Abstract
Objective
To compare radius of curvature (RoC) of distal femur osteochondral autograft transfer (OAT) donor sites from the intercondylar notch and trochlear ridge with recipient sites on the distal and posterior condyles and evaluate differences between recipient sites.
Design
Nineteen cadaveric femurs were scanned with a 3-dimensional high-resolution sensor. Donor regions included the lateral (LTR) and medial trochlear ridges (MTR), and the lateral (LICN) and medial intercondylar notch (MICN). Recipient regions analyzed were the distal medial (DMFC), posterior medial (PMFC), distal lateral (DLFC), and posterior lateral femur condyle (PLFC). Six-millimeter OAT grafts were simulated, and average RoC of all regions was compared using an analysis of variance. Post hoc testing was performed using Fisher’s least significant difference.
Results
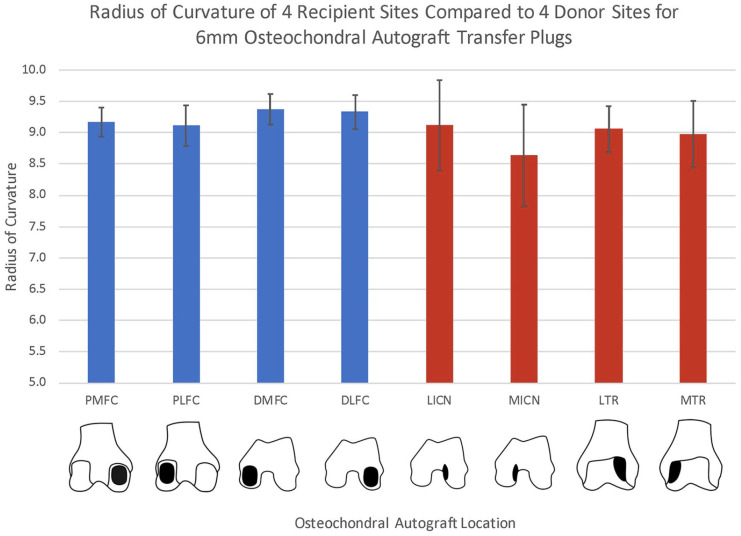
We found no significant differences in RoC of the LICN compared with all 4 recipient sites (P = 0.19, 0.97, 0.11, and 0.75 for DLFC, PLFC, DMFC, and PMFC, respectively) or the LTR and MTR to the posterior condyles (LTR vs. PLFC and PMFC; P = 0.72, 0.47, MTR vs. PLFC and PMFC P = 0.39, 0.22, respectively). Significant differences were found for RoC of the MICN compared with each recipient site (P < 0.001) and between distal and posterior femoral condyles (DLFC vs. PLFC, P = 0.016; DMFC vs. PMFC, P = 0.023).
Conclusion
The LICN is the ideal donor option for all recipient sites on the femoral condyles with respect to RoC of 6-mm OAT plugs. The MTR and LTR were acceptable donor sources for the posterior condyles, while the MICN was a poor match for all recipient sites. Additionally, the distal femur condyle and posterior femur condyle have different RoCs.
Keywords: osteochondral lesion, autograft, articular cartilage, knee
Introduction
Osteochondral lesions of the distal femur are potentially devastating injuries that are prevalent across a wide range of patient populations.1-5 Focal lesions at the articular surface of the knee have been shown to be as debilitating as end-stage osteoarthritis. 6 When injured, the hyaline cartilage of the articular surface has a very limited capacity to regenerate itself. Two broad surgical strategies exist to address articular cartilage lesions: repair or restoration. Reparative techniques include microfracture, subchondral drilling, and abrasion chondroplasty. These techniques aim to stimulate beneficial growth factors from the subchondral bone, resulting in fibrocartilaginous healing. 7 The incorporation of fibrocartilage, which is known to have a higher composition of type I cartilage, leads to inferior wear characteristics compared with the native hyaline cartilage.8-10 While there has been proven benefits to these reparative techniques, particularly in short-term outcomes, there remains concerns for residual pain and progression of chondral injury, which has resulted in the development of several other surgical techniques aimed at improving outcomes.11-15 Restorative cartilage techniques include autograft transfer, allograft transfer, and autologous chondrocyte implantation. These techniques aim to restore hyaline cartilage to articular lesions with minimal fibrocartilaginous healing.
Osteochondral autograft transfer (OAT) involves harvesting of a cylindrical plug from a lesser load-bearing portion of an articular surface and inserting it into a prepared area at the site of an articular defect. OAT has evolved into a viable treatment option for many osteochondral injuries. Benefits to the OAT procedure include a single surgical event with immediate access to native hyaline cartilage without risk of an immune response. Favorable results have been reported across a wide range of patient groups.16-22 Many consider OAT the standard for treating high grade articular lesions with surface area less than 2 cm2, particularly for high-demand patients; it has also been shown to be a useful option for some patients with lesions between 2 and 4 cm. 23 Despite its advantages, issues and challenges do arise. Size limitations on the amount of cartilage available exist due to concerns of donor site morbidity. Also, matching donor and recipient sites can be difficult and multifactorial as size, contact stresses, cartilage thickness, and curvature are all to be considered. When OAT donor grafts do not match the curvature of the recipient region, this can lead to significantly poorer clinical outcomes. 24
Previous authors have investigated matching of donor and recipient sites of the femoral condyles.25-28 Some studies show the medial trochlea or lateral trochlea to be the ideal donor site for weightbearing portions of the distal femur.25-28 These studies did not evaluate the medial intercondylar notch as a donor site. Additionally, while most studies focus on the distal weight bearing portion of the femur as a recipient site, the posterior femoral condyles can also be an area of chondral injury.
