Abstract
Objective
The aim of this systematic review was to analyze the evidence about the efficacy of the several synovial fluid (SF) biomarkers proposed for knee osteoarthritis (OA), categorizing them by both molecular characteristics and clinical use according to the BIPEDs criteria, to provide a comprehensive and structured overview of the current literature.
Design
A systematic review was performed in May 2020 on PubMed, Cochrane Library, and Embase databases about SF biomarkers in patients with knee OA. The search was limited to articles in the last 20 years on human studies, involving patients with knee OA, reporting SF biomarkers. The evidence for each selected SF biomarker was quantified according to the 6 categories of BIPEDs classification.
Results
A total of 159 articles were included in the qualitative data synthesis and 201 different SF biomarkers were identified. Among these, several were investigated multiple times in different articles, for a total of 373 analyses. The studies included 13,557 patients with knee OA. The most promising SF biomarkers were C4S, IL-6, IL-8, Leptin, MMP-1/3, TIMP-1, TNF-α, and VEGF. The “burden of disease” and “diagnostic” categories were the most represented with 132 and 106 different biomarkers, respectively.
Conclusions
The systematic review identified numerous SF biomarkers. However, despite the high number of studies on the plethora of identified molecules, the evidence about the efficacy of each biomarker is supported by limited and often conflicting findings. Further research efforts are needed to improve the understanding of SF biomarkers for a better management of patients with knee OA.
Keywords: biomarker, synovial fluid, knee, osteoarthritis, cartilage
Introduction
Osteoarthritis (OA) is a degenerative joint disease characterized by progressive deterioration and loss of articular cartilage with concomitant structural and functional changes in the entire joint, including synovium, meniscus (in the knee), periarticular ligaments, and subchondral bone. 1 Clinical features of OA are mostly determined by signs and symptoms of inflammation including pain, effusions and secondarily resulting in stiffness and loss of mobility, often associated with significant functional impairment. 2 The diagnosis of OA is currently based on clinical symptoms and radiographic criteria (e.g., joint space narrowing, osteophytes, subchondral sclerosis). 3 However, these parameters are difficult to detect at early stages of the disease and most often are recognized only in the advanced stages; thus, the diagnosis of OA is often delayed, when joint tissue destruction is irreversible and conservative treatments are less effective. 4 Moreover, monitoring the progression, as well as therapeutic management of OA, are also complex. Patients are followed by radiographs and magnetic resonance to evaluate the imaging progression of joint degeneration, and there is no examination that can predict the effects and/or the benefits of the current treatments used for OA, such as exercise, nonsteroidal anti-inflammatory drugs (NSAIDs), or injective therapies with corticosteroids, hyaluronic acid, or platelet-rich plasma.5,6 Considering these limitations, alternative parameters, that is, biomarkers, that may be more sensitive in early stages of OA have been pursued as diagnostic tools and to evaluate disease progression. 7
A biomarker is defined as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention,” 8 and it can be represented by radiographic, histologic, physiologic, or molecular characteristics. 9 In particular, molecular biomarkers are widely studied in knee OA, as their values could reflect dynamic and quantitative changes in joint remodeling and therefore disease progression. The BIPEDs classification has been introduced to identify key parameters for the evaluation and qualification of the utility of these biomarkers, aimed at providing a common language and structure to communicate knowledge and advances related to biomarkers for both clinical and research applications. 10 Accordingly, biomarkers in knee OA can be extracted from serum, urine, or synovial fluid (SF) samples, and significant research efforts have been spent to identify the most suitable molecules, though often controversial findings on their efficacy have been reported among the different studies in the literature. 11 In this light, while serum and urine parameters are prone to systemic influences that hinder the identification of local changes, measuring biomarkers in SF of the knee could provide more information about the condition of the diseased joint, being a direct representation of the changes in the intraarticular environment. Moreover, SF biomarkers are in higher concentrations than in blood or urine, thus able to prove higher sensitivity and specificity. 12 For this reason, evidence on several SF biomarkers is rapidly increasing, with many recent publications aimed either at confirming the potential of previous preliminary findings or identifying new molecules as suitable knee OA biomarkers.
The aim of this systematic review was to analyze the evidence about the efficacy of the several SF biomarkers proposed for knee OA, categorizing them according to both molecular characteristics and clinical use by applying the BIPEDs criteria, in order to provide a comprehensive and structured overview of the current evidence about SF biomarkers in knee OA.
Methods
Search Strategy and Screening Criteria
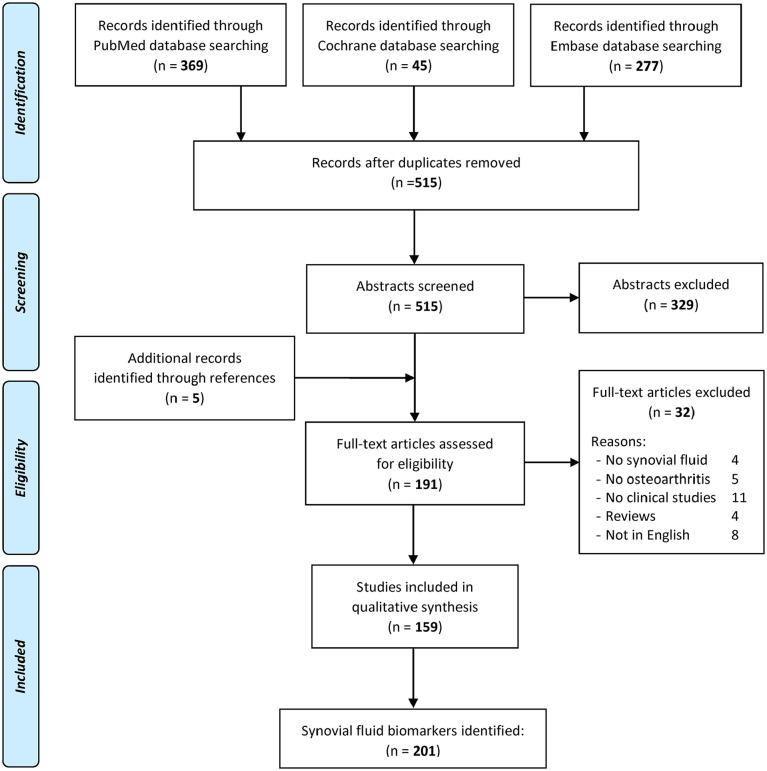
A systematic review was performed on the literature about SF biomarkers in patients with knee OA. The search was conducted on PubMed, Cochrane Library, and Embase databases on May 18, 2020, by using the following parameters: “synovial fluid AND biomarker AND knee AND osteoarthritis.” Literature search was limited to the last 20 years (2000-2020). The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines were used; 13 a flowchart of the study selection for qualitative data synthesis is reported in Figure 1 . The screening process was conducted separately by 2 independent observers (AB and GM). In the first step, the articles were screened by title and abstract. The following inclusion criteria for relevant articles were used during the initial screening of titles and abstracts: human studies, involving patients with knee OA, reporting SF biomarkers, written in English language. Exclusion criteria were articles written in other languages, preclinical studies, biomarkers from other samples (e.g., serum, urine), studies reporting other diseases and other joints without evaluating knee OA patients, and reviews. In the second step, the full texts of the selected papers were screened according to the previously mentioned inclusion/exclusion criteria. Moreover, the reference lists from the selected papers and from the systematic reviews found during the screening were also checked, and all selected studies were included in the qualitative data synthesis.
Figure 1.
Flowchart of the study selection.
Classification of Biomarkers
Afterwards, selected SF biomarkers were categorized according to Lotz et al., 7 based on their nature and molecular origin, into 4 classes: (1) biomarkers associated with the metabolism of collagen in cartilage (type II collagen) or subchondral bone (type I collagen); (2) biomarkers associated with the metabolism of aggrecan in cartilage; (3) biomarkers related to a range of non-collagenous proteins that have a role in other metabolic pathways in the joint, including glycoproteins, proteoglycans, metalloproteinases, and advanced glycation end-products, as well as hyaluronan, which is a constituent of both cartilage and synovium; and (4) biomarkers associated with other processes, such as inflammation or fibrosis.
Moreover, all SF biomarkers were classified in the 6 categories of BIPEDs classification and each of these was summarized in separate paragraphs and tables. BIPEDs classification was initially proposed by Bauer et al. in 2006 10 (later modified by Kraus et al. 14 ) to provide specific biomarker definitions with the goal of improving the ability to develop and analyze OA biomarkers and to communicate these advances within a common framework. In detail, 6 categories of biomarkers have been identified:
Burden of disease biomarkers: assess the severity or extent of disease, typically at a single point in time, among individuals with OA. Studies of these markers require comparison with one or more gold standard methods of determining disease severity, such as clinical or radiographic criteria (i.e., Kellgren-Lawrence classification).
Investigative biomarkers: classified investigative if there is insufficient information to allow inclusion into one of other categories.
Prognostic biomarkers: a prognostic biomarker predicts OA onset in patients without the disease or OA progression in those with existing disease. Their evaluation requires longitudinal studies showing an association of the marker at baseline with the risk of development or progression of OA.
Efficacy of intervention biomarkers: those that can be applied in randomized controlled trials to assess the changes associated with pharmacological treatments. These markers can be indicative or predictive of treatment efficacy.
Diagnostic biomarkers: a diagnostic biomarker can distinguish between individuals with and without OA with good sensitivity and specificity. Studies of these markers must include individuals with and without OA, and those with conditions sometimes confused with OA, such as rheumatoid arthritis.
Safety biomarkers: those able to reflect tissue and/or organ toxicity following a specific treatment, to monitor for local and systemic adverse effects both early and advanced, to set therapeutic dosages that do not impact on physiology and to understand what “safe” ranges are for joint tissue biomarkers.
According to this classification, a single biomarker may meet multiple criteria for different uses; thus, biomarkers have been classified into one or more categories.
Results
In the initial search, 515 studies were identified. Subsequently, all abstracts were screened and selected according to the inclusion and exclusion criteria ( Fig. 1 ): 329 abstracts were excluded and 5 articles were identified through the reference lists, which gave a total of 191 full-text articles assessed for eligibility. Afterwards, further 32 articles were excluded, either because they were reviews, studies not written in English, studies that described biomarkers not obtained from SF samples, or studies that included other diseases without reporting about OA. Thus, 159 articles12,15-172 were included in the qualitative data synthesis; among these, 138 within cross-sectional and 21 within longitudinal studies.
The studies included 13,557 patients. A total of 789 patients with knee injury (mainly meniscal or anterior cruciate lesions), 755 controls (donors or volunteers), and 431 with knee rheumatoid arthritis were also reported as comparative group. In the included studies, 201 different SF biomarkers were identified. Among these, several were investigated multiple times in different articles, for a total of 373 analyses of SF biomarkers reported in the literature in the last 20 years. In the following paragraphs, the identified biomarkers are grouped according to their molecular characteristics (Lotz et al. 7 ), and the results documented for each biomarker are summarized according to the BIPEDs classification.
Molecular Characteristics
SF biomarkers are categorized into 4 classes based on their nature and molecular origin: the most represented biomarkers were those related to “other processes” as inflammation or fibrosis. In particular, inflammation biomarkers were the most studied, with cytokines and chemokines above all: IL-6 was the most studied biomarker (17 articles), followed by IL-8 (12 articles), TNF-α (9 articles), and other less reported ones. Other SF biomarkers included in this category were growth factors (GFs), adipokines, metabolites, and other proteins related to bone or cartilage metabolism. Other articles focused on SF biomarkers related to collagen metabolism, with the most studied being CTX-II (5 articles). In the category of SF biomarkers related to aggrecan metabolism, the most considered were chondroitin 4-sulfate (7 articles), chondroitin 6-sulfate (6 articles), and keratan sulfate (4 articles). Finally, the most common SF biomarkers related to other non-collagenous proteins included COMP and MMP-3 (10 articles for each), TIMP-1 and MMP-1 (9 articles for each), MMP-13 (6 articles), and hyaluronan (5 articles). More details are reported in Table 1 .
Table 1.
Selected SF Biomarkers Classified Based on Their Molecular Characteristics.
| Biomarkers related to collagen metabolism |
| CIM; CIIM; Collagenase 2; CTXI**; CTXII****; Deoxypyridinoline*; PICP; PIICP*; Pyridinoline* |
| Biomarkers related to aggrecan metabolism |
| ADAMTS-4; ADAMTS-5; Aggrecan; Aggrecan Epitope 846*; ARGS-aggrecan**; Chondroitin 4-sulfate******; Chondroitin 6-sulfate*****; Dermatan sulfate; Keratin sulfate*** |
| Biomarkers related to other non-collagenous proteins |
| COMP*********; Fibulin-3; Hyaluronan****; MWHA;
Osteocalcin*; Osteogenic Protein 1***; Osteopontin*; sRAGE;
YKL-40 Matrix metalloproteinases: MMP-1********; MMP-2*; MMP-3*********; MMP-7; MMP-8; MMP-9; MMP-12; MMP-13***** Tissue inhibitors of metalloproteinases: TIMP-1********; TIMP-2*; TIMP-3; TIMP-4 Extracellular matrix: Glypican-3; Syndecan4; sBGN; sDCN; ucMGP; Tenascin C*; TSG-6** |
| Biomarkers related to other processes |
| Cytokines: Activin A*; Chemerin**; HMGB-1; IFNγ; IL-1*;
IL-1β***; IL-2; IL-5; IL-6****************; IL-7;
IL-8***********; IL-10**; IL-11; IL-12p70; IL-13; IL-15;
IL-17; IL-18***; IL-34; Inhibin A; MIF;
TNF-α******** Chemokines: CCL2****; CCL3*; CCL4; CCL5***; CCL11; CCL13; CCL20; CCL22; CX3CL1*; CXCL10**; CXCL12*; CXCL13 Complement system: C3 fragment; C3f Des-Arg; C3bBbP; C4d; sTCC Growth factors: Adrenomedullin; BDNF; CTGF; FGF-2*; FGF-21; HGF; Hif-1α*; G-CSF; LIF; LTBP2; M-CSF; PDGF-AA; sCD40L; TGF-β1**; VEGF****** Adipokines: Adiponectin; FABP4; Leptin****; Omentin-1*; RBP-4; Resistin***; Visfatin Bone or cartilage metabolism: BMP-2; CD-RAP*; DKK1***; Exosomal lncRNA PCGEM1; Exosomal lncRNA HOTAIR; Exosomal lncRNA GAS5; FRZB*; Ghrelin; Glypican-3; Gremlin-1**; Irisin; Lubricin; NTx; OPG; OPG/OCIF; Sclerostin*; Thymosin β4 Metabolites: 3-Hydroxybutyrate; Citrate; Creatine; Ethanol; Ethanolamine; Fructose; Hexanolycarnitine; Malate; Methionine; N-Phenylacetylglycine; O-Acetylcarnitine MicroRNA: MicroRNA-140-3p; MicroRNA-140-5p; miR-23a-3p*; miR-24-3p; miR-27a-3p; miR-27b-3p; miR-29c-3p; miR-30c-5p; miR-34a-5p; miR-100-5p; miR-186-5p; miR-200c-3p; miR-1826 Oxidative or Nitrosative stress: 3-Nitrotyrosine; 8-Isoprostane F2α; GPx; GSH; Iron; MPO; Nitrotyrosine; SOD; TBARs; sVAP-1; Vitamin E OTHER: α-MSH; α(II)CB11B; ADA; Albumin; anti-CCP; ATP; Autotaxin; Bradykinin*; Calprotectin; Camp; CD14*; CD163; CGRP*; Chlorinated peptides; Endoglin; Haptoglobin; Hs-CRP; HSPA1A; iCAM-1; IgA-RF; IgG-RF; IgM-RF; ITI-H4; LBP; LPS; Neuropeptide Y*; Oncostatin M; P Selectin; Pentosidine; Periostin*; PGE2*; Phospholipids; RANKL; S100A12; Salic Acid; Sphingolipids; sTNFR-1; sTNFR-2; Substance P; TWEAK; UCMA; vCAM-1; White blood cells |
Asterisks indicate the number of further articles analyzing the specific biomarker. α-MSH = melanocyte stimulating hormone; α(II)CB11B = cluster of differentiation molecule 11B; ADA = adenosine deaminase; ADAMTS-4 = aggrecanase-1; ADAMTS-5 = aggrecanase-2; anti-CCP = anti–cyclic citrullinated peptide; BDNF = brain-derived neurotrophic factor; BMP = bone morphogenetic protein; CCL = chemokine (C-C motif) ligand; CD = cluster of differentiation; CD-RAP = cartilage derived retinoic acid sensitive protein; CGRP = calcitonin gene-related peptide; CIM = collagen type I-specific neoepitope; CIIM = collagen type II-specific neoepitope; COMP = cartilage oligomeric matrix protein; CTGF = connective tissue growth factor; CTX = C-terminal telopeptide; CXCL = chemokine (C-X-C motif) ligand; DKK = dickkopf-1; FABP4 = fatty acid-binding protein 4; FGF-2 = basic fibroblast growth factor; FGF-21 = fibroblast growth factor-21; FRZB = frizzled-related protein; G-CSF = granulocyte-colony stimulating factor; GPx = glutathione peroxidase; GSH = glutathione; HGF = hepatocyte growth factor; Hif-1α = hypoxia-inducible factor-1α; HMGB-1 = high-mobility group box 1; Hs-CRP = high sensitivity C-reactive protein; HSPA1A = heat shock protein family A (Hsp70) member 1A; iCAM-1 = intercellular adhesion molecule 1; IFNγ = interferon-γ; IgA-RF = IgA rheumatoid factor; IgG-RF = IgG rheumatoid factor; IgM-RF = IgM rheumatoid factor; IL = interleukin; ITI-H4 = inter-alpha-trypsin inhibitor heavy chain 4; LBP = LPS binding protein; LIF = leukemia-inhibitory factor; LPS = lipopolysaccharide; LTBP2 = latent-transforming growth factor beta-binding protein 2; M-CSF = macrophage-colony stimulating factor; MIF = macrophage migration inhibitory factor; MMP = matrix metalloproteinases; MPO = myeloperoxidase; MWHA = high-molecular-weight HA; NTx = N-terminal telopeptide; OPG = osteoprotegerin; OPG/OCIF = osteoprotegerin/osteoclastogenesis inhibitory factor; PDGF-AA = platelet-derived growth factor; PGE2 = prostaglandin E2; PICP = procollagen type II carboxy-terminal propeptide; PIICP = procollagen type II carboxy-terminal propeptide; RANKL = receptor activator of nuclear factor kappa-Β ligand; RBP-4 = retinol binding protein 4; S100A12 = S100 calcium-binding protein A12; sBGN = soluble biglycan; sDCN = soluble decorin; sCD40L = soluble CD40 ligand; SOD = superoxide dismutase; sRAGE = soluble receptor for advanced glycation end products; sTCC = soluble terminal complement complex; sTNFR = soluble tumor necrosis factor receptor; sTCC = soluble terminal complement complex; sVAP-1 = soluble vascular adhesion protein-1; TBARs = thiobarbituric acid reactive substances; TGF-β1 = transforming growth factor-β; TIMP = tissue inhibitors of metalloproteinases; TNF-α = tumor necrosis factor-α; TSG-6 = TNF-stimulated gene 6 protein; TWEAK = TNF-like weak inducer of apoptosis; UCMA = upper zone of growth plate and cartilage matrix associated; ucMGP = uncarboxylated matrix Gla-protein; vCAM-1 = vascular cell adhesion molecule-1; VEGF = vascular endothelial growth factor; YKL-40 = chitinase-3-like protein 1.
BIPEDs Classification
Selected biomarkers were classified according to BIPEDs classification in 6 categories, as reported in the following paragraphs and tables, summarizing both their clinical and imaging results.
Burden of Disease Biomarkers
The “burden of disease” category is the most represented in the literature of SF biomarkers: 132 different biomarkers reported in 96 studies were identified, for a total of 190 analyses on burden of disease SF biomarkers. Kellgren-Lawrence classification was the most commonly used reference parameter for the radiographic severity, applied in 82 studies; other less commonly used scales were Ahlbäck classification (2 articles) and Tomihisa Koshino’s scoring system (1 article). Moreover, in some articles also arthroscopic severity was evaluated with the Outerbridge classification (2 articles) or with the International Cartilage Repair Society classification (1 article). Last, the knee OA severity was also respectively evaluated with scintigraphy imaging, ultrasonographic findings, macroscopy and histology (1 article each).
For clinical severity, the most used reference parameter was the WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index) scale (26 articles); other less commonly used parameters were VAS (visual analogue scale) in 7 articles, followed by Numeric Rating Scale (NRS), NPQ (Neurophysiology of Pain Questionnaire) scale, KSS (Karolinska Sleepiness Scale) scale, and Watanabe’s pain score (1 article each).
A positive correlation with the knee OA severity was found for 101 analyses of SF biomarkers, a negative correlation for 32, and no correlation for 57 analyses of SF biomarkers. Among the most studied biomarkers of this category, IL-6 was analyzed in 8 studies, reporting controversial results: a negative correlation with radiographic or clinical severity of knee OA were reported in 4 cases, but a positive correlation was reported in 2 cases, with no correlation in the remaining 2 cases. IL-8 was analyzed in 6 studies, showing a positive correlation in only one case for both radiographic and clinical severity, while no correlation in the other 5 cases. MMP-1 levels in SF were analyzed in 6 studies, showing a negative correlation with radiographic OA severity in 2 cases, no correlation in 2 cases, while a positive correlation was reported in 2 cases. Similarly, MMP-3 and MMP-13 were investigated in 5 articles each, showing correlation with OA severity in only 2 cases, while no correlation was reported in the other cases. Conversely, consistent results have been found for some biomarkers: SF levels of VEGF were investigated in 7 studies, showing a positive correlation with OA severity in 6 cases and no correlation in the last one. CCL2, Leptin, and TIMP-1 were analyzed in 4 studies each, showing a positive correlation with OA severity in 3 cases and no correlation in the last one. Results for each biomarker are summarized in Table 2 , while more detailed findings are reported in the supplementary material.
Table 2.
Burden of Disease SF Biomarkers.
| Biomarker | References | Imaging | Clinical | Biomarker | References | Imaging | Clinical |
|---|---|---|---|---|---|---|---|
| α-MSH | 25 | ↓ | ↓ | KS | 42 | \ | \ |
| ADAMTS-5 | 171 | − | \ | LBP | 55 | \ | ↑ |
| Adiponectin | 20 | \ | \ | Leptin | 39, 77, 94, 130 | ↑ \ \ ↑ | − \ ↑ − |
| Adrenomedullin | 51 | ↑ | − | LPS | 55 | ↑ | ↑ |
| ATP | 119 | − | ↑ | Lubricin | 53 | \ | \ |
| Autotaxin | 72 | ↑ | ↑ | M-CSF | 171 | − | \ |
| BDNF | 90 | \ | − | MiRNA-140-3p | 38 | ↓ | − |
| BMP-2 | 74 | ↑ | ↑ | MiRNA-140-5p | 38 | ↓ | − |
| Bradykinin | 171 | − | \ | MIF | 54 | ↑ | ↑ |
| C3 fragment | 36 | ↓ | − | miR-186-5p | 56 | ↑ | − |
| C3f Des-Arg | 36 | ↓ | − | miR-23a-3p | 56 | ↑ | − |
| Calprotectin | 157 | − | ↑ | miR-24-3p | 56 | ↑ | − |
| CCL2 | 12, 21, 81, 109 | \ ↑ \ \ | ↑ − ↑ − | miR-27a-3p | 56 | ↑ | − |
| CCL3 | 109 | \ | − | miR-27b-3p | 56 | ↑ | − |
| CCL5 | 68 | \ | − | miR-29c-3p | 567 | ↑ | − |
| CCL13 | 73 | ↑ | − | miR-34a-5p | 56 | ↑ | − |
| CCL20 | 18 | ↑ | ↑ | MMP-1 | 32, 68, 100, 106, 126, 150 | ↓ \ ↑ ↓ \ \ | − − − − − − |
| CD-RAP | 148 | ↓ | − | MMP-2 | 68, 100 | \ ↑ | − − |
| CGRP | 70, 171 | ↑ − | ↑ \ | MMP-3 | 21, 100, 126, 150, 171 | \ ↑ \ \ − | ↑ − − − \ |
| Chemerin | 109, 164 | ↑ ↑ | − − | MMP-7 | 100 | \ | − |
| Cl-peptides | 138 | ↓ | − | MMP-8 | 100 | ↑ | − |
| C4S | 137 | ↓ | − | MMP-9 | 100 | ↑ | − |
| C6S | 137 | ↓ | − | MMP-12 | 100 | \ | − |
| CIM | 46 | − | ↑ | MMP-13 | 40, 77, 100, 126, 171 | \ \ ↑ \ − | − − \ − ↑ |
| CIIM | 165 | ↑ | − | MPO | 138 | ↓ | − |
| Collagenase 2 | 40 | \ | \ | Neopterin | 96, 171 | ↑ − | ↑ \ |
| COMP | 26, 77, 128, 137 | ↑ \ \ ↓ | − \ − − | Neuropeptide Y | 80 | ↑ | ↑ |
| CTGF | 103 | ↑ | − | NTx | 150 | \ | − |
| CTXI | 44 | \ | \ | Omentin-1 | 98, 105 | − ↓ | − ↓ |
| CTXII | 44 | ↑ | \ | OPG | 135 | ↑ | − |
| CX3CL1 | 71, 93 | ↑ ↑ | ↑ − | OPG/OCIF | 152 | ↑ | − |
| CXCL10 | 110 | ↓ | − | OP-1 | 42, 132 | ↑ ↑ | − − |
| CXCL12 | 63, 101 | ↑ ↑ | − − | Osteopontin | 116, 129 | ↑ ↑ | − − |
| Deoxypyridinoline | 150 | ↑ | − | P selectin | 122 | ↑ | − |
| Dermatansulfate | 137 | ↓ | − | Periostin | 69 | ↑ | − |
| DKK1 | 43, 115 | ↑ ↓ | − − | PICP | 150 | \ | − |
| Endoglin | 127 | ↑ | − | Pyridinoline | 150 | \ | − |
| Exosomal lncRNA GAS5 |
28 | \ | \ | RANKL | 113 | ↑ | − |
| Exosomal lncRNA HOTAIR |
28 | \ | \ | Resistin | 62, 77 | ↑ \ | ↑ \ |
| Exosomal lncRNA PCGEM1 |
28 | ↑ | ↑ | S100A12 | 97 | ↑ | ↑ |
| FGF-21 | 75 | ↑ | − | Salic Acid | 83 | ↑ | − |
| Fibulin-3 | 35 | ↑ | − | Sclerostin | 43, 86 | \ ↓ | − − |
| Ghrelin | 45 | ↓ | ↓ | sICAM-1 | 21 | ↑ | ↑ |
| Glypican-3 | 24 | ↓ | − | sRAGE | 120 | ↓ | − |
| GREM 1 | 48 | ↑ | − | sTNFR-1 | 85 | − | ↓ |
| HA | 42, 126 | \ \ | − − | sTNFR-2 | 85 | − | ↓ |
| Haptoglobin | 60 | ↑ | − | Substance P | 171 | − | \ |
| HGF | 126 | \ | − | sVAP1 | 22 | ↑ | − |
| HIF-1α | 47, 78 | ↑ ↑ | − − | sVCAM-1 | 21 | ↑ | ↑ |
| HMGB-1 | 65 | ↑ | ↑ | Syndecan 4 | 160 | ↑ | − |
| IFNγ | 109 | \ | − | Tenascin-C | 143 | ↑ | − |
| IL-1 | 40 | \ | − | TGF-β1 | 126 | \ | − |
| IL-1β | 44, 109, 171 | − \ ↓ | ↓ \ ↓ | Thymosin β4 | 95 | ↑ | − |
| IL-2 | 109 | \ | − | TIMP-1 | 21, 100, 126, 150 | ↑ ↑ \ ↑ | ↑ − − − |
| IL-5 | 109 | \ | − | TIMP-2 | 100 | \ | − |
| IL-6 | 40, 44, 46, 68, 109, 153, 166, 171 | \ \ − ↑ \ ↓ − ↓ | − \ ↑ − \ − ↓ ↓ | TIMP-3 | 100 | ↑ | − |
| IL-7 | 106 | \ | − | TIMP-4 | 100 | \ | - |
| IL-8 | 40, 44, 68, 109, 153, 166 | \ \ ↑ \ \ − | − \ − − − ↑ | TNF-α | 40, 44, 171 | \ − \ | − ↑ ↓ |
| IL-10 | 40 | \ | − | TWEAK | 32 | ↓ | − |
| IL-12p70 | 109 | \ | − | UCMA | 19 | ↑ | ↑ |
| IL-15 | 133 | ↓ | − | ucMGP | 57 | ↓ | − |
| IL-17 | 66 | \ | ↑ | VEGF | 21, 30, 40, 77, 82, 106, 126 | ↑ \ \ ↑ ↑ ↑ \ | ↑ ↑ − \ − − − |
| IL-18 | 39 | ↑ | \ | Visfatin | 114 | ↑ | − |
| IL-34 | 27 | ↑ | ↑ | YKL-40 | 64 | − | ↑ |
| Irisin | 59 | ↓ | − |
“↑” = positive correlation; “↓” = negative correlation; “\” = no correlation; “−” = not evaluated.
Investigative Biomarkers
This systematic review found 41 different SF biomarkers without sufficient information to allow inclusion into one of the other categories (among these, some biomarkers have SF levels correlated with serum levels, others changed in SF following exercise or arthroscopic surgery or high tibial osteotomy, and others had levels correlating to other biomarkers). For this reason, these biomarkers were classified in the “investigative” category. Among these, CD-RAP, COMP, MMP-1, MMP-3, MMP-13, RBP-4, and TIMP-1 showed higher levels in SF samples compared to serum. Conversely, Pentosidine showed lower levels in SF samples compared to serum. Moreover, the SF levels of Adiponectin, Bradykinin, IL-6, IL-18, Oncostatin M, PGE2, Resistin, TNF-α, and TSSG-6 showed a significant correlation with the SF levels of other biomarkers for cartilage degradation or inflammation. More detailed findings for investigative SF biomarkers are reported in the supplementary material.
Prognostic Biomarkers
Only 8 studies described “prognostic” SF biomarkers for knee OA, identifying 9 biomarkers, out of the 14 investigated ones, correlating with OA progression.
CD14 and CD163 were positively associated with osteophyte progression at 3 years of follow-up, supporting the central role of inflammation as a determinant of progression risk of OA. Similarly, baseline PIICP level showed a direct positive correlation with radiographic joint space narrowing over 4 years. TSG-6 at baseline predicted OA progression over a period of 3 years. IL-6, IL-8, and TNFα were investigated in subjects with previous meniscectomy; higher or over time increasing levels of IL-6 and TNF-α, but not IL-8, were associated with an increased risk of radiographic OA progression after 18 years. Following meniscectomy, ARGS concentrations were inversely associated with both future loss of joint space and pain worsening after 18 years with low levels associated with increased risk of progression and higher levels with decreased risk. With regard to biomarkers associated to anterior cruciate ligament (ACL) rupture, acute and chronic SF concentrations of aggrecan, COMP, MMP-3, and TIMP-1, collected at the time of ACL reconstruction, failed to predict knee OA 16 years after ACL injury. Finally, CTXI and CTXII concentrations were associated with a rapid clinical progression in OA patients with obesity and depression with respect to patients without comorbidities. More detailed findings of the studies on each of these biomarkers are reported in Table 3 .
Table 3.
Prognostic SF Biomarkers.
| Biomarker | References | Patients | Follow-up | Results |
|---|---|---|---|---|
| Aggrecan | 50 | 88 patients post ACL injury | 16 Years | Neither acute nor chronic concentrations of SF aggrecan were associated with the development of radiographic knee OA at the 16-year follow-up. |
| ARGS aggrecan | 104 | 141 patients post meniscectomy | 18 Years | Having decreasing levels of SF ARGS over time was associated with an increased risk of loss of joint space and pain worsening but showed no association with other patient-reported outcomes or osteophyte progression. |
| CD14 | 76 | 159 OA | 3 Years | SF CD14 levels were positively associated with osteophyte progression at 3 years. |
| CD163 | 76 | 159 OA | 3 Years | SF CD163 levels were positively associated with osteophyte progression at 3 years. |
| COMP | 50 | 88 patients post ACL injury | 16 Years | Neither acute nor chronic concentrations of SF COMP were associated with the development of radiographic knee OA at the 16-year follow-up. |
| CTXI | 169 | 600 OA | 2 Years | CTXI was associated with a rapid clinical progression in OA patients with obesity and depression respect to patients without comorbidities. |
| CTXII | 169 | 600 OA | 2 Years | CTXII was associated with a rapid clinical progression in OA patients with obesity and depression respect to patients without comorbidities. |
| IL-6 | 61 | 132 patients post meniscectomy | 18 Years | In subjects with previous meniscectomy, higher or over time increasing SF levels of IL-6 seems to be associated with increased risk for progression of radiographic OA. |
| IL-8 | 61 | 132 patients post meniscectomy | 18 Years | No correlation of SF IL-8 levels and increased risk for progression of radiographic OA. |
| MMP-3 | 50 | 88 patients post AC -injury | 16 Years | Neither acute nor chronic concentrations of SF MMP-3 were associated with the development of radiographic knee OA at the 16-year follow-up. |
| PIICP | 147 | 110 OA | 4 Years | Quantification of SF PIICP was able to predict subsequent radiographic progression in early knee OA. |
| TIMP-1 | 50 | 88 patients post ACL injury | 16 Years | Neither acute nor chronic concentrations of SF TIMP-1 were associated with the development of radiographic knee OA at the 16-year follow-up. |
| TNF-α | 61 | 132 patients post meniscectomy | 18 Years | In subjects with previous meniscectomy, higher or over time increasing SF levels of TNF-α seems to be associated with increased risk for progression of radiographic OA. |
| TSG-6 | 91 | 132 OA | 3 Years | TSG-6 is a promising biomarker for OA progression, for the correlation with the joint space narrowing. |
| 163 | 813 OA | 17 Months | TSG-6 activity can potentially be used to track longitudinal changes in disease status and inform treatment decisions. |
OA = osteoarthritis; SF = synovial fluid; ACL = anterior cruciate ligament.
Efficacy of Intervention Biomarkers
Only 7 studies described “efficacy of intervention” SF biomarkers for knee OA, identifying a total of 20 different markers with composite findings.
Biomarkers related to aggrecan metabolism were the most represented, such as C6S, C4S, aggrecan, and keratan sulfate. C4S and Tenascin-C levels in SF before HA injection showed a significant inverse correlation with the improvement of VAS pain after treatment in knee OA patients. Moreover, positive correlations were noted between the concentrations of C6S and aggrecan before HA injection and the improvement of the Japanese Orthopaedic Association (JOA) score 1 month after injection. SF levels of white blood cells before steroid injection showed a significant correlation with the reduction in KOOS (Knee Injury and Osteoarthritis Outcome Score) pain score after treatment, identifying persons more likely to benefit from an intra-articular steroid injection.
Looking at changes in SF biomarkers after treatment, concentrations of α(II)CB11B, C4S, C6S, IL-6, MMP-1, and MMP-9 showed a significant decrease after HA injections. Conversely, SF concentrations of COMP, HA, and osteocalcin increased significantly after HA injections. SF CTXI were significantly reduced in knee OA patients treated with oral colchicine compared to placebo. More detailed findings of the studies on each of these biomarkers are reported in Table 4 .
Table 4.
Efficacy of Intervention SF Biomarkers.
| Biomarker | References | Type of treatment | Results |
|---|---|---|---|
| α(II)CB11B | 151 | 20 OA treated with HA injection | SF α(II)CB11B concentration showed a significant decrease after HA injections. |
| Aggrecan | 139 | 38 OA treated with HA injection | Positive correlations were noted between the concentrations of aggrecan before HA injection and the improvement of the JOA score after injection (after 1 month). |
| C4S | 79 | 55 OA treated with HA injection | C4S and the SF volume were significantly decreased after intraarticular (IA) HA treatment. These improvements of SF properties indicated that IA HA had potential of affecting for the joint cartilage and synovium with knee OA. |
| 125 | 51 OA treated with HA or steroid | No significant differences between 2 groups. C4S Concentrations in SF decreased slightly after injection therapy in both groups. There was no correlation between changes in the biomarkers and clinical results. | |
| 136 | 28 OA treated with HA injection | Inverse correlations were observed between the levels of C4S before HA injection and improvement of VAS after the 5 injections. | |
| 139 | 38 OA treated with HA injection | C4S decreased after injection, although these decreases were not significant. | |
| C6S | 79 | 55 OA treated with HA injection | C6S and the SF volume were significantly decreased after IA HA treatment. These improvements of SF properties indicated that IA HA had potential of affecting for the joint cartilage and synovium membrane with knee OA. |
| 125 | 51 OA treated with HA or steroid | No significant differences between 2 groups. C6S Concentrations in SF decreased slightly after injection therapy in both groups. There was no correlation between changes in the biomarkers and clinical results. | |
| 136 | 28 OA treated with HA injection | No significant correlation was seen between levels of C6S before injections and improvement of VAS after the 5 injections. | |
| 139 | 38 OA treated with HA injection | Positive correlations were noted between the concentrations of C6S before HA injection and the improvement of the JOA score after injection (after 1 month). | |
| CD14 | 33 | 109 OA treated with Colchicine or placebo | No significant differences between 2 groups. |
| COMP | 152 | 20 OA treated with HA injection | COMP levels were significantly higher after HA injections. |
| CTXI | 33 | 109 OA treated with Colchicine or placebo | SF CTXI were significantly reduced in the Colchicine group, but not the placebo-treated group after 16 weeks. |
| CTXII | 33 | 109 OA treated with Colchicine or placebo | No significant differences between 2 groups. |
| HA | 79 | 55 OA treated with HA injection | No correlation. |
| 125 | 51 OA treated with HA or steroid | HA concentrations in SF increased significantly after therapy in the Na-HA group but remained unchanged in the CS group. There was no correlation between changes in the biomarkers and clinical results. | |
| IL-6 | 33 | 109 OA treated with Colchicine or placebo | No significant differences between 2 groups. |
| 79 | 55 OA treated with HA injection | IL-6 and the SF volume were significantly decreased after IA HA treatment. These improvements of SF properties indicated that IA HA had potential of affecting for the joint cartilage and synovium membrane with knee OA. | |
| IL-8 | 33 | 109 OA treated with Colchicine or placebo | No significant differences between 2 groups. |
| IL-18 | 33 | 109 OA treated with Colchicine or placebo | No significant differences between 2 groups. |
| Keratansulfate | 136 | 28 OA treated with HA injection | No significant correlation was seen between levels of KS before injections and improvement of VAS after the 5 injections. |
| 79 | 55 OA treated with HA injection | No correlation. | |
| MMP-1 | 151 | 20 OA treated with HA injection | SF MMP-1 concentration showed a significant decrease after HA injections. |
| MMP-9 | 125 | 51 OA treated with HA or steroid | MMP-9 concentrations in SF decreased after injection therapy in the Na-HA group but were unchanged in the CS group. There was no correlation between changes in the biomarkers and clinical results. |
| Osteocalcin | 151 | 20 OA treated with HA injection | Osteocalcin levels were significantly higher after HA injections. |
| Tenascin-C | 136 | 28 OA treated with HA injection | Inverse correlations were observed between the levels of TN-C before HA injection and improvement of VAS after the 5 injections. |
| TNF-α | 33 | 109 OA treated with Colchicine or placebo | No significant differences between 2 groups. |
| TIMP-1 | 125 | 51 OA treated with HA or steroid | TIMP-1 concentrations did not change significantly after therapy in either group. There was no correlation between changes in the biomarker and clinical results. |
| White blood cells | 51 | 55 OA treated with steroid injection | With each category increase in SF WBC there was a greater mean reduction in KOOS pain score after steroid injection. Total SF WBC count may predict response to anti-inflammatory treatment. |
OA = osteoarthritis; HA = hyaluronan; SF = synovial fluid; JOA = Japanese Orthopaedic Association; VAS = Visual Analogue Scale; WBC = white blood cells; KOOS = Knee Injury and Osteoarthritis Outcome Score.
Diagnostic Biomarkers
“Diagnostic” SF biomarkers were studied in 45 articles focusing on 106 different biomarkers, for a total of 139 analyses on this category. Seventy-eight biomarkers were studied for the diagnosis between OA patients and healthy controls; among these, 42 presented significantly higher levels in the SF of OA patients compared with healthy controls, 21 presented significantly lower levels in OA compared to healthy controls, while for 15 biomarkers no differences were found with the control. Fifty-five biomarkers were investigated for the differential diagnosis between OA and immune-mediated inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, pseudogout), among these, 5 presented significantly higher levels in OA patients versus inflammatory arthritis, 35 presented significantly lower levels in OA patients versus inflammatory arthritis, while 15 showed no statistically significant differences versus control groups. Finally, 49 biomarkers were studied to differentiate the SF of OA patients from the SF of knee injured patients; among these, 17 presented significantly higher concentrations in SF of OA patients, 13 presented lower concentration in SF of OA patients, while no differences were found for 19 biomarkers. IL-6 was the most studied biomarker also for this category, with 7 studies evaluating its diagnostic usefulness: this cytokine showed significantly higher levels in OA patients when compared with healthy controls or knee injury patients (4 studies). Moreover, SF levels of IL-6 were significantly lower in OA patients when compared with rheumatic patients (3 studies). Similarly, IL-8 showed higher levels in SF of patients with knee OA when compared with healthy controls or knee injury patients (3 studies), and lower levels when compared with rheumatic patients (3 studies). Furthermore, MMP-1, MMP-3, and CCL5 were significantly increased in the SF of OA patients compared with healthy controls and knee injury patients (3 studies for each one). Conversely, some SF biomarkers, including cAMP, CD-RAP, COMP, ITI-H4, and MMP-7, were significantly higher in OA patients compared with rheumatic patients (one study for each one). Results for each biomarker are summarized in Table 5 , while more detailed findings are reported in the supplementary material.
Table 5.
Diagnostic SF Biomarkers.
| Biomarker | References | Controls | Knee Injury | RA | Biomarker | References | Controls | Knee Injury | RA |
|---|---|---|---|---|---|---|---|---|---|
| 3-Hydroxybutyrate | 67 | ↓ | − | − | IL-11 | 111 | ↑ | − | \ |
| 3-Nitrotyrosine | 29 | − | \ | − | IL-13 | 167 | ↑ | − | − |
| 8-Isoprostane F2α | 29 | − | ↑ | − | IL-18 | 134 | − | − | ↓ |
| Activin A | 118 | − | − | ↓ | Inhibin A | 118 | − | − | ↓ |
| ADA | 117 | − | − | ↓ | Iron | 124 | − | \ | |
| ADAMTS-4 | 12 | ↑ | ↑ | − | ITI-H4 | 161 | − | − | ↑ |
| Aggrecan ARGS | 131 | ↑ | − | − | Leptin | 118, 124, 161 | − − ↑ | − ↑ − | ↓ − − |
| anti-CCP | 140 | − | − | ↓ | LIF | 112 | ↓ | − | \ |
| C3bBbP | 49 | ↑ | \ | ↓ | Malate | 67 | ↓ | − | − |
| C4d | 49 | ↑ | \ | ↓ | Methionine | 67 | ↓ | − | − |
| cAMP | 134 | − | − | ↑ | MicroRNA-140-3p | 38 | ↓ | − | ↓ |
| CCL2 | 68 | \ | − | − | MicroRNA-140-5p | 38 | ↓ | − | ↓ |
| CCL3 | 167 | ↑ | − | − | miR-100-5p | 17 | ↓ | − | − |
| CCL4 | 167 | ↑ | − | − | miR-1826 | 17 | ↓ | − | − |
| CCL5 | 68, 112, 167, 172 | ↑ − ↑ − | − \ − ↑ | − − − − | miR-200c-3p | 17 | ↓ | − | − |
| CCL11 | 167 | ↓ | − | − | MMP-1 | 68, 100, 172 | ↑ ↑ − | − − ↑ | − \ − |
| CCL22 | 167 | ↑ | − | − | MMP-2 | 68, 100 | \ ↑ | − − | − \ |
| CD-RAP | 148 | − | − | ↑ | MMP-3 | 100, 155, 172 | ↑ − − | − − ↑ | \ \ − |
| Chemerin | 89 | − | − | \ | MMP-7 | 100 | ↑ | − | ↑ |
| Cl-peptides | 138 | ↑ | − | − | MMP-8 | 100 | ↑ | − | \ |
| CIIM | 165 | ↑ | − | − | MMP-9 | 100 | ↑ | − | \ |
| Citrate | 67 | ↑ | − | − | MMP-12 | 100 | \ | − | \ |
| Collagenase 2 | 40 | − | \ | − | MMP-13 | 40, 100 | − ↑ | \ − | − \ |
| COMP | 26, 111, 168 | ↑ \ − | − − − | − \ ↑ | MPO | 138 | ↑ | − | − |
| Creatine | 67 | \ | − | − | Neuropeptide Y | 80 | ↑ | − | − |
| CTXII | 144 | ↑ | − | − | Nitrotyrosine | 99 | ↑ | ↓ | ↓ |
| CXCL10 | 167, 172 | ↑ − | − ↑ | − − | O-Acetylcarnitine | 66 | ↓ | − | − |
| Deoxypyridinoline | 146 | − | − | \ | Osteocalcin | 111 | \ | − | \ |
| DKK1 | 31, 43 | \ − | − ↑ | − − | OP-1 | 111, 172 | ↑ − | − ↑ | ↓ − |
| Ethanol | 67 | ↓ | − | − | Osteopontin | 129 | ↑ | − | − |
| Ethanolamine | 67 | ↓ | − | − | PDGF-AA | 167 | ↑ | − | − |
| Exosomal lncRNA GAS5 | 28 | \ | − | − | Periostin | 162 | − | − | ↓ |
| Exosomal lncRNA HOTAIR | 28 | \ | − | − | PGE2 | 134 | − | − | ↓ |
| Exosomal lncRNA PCGEM1 | 28 | ↑ | − | − | Phenylacetylglycine | 67 | ↓ | − | − |
| FABP4 | 34 | ↑ | − | − | Pyridinoline | 146 | − | − | ↓ |
| FGF-2 | 167 | \ | − | − | RANKL | 113 | − | − | \ |
| Fructose | 67 | ↑ | − | − | Sbgn | 58 | − | ↑ | ↓ |
| FRZB | 31 | ↓ | − | − | sCD40L | 167 | ↑ | − | − |
| G-CSF | 167 | ↓ | − | − | Sclerostin | 43 | − | \ | − |
| GPx | 124 | − | \ | − | sDCN | 58 | − | \ | \ |
| GREM 1 | 31 | \ | − | − | SOD | 124 | − | \ | − |
| GSH | 124 | − | \ | − | sTCC | 49 | ↑ | \ | ↓ |
| Haptoglobin | 60 | − | ↑ | − | TBARs | 124 | − | \ | − |
| Hexanolycarnitine | 67 | ↓ | − | − | TGF-β1 | 167 | \ | − | − |
| Hif-1α | 47 | ↑ | − | − | TIMP-1 | 100, 172 | ↓ − | − \ | ↓ − |
| hs-CRP | 118 | − | ↓ | − | TIMP-2 | 100 | \ | − | ↓ |
| HSPA1A | 172 | − | ↑ | − | TIMP-3 | 100 | ↓ | − | ↓ |
| IgA-RF | 141 | − | ↓ | − | TIMP-4 | 100 | ↓ | − | ↓ |
| IgM-RF | 156 | − | ↓ | − | TNF-α | 40, 89, 172 | − − − | \ − \ | − ↓ − |
| IL-1 | 40, 111 | − ↓ | \ | − ↓ | VEGF | 40, 77 | − − | ↑ − | − ↓ |
| IL-6 | 40, 68, 89, 111, 153, 167, 172 | − ↑− \ − ↑ − | ↑ − − − − − ↑ | − − ↓ ↓ ↓ − − | Visfatin | 114 | ↑ | − | − |
| IL-8 | 40, 68, 89, 111, 112, 153, 172 | − ↑ − \ − − − | \ − − − ↑ − ↑ | − − ↓ ↓ − ↓− | Vitamin E | 124 | − | ↓ | − |
| IL-10 | 40 | − | ↓ | − |
“↑” = biomarker levels were significant higher in OA patients; “↓” = biomarker levels were significant lower in OA patients; “\” = no significant differences; “−” = not evaluated; RA, rheumatoid arthritis.
Discussion
The main finding of this systematic review is that in the literature SF biomarkers related to knee OA are numerous, with 201 identified molecules, and “burden of disease” and “diagnostic” categories being the most studied, compared to “prognostic” and “therapeutic” ones. However, while many biomarkers were found to correlate with disease features, each result is supported by limited and sometimes controversial findings.
The research on SF biomarkers can foster the scientific progress in the field of knee OA. In fact, SF presents potential key features because it is in direct contact with cartilage and synovial layer of the involved joint. In a joint with OA, the disease alters the composition of SF, with abnormal matrix turnover resulting in the release into the joint fluid of many molecules and fragments of matrix components derived from tissues such as the articular cartilage, bone, and synovium. 173 Some of these fragments can be assayed before they are even detectable in other samples such as serum and urine. 174 Therefore, SF can reflect more closely the alterations caused by OA in a singular joint, without undergoing systemic alterations due to other physiological or pathological processes originating from other tissues. 175 Moreover, many biomarkers produced by collagen II synthesis or degradation or by synovium turnover are found in the SF in higher concentrations than serum or urine, such as CD-RAP, COMP, MMP-1, MMP-3, MMP-13, and TIMP-1,141,142 offering the possibility of having a higher sensitivity and specificity of these markers in SF samples. Accordingly, the detection of biomarkers in SF seems more favorable than in other body fluids. On the other hand, the evaluation of SF also presents some limitations; while it bathes the intrinsic structures of diarthrodial joints offering the unique opportunity to study the entire joint, it is more difficult to obtain in the clinical practice, and its evaluation sometimes limited by the small quantity. 4 For this reason, researchers are looking for new tools that can improve biomarkers detection and measurement in SF, such as a recent permanent magnet introduced by Garraud et al. 176 With this technique, biomarkers may be collected from SF by injecting magnetic particles that are functionalized to bind with biomarker molecules within the fluid and then collecting these particle-biomarker conjugates from the fluid via a permanent magnet. Thus, this approach could allow for a more reliable collection of biomarkers from highly viscous biological fluids or complex anatomical locations without the need to remove the fluid from the patient.
The study of SF biomarkers is paramount also for other reasons, as it may allow to shed some light about OA pathogenesis, thanks to insights into the interaction of different biomarkers in the complex cascade which finally leads to OA. Following this concept, inflammatory biomarkers were the most studied, confirming the interest on these mediators produced by articular tissues in OA, being implicated in the pathogenesis. 177 In fact, IL-6 and IL-8 were the most studied, with 17 and 12 studies, respectively. Unfortunately, despite the high research interest due to the direct involvement in the disease processes, research led to often conflicting results, and the same was found for the other molecular categories. For example, MMPs are implicated in the loss of articular cartilage in OA, 178 but while they have been found to be often significantly higher in SF of advanced stage of knee OA compared with early stages, they were at times unrelated to knee OA severity or even to OA presence.68,126,150 Among other molecules correlated with the pathogenesis, adipokine leptin showed in most studies a positive correlation with both diagnosis and severity of knee OA.39,94,118,130 Moreover, VEGF showed in many studies a positive correlation with radiographic grading, ultrasonographic findings and functional status in knee OA, confirming the role of angiogenesis in the pathogenesis of OA.21,40,77,82,106
Overall, the literature analysis underlined a plethora of molecules investigated as SF biomarkers, but each one with a limited number of studies. Accordingly, while some molecules emerged as possible suitable candidates, most of them have either weak or even controversial findings. In this regard, biomarkers in serum and urine have often shown discordant results in SF. For example, COMP is a constituent of hyaline articular cartilage released from damaged cartilage into SF and hence into the circulation, and as such it was frequently studied in serum, with a recent meta-analysis showing elevated serum COMP in patients with knee OA where it was sensitive to OA disease severity. 179 However, the few studies on COMP levels in SF could not confirm such positive results, with the majority of the studies being not able to correlate OA severity and prognosis.50,77,111,128 Similarly, CTX-II, a marker related to collagen type II degradation, was the molecule most extensively investigated in urinary samples, 66 times in total as reported in a review by Van Spil et al., 11 with a strong correlation with knee OA grade and diagnosis. However, these results are weakly confirmed in SF, where the literature analysis showed only 2 out of 3 studies supporting a similar correlation.44,144
Controversial results are probably due to the current literature limitations, but they can be also related to the complexity of the OA processes, which are complicated and multidimensional and may affect differently the whole articular environment. In this light, evaluating one biomarker in the absence of a clear understanding of its interaction with other molecules can lead to erroneous conclusions. Each biomarker could play a small role in the summative outcome of interest; thus, the measurement of complex, composite biomarkers may enable better predictions. Different molecules or, even more, different modalities to assess OA biomarkers may offer complementary information, and aggregate markers may provide superior performance. New technologies are being develop such as interactome test, a method that allows the visualization and interpretation of complex interactions among large numbers of molecules and could therefore be used as a method of integrating multiple “omic” datasets to provide an understanding of the organizational complexity within the biological system. 180 The study of groups of proteins or proteins and metabolites that are highly interconnected can be used to identify key functions within an interactome network. 181 Accordingly, several studies are now focused on applying metabolomics in serum and in urine samples to detect a metabolic fingerprint of OA.182,183 However, this aspect is still not investigated in SF samples, with only preliminary evidence showing the ability of the metabolic profiles to distinguish OA patients from the controls in a small population, thus supporting the need for further efforts to develop this promising biomarker approach in large-scale metabolomic studies. 67 Similarly, the association between biological markers and imaging markers could provide better results. For example, Dam et al. 184 suggested that a combination of biochemical and MRI-based markers can improve diagnosis and prognosis of knee OA and may be useful to select high-risk patients for inclusion in disease-modifying osteoarthritis drugs (DMOAD) clinical trials. Although promising, the analysis of such heterogeneous aspects could seem challenging with the current technologies, but the emerging advances in machine learning protocols may open a completely new horizon for a multidimensional evaluation of OA patients. 185
The limitations of the current systematic review reflect those of the included studies and their limited quality. In fact, while the literature analysis was able to identify a large number of studies and 201 molecules, only a few papers supported each biomarker, thus impairing the possibility to draw definitive conclusions in this field. High-quality studies for a large-scale investigation of these SF biomarkers are crucial in order to increase the knowledge about the applicability of these biomarkers and even favor the understanding of OA pathogenesis. In particular, this systematic review underlined the paucity of longitudinal studies on biomarkers. In fact, only 21 studies, out of the 159 included, evaluated the biomarker changes over time. Considering that the very high interindividual variation in biomarker levels can most accurately be determined in a longitudinal fashion, research efforts should focus on further studies of this type. Moreover, further studies are needed to better understand the biomarker patterns in the early stages of OA, including the evaluation of biomarker changes in joints affected by ACL injuries, meniscal tears, and focal chondral lesions. In these clinical settings, changes in biomarker patterns have been demonstrated, showing the possibility to identify subsets of patients exhibiting dysregulation of the inflammatory response and an increased risk of posttraumatic osteoarthritis.186-188 Another limitation is that the literature presents scattered and incomplete information about several aspects, such as sex, type of OA, BMI, age, and their role in biomarker OA correlation. All these factors might influence the biomarker results, and future studies should investigate how these key aspects can affect the role of the plethora of molecules as synovial OA biomarkers.
Research efforts could identify new and more sensitive/specific biomarkers, or improve existing biomarker assays, with better technologies and platforms favoring a more consistent and standardized detection and measurement. In fact, most biomarkers are currently in an explorative stage, and there are still no “normal ranges” used by the various authors to foster study comparisons and the adoption of a common reference for different studies. Moreover, most of the literature retrieved did not provide conclusions on the sensitivity and specificity of the selected biomarkers. Due to the limitations and heterogeneity in data reporting, it was not possible to perform a meta-analysis of the results. On the other hand, the systematic review documented a rapidly evolving field, underlining potential and limitations of several molecules and identifying promising areas for future studies on biomarkers. To our knowledge, this is the first systematic evaluation of the literature on SF biomarkers carried out in accordance with the BIPEDs classification. BIPEDs classification aims at improving our collective ability to match a biomarker with its appropriate purpose to enable greater speed, efficiency, and precision in the development of useful diagnostic and therapeutic technologies and strategies, in the end providing a sound foundation for the development and implementation of public health policies. In this light, the BIPEDs classification in this systematic review could simplify the use of SF biomarkers for therapeutic development, clinical research, and patient care.
In conclusion, the systematic review of the literature identified numerous SF biomarkers. According to BIPEDs classification, out of the 201 SF biomarkers identified, the “burden of disease” category is the most represented with 132 different biomarkers, followed by the 106 “diagnostic” biomarkers, while “efficacy of intervention” and “prognostic” SF biomarkers were less investigated, being only 20 and 14, respectively. The most promising SF biomarkers were C4S, IL-6, IL-8, Leptin, MMP-1/3, TIMP-1, TNF-α, and VEGF. However, despite the large number of studies on the plethora of identified molecules, the evidence about the efficacy of each biomarker is supported by limited and often conflicting findings, and further research efforts are needed to improve the understanding of SF biomarkers for a better management of patients affected by knee OA.
Supplemental Material
Supplemental material, Table_S1_new for Synovial Fluid Biomarkers in Knee Osteoarthritis: A Systematic Review and Quantitative Evaluation Using BIPEDs Criteria by Angelo Boffa, Giulia Merli, Luca Andriolo, Christian Lattermann, Gian M. Salzmann and Giuseppe Filardo in CARTILAGE
Supplemental material, Table_S2_new for Synovial Fluid Biomarkers in Knee Osteoarthritis: A Systematic Review and Quantitative Evaluation Using BIPEDs Criteria by Angelo Boffa, Giulia Merli, Luca Andriolo, Christian Lattermann, Gian M. Salzmann and Giuseppe Filardo in CARTILAGE
Supplemental material, Table_S3_new_1 for Synovial Fluid Biomarkers in Knee Osteoarthritis: A Systematic Review and Quantitative Evaluation Using BIPEDs Criteria by Angelo Boffa, Giulia Merli, Luca Andriolo, Christian Lattermann, Gian M. Salzmann and Giuseppe Filardo in CARTILAGE
Footnotes
Supplementary material for this article is available on the Cartilage website at https://journals.sagepub.com/home/cara
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Angelo Boffa  https://orcid.org/0000-0002-1523-6900
https://orcid.org/0000-0002-1523-6900
Giulia Merli  https://orcid.org/0000-0001-7895-5606
https://orcid.org/0000-0001-7895-5606
Luca Andriolo  https://orcid.org/0000-0001-6352-9671
https://orcid.org/0000-0001-6352-9671
References
- 1. Mobasheri A, Batt M. An update on the pathophysiology of osteoarthritis. Ann Phys Rehabil Med. 2016;59(5-6):333-9. doi: 10.1016/j.rehab.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 2. Goldring SR, Goldring MB. Clinical aspects, pathology and pathophysiology of osteoarthritis. J Musculoskelet Neuronal Interact. 2006;6(4):376-8. [PubMed] [Google Scholar]
- 3. Bijlsma JW, Berenbaum F, Lafeber FPJG. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115-26. [DOI] [PubMed] [Google Scholar]
- 4. Madry H, Kon E, Condello V, Peretti GM, Steinwachs M, Seil R, et al. Early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1753-62. doi: 10.1007/s00167-016-4068-3 [DOI] [PubMed] [Google Scholar]
- 5. Kan HS, Chan PK, Chiu KY, Yan CH, Yeung SS, Ng YL, et al. Non-surgical treatment of knee osteoarthritis. Hong Kong Med J. 2019;25(2):127-33. doi: 10.12809/hkmj187600 [DOI] [PubMed] [Google Scholar]
- 6. Filardo G, Kon E, Longo UG, Madry H, Marchettini P, Marmotti A, et al. Non-surgical treatments for the management of early osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1775-85. doi: 10.1007/s00167-016-4089-y [DOI] [PubMed] [Google Scholar]
- 7. Lotz M, Martel-Pelletier J, Christiansen C, Brandi ML, Bruyère O, Chapurlat R, et al. Value of biomarkers in osteoarthritis: current status and perspectives. Ann Rheum Dis. 2013;72(11):1756-63. doi: 10.1136/annrheumdis-2013-203726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89-95. [DOI] [PubMed] [Google Scholar]
- 9. Califf RM. Biomarker definitions and their applications. Exp Biol Med (Maywood). 2018;243(3):213-21. doi: 10.1177/1535370217750088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bauer DC, Hunter DJ, Abramson SB, Attur M, Corr M, Felson D, et al. ; Osteoarthritis Biomarkers Network. classification of osteoarthritis biomarkers: a proposed approach. Osteoarthritis Cartilage. 2006;14(8):723-7. [DOI] [PubMed] [Google Scholar]
- 11. van Spil WE, DeGroot J, Lems WF, Oostveen JC, Lafeber FPJG. Serum and urinary biochemical markers for knee and hip-osteoarthritis: a systematic review applying the consensus BIPED criteria. Osteoarthritis Cartilage. 2010;18(5):605-12. doi: 10.1016/j.joca.2010.01.012 [DOI] [PubMed] [Google Scholar]
- 12. Roberts S, Evans H, Wright K, van Niekerk L, Caterson B, Richardson JB, et al. ADAMTS-4 activity in synovial fluid as a biomarker of inflammation and effusion. Osteoarthritis Cartilage. 2015;23(9):1622-6. doi: 10.1016/j.joca.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PZ, Ionnidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kraus VB, Burnett B, Coindreau J, Cottrell S, Eyre D, Gendreau M, et al. ; OARSI FDA Osteoarthritis Biomarkers Working Group. Application of biomarkers in the development of drugs intended for the treatment of osteoarthritis. Osteoarthritis Cartilage. 2011;19(5):515-42. doi: 10.1016/j.joca.2010.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kwak YH, Kwak DK, Kim NY, Kim YJ, Lim JS, Yoo JH. Significant changes in synovial fluid microRNAs after high tibial osteotomy in medial compartmental knee osteoarthritis: identification of potential prognostic biomarkers. PLoS One. 2020;15(1_suppl):e0227596. doi: 10.1371/journal.pone.0227596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Watt FE, Hamid B, Garriga C, Judge A, Hrusecka R, Custers RJH, et al. The molecular profile of synovial fluid changes upon joint distraction and is associated with clinical response in knee osteoarthritis. Osteoarthritis Cartilage. 2020;28(3):324-33. doi: 10.1016/j.joca.2019.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lai Z, Cao Y. Plasma miR-200c-3p, miR-100-5p, and miR-1826 serve as potential diagnostic biomarkers for knee osteoarthritis: randomized controlled trials. Medicine (Baltimore). 2019;98(51):e18110. doi: 10.1097/MD.0000000000018110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guan J, Li Y, Ding LB, Liu GY, Zheng XF, Xue W, et al. Relationship between serum and synovial fluid CCL20 concentrations with disease severity in primary knee osteoarthritis. J Musculoskelet Neuronal Interact. 2019;19(3):326-32. [PMC free article] [PubMed] [Google Scholar]
- 19. Okuyan HM, Terzi MY, Ozcan O, Kalaci A. Association of UCMA levels in serum and synovial fluid with severity of knee osteoarthritis. Int J Rheum Dis. 2019;22(10):1884-90. doi: 10.1111/1756-185X.13682 [DOI] [PubMed] [Google Scholar]
- 20. Nugzar O, Zandman-Goddard G, Oz H, Lakstein D, Feldbrin Z, Shargorodsky M. The role of ferritin and adiponectin as predictors of cartilage damage assessed by arthroscopy in patients with symptomatic knee osteoarthritis. Best Pract Res Clin Rheumatol. 2018;32(5):662-68. doi: 10.1016/j.berh.2019.04.004 [DOI] [PubMed] [Google Scholar]
- 21. Haraden CA, Huebner JL, Hsueh MF, Li YJ, Kraus VB. Synovial fluid biomarkers associated with osteoarthritis severity reflect macrophage and neutrophil related inflammation. Arthritis Res Ther. 2019;21(1_suppl):146. doi: 10.1186/s13075-019-1923-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bournazou E, Samuels J, Zhou H, Krasnokutsky S, Patel J, Han T, et al. Vascular adhesion protein-1 (VAP-1) as predictor of radiographic severity in symptomatic knee osteoarthritis in the New York University Cohort. Int J Mol Sci. 2019;20(11):2642. doi: 10.3390/ijms20112642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang X, Zhong L, van Helvoort E, Lafeber F, Mastbergen S, Hendriks J, et al. The expressions of Dickkopf-related protein 1 and frizzled-related protein are negatively correlated to local inflammation and osteoarthritis severity. Cartilage. Epub 2019 Apr 4. doi: 10.1177/1947603519841676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Udomsinprasert W, McConachie E, Ngarmukos S, Theerawattanapong N, Tanavalee A, Honsawek S. Plasma and joint fluid glypican-3 are inversely correlated with the severity of knee osteoarthritis. Cartilage. Epub 2019 Apr 4. doi: 10.1177/1947603519841679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guan J, Li Y, Ding LB, Liu GY, Zheng XF, Xue W, et al. Synovial fluid alpha-melanocyte-stimulating hormone may act as a protective biomarker for primary knee osteoarthritis. Discov Med. 2019;27(146):17-26. [PubMed] [Google Scholar]
- 26. Vertti RDAP, Muñiz LSA, Martínez JM, Galarza FFG, Astorga RA. Cartilage oligomeric matrix protein levels in type 2 diabetes associated with primary knee osteoarthritis patients. Genet Test Mol Biomarkers. 2019;23(1_suppl):16-22. doi: 10.1089/gtmb.2018.0184 [DOI] [PubMed] [Google Scholar]
- 27. Wang SL, Zhang R, Hu KZ, Li MQ, Li ZC. Interleukin-34 synovial fluid was associated with knee osteoarthritis severity: a cross-sectional study in knee osteoarthritis patients in different radiographic stages. Dis Markers. 2018;2018:2095480. doi: 10.1155/2018/2095480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao Y, Xu J. Synovial fluid-derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int Orthop. 2018;42(12):2865-72. doi: 10.1007/s00264-018-4093-6 [DOI] [PubMed] [Google Scholar]
- 29. Franz A, Joseph L, Mayer C, Harmsen JF, Schrumpf H, Fröbel J, et al. The role of oxidative and nitrosative stress in the pathology of osteoarthritis: novel candidate biomarkers for quantification of degenerative changes in the knee joint. Orthop Rev (Pavia). 2018;10(2):7460. doi: 10.4081/or.2018.7460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takano S, Uchida K, Inoue G, Matsumoto T, Aikawa J, Iwase D, et al. Vascular endothelial growth factor expression and their action in the synovial membranes of patients with painful knee osteoarthritis. BMC Musculoskelet Disord. 2018;19(1_suppl):204. doi: 10.1186/s12891-018-2127-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang X, Post JN, Zhong L, Leijten J, Larsson S, Karperien M, et al. Dickkopf-related protein 1 and gremlin 1 show different response than frizzled-related protein in human synovial fluid following knee injury and in patients with osteoarthritis. Osteoarthritis Cartilage. 2018;26(6):834-43. doi: 10.1016/j.joca.2018.02.904 [DOI] [PubMed] [Google Scholar]
- 32. Hwang IY, Youm YS, Cho SD, Choi SW, Bae MH, Park SJ, et al. Synovial fluid levels of TWEAK and matrix metallo-proteinase 1 in patients with ostearthritis, and associations with disease severity. J Orthop Surg (Hong Kong). 2018;26(1_suppl):2309499018760112. doi: 10.1177/2309499018760112 [DOI] [PubMed] [Google Scholar]
- 33. Leung YY, Haaland B, Huebner JL, Wong SBS, Tjai M, Wang C, et al. Colchicine lack of effectiveness in symptom and inflammation modification in knee osteoarthritis (COLKOA): a randomized controlled trial. Osteoarthritis Cartilage. 2018;26(5):631-40. doi: 10.1016/j.joca.2018.01.026 [DOI] [PubMed] [Google Scholar]
- 34. Zhang C, Li T, Chiu KY, Wen C, Xu A, Yan CH. FABP4 as a biomarker for knee osteoarthritis. Biomark Med. 2018;12(2):107-18. doi: 10.2217/bmm-2017-0207 [DOI] [PubMed] [Google Scholar]
- 35. Wu Q, Sun X, Du L. Association of fibulin-3 concentrations with the presence and severity of knee osteoarthritis: a cross-sectional study. Knee. 2017;24(6):1369-73. doi: 10.1016/j.knee.2017.04.018 [DOI] [PubMed] [Google Scholar]
- 36. Corigliano A, Preianò M, Terracciano R, Savino R, De Gori M, Galasso O, et al. C3f is a potential tool for the staging of osteoarthritis. J Biol Regul Homeost Agents. 2017;31(4 Suppl 1):29-35. [PubMed] [Google Scholar]
- 37. Chou CH, Attarian DE, Wisniewski HG, Band PA, Kraus VB. TSG-6—a double-edged sword for osteoarthritis (OA). Osteoarthritis Cartilage. 2018;26(2):245-54. doi: 10.1016/j.joca.2017.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yin CM, Suen WC, Lin S, Wu XM, Li G, Pan XH. Dysregulation of both miR-140-3p and miR-140-5p in synovial fluid correlate with osteoarthritis severity. Bone Joint Res. 2017;6(11):612-8. doi: 10.1302/2046-3758.611.BJR-2017-0090.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Panina SB, Krolevets IV, Milyutina NP, Sagakyants AB, Kornienko IV, Ananyan AA, et al. Circulating levels of proinflammatory mediators as potential biomarkers of post-traumatic knee osteoarthritis development. J Orthop Traumatol. 2017;18(4):349-57. doi: 10.1007/s10195-017-0473-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ding J, Niu X, Su Y, Li X. Expression of synovial fluid biomarkers in patients with knee osteoarthritis and meniscus injury. Exp Ther Med. 2017;14(2):1609-13. doi: 10.3892/etm.2017.4636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li ZM, Li M. Improvement in orthopedic outcome score and reduction in IL-1β, CXCL13, and TNF-α in synovial fluid of osteoarthritis patients following arthroscopic knee surgery. Genet Mol Res. 2017;16(3). doi: 10.4238/gmr16039487 [DOI] [PubMed] [Google Scholar]
- 42. Bhutia SC, Sherpa ML, Dewan SK, Singh TA. Correlation of cartilage metabolic markers & antioxidants with the severity of knee osteoarthritis. Indian J Med Res. 2016;144(6):932-4. doi: 10.4103/ijmr.IJMR_1235_14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Theologis T, Efstathopoulos N, Nikolaou V, Charikopoulos I, Papapavlos I, Kokkoris P, et al. Association between serum and synovial fluid Dickkopf-1 levels with radiographic severity in primary knee osteoarthritis patients. Clin Rheumatol. 2017;36(8):1865-72. doi: 10.1007/s10067-017-3640-7 [DOI] [PubMed] [Google Scholar]
- 44. Leung YY, Huebner JL, Haaland B, Wong SBS, Kraus VB. Synovial fluid pro-inflammatory profile differs according to the characteristics of knee pain. Osteoarthritis Cartilage. 2017;25(9):1420-7. doi: 10.1016/j.joca.2017.04.001 [DOI] [PubMed] [Google Scholar]
- 45. Zou YC, Deng HY, Mao Z, Zhao C, Huang J, Liu G. Decreased synovial fluid ghrelin levels are linked with disease severity in primary knee osteoarthritis patients and are increased following laser therapy. Clin Chim Acta. 2017;470:64-9. doi: 10.1016/j.cca.2017.04.014 [DOI] [PubMed] [Google Scholar]
- 46. Radojčić MR, Thudium CS, Henriksen K, Tan K, Karlsten R, Dudley A, et al. Biomarker of extracellular matrix remodelling C1M and proinflammatory cytokine interleukin 6 are related to synovitis and pain in end-stage knee osteoarthritis patients. Pain. 2017;158(7):1254-63. doi: 10.1097/j.pain.0000000000000908 [DOI] [PubMed] [Google Scholar]
- 47. Qing L, Lei P, Liu H, Xie J, Wang L, Wen T, et al. Expression of hypoxia-inducible factor-1α in synovial fluid and articular cartilage is associated with disease severity in knee osteoarthritis. Exp Ther Med. 2017;13(1_suppl):63-8. doi: 10.3892/etm.2016.3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yi J, Jin Q, Zhang B, Wu X, Ge D. Gremlin-1 concentrations are correlated with the severity of knee osteoarthritis. Med Sci Monit. 2016;22:4062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Struglics A, Okroj M, Swärd P, Frobell R, Saxne T, Lohmander LS, et al. The complement system is activated in synovial fluid from subjects with knee injury and from patients with osteoarthritis. Arthritis Res Ther. 2016;18(1_suppl):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Neuman P, Dahlberg LE, Englund M, Struglics A. Concentrations of synovial fluid biomarkers and the prediction of knee osteoarthritis 16 years after anterior cruciate ligament injury. Osteoarthritis Cartilage. 2017;25(4):492-8. doi: 10.1016/j.joca.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 51. Liu L, Huang R, Ma D, Cheng W, Feng W, Xing D, et al. Correlation of adrenomedullin concentrations with knee osteoarthritis grade. Med Sci Monit. 2016;22:2775-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McCabe PS, Parkes MJ, Maricar N, Hutchinson CE, Freemont A, O’Neill TW, et al. Brief report: synovial fluid white blood cell count in knee osteoarthritis: association with structural findings and treatment response. Arthritis Rheumatol. 2017;69(1_suppl):103-7. doi: 10.1002/art.39829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ogawa H, Matsumoto K, Terabayashi N, Kawashima K, Takeuchi K, Akiyama H. Association of lubricin concentration in synovial fluid and clinical status of osteoarthritic knee. Mod Rheumatol. 2017;27(3):489-92. doi: 10.1080/14397595.2016.1209829 [DOI] [PubMed] [Google Scholar]
- 54. Zhang PL, Liu J, Xu L, Sun Y, Sun XC. Synovial fluid macrophage migration inhibitory factor levels correlate with severity of self-reported pain in knee osteoarthritis patients. Med Sci Monit. 2016;22:2182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huang ZY, Stabler T, Pei FX, Kraus VB. Both systemic and local lipopolysaccharide (LPS) burden are associated with knee OA severity and inflammation. Osteoarthritis Cartilage. 2016;24(10):1769-75. doi: 10.1016/j.joca.2016.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li YH, Tavallaee G, Tokar T, Nakamura A, Sundararajan K, Weston A, et al. Identification of synovial fluid microRNA signature in knee osteoarthritis: differentiating early- and late-stage knee osteoarthritis. Osteoarthritis Cartilage. 2016;24(9):1577-86. doi: 10.1016/j.joca.2016.04.019 [DOI] [PubMed] [Google Scholar]
- 57. Bing W, Feng L. Attenuate synovial fluid uncarboxylated matrix Gla-protein (ucMGP) concentrations are linked with radiographic progression in knee osteoarthritis. Adv Clin Exp Med. 2015;24(6):1013-7. doi: 10.17219/acem/33824 [DOI] [PubMed] [Google Scholar]
- 58. Barreto G, Soininen A, Ylinen P, Sandelin J, Konttinen YT, Nordström DC, et al. Soluble biglycan: a potential mediator of cartilage degradation in osteoarthritis. Arthritis Res Ther. 2015;17:379. doi: 10.1186/s13075-015-0902-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mao Y, Xu W, Xie Z, Dong Q. Association of irisin and CRP levels with the radiographic severity of knee osteoarthritis. Genet Test Mol Biomarkers. 2016;20(2):86-9. doi: 10.1089/gtmb.2015.0170 [DOI] [PubMed] [Google Scholar]
- 60. Liao W, Li Z, Zhang H, Li J, Wang K, Yang Y. Proteomic analysis of synovial fluid as an analytical tool to detect candidate biomarkers for knee osteoarthritis. Int J Clin Exp Pathol. 2015;8(9):9975-89. [PMC free article] [PubMed] [Google Scholar]
- 61. Larsson S, Englund M, Struglics A, Lohmander LS. Interleukin-6 and tumor necrosis factor alpha in synovial fluid are associated with progression of radiographic knee osteoarthritis in subjects with previous meniscectomy. Osteoarthritis Cartilage. 2015;23(11):1906-14. doi: 10.1016/j.joca.2015.05.035 [DOI] [PubMed] [Google Scholar]
- 62. Song YZ, Guan J, Wang HJ, Ma W, Li F, Xu F, et al. Possible involvement of serum and synovial fluid resistin in knee osteoarthritis: cartilage damage, clinical, and radiological links. J Clin Lab Anal. 2016;30(5):437-43. doi: 10.1002/jcla.21876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. He W, Wang M, Wang Y, Wang Q, Luo B. Plasma and synovial fluid CXCL12 levels are correlated with disease severity in patients with knee osteoarthritis. J Arthroplasty. 2016;31(2):373-7. doi: 10.1016/j.arth.2015.08.018 [DOI] [PubMed] [Google Scholar]
- 64. Guan J, Liu Z, Li F, Feng JS, Wang HJ, Chu JG, et al. Increased synovial fluid YKL-40 levels are linked with symptomatic severity in knee osteoarthritis patients. Clin Lab. 2015;61(8):991-7. [DOI] [PubMed] [Google Scholar]
- 65. Ke X, Jin G, Yang Y, Cao X, Fang R, Feng X, et al. Synovial fluid HMGB-1 levels are associated with osteoarthritis severity. Clin Lab. 2015;61(7):809-18. [DOI] [PubMed] [Google Scholar]
- 66. Liu Y, Peng H, Meng Z, Wei M. Correlation of IL-17 level in synovia and severity of knee osteoarthritis. Med Sci Monit. 2015;21:1732-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mickiewicz B, Kelly JJ, Ludwig TE, Weljie AM, Wiley JP, Schmidt TA, et al. Metabolic analysis of knee synovial fluid as a potential diagnostic approach for osteoarthritis. J Orthop Res. 2015;33(11):1631-8. doi: 10.1002/jor.22949 [DOI] [PubMed] [Google Scholar]
- 68. Monibi F, Roller BL, Stoker A, Garner B, Bal S, Cook JL. Identification of synovial fluid biomarkers for knee osteoarthritis and correlation with radiographic assessment. J Knee Surg. 2016;29(3):242-7. doi: 10.1055/s-0035-1549022 [DOI] [PubMed] [Google Scholar]
- 69. Honsawek S, Wilairatana V, Udomsinprasert W, Sinlapavilawan P, Jirathanathornnukul N. Association of plasma and synovial fluid periostin with radiographic knee osteoarthritis: cross-sectional study. Joint Bone Spine. 2015;82:352-5. doi: 10.1016/j.jbspin.2015.01.023 [DOI] [PubMed] [Google Scholar]
- 70. Dong T, Chang H, Zhang F, Chen W, Zhu Y, Wu T, et al. Calcitonin gene-related peptide can be selected as a predictive biomarker on progression and prognosis of knee osteoarthritis. Int Orthop. 2015;39(6):1237-43. doi: 10.1007/s00264-015-2744-4 [DOI] [PubMed] [Google Scholar]
- 71. Huo LW, Ye YL, Wang GW, Ye YG. Fractalkine (CX3CL1): a biomarker reflecting symptomatic severity in patients with knee osteoarthritis. J Investig Med. 2015;63(4):626-31. doi: 10.1097/JIM.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 72. Mabey T, Taleongpong P, Udomsinprasert W, Jirathanathornnukul N, Honsawek S. Plasma and synovial fluid autotaxin correlate with severity in knee osteoarthritis. Clin Chim Acta. 2015;444:72-7. doi: 10.1016/j.cca.2015.01.032 [DOI] [PubMed] [Google Scholar]
- 73. Gao F, Tian J, Pan H, Gao J, Yao M. Association of CCL13 levels in serum and synovial fluid with the radiographic severity of knee osteoarthritis. J Investig Med. 2015;63(3):545-7. doi: 10.1097/JIM.0000000000000150 [DOI] [PubMed] [Google Scholar]
- 74. Liu Y, Hou R, Yin R, Yin W. Correlation of bone morphogenetic protein-2 levels in serum and synovial fluid with disease severity of knee osteoarthritis. Med Sci Monit. 2015;21:363-70. doi: 10.12659/MSM.892160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Li ZC, Xiao J, Wang G, Li MQ, Hu KZ, Ma T, et al. Fibroblast growth factor-21 concentration in serum and synovial fluid is associated with radiographic bone loss of knee osteoarthritis. Scand J Clin Lab Invest. 2015;75(2):121-5. doi: 10.3109/00365513.2014.992942 [DOI] [PubMed] [Google Scholar]
- 76. Daghestani HN, Pieper CF, Kraus VB. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol. 2015;67(4):956-65. doi: 10.1002/art.39006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kim HR, Lee JH, Kim KW, Kim BM, Lee SH. The relationship between synovial fluid VEGF and serum leptin with ultrasonographic findings in knee osteoarthritis. Int J Rheum Dis. 2016;19(3):233-40. doi: 10.1111/1756-185X.12486 [DOI] [PubMed] [Google Scholar]
- 78. Chu H, Xu ZM, Yu H, Zhu KJ, Huang H. Association between hypoxia-inducible factor-1a levels in serum and synovial fluid with the radiographic severity of knee osteoarthritis. Genet Mol Res. 2014;13(4):10529-36. doi: 10.4238/2014.December.12.15 [DOI] [PubMed] [Google Scholar]
- 79. Kusayama Y, Akamatsu Y, Kumagai K, Kobayashi H, Aratake M, Saito T. Changes in synovial fluid biomarkers and clinical efficacy of intra-articular injections of hyaluronic acid for patients with knee osteoarthritis. J Exp Orthop. 2014;1(1_suppl):16. doi: 10.1186/s40634-014-0016-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang L, Zhang L, Pan H, Peng S, Lv M, Lu WW. Levels of neuropeptide Y in synovial fluid relate to pain in patients with knee osteoarthritis. BMC Musculoskelet Disord. 2014;15:319. doi: 10.1186/1471-2474-15-319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li L, Jiang BE. Serum and synovial fluid chemokine ligand 2/monocyte chemoattractant protein 1 concentrations correlates with symptomatic severity in patients with knee osteoarthritis. Ann Clin Biochem. 2015;52(Pt 2):276-82. doi: 10.1177/0004563214545117 [DOI] [PubMed] [Google Scholar]
- 82. Mabey T, Honsawek S, Saetan N, Poovorawan Y, Tanavalee A, Yuktanandana P. Angiogenic cytokine expression profiles in plasma and synovial fluid of primary knee osteoarthritis. Int Orthop. 2014;38(9):1885-92. doi: 10.1007/s00264-014-2406-y [DOI] [PubMed] [Google Scholar]
- 83. Cui Z, Liu K, Wang A, Liu S, Wang F, Li J. Correlation between sialic acid levels in the synovial fluid and the radiographic severity of knee osteoarthritis. Exp Ther Med. 2014;8(1_suppl):255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Koskinen A, Vuolteenaho K, Moilanen T, Moilanen E. Resistin as a factor in osteoarthritis: synovial fluid resistin concentrations correlate positively with interleukin 6 and matrix metalloproteinases MMP-1 and MMP-3. Scand J Rheumatol. 2014;43(3):249-53. doi: 10.3109/03009742.2013.853096 [DOI] [PubMed] [Google Scholar]
- 85. Simão AP, Almeida TM, Mendonça VA, Santos SA, Gomes WF, Coimbra CC, et al. Soluble TNF receptors are produced at sites of inflammation and are inversely associated with self-reported symptoms (WOMAC) in knee osteoarthritis. Rheumatol Int. 2014;34(12):1759-63. doi: 10.1007/s00296-014-3016-0 [DOI] [PubMed] [Google Scholar]
- 86. Mabey T, Honsawek S, Tanavalee A, Wilairatana V, Yuktanandana P, Saetan N, et al. Plasma and synovial fluid sclerostin are inversely associated with radiographic severity of knee osteoarthritis. Clin Biochem. 2014;47(7-8):547-51. doi: 10.1016/j.clinbiochem.2014.03.011 [DOI] [PubMed] [Google Scholar]
- 87. Kosinska MK, Liebisch G, Lochnit G, Wilhelm J, Klein H, Kaesser U, et al. Sphingolipids in human synovial fluid—a lipidomic study. PLoS One. 2014;9(3):e91769. doi: 10.1371/journal.pone.0091769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Germaschewski FM, Matheny CJ, Larkin J, Liu F, Thomas LR, Saunders JS, et al. Quantitation OF ARGS aggrecan fragments in synovial fluid, serum and urine from osteoarthritis patients. Osteoarthritis Cartilage. 2014;22(5):690-7. doi: 10.1016/j.joca.2014.02.930 [DOI] [PubMed] [Google Scholar]
- 89. Valcamonica E, Chighizola CB, Comi D, De Lucia O, Pisoni L, Murgo A, et al. Levels of chemerin and interleukin 8 in the synovial fluid of patients with inflammatory arthritides and osteoarthritis. Clin Exp Rheumatol. 2014;32(2):243-50. [PubMed] [Google Scholar]
- 90. Simão AP, Mendonça VA, de Oliveira Almeida TM, Santos SA, Gomes WF, Coimbra CC, et al. Involvement of BDNF in knee osteoarthritis: the relationship with inflammation and clinical parameters. Rheumatol Int. 2014;34(8):1153-7. doi: 10.1007/s00296-013-2943-5 [DOI] [PubMed] [Google Scholar]
- 91. Wisniewski HG, Colón E, Liublinska V, Karia RJ, Stabler TV, Attur M, et al. TSG-6 activity as a novel biomarker of progression in knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(2):235-41. doi: 10.1016/j.joca.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bellucci F, Meini S, Cucchi P, Catalani C, Nizzardo A, Riva A, et al. Synovial fluid levels of bradykinin correlate with biochemical markers for cartilage degradation and inflammation in knee osteoarthritis. Osteoarthritis Cartilage. 2013;21(11):1774-80. doi: 10.1016/j.joca.2013.08.014 [DOI] [PubMed] [Google Scholar]
- 93. Zou Y, Li Y, Lu L, Lin Y, Liang W, Su Z, et al. Correlation of fractalkine concentrations in serum and synovial fluid with the radiographic severity of knee osteoarthritis. Ann Clin Biochem. 2013;50(Pt 6):571-5. doi: 10.1177/0004563213480494 [DOI] [PubMed] [Google Scholar]
- 94. Lübbeke A, Finckh A, Puskas GJ, Suva D, Lädermann A, Bas S, et al. Do synovial leptin levels correlate with pain in end stage arthritis? Int Orthop. 2013;37(10):2071-9. doi: 10.1007/s00264-013-1982-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wei M, Duan D, Liu Y, Wang Z, Li Z. Increased thymosin β4 levels in the serum and SF of knee osteoarthritis patients correlate with disease severity. Regul Pept. 2013;185:34-6. doi: 10.1016/j.regpep.2013.06.011 [DOI] [PubMed] [Google Scholar]
- 96. Zhou SJ, Sun ZX, Liu J. Neopterin concentrations in synovial fluid may reflect disease severity in patients with osteoarthritis. Scand J Clin Lab Invest. 2013;73(4):344-8. doi: 10.3109/00365513.2013.783228 [DOI] [PubMed] [Google Scholar]
- 97. Wang LC, Zhang HY, Shao L, Chen L, Liu ZH, He X, et al. S100A12 levels in synovial fluid may reflect clinical severity in patients with primary knee osteoarthritis. Biomarkers. 2013;18(3):216-20. [DOI] [PubMed] [Google Scholar]
- 98. Li ZG, Zhao DW, Xia CJ, Wang TN, Liu YP, Zhang Y, et al. Decreased synovial fluid omentin-1 concentrations reflect symptomatic severity in patients with knee osteoarthritis. Scand J Clin Lab Invest. 2012;72(8):623-8. doi: 10.3109/00365513.2012.726370 [DOI] [PubMed] [Google Scholar]
- 99. Misko TP, Radabaugh MR, Highkin M, Abrams M, Friese O, Gallavan R, et al. Characterization of nitrotyrosine as a biomarker for arthritis and joint injury. Osteoarthritis Cartilage. 2013;21(1_suppl):151-6. doi: 10.1016/j.joca.2012.09.005 [DOI] [PubMed] [Google Scholar]
- 100. Heard BJ, Martin L, Rattner JB, Frank CB, Hart DA, Krawetz R. Matrix metalloproteinase protein expression profiles cannot distinguish between normal and early osteoarthritic synovial fluid. BMC Musculoskelet Disord. 2012;13:126. doi: 10.1186/1471-2474-13-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Xu Q, Sun XC, Shang XP, Jiang HS. Association of CXCL12 levels in synovial fluid with the radiographic severity of knee osteoarthritis. J Investig Med. 2012;60(6):898-901. doi: 10.2310/JIM.0b013e31825f9f69 [DOI] [PubMed] [Google Scholar]
- 102. Nakajima A, Nakagawa K, Aoki Y, Sonobe M, Shibata Y, Yamazaki M, et al. Changes in synovial fluid biochemical markers following arthroscopic surgery in patients with knee osteoarthritis. Rheumatol Int. 2013;33(1_suppl):209-14. doi: 10.1007/s00296-012-2374-8 [DOI] [PubMed] [Google Scholar]
- 103. Honsawek S, Yuktanandana P, Tanavalee A, Chirathaworn C, Anomasiri W, Udomsinprasert W, et al. Plasma and synovial fluid connective tissue growth factor levels are correlated with disease severity in patients with knee osteoarthritis. Biomarkers. 2012;17(4):303-8. doi: 10.3109/1354750X.2012.666676 [DOI] [PubMed] [Google Scholar]
- 104. Larsson S, Englund M, Struglics A, Lohmander LS. The association between changes in synovial fluid levels of ARGS-aggrecan fragments, progression of radiographic osteoarthritis and self-reported outcomes: a cohort study. Osteoarthritis Cartilage. 2012;20(5):388-95. doi: 10.1016/j.joca.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 105. Xu L, Zhu GB, Wang L, Wang DF, Jiang XR. Synovial fluid omentin-1 levels are inversely correlated with radiographic severity of knee osteoarthritis. J Investig Med. 2012;60(3):583-6. doi: 10.2310/JIM.0b013e31824443cb [DOI] [PubMed] [Google Scholar]
- 106. Rübenhagen R, Schüttrumpf JP, Stürmer KM, Frosch KH. Interleukin-7 levels in synovial fluid increase with age and MMP-1 levels decrease with progression of osteoarthritis. Acta Orthop. 2012;83(1_suppl):59-64. doi: 10.3109/17453674.2011.645195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Cattano NM, Driban JB, Balasubramanian E, Barbe MF, Amin M, Sitler MR. Biochemical comparison of osteoarthritic knees with and without effusion. BMC Musculoskelet Disord. 2011;12:273. doi: 10.1186/1471-2474-12-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Huang K, Du G, Li L, Liang H, Zhang B. Association of chemerin levels in synovial fluid with the severity of knee osteoarthritis. Biomarkers. 2012;17(1_suppl):16-20. doi: 10.3109/1354750X.2011.634028 [DOI] [PubMed] [Google Scholar]
- 109. Vangsness CT, Jr, Burke WS, Narvy SJ, MacPhee RD, Fedenko AN. Human knee synovial fluid cytokines correlated with grade of knee osteoarthritis—a pilot study. Bull NYU Hosp Jt Dis. 2011;69(2):122-7. [PubMed] [Google Scholar]
- 110. Saetan N, Honsawek S, Tanavalee A, Tantavisut S, Yuktanandana P, Parkpian V. Association of plasma and synovial fluid interferon-γ inducible protein-10 with radiographic severity in knee osteoarthritis. Clin Biochem. 2011;44(14-15):1218-22. doi: 10.1016/j.clinbiochem.2011.07.010 [DOI] [PubMed] [Google Scholar]
- 111. Kokebie R, Aggarwal R, Lidder S, Hakimiyan AA, Rueger DC, Block JA, et al. The role of synovial fluid markers of catabolism and anabolism in osteoarthritis, rheumatoid arthritis and asymptomatic organ donors. Arthritis Res Ther. 2011;13(2):R50. doi: 10.1186/ar3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Pierzchala AW, Kusz DJ, Hajduk G. CXCL8 and CCL5 expression in synovial fluid and blood serum in patients with osteoarthritis of the knee. Arch Immunol Ther Exp (Warsz). 2011;59(2):151-5. doi: 10.1007/s00005-011-0115-4 [DOI] [PubMed] [Google Scholar]
- 113. Ellabban AS, Kamel SR, Ahmed SS, Osman AM. Receptor activator of nuclear factor kappa B ligand serum and synovial fluid level. A comparative study between rheumatoid arthritis and osteoarthritis. Rheumatol Int. 2012;32(6):1589-96. doi: 10.1007/s00296-011-1831-0 [DOI] [PubMed] [Google Scholar]
- 114. Duan Y, Hao D, Li M, Wu Z, Li D, Yang X, et al. Increased synovial fluid visfatin is positively linked to cartilage degradation biomarkers in osteoarthritis. Rheumatol Int. 2012;32(4):985-90. doi: 10.1007/s00296-010-1731-8 [DOI] [PubMed] [Google Scholar]
- 115. Honsawek S, Tanavalee A, Yuktanandana P, Ngarmukos S, Saetan N, Tantavisut S. Dickkopf-1 (Dkk-1) in plasma and synovial fluid is inversely correlated with radiographic severity of knee osteoarthritis patients. BMC Musculoskelet Disord. 2010;11:257. doi: 10.1186/1471-2474-11-257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hasegawa M, Segawa T, Maeda M, Yoshida T, Sudo A. Thrombin-cleaved osteopontin levels in synovial fluid correlate with disease severity of knee osteoarthritis. J Rheumatol. 2011;38(1_suppl):129-34. doi: 10.3899/jrheum.100637 [DOI] [PubMed] [Google Scholar]
- 117. Zamani B, Jamali R, Ehteram H. Synovial fluid adenosine deaminase and high-sensitivity C-reactive protein activity in differentiating monoarthritis. Rheumatol Int. 2012;32(1_suppl):183-8. doi: 10.1007/s00296-010-1602-3 [DOI] [PubMed] [Google Scholar]
- 118. El-Gendi SS, Moniem AE, Tawfik NM, Ashmawy MM, Mohammed OA, Mostafa AK, et al. Value of serum and synovial fluid activin A and inhibin A in some rheumatic diseases. Int J Rheum Dis. 2010;13(3):273-9. doi: 10.1111/j.1756-185X.2010.01532.x [DOI] [PubMed] [Google Scholar]
- 119. Kumahashi N, Naitou K, Nishi H, Oae K, Watanabe Y, Kuwata S, et al. Correlation of changes in pain intensity with synovial fluid adenosine triphosphate levels after treatment of patients with osteoarthritis of the knee with high-molecular-weight hyaluronic acid. Knee. 2011;18(3):160-4. doi: 10.1016/j.knee.2010.04.013 [DOI] [PubMed] [Google Scholar]
- 120. Chayanupatkul M, Honsawek S. Soluble receptor for advanced glycation end products (sRAGE) in plasma and synovial fluid is inversely associated with disease severity of knee osteoarthritis. Clin Biochem. 2010;43(13-14):1133-7. doi: 10.1016/j.clinbiochem.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 121. Helmark IC, Mikkelsen UR, Børglum J, Rothe A, Petersen MC, Andersen O, et al. Exercise increases interleukin-10 levels both intraarticularly and peri-synovially in patients with knee osteoarthritis: a randomized controlled trial. Arthritis Res Ther. 2010;12(4):R126. doi: 10.1186/ar3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Cheng T, Li FF, Zhao S, Peng XC, Zhang XL. Soluble P selectin in synovial fluid level is correlated with the radiographic severity of knee osteoarthritis. Clin Chim Acta. 2010;411(19-20):1529-31. doi: 10.1016/j.cca.2010.05.009 [DOI] [PubMed] [Google Scholar]
- 123. Hao D, Li M, Wu Z, Duan Y, Li D, Qiu G. Synovial fluid level of adiponectin correlated with levels of aggrecan degradation markers in osteoarthritis. Rheumatol Int. 2011;31(11):1433-7. doi: 10.1007/s00296-010-1516-0 [DOI] [PubMed] [Google Scholar]
- 124. Sutipornpalangkul W, Morales NP, Charoencholvanich K, Harnroongroj T. Lipid peroxidation, glutathione, vitamin E, and antioxidant enzymes in synovial fluid from patients with osteoarthritis. Int J Rheum Dis. 2009;12(4):324-8. doi: 10.1111/j.1756-185X.2009.01430.x [DOI] [PubMed] [Google Scholar]
- 125. Shimizu M, Higuchi H, Takagishi K, Shinozaki T, Kobayashi T. Clinical and biochemical characteristics after intra-articular injection for the treatment of osteoarthritis of the knee: prospective randomized study of sodium hyaluronate and corticosteroid. J Orthop Sci. 2010;15(1_suppl):51-6. doi: 10.1007/s00776-009-1421-0 [DOI] [PubMed] [Google Scholar]
- 126. Anitua E, Sánchez M, de la Fuente M, Azofra J, Zalduendo M, Aguirre JJ, et al. Relationship between investigative biomarkers and radiographic grading in patients with knee osteoarthritis. Int J Rheumatol. 2009;2009:747432. doi: 10.1155/2009/747432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Honsawek S, Tanavalee A, Yuktanandana P. Elevated circulating and synovial fluid endoglin are associated with primary knee osteoarthritis severity. Arch Med Res. 2009;40(7):590-4. doi: 10.1016/j.arcmed.2009.07.010 [DOI] [PubMed] [Google Scholar]
- 128. Addison S, Coleman RE, Feng S, McDaniel G, Kraus VB. Whole-body bone scintigraphy provides a measure of the total-body burden of osteoarthritis for the purpose of systemic biomarker validation. Arthritis Rheum. 2009;60(11):3366-73. doi: 10.1002/art.24856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Gao SG, Li KH, Zeng KB, Tu M, Xu M, Lei GH. Elevated osteopontin level of synovial fluid and articular cartilage is associated with disease severity in knee osteoarthritis patients. Osteoarthritis Cartilage. 2010;18(1_suppl):82-7. doi: 10.1016/j.joca.2009.07.009 [DOI] [PubMed] [Google Scholar]
- 130. Ku JH, Lee CK, Joo BS, An BM, Choi SH, Wang TH, et al. Correlation of synovial fluid leptin concentrations with the severity of osteoarthritis. Clin Rheumatol. 2009;28(12):1431-5. doi: 10.1007/s10067-009-1242-8 [DOI] [PubMed] [Google Scholar]
- 131. Larsson S, Lohmander LS, Struglics A. Synovial fluid level of aggrecan ARGS fragments is a more sensitive marker of joint disease than glycosaminoglycan or aggrecan levels: a cross-sectional study. Arthritis Res Ther. 2009;11(3):R92. doi: 10.1186/ar2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Honsawek S, Chayanupatkul M, Tanavalee A, Sakdinakiattikoon M, Deepaisarnsakul B, Yuktanandana P, et al. Relationship of plasma and synovial fluid BMP-7 with disease severity in knee osteoarthritis patients: a pilot study. Int Orthop. 2009;33(4):1171-5. doi: 10.1007/s00264-009-0751-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Scanzello CR, Umoh E, Pessler F, Diaz-Torne C, Miles T, Dicarlo E, et al. Local cytokine profiles in knee osteoarthritis: elevated synovial fluid interleukin-15 differentiates early from end-stage disease. Osteoarthritis Cartilage. 2009;17(8):1040-8. doi: 10.1016/j.joca.2009.02.011 [DOI] [PubMed] [Google Scholar]
- 134. Morovic-Vergles J, Culo MI, Gamulin S, Culo F. Cyclic adenosine 5′-monophosphate in synovial fluid of rheumatoid arthritis and osteoarthritis patients. Rheumatol Int. 2008;29(2):167-71. doi: 10.1007/s00296-008-0663-z [DOI] [PubMed] [Google Scholar]
- 135. Pilichou A, Papassotiriou I, Michalakakou K, Fessatou S, Fandridis E, Papachristou G, et al. High levels of synovial fluid osteoprotegerin (OPG) and increased serum ratio of receptor activator of nuclear factor-kappa B ligand (RANKL) to OPG correlate with disease severity in patients with primary knee osteoarthritis. Clin Biochem. 2008;41(9):746-9. doi: 10.1016/j.clinbiochem.2008.02.011 [DOI] [PubMed] [Google Scholar]
- 136. Hasegawa M, Nakoshi Y, Tsujii M, Sudo A, Masuda H, Yoshida T, et al. Changes in biochemical markers and prediction of effectiveness of intra-articular hyaluronan in patients with knee osteoarthritis. Osteoarthritis Cartilage. 2008;16(4):526-9. [DOI] [PubMed] [Google Scholar]
- 137. Momohara S, Okada N, Ikari K, Mizuno S, Okamoto H. Dermatan sulfate in the synovial fluid of patients with knee osteoarthritis. Mod Rheumatol. 2007;17(4):301-5. [DOI] [PubMed] [Google Scholar]
- 138. Steinbeck MJ, Nesti LJ, Sharkey PF, Parvizi J. Myeloperoxidase and chlorinated peptides in osteoarthritis: potential biomarkers of the disease. J Orthop Res. 2007;25(9):1128-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Sugimoto H, Yamada H, Terada N, Kanaji A, Kato S, Date H, et al. Intraarticular injection of high molecular weight hyaluronan for osteoarthritis of the knee—prediction of effectiveness with biological markers. J Rheumatol. 2006;33(12):2527. [PubMed] [Google Scholar]
- 140. Caspi D, Anouk M, Golan I, Paran D, Kaufman I, Wigler I, et al. Synovial fluid levels of anti-cyclic citrullinated peptide antibodies and IgA rheumatoid factor in rheumatoid arthritis, psoriatic arthritis, and osteoarthritis. Arthritis Rheum. 2006;55(1_suppl):53-6. [DOI] [PubMed] [Google Scholar]
- 141. Andereya S, Streich N, Schmidt-Rohlfing B, Mumme T, Müller-Rath R, Schneider U. Comparison of modern marker proteins in serum and synovial fluid in patients with advanced osteoarthrosis and rheumatoid arthritis. Rheumatol Int. 2006;26(5):432-8. [DOI] [PubMed] [Google Scholar]
- 142. Senolt L, Braun M, Olejárová M, Forejtová S, Gatterová J, Pavelka K. Increased pentosidine, an advanced glycation end product, in serum and synovial fluid from patients with knee osteoarthritis and its relation with cartilage oligomeric matrix protein. Ann Rheum Dis. 2005;64(6):886-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Hasegawa M, Hirata H, Sudo A, Kato K, Kawase D, Kinoshita N, et al. Tenascin-C concentration in synovial fluid correlates with radiographic progression of knee osteoarthritis. J Rheumatol. 2004;31(10):2021-6. [PubMed] [Google Scholar]
- 144. Lohmander LS, Atley LM, Pietka TA, Eyre DR. The release of crosslinked peptides from type II collagen into human synovial fluid is increased soon after joint injury and in osteoarthritis. Arthritis Rheum. 2003;48(11):3130-9. [DOI] [PubMed] [Google Scholar]
- 145. Miyaguchi M, Kobayashi A, Kadoya Y, Ohashi H, Yamano Y, Takaoka K. Biochemical change in joint fluid after isometric quadriceps exercise for patients with osteoarthritis of the knee. Osteoarthritis Cartilage. 2003;11(4):252-9. [DOI] [PubMed] [Google Scholar]
- 146. Kaufmann J, Mueller A, Voigt A, Carl HD, Gursche A, Zacher J, et al. Hydroxypyridinium collagen crosslinks in serum, urine, synovial fluid and synovial tissue in patients with rheumatoid arthritis compared with osteoarthritis. Rheumatology (Oxford). 2003;42(2):314-20. [DOI] [PubMed] [Google Scholar]
- 147. Sugiyama S, Itokazu M, Suzuki Y, Shimizu K. Procollagen II C propeptide level in the synovial fluid as a predictor of radiographic progression in early knee osteoarthritis. Ann Rheum Dis. 2003;62(1_suppl):27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Saito S, Kondo S, Mishima S, Ishiguro N, Hasegawa Y, Sandell LJ, et al. Analysis of cartilage-derived retinoic-acid-sensitive protein (CD-RAP) in synovial fluid from patients with osteoarthritis and rheumatoid arthritis. J Bone Joint Surg Br. 2002;84(7):1066-9. [DOI] [PubMed] [Google Scholar]
- 149. Futani H, Okayama A, Matsui K, Kashiwamura S, Sasaki T, Hada T, et al. Relation between interleukin-18 and PGE2 in synovial fluid of osteoarthritis: a potential therapeutic target of cartilage degradation. J Immunother. 2002;25(Suppl 1):S61-S64. [DOI] [PubMed] [Google Scholar]
- 150. Schmidt-Rohlfing B, Thomsen M, Niedhart C, Wirtz DC, Schneider U. Correlation of bone and cartilage markers in the synovial fluid with the degree of osteoarthritis. Rheumatol Int. 2002;21(5):193-9. [DOI] [PubMed] [Google Scholar]
- 151. Herrero-Beaumont G, Guerrero R, Sánchez-Pernaute O, Acebes C, Palacios I, Mas S, et al. Cartilage and bone biological markers in the synovial fluid of osteoarthritic patients after hyaluronan injections in the knee. Clin Chim Acta. 2001;308(1-2):107-15. [DOI] [PubMed] [Google Scholar]
- 152. Takemura M, Harada A, Mizuno M, Yano K, Yamada Y. Relationship between osteoprotegerin/osteoclastogenesis inhibitory factor concentration in synovial fluid and disease severity in individuals with osteoarthritis of the knee. Metabolism. 2001;50(1_suppl):1-2. [DOI] [PubMed] [Google Scholar]
- 153. Kaneko S, Satoh T, Chiba J, Ju C, Inoue K, Kagawa J. Interleukin-6 and interleukin-8 levels in serum and synovial fluid of patients with osteoarthritis. Cytokines Cell Mol Ther. 2000;6(2):71-9. [DOI] [PubMed] [Google Scholar]
- 154. Kobayashi T, Yoshihara Y, Yamada H, Fujikawa K. Procollagen IIC-peptide as a marker for assessing mechanical risk factors of knee osteoarthritis: effect of obesity and varus alignment. Ann Rheum Dis. 2000;59(12):982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Kageyama Y, Miyamoto S, Ozeki T, Hiyohsi M, Suzuki M, Nagano A. Levels of rheumatoid factor isotypes, metalloproteinase-3 and tissue inhibitor of metalloproteinase-1 in synovial fluid from various arthritides. Clin Rheumatol. 2000;19(1_suppl):14-20. [DOI] [PubMed] [Google Scholar]
- 156. Manicourt DH, Poilvache P, Van Egeren A, Devogelaer JP, Lenz ME, Thonar EJ. Synovial fluid levels of tumor necrosis factor alpha and oncostatin M correlate with levels of markers of the degradation of crosslinked collagen and cartilage aggrecan in rheumatoid arthritis but not in osteoarthritis. Arthritis Rheum. 2000;43(2):281-8. [DOI] [PubMed] [Google Scholar]
- 157. Calvet J, Orellana C, Navarro N, Galisteo C, Gratacós J, Larrosa M. Calprotectin as a useful marker of inflammation in knee osteoarthritis. Ann Rheum Dis. 2018;77(Suppl 2):1605-6. [Google Scholar]
- 158. Scotece M, Koskinen-Kolasa A, Moilanen T, Moilanen E, Vuolteenaho K. Novel adipokines associated with osteoarthritis: retinol binding protein 4 is produced by cartilage and correlates with matrix metalloproteinases in osteoarthritis patients. Osteoarthritis Cartilage. 2018;26(Suppl 1):S126-S127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Gamal M, Bassiouni H, Eldahan M, Zaky K, Ali A. Serum and synovial fluid levels of resistin as biomarker for primary knee osteoarthritis flare ups. Int J Rheum Dis. 2017;20(Suppl 1):50. [Google Scholar]
- 160. Bollmann M, Gronau T, Papz T, Lohmann CH, Bertrand J. Shed syndecan 4 in synovial fluid as a biomarker for OA severity. Osteoarthritis Cartilage. 2017;25(Suppl 1):S93-S94. [Google Scholar]
- 161. Anping C, Yan X, Jia F, Yuan L, Yang X. Inter-alpha-trypsin inhibitor heavy chain 4 (ITI-H4) is expressed in chondrocytes and a biomarker of OA in synovial fluid. Int J Rheum Dis. 2016;19(Suppl 2):135-6. [Google Scholar]
- 162. Athanasou N, Mantoku A, Kudo A, Inagaki Y, Mahoney D, Kashima T. Periostin expression in inflammatory and noninflammatory osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2016;24(Suppl 1):S69. [Google Scholar]
- 163. Yu S, Wisniewski HG., Lee E, Deakin T, Zhou XS, Karia R, et al. A pragmatic knee preservation registry to follow synovial fluid biomarkers and clinical outcome in patients with degenerative joint disease. J Orthop Res. 2016;34. [Google Scholar]
- 164. Duan GQ, Ren CF. Correlation between the severity of knee osteoarthritis and levels of chemerin in serum and synovial. Chin J Tissue Eng Res. 2015;19(2):177-81. [Google Scholar]
- 165. Gai P, Sun H, Wang G, Xu Q, Qi X, Zhang Z, et al. Collagen type II-specific neoepitope (CIIM) in synovial fluid as a potential biomarker for osteoarthritis. Gene Ther Mol Biol. 2014;16(1_suppl):227-35. [Google Scholar]
- 166. Kuziora M, Liu Z, Higgs BW, Brohawn P, Lehmann K, Pilataxi F, et al. A subset of osteoarthritis individuals has elevated IL-6 pathway activation associated with worse symptoms. Arthritis Rheum. 2013;65(Suppl 10):S59. [Google Scholar]
- 167. Beekhuizen M, Gierman L, Creemers LB, Dhert WJ, Huizinga TW, Van Osch GJ, et al. A descriptive study of synovial fluid changes in cytokine, chemokine and growth factor levels between osteoarthritis patients and healthy donors. Osteoarthritis Cartilage; 2012;20(Suppl 1):S79-S80. [Google Scholar]
- 168. Śenolt L, Gatterová J, Pavelka K. Cartilage oligomeric matrix protein (COMP) in serum and synovial fluid from patients with osteoarthritis and rheumatoid arthritis. Rheumatologia. 2005;19(3):123-28. [Google Scholar]
- 169. Jacobs CA, Vranceanu AM, Thompson KL, Lattermann C. Rapid progression of knee pain and osteoarthritis biomarkers greatest for patients with combined obesity and depression: data from the osteoarthritis initiative. Cartilage. 2020;11(1_suppl):38-46. doi: 10.1177/1947603518777577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Sano Y, Toyoshima S, Miki Y, Taketomi Y, Ito M, Lee H, et al. Activation of inflammation and resolution pathways of lipid mediators in synovial fluid from patients with severe rheumatoid arthritis compared with severe osteoarthritis. Asia Pac Allergy. 2020;10(2):e21. doi: 10.5415/apallergy.2020.10.e21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Li L, Li Z, Li Y, Hu X, Zhang Y, Fan P. Profiling of inflammatory mediators in the synovial fluid related to pain in knee osteoarthritis. BMC Musculoskelet Disord. 2020;21(1_suppl):99. doi: 10.1186/s12891-020-3120-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Alonso B, Bravo B, Mediavilla L, Gortazar AR, Forriol F, Vaquero J, et al. Osteoarthritis-related biomarkers profile in chronic anterior cruciate ligament injured knee. Knee. 2020;27(1_suppl):51-60. doi: 10.1016/j.knee.2019.12.007 [DOI] [PubMed] [Google Scholar]
- 173. Garnero P, Rousseau JC, Delmas PD. Molecular basis and clinical use of biochemical markers of bone, cartilage, and synovium in joint diseases. Arthritis Rheum. 2000;43(5):953-68. [DOI] [PubMed] [Google Scholar]
- 174. Blanco FJ. Osteoarthritis year in review 2014: we need more biochemical biomarkers in qualification phase. Osteoarthritis Cartilage. 2014;22(12):2025-32. doi: 10.1016/j.joca.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 175. Felson DT. The current and future status of biomarkers in osteoarthritis. J Rheumatol. 2014;41(5):834-6. doi: 10.3899/jrheum.140094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Garraud A, Velez C, Shah Y, Garraud N, Kozissnik B, Yarmola EG, et al. Investigation of the capture of magnetic particles from high-viscosity fluids using permanent magnets. IEEE Trans Biomed Eng. 2016;63(2):372-8. doi: 10.1109/TBME.2015.2458783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Daghestani HN, Kraus VB. Inflammatory biomarkers in osteoarthritis. Osteoarthritis Cartilage. 2015; 23(11):1890-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Takaishi H, Kimura T, Dalal S, Okada Y, D’Armiento J. Joint diseases and matrix metalloproteinases: a role for MMP-13. Curr Pharm Biotechnol. 2008;9:47-54. [DOI] [PubMed] [Google Scholar]
- 179. Hoch JM, Mattacola CG, McKeon JMM, Howard JS, Lattermann C. Serum cartilage oligomeric matrix protein (sCOMP) is elevated in patients with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2011;19(12):1396-404. doi:10.1016/j.joca. 2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Hulme CH, Stevens A, Dunn W, Heazell AEP, Hollywood K, Begley P, et al. Identification of the functional pathways altered by placental cell exposure to high glucose: lessons from the transcript and metabolite interactome. Sci Rep. 2018;8(1_suppl):5270. doi: 10.1038/s41598-018-22535-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Vidal M, Cusick ME, Barabási AL. Interactome networks and human disease. Cell. 2011;144(6):986-98. doi: 10.1016/j.cell.2011.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Zhai G, Wang-Sattler R, Hart DJ, Arden NK, Hakim AJ, Illig T, et al. Serum branched-chain amino acid to histidine ratio: a novel metabolomic biomarker of knee osteoarthritis.Ann Rheum Dis. 2010;69(6):1227-31. doi: 10.1136/ard.2009.120857 [DOI] [PubMed] [Google Scholar]
- 183. Lamers RJ, van Nesselrooij JH, Kraus VB, Jordan JM, Renner JB, Dragomir AD, et al. Identification of an urinary metabolite profile associated with osteoarthritis. Osteoarthritis Cartilage. 2005;13(9):762-8. [DOI] [PubMed] [Google Scholar]
- 184. Dam EB, Loog M, Christiansen C, Byrjalsen I, Folkesson J, Nielsen M, et al. Identification of progressors in osteoarthritis by combining biochemical and MRI-based markers. Arthritis Res Ther. 2009;11(4):R115. doi: 10.1186/ar2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Nehrer S, Ljuhar R, Steindl P, Simon R, Maurer D, Ljuhar D, et al. Automated knee osteoarthritis assessment increases physicians’ agreement rate and accuracy: data from the osteoarthritis initiative. Cartilage. Epub 2019 Nov 24. doi: 10.1177/1947603519888793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Jacobs CA, Hunt ER, Conley CE, Johnson DL, Stone AV, Huebner JL, et al. Dysregulated inflammatory response related to cartilage degradation after ACL injury. Med Sci Sports Exerc. 2020;52(3):535-41. doi: 10.1249/MSS.0000000000002161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Lattermann C, Jacobs CA, Bunnell MP, Jochimsen KN, Abt JP, Reinke EK, et al. Logistical challenges and design considerations for studies using acute anterior cruciate ligament injury as a potential model for early posttraumatic osteoarthritis. J Orthop Res. 2017;35(3):641-50. doi: 10.1002/jor.23329 [DOI] [PubMed] [Google Scholar]
- 188. King JD, Rowland G, Tezanos AGV, Warwick J, Kraus VB, Lattermann C, et al. Joint fluid proteome after anterior cruciate ligament rupture reflects an acute posttraumatic inflammatory and chondrodegenerative state. Cartilage. 2020;11(3):329-37. doi: 10.1177/1947603518790009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Table_S1_new for Synovial Fluid Biomarkers in Knee Osteoarthritis: A Systematic Review and Quantitative Evaluation Using BIPEDs Criteria by Angelo Boffa, Giulia Merli, Luca Andriolo, Christian Lattermann, Gian M. Salzmann and Giuseppe Filardo in CARTILAGE
Supplemental material, Table_S2_new for Synovial Fluid Biomarkers in Knee Osteoarthritis: A Systematic Review and Quantitative Evaluation Using BIPEDs Criteria by Angelo Boffa, Giulia Merli, Luca Andriolo, Christian Lattermann, Gian M. Salzmann and Giuseppe Filardo in CARTILAGE
Supplemental material, Table_S3_new_1 for Synovial Fluid Biomarkers in Knee Osteoarthritis: A Systematic Review and Quantitative Evaluation Using BIPEDs Criteria by Angelo Boffa, Giulia Merli, Luca Andriolo, Christian Lattermann, Gian M. Salzmann and Giuseppe Filardo in CARTILAGE