Abstract
Objective
The purpose of this systematic review is to determine (1) current reported treatment options for isolated tibial plateau (TP) cartilage lesions, (2) patient reported outcomes following various treatments, and (3) complication rate and survivorship following various treatments.
Design
A literature search of PubMed, the Cochrane Library, and CINAHL was conducted adhering to PRISMA guidelines. Patients were included if they had TP cartilage lesions treated with surgery. Lesion characteristics, surgical procedure details, patient reported outcomes, complication, and failure rates were collected.
Results
Thirteen studies yielded 205 knees with TP cartilage lesions treated surgically. Ages ranged from 12 to 77 years. Surgical techniques included 138 treated with osteochondral allograft transplantation (OCA), 37 treated with osteochondral autograft transfer system (OATS), 11 treated with microfracture, 11 treated with an osteochondral scaffold, and 8 treated with autologous chondrocyte implantation (ACI). The patient-reported outcome measures were heterogeneous, but all reported improvements with the notable exception of one study evaluating microfracture. The rate of complications ranged from 0% to 4.6%. Failure rate ranged from 22% to 46% for OCA and 0% to 16% for OATS. No failures were reported for the additional techniques.
Conclusions
Various surgical techniques have been utilized for the treatment of TP cartilage lesions. Patient-reported outcome measures were heterogeneous, but improvements were reported following all surgical treatments except for microfracture, which resulted in decreased scores at mid-term follow-up. The complication rate was low for all techniques described. However, the failure rate was higher following unicondylar OCA for salvage treatment of posttraumatic deformities.
Keywords: tibial plateau cartilage lesion, osteochondral allograft, osteochondral autograft
Introduction
Cartilage defects involving the knee were found in 63% of 31,516 patients undergoing knee arthroscopy by Curl et al. 1 between 1991 and 1995. It is suspected that the incidence is only increasing. 2 Unfortunately, these defects can be difficult to treat due to their low healing potential. 3 Lesions of the femur and patella articular surfaces have been reported as the most common and a variety of surgical treatments exist.1,4 Autologous chondrocyte implantation (ACI) and osteochondral allograft transplantation (OCA) are good options for lesions larger than 2 cm2.4,5 Osteochondral autograft transfer system (OATS) has demonstrated improved results compared to microfracture for smaller lesions.6,7 These treatments have been thoroughly studied for cartilage lesions involving the femur, but there is limited data available regarding the use of these modalities for cartilage lesions isolated to the tibial plateau (TP).
Surgical treatment of TP cartilage lesions is fraught with unique challenges primarily associated with achieving adequate exposure. 8 Hannon et al. 5 recently demonstrated improvements in outcomes with OCA of the femur for tibiofemoral bipolar lesions regardless of whether the reciprocal TP lesion was treated. Bugbee 8 further emphasized the need to determine when and how TP cartilage lesions should be treated in his editorial commentary. Although the proper management of bipolar lesions is still unsolved, even less is understood about the optimal approach to isolated TP cartilage lesions. Yabumoto et al. 9 reported improvements in International Knee Documentation Committee (IKDC) scores for osteochondral TP lesions treated with retrograde OATS. OCA has demonstrated mixed results when used as a salvage procedure for treatment of posttraumatic TP cartilage lesions.10,11 Kreuz et al. 12 reported generally poor results at 3 years postoperatively following microfracture for TP lesions. ACI with concurrent high tibial osteotomy (HTO) has demonstrated quality outcomes in a small series of 8 patients at minimum 2-year follow-up. 13 At this time, it is unclear whether TP cartilage lesions should be treated and which surgical treatment option is best.
The purpose of this systematic review of the literature is to determine (1) current reported treatment options for isolated TP cartilage lesions, (2) patient-reported outcomes following various treatments, and (3) complication rate and survivorship following various treatments.
Methods
Literature Search
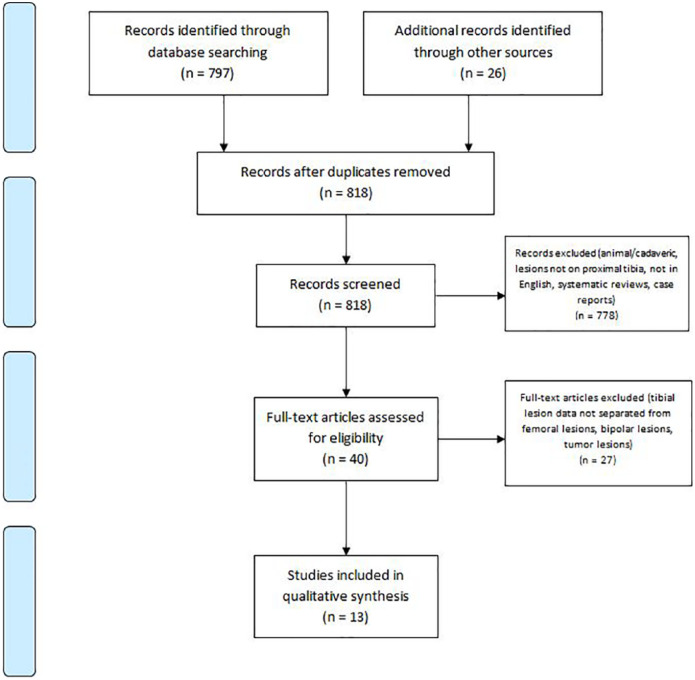
A comprehensive search of the available literature was performed on September 17, 2018 following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines ( Fig. 1 ). The databases that were searched include PubMed (MEDLINE), the Cochrane Central Register of Controlled Trials & Cochrane Library, and CINAHL (Cumulative Index for Nursing and Allied Health Literature) and the search period parameters were set from January 1, 1995 to September 17, 2018. A Boolean search was performed utilizing the following terms: (proximal tibia cartilage) OR (tibial plateau chondral) OR (tibial plateau lesions) OR (bipolar chondral) and articles were catalogued using Microsoft Excel (2010; Microsoft Corp, Redmond, WA). The initial search yielded 797 articles from PubMed, 3 articles from CINAHL, and 23 articles from the Cochrane Library. The reference list of articles was reviewed for any missed articles and an additional 5 articles were included. After removal of duplicates, 818 articles underwent screening for inclusion in this study.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) flow diagram demonstrating the study selection process.
Selection Criteria
Titles and abstracts of these 818 studies were independently reviewed by 2 authors (CDB, HPM) and only studies eliminated in consensus were removed from the list with any disagreements being resolved by consensus discussion between those authors.
Inclusion criteria for the evaluation of full-text articles were the following: a confirmed series or cohort of patients with a tibial plateau cartilage lesion, operative treatment of the lesion and documented patient reported, clinical, functional, and/or radiographic outcome measures. Exclusion criteria included systematic reviews, case reports, cadaveric and animal studies, studies not pertaining to the TP, studies with unavailable full English texts, bipolar lesions, lesions created due to tumor resection, and isolated osteotomy procedure of the knee.
Several studies contained cohorts of patients that included both cartilage lesions of the tibial plateau as well as other cartilage lesions of the knee. Only cohorts of patients that explicitly fulfilled the inclusion/exclusion criteria were reported in our analysis. If a study provided a table containing individual patient demographics, surgical treatment, and outcomes, those individual patients were included for analysis. In the case that a study failed to distinguish between patient cohorts and a table with each individual patient’s data were not provided, it was excluded.
Quality Assessment
As there were no randomized controlled trials identified throughout the search, each study was assessed using the methodologic index for nonrandomized studies (MINORS) scoring system. MINORS is a validated tool designed for assessing the quality of nonrandomized surgical studies based on a scoring scale. This scoring scale allows for a maximum score of 16 for noncomparative studies (8-item checklist scored from 0 to 2) and a maximum score of 24 for comparative studies (12-item checklist scored from 0 to 2) in which higher scores represent a lower level of bias. Each study was independently reviewed and scored by 2 authors (CDB, HPM) and any disagreements were resolved by consensus discussion. MINORS score results of each study are displayed in Table 1 and are presented as percentages for normalization between comparative and noncomparative studies. Level of evidence was determined based on criteria from the Oxford Centre for Evidence-Based Medicine.
Table 1.
Characteristics of Included Studies (n = 13).
| Authors, Year | MINORS Score, % | Level of Evidence | Procedure | Outcome Measures Reported | No. of Tibias (M/F) | Lesion Location | Lesion Size | Lesion Etiology | Age (y) | Follow-up (mo) |
|---|---|---|---|---|---|---|---|---|---|---|
| Bakay et al., 1998 | 63 | RCS, level IV | OCA | XR | 5 (NR) | NR | Unicondylar | Posttraumatic | 48 (21-64) a | 19 (10-38) a |
| Drexler et al., 2015 | 75 | RCS, level IV | OCA + DFO | KS-F, KSKS | 27 (12 M/15 F) | 27 LTP | Unicondylar | Posttraumatic | 42 (17-62) | 156 (24-372) |
| Fanceshchi et al., 2008 | 75 | RCS, level IV | ACI + HTO | IKDC, Lysholm, Tegner, VAS | 8 (4 M/4 F) | 8 MTP | 3 cm2 (2-4) | NR | 49 (42-58) | 28 (25-31) |
| Gracitelli et al., 2017 | 75 | RCS, level IV | OCA | IKDC, KS-F, Modified Merle d’Aubigné-Postel | 29 (NR) | 6 MTP, 23 LTP | 14 cm2 (2-33) a | Posttraumatic | 34 (16-54) a | 69.6 (22.8-283.2) |
| Hangody et al., 2010 | 69 | PCS, level IV | OATS | Modified HSS | 16 (NR) | 15 LTP, 1 MTP | 1 cm2 (1-2) | NR | 24 (14-49) | 115.2 (24-204) |
| Kon et al., 2014 | 81 | PCS, level IV | OCS | IKDC, Tegner, Subjective | 11 (6 M/5 F) | NR | 5 cm2 (3-12) | 10 posttraumatic, 1 DIC | 37 ± 11.0 | 24 |
| Kreuz et al., 2006 | 81 | PCS, level IV | Microfracture | Cincinnati, ICRS, MRI | 11 (3 M/8 F) | NR | 2 cm2 (1-4) | NR | 39 (22-55) | 36 |
| Locht et al., 1984 | 63 | RCS, level IV | OCA | Subjective, ROM, XR | 12 (NR) | 11 LTP, 1MTP | Unicondylar | Posttraumatic | 50 (21-70) | 45.9 (27-105) |
| Ma et al., 2004 | 69 | RCS, level IV | OATS | Lysholm, Arthroscopy, XR | 2 (NR) | 2 LTP | 5 cm2 | Posttraumatic | 41 (32-51) | 46.5 (46-47) |
| Shasha et al., 2003 | 75 | RCS, level IV | OCA | HSS, XR | 65 (29 M/36 F) | 54 LTP, 11 MTP | Unicondylar | Posttraumatic | 42 (26-69) | 141.6 (60-288) |
| Ueblacker et al., 2004 | 56 | RCS, level IV | Retrograde OATS | Arthroscopy, XR, MRI | 3 (NR) | 2 LTP, 1 MTP | NR | 1 posttraumatic, 2 unknown | 38 (34-40) | 16 (6-35) |
| Wajsfisz et al., 2013 | 69 | PCS, level IV | OATS | IKDC, Tegner, KOOS | 4 (3 M/1 F) | 1 MTP, 3 LTP | 9 mm (8-10) | Posttraumatic | 31 (17-41) | 55 (52-60) |
| Yabumoto et al., 2017 | 75 | RCS, level IV | OATS | IKDC, Arthroscopy, ICRS, JOA, | 12 (6 M/6 F) | 3 MTP, 9 LTP | 220 mm2 (100-500) | 8 posttraumatic, 4 osteonecrosis | 38 (12-77) | 75 (24-126) |
OCS = osteochondral scaffold (Maioregen, Fin-Ceramica, Faenza, Italy) was a biomimetic nanostructured implant; OCA = osteochondral allograft; OATS = osteochondral autograft transfer system; ACI = autologous chondrocyte implantation; MTP = medial tibial plateau; LTP = lateral tibial plateau; DFO = distal femur osteotomy; HTO = high tibial osteotomy; IKDC = International Knee Documentation Committee; KS-F = Knee Society–Function; KSKS = Knee Society Knee Score; ICRS = International Cartilage Repair Society; JOA = Japanese Orthopaedic Association; KOOS = Knee injury and Osteoarthritis Outcome Score; VAS = visual analogue score; HSS = Hospital for Special Surgery; ROM = range of motion; XR = X-ray; MRI = magnetic resonance imaging; RCS = retrospective case series; PCS = prospective case series; M = male; F = female; DIC = disseminated intravascular coagulation; NR = not reported.
Includes additional patients without isolated tibial lesions.
Data Extraction and Analysis
Following final selection of studies for inclusion the data extracted included study properties (year, level of evidence, number of knees), patient demographics (age, lesion etiology, lesion location, lesion size, follow-up period), surgical procedure, concomitant procedures, outcomes (patient-reported, functional, clinical, radiographic), complications, failures, survivorship, and reoperation. Because of an inadequate number of comparative studies and heterogeneity of reported outcomes, pooling of results was not performed and instead ranges were reported. In studies with cohorts of both cartilage lesions of the TP and other regions of the knee, only patients with cartilage lesions of the TP were included in our analysis and any data which utilized a combination of those cohorts was excluded or noted.
Results
Forty full texts were manually reviewed for inclusion. Ultimately, 13 studies met all inclusion criteria and were analyzed in this systematic review. Characteristics related to these studies are reported in Table 1 . MINORS scores ranged from 56% to 81% and all studies (13/13) were best classified as level of evidence IV. The 13 studies yielded 205 knees with isolated TP cartilage lesions that were treated surgically. Patient age ranged from 12 to 77 years. A variety of surgical techniques were utilized including 138 treated with OCA, 37 treated with OATS, 11 treated with microfracture, 11 treated with OCS (osteochondral scaffold; Maioregen, Fin-Ceramica, Faenza, Italy), and 8 treated with ACI.
Surgical Treatments and Indications
Surgical treatments and lesion etiology are reported in Table 1 . The most commonly studied techniques were OCA and OATS, with 5 studies for each (n = 138 knees and n = 37 knees, respectively). All cases of OCA were bulk allografts replacing entire hemi-plateaus rather than small dowels.
Patient-Reported Outcomes
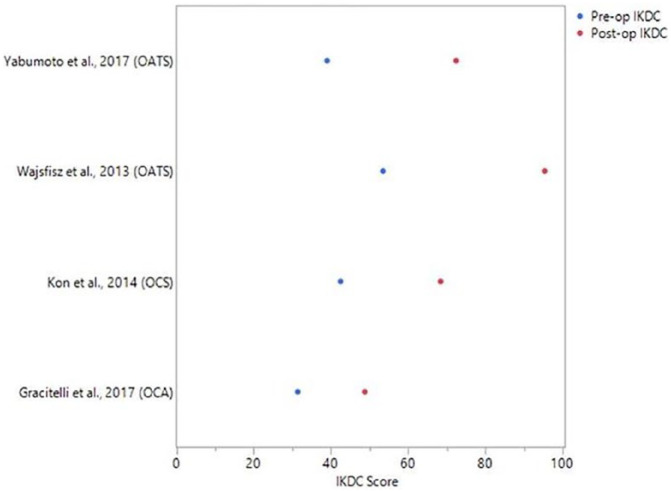
There was significant heterogeneity in patient reported outcomes as shown in Table 2 . All studies reported postoperative improvements in subjective outcome scores at follow up ranging from 6 to 372 months except for 1 study evaluating microfracture at 36-month follow-up, which reported decreased scores. Across all treatment types, 4 studies reported IKDC subjective outcome scores with a mean improvement ranging from 17.4 to 41.9 points ( Fig. 2 ).
Table 2.
Complications, Reoperations, and Failures.
| Authors, Year | Procedure | Concomitant Procedure | Significant Complications | Reoperations | Failures | Survivorship |
|---|---|---|---|---|---|---|
| Bakay et al., 1998 | OCA | NR | NR | NR | 2 | NR |
| Drexler et al., 2015 | OCA + DFO | DFVO | None | 6 | 1 revision OCA, 5 TKA conversion | 89% at 10years, 23.8% at 20 years |
| Fanceshchi et al., 2008 | ACI + HTO | HTO for varus knee | None | None | None | NR |
| Gracitelli et al., 2017 | OCA | NR | None | 13 | 1 revision OCA, 5 TKA conversion | 66.8% at 10 years |
| Hangody et al., 2010 | OATS | 1 ACLR, 3 meniscus resections | 1 donor site morbidity | NR | None | NR |
| Kon et al., 2014 | OCS | 3 hardware removals, 4 osteotomies (3 in varus, 1 in valgus), 1 lateral meniscectomy, and 3 tibial plateau elevation osteotomies | 3 postoperative fevers | None | None | 100% at 2 years |
| Kreuz et al., 2006 | Microfracture | NR | None | None | None | NR |
| Locht et al., 1984 | OCA | 4 meniscal graft, 1 femoral surface graft with a meniscus | None | NR | 4 < PRO | NR |
| Ma et al., 2004 | OATS | none | None | 8 repeat scopes | None | NR |
| Shasha et al., 2003 | OCA | 39 meniscal allograft, 26 DFVO, 12 Closing wedge HTO | 1 DVT, 1 loosening,1 fracture | 54 | 21 TKA conversion, 2 < PRO | 80% at 10 years, 46% at 20 years |
| Ueblacker et al., 2004 | Retrograde OATS | 1 HTO for varus knee | None | None | None | NR |
| Wajsfisz et al., 2013 | OATS | NR | 1 hardware irritation | 1 hardware removal | None | NR |
| Yabumoto et al., 2017 | OATS | 1 HTO, 5 Femoral OAT | None | 7 repeat scopes | 1 TKA, 1 DFO | NR |
OCS = osteochondral scaffold (Maioregen, Fin-Ceramica, Faenza, Italy) was a biomimetic nanostructured implant; OCA = osteochondral allograft; OATS = osteochondral autograft transfer; ACI = autologous chondrocyte implantation; ACLR = anterior cruciate ligament reconstruction; MTP = medial tibial plateau; LTP = lateral tibial plateau; DFO = distal femur osteotomy; DFVO = distal femoral varus osteotomy; HTO = high tibial osteotomy; PRO = patient-reported outcomes; DVT = deep vein thrombosis; TKA = total knee arthroplasty; NR = not reported.
Figure 2.
Reported International Knee Documentation Committee (IKDC) scores. Blue dots represent mean preoperative IKDC scores and red dots represent mean postoperative IKDC scores. Surgical treatment is listed in parentheses.
Complications and Survivorship
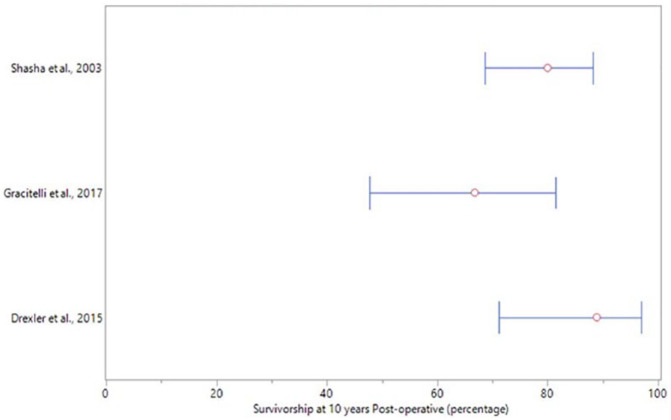
The rate of complications ranged from 0% to 4.6% following any surgical treatment of TP cartilage lesions as presented in Table 3 . Three studies reported survivorship ranging from 66.8% to 89% 10 years following treatment with OCA ( Fig. 3 ). One study reported 2 failures following treatment with OATS, while the additional 4 studies evaluating OATs demonstrated no failures. No failures were reported for all remaining surgical treatments ( Table 3 ).
Table 3.
Reported Outcome Scores, Imaging, and Arthroscopy.
| Authors, Year | Outcome Measures Reported | Outcome Scores | Imaging and Arthroscopy |
|---|---|---|---|
| Bakay et al., 1998 | XR | NR | XR: 1 excellent, 2 good, 1 fair, 1 poor result |
| Drexler et al., 2015 | KS-F, KSKS | KS-F: Preoperatively 50.6, postoperatively 61.9
(P < 0.01) (median
scores) KSKS: Preoperatively 54.6, postoperatively 72.6 (P < 0.01) (median scores) |
NR |
| Fanceshchi et al., 2008 | IKDC, Tegner, Lysholm, VAS | IKDC: Preoperatively all had abnormal and severely abnormal
scores. Postoperatively 7 patients had normal and nearly
normal values, and 1 had abnormal value. Tegner: Preoperatively 3.7 (3-5), post-operatively 7 (5-8) (P < 0.05) Lysholm: Preoperatively 65.7 (49–88), post-operatively 94.6 (89-100) (P < 0.05) VAS: Preoperatively 7.2 (6-9), postoperatively 2 (0-5) (P < 0.05) |
NR |
| Gracitelli et al., 2017 | IKDC, KS-F, Modified Merle d’Aubigné-Postel | IKDC total: Preoperatively 31.4 ± 12.6, post-operatively
48.8 ± 23.8 (P = 0.012) IKDC Pain: Preoperatively 6.2 ± 2.0, postoperatively 4.6 ± 3.2 (P = 0.111) IKDC function: Preoperatively 2.6 ± 1.9, postoperatively 5.3 ± 2.5 (P = 0.005) KS-F: Preoperatively 51.8 ± 23.7, postoperatively 71.7 ± 20.0 (P = 0.007) Modified Merle: Preoperatively 11.3 ± 1.7, postoperatively 14.5 ± 2.7 (P = 0.001) |
NR |
| Hangody et al., 2010 | Modified HSS | Modified HSS: Preoperatively 52, postoperatively 86 (P = 0.015) | NR |
| Kon et al., 2014 | IKDC, Tegner, Subjective | IKDC Subjective score: Preoperatively 42.5 ± 10.2,
postoperatively 68.4 ± 17.0. IKDC Objective score: Preoperatively had 27.3% normal and nearly normal knees, postoperatively had 85.7% normal and nearly normal knees Tegner: Preinjury 5.3 ± 2.5, pre-operatively 2.3 ± 2.1, post-operatively 4.4 ± 1.9 Subjective: 10 of the 11 patients (90.9%) reported a subjective improvement of symptoms and function after treatment |
NR |
| Kreuz et al., 2006 | Cincinnati, ICRS, MRI | Cincinnati: Preoperatively 4.0 ± 0.0, post-operatively 2.55
± 1.04 (P = 0.008) ICRS: Preoperatively 3.91 ± 0.3, Postoperatively 3.0 ± 0.77 (P = 0.008) |
MRI: Defect filling 2.64 ± 0.92, subchondral edema 2.55 ± 0.93, cartilage signal 2.55 ± 0.93, effusion 2.36 ± 1.03, overall score 2.64 ± 0.92 (used previously published scoring system 14 ) |
| Locht et al., 1984 | Subjective, ROM, XR | Subjective: Preoperatively 3.58, postoperatively
7.50 ROM: Either improved or stayed the same in all but 1 patient. |
XR: All knees showed at least slight collapse of the osseous component of the graft. The collapse measured three millimeters or less in 10 knees. |
| Ma et al., 2004 | Lysholm, arthroscopy, XR | Lysholm: Preoperatively 47.5 (46-49), postoperatively 80 (79-81) | Arthroscopy was performed in 2 patients: Showed uneven
surface and degenerative change with fibrillation XR: Narrowing of joint space over the lateral compartment |
| Shasha et al., 2003 | HSS, XR | HSS: Postoperatively 85.3 ± 11.0 points | XR: Radiographic evidence of allograft union to host bone in all cases, 3 cases of graft collapse in excess of 3 mm. There was no or mild degenerative changes in 21 patients and moderate or severe degenerative changes in 14. |
| Ueblacker et al., 2004 | Arthroscopy, XR, MRI | NR | Arthroscopy was performed in 1 patient: The osteochondral
cylinder was well integrated and flush with the articular
surface. XR: The osteochondral cylinders were congruent in the mediolateral tibial plateau. MRI: Vital cylinders, healing of the implanted plug and a congruent chondral surface was observed in all patients. |
| Wajsfisz et al., 2013 | IKDC, Tegner, KOOS | IKDC: Preoperatively 53.5 (37-66), postoperatively 95.4
(93.1-97.7) Tegner: Preoperatively 7 (6-8), postoperatively 7 (6-8) KOOS: Reported significant improvement in all patients |
NR |
| Yabumoto et al., 2017 | IKDC, Arthroscopy, ICRS, JOA | IKDC: Preoperatively 39.0 (13.0-57.1), post-operatively 72.4
(33.3-100) (P = 0.0022) JOA: Preoperatively 65.8 (30.0-85.0), postoperatively 85.8 (50.0-100) (P = 0.0022) |
Arthroscopy was performed in 7 patients at mean 14.9 months (range, 12-27 months): ICRS Cartilage Repair Assessment Score: 8.57 (3-12); with 2 patients needing second operation removed: 10.6 (9-12) |
IKDC = International Knee Documentation Committee; KS-F = Knee Society–Function; KSKS = Knee Society Knee Score; ICRS = International Cartilage Repair Society; JOA = Japanese Orthopaedic Association; KOOS = Knee injury and Osteoarthritis Outcome Score; VAS = visual analogue score; HSS = Hospital for Special Surgery; ROM = range of motion; XR = X-ray; MRI = magnetic resonance imaging; NR = not reported.
Figure 3.
Survivorship at 10 years. The red circle represent the survivorship rate of the osteochondral allograft at 10 years postoperatively for each study that reported this outcome. The 95% confidence intervals were calculated using the adjusted Wald method.
Postoperative imaging or second-look arthroscopy was performed and reported in 7 studies ( Table 3 ). Five studies reported radiographic results, 3 studies reported second-look arthroscopy findings, and 2 studies reported MRI results.
Discussion
The main finding in this systematic review was that a variety of surgical techniques to address TP cartilage lesions have been described. Reported outcome measures were very heterogeneous, but in general, patient-reported outcomes increased postoperatively for all techniques with the exception of microfracture. The failure rate was highest when unicondylar OCA was used as a salvage procedure for treatment of posttraumatic deformities.
Osteochondral allograft transplantation was the most common technique utilized in the studies included in this review. This technique was used in 5 studies including 138 patients and all lesions were unicondylar requiring transplant of an entire hemi-tibial plateau.10,11,15-17 OCA is more commonly used on the femoral articular surface but often times does not include transplant of the entire hemi-articular surface. 18 Our review of the literature suggests that when OCA is used on the tibia for an isolated tibial lesion it consists of an entire hemi-plateau. OATS was the next most common technique used in 5 studies including 37 patients with lesions ranging from 9 mm to 5 cm2.9,19-22 Lesions treated with OATS were smaller than those treated with OCA. Larger lesions are more appropriately treated with OCA to avoid donor site morbidity. This is consistent with the indications for femoral OCA and OATS.4,6 Yabumoto et al. 9 found the reoperation rate following retrograde OATs for TP lesions to be significantly higher for lesions ≥400 mm2. Microfracture and ACI are 2 surgical procedures that have been extensively studied for treatment of femoral cartilage defects. 23 Only 1 study evaluating each technique was identified for treatment of TP cartilage lesions.12,13 Finally, 1 study followed 11 patients after treatment of TP cartilage lesions with a cell-free biomimetic osteochondral scaffold (OCS). 24 This technique previously led to successful treatment of osteochondritis dissecans (OCD) lesions involving the femoral condyles.25,26
The patient reported outcomes evaluated by the studies included in this systematic review varied significantly. This is likely related to many factors. The studies were published over many years ranging from 1984 to 2017. Patient-reported outcome measures have changed considerably during this time. Additionally, procedures were performed for multiple indications including focal and contained cartilage lesions and entire hemi-plateau posttraumatic deformities. Gauging a successful outcome following each of these may differ and require variable outcome measures. Overall, follow-up ranged from 6 to 372 months and a mean improvement in subjective outcome scores was observed following all surgical treatments except microfracture. Kreuz et al. 12 observed a decrease in International Cartilage Repair Society (ICRS) and modified Cincinnati knee scores at a mean 36 months postoperatively following microfracture. In this study, worse outcomes were noted for lesions involving the patellofemoral or TP surfaces. Interestingly, this decrease has not been observed at mid- and long-term follow up of microfracture for treatment of other cartilage lesions throughout the knee. 23 The decrease may be related to the relatively increased age (mean 39.7 years) and larger defect size (mean 2.39 cm2) in the study by Kreuz et al. 12 Following their study, the authors now consider larger defects (>2 cm2) in older patients to be a contraindication for use of microfracture. Five studies included in this review evaluated outcomes following OATS and all reported postoperative improvements. Unfortunately, there were not 3 studies evaluating OATS that reported the same outcome measures. Postoperative improvements were seen for the 5 studies evaluating OCA as a surgical treatment, but again, there were not 3 studies evaluating OCA that reported the same outcome measures. Franceschi et al 13 reported mean postoperative Lysholm scores of 94.6 following combined ACI and HTO at a mean of 28 months. These results are comparable to or better than additional studies evaluating ACI for treatment of cartilage lesions throughout the knee.27,28 More research is needed to fully evaluate the long-term efficacy of ACI for treatment of TP cartilage lesions. Additionally, there is a need for more uniform patient-reported outcome measures, especially when evaluating surgical treatment options for a particular pathology.
There was a low rate of complications for surgical treatment of isolated TP cartilage lesions. For the 5 studies evaluating OCA, no major intraoperative complications were reported. Three studies reported a 10-year survivorship ranging from 66.8% to 89%. Variability may be attributed to concomitant procedures. For example, Drexler et al. 16 reported a survivorship of 89% at 10 years, but this was the only study that included concomitant distal femoral osteotomy in all patients. Additionally, definition of failure varied across studies. The majority of studies defined failure as need for revision or conversion to total knee arthroplasty. Two studies included low patient-reported outcomes as failure.11,17 Complications following OATS included 1 case of hardware irritation and 1 case of donor site morbidity. No intraoperative complications were reported. Yabumoto et al. 9 disclosed 2 cases of failure while no other studies reported failures for OATS. In each of these, the lesion size was ≥400 mm2, which the authors consider a relative contraindication for OATS. In the remaining studies evaluating OCS, microfracture, and ACI no failures or complications were reported, and all had a presumed survivorship of 100%. There are multiple possibilities that could explain why OCA resulted in more failures. In most cases, OCA was chosen due to more severe pathology involving the articular surface as a result of posttraumatic deformities. Additionally, there were more concomitant procedures. For example, Shasha et al. 11 performed 65 OCA procedures with multiple concomitant procedures consisting of 39 meniscal allografts, 26 distal femoral osteotomies, and 12 closing wedge HTOs. All cases of OCA were bulk hemi-plateau allografts, which requires a significant amount of creeping substitution as opposed to cases involving a smaller defect requiring a single dowel with only 5 to 6 mm of composite bone and cartilage thickness that would be expected to incorporate sooner. 29
Limitations
A number of limitations should be considered for this systematic review. Although PRISMA guidelines were strictly adhered to, it is possible that studies evaluating surgical treatment of isolated tibial plateau cartilage lesions were excluded during the selection process. Possibly due to the relative infrequency of isolated TP lesions, all included studies were level IV case series lacking randomization, blinding, or comparative control groups. The cartilage lesion type and size, outcome measures, and follow-up time were very heterogeneous. Additionally, the definition of treatment failure was variable across studies. Because of this, data were not pooled, making it difficult to draw widely applicable conclusions regarding indications and surgical management. 30 Nevertheless, this systematic review does provide the first comprehensive presentation of available data evaluating surgical management of TP cartilage lesions.
Conclusions
A variety of surgical techniques have been utilized for the treatment of isolated tibial plateau cartilage lesions. Patient reported outcome measures were heterogeneous in nature, but improvements were reported following all surgical treatments except for microfracture, which resulted in decreased scores at mid-term follow-up. In general, the complication rate was low for all techniques described. However, the failure rate was higher following unicondylar allograft transplantation for salvage treatment of posttraumatic deformities.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflict of Interest: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Christopher L. Camp: Arthrex Inc.: travel and lodging. Michael J. Stuart: Arthrex, Inc.: consultancy, IP royalties; Stryker: research support; American Journal of Sports Medicine: board membership. Norimasa Nakamura: Biomet: paid consultant; International Cartilage Repair Society: board or committee member; International Society of Arthroscopy, Knee Surgery, and Orthopaedic Sports Medicine: board or committee member; Journal of Experimental Orthopaedics (Springer): editorial or governing board; Journal of Orthopaedic Science (Springer): editorial or governing board; Novartis: paid consultant; Cartilage (Sage): editorial or governing board. Daniel B.F. Saris: Cartilage: editorial or governing board; International Cartilage Repair Society, Dutch Orthopaedic Society: board or committee member; Knee Surgery, Sports Traumatology, Arthroscopy: editorial or governing board; Smith & Nephew: paid presenter or speaker; Smith & Nephew, Genzyme, Tigenix: paid consultant. Aaron J. Krych: Aesculap/B.Braun: research support; American Journal of Sports Medicine: editorial or governing board; Arthrex, Inc.: IP royalties; paid consultant; Arthritis Foundation: research support; Ceterix: research support; Histogenics: research support; International Cartilage Repair Society: board or committee member; International Society of Arthroscopy, Knee Surgery, and Orthopaedic Sports Medicine: board or committee member; Minnesota Orthopedic Society: board or committee member; Musculoskeletal Transplantation Foundation: board or committee member; Vericel: paid consultant; Open Payments Database: General Payments from Arthrex Inc., Musculoskeletal Transplant Foundation, Ceterix, Orthopedics, Inc., Gemini Medical LLC, and Gemini Mountain Medical.
ORCID iDs: Christopher L. Camp  https://orcid.org/0000-0003-3058-7327
https://orcid.org/0000-0003-3058-7327
Aaron J. Krych  https://orcid.org/0000-0003-3248-8007
https://orcid.org/0000-0003-3248-8007
References
- 1. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-60. [DOI] [PubMed] [Google Scholar]
- 2. Mithoefer K, McAdams TR, Scopp JM, Mandelbaum BR. Emerging options for treatment of articular cartilage injury in the athlete. Clin Sports Med. 2009;28(1_suppl):25-40. [DOI] [PubMed] [Google Scholar]
- 3. Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64(3):460-6. [PubMed] [Google Scholar]
- 4. Camp CL, Stuart MJ, Krych AJ. Current concepts of articular cartilage restoration techniques in the knee. Sports Health. 2014;6(3):265-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hannon CP, Weber AE, Gitelis M, Meyer MA, Yanke AB, Cole BJ. Does treatment of the tibia matter in bipolar chondral defects of the knee? clinical outcomes with greater than 2 years follow-up. Arthroscopy. 2018;34(4):1044-51. [DOI] [PubMed] [Google Scholar]
- 6. Krych AJ, Harnly HW, Rodeo SA, Williams RJ, 3rd. Activity levels are higher after osteochondral autograft transfer mosaicplasty than after microfracture for articular cartilage defects of the knee: a retrospective comparative study. J Bone Joint Surg Am. 2012;94(11):971-8. [DOI] [PubMed] [Google Scholar]
- 7. Lynch TS, Patel RM, Benedick A, Amin NH, Jones MH, Miniaci A. Systematic review of autogenous osteochondral transplant outcomes. Arthroscopy. 2015;31(4):746-754. [DOI] [PubMed] [Google Scholar]
- 8. Bugbee W. Editorial Commentary: To treat or not to treat? Are we any closer to knowing what to do with cartilage lesions of the tibia? Arthroscopy. 2018;34(4):1052-3. [DOI] [PubMed] [Google Scholar]
- 9. Yabumoto H, Nakagawa Y, Mukai S, Saji T, Nakamura T. Surgical technique and clinical outcomes of retrograde osteochondral autograft transfer for osteochondral lesions of the tibial plateau. Arthroscopy. 2017;33(6):1241-7. [DOI] [PubMed] [Google Scholar]
- 10. Gracitelli GC, Tirico LE, McCauley JC, Pulido PA, Bugbee WD. Fresh osteochondral allograft transplantation for fractures of the knee. Cartilage. 2017;8(2):155-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shasha N, Krywulak S, Backstein D, Pressman A, Gross AE. Long-term follow-up of fresh tibial osteochondral allografts for failed tibial plateau fractures. J Bone Joint Surg Am. 2003;85-A(Suppl 2):33-39. [DOI] [PubMed] [Google Scholar]
- 12. Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14(11):1119-25. [DOI] [PubMed] [Google Scholar]
- 13. Franceschi F, Longo UG, Ruzzini L, Marinozzi A, Maffulli N, Denaro V. Simultaneous arthroscopic implantation of autologous chondrocytes and high tibial osteotomy for tibial chondral defects in the varus knee. Knee. 2008;15(4):309-13. [DOI] [PubMed] [Google Scholar]
- 14. Henderson IJ, Tuy B, Connell D, Oakes B, Hettwer WH. Prospective clinical study of autologous chondrocyte implantation and correlation with MRI at three and 12 months. J Bone Joint Surg Br. 2003;85(7):1060-6. [DOI] [PubMed] [Google Scholar]
- 15. Bakay A, Csönge L, Papp G, Fekete L. Osteochondral resurfacing of the knee joint with allograft. Clinical analysis of 33 cases. Int Orthop. 1998;22(5):277-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drexler M, Gross A, Dwyer T, Safir O, Backstein D, Chaudhry H, et al. Distal femoral varus osteotomy combined with tibial plateau fresh osteochondral allograft for post-traumatic osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2015;23(5):1317-23. [DOI] [PubMed] [Google Scholar]
- 17. Locht RC, Gross AE, Langer F. Late osteochondral allograft resurfacing for tibial plateau fractures. J Bone Joint Surg Am. 1984;66(3):328-35. [PubMed] [Google Scholar]
- 18. Gortz S, Bugbee WD. Allografts in articular cartilage repair. Instr Course Lect. 2007;56:469-80. [PubMed] [Google Scholar]
- 19. Hangody L, Dobos J, Baló E, Pánics G, Hangody LR, Berkes I. Clinical experiences with autologous osteochondral mosaicplasty in an athletic population: a 17-year prospective multicenter study. Am J Sports Med. 2010;38(6):1125-33. [DOI] [PubMed] [Google Scholar]
- 20. Ma HL, Hung SC, Wang ST, Chang MC, Chen TH. Osteochondral autografts transfer for post-traumatic osteochondral defect of the knee-2 to 5 years follow-up. Injury. 2004;35(12):1286-92. [DOI] [PubMed] [Google Scholar]
- 21. Ueblacker P, Burkart A, Imhoff AB. Retrograde cartilage transplantation on the proximal and distal tibia. Arthroscopy. 2004;20(1_suppl):73-8. [DOI] [PubMed] [Google Scholar]
- 22. Wajsfisz A, Makridis KG, Djian P. Arthroscopic retrograde osteochondral autograft transplantation for cartilage lesions of the tibial plateau: a prospective study. Am J Sports Med. 2013;41(2):411-5. [DOI] [PubMed] [Google Scholar]
- 23. Kraeutler MJ, Belk JW, Purcell JM, McCarty EC. Microfracture versus autologous chondrocyte implantation for articular cartilage lesions in the knee: a systematic review of 5-year outcomes. Am J Sports Med. 2018;46(4):995-9. [DOI] [PubMed] [Google Scholar]
- 24. Kon E, Filardo G, Venieri G, Perdisa F, Marcacci M. Tibial plateau lesions. Surface reconstruction with a biomimetic osteochondral scaffold: results at 2 years of follow-up. Injury. 2014;45(Suppl 6):S121-S125. [DOI] [PubMed] [Google Scholar]
- 25. Filardo G, Kon E, Di Martino A, Busacca M, Altadonna G, Marcacci M. Treatment of knee osteochondritis dissecans with a cell-free biomimetic osteochondral scaffold: clinical and imaging evaluation at 2-year follow-up. Am J Sports Med. 2013;41(8):1786-93. [DOI] [PubMed] [Google Scholar]
- 26. Perdisa F, Kon E, Sessa A, Andriolo L, Busacca M, Marcacci M, et al. Treatment of knee osteochondritis dissecans with a cell-free biomimetic osteochondral scaffold: clinical and imaging findings at midterm follow-up. Am J Sports Med. 2018;46(2):314-21. [DOI] [PubMed] [Google Scholar]
- 27. Ossendorf C, Steinwachs MR, Kreuz PC, Osterhoff G, Lahm A, Ducommun PP, et al. Autologous chondrocyte implantation (ACI) for the treatment of large and complex cartilage lesions of the knee. Sports Med Arthrosc Rehabil Ther Technol. 2011;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38(6):1117-24. [DOI] [PubMed] [Google Scholar]
- 29. Khan SN, Cammisa FP, Jr, Sandhu HS, Diwan AD, Girardi FP, Lane JM. The biology of bone grafting. J Am Acad Orthop Surg. 2005;13(1_suppl):77-86. [PubMed] [Google Scholar]
- 30. Cote MP, Lubowitz JH, Rossi MJ, Brand JC. Reviews pooling heterogeneous, low-evidence, high-bias data result in incorrect conclusions: but heterogeneity is an opportunity to explore. Arthroscopy. 2018;34(12):3126-8. [DOI] [PubMed] [Google Scholar]