Abstract
Meniscus tissue deficiency resulting from primary meniscectomy or meniscectomy after failed repair is a clinical challenge because the meniscus has little to no capacity for regeneration. Loss of meniscus tissue has been associated with early-onset knee osteoarthritis due to an increase in joint contact pressures in meniscectomized knees. Clinically available replacement strategies range from allograft transplantation to synthetic implants, including the collagen meniscus implant, ACTIfit, and NUSurface. Although short-term efficacy has been demonstrated with some of these treatments, factors such as long-term durability, chondroprotective efficacy, and return to sport activities in young patients remain unpredictable. Investigations of cell-based and tissue-engineered strategies to treat meniscus tissue deficiency are ongoing.
Keywords: meniscus, meniscus augmentation, meniscus transplantation, procedures, tissue
Introduction
The meniscus plays a vital role in optimizing force transmission and providing stability in the knee. These fibrocartilaginous tissues are semilunar in shape and consist of a sparse distribution of cells surrounded by an abundant extracellular matrix that imparts the tissue’s mechanical function. Meniscal tears are common, and primary surgical options include partial meniscectomy or meniscal repair. As arthroscopic techniques have advanced and biologic augmentation strategies are being investigated, meniscal repairs are now being performed for all tear types, including those in the avascular (white-white) zone that have traditionally been treated with partial meniscectomy (e.g., radial and horizontal cleavage tears).
When meniscal repair fails or is not a valid option resulting in surgical meniscectomy, the loss of meniscal tissue results in a difficult challenge. The meniscus has little capacity for tissue regeneration, and meniscus tissue deficiency has been associated with early-onset knee osteoarthritis due to a decrease in tibiofemoral contact area and an increase in joint contact pressures, particularly among the active population. 1 Several treatment options exist for restoring the deficient meniscus, from allograft transplantation to artificial implants. Indications and contraindications for these treatment options are listed in Table 1 . Despite the improvement in clinical symptoms, the long-term chondroprotective effects from meniscal transplantation or synthetic implants are unclear. A few cell-based meniscal tissue replacement options are being investigated under clinical trials, but none are currently available to date.
Table 1.
Meniscus Replacement Strategies—Indications and Contraindications.
| Method | Indications | Contraindications |
|---|---|---|
| Meniscus allograft transplantation (MAT) | • Considerable meniscus deficiency with symptoms due to early compartment overload | • Diffuse chondral degeneration (grade 3 or 4)
and/or flattening of femoral condyle • Inflammatory arthropathy • Unaddressed ligamentous insufficiency • Unaddressed axial malalignment • Obesity |
| Collagen meniscus implant (CMI) and ACTIfit | • Segmental/partial meniscus deficiency with intact anterior and posterior horns and meniscal rim | |
| NUSurface | • Considerable meniscus deficiency with intact ≥2 mm meniscal rim |
Meniscus Allograft Transplantation
Meniscus allograft transplantation (MAT) has proven to be an effective solution for young and active symptomatic patients who have undergone meniscectomy ( Fig. 1 ).2-6 Ideally, MAT should be performed when there is absent or only mild preexisting arthrosis due to the graft’s capacity to reduce peak tibiofemoral contact pressures and potentially slow the rate of articular cartilage degeneration. Focal articular cartilage lesions in the same compartment, limb alignment, and ligamentous stability are all necessary clinical considerations that can be concurrently addressed to preserve the longevity of the meniscal allograft and optimize patient outcomes.
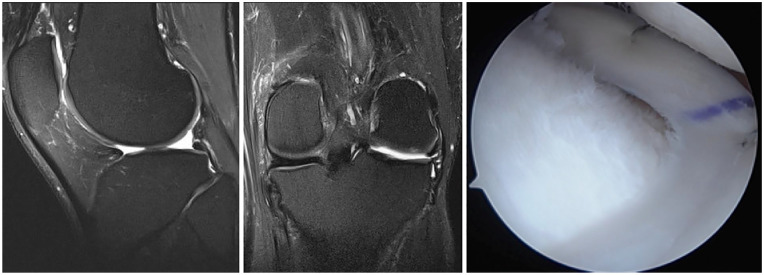
Figure 1.
Sagittal (left) and coronal (middle) magnetic resonance imaging of the left knee shows absent lateral meniscus after previous subtotal lateral meniscectomy. The patient was treated with lateral meniscus allograft transplantation (right) using bone plug fixation.
Graft Processing
In the United States, meniscus allograft tissue is most commonly distributed in fresh and fresh-frozen forms, while cryopreserved allografts are infrequently offered but remain an option in other countries ( Table 2 ). Lyophilization (freeze-drying) has fallen out of favor because of its deleterious effects on the mechanical properties of the allograft and graft shrinkage. Although some believe that preservation methods that maintain cell viability (i.e., fresh and cryopreserved) enhance graft survival and function, there has been no evidence to date demonstrating this supposed benefit. Data from animal models have shown a relatively rapid repopulation of donor graft tissue with recipient cells within a few weeks after transplantation, 7 thereby raising questions about the necessity of cell viability in optimizing graft survival and clinical outcomes. However, fresh-frozen grafts seem to have diminished collagen fiber architecture and biomechanics compared with fresh and cryopreserved grafts. 8 The lower cost and logistical benefits of fresh-frozen grafts account for their greater popularity at most centers.
Table 2.
Meniscus Allograft Preservation Methods.
| Preservation Method | Technique | Cell Viability | Immunogenicity | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Fresh | Storage at 4 °C | Yes | Yes | Native microarchitecture and material properties | Risk of disease transmission, short storage time, and logistical planning |
| Fresh-frozen | Deep freezing to −80 °C | No | Reduced | Prolonged storage, cost-effective | Altered collagen fiber architecture |
| Cryopreserved | Slow-freezing to −196 °C in an anhydrous environment to prevent intracellular water crystallization | Yes (4%-54%) | Yes | Preservation of collagen fiber architecture, prolonged storage | Expensive, decreased viability, and changes to cell metabolism |
| Lyophilized | Freeze-drying and storage at room temperature | No | Reduced | Unlimited storage | Deleterious effects on mechanical properties and graft shrinkage |
Treatment of meniscus allografts with gamma irradiation or chemical processes can be performed to reduce the risk of bacterial, fungal, and viral transmission. Sterilization typically results in killing of viable cells and is thus not performed on fresh and cryopreserved grafts. Dosages of radiation required to kill viruses (i.e., 1.5-2.0 mrad) can cause deleterious changes to the meniscus tissue biomechanical properties, 8 and therefore, use of nonirradiated grafts is preferable. Ethylene oxide gas sterilization, which is commonly used to sterilize medical devices, produces a metabolic byproduct (ethylene chlorohydrin) that causes significant synovitis and is therefore not recommended as a sterilization agent. 9 Other sterilization techniques such as supercritical CO2, which are purported to better preserve tissue properties over gamma irradiation, are being investigated. These emerging and proprietary sterilization techniques may be more appropriate for synthetic materials rather than biologic tissue grafts.
Graft Fixation
Peripheral fixation of meniscal allografts is traditionally performed using vertical-mattress sutures along with accurate reestablishment of the meniscal horns and roots. Both inside-out mattress sutures tied over the joint capsule and all-inside sutures are widely used. Some native peripheral rim tissue should be retained to decrease peripheral extrusion and provide a firm base to which the allograft is secured. Secure fixation of the horns and roots is crucial for permitting optimal distribution of hoop stresses throughout the meniscus allograft. Three main fixation methods for securing the graft horns and roots include (1) soft tissue only, (2) bone plugs, and (3) bone bridge. The optimal method for horn fixation continues to be debated. Recent cadaveric studies have suggested that bone plug fixation more closely reproduces the normal function of the meniscus compared with soft tissue fixation for medial meniscal allografts.10,11 For lateral meniscal allografts, Brial et al. 12 showed that although both bridge and bone plug fixation methods improved lateral tibiofemoral compartment contact mechanics compared with the meniscectomized state, bone bridge fixation better restored contact mechanics to that of the intact knee. Conversely, Novaretti et al. 13 found no differences between bone bridge and soft tissue fixation methods for lateral meniscal allografts regarding kinematics and forces experienced during applied loads. Clinical studies have yet to demonstrate superiority of bone fixation techniques, which are technically more demanding, over soft tissue fixation. 14 In a meta-analysis, Jauregui et al. 14 did not find significant differences in meniscal allograft tear rates, failure rates, or patient-reported outcomes between soft tissue suture and bone fixation methods. However, several of the studies included historical data that used lyophilized and/or irradiated grafts and older surgical techniques. Further studies are needed to determine the optimal fixation technique for MAT.
Clinical Outcomes
Many studies reporting on the outcomes of MAT are limited by low level of evidence; heterogeneity of data including patients, graft types, and techniques; inconsistent exclusion criteria for transplantation; and concomitant procedures that may confound results. As graft preparation and fixation techniques continue to advance, the clinical outcomes of MAT may be further optimized.
In studies evaluating the clinical outcomes of MAT, clinically significant improvements in patient-reported outcome scores, including the Lysholm, Knee Injury and Osteoarthritis Outcome Score (KOOS), and International Knee Documentation Committee (IKDC) scores, are noted at mid-term follow-up.2-5 Although functional improvement can be maintained up to 10 years, activity and sport-specific scores seem to decline during the interval between short-term/mid-term and long-term follow-up.3,15 For fresh-frozen grafts, 5-year graft survivorship is high, ranging from 84% to 95% ( Table 3 ). For cryopreserved grafts, 10+-year graft survivorship is reported to range from 45% to 71%.17-19 The presence of grade 4 articular cartilage loss or bipolar lesions at the time of MAT seems to portend worse graft survival,2,4 suggesting that early treatment of symptomatic patients may be optimal before significant chondrosis is sustained. Postoperatively, the incidence of graft tears ranges from 11% to 16%, although the majority of these cases do not necessitate full graft removal. 3 The wide variability in rate of graft tears reported is likely due to the use of different outcome measures for graft assessment, including MRI scan and second-look arthroscopy. When comparing medial MAT with lateral MAT, the functional outcomes and long-term survival rates appear to be similar. 20
Table 3.
Published Success Rates and Survivorship Following Meniscus Allograft Transplantation With Fresh-Frozen Grafts.
| Study | No. of Patients | Mean Follow-up (y) | Clinical Success Rates (%) | Graft Survivorship |
|---|---|---|---|---|
| Grassi et al. 3 | 46 | 10.8 | 60-82 | 86% at 10 y |
| Searle et al. 5 | 43 | 3.4 | 79 | 91% at 3.4 y |
| Zaffagnini et al. 6 | 147 | 4.0 | 84 | 95% at 6 y |
| Lee et al. 4 | 222 | 3.7 | 91 | 83.5% at 5 y |
| Bloch et al. 2 | 240 | 3.4 | — | 87.4% at 5 y |
| McCormick et al. 16 | 172 | 4.9 | — | 95% at 5 y |
Probability of returning to work after MAT can be high (>85%), 21 although this is likely dependent on the intensity of loading required on the knee for the specific occupation. In a cohort of active-duty military patients, only 20% were able to return to full duty, and 46% had permanent profile activity restrictions. 22 Although MAT is generally considered a salvage procedure with return to repetitive impact activities such as running and jumping generally being discouraged, recent studies suggest that return to modest sports activities in the short-term is a reasonable goal. In a meta-analysis, the majority of athletes and physically active patients (77%) were able to return to sport after MAT at a mean of 9.2 months postoperatively, and two-thirds of athletes were able to return to preinjury levels. 23 Graft-related reoperations, which were mostly partial meniscectomies, were reported in 13% of patients. 23
Although MAT appears to decrease tibiofemoral pain in the short-term, the long-term chondroprotective effects of the procedure remain unclear. In sheep and rabbits, MAT has been shown to protect the articular cartilage from degeneration. 24 While human cadaveric studies demonstrate the biomechanical benefits of MAT with reduced peak contact pressures compared with meniscectomized knees, MAT does not fully restore contact mechanics and kinematics to that of the intact knee.13,25 Articular cartilage benefits in animals and human biomechanical studies seem to agree with some clinical studies, but other clinical studies in humans have not found clear chondroprotective benefits. At a mean of 12 years, Verdonk et al. 26 found that 52% of patients did not show any change in joint space width, whereas all failure cases that were converted to total knee arthroplasty (TKA) were characterized by an increase in joint space narrowing. A systematic review evaluating the chondroprotective effects of MAT reported a weighted mean joint space loss of 0.032 mm at 4.5 years across 11 studies. 27 Although there is evidence to support the theory that MAT reduces the progression of osteoarthritis, the current data suggest that it is unlikely to be as effective as the native meniscus.
Synthetic Options
Two synthetic, scaffold-based meniscal substitutes for partial meniscus replacement are currently commercially available for clinical use: collagen meniscus implant (CMI; Stryker Corporation, Kalamazoo, MI, USA) and ACTIfit (Orteq Sports Medicine Ltd., London, UK). Both have demonstrated promising results in early clinical trials.28-30 In contrast to MAT, both can replace segmental meniscal defects, thereby preserving intact native meniscus tissue, and the off-the-shelf nature of synthetic grafts makes surgical planning easier. However, lack of cell migration, stress shielding, and degradation products causing chronic synovitis remain a concern with any scaffold-based treatment options. Another synthetic option, the NUSurface Meniscus Implant (Active Implants LLC, Memphis, TN, USA), is a non-anchored, non-absorbable meniscal prosthesis designed for total replacement of the medial meniscus. Although these options may be clinically available, lack of third-party insurance reimbursement has limited their clinical utilization.
Collagen Meniscus Implant
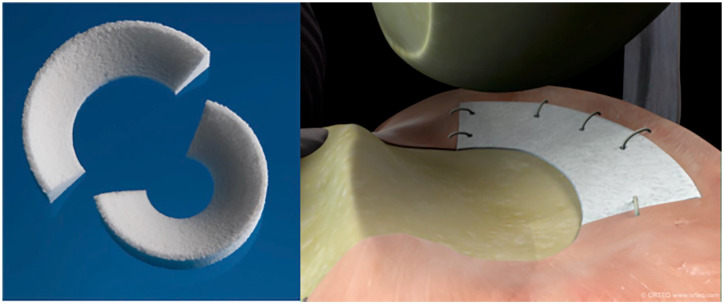
The CMI, which consists of type I collagen fibers derived from bovine Achilles tendons, has gained attention since the first clinical trial was published in 1997. It received US Food and Drug Administration (FDA) 510(k) clearance in 2008. Designed for segmental meniscal replacement, the implant is sized to match the prepared meniscal defect and sutured to the surrounding intact meniscus tissue ( Fig. 2 ). The bioresorbable scaffold is very porous, facilitating tissue ingrowth via proliferation of both fibroblasts and fibrochondrocytes as well as production of extracellular matrix. Second-look arthroscopy after CMI implantation has demonstrated formation of meniscus-like tissue grossly with variable degrees of maturity and integration to the host rim. 28 On MRI follow-up, remodeling of the CMI occurs up to 5 years after implantation as indicated by decreasing signal intensity and size; however, the majority of knees show persistent hyperintense signal compatible with myxoid degeneration. 31
Figure 2.
Photograph of collagen meniscal implant (CMI).
The majority of published clinical outcomes on the CMI are limited to treatment of medial meniscal defects, although short-term results after CMI treatment for lateral meniscal defects are available. 32 Ten-year data demonstrate significant clinical improvements after CMI, 33 but comparative studies are scarce. In a systematic review of 311 patients treated with CMI, the failure rate was 6.7% at a mean follow-up of 44 months. 29 Visual Analog Scale (VAS) pain, Lysholm, and Tegner scores were significantly improved at final follow-up, with most studies demonstrating improvement in Lysholm scores above minimal clinically important difference (MCID) and patient acceptable symptom state (PASS) thresholds. 29 A randomized, controlled, multicenter clinical trial showed that in patients with prior medial meniscal procedures, those treated with CMI regained significantly higher activity (Tegner Activity Scale) and were more satisfied compared with patients treated with repeat partial medial meniscectomy. However, in patients who had no prior meniscal surgery, no difference could be observed between CMI and partial meniscectomy treatment groups. 28 In another comparative study with a minimum of 10 years of follow-up, CMI-treated patients had better pain, activity level, and radiographic outcomes (less medial joint space narrowing) compared with patients treated with partial meniscectomy. 33
ACTIfit
The ACTIfit ( Fig. 3 ), composed of a synthetic hybrid of polycaprolactone (80%) and polyurethane (20%), was first described in a clinical trial in 2011 34 and was granted FDA Breakthrough Designation in 2020. Similar to the CMI, it was designed for segmental meniscal replacement. The ACTIfit scaffold degrades slowly over a 5-year period, starting with hydrolysis of the softer polycaprolactone segments, while the more rigid polyurethane is slowly removed by macrophages and giant cells. 35 At time zero, both the CMI and ACTIfit demonstrate significantly lower stiffness compared with native meniscus specimens and are absent of viscoelastic properties, with no notable biomechanical differences between the two artificial implants. 36 Tissue ingrowth and formation of meniscus-like fibrocartilage tissue are evident according to histological analysis and second-look arthroscopy. 34 At long-term follow-up, viability remains low in the resultant repair tissue, and the biomechanical properties of the remodeled implants do not approach that of the native meniscus. 37
Figure 3.
Photograph and in vivo illustration of ACTIfit implant (courtesy of Orteq Sports Medicine, London, UK).
To date, clinical studies on the ACTIfit are limited to short-term and mid-term case series. In the largest available series consisting of 155 patients, pain and knee scores improved postoperatively at 2 years and were stable at 5 years of follow-up. 30 Postoperative MRI demonstrated a small-sized implant with an irregular surface in the majority of cases. The overall surgical failure rate was 12.4% at 5 years, with no difference in failure rates between medial and lateral implants. 30 A few studies have attempted to compare the clinical outcomes of CMI versus ACTIfit and have demonstrated no differences in failure rate or improvement in patient-reported outcomes between groups.29,38
NUSurface
The NUSurface Meniscus Implant is a non-anatomical, discoid-shaped, free-floating meniscal substitute designed for total replacement of the medial meniscus. It is made of polycarbonate-urethane, a medical grade plastic. A biomechanical study showed that implantation of the NUSurface Meniscus Implant restores the average and peak tibiofemoral contact pressures to 93% and 92%, respectively, compared with the native medial meniscus. 39 The NUSurface was granted FDA Breakthrough Designation in 2019, and 2 Investigational Device Exemption (IDE) clinical trials are currently ongoing in the United States. In Europe and Israel, clinical use of the NUSurface has been ongoing since 2008, but there are minimal published outcomes data available. Preliminary results have demonstrated significant improvements in pain and KOOS scores at 12 months for the NUSurface compared with non-surgical therapy and a similar adverse event rate.40,41
Cell-Based Options
Several cell-based meniscal replacement options are being developed and tested in clinical trials. Although the following cell-based options are not currently approved by the FDA, orthopedic surgeons could benefit from knowledge of these options that may be commercially available in the future.
Cell Bandage (Azellon)
The Cell Bandage consists of expanded (passage 1) autologous bone marrow–derived mesenchymal stromal cells (MSCs) embedded in a collagen matrix. It is a 2-stage procedure, with the first stage involving harvesting of cells from host bone marrow, isolation, culture, and seeding onto the collagen matrix, followed by the second stage implantation. This treatment was designed for repair of meniscus tears in the avascular (white-white) zone that would otherwise be an indication for meniscectomy; the seeded scaffold is placed between torn edges of the meniscus, and the tissue is reapproximated using sutures. 42
Preclinical studies for the Cell Bandage used an ovine model, in which autologous bone marrow–derived MSCs were used. 42 Three out of 5 sheep showed successful healing in the white-white region of the meniscus at 13 weeks; however, no animals showed healed lesions after 6 months. In comparison, no animals in the collagen sponge-only and suture-only control groups showed signs of healing at either time point.
In a first in-human study (phase I clinical trial in the United Kingdom), implantation of the Cell Bandage appeared to be safe as no adverse local or systemic immune responses were reported. 42 The implant survived in 3 out of 5 patients at 24-month follow-up as indicated on MRI without any further treatments. Clinically significant improvement in IKDC and Tegner-Lysholm scores was observed at 12 months and maintained at 24 months. The two other patients developed recurrent symptoms due to retear or nonhealing of the meniscus before ultimately receiving subsequent meniscectomy. As the phase I clinical trial is still ongoing (EU Clinical Trials Register, 2010-024162-22), no results with longer follow-up have been published.
Chondrogen (Mesoblast)
Chondrogen consists of expanded (passage 2) allogeneic adult bone marrow–derived MSCs suspended in a sodium hyaluronate solution that is injected intra-articularly following partial meniscectomy. 43 As opposed to being a meniscus tissue substitute, Chondrogen is an augmentation biologic injectable therapy that attempts to enhance meniscus regeneration and tissue volume after meniscectomy. Human MSCs are derived from bone marrow aspirates collected from unrelated donors (18-30 years of age) and are not human leukocyte antigen (HLA)-matched to recipients.
A phase I/II, randomized, double-blind, controlled study on Chondrogen consisting of 55 patients has been reported. 43 After partial meniscectomy, patients received a single intra-articular injection of 50 million MSCs, 150 million MSCs, or a vehicle control. No patients in any of the treatment groups exhibited abnormal immune or hematologic responses. At 24 months, 3 patients (18%) from the 50 million MSC group exhibited a >15% increase in meniscus volume as found on MRI, while this was not observed in patients in the 150 million and control groups. Decreased VAS pain and increased Lysholm scores were seen at 24 months in all treatment groups with respect to baseline. This phase I/II study in the United States has been completed (ClinicalTrials.gov Identifier NCT00702741), although results have not yet been published.
Future Directions
Although MAT and synthetic meniscus replacement options may be effective in alleviating knee joint pain in the short-term, their long-term durability and chondroprotective effects are questionable. Inferior mechanical properties of these grafts presumably lead to their eventual failure over time. This motivates the development of tissue-engineered meniscus replacement and regeneration options that recapitulate the mechanical, structural, and compositional properties of native meniscus tissue. Tissue engineering researchers have proposed both scaffold-based and scaffold-free meniscus replacement options. Scaffold-based technologies, including 3-dimensional (3D)-printed biomimetic constructs, have been used in vivo to successfully replace the knee meniscus in a rabbit model after a total meniscectomy. 44 The unique advantage of 3D-printed biomimetic constructs is that it can be personalized to the patient anatomy with the use of MRI. 45 Furthermore, 3D-printed meniscus constructs can be fabricated with a variety of materials that may prevent immunorejection upon implantation, including bio-ink containing collagen and cells such as MSCs or meniscus fibrochondrocytes from autologous sources.45,46
Scaffold-free neomenisci ( Fig. 4 ) can be engineered with a self-assembling process using an abundant cell source and combined with external biochemical and biomechanical stimuli to enhance its mechanical and microstructural properties to approach those of native tissue. 47 The addition of mechanical and chemical stimuli during culture, alone or in combination, has been used to augment the mechanical and biochemical properties of self-assembled, tissue-engineered constructs.44,48 For example, using a combination of TGF-β1, chondroitinase ABC, lysyl oxidase-like protein 2, and lysophosphatidic acid induced matrix augmentation and directional remodeling in self-assembled neomenisci constructs, with synergistic increases in mechanical properties, biochemical content, and mechanical anisotropy. 47 Following the FDA paradigm for the translation of engineered tissues, large animal preclinical studies will have to be conducted to show the safety and efficacy of these meniscus replacement options before their implantation in human patients.
Figure 4.