The purpose of this study was to compare radius of curvature (RoC) of distal femur OAT donor sites from the intercondylar notch and trochlear ridge with recipient sites on both the distal and posterior condyles. A secondary objective was to compare the RoC of the distal and posterior femoral condyles. We hypothesize that there will be no significant difference between the RoC of medial or lateral intercondylar notch RoC and recipient regions of the distal femur for 6-mm OAT autograft plugs. Furthermore, we also hypothesize that sites on the posterior femoral condyles would have a curvature different than those on the distal femoral condyles.
Methods
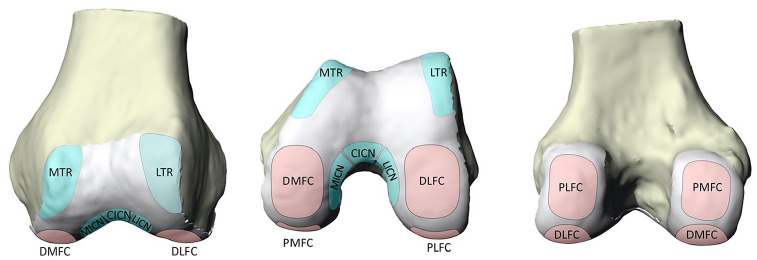
A determination of not human research was made by the institutional review board. The condylar region of 19 distal femurs were harvested from 13 cadavers provided by the institution’s biomechanics lab for analysis. Six were male femurs, and 13 were female. Specimens ranged from 52 to 67 years in age; average age was 62.1 ± 4.1 years. Specimens were grossly examined for visible cartilage defects and no cadavers were excluded. Donor and recipient regions for OAT were identified and marked on each femur based on previously described regions.25-28 Plastalina Modeling Clay (Van Aken International, Dalton, GA) bounded the segmented regions. The recipient articular surfaces were segmented into four regions: the distal medial femoral condyle (DMFC), distal lateral femoral condyles (DLFC), posterior medial femoral condyle (PMFC), and posterior lateral femoral condyles (PLFC). Four donor regions were also marked out: the lateral trochlear (LTR), medial trochlear ridges (MTR), lateral intercondylar notch (LICN), and medial intercondylar notch (MICN). Additionally, the central intercondylar notch was bounded. Fig. 1 illustrates our designated regions from the inferior aspect of a model femur, while Fig. 2 provides a 3-dimensional (3D) visual of our highlighted donor and recipient sites.
Figure 1.
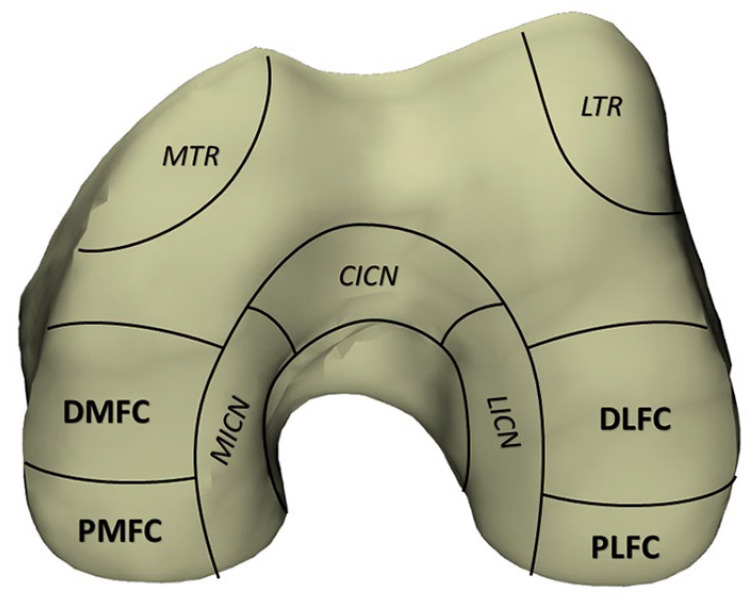
A right distal femur model which shows 4 donor locations (italics): the lateral trochlear ridge (LTR), medial trochlear ridge (MTR), lateral intercondylar notch (LICN), and medial intercondylar notch (MICN) and 4 recipient (bold) regions: the distal medial femoral condyle (DMFC), distal lateral femoral condyle (DLFC), posterior medial femoral condyle (PMFC), and posterior lateral femoral condyle (PLFC).
Figure 2.
A left distal femur model demonstrating 5 donor locations (blue): the lateral trochlear ridge (LTR), medial trochlear ridge (MTR), lateral intercondylar notch (LICN), and medial intercondylar notch (MICN) and 4 recipient (pink) regions: the distal medial femoral condyle (DMFC), distal lateral femoral condyle (DLFC), posterior medial femoral condyle (PMFC), and posterior lateral femoral condyle (PLFC). (A) Anterior view which best visualizes the trochlear donor regions. (B) Distal view which best visualizes the distal femoral condyles recipient regions and the intercondylar notch donor regions. (C) Posterior view which best visualizes the posterior condyles recipient regions.
A 3D snapshot sensor Gocator 3506 (LMI Technologies Inc., Burnaby, British Columbia, Canada) was configured to capture a field of view 27 × 45 mm with a resolution of 100 µm. An accuracy of 12 µm was utilized to scan these specimens. Zinc stearate (Weld-Aid Products, Detroit, MI) was applied to tissues to reduce glare and improve data capture. Fig. 3 demonstrates a cadaveric specimen and corresponding topography images captured from the 3D scanner.
Figure 3.
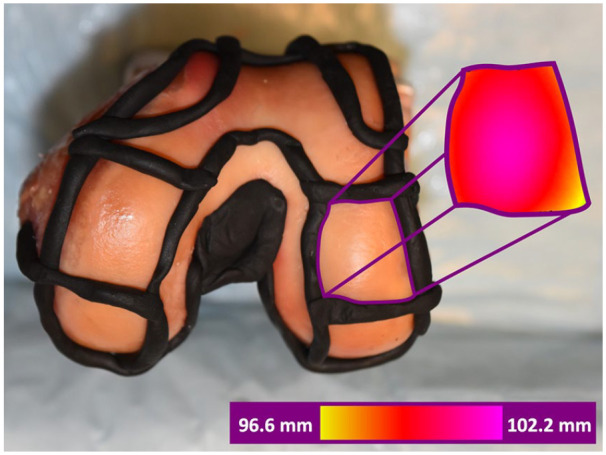
Top: Distal view of a cadaveric left distal femur with sectioned regions. Plastalina Modeling Clay (Van Aken International, Dalton, GA) bounds the segmented regions. Right colored section: Corresponding scanned images of the distal lateral femur condyle captured with the Gocator scanner. Coloring denotes the distance between the scanner camera and the articular surface.
The resultant clouds of data points obtained from the 3D scanner were exported to Rhinoceros 3D software (Robert McNeel & Associates, Seattle, WA) and analyzed using a custom script. The direction perpendicular to the plane tangential to the surface of the femur was used as the direction for simulated graft harvesting. A honeycomb pattern of cylinders was generated on the digital 3D cartilage surface, simulating the harvesting of 6-mm grafts. The honeycomb pattern of potential grafts randomly created juxtaposed circles to ensure there was no overlap of plugs (see Fig. 4 with blue honeycomb pattern).
Figure 4.
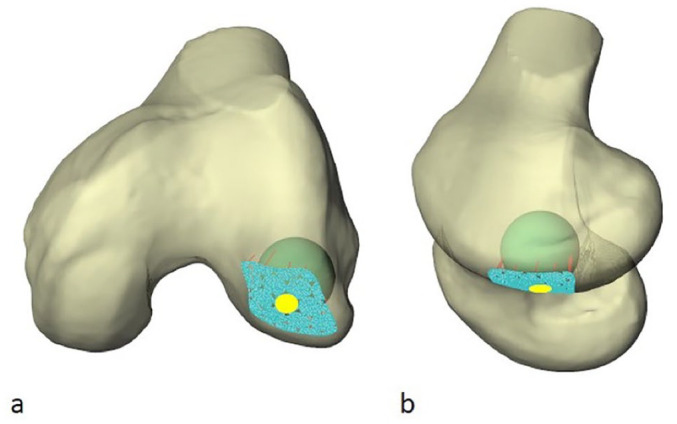
Two views (a, semiaxial; b, semisagittal) of a left distal femur illustrating a “best fit” radius of curvature (RoC) sphere within the distal lateral femoral condyle. The honeycomb pattern of juxtaposed circles is formed by blue nodes. The contacted articular surface highlighted in yellow represents one simulated graft recipient site. The peripheral partial blue circles were excluded.
From this point, a best fit sphere was generated for each randomly selected point of interest (simulated donor or recipient sites). The calculation of the best fit sphere was obtained with the summed square errors calculation with the fit sphere in the direction perpendicular to the articular surface ( Fig. 4 ). The software provided a measurement of curvature, number of points used in the generation, coefficient of determination (R2), and the digital cartilage surface. The coefficient of determination (R2) calculated for each OAT indicates how closely the surface of the OAT can be described as a spherical surface. A threshold of R2 ≥ 0.6 was established for inclusion of individual harvest sites. The sites below this criterion were not suitable for characterization of the surface. To eliminate partial plugs on peripheral region borders and preserve the clinically relevant application of the grafts, all harvest sites with fewer than 800 nodes (0.10 mm nodal mesh spacing) used in determination of the best-fit sphere were eliminated. After the data were filtered based on the coefficient of determination for the best fit sphere and nodal criteria, the mean RoC was calculated for each donor/recipient region. While some previous studies have compared the entire RoC of a region, the average RoC of multiple 6-mm potential plugs was utilized as this was more applicable to arthroscopic OAT mosaicplasty.29,30
Statistics
The RoC was compared for each of the donor and recipient locations using an analysis of variance (ANOVA) test. Post hoc testing was performed to determine specific differences using Fisher’s least significant difference (LSD). Each region was matched and compared with regions on the same knee. Quantitative RoC difference as well as statistical significance of the difference was determined. Significance was defined as P < 0.05. An a priori power analysis utilizing data from a previously published study comparing curvature of OAT locations determined that there were at least 11 samples needed in each group to yield a 1 – β of 0.80. 25 Post hoc power analysis was performed using previously published formulas. 31 All statistical analyses were completed with IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, NY).
Results
After analyzing 5 donor and 4 recipient regions of 19 cadaveric femurs, a total of 1,897 OAT grafts met inclusion criteria. RoC was evaluated for a total of 641 donor grafts and 1,256 recipient grafts. The average number of grafts per region can be seen in Table 1 . There was a significant difference in the number of grafts analyzed among the donor and recipient sites (P < 0.001). Post hoc power analysis comparing RoC for different locations for the 6 mm plug revealed a 1 – β of 0.90.
Table 1.
Mean Number of Donor and Recipient Grafts per Region for Osteochondral Autograft Transfer of the Distal Femur.
| No. of Grafts, Mean ± SD | |
|---|---|
| Recipient region | |
| Distal medial FC | 15.5 ± 2.6 |
| Distal lateral FC | 14.2 ± 3.7 |
| Posterior medial FC | 17.4 ± 4.3 |
| Posterior lateral FC | 18.9 ± 5.3 |
| Donor region | |
| Lateral trochlear ridge | 10.8 ± 3.6 |
| Medial trochlear ridge | 7.4 ± 3.4 |
| Lateral IC notch | 5.4 ± 3.3 |
| Medial IC notch | 6.0 ± 3.3 |
| Central IC notch | 4.1 ± 3.1 |
FC, femoral condyle; IC, intercondylar.
Average RoC and average coefficient of determination (R2) for the donor and recipient sites were determined (see Table 2 ). The average RoC of the central intercondylar notch (CIN) was −9.51 ± 0.77. The RoC of the CIN donor site was significantly different than all 4 recipient sites (P < 0.001). Because of the concavity of the CIN, it was excluded from further comparisons. We also analyzed the amount of variance in each region, with respect to RoC, for both donor and recipient sites. We found no significant difference in the variance within regions when comparing donor regions (P = 0.08) and recipient regions (P = 0.53, see Table 3 ).
Table 2.
Mean Radius of Curvature (RoC) and Coefficient of Determination (R2) of Donor and Recipient Regions for Osteochondral Autograft Transfer of the Distal Femur.
| RoC, mm, Mean [range] ± SD | R2, Mean ± SD | |
|---|---|---|
| Recipient region | ||
| Distal medial FC | 9.37 [5.31-10.89] ± 0.24 | 0.94 ± 0.07 |
| Distal lateral FC | 9.33 [5.48-10.51] ± 0.27 | 0.93 ± 0.06 |
| Posterior medial FC | 9.17 [6.60-10.77] ± 0.23 | 0.95 ± 0.05 |
| Posterior lateral FC | 9.11 [6.34-11.13] ± 0.33 | 0.95 ± 0.06 |
| Donor region | ||
| Lateral trochlear ridge | 9.05 [6.31-11.28] ± 0.37 | 0.92 ± 0.08 |
| Medial trochlear ridge | 8.98 [6.04-12.02] ± 0.53 | 0.91 ± 0.08 |
| Lateral IC notch | 9.12 [5.10-10.57] ± 0.72 | 0.94 ± 0.06 |
| Medial IC notch | 8.64 [5.21-10.43] ± 0.81 | 0.91 ± 0.08 |
FC, femoral condyle; IC, intercondylar.
Table 3.
Average Variance of Radius of Curvature Within Each Donor and Recipient Regions for Osteochondral Autograft Transfer of the Distal Femur.
| Variance | |
|---|---|
| Recipient region a | |
| Distal medial FC | 0.63 |
| Distal lateral FC | 0.53 |
| Posterior medial FC | 0.53 |
| Posterior lateral FC | 0.68 |
| Donor region b | |
| Lateral trochlear ridge | 0.47 |
| Medial trochlear ridge | 1.36 |
| Lateral IC notch | 0.66 |
| Medial IC notch | 1.02 |
FC, femoral condyle; IC, intercondylar.
P = 0.53.
P = 0.08.
The average RoC difference or mismatch, in mm, between each donor and recipient site is displayed in Table 4 . This was calculated by averaging the difference in RoC for each matched comparison between regions.
Table 4.
Comparison of Distal Femur RoC Mismatch Between Each Donor and Recipient Region Using 6-mm Osteochondral Autograft Transfer Grafts. a
| Respective Average RoC Difference, mm | ||||
|---|---|---|---|---|
| Recipient Regions | ||||
| Distal Medial FC | Distal Lateral FC | Posterior Medial FC | Posterior Lateral FC | |
| Donor regions | ||||
| Lateral trochlear ridge | 0.32 b | 0.28 | 0.12 | 0.06 |
| Medial trochlear ridge | 0.39 b | 0.35 b | 0.19 | 0.13 |
| Lateral IC notch | 0.25 | 0.21 | 0.07 | 0.01 |
| Medial IC notch | 0.73 b | 0.69 b | 0.53 b | 0.47 b |
RoC, radius of curvature; FC, femoral condyle; IC, intercondylar.
Boldfaced values identify the areas of best match.
Statistical significance (P < 0.05).
We found no significant difference for RoC of the LICN compared to all 4 recipient sites (P = 0.19, 0.97, 0.11, and 0.75, for DLFC, PLFC, DMFC, and PMFC, respectively). There was also no difference between the LTR and the PLFC and PMFC (P = 0.72 and 0.47, respectively) or the MTR and the PLFC and PMFC (P = 0.39 and 0.22, respectively). A significant difference was found between the MTR and both the DMFC and DLFC (P = 0.013, 0.027). The MICN had a significantly different RoC than each recipient site (P < 0.001) ( Fig. 5 ). When comparing only recipient sites, there was a difference in RoC between the DLFC and PLFC (P = 0.016). Significant differences also existed in the comparison of the DMFC to the PLFC and PMFC (P = 0.004 and 0.023, respectively). Significant differences were not found in comparison of the DLFC with the DMFC or PMFC (P = 0.61 and 0.07, respectively).
Figure 5.
Bar graph with average radius of curvature (RoC) of 4 recipient locations (blue) and 4 donor locations (red) for 6-mm diameter osteochondral autograft transfers. Donor regions included the lateral trochlear ridge (LTR) and medial trochlear ridge (MTR), as well as the lateral intercondylar notch (LICN) and medial intercondylar notch (MICN). Recipient regions analyzed were the distal medial (DMFC), posterior medial (PMFC), distal lateral (DLFC), and posterior lateral femur condyle (PLFC).
Discussion
The current study reports that the LICN is an acceptable donor source for all recipient locations based on RoC. The MTR and LTR were acceptable donor sources for the posterior condyles while the MICN was a poor match for all recipient sites. The topic of curvature matching has previously been investigated, and helpful insight has been provided.25-28 However, previous studies have evaluated only the trochlear ridges as a donor site or only the distal or anterior femoral condyles as a recipient site. 28 These previous studies have not evaluated all potential donor and recipient site combinations.25-28 Additionally, some of these previous studies evaluated RoC in a 2D manner either in the coronal or sagittal plane. 27 Our study compared each possible autograft donor site to all portions of the weightbearing surface of the distal femur using 3D analyzation.
Three-dimensional RoC allograft matching techniques in the coronal and sagittal planes have been shown to successfully restore native articular surface dimensions and produce a greater number of compatible donor sites than linear techniques. 32 In our study, a high-resolution 3D scanner and advanced software was used to obtain precise data for comparison. This provided a 3D model to obtain a RoC based on a sphere of best fit instead of a 2D circle. The resolution of our 3D scans was found to be 0.10 mm with a small standard deviation demonstrating the precision of our measurements. We believe that the 3D nature of our study provides a more complete analysis of all available donor and recipient combinations, compared to a 2D analysis only in the sagittal or coronal plane. The advanced imaging and software used delivers a thorough evaluation of RoC to supplement the current literature on autograft matching.
In addition to focal anatomy, procedural technique can also affect the congruency of the restored articular surface. A small amount of recession or prominence in an autograft plug leads to significant alterations in contact pressures at the grafted site. It has been reported that there is a 21% to 57% increase in contact stress, with grafts that were only 1 mm proud.33,34 Countersinking grafts may be more forgiving in terms of effecting contact pressures, but grafts sunk as little as 2 mm can lead to necrosis and fibrocartilage overgrowth.34,35
The above discussion demonstrates the importance of restoring articular surface congruity to minimize alterations in load distribution about the grafted areas. While there are many donor site characteristics to consider, we believe that the importance of curvature matching should certainly not be overlooked. Theoretically, grafts with significantly more curvature than the surrounding cartilage may have an effect similar to a graft that was left excessively proud. Mismatched graft curvature can produce at least a moderate increase in peak contact stresses about grafted site and has been shown to be associated with worse clinical outcomes.24,36
Radius of curvature for the distal femur of both donor and recipient locations has been reported. Ahmed et al. 26 reported the lateral trochlea to have a curvature of 77.1 m−1 or an RoC of 13 mm. While in that study, the LICN was not evaluated, the RoC for the LTR is similar to our measured values. Additionally, their study demonstrated the LTR RoC poorly matched the distal medial and lateral femur condyle RoC, which is comparable to our findings. Another study by Hohe et al. 37 reported the trochlear average maximum curvature to be 85.5 m−1 or a RoC of 11.7 mm. These RoC values are similar to our MTR and LTR donor regions where the trochlear curvature is highest. While these studies reported the curvature of regions, other studies have compared regions without specifically reporting the curvature or RoC.25,27,28
Bartz et al. 25 used a laser scanner and custom software to analyze 7 cadaveric femurs and assessed autografts of 4, 6, and 8 mm in size. They found that the inferior portions of the medial and lateral patellar groove restored the natural curvature of the femoral condyles well when using all 3 sizes. Their study also found the lateral intercondylar notch restored curvature well when using 4- and 6-mm grafts. This is comparable to our study, which found the LICN matched the distal femur and posterior condyles well, for smaller graft sizes. Similar to our study, Bartz et al. 25 found the central intercondylar notch to have a concave topography, making it a poor donor choice for femur condyle OAT.
Terukina et al. 27 also contributed to the topic with their cadaveric study, which examined 8 femoral condyles. They reported that the anterior portion of the lateral trochlea provided the best match for the femoral condyles, but likewise there was not distinction between different regions of the femoral condyle recipient sites. Additionally, this analysis was done in 2 dimensions, examining distal femur specimens that were sectioned only in the sagittal plane.
Our study expanded on these findings by considering the weightbearing femoral condyles as 2 separate regions, distal and posterior. A previous study, while not evaluating the intercondylar notch as a donor site, did analyze OAT matching with grafts harvested from the trochlea and transferred to both the anterior and posterior condyles separately. 28 The authors simulated 6-, 8-, and 10-mm grafts, comparing curvature of donor sites from various regions of the trochlear groove to various regions of the femoral condyles. Eleven cadaveric knees and a 3D laser scanner were utilized in this study which used a “vertical interval” as a quantitative measurement of surface curvature. The results showed that peripheral areas of the trochlea best matched the posterior portion of the condyles. They found grafts from the more central portion of the trochlea better matched the curvature of the distal or anterior aspects of the condyles. Our study only examined the peripheral areas of the trochlea as these are the typical areas where OAT plugs are harvested. Our results are comparable to the study by Nishizawa et al. 28 in that they also demonstrated the peripheral trochlea 6 mm OAT plugs matched the posterior condyles more closely.
Appropriate graft selection is believed to improve patient outcomes following OAT procedure and most agree that achieving anatomic restoration of the articular surface is of significant importance.33,38,39 Attaining restoration requires consideration of many factors to include donor site morbidity, available surface area, and articular cartilage thickness. Donor site morbidity can be minimized by avoiding harvest in highly stressed areas. While some areas, such as the medial trochlea, have been shown to undergo less contact stress,26,38 other investigators have suggested that no potential donor area is completely non-load-bearing. 39 While our study found the medial trochlea and lateral intercondylar notch were excellent donor sites with regard to RoC, there are other factors to consider. One potential drawback of the medial trochlea is a considerably smaller area than its lateral counterpart, limiting available graft harvest. 38 It should also be noted that the lateral intercondylar notch was described by Thaunat et al. 40 as having the thinnest articular cartilage when compared with all other potential donor sites within the trochlea and intercondylar notch. However, other studies have determined that the intercondylar notch has similar cartilage thickness to other donor regions.26,41
Curvature is but one donor site characteristic which must be considered for optimal outcomes. Prior articular injury, unexpected characteristic of chondral lesions, or new exam findings while under anesthesia may lead to changes in the operative plan. With many variables to consider on a patient-by-patient or lesion-by-lesion basis, it is imperative to have multiple options available for harvest when entering the operating room. Though prior evidence has suggested that the peripheral portions of trochlear groove are the ideal donor sites to restore curvature with distal femoral articular lesions, we have demonstrated that the lateral intercondylar notch can also provide a very accurate restoration of curvature, particularly when smaller grafts are needed.
Our study is not without limitations. Most OAT procedures are performed on young and active patients, but the average age of our specimens was 62.1 years. It is possible that there are age-related changes in the contour of the knee’s articular surface. The authors accounted for this through gross examination of the specimens. There were also limitations involving the software we used, which randomized simulated graft locations within each region of the distal femur. Though we excluded grafts which contained less than 800 nodes, there were still several grafts which did not fall completely within the boundaries of each region. While we could have manually selected each simulated graft, we felt it was important to randomize this process to get a true average RoC. Additionally, we compared the average RoC of each donor or recipient region for each knee. There may be differences in the RoC of each subregion within each region. These subregions should be specifically analyzed in future studies. There have also been conflicting descriptions of cartilage thickness in various regions.26,40,41 Our study could have evaluated the cartilage thickness in each region but was limited to a topographic analysis only. Last, we did not include the central trochlea involved in the patellofemoral joint. Our study could be expanded to evaluate this region as well as the central portion of the intercondylar notch as their concavity may allow for close curvature matching of lesions in this region.
In conclusion, the LICN is the ideal donor option for all recipient sites on the femoral condyles with respect to RoC of 6-mm OAT plugs. The MTR and LTR were acceptable donor sources for the posterior condyles, while the MICN was a poor match for all recipient sites. Additionally, the distal femur condyle and posterior femur condyle have a different RoC.
Footnotes
Author’s Note: This study was presented at the 2019 ICRS World Congress.
Acknowledgments and Funding: The authors would like to thank Alan Ogden for his contribution preparing and analyzing our specimens. We would also like to thank Shelia Rogers for her help organizing and processing our data and Anna Hoefler for her assistance with manuscript preparation. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was funded by the LSU Health Biomechanics Lab.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Not applicable. A determination of not human research was made by the institutional review board.
References
- 1. Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18(7):730-4. doi: 10.1053/jars.2002.32839 [DOI] [PubMed] [Google Scholar]
- 2. Flanigan DC, Harris JD, Trinh TQ, Siston RA, Brophy RH. Prevalence of chondral defects in athletes’ knees: a systematic review. Med Sci Sports Exerc. 2010;42(10):1795-801. doi: 10.1249/MSS.0b013e3181d9eea0 [DOI] [PubMed] [Google Scholar]
- 3. Andrade R, Vasta S, Papalia R, Pereira H, Oliveira JM, Reis RL, et al. Prevalence of articular cartilage lesions and surgical clinical outcomes in football (soccer) players’ knees: a systematic review. Arthroscopy. 2016;32(7):1466-77. doi: 10.1016/j.arthro.2016.01.055 [DOI] [PubMed] [Google Scholar]
- 4. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13:456-60. [DOI] [PubMed] [Google Scholar]
- 5. Åroøen A, Løken S, Heir S, Alvik E, Ekeland A, Granlund OG, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32(1_suppl):211-5. doi: 10.1177/0363546503259345 [DOI] [PubMed] [Google Scholar]
- 6. Heir S, Nerhus TK, Røtterud JH, Løken S, Ekeland A, Engebretsen L, et al. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am J Sports Med. 2010;38(2):231-7. doi: 10.1177/0363546509352157 [DOI] [PubMed] [Google Scholar]
- 7. Freedman KB, Nho SJ, Cole BJ. Marrow stimulating technique to augment meniscus repair. Arthroscopy. 2003;19(7):794-8. doi: 10.1016/S0749-8063(03)00695-9 [DOI] [PubMed] [Google Scholar]
- 8. Nehrer S, Spector M, Minas T. Histologic analysis of tissue after failed cartilage repair procedures. Clin Orthop Relat Res. 1999;(365):149-62. doi: 10.1097/00003086-199908000-00020 [DOI] [PubMed] [Google Scholar]
- 9. Coletti JM, Jr, Akeson WH, Woo SL. A comparison of the physical behavior of normal articular cartilage and the arthroplasty surface. J Bone Joint Surg Am. 1972;54(1_suppl):147-60. [PubMed] [Google Scholar]
- 10. Lane JM, Brighton CT, Ottens HR, Lipton M. Joint resurfacing in the rabbit using an autologous osteochondral graft. A biomedical and metabolic study of cartilage viability. J Bone Joint Surg Am. 1977;59(2):218-22. doi: 10.2106/00004623-197759020-00015 [DOI] [PubMed] [Google Scholar]
- 11. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-84. doi: 10.1053/jars.2003.50112 [DOI] [PubMed] [Google Scholar]
- 12. Welton KL, Logterman S, Bartley JH, Vidal AF, McCarty EC. Knee cartilage repair and restoration: common problems and solutions. Clin Sports Med. 2018;37(2):307-30. doi: 10.1016/j.csm.2017.12.008 [DOI] [PubMed] [Google Scholar]
- 13. Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14(11):1119-25. doi: 10.1016/j.joca.2006.05.003 [DOI] [PubMed] [Google Scholar]
- 14. Mithoefer K, Mcadams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053-63. doi: 10.1177/0363546508328414 [DOI] [PubMed] [Google Scholar]
- 15. Gudas R, Gudaite A, Mickevičius T, Masiulis N, Simonaitytė R, Cekanauskas E, et al. Comparison of osteochondral autologous transplantation, microfracture, or debridement techniques in articular cartilage lesions associated with anterior cruciate ligament injury: a prospective study with a 3-year follow-up. Arthroscopy. 2013;29(1_suppl):89-97. doi: 10.1016/j.arthro.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 16. Gudas R, Gudaite A, Pocius A, Gudiene A, Cekanauskas E, Monastyreckiene E, et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40(11):2499-508. doi: 10.1177/0363546512458763 [DOI] [PubMed] [Google Scholar]
- 17. Gudas R, Kalesinskas RJ, Kimtys V, Stankevic̆ius E, Tolius̆is V, Bernotavic̆ius G, et al. A prospective randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint in young athletes. Arthroscopy. 2005;21:1066-75. [DOI] [PubMed] [Google Scholar]
- 18. Harris JD, Brophy RH, Siston RA, Flanigan DC. Treatment of chondral defects in the athlete’s knee. Arthroscopy. 2010;26(6):841-52. doi: 10.1016/j.arthro.2009.12.030 [DOI] [PubMed] [Google Scholar]
- 19. Hangody L, Dobos J, Balo E, Panics G, Hangody LR, Berkes I. Clinical experiences with autologous osteochondral mosaicplasty in an athletic population: a 17-year prospective multicenter study. Am J Sports Med. 2010;38(6):1125-33. doi: 10.1177/0363546509360405 [DOI] [PubMed] [Google Scholar]
- 20. Muller S, Breederveld RS, Tuinebreijer WE. Results of osteochondral autologous transplantation in the knee. Open Orthop J. 2010;4(2):111-4. doi: 10.2174/1874325001004020111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oztürk A, Ozdemir MR, Ozkan Y. Osteochondral autografting (mosaicplasty) in grade IV cartilage defects in the knee joint: 2- to 7-year results. Int Orthop. 2006;30(3):200-4. doi: 10.1007/s00264-005-0068-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pareek A, Reardon PJ, Maak TG, Levy BA, Stuart MJ, Krych AJ. Long-term outcomes after osteochondral autograft transfer: a systematic review at mean follow-up of 10.2 years. Arthroscopy. 2016;32(6):1174-84. doi: 10.1016/j.arthro.2015.11.037 [DOI] [PubMed] [Google Scholar]
- 23. Richter DL, Schenck RC, Jr, Wascher DC, Treme G. Knee articular cartilage repair and restoration techniques: a review of the literature. Sports Health. 2016;8(2):153-60. doi: 10.1177/1941738115611350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gursoy S, Simsek ME, Akkaya M, Kaya O, Bozkurt M. Local curvature mismatch may worsen the midterm functional outcomes of osteochondral allograft transplantation. Knee Surg Sport Traumatol Arthrosc. Epub 2020. October 12. doi: 10.1007/s00167-020-06319-4 [DOI] [PubMed] [Google Scholar]
- 25. Bartz RL, Kamaric E, Noble PC, Lintner D, Bocell J. Topographic matching of selected donor and recipient sites for osteochondral autografting of the articular surface of the femoral condyles. Am J Sports Med. 2001;29(2):207-12. doi: 10.1177/03635465010290021501 [DOI] [PubMed] [Google Scholar]
- 26. Ahmad CS, Cohen ZA, Levine WN, Ateshian GA, Mow VC. Biomechanical and topographic considerations for autologous osteochondral grafting in the knee. Am J Sports Med. 2001;29(2):201-6. doi: 10.1177/03635465010290021401 [DOI] [PubMed] [Google Scholar]
- 27. Terukina M, Fujioka H, Yoshiya S, Kurosaka M, Makino T, Matsui N, et al. Analysis of the thickness and curvature of articular cartilage of the femoral condyle. Arthroscopy. 2003;19(9):969-73. doi: 10.1016/j.arthro.2003.09.006 [DOI] [PubMed] [Google Scholar]
- 28. Nishizawa Y, Matsumoto T, Araki D, Nagamune K, Matsushita T, Kurosaka M, et al. Matching articular surfaces of selected donor and recipient sites for cylindrical osteochondral grafts of the femur: quantitative evaluation using a 3-dimensional laser scanner. Am J Sports Med. 2014;42(3):658-64. doi: 10.1177/0363546513518005 [DOI] [PubMed] [Google Scholar]
- 29. Du PZ, Markolf KL, Levine BD, McAllister DR, Jones KJ. Differences in the radius of curvature between femoral condyles: implications for osteochondral allograft matching. J Bone Joint Surg Am. 2018;100(15):1326-31. doi: 10.2106/JBJS.17.01509 [DOI] [PubMed] [Google Scholar]
- 30. Yanke AB, Urita A, Shin JJ, Cvetanovich GL, Moran EK, Bach BR, Jr, et al. Topographic analysis of the distal femoral condyle articular cartilage surface: adequacy of the graft from opposite condyles of the same or different size for the osteochondral allograft transplantation. Cartilage. 2019;10(2):205-13. doi: 10.1177/1947603517752056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rosner B. Fundamentals of Biostatistics. 7th ed. Brooks/Cole Cengage Learning; 2011. [Google Scholar]
- 32. Bernstein DT, O’Neill CA, Kim RS, Jones HL, Noble PC, Harris JC, et al. Osteochondral allograft donor-host matching by the femoral condyle radius of curvature. Am J Sports Med. 2017;45(2):403-9. doi: 10.1177/0363546516671519 [DOI] [PubMed] [Google Scholar]
- 33. Harris JD, Solak KK, Siston RA, Litsky A, Richards J, Flanigan DC. Contact pressure comparison of proud osteochondral autograft plugs versus proud synthetic plugs. Orthopedics. 2011;34(2):97. doi: 10.3928/01477447-20101221-06 [DOI] [PubMed] [Google Scholar]
- 34. Koh JL, Wirsing K, Lautenschlager E, Zhang LO. The effect of graft height mismatch on contact pressure following osteochondral grafting: a biomechanical study. Am J Sports Med. 2004;32(2):317-20. doi: 10.1177/0363546503261730 [DOI] [PubMed] [Google Scholar]
- 35. Huang FS, Simonian PT, Norman AG, Clark JM. Effects of small incongruities in a sheep model of osteochondral autografting. Am J Sports Med. 2004;32(8):1842-8. doi: 10.1177/0363546504264895 [DOI] [PubMed] [Google Scholar]
- 36. D’Lima DD, Chen PC, Colwell CW., Jr. Osteochondral grafting: effect of graft alignment, material properties, and articular geometry. Open Orthop J. 2009;3(1_suppl):61-8. doi: 10.2174/1874325000903010061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hohe J, Ateshian G, Reiser M, Englmeier KH, Eckstein F. Surface size, curvature analysis, and assessment of knee joint incongruity with MRI in vivo. Magn Reson Med. 2002;47(3):554-61. doi: 10.1002/mrm.10097 [DOI] [PubMed] [Google Scholar]
- 38. Garretson RB, 3rd, Katolik LI, Verma N, Beck PR, Bach BR, Cole BJ. Contact pressure at osteochondral donor sites in the patellofemoral joint. Am J Sports Med. 2004;32(4):967-74. doi: 10.1177/0363546503261706 [DOI] [PubMed] [Google Scholar]
- 39. Simonian PT, Sussmann PS, Wickiewicz TL, Paletta GA, Warren RF. Contact pressures at osteochondral donor sites in the knee. Am J Sports Med. 1998;26(4):491-4. doi: 10.1177/03635465980260040201 [DOI] [PubMed] [Google Scholar]
- 40. Thaunat M, Sophie C, Lunn J, Charrois O, Fallet L, Beaufils P. Cartilage thickness matching of selected donor and recipient sites for osteochondral autografting of the medial femoral condyle. Knee Surg Sports Traumatol Arthrosc. 2007;15(4):381-6. doi: 10.1007/s00167-006-0222-7 [DOI] [PubMed] [Google Scholar]
- 41. Schub DL, Frisch NC, Bachmann KR, Winalski C, Saluan PM. Mapping of cartilage depth in the knee and elbow for use in osteochondral autograft procedures. Am J Sports Med. 2013;41(4):903-7. doi: 10.1177/0363546513475343 [DOI] [PubMed] [Google Scholar]