFAS between 24 and 48 months with a direct correlation between clinical and MRI results followed by mid-term results. 186 Buda et al. 187 corroborated these results with those obtained in 64 patients and summarized that clinical results peaked at 24 months, before declining gradually to settle at a score of around 80 at the maximum follow-up of 72 months.
A combination of OAT and BMAC has been analyzed by Kennedy and Murawski 188 in 72 patients treated. After a mean follow-up time of 28.02 months, the authors had found a significant improvement in the FAOS-score as well as in the SF-20 scores. But 3 patients exhibited a donor-site morbidity. Unfortunately, the authors did not compare these patients with those treated with OAT only to evaluate the influence of BMAC on OATS. A comparison of BMAC (40 patients) versus ACI (40 patients) 105 exhibited similar results in both groups without any statistically significant differences at 48 months. But the authors preferred bone marrow–derived cells over ACI for the single step procedure, owing the patients’ comfort and lower costs.
A case-control study reported by Hanon et al. 189 compared 22 patients treated with a combination of BMS and BMAC, who were retrospectively compared with 12 patients who received BMS without an adjunct of BMAC. After a mean follow-up 48.3 months (+BMAC) and 77.3 months (without BMAC) those patients who had received the adjunct exhibited a significantly improvement examined with the FAOS (Foot and Ankle Outcome Score) and SF-12 Physical Component Summary score. But, patients treated with the adjunct had a significant better MOCART score in their MRIs.
Most recently, Vannini et al. 190 reported on return to sports evaluated in a further level IV study in 140 patients after treatment of talar chondral or osteochondral lesions with bone marrow–derived cell transplantation. Confusing was the fact that the authors had used 3 different methods to apply BMAC (Spongostan powder in 25 patients, HA membrane [HYAFF-11] in 70 patients, collagen membrane [Biopad] in 45patients). 190
In a prospective study, Murphy et al. 191 have compared results obtained from 49 patients who received MFX with BMAC and fibrin glue as an adjunct versus 52 patients treated with MFX alone for repair of talar OCL. This level III study showed that both groups had a significant improvement in pain scores, quality of life and activities of daily living as well as in sports activities. But the MFX group with adjuncts had a significantly less revision rate (12.2%) than the MFX-group without adjunct (28.8%). Another group 192 did an interesting comparison using a method they called MAST (matrix associated stem-cell transplantation) with AMIC in combination with peripheral blood concentrate (AMIC + PBC) versus only AMIC. In a total of 120 patients suffering from 136 talar lesions located in different areas of the talus and a smaller subgroup with lesions at the distal tibia the results after a mean follow-up of 2 years both groups exhibited similar clinical outcomes and a significant pain reduction.
The use of BMAC in juvenile patients suffering from JOCD has first been studied by Pagliazzi et al. 193 In their preliminary analysis they had sampled clinical and radiological data (radiographs and MRI) from 4 JOCD patients with advanced talar lesions (6 stage II, 1 stage IV [Berndt and Harty stage]). After a mean follow-up of 4 years, patients exhibited a significant improvement in terms of AOFAS scores. A complete bony filling was seen in 3 of 7 cases in radiographs and in 4 of 7 in MRI controls. In a systematic review, Chahla et al. 194 summarized the recent literature on BMAC for treatment of taller OCL. Although there were several studies on this topic, they found only 4 studies out of 47 that could be considered for an “insightful analysis.” Three of these were retrospective level IV studies185-187 and only 1 fulfilled criteria of a prospective comparative analysis. 189
The International Consensus Group did not make any analysis on the use of BMAC for different ankle osteochondral lesions and there is not yet any consensus or strong evidence that a BMAC procedure for ankle lesions will speed up return to play.
Mini-Metallic Implants
A relatively new method is implantation of metallic substitutes to treat OCD-lesions in failed previous surgeries. Van Bergen et al. 195 could demonstrate an excellent fit of these implants in a cadaveric study.
Currently, there are at least 4 mid-term follow-up studies on implantation of hemi-caps as a salvage procedure after failed previous interventions in the ankle joint available on 10 to 38 patients.196-199 Three groups reported on a rather large number of revision surgeries with a revision rate up to 70% 196 and/or high rate of unsatisfied or only moderately satisfied patients (8 of 12, 66%). 199 Only 2 groups with rather large cohorts of 31 patients 198 and 38 patients 197 reported on good clinical results, but a large rate of additional surgeries, 42% 198 and 55.3%. 197 Nevertheless, 77% patients regained their sports activities postoperatively after 4.1 months (SD, ±3). 197
In contrast, 2 groups stated that “metal resurfacing might not be considered a definitely valid alternative for treatment of OCDs after failed previous surgery” 199 and “that implantation of the Hemi CAP® as a salvage procedure for OCDs of the talus is challenging and does not consistently lead to good clinical results.” 196
An alternative mini-metal implant might be the Episealer (Episurf AB, Stockholm, Sweden). It is a custom-made patient-specific resurfacing implant developed for local cartilage repair first for the knee in clinical use. For the ankle joint now CE-marked implants exist for the ankle but still not any clinical results. It aims to treat patients suffering from pain and reduced mobility due to focal cartilage lesions and underlying bone damage. 200
Long-Term Follow-ups
Long-term results with a minimum of 5 years follow-up up to 20 years including palliative and reconstructive procedures are numerous.20,63,89,95,96,108,112,201-209
There is only 1 article on exclusively conservative treatment in a reasonable number of 22 patients with 24 OCL out of initially 48 that have been reexamined after 14 years (range, 11-20 years). 201 Regarding pain 75% reported pain reduction and 13% each an unchanged or increased pain, 73% of the patients showed no progression of ankle osteoarthritis and 27% progression by one grade. Another article described a rate of good results (42%) after conservative treatment of the initial OCD stages and, later, surgical removal of the lesions (33%). They stated that the longer the symptoms persisted, the worse the results. 9
Comparison of long-term results after surgical therapies is difficult owing to the different used procedures and the lack of studies comparing different surgical strategies prospectively. In older publications authors report only on excision and drilling.20,63,89,204-206 After excision and drilling most of the reports with long-term results mentioned an excellent or good outcome is achieved in between 65% and 88% of the patients after removal of the lesion plus a BMS.20,63,89,204-206
In 2013 and 2016 there were 2 article207,208 reporting exclusively on arthroscopic debridement and BMS. Van Bergen et al. 203 found in their follow-up of 88% of 57 eligible patients and found excellent results in 20%, good in 58%, and fair in 22%. Similar results were seen in the Berndt and Harty outcome question. Ninety-four percent of patients had resumed work and 88% had resumed sports. Radiographs have exhibited osteoarthritis grade 0 in 33%, grade I in 63%, and grade II in 4% and in the majority (67%) radiographs showed no progression. A rather large group of patients (82 patients) has been followed up by Polat et al. 208 They found in patients with a minimum period of 5 years and a mean follow-up of 10 years rather good clinical results and only a minor rate of osteoarthritis. In another long-term analysis from the same institution with a maximum follow-up period of 22.6 years (mean, 118 months) van Eekeren et al. 207 reported on their results about return to sports after arthroscopic debridement and BMS.
They found that in 76% of 93 reexamined patients continued participating in sports at final follow-up but did not reach the level before surgery.
More recently, there are more long-term results on reconstructive procedures such as ACL/MACI95,96,202 or OATS/mosaicplasty108,112 available. Giannini et al. 96 published data of a long-term follow-up 10 years after ACL. They found in a rather small number of patients (n = 10) a significant increase of the AOFAS score and in MRI analyses a good integration of the newly developed tissue in T2-mapping and MOCART. Anders et al. 95 reported similar results on the MACI variant of ACL after a mean follow-up 5 years. The most benefit was observed within the first postoperative year. In addition, they did not find any differences between OCD lesions and traumatic ones as well as for a first-line treatment compared with a second-line use. Pagliazzi et al. 202 demonstrated in a longitudinal MRI analysis of 20 patients treated by ACI at a 7-year follow-up compared with the MRI T2-mapping at a 5-year follow-up. Patients who experienced an improvement between 5 and 7 years after surgery had a significant greater percentage of T2-map value of 35 to 45 ms (hyaline cartilage) compared with those patients who did not improve. Kwak et al. 208 had seen similar results at a mean of 5.8 years postoperatively: 9 patients were classified as excellent, 14 as good, 5 as fair, and 1 as poor using the same AOFAS.
Regarding autologous osteochondral transplantation either OATS or mosaicplasty there are three reports with rather different results. While Hangody et al. 108 with the mosaicplasty reported on good and excellent results in 94% and Imhoff et al. 209 with the OATS procedure achieved rather good results, for example, significant improvement of AOFAS score and significant postoperative pain reduction in 26 ankles of 25 patients at a mean follow-up of 7 years. Regarding postoperative MRI analyses they found that patients with a normal graft integration or a minor incongruity (81%) exhibited significantly better AOFAS scores.
In contrast, Valderrabano et al. 112 had found rather inconsistent results: While the satisfaction rate in 92% (11 of 12) after a mean of 6 years postoperatively was good or excellent, all patients demonstrated a significantly decreased postoperative sports activity score and a significantly reduced dorsiflexion. Furthermore, in 100% of the follow-up patients radiologically recurrent lesions had been detected.
Comorbidities
Little is known about comorbidities of talar OLTs. Since ankle instability with supination and/or pronation sprain is supposed to be an important etiological factor in talar OCD lesions, in principle, it should be considered to treat any ankle instability as well. Lee et al. 210 compared OLT in ankles without any chronic lateral ankle instability (CLAI) with those with CLAI and found that patients with CLAI had an increased proportion of larger lesions and additional chondral lesions at the tip of the medial malleolus and the tibia plafond than patients with stable ankle joints. In addition, CLAI patients displayed an increased clinical failure rate (AOFAS score <80) and an inferior performance in sport and recreational activities. Gregush and Ferkel 211 had looked after the influence of concomitant ankle instability treated simultaneously with the OCL and stated, “In sum, 74% of patients had a good result on the Berndt and Harty Scale, 23% had a fair result, and 3% had a poor result.” In addition, hind foot malalignment should be taken into account in therapeutical procedures.212,213
Discussion and Conclusion
Treatment of an OLT (OCD/OCL) depends mainly on the symptoms, the location of the lesion, and the imaging evaluation. Patients with medial or lateral lesions and radiographic findings that reveal no detached osteochondral fragment may be treated conservatively. Conservative treatment in these patients includes immobilization of the limb in a brace and reduced weightbearing for up to 3 months. Patients with lesions and signs of a partially or completely detached fragment even if still “in situ” most often require surgical interventions. Best results will be achieved by the use of reconstructive techniques. In a recent systematic review by Dahmen et al., 214 the authors looked at final 52 studies with 1236 primary treated osteochondral defects. None of the repair techniques used showed superiority over others but as only 2 studies were randomized comparisons, it is very difficult to tell which treatment alternative to use. 214 But one has to consider that this statement is basing on a review of different already published data. A prospective, comparative, and randomized study analyzing several kinds of surgical procedures is still missing.
However, based on the available literature, one may summarize the following treatment alternatives without any ranking:
Conservative treatment could be the choice for small, stable osteochondral lesions
Operative treatment when conservative treatment has failed
A healthy osteochondral fragment may be refixated either retro- or antegrade
A necrotic osteochondral fragment is excised and for small defects BMS may be used
For larger and deeper lesions more complex surgeries are used where the treatment choice is up to the surgeon’s preference
Medium sized and deep lesions may be treated with osteochondral autografts, BMAC, AMIC, particulated auto- or allografts, or ACI
Large sized and deep lesions with bone grafts, osteochondral allografts
Failed biological repairs could be candidates for mini-metal implants
Augmentation of graft alternatives may be done with PRP.
In summary, the scientific situation is still poor as more than 90% of the articles published on talar OCL are level III and IV studies. Thus, regarding the scientific value and consequently on the impact on the clinical application of any particular decision is arbitrary and not scientifically based. Furthermore, the evaluation of preoperative status and the postoperative results per se is under debate. Servlet et al. 215 have discussed the weaknesses with the most commonly used outcome score; the AOFAS score. Subsequently, a validated OCL score for both the OCLs and the OCDs is needed for the future. Thus, there is a tremendous need for a better scientific based on how to choose the appropriate repair technique for each single case.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Juergen Bruns  https://orcid.org/0000-0001-8802-1022
https://orcid.org/0000-0001-8802-1022
References
- 1. Flick A, Gould N. Osteochondritis dissecans of the talus (transchondral fractures of the talus): review of the literature and new surgical approach for medial dome lesions. Foot Ankle. 1985;5:165-85. [DOI] [PubMed] [Google Scholar]
- 2. Parisien J. Arthroscopic treatment of osteochondral lesions of the talus. Am J Sports Med. 1986;14:211-7. [DOI] [PubMed] [Google Scholar]
- 3. Thompson J, Loomer RL. Osteochondral lesions of the talus in a sports medicine clinic. A new radiographic technique and surgical approach. Am J Sports Med. 1984;12:460-3. [DOI] [PubMed] [Google Scholar]
- 4. Bruns J, Rosenbach B. Osteochondrosis dissecans of the talus. Arch Orthop Trauma Surg. 1992;112:23-7. [DOI] [PubMed] [Google Scholar]
- 5. Shearer C, Loomer R, Clement D. Nonoperatively managed stage 5 osteochondral talar lesions. Foot Ankle Int. 2002;23:651-4. [DOI] [PubMed] [Google Scholar]
- 6. Baker C, Andrews JR, Ryan JB. Arthroscopic treatment of transchondral talar dome fractures. Arthroscopy. 1986;2:82-7. [DOI] [PubMed] [Google Scholar]
- 7. Pritsch M, Horoshovski H, Farine I. Arthroscopic treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 1986;68:862-5. [PubMed] [Google Scholar]
- 8. Kumai T, Takakura Y, Higashiyama Y, Tamai S. Arthroscopic drilling for the treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 1999;81:1229-35. [DOI] [PubMed] [Google Scholar]
- 9. Tol JL, Struijs PAA, Bossuyt PMM, Verhagen RAW, van Dijk CN. Treatment strategies in osteochondral defects of the talar dome: a systematic review. Foot Ankle Int. 2000;21:119-26. [DOI] [PubMed] [Google Scholar]
- 10. Perumal V, Wall E, Babekir N. Juvenile osteochondritis dissecans of the talus. J Pediatr Orthop. 2007;27:821-5. [DOI] [PubMed] [Google Scholar]
- 11. Zengerink M, Struijs PAA, Tol JL, van Dijk CN. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2010;18:238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lam KY, Siow HM. Conservative treatment for juvenile osteochondritis dissecans of the talus. J Orthop Surg (Hong Kong). 2012;20:176-80. [DOI] [PubMed] [Google Scholar]
- 13. Zanon G, Di Vico G, Marullo M. Osteochondritis dissecans of the talus. Joints. 2014;2:115-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coltart WD. Sprained ankle. Brit Med J. 1951;2: 957-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kessler J, Weiss JM, Nikizad H, Gyurdzhyan S, Jacobs JC, Jr, Bebchuk JD, et al. Osteochondritis dissecans of the ankle in children and adolescents: demographics and epidemiology. Am J Sports Med 2014; 42:2165-71. [DOI] [PubMed] [Google Scholar]
- 16. Weiss JM, Nikizad H, Shea KG, Gyurdzhyan S, Jacobs JC, Cannamela PC, et al. The incidence of surgery in osteochondritis dissecans in children and adolescents. Orthop J Sports Med. 2016;4:2325967116635515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zwingmann J, Südkamp NP, Schmal H, Niemeyer P. Surgical treatment of osteochondritis dissecans of the talus: a systematic review. Arch Orthop Trauma Surg. 2012;132:1241-50. [DOI] [PubMed] [Google Scholar]
- 18. Berndt A, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1951;41:988-1020. [PubMed] [Google Scholar]
- 19. McCullough C, Venugopal V. Osteochondritis dissecans of the talus: the natural history. Clin Orthop Relat Res. 1979;144:264-8. [PubMed] [Google Scholar]
- 20. Alexander A, Lichtman DM. Surgical treatment of transchondral talar dome fractures (osteochondritis dissecans). J Bone Joint Surg Am. 1980;62:646-52. [PubMed] [Google Scholar]
- 21. Naumetz V, Schweigel JF. Osteocartilagenous lesions of the talar dome. J Trauma. 1980;20:924-7. [DOI] [PubMed] [Google Scholar]
- 22. van Dijk C, Reilingh ML, Zengerink M, van Bergen CJ. Osteochondral defects in the ankle: why painful? Knee Surg Sports Traumatol Arthrosc. 2010;18:570-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haene R, Qamirani E, Story RA, Pinsker E, Daniels TR. Intermediate outcomes of fresh talar osteo-chondral allografts for treatment of large osteochondral lesions of the talus. J Bone Joint Surg Am. 2012;94:1105-10. [DOI] [PubMed] [Google Scholar]
- 24. Elias I, Zoga AC, Morrison WB, Schweitzer ME, Besser MP, Raikin SM. Osteochondral lesions of the talus: localization and morphologic data from 424 patients using a novel anatomical grid scheme. Foot Ankle Int. 2007;28:154-61. [DOI] [PubMed] [Google Scholar]
- 25. Verhagen RA, Struijs PA, Bossuyt PM, van Dijk CN. Systematic review of treatment strategies for osteochondral defects of the talar dome. Foot Ankle Clin. 2003;8:233-42. [DOI] [PubMed] [Google Scholar]
- 26. Orr JD, Dutton JR, Fowler JT. Anatomic location and morphology of symptomatic, operatively treated osteochondral lesions of the talus. Foot Ankle Int. 2012;33:1051-57. [DOI] [PubMed] [Google Scholar]
- 27. Irwin R, Shimozono Y, Yasui Y, Megill R, Deyer TW, Kennedy JG. Incidence of coexisting talar and tibial osteochondral lesions correlates with patient age and lesion location. Orthop J Sports Med. 2018;6:2325967118790965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yurttas Y, Kilic C, Akpancar S, Celikkanat S, Hanifi Gemci M, Hamcan S, et al. The relation between ankle morphology and osteochondritis dissecans of talus. Acta Orthop Belg. 2018;84:229-34. [PubMed] [Google Scholar]
- 29. Teramoto A, Shoji H, Kura H, Sakakibara Y, Kamiya T, Watanabe K, et al. Investigation of factors related to the occurrence of osteochondral lesions of the talus by 3D bone morphology of the ankle. Bone Joint J. 2018;100-B:1487-90. [DOI] [PubMed] [Google Scholar]
- 30. Yavuz İA, Yildirim AO, Oken OF, Ceyhan E, İnci F, Yavuz OO, et al. Is it an over-looked injury? Magnetic resonance imaging examination of occult talus lesions concomitant to tibial shaft fracture. J Foot Ankle Surg. 2019;58(3):447-52. doi: 10.1053/j.jfas.2018.09.007 [DOI] [PubMed] [Google Scholar]
- 31. Procter P, Paul JP. Ankle joint biomechanics. J Biomech. 1982;15:627-34. [DOI] [PubMed] [Google Scholar]
- 32. Mow V, Flatow EL, Ateshian GA. Biomechanics. In: Buckwalter JA, Einhorn TA, Simon SR, editors. Orthopaedic basic science: biology and biomechanics of the musculoskeletal system. 2nd ed. American Academy of Orthopaedic Surgeons; 2000:133-80. [Google Scholar]
- 33. Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58:356-7. [PubMed] [Google Scholar]
- 34. Bruns J, Rosenbach B. Pressure distribution at the ankle joint. Clin Biomech (Bristol, Avon). 1990;5:153-61. [DOI] [PubMed] [Google Scholar]
- 35. Athanasiou K, Niederauer GG, Schenck RC., Jr. Biomechanical topography of human ankle cartilage. Ann Biomed Eng. 1995;23:697-704. [DOI] [PubMed] [Google Scholar]
- 36. Simon W, Friedenberg S, Richardson S. Joint congruence. A correlation of joint congruence and thickness of articular cartilage in dogs. J Bone Joint Surg Am. 1973;55:1614-20. [PubMed] [Google Scholar]
- 37. Shepherd DE, Seedhom BB. Thickness of human articular cartilage in joints of the lower limb. Ann Rheum Dis. 1999;58:27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hunt KJ, Lee AT, Lindsey DP, Slikker W, 3rd, Chou LB. Osteochondral lesions of the talus: effect of defect size and plantarflexion angle on ankle joint stresses. Am J Sports Med. 2012;40:895-901. [DOI] [PubMed] [Google Scholar]
- 39. Fansa AM, Murawski CD, Imhauser CW, Nguyen JT, Kennedy JG. Autologous osteochondral transplantation of the talus partially restores contact mechanics of the ankle joint. Am J Sports Med. 2011;39:2457-65. [DOI] [PubMed] [Google Scholar]
- 40. Nishimura G, Yamato M, Togawa M. Trabecular trauma of the talus and medial malleolus concurrent with lateral collateral ligamentous injuries of the ankle: evaluation with MR imaging. Skeletal Radiol. 1996;25:49-54. [DOI] [PubMed] [Google Scholar]
- 41. Bruns J, Werner M, Soyka M. Is vitamin D insufficiency or deficiency related to the development of osteochondritis dissecans? Knee Surg Sports Traumatol Arthrosc. 2016;24:1575-79. [DOI] [PubMed] [Google Scholar]
- 42. Maier GS, Lazovic D, Maus U, Roth KE, Horas K, Seeger JB. Vitamin D deficiency: the missing etiological factor in the development of juvenile osteochondrosis dissecans? J Pediatr Orthop. 2019;39:51-4. [DOI] [PubMed] [Google Scholar]
- 43. Fraissler L, Boelch SP, Schäfer T, Walcher M, Arnholdt J, Maier G, et al. Vitamin D deficiency in patients with idiopathic and traumatic osteochondritis dissecans of the talus. Foot Ankle Int. 2019;40:1309-18. [DOI] [PubMed] [Google Scholar]
- 44. Bruns J, Werner M, Habermann C. Osteochondritis dissecans: etiology, pathology, and imaging with a special focus on the knee joint. Cartilage. 2018;9:346-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bruns J, Werner M, Habermann CR. Osteochondritis dissecans of smaller joints: the elbow. Cartilage. Published online May 21, 2019. doi: 10.1177/1947603519847735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stroud CC, Marks RM. Imaging of osteochondral lesions of the talus. Foot Ankle Clin. 2000;5:119-33. [PubMed] [Google Scholar]
- 47. van Bergen CJA, Gerards RM, Opdam KTM, Terra MP, Kerkhoffs GMMJ. Diagnosing, planning and evaluating osteochondral ankle defects with imaging modalities. World J Orthop. 2015;6:944-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Seo SG, Kim JS, Seo DK, Kim YJ, Lee SH, Lee HS. Osteochondral lesions of the talus. Few patients require surgery. Acta Orthop. 2018;89:462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Looze CA, Capo J, Ryan MK, Begly JP, Chapman C, Swanson D, et al. Evaluation and management of osteochondral lesions of the talus. Cartilage. 2017;8:19-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hassink G, Testa EA, Leumann A, Hügle T, Rasch H, Hirschmann MT. Intra- and inter-observer reliability of a new standardized diagnostic method using SPECT/CT in patients with osteochondral lesions of the ankle joint. BMC Med Imaging. 2016;16:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Upadhyay B, Mo J, Beadsmoore C, Marshall T, Toms A, Buscombe J. Technetium-99 m methylene diphosphonate single-photon emission computed tomography/computed tomography of the foot and ankle. World J Nucl Med. 2017;16:88-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mintz DN, Tashjian GS, Connell DA, Deland JT, O’Malley M, Potter HG. Osteochondral lesions of the talus: a new magnetic resonance grading system with arthroscopic correlation. Arthroscopy. 2003;19:353-9. [DOI] [PubMed] [Google Scholar]
- 53. Schmid MR, Pfirrmann CWA, Hodler J, Vienne P, Zanetti M. Cartilage lesions in the ankle joint: comparison of MR arthrography and CT arthrography. Skeletal Radiol. 2003;32:259-65. [DOI] [PubMed] [Google Scholar]
- 54. Verhagen RAW, Maas M, Dijkgraaf MGW, Tol JL, Krips R, van Dijk CN. Prospective study on diagnostic strategies in osteochondral lesions of the talus. Is MRI superior to helical CT? J Bone Joint Surg Br. 2005;87:41-6. [PubMed] [Google Scholar]
- 55. Kirschke JS, Braun S, Baum T, Holwein C, Schaeffeler C, Imhoff AB, et al. Diagnostic value of CT arthrography for evaluation of osteochondral lesions at the ankle. Biomed Res Int. 2016;2016:3594253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yasui Y, Hannon CP, Fraser EJ, Ackermann J, Boakye L, Ross KA, et al. Lesion size measured on MRI does not accurately reflect arthroscopic measurement in talar osteochondral lesions. Orthop J Sports Med. 2019;7(2):2325967118825261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Görmeli G, Karakaplan M, Görmeli CA, Sarıkaya B, Elmalı N, Ersoy Y. Clinical effects of platelet-rich plasma and hyaluronic acid as an additional therapy for talar osteochondral lesions treated with microfracture surgery: a prospective randomized clinical trial. Foot Ankle Int. 2015;36:891-900. [DOI] [PubMed] [Google Scholar]
- 58. Yausep OE, Madhi I, Trigkilidas D. Platelet rich plasma for treatment of osteochondral lesions of the talus: a systematic review of clinical trials. J Orthop. 2020;18:218-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Struckmann VF, Harhaus L, Simon R, von Recum J, Woelfl C, Kneser U, et al. Vascularized medial femoral condyle autografts for osteochondral lesions of the talus: a preliminary prospective randomized controlled trial. J Foot Ankle Surg. 2020;59:307-13. [DOI] [PubMed] [Google Scholar]
- 60. Guney A, Akar M, Karaman I, Oner M, Guney B. Clinical outcomes of platelet rich plasma (PRP) as an adjunct to microfracture surgery in osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2015;23:2384-89. [DOI] [PubMed] [Google Scholar]
- 61. Guney A, Yurdakul E, Karaman I, Bilal O, Kafadar IH, Oner M. Medium-term outcomes of mosaicplasty versus arthroscopic microfracture with or without platelet-rich plasma in the treatment of osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2016;24:1293-8. [DOI] [PubMed] [Google Scholar]
- 62. Gobbi A, Francisco RA, Lubowitz JH, Allegra F, Canata G. Osteochondral lesions of the talus: randomized controlled trial comparing chondroplasty, microfracture, and osteochondral autograft transplantation. Arthroscopy. 2006;22:1085-92. [DOI] [PubMed] [Google Scholar]
- 63. Pettine K, Morrey BF. Osteochondral fractures of the talus. A long-term follow-up. J Bone Joint Surg Br. 1987;69:89-92. [DOI] [PubMed] [Google Scholar]
- 64. Higuera J, Laguna R, Peral M, Aranda E, Soleto J. Osteochondritis dissecans of the talus during childhood and adolescence. J Pediatr Orthop. 1998;18:328-32. [PubMed] [Google Scholar]
- 65. Letts M, Davidson D, Ahmer A. Osteochondritis dissecans of the talus in children. J Pediatr Orthop. 2003;23:617-25. [DOI] [PubMed] [Google Scholar]
- 66. Badekas T, Takvorian M, Souras N. Treatment principles for osteochondral lesions in foot and ankle. Int J Orthop. 2013;37:1697-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Thiele S, Thiele R, Gerdesmeyer L. Lateral epicondylitis: this is still a main indication for extracorporeal shockwave therapy. Int J Surg. 2015;24(Pt B):165-70. [DOI] [PubMed] [Google Scholar]
- 68. Zhang C, Huang H, Yang L, Duan X. Extracorporeal shock wave therapy for pain relief after arthroscopic treatment of osteochondral lesions of talus. J Foot Ankle Surg. 2020;59:190-4. [DOI] [PubMed] [Google Scholar]
- 69. Heyse TJ, Schüttler KF, Schweitzer A, Timmesfeld N, Efe T, Paletta JR, et al. Juvenile osteochondritis dissecans of the talus: predictors of conservative treatment failure. Arch Orthop Trauma Surg. 2015;135:1337-41. [DOI] [PubMed] [Google Scholar]
- 70. Dombrowski ME, Yasui Y, Murawski CD, Fortier LA, Giza E, Haleem AM, et al. Conservative management and biological treatment strategies: proceedings of the International Consensus Meeting on Cartilage Repair of the Ankle International Consensus Group on Cartilage Repair of the Ankle. Foot Ankle Int. 2018;39(1 suppl):9S-15S. [DOI] [PubMed] [Google Scholar]
- 71. Kim JY, Reyes FJV, Yi Y, Lee WC. Is antegrade transmalleolar drilling method for osteochondral lesion of talus necessary? Iatrogenic cystic formation at the tibia: a report of five cases. Clin Orthop Surg. 2016;8:119–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Donnenwerth MP, Roukis TS. Outcome of arthroscopic debridement and microfracture as the primary treatment for osteochondral lesions of the talar dome. Arthroscopy. 2012;28:1902-7. [DOI] [PubMed] [Google Scholar]
- 73. Goh GS, Bin Abd Razak HR, Mitra AK. Outcomes are favorable after arthroscopic treatment of osteochondritis dissecans of the talus. J Foot Ankle Surg. 2015;54:57-60. [DOI] [PubMed] [Google Scholar]
- 74. Schmalzried TP, Akizuki KH, Fedenko AN, Mirra J. The role of access of joint fluid to bone in periarticular osteolysis. A report of four cases. J Bone Joint Surg Am. 1997;79:447-52. [DOI] [PubMed] [Google Scholar]
- 75. Reilingh ML, Blankevoort L, van Eekeren IC, van Dijk CN. Morphological analysis of subchondral talar cysts on micro CT. Knee Surg Sports Traumatol Arthrosc. 2013;21:1409-17. [DOI] [PubMed] [Google Scholar]
- 76. Cox LGE, Lagemaat MW, van Donkelaar, van Rietbergen B, Reilingh ML, Blankevoort L, et al. The role of pressurized fluid in subchondral bone cyst growth. Bone. 2011;49:762-8. [DOI] [PubMed] [Google Scholar]
- 77. Shimozono Y, Coale M, Yasui Y, O’Halloran A, Deyer TW, Kennedy JG. Subchondral bone degradation after microfracture for osteochondral lesions of the talus: an MRI analysis. Am J Sports Med. 2018;46:642-8. [DOI] [PubMed] [Google Scholar]
- 78. Chen H, Chevrier A, Hoemann CD, Sun J, Ouyang W, Buschmann MD. Characterization of subchondral bone repair for marrow-stimulated chondral defects and its relationship to articular cartilage resurfacing. Am J Sports Med. 2011;39:1731-40. [DOI] [PubMed] [Google Scholar]
- 79. Seow D, Yasui Y, Hutchinson ID, Hurley ET, Shimozono Y, Kennedy JG. The subchondral bone is affected by bone marrow stimulation: a systematic review of preclinical animal studies. Cartilage. 2019;10:70-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gianakos AL, Yasui Y, Fraser EJ, Ross KA, Prado MP, Fortier LA, et al. The effect of different bone marrow stimulation techniques on human talar subchondral bone: a micro-computed tomography evaluation. Arthroscopy. 2016;32:2110-7. [DOI] [PubMed] [Google Scholar]
- 81. Lambers KTA, Dahmen J, Reilingh ML, van Bergen CJA, Stufkens SAS, Kerkhoffs GMMJ. Arthroscopic lift, drill, fill and fix (LDFF) is an effective treatment option for primary talar osteochondral defects. Knee Surg Sports Traumatol Arthrosc. 2020;28:141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Reilingh ML, Lambers KTA, Dahmen J, Opdam KTM, Kerkhoffs GMMJ. The subchondral bone healing after fixation of an osteochondral talar defect is superior in comparison with microfracture. Knee Surg Sports Traumatol Arthrosc. 2018;26:2177-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cuttica D, Smith WB, Hyer CF, Philbin TM, Berlet GC. Osteochondritis dissecans of the talus: predictors of clinical outcome. Foot Ankle Int. 2011;11:1045-51. [DOI] [PubMed] [Google Scholar]
- 84. Ramponi L, Yasui Y, Murawski CD, Ferkel RD, DiGiovanni CW, Kerkhoffs GMMJ, et al. Lesion size is a predictor of clinical outcomes after bone marrow stimulation for osteochondral lesions of the talus: a systematic review. Am J Sports Med. 2017;45:1698-705. [DOI] [PubMed] [Google Scholar]
- 85. Yasui Y, Ramponi L, Seow D, Hurley ET, Miyamoto W, Shimozono Y, et al. Systematic review of bone marrow stimulation for osteochondral lesion of talus—evaluation for level and quality of clinical studies. World J Orthop. 2017;8:956-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Becher C, Malahias MA, Ali MM, Maffulli N, Thermann H. Arthroscopic microfracture vs. arthroscopic autologous matrix-induced chondrogenesis for the treatment of articular cartilage defects of the talus. Knee Surg Sports Traumatol Arthrosc. 2019;27:2731-6. [DOI] [PubMed] [Google Scholar]
- 87. Weigelt L, Hartmann R, Pfirrmann C, Espinosa N, Wirth SH. Autologous matrix-induced chondrogenesis for osteochondral lesions of the talus: a clinical and radiological 2- to 8-year follow-up study. Am J Sports Med. 2019;47:1679-86. [DOI] [PubMed] [Google Scholar]
- 88. Baums MH, Schultz W, Kostuj T, Klinger HM. Cartilage repair techniques of the talus: an update. World J Orthop. 2014;18:171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ferkel RD, Zanotti RM, Komenda GA, Sgaglione NA, Cheng MS, Applegate GR, et al. Arthroscopic treatment of chronic osteochondral lesions of the talus: long-term results. Am J Sports Med. 2008;36:1750-62. [DOI] [PubMed] [Google Scholar]
- 90. Vannini F, Cavallo M, Baldassarri M, Castagnini F, Olivieri A, Ferranti E, et al. Treatment of juvenile osteochondritis dissecans of the talus: current concepts review. Joints. 2015;2:188-91. [PMC free article] [PubMed] [Google Scholar]
- 91. Toale J, Shimozono Y, Mulvin C, Dahmen J, Kerkhoffs GMMJ, Kennedy JG. Midterm outcomes of bone marrow stimulation for primary osteochondral lesions of the talus. A systematic review. Orthop J Sports Med. 2019;7:2325967119879127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gao L, Orth P, Cucchiarini M, Madry H. Autologous matrix-induced chondrogenesis: a systematic review of the clinical evidence. Am J Sports Med. 2019;47:222-31. [DOI] [PubMed] [Google Scholar]
- 93. Hannon CP, Bayer S, Murawski CD, Canata GL, Clanton TO, Haverkamp D, et al. Debridement, curettage, and bone marrow stimulation: Proceedings of the International Consensus Meeting on Cartilage Repair of the Ankle. Foot Ankle Int. 2018;39(1 suppl):16S-22S. [DOI] [PubMed] [Google Scholar]
- 94. Seijas R, Alvarez P, Ares O, Steinbacher G, Cuscó X, Cugat R. Osteocartilaginous lesions of the talus in soccer players. Arch Orthop Trauma Surg. 2010;130:329-33. [DOI] [PubMed] [Google Scholar]
- 95. Anders S, Goetz J, Schubert T, Grifka J, Schaumburger J. Treatment of deep articular talus lesions by matrix associated autologous chondrocyte implantation—results at five years. Int Orthop. 2012;36:2279-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Giannini S, Battaglia M, Buda R, Cavallo M, Ruffilli A, Vannini F. Surgical treatment of osteochondral lesions of the talus by open-field autologous chondrocyte implantation: a 10-year follow-up clinical and magnetic resonance imaging T2-mapping evaluation. Am J Sports Med. 2009;37(suppl 1):112S-118S. [DOI] [PubMed] [Google Scholar]
- 97. Schneider T, Karaikudi S. Matrix-induced autologous chondrocyte implantation (MACI) grafting for osteochondral lesions of the talus. Foot Ankle Int. 2009;30:810-4. [DOI] [PubMed] [Google Scholar]
- 98. Giannini S, Buda R, Grigolo B, Vannini F. Autologous chondrocyte transplantation in osteochondral lesions of the ankle joint. Foot Ankle Int. 2001;22:513-7. [DOI] [PubMed] [Google Scholar]
- 99. Giannini S, Vannini F, Buda R. Osteoarticular grafts in the treatment of OCD of the talus: mosaicplasty versus autologous chondrocyte transplantation. Foot Ankle Clin. 2002;7:621-33. [DOI] [PubMed] [Google Scholar]
- 100. Giannini S, Buda R, Faldini C, Vannini F, Bevoni R, Grandi G, et al. Surgical treatment of osteochondral lesions of the talus in young active patients. J Bone Joint Surg Am. 2005;87(Suppl 2):28-41. [DOI] [PubMed] [Google Scholar]
- 101. Giannini S, Buda R, Grigolo B, Vannini F, De Franceschi L, Facchini A. The detached osteochondral fragment as a source of cells for autologous chondrocyte implantation (ACI) in the ankle joint. Osteoarthritis Cartilage. 2008;13:601-7. [DOI] [PubMed] [Google Scholar]
- 102. Giannini S, Buda R, Vannini F, Di Caprio F, Grigolo B. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: surgical technique and results. Am J Sports Med. 2008;36:873-80. [DOI] [PubMed] [Google Scholar]
- 103. Erickson B, Fillingham Y, Hellman M, Parekh SG, Gross CE. Surgical management of large talar osteochondral defects using autologous chondrocyte implantation. Foot Ankle Surg. 2018;24:131-6. [DOI] [PubMed] [Google Scholar]
- 104. Valderrabano V, Miska M, Leumann A, Wiewiorski M. Reconstruction of osteochondral lesions of the talus with autologous spongiosa grafts and autologous matrix-induced chondrogenesis. Am J Sports Med. 2013;41:519-27. [DOI] [PubMed] [Google Scholar]
- 105. Buda R, Vannini F, Castagnini F, Cavallo M, Ruffilli A, Ramponi L, et al. Regenerative treatment in osteochondral lesions of the talus: autologous chondrocyte implantation versus one-step bone marrow derived cells transplantation. Int Orthop. 2015;39:893-900. [DOI] [PubMed] [Google Scholar]
- 106. Rothrauff BB, Murawski CD, Angthong C, Becher C, Nehrer S, Niemeyer P, et al. ; International Consensus Group on Cartilage Repair of the Ankle. Scaffold-based therapies: Proceedings of the International Consensus Meeting on Cartilage Repair of the Ankle. Foot Ankle Int. 2018;39(1 suppl):41S-47S. [DOI] [PubMed] [Google Scholar]
- 107. Sabaghzadeh A, Mirzaee F, Shahriari Rad H, Bahramian F, Alidousti A, Aslani H. Osteochondral autograft transfer (mosaicplasty) for treatment of patients with osteochondral lesions of talus. Chin J Traumatol. 2020;23:60-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hangody L, Kish G, Módis L, Szerb I, Gáspár L, Diószegi Z, et al. Mosaicplasty for the treatment of osteochondritis dissecans of the talus: two to seven year results in 36 patients. Foot Ankle Int. 2001;22:552-8. [DOI] [PubMed] [Google Scholar]
- 109. Haasper C, Zelle BA, Knobloch K, Jagodzinski M, Citak M, Lotz J, et al. No mid-term difference in mosaicplasty in previously treated versus previously untreated patients with osteochondral lesions of the talus. Arch Orthop Trauma Surg. 2008;128:499-504. [DOI] [PubMed] [Google Scholar]
- 110. Scranton PJ, Frey CC, Feder KS. Outcome of osteochondral autograft transplantation for type-V cystic osteochondral lesions of the talus. J Bone Joint Surg Br. 2006;88:614-9. [DOI] [PubMed] [Google Scholar]
- 111. Emre T, Ege T, Cift HT, Demircioglu DT, Seyhan B, Uzun M. Open mosaicplasty in osteochondral lesions of the talus: a prospective study. J Foot Ankle Surg. 2012;51:556-60. [DOI] [PubMed] [Google Scholar]
- 112. Valderrabano V, Leumann A, Rasch H, Egelhof T, Hintermann B, Pagenstert G. Knee-to-ankle mosaicplasty for the treatment of osteochondral lesions of the ankle joint. Am J Sports Med. 2009;37(suppl 1):105S-111S. [DOI] [PubMed] [Google Scholar]
- 113. Gül M, Çetinkaya E, Aykut ÜS, Özkul B, Saygılı MS, Akman YE, et al. Effect of the presence of subchondral cysts on treatment results of autologous osteochondral graft transfer in osteochondral lesions of the talus. J Foot Ankle Surg. 2016;55:1003-6. [DOI] [PubMed] [Google Scholar]
- 114. Schöttle PB, Oettl GM, Agneskirchner JD, Imhoff AB. Operative therapy of osteochondral lesions of the talus with autologous cartilage-bone transplantation [in Germany]. Orthopäde. 2001;30:53-8. [DOI] [PubMed] [Google Scholar]
- 115. Baltzer A, Arnold JP. Bone-cartilage transplantation from the ipsilateral knee for chondral lesions of the talus. Arthroscopy. 2005;21:159-66. [DOI] [PubMed] [Google Scholar]
- 116. Woelfle J, Reichel H, Nelitz M. Indications and limitations of osteochondral autologous transplantation in osteochondritis dissecans of the talus. Knee Surg Sports Traumatol Arthrosc. 2013;21:1925-30. [DOI] [PubMed] [Google Scholar]
- 117. Park KH, Hwang Y, Han SH, Park YJ, Shim DW, Choi WJ, et al. Primary versus secondary osteochondral autograft transplantation for the treatment of large osteochondral lesions of the talus. Am J Sports Med. 2018;46:1389-96. [DOI] [PubMed] [Google Scholar]
- 118. Shimozono Y, Hurley ET, Myerson CL, Kennedy JG. Good clinical and functional outcomes at mid-term following autologous osteochondral transplantation for osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2018;26:3055-62. [DOI] [PubMed] [Google Scholar]
- 119. Shimozono Y, Seow D, Yasui Y, Fields K, Kennedy JG. Knee-to-talus donor-site morbidity following autologous osteochondral transplantation: a meta-analysis with best-case and worst-case analysis. Clin Orthop Relat Res. 2019;477:1915-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sammarco GJ, Makwana NK. Treatment of talar osteochondral lesions using local osteochondral graft. Foot Ankle Int. 2002;23:693-8. [DOI] [PubMed] [Google Scholar]
- 121. Kreuz P, Steinwachs M, Erggelet C, Lahm A, Henle P, Niemeyer P. Mosaicplasty with autogenous talar autograft for osteochondral lesions of the talus after failed primary arthroscopic management: a prospective study with a 4-year follow-up. Am J Sports Med. 2006;34:55-63. [DOI] [PubMed] [Google Scholar]
- 122. Kreuz P, Lahm A, Haag M, Köstler W, Konrad G, Zwingmann J, et al. Tibial wedge osteotomy for osteochondral transplantation in talar lesions. Int J Sports Med. 2008;29:584-9. [DOI] [PubMed] [Google Scholar]
- 123. Assenmacher J, Kelikian AS, Gottlob C, Kodros S. Arthroscopically assisted autologous osteochondral transplantation for osteochondral lesions of the talar dome: an MRI and clinical follow-up study. Foot Ankle Int. 2001;22:544-51. [DOI] [PubMed] [Google Scholar]
- 124. Zhu Y, Xu X. Osteochondral autograft transfer combined with cancellous allografts for large cystic osteochondral defect of the talus. Foot Ankle Int. 2016;37:1113-8. [DOI] [PubMed] [Google Scholar]
- 125. Nguyen A, Ramasamy A, Walsh M, McMenemy L, Calder JDF. Autologous osteochondral transplantation for large osteochondral lesions of the talus is a viable option in an athletic population. Am J Sports Med. 2019;47:3429-35. [DOI] [PubMed] [Google Scholar]
- 126. Fraser EJ, Harris MC, Prado MP, Kennedy JG. Autologous osteochondral transplantation for osteochondral lesions of the talus in an athletic population. Knee Surg Sports Traumatol Arthrosc. 2016;24:1272-9. [DOI] [PubMed] [Google Scholar]
- 127. Steman JAH, Dahmen J, Lambers KTA, Kerkhoffs GMMJ. Return to sports after surgical treatment of osteochondral defects of the talus. Orthop J Sports Medicine. 2019;7:2325967119876238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kolker D, Murray M, Wilson M. Osteochondral defects of the talus treated with autologous bone grafting. J Bone Joint Surg Br. 2004;86:521-6. [PubMed] [Google Scholar]
- 129. Shimozono Y, Brown AJ, Batista JP, Murawski CD, Gomaa M, Kong SE, et al. ; International Consensus Group on Cartilage Repair of the Ankle. Subchondral pathology: Proceedings of the International Consensus Meeting on Cartilage Repair of the Ankle. Foot Ankle Int. 2018;39(1 suppl):48S-53S. [DOI] [PubMed] [Google Scholar]
- 130. Mulholland RS, Gross AE. Osteocartilagenous lesions of the talus treated with fresh osteochondral allograft transplantation. Transplantation Today. 1990;7:15-23. [Google Scholar]
- 131. Gross AE, Agnidis Z, Hutchison CR. Osteochondral defects of the talus treated with fresh osteochondral allograft transplantation. Foot Ankle Int. 2001;22:385-91. [DOI] [PubMed] [Google Scholar]
- 132. Vannini F, Buda R, Pagliazzi G, Ruffilli A, Cavallo M, Giannini S. Osteochondral allografts in the ankle joint: state of the art. Cartilage. 2013;4:204-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Berlet GC, Hyer CF, Philbin TM, Hartman JF, Wright ML. Does fresh osteochondral allograft transplantation of talar osteochondral defects improve function? Clin Orthop Relat Res. 2011;469:2356-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Görtz S, De Young AJ, Bugbee WD. Fresh osteochondral allografting for osteochondral lesions of the talus. Foot Ankle Int. 2010;31:283-90. [DOI] [PubMed] [Google Scholar]
- 135. Hahn DB, Aanstoos ME, Wilkins RM. Osteochondral lesions of the talus treated with fresh talar allografts. Foot Ankle Int. 2010;31:277-82. [DOI] [PubMed] [Google Scholar]
- 136. Janis L, Kaplansky DB, DeCarbo WT. Early clinical experience with a fresh talar transplant inlay allograft for the treatment of osteochondral lesions of the talus. J Am Podiatr Med Assoc. 2010;100:25-34. [DOI] [PubMed] [Google Scholar]
- 137. El-Rashidy H, Villacis D, Omar I, Kelikian AS. Fresh osteochondral allograft for the treatment of cartilage defects of the talus: a retrospective review. J Bone Joint Surg Am. 2011;93:1634-40. [DOI] [PubMed] [Google Scholar]
- 138. Adams SB, Dekker TJ, Schiff AP, Gross CP, Nunley JA, Easley ME. Prospective evaluation of structural allograft transplantation for osteochondral lesions of the talar shoulder. Foot Ankle Int. 2018;39:28-34. [DOI] [PubMed] [Google Scholar]
- 139. Orr JD, Dunn JC, Heida KA, Jr, Kusnezov NA, Waterman BR, Belmont PJ., Jr. Results and functional outcomes of structural fresh osteochondral allograft transfer for treatment of osteochondral lesions of the talus in a highly active population. Foot Ankle Spec. 2017;10:125-32. [DOI] [PubMed] [Google Scholar]
- 140. Jackson AT, Drayer NJ, Samona J, Dukes CA, Chen CS, Arrington EA, et al. Osteochondral allograft transplantation surgery for osteochondral lesions of the talus in athletes. J Foot Ankle Surg. 2019;58:623-7. [DOI] [PubMed] [Google Scholar]
- 141. Van Tienderen RJ, Dunn JC, Kusnezov N, Orr JD. Osteochondral allograft transfer for treatment of osteochondral lesions of the talus: a systematic review. Arthroscopy. 2017;33:217-22. [DOI] [PubMed] [Google Scholar]
- 142. Ahmad J, Jones K. Comparison of osteochondral autografts and allografts for treatment of recurrent or large talar osteochondral lesions. Foot Ankle Int. 2016;37:40-50. [DOI] [PubMed] [Google Scholar]
- 143. Shimozono Y, Hurley ET, Nguyen JT, Deyer TW, Kennedy JG. Allograft compared with autograft in osteochondral transplantation for the treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 2018;100:1838-44. [DOI] [PubMed] [Google Scholar]
- 144. Smyth NA, Murawski CD, Adams SB, Jr, Berlet GC, Buda R, Labib SA, et al. ; International Consensus Group on Cartilage Repair of the Ankle. Osteochondral allograft: Proceedings of the International Consensus Meeting on Cartilage Repair of the Ankle. Foot Ankle Int. 2018;39(1 suppl):35S-40S. [DOI] [PubMed] [Google Scholar]
- 145. Seow D, Yasui Y, Hurley ET, Ross AW, Murawski CD, Shimozono Y, et al. Extracellular matrix cartilage allograft and particulate cartilage allograft for osteochondral lesions of the knee and ankle joints: a systematic review. Am J Sports Med. 2018;46:1758-66. [DOI] [PubMed] [Google Scholar]
- 146. Ryan P, Turner RC, Anderson CD, Groth AT. Comparative outcomes for the treatment of articular cartilage lesions in the ankle with a DeNovo NT Natural Tissue Graft: open versus arthroscopic treatment. Orthop J Sports Med. 2018;6(12):2325967118812710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Benders KEM, van Weeren PR, Badylak SF, Saris DBF, Dhert WJA, Malda J. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol. 2013;31:169-76. [DOI] [PubMed] [Google Scholar]
- 148. Sutherland AJ, Beck EC, Dennis SC, Converse GL, Hopkins RA, Berkland CJ, et al. Decellularized cartilage may be a chondroinductive material for osteochondral tissue engineering. PLoS One. 2015;10:e0121966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kiyotake EA, Beck EC, Detamore MS. Cartilage extracellular matrix as a biomaterial for cartilage regeneration. Ann N Y Acad Sci. 2016;1383:139-59. [DOI] [PubMed] [Google Scholar]
- 150. Teng Y, Li X, Chen Y, Cai H, Cao W, Sun Y, et al. Extracellular matrix powder from cultured cartilage-like tissue as cell carrier for cartilage repair. J Mater Chem B. 2017;5:3283-92. [DOI] [PubMed] [Google Scholar]
- 151. Das P, Singh YP, Mandal BB, Nandi SK. Tissue-derived decellularized extracellular matrices toward cartilage repair and regeneration. Methods Cell Biol. 2020;157:185-221. [DOI] [PubMed] [Google Scholar]
- 152. Ahmad J, Maltenfort M. Arthroscopic treatment of osteochondral lesions of the talus with allograft cartilage matrix. Foot Ankle Int. 2017;38:855-62. [DOI] [PubMed] [Google Scholar]
- 153. Saltzman BM, Lin J, Lee S. Particulated juvenile articular cartilage allograft transplantation for osteochondral talar lesions. Cartilage. 2017;8:61-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Karnovsky SC, DeSandis B, Haleem AM, Sofka CM, O’Malley M, Drakos MC. Comparison of juvenile allogenous articular cartilage and bone marrow aspirate concentrate versus microfracture with and without bone marrow aspirate concentrate in arthroscopic treatment of talar osteochondral lesions. Foot Ankle Int. 2018;39:393-405. [DOI] [PubMed] [Google Scholar]
- 155. Dekker TJ, Steele JR, Federer AE, Easley ME, Hamid KS, Adams SB. Efficacy of particulated juvenile cartilage allograft transplantation for osteochondral lesions of the talus. Foot Ankle Int. 2018;39:278-83. [DOI] [PubMed] [Google Scholar]
- 156. DeSandis BA, Haleem AM, Sofka CM, O’Malley MJ, Drakos MC. Arthroscopic treatment of osteochondral lesions of the talus using juvenile articular cartilage allograft and autologous bone marrow aspirate concentration. J Foot Ankle Surg. 2018;57:273-80. [DOI] [PubMed] [Google Scholar]
- 157. Heida KA, Jr, Tihista MC, Kusnezov NA, Dunn JC, Orr JD. Outcomes and predictors of postoperative pain improvement following particulated juvenile cartilage allograft transplant for osteochondral lesions of the talus. Foot Ankle Int. 2020;41(5):572-81. [DOI] [PubMed] [Google Scholar]
- 158. Aldawsari K, Alrabai HM, Sayed A, Alrashidi Y. Role of particulated juvenile cartilage allograft transplantation in osteochondral lesions of the talus: a systematic review. Foot Ankle Surg. Published online February 26, 2020. doi: 10.1016/j.fas.2020.02.011 [DOI] [PubMed] [Google Scholar]
- 159. Shimozono Y, Yasui Y, Ross AW, Miyamoto W, Kennedy JG. Scaffolds based therapy for osteochondral lesions of the talus: a systematic review. World J Orthop. 2017;18:798-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Cadossi M, Buda RE, Ramponi L, Sambri A, Natali S, Giannini S. Bone marrow-derived cells and biophysical stimulation for talar osteochondral lesions: a randomized controlled study. Foot Ankle Int. 2014;35:981-7. [DOI] [PubMed] [Google Scholar]
- 161. Domayer SE, Apprich S, Stelzeneder D, Hirschfeld C, Sokolowski M, Kronnerwetter C, et al. Cartilage repair of the ankle: first results of T2 mapping at 7.0 T after microfracture and matrix associated autologous cartilage transplantation. Osteoarthritis Cartilage. 2012;20:829-36. [DOI] [PubMed] [Google Scholar]
- 162. Giannini S, Buda R, Cavallo M, Ruffilli A, Cenacchi A, Cavallo C, et al. Cartilage repair evolution in post-traumatic osteochondral lesions of the talus: from open field autologous chondrocyte to bone-marrow-derived cells transplantation. Injury. 2010;41:1196-203. [DOI] [PubMed] [Google Scholar]
- 163. Christensen BB, Foldager CB, Jensen J, Jensen NC, Lind M. Poor osteochondral repair by a biomimetic collagen scaffold: 1- to 3-year clinical and radiological follow-up. Knee Surg Sports Traumatol Arthrosc. 2016;24:2380-7. [DOI] [PubMed] [Google Scholar]
- 164. Albano D, Martinelli N, Bianchi A, Messina C, Malerba F, Sconfienza LM. Clinical and imaging outcome of osteochondral lesions of the talus treated using autologous matrix-induced chondrogenesis technique with a biomimetic scaffold. BMC Musculoskelet Disord. 2017;18:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. McGoldrick NP, Murphy EP, Kearns SR. Osteochondral lesions of the ankle: the current evidence supporting scaffold-based techniques and biological adjuncts. Foot Ankle Surg. 2018;24:86-91. [DOI] [PubMed] [Google Scholar]
- 166. Gianakos AL, Yasui Y, Hannon CP, Kennedy JG. Current management of talar osteochondral lesions. World J Orthop. 2017;8:12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Yamaguchi FSM, Shams S, Silva EA, Stilhano RS. PRP and BMAC for musculoskeletal conditions via biomaterial carriers. Int J Mol Sci. 2019;20:5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Yasui Y, Ross AW, Kennedy JG. Platelet-rich plasma and concentrated bone marrow aspirate in surgical treatment for osteochondral lesions of the talus. Foot Ankle Clin. 2016;21:869-84. [DOI] [PubMed] [Google Scholar]
- 169. Elghawy AA, Sesin C, Rosselli M. Osteochondral defects of the talus with a focus on platelet-rich plasma as a potential treatment option: a review. BMJ Open Sport Exerc Med. 2018;4:e000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Smyth NA, Murawski CD, Haleem AM, Hannon CP, Savage-Elliott I, Kennedy JG. Establishing proof of concept: platelet-rich plasma and bone marrow aspirate concentrate may improve cartilage repair following surgical treatment for osteochondral lesions of the talus. World J Orthop. 2012;3:101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Akeda K, An HS, Okuma M, Attawia M, Miyamoto K, Thonar EJ, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006;14:1272-80. [DOI] [PubMed] [Google Scholar]
- 172. Krüger JP, Hondke S, Endres M, Pruss A, Siclari A, Kaps C. Human platelet-rich plasma stimulates migration and chondrogenic differentiation of human subchondral progenitor cells. J Orthop Res. 2012;30:845-52. [DOI] [PubMed] [Google Scholar]
- 173. Belk JW, Kraeutler MJ, Houck DA, Goodrich JA, Dragoo JL, McCarty EC. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med. Published online April 17, 2020. doi: 10.1177/0363546520909397 [DOI] [PubMed] [Google Scholar]
- 174. Repetto I, Biti B, Cerruti P, Trentini R, Felli L. Conservative treatment of ankle osteoarthritis: can platelet-rich plasma effectively postpone surgery? J Foot Ankle Surg. 2017;56:362-5. [DOI] [PubMed] [Google Scholar]
- 175. Chen Z, Wang C, You D, Zhao S, Zhu Z, Xu M. Platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: a meta-analysis. Medicine (Baltimore). 2020;99(11):e19388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Filardo G, Di Matteo B, Kon E, Merli G, Marcacci M. Platelet-rich plasma in tendon-related disorders: results and indications. Knee Surg Sports Traumatol Arthrosc. 2018;26:1984-99. [DOI] [PubMed] [Google Scholar]
- 177. Hennig PR, Grear BJ. Platelet-rich plasma in the foot and ankle. Curr Rev Musculoskelet Med. 2018;11:616-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Moraes VY, Lenza M, Tamaoki MJ, Faloppa F, Belloti JC. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst Rev. 2014;29:CD010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Akpancar S, Gül D. Comparison of platelet rich plasma and prolotherapy in the management of osteochondral lesions of the talus: a retrospective cohort study. Med Sci Monit. 2019;25:5640-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Mei-Dan O, Carmont MR, Laver L, Mann G, Maffulli N, Nyska M. Platelet-rich plasma or hyaluronate in the management of osteochondral lesions of the talus. Am J Sports Med. 2012;40:534-41. [DOI] [PubMed] [Google Scholar]
- 181. Gu W, Li T, Shi Z, Mei G, Xue J, Zou J, et al. Management of Hepple stage V osteochondral lesion of the talus with a platelet-rich plasma Scaffold. BioMed Res Int. 2017;2017:6525373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381-90. [PubMed] [Google Scholar]
- 183. Betsch M, Schneppendahl J, Thuns S, Herten M, Sager M, Jungbluth P, et al. Bone marrow aspiration concentrate and platelet rich plasma for osteochondral repair in a porcine osteochondral defect model. PLoS One 2013;8(8):e71602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Cotter EJ, Wang KC, Yanke AB, Chubinskaya S. Bone marrow aspirate concentrate for cartilage defects of the knee: from bench to bedside evidence. Cartilage. 2018;9:161-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Giannini S, Buda R, Vannini F, Cavallo M, Grigolo B. One-step bone marrow-derived cell transplantation in talar osteochondral lesions. Clin Orthop Relat Res. 2009;467:33307-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Giannini S, Buda R, Battaglia M, Cavallo M, Ruffilli A, Ramponi L, et al. One-step repair in talar osteochondral lesions: 4-year clinical results and t2-mapping capability in outcome prediction. Am J Sports Med. 2013;41:511-8. [DOI] [PubMed] [Google Scholar]
- 187. Buda R, Vannini F, Cavallo M, Baldassarri M, Natali S, Castagnini F, et al. One-step bone marrow-derived cell transplantation in talarosteochondral lesions: mid-term results. Joints. 2014;8:102-7. [PMC free article] [PubMed] [Google Scholar]
- 188. Kennedy JG, Murawski CD. The treatment of osteochondral lesions of the talus with autologous osteochondral transplantation and bone marrow aspirate concentrate: surgical technique. Cartilage. 2011;2:327-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Hannon CP, Ross KA, Murawski CD, Deyer TW, Smyth NA, Hogan MV, et al. Arthroscopic bone marrow stimulation and concentrated bone marrow aspirate for osteochondral lesions of the talus: a case-control study of functional and magnetic resonance observation of cartilage repair tissue outcomes. Arthroscopy. 2016;32:339-47. [DOI] [PubMed] [Google Scholar]
- 190. Vannini F, Cavallo M, Ramponi L, Castagnini F, Massimi S, Giannini S, et al. Return to sports after bone marrow derived cell transplantation for osteochondral lesions of the talus. Cartilage. 2017;8:80-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Murphy EP, McGoldrick NP, Curtin M, Kearns SR. A prospective evaluation of bone marrow aspirate concentrate and microfracture in the treatment of osteochondral lesions of the talus. Foot Ankle Surg. 2019;25:441-8. [DOI] [PubMed] [Google Scholar]
- 192. Richter M, Zech S, Meissner S, Naef I. Comparison matrix-associated stem cell transplantation (MAST) with autologous matrix induced chondrogenesis plus peripheral blood concentrate (AMIC + PBC) in chondral lesions at the ankle—a clinical matched-patient analysis. Foot Ankle Surg. 2020;26:669-75. [DOI] [PubMed] [Google Scholar]
- 193. Pagliazzi G, Baldassarri M, Perazzo L, Vannini F, Castagnini F, Buda R. Tissue bioengineering in the treatment of osteochondritis dissecans of the talus in children with open physis: preliminary results. J Pediatr Orthop. 2018;38:375-81. [DOI] [PubMed] [Google Scholar]
- 194. Chahla J, Cinque ME, Shon JM, Liechti DJ, Matheny LM, LaPrade RF, et al. Bone marrow aspirate concentrate for the treatment of osteochondral lesions of the talus: a systematic review of outcomes. J Exp Orthop. 2016;3:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. van Bergen CJ, Zengerink M, Blankevoort L, van Sterkenburg MN, van Oldenrijk J, van Dijk CN. Novel metallic implantation technique for osteochondral defects of the medial talar dome. A cadaver study. Acta Orthop. 2010;81:495-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Ettinger S, Stukenborg-Colsman C, Waizy H, Becher C, Yao D, Claassen L, et al. Results of HemiCAP® implantation as a salvage procedure for osteochondral lesions of the talus. J Foot Ankle Surg. 2017;56:788-92. [DOI] [PubMed] [Google Scholar]
- 197. Vuurberg G, Reilingh ML, van Bergen CJA, van Eekeren ICM, Gerards RM, van Dijk CN. Metal resurfacing inlay implant for osteochondral talar defects after failed previous surgery: a midterm prospective follow-up study. Am J Sports Med. 2018;46:1685-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Ebskov LB, Hegnet Andersen K, Bro Rasmussen P, Johansen JK, Benyahia M. Mid-term results after treatment of complex talus osteochondral defects with HemiCAP implantation. Foot Ankle Surg. 2020;26:384-90. [DOI] [PubMed] [Google Scholar]
- 199. Maiorano E, Bianchi A, Hosseinzadeh MK, Malerba F, Martinelli N, Sansone V. HemiCAP® implantation after failed previous surgery for osteochondral lesions of the talus. Foot Ankle Surg. Published online February 21, 2020. doi: 10.1016/j.fas.2020.02.008 [DOI] [PubMed] [Google Scholar]
- 200. Stålman A, Sköldenberg O, Martinez-Carranza N, Roberts D, Högström M, Ryd L. No implant migration and good subjective outcome of a novel customized femoral resurfacing metal implant for focal chondral lesions. Knee Surg Sports Traumatol Arthrosc. 2018;26:2196-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Weigelt L, Laux CJ, Urbanschitz L, Espinosa N, Klammer G, Götschi T, et al. Long-term prognosis after successful nonoperative treatment of osteochondral lesions of the talus. An observational 14-year follow-up study. Orthop J Sports Med. 2020;8(6):2325967120924183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Pagliazzi G, Vannini F, Battaglia M, Ramponi L, Buda R. Autologous chondrocyte implantation for talar osteochondral lesions: comparison between 5-year follow-up magnetic resonance imaging findings and 7-year follow-up clinical results. J Foot Ankle Surg. 2018;57:221-5. [DOI] [PubMed] [Google Scholar]
- 203. van Bergen CJ, Kox LS, Maas M, Sierevelt IN, Kerkhoffs GMMJ, van Dijk CN. Arthroscopic treatment of osteochondral defects of the talus. Outcomes at eight to twenty years of follow-up. J Bone Joint Surg Am. 2013;95:519-25. [DOI] [PubMed] [Google Scholar]
- 204. Bauer M, Johnsson K, Linden B. Osteochondritis dissecans of the ankle. A 20-year follow-up study. J Bone Joint Surg Br. 1987;69:93-6. [DOI] [PubMed] [Google Scholar]
- 205. Angermann P, Jensen P. Osteochondritis dissecans of the talus: long-term results of surgical treatment. Foot Ankle. 1989;10:161-3. [DOI] [PubMed] [Google Scholar]
- 206. Hankemeier S, Müller EJ, Kaminski A, Muhr G. 10-year results of bone marrow stimulating therapy in the treatment of osteochondritis dissecans of the talus [in German]. Unfallchirurg. 2003;106:461-6. [DOI] [PubMed] [Google Scholar]
- 207. van Eekeren ICM, van Bergen CJA, Sierevelt IN, Reilingh ML, an Dijk CN. Return to sports after arthroscopic debridement and bone marrow stimulation of osteochondral talar defects: a 5- to 24-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2016;24:1311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Kwak SK, Kern BS, Ferkel RD, Chan KW, Kasraeian S, Applegate GR. Autologous chondrocyte implantation of the ankle: 2- to 10-year results. Am J Sports Med. 2014;42:2156-64. [DOI] [PubMed] [Google Scholar]
- 209. Imhoff AB, Paul J, Ottinger B, Wörtler K, Lämmle L, Spang J, et al. Osteochondral transplantation of the talus: long-term clinical and magnetic resonance imaging evaluation. Am J Sports Med. 2011;39:1487-93. [DOI] [PubMed] [Google Scholar]
- 210. Lee M, Kwon JW, Choi WJ, Lee JW. Comparison of outcomes for osteochondral lesions of the talus with and without chronic lateral ankle instability. Foot Ankle Int. 2015;36:1050-7. [DOI] [PubMed] [Google Scholar]
- 211. Gregush RV, Ferkel RD. Treatment of the unstable ankle with an osteochondral lesion: results and long-term follow-up. Am J Sports Med. 2010;38:782-90. [DOI] [PubMed] [Google Scholar]
- 212. Slullitel PAI, Tripodi ML, Bosio ST, Puigdevall M, Maenza R. Massive osteochondral lesion of the talus in a skeletally immature patient associated with a tarsal coalition and valgus hindfoot. J Foot Ankle Surg. 2017;56:1257-62. [DOI] [PubMed] [Google Scholar]
- 213. Hotfiel T, Engelhardt M. Osteochondritis dissecans of the talus with hindfoot malalignment—autologous matrix-induced chondrogenesis with lateral calcaneal distraction osteotomy in an internationally successful young female ski racer. Sportverletz Sportschaden. 2015;29:118-21. [DOI] [PubMed] [Google Scholar]
- 214. Dahmen J, Lambers KTA, Reiling ML, van Bergen CJA, Stufkens SAS, Kerkhoffs GMMJ. No superior treatment for primary osteochondral defects of the talus. Knee Surg Sports Traumatol Arthrosc. 2018;26:2142-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Servlet IN, van Bergen CJ, Silbernagel KN, Haverkamp D, Karlsson J. Outcomes scores. In: van Dijk CN, Kennedy JG, editors. Talar osteochondral defects: diagnosis, planning, treatment and rehabilitation. Springer; 2014:95-104. [Google Scholar]