Abstract
Objective. BioCartilage is a desiccated, particulated cartilage allograft used for repair of focal cartilage defects. It is mixed with a biologic such as bone marrow concentrate (BMC), pressed into a contained defect, and sealed with fibrin glue. The objective of this study was to assess if BioCartilage could serve as a bioactive scaffold by affecting cellular adhesion, cellular migration, or the release interleukin-1 receptor antagonist protein (IL-1RA), and to identify its full proteomic makeup. Design. Cartilage explants were used to model confined defects. BioCartilage was mixed with BMC, grafted into defects, and sealed with 1 of 5 fibrin glues. Constructs were cultured for 24 or 48 hours and then processed for live/dead microscopy. Chondrocyte and mesenchymal stem cell (MSC) adhesion on BioCartilage was assessed using scanning electron microscopy. Conditioned medium from cultures and the biologics used in the study were assayed for IL-1RA. The protein footprint of BioCartilage was determined using bottom-up proteomics. Results. BioCartilage supported chondrocyte and MSC attachment within 24 hours, and cell viability was retained in all constructs at 24 and 48 hours. Fibrin glue did not inhibit cell attachment. BMC had the highest concentration of IL-1RA. Proteomics yielded 254 proteins, including collagens, proteoglycans, and several bioactive proteins with known anabolic roles including cartilage oligomeric matrix protein. Conclusions. This study suggests that BioCartilage has the chemical composition and architecture to support cell adherence and migration and to provide bioactive proteins, which together should have biologics advantages in cartilage repair beyond its role as a scaffold.
Keywords: fibrin glue, proteomics, interleukin-1 receptor antagonist protein, scaffold, bioactive
Introduction
There are several cell- and scaffold-based methods used for the repair of isolated cartilage defects.1-3 Each of these methods has advantages and challenges, and no single method has proven superior to the others with repair tissue composition and mechanical properties remaining inferior to native articular cartilage. BioCartilage (Arthrex Inc., Naples, FL) is a particulated allograft of cartilage extracellular matrix (ECM), has demonstrated good clinical results, and offers several advantages compared with other cartilage repair techniques because it provides an off-the-shelf, micronized scaffold to support chondrogenesis in a single-stage procedure.
In surgery, BioCartilage is placed over a microfractured defect, created to theoretically access mesenchymal cells within the subchondral bone and provide a relatively simple and inexpensive option for cartilage repair. BioCartilage is typically mixed with a blood product such as platelet-rich plasma (PRP) or bone marrow concentrate (BMC), and secured in the defect site with a fibrin glue sealant. 4 Rehydration of BioCartilage with PRP or BMC serves to facilitate cartilage repair4,5 and patient return to function by providing a source of growth factors,6-9 decreasing articular pain, 10 and, in the case of BMC,11-13 by providing or recruiting mesenchymal stem cells (MSCs) to the site of repair. 14 However, it is unclear if BioCartilage also has biological affects beyond that of an inert collagen scaffold due to its hyaline cartilage composition and structure.
BioCartilage mixed with PRP or BMC and retained with a fibrin glue sealant has been used in the shoulder, 15 knee, 16 ankle,17-19 and first metatarsal phalangeal joint 20 with reported success compared with microfracture alone. When used in an equine model to treat full thickness cartilage defects in the knee, BioCartilage and PRP grafting of microfractured defects, which were sealed with commercial fibrin glue (Tisseel, Baxter Healthcare Corp., Deerfield, IL) had better repair-host integration, base-integration, and increased type II collagen compared with microfracture alone. 16
Fibrin glue sealants are used surgically to seal BioCartilage into a cartilage defect, and are formed when fibrinogen is cleaved by thrombin, resulting in assembly of fibrin monomers into fibrils forming a 3-dimensional network. 21 Typically, a dual syringe applicator system is used to combine fibrinogen with thrombin. 21 Autologous and commercial (allogeneic) fibrin sealants are both available materials to seal BioCartilage into the defect site, but it is unknown if they have differing effects on cartilage repair. Commercial fibrin sealants form dense fibrin networks, inhibiting cellular migration, proliferation, and differentiation.22-24 In contrast, autologous fibrin sealants have an order of magnitude lower fibrinogen concentration compared with commercial, allogeneic fibrin sealants,25-27 but how these 2 very different fibrin sealants comparatively affect the biology of scaffold-based cartilage repair procedures is unknown.
Although BioCartilage is primarily viewed as a cell-free scaffold, there have been no studies investigating potential biological mechanisms by which it might also enhance cartilage repair. This study sought to fill this knowledge gap by assessing adhesion and migration of chondrocytes and MSCs on BioCartilage, cell viability in a BioCartilage graft with autologous or commercial fibrin sealants, the effect of BioCartilage on release of the anti-inflammatory protein interleukin-1 receptor antagonist protein (IL-1RA) from BMC, and the presence of bioactive proteins in BioCartilage using proteomic analysis.
Methods
Study Overview
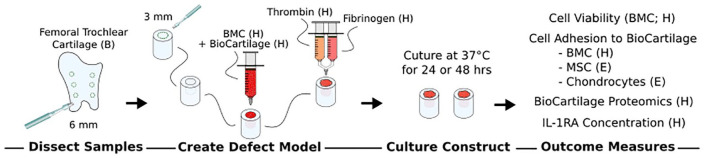
To model a confined articular cartilage defect, circular cartilage explants were isolated from bovine femoral trochlear cartilage, and a central defect was created in each explant. To simulate cartilage repair, the defect was grafted with a BioCartilage and BMC mixture and sealed with either allogeneic/commercial or all-autologous fibrin glue that was generated using blood-based biologics ( Fig. 1 ). Repair constructs were assessed for cell viability and the ability of cells to adhere to BioCartilage. Biologics were assessed for IL-1RA concentration, and bottom-up proteomics was performed to determine the full protein footprint of BioCartilage. The experiment was repeated 5 times using cartilage and biologics from separate donors. Results for cell viability, cell adhesion, and IL-1RA concentration were compared between donors and fibrin sealants.
Figure 1.
Study design. Cartilage constructs with central defects were generated through dissection of articular cartilage to model a confined cartilage defect. The central defects were grafted with a BioCartilage/BMC mixture which was sealed with 1 of 5 types of fibrin glue to model cartilage repair. Repair constructs were cultured for 24 or 48 hours then imaged using live/dead confocal microscopy and SEM. For SEM, cartilage defects were also grafted with isolated bone marrow-derived MSC or articular chondrocytes. IL-1RA was measured in medium and biologics (PRP, PPP, BMC, commercial fibrin glue). Proteomics was performed on BioCartilage. BMC, bone marrow concentrate; SEM, scanning electron microscopy; PRP, platelet-rich plasma; PPP, platelet-poor plasma; IL-1RA, interleukin-1 receptor antagonist. Letters in parentheses denote source: H, human; E, equine.
Biologics: BMC, PRP, Platelet-Poor Plasma, and Autologous Thrombin
Blood and bone marrow aspirate in 14% anticoagulant citrate dextrose solution, formula A (ACD-A; N = 5), matched from the same donor, were purchased from HemaCare Bioresearch Products, Van Nuys, CA. Samples were used within 24 hours of collection. Donor demographics are presented in Table 1 . Blood was processed to generate PRP and platelet-poor plasma (PPP); and bone marrow aspirate was processed to generate BMC using the Angel System (Arthex Inc., Naples, FL) according to manufacturer directions. PRP was generated using a 7% hematocrit setting, and BMC was generated using a 15% hematocrit setting. Complete blood counts (CBC) on blood, bone marrow, and respective concentrates were evaluated. Autologous thrombin was generated from PPP using the Thrombinator System (Arthrex Inc.) according to manufacturer directions. Briefly 10% CaCl2 and PPP were injected into the Thrombinator device, which contains glass beads to activate coagulation. When thrombin was needed, an additional aliquot of 10% CaCl2 and PPP was added. The device was incubated for an additional minute and the activated serum was extracted through an 18 µm filter.
Table 1.
Demographics of Blood and Bone Marrow Aspirate Donors.
| Donor |
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Age (years) | 45 | 49 | 18 | 59 | 35 |
| Sex | Male | Male | Male | Male | Male |
Ex Vivo Model of Cartilage Repair
Fresh neonatal bovine femoral cartilage was dissected using sterile technique. Trochlear cartilage was cut to a uniform thickness of 2 mm using a double-edged razor blade lubricated with normal equine synovial fluid to minimize cell death. From these cartilage slabs, 6-mm diameter biopsy punches were made. In the center of the cartilage punch, a 3-mm diameter punch biopsy was used to create a central cartilage defect to model a confined articular cartilage defect. Cartilage constructs were adhered to microscope slides using tissue glue. To model cartilage repair, the central defect was grafted with 0.1 mL (approximately ¾ depth of the defect) of a 1:1 mixture of BioCartilage:BMC, which was gently tapped into the defect to level the surface of the BioCartilage/BMC graft. The BioCartilage/BMC graft was sealed with 1 of 5 different types of fibrin glues formed from combinations of either allogeneic or autologous sources of fibrinogen and thrombin ( Table 2 ). Repair constructs were cultured in 1.0 mL of Dulbecco’s modified Eagle’s medium (DMEM) low glucose media with 5% antibiotic-antimycotic solution containing penicillin, streptomycin and amphotericin, in 48-well plates at 37°C for 24 or 48 hours. After culture, repair constructs were fixed or processed for live/dead confocal microscopy. Tissue culture media from 24 and 48 hours was stored at −80°C for enzyme-linked immunosorbent assay (ELISA) analysis of IL-1RA.
Table 2.
Fibrin Glue Contents. a
| Group No. | Thrombin Source | Fibrinogen Source |
|---|---|---|
| 1 | Commercial | Commercial |
| 2 | Commercial | PPP |
| 3 | Commercial | PRP |
| 4 | Autologous | PPP |
| 5 | Autologous | PRP |
| 6 | No sealant (control) | |
PRP = platelet-rich plasma; PPP = platelet-poor plasma.
Fibrin glue was used to seal the BioCartilage/bone marrow aspirate (BMC) into cartilage defect constructs. Fibrin glue was generated by a 4:1 mixture of fibrinogen and thrombin. Fibrin and thrombin were from a commercial source (Tisseel) or generated from processing of blood to generate PRP or PPP, and PPP to generate autologous thrombin.
Cell Viability Assay
Constructs were rinsed in PBS, then stained with calcein AM (1 μg/mL) and ethidium homodimer-1 (1 μg/mL; Live/Dead Viability/Cytotoxicity Kit, Thermo Fisher Scientific, Waltham, MA) to visual live and dead cells, respectively. Constructs were stained for 30 minutes, and then rinsed 3 times in PBS. Imaging was conducted at 25× on a confocal microscope (Zeiss LSM 710 Confocal; Oberkochen, Germany) using a 3-channel sequential scan. Excitation/emission spectra settings for each of the fluorescent dyes were as follows: calcein AM 488/499-550 nm, ethidium homodimer-1 561/590-696 nm. Reflectance was used to visualize collagen, in order to assess BioCartilage architecture. Digital images were captured, and assessed qualitatively for cell viability.
Ex Vivo Model of Cartilage Repair: Construct Preparation to Assess Cell Adhesion
To test if MSCs or articular chondrocytes would adhere and/or migrate on BioCartilage, cartilage defects were filled with BioCartilage and passage 2 bone marrow–derived MSCs or chondrocytes mixed with PPP. PPP was utilized here because the abundance of red and white blood cells in BMC makes identification of MSCs or chondrocytes difficult. Equine MSCs (0.5 × 106) or chondrocytes (3.36 × 106) from a 2-year-old (young adult) horse were mixed with BioCartilage to achieve an approximate 30% confluence based on surface area estimations. To ensure that native cells in the bovine cartilage could not contribute to cells identified on the BioCartilage, the cartilage was frozen at −80°C for 48 hours to lyse native chondrocytes. Repair constructs were imaged using scanning electron microscopy (SEM). Constructs were cultured for 48 hours as described above.
Scanning Electron Microscopy
SEM was performed to assess MSC and chondrocyte adherence to and migration on BioCartilage. Repair constructs used for SEM imaging were fixed in 2% glutaraldehyde for 2 hours and then rinsed thrice 5 minutes with 0.05 M cocadylate buffer. Osmium tetroxide (OsO4) heavy metal stain was added for 1 hour and samples were again rinsed thrice 5 minutes in cocadylate buffer. Constructs were dehydrated in routine serial ethanol concentrations, critical point dried (BAL-TEC CPD 030; Balzers, Liechtenstein), mounted for imaging using colloidal silver, and were sputter coated with gold. SEM imaging was conducted (LEO 1550 FESEM Keck SEM; Oberkochen, Germany) at an accelerating voltage of 5 keV and an aperture size of 30 μm.
IL-1RA Quantification
IL-1RA was quantified in biologics (bone marrow aspirate, BMC, blood, PRP, PPP, autologous thrombin, and allogeneic thrombin) and in conditioned media from repair constructs cultured for 24 or 48 hours. Samples were thawed, centrifuged at 330 × g for 2 minutes, and 100 µL of supernatant was used for ELISA according to manufacturer’s instructions (Human IL-1RA/IL-1F3 Quantikine ELISA Kit, R&D Systems, Minneapolis, MN). Samples were read on a spectrophotometric microplate reader (Tecan Safire; Männedorf, Switzerland).
BioCartilage Proteomics
BioCartilage samples were pulverized in liquid nitrogen using a mortar and pestle to obtain approximately 250 mg of homogenous powder. The sample was weighed and extracted using 15 volumes (v/w) of chaotropic extraction buffer (4 M GdnHCl, 50 mM NaAc, 100 mM 6-aminocaproic acid, 5 mM benzamidine, 5 mM N-ethylmaleimide, pH 5.8) for 24 hours on an orbital shaker at 4°C. Samples were centrifuged at 13,200 × g for 30 minutes, the supernatant was aspirated, the extraction pellet was resuspended in the same 15 volume (v/w) of chaotropic extraction buffer, and allowed to extract overnight. Samples were washed in chaotropic extraction buffer for 2 hours, then 1 hour, with centrifugation between washes.
Samples were submitted to the Proteomics Core of Cornell University where they were trypsin-digested and the resulting peptide fragments were analyzed using an Orbitrap Velos on-line LC/MS/MS (liquid chromatography–tandem mass spectrometry) system (Applied Biosystems/MDS Sciex). The MS/MS raw data files were submitted to Mascot 2.2 (Matrix Science) for NCBI database searching, and the results were further analyzed using MaxQuant label free quantification and PANTHER software.
Statistical Analysis
IL-1RA concentration in biologics (blood, PRP, PPP, autologous thrombin, bone marrow aspirate, BMC, and commercial thrombin) were compared using a Kruskal-Wallis 1-way analysis of variance with a P value <0.05 considered as significant.
Results
Generation of Biologics
Centrifugation of blood and BMC successfully resulted in generation of PRP, PPP, and BMC in all samples. There was an average 7.6-fold (±5.4) increase in platelet concentration and an average 3.6-fold (±1.4) increase in nucleated cell count in PRP compared with starting blood samples. For bone marrow aspirate, processing resulted in a 5.8-fold (±0.7) increase in platelets and an average 3.8-fold (±0.9) increase in total nucleated cell count in BMC.
Cell Viability
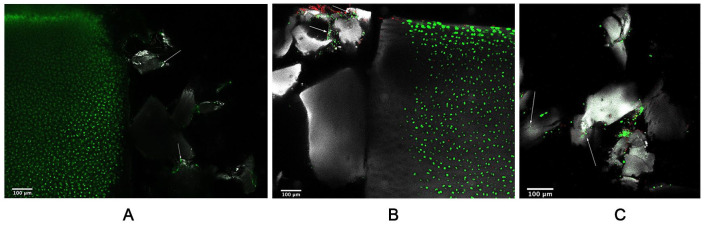
Live/dead confocal microscopy was conducted to assess cell viability in repair constructs with BioCartilage/BMC grafts after 24 or 48 hours of culture. Cell viability was retained with no difference samples cultured for 24 hours ( Fig. 2A ) or 48 hours ( Fig. 2B ). At both time points, native cartilage contained primarily live cells, indicating that BioCartilage/BMC grafts support cell viability in the surrounding cartilage. At 48 hours, few dead cells were noted along the articular surface of the cartilage, which can likely be attributed to cartilage damage from dissection. In all samples, live cells were present within BioCartilage lacunae indicating that cells from the BMC adhered to BioCartilage ( Fig. 2C ).
Figure 2.
Cell viability is retained in BioCartilage/bone marrow concentrate (BMC)/fibrin glue grafts. Repair constructs cultured for (A) 24 hours, or (B) 48 hours, and (C) live cells within BioCartilage lacunae at 48 hours. Native cartilage contains primarily live cells; few dead cells can be seen. Arrows indicate scattered live cells within the BioCartilage graft region. Calcein AM/ethidium-1 homodimer (live/dead; green/red) staining of the interface between native cartilage (left) and BioCartilage (right), with articular cartilage oriented upward. Gray represents reflectance of collagen in native cartilage and that of BioCartilage. When samples were bifurcated the BioCartilage/BMC graft did not result in an even plane, resulting in dark/empty regions. Imaging was conducted at 25×.
Cell Morphology
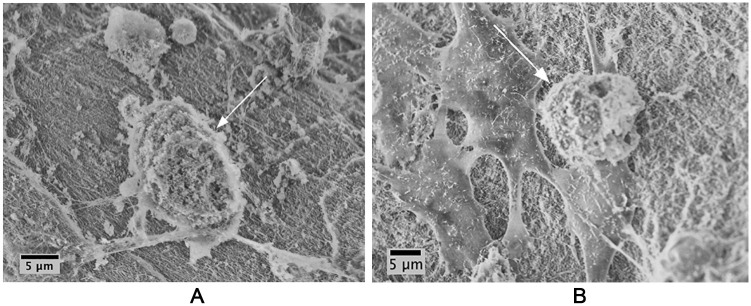
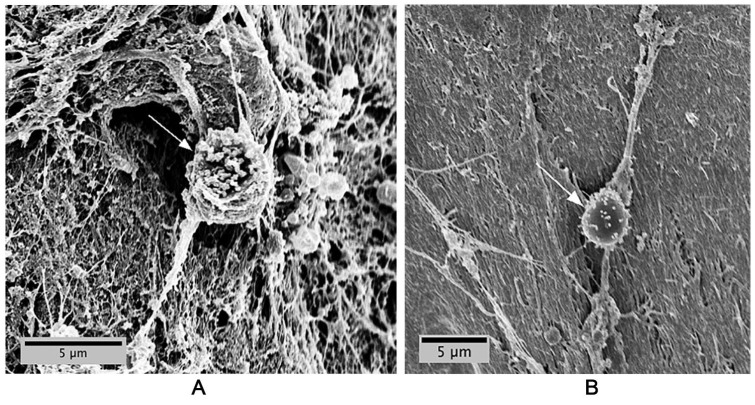
SEM enabled 3-dimensional visualization of MSC and chondrocyte morphology when grafted with BioCartilage in PPP. Both MSCs ( Fig. 3A ) and chondrocytes ( Fig. 3B ) adhered to and migrated on BioCartilage, as indicated by cell spreading on the scaffold. Samples containing BioCartilage:BMC grafts and sealed with fibrin glue exhibited cell spreading indicative of cellular attachment and migration ( Fig. 4A and B ) similar to isolated MSC and chondrocytes.
Figure 3.
Mesenchymal stem cells (MSCs) and chondrocytes adhere and migrate on BioCartilage. (A) MSCs adhere to and spread (arrow) on BioCartilage; (B) chondrocytes adhere to and migrate (arrow) on BioCartilage. BioCartilage was mixed with MSCs or chondrocytes in platelet-poor plasma (PPP) and grafted into cartilage defects to model cartilage repair. Repair constructs were cultured for 48 hours, then fixed and dehydrated prior to scanning electron microscopy (SEM) imaging. SEM was conducted at an accelerating voltage of 5 keV and an aperture size of 30 μm.
Figure 4.
Cells in bone marrow concentrate (BMC) adhere to BioCartilage. Cells adhere and spread (arrows) to BioCartilage after (A) 24 hours of cultures and (B) 48 hours of culture. BioCartilage was mixed with BMC and sealed with commercial fibrin glue (Tisseel). Scanning electron microscopy (SEM) was conducted at an accelerating voltage of 5 keV and an aperture size of 30 μm.
IL-1RA ELISA in Biologics and in Conditioned Medium from Constructs
IL-1RA was detected in all biologics. The highest concentration was found in BMC, which was significantly greater than all other groups ( Fig. 5 ). There was no significant difference in IL-1RA concentration between PRP and bone marrow aspirate, which were both greater than PPP, autologous thrombin, and allogeneic thrombin. In conditioned medium from BioCartilage/BMC constructs, very low (<10 pg/mL) to undetectable concentrations of IL-1RA were detected, regardless of the fibrin glue sealant (data not presented). From the 50 medium samples (5 fibrin glue sealant groups × 5 donors each cultured for 24 or 48 hours) 19 samples were below the lower limit of detection on the IL-1RA ELISA. Of the remaining 31 samples, only 9 had a concentration of IL-1RA >10 pg/mL.
Figure 5.
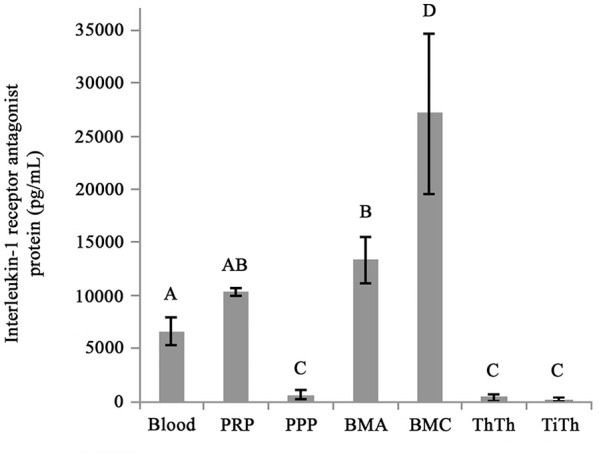
Interleukin-1 receptor antagonist (IL-1RA) concentration in bone marrow concentrate (BMC) is greater than that of the other biologics.
BioCartilage Proteomics
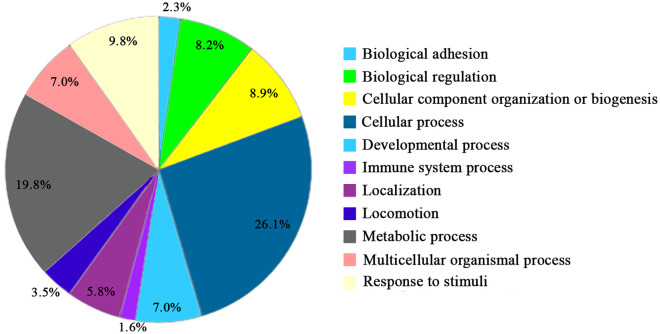
Proteomic analyses yielded a total of 214 total proteins in BioCartilage (Supplemental Table 1). Unsurprisingly, proteins identified in this micronized, dehydrated cartilage product were primarily ECM proteins, including proteoglycans and collagens. There were also several bioactive proteins with known anabolic roles in maintenance of articular cartilage, including cartilage oligomeric matrix protein (COMP) as the most abundant protein in BioCartilage. Lumican, α-2 macroglobulin (A2M), growth and differentiation factor-1 (GDF-1), stromal cell–derived factor (SDF-1), platelet-derived growth factor (PDGF), and protein S100 were also detected. Categorization of these 214 proteins by their biological processes is shown in Figure 6 . The majority of detected proteins belonged to the categories of cellular and metabolic processing at 25% and 20%, respectively. This suggests that BioCartilage may function as more than an inert scaffold when used in cartilage repair.
Figure 6.
Proteomic analysis of BioCartilage. Proteins (214) present in BioCartilage categorized by their biological processes. Categorical percentages are out of 100%.
Discussion
BioCartilage has been shown to be a safe and biocompatible option for cartilage repair applications in several joints.15-20 It is widely assumed that BioCartilage functions as an inert scaffold and this study aimed to further investigate biological mechanisms by which BioCartilage might effectuate cartilage repair. We showed that chondrocytes and MSCs adhere and migrate on BioCartilage with retained cell viability. Proteomic analysis supports BioCartilage contribution to repair by release of proteins involved in cartilage homeostasis due to the presence of several bioactive proteins. These data suggest BioCartilage has bioactive repair potential by supplying bioactive proteins to the site of repair and serving as a scaffold for cell adhesion and migration.
BioCartilage is produced by subjecting articular cartilage to a process known as hypothermic dehydration,25,26 which allows more precise control of crystal size and nucleation rate compared to lyophilization, which is free drying under vacuum. 28 Hypothermic dehydration also retains ECM structure. Although we did not compare BioCartilage prepared by hypothermic dehydration versus lyophilization, both the scanning electron and confocal microscopy images demonstrate retention of cartilage matrix structure, including chondrocyte lacunae.
Multiple fibrin sealants were tested in this study to determine if they had an effect on viability, adhesion, and migration of cells in a BioCartilage composite graft. After mixing BioCartilage with a blood product (BMC in this present study) the resultant combination is comparable to a grout in terms of its sand-like composition. Fibrin sealants should be able to penetrate into the mixture to a depth which is likely dependent on how firmly the mixture is packed into the cartilage defect. Several studies have demonstrated that fibrin can inhibit cellular migration, proliferation, and differentiation,22-24 prompting our investigation into the use of several fibrin glue combinations, including an all-autologous fibrin made from PPP. Use of an all-autologous fibrin glue would avoid the rarely reported, but possible immune response to commercial fibrin that is generated from pool plasma, 21 and obviate the need for storage of frozen fibrin glues. None of the 5 fibrin sealant combinations tested had a negative effect on cell viability, adhesion, or migration suggesting that the type of fibrin sealant used in clinical cases of articular cartilage repair may be dictated by surgeon preference.
BioCartilage is commonly mixed with BMC in cartilage repair procedures. 4 BMC provides growth factors with anabolic effects on cartilage metabolism, as well as stimulation of cell replication and recruitment of MSCs.11,14,27 While other blood-based biologics such as PRP contain similar growth factors, BMC has the added advantages of containing MSCs and high concentrations of the anti-inflammatory protein IL-1RA.11,29 In this study, we attempted to determine if BioCartilage had an effect on IL-1RA release from BMC, but the volume of BMC contained (0.05 µL) in the defects contained insufficient IL-1RA for ELISA detection.
Proteomic analysis of BioCartilage was performed to determine if bioactive proteins were retained during hypothermic dehydration processing and storage. As expected, ECM components such as proteoglycans and collagens were also abundantly present in BioCartilage. The most abundant ECM protein in BioCartilage was cartilage oligomeric matrix peptide (COMP), and this is also the second most abundant protein in native articular cartilage. 30 In articular cartilage, COMP has known roles in collagen secretion and fibrillogenesis as well as inducing chondrocyte proliferation. None of the biological functions of COMP were tested in the present study, but the high abundance of COMP and other proteins with known roles in cartilage homeostasis indicate that the proteome of the cartilage ECM was largely preserved during generation of BioCartilage.28,31 Overall, BioCartilage shares 6 of the 10 most abundant proteins found in native articular cartilage. 30
This study investigated the biological mechanisms by which BioCartilage may effectuate cartilage repair. Proteomics characterization identified ECM components as well as several bioactive proteins that are abundant in native cartilage tissue. Chondrocytes and MSCs were both found to adhere and migrate onto BioCartilage, as indicated by cell spreading on the scaffold. Collectively, the results of this study suggest that BioCartilage has the chemical composition and architecture to support cell adherence and migration, and to provide bioactive proteins. Together, these properties of BioCartilage should have biological advantages in cartilage repair that augment its role as a scaffold in cartilage repair.
Supplemental Material
Supplemental material, Table_S1_full_proteomic_analysis for Biological Mechanisms for Cartilage Repair Using a BioCartilage Scaffold: Cellular Adhesion/Migration and Bioactive Proteins by Jacqueline Commins, Rebecca Irwin, Andrea Matuska, Margaret Goodale, Michelle Delco and Lisa Fortier in CARTILAGE
Footnotes
Supplementary material for this article is available on the Cartilage website at https://journals.sagepub.com/home/car.
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Arthrex Inc., Naples, FL. The electron microscopy facility of the Cornell Center for Materials Research (CCMR) is supported by the National Science Foundation Materials Research Science and Engineering Centers (MRSEC) program (DMR 1120296). Research reported in this publication was supported by Cornell University Biotechnology Resource Center (BRC) and the National Institutes of Health under award number S10RR025502.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Andrea Matuska is an employee of Arthrex Inc. and Lisa Fortier is a consultant for Arthrex Inc.
Ethical Approval: Ethical approval for this study was obtained from the Institutional Animal Care and Use Committee at Cornell University (Approval Number 2006-0026).
Informed Consent: Not applicable.
Trial Registration: Not applicable.
ORCID iD: Lisa Fortier  https://orcid.org/0000-0003-1072-5059
https://orcid.org/0000-0003-1072-5059
References
- 1. Lamplot JD, Schafer KA, Matava MJ. Treatment of failed articular cartilage reconstructive procedures of the knee: a systematic review. Orthop J Sport Med. 2018;6:2325967118761871. doi: 10.1177/2325967118761871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Welton KL, Logterman S, Bartley JH, Vidal AF, McCarty EC. Knee cartilage repair and restoration: common problems and solutions. Clin Sports Med. 2018;37:307-30. [DOI] [PubMed] [Google Scholar]
- 3. Li MH, Xiao R, Li JB, Zhu Q. Regenerative approaches for cartilage repair in the treatment of osteoarthritis. Osteoarthritis Cartilage. 2017;25:1577-87. [DOI] [PubMed] [Google Scholar]
- 4. Abrams GD, Mall NA, Fortier LA, Roller BL, Cole BJ. BioCartilage: background and operative technique. Oper Tech Sports Med. 2013;21:116-24. [Google Scholar]
- 5. Batista MA, Leivas TP, Rodrigues CJ, Arenas GCF, Belitardo DR, Guarniero R. Comparison between the effects of platelet-rich plasma and bone marrow concentrate on defect consolidation in the rabbit tibia. Clinics (Sao Paulo). 2011;66:1787-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kraeutler MJ, Chahla J, LaPrade RF, Pascual-Garrido C. Biologic options for articular cartilage wear (platelet-rich plasma, stem cells, bone marrow aspirate concentrate). Clin Sports Med. 2017;36:457-68. [DOI] [PubMed] [Google Scholar]
- 7. Shi WJ, Tjoumakaris FP, Lendner M, Freedman KB. Biologic injections for osteoarthritis and articular cartilage damage: can we modify disease? Phys Sportsmed. 2017;45:203-23. doi: 10.1080/00913847.2017.1357421 [DOI] [PubMed] [Google Scholar]
- 8. Mascarenhas R, Saltzman BM, Fortier LA, Cole BJ. Role of platelet-rich plasma in articular cartilage injury and disease. J Knee Surg. 2014;28:3-10. [DOI] [PubMed] [Google Scholar]
- 9. Sampson S, Bemden AB, Aufiero D. Autologous bone marrow concentrate: review and application of a novel intra-articular orthobiologic for cartilage disease. Phys Sportsmed. 2013;41:7-18. [DOI] [PubMed] [Google Scholar]
- 10. Sundman EA, Cole BJ, Karas V, Valle CD, Tetreault MW, Mohammed HO, et al. The anti-inflammatory and matrix restorative mechanisms of platelet-rich plasma in osteoarthritis. Am J Sports Med. 2014;42:35-41. [DOI] [PubMed] [Google Scholar]
- 11. Cassano JM, Kennedy JG, Ross KA, Fraser EJ, Goodale MB, Fortier LA. Bone marrow concentrate and platelet-rich plasma differ in cell distribution and interleukin 1 receptor antagonist protein concentration. Knee Surg Sports Traumatol Arthrosc. 2018;26:333-42. doi: 10.1007/s00167-016-3981-9 [DOI] [PubMed] [Google Scholar]
- 12. Fortier LA, Potter HG, Rickey EJ, Schnabel LV, Foo LF, Chong LR, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92:1927-37. [DOI] [PubMed] [Google Scholar]
- 13. Chu CR, Fortier LA, Williams A, Payne KA, McCarrel TM, Bowers ME, et al. Minimally manipulated bone marrow concentrate compared with microfracture treatment of full-thickness chondral defects: a one-year study in an equine model. J Bone Joint Surg Am. 2018;100:138-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holmes HL, Wilson B, Goerger JP, Silverberg JL, Cohen I, Zipfel WR, et al. Facilitated recruitment of mesenchymal stromal cells by bone marrow concentrate and platelet rich plasma. PLoS One. 2018;13:e0194567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shin JJ, Mellano C, Cvetanovich GL, Frank RM, Cole BJ. Treatment of glenoid chondral defect using micronized allogeneic cartilage matrix implantation. Arthrosc Tech. 2014;3:e519-e522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fortier LA, Chapman HS, Pownder SL, Roller BL, Cross JA, Cook JL, et al. BioCartilage improves cartilage repair compared with microfracture alone in an equine model of full-thickness cartilage loss. Am J Sports Med. 2016;44:2366-74. doi: 10.1177/0363546516648644 [DOI] [PubMed] [Google Scholar]
- 17. Clanton TO, Johnson NS, Matheny LM. Use of cartilage extracellular matrix and bone marrow aspirate concentrate in treatment osteochondral lesions of the talus. Tech Foot Ankle Surg. 2014;13:212-20. [Google Scholar]
- 18. Desai S. Surgical treatment of a tibial osteochondral defect with debridement, marrow stimulation, and micronized allograft cartilage matrix: report of an all-arthroscopic technique. J Foot Ankle Surg. 2016;55:279-82. [DOI] [PubMed] [Google Scholar]
- 19. Desai S. Treatment of osteochondral lesions of the talus with marrow stimulation and micronized allograft cartilage matrix: an all-arthroscopic technique. Tech Foot Ankle Surg. 2014;13:167-73. [DOI] [PubMed] [Google Scholar]
- 20. Cole W. BioCartilage use in the 1st MPJ. Podiatry Manage. 2013;32:135-8. [Google Scholar]
- 21. Noori A, Ashrafi SJ, Vaez-Ghaemi R, Hatamian-Zaremi A, Webster TJ. A review of fibrin and fibrin composites for bone tissue engineering. Int J Nanomedicine. 2017;12:4937-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hale BW, Goodrich LR, Frisbie DD, McIlwraith CW, Kisiday JD. Effect of scaffold dilution on migration of mesenchymal stem cells from fibrin hydrogels. Am J Vet Res. 2012;73:313-8. [DOI] [PubMed] [Google Scholar]
- 23. Brittberg M, Sjögren-Jansson E, Lindahl A, Peterson L. Influence of fibrin sealant (Tisseel) on osteochondral defect repair in the rabbit knee. Biomaterials. 1997;18:235-42. [DOI] [PubMed] [Google Scholar]
- 24. Catelas I, Sese N, Wu BM, Dunn JCY, Helgerson S, Tawil B. Human mesenchymal stem cell proliferation and osteogenic differentiation in fibrin gels in vitro. Tissue Eng. 2006;12:2385-96. [DOI] [PubMed] [Google Scholar]
- 25. O’Brien FJ, Harley BA, Yannas IV, Gibson LJ. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 2005;26:433-41. [DOI] [PubMed] [Google Scholar]
- 26. Polak R, Pitombo RNM. Care during freeze-drying of bovine pericardium tissue to be used as a biomaterial: a comparative study. Cryobiology. 2011;63:61-6. [DOI] [PubMed] [Google Scholar]
- 27. Oliver K, Awan T, Bayes M. Single- versus multiple-site harvesting techniques for bone marrow concentrate: evaluation of aspirate quality and pain. Orthop J Sport Med. 2017;5:2325967117724398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ruthard J, Hermes G, Hartmann U, Sengle G, Pongratz G, Ostendorf B, et al. Identification of antibodies against extracellular matrix proteins in human osteoarthritis. Biochem Biophys Res Commun. 2018;503:1273-7. [DOI] [PubMed] [Google Scholar]
- 29. Baltzer AWA, Moser C, Jansen SA, Krauspe R. Autologous conditioned serum (Orthokine) is an effective treatment for knee osteoarthritis. Osteoarthritis Cartilage. 2009;17:152-60. [DOI] [PubMed] [Google Scholar]
- 30. Folkesson E, Turkiewicz A, Englund M, Önnerfjord P. Differential protein expression in human knee articular cartilage and medial meniscus using two different proteomic methods: a pilot analysis. BMC Musculoskelet Disord. 2018;19:416. doi: 10.1186/s12891-018-2346-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Posey KL, Coustry F, Hecht JT. Cartilage oligomeric matrix protein: COMPopathies and beyond. Matrix Biol. 2018;71-72:161-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Table_S1_full_proteomic_analysis for Biological Mechanisms for Cartilage Repair Using a BioCartilage Scaffold: Cellular Adhesion/Migration and Bioactive Proteins by Jacqueline Commins, Rebecca Irwin, Andrea Matuska, Margaret Goodale, Michelle Delco and Lisa Fortier in CARTILAGE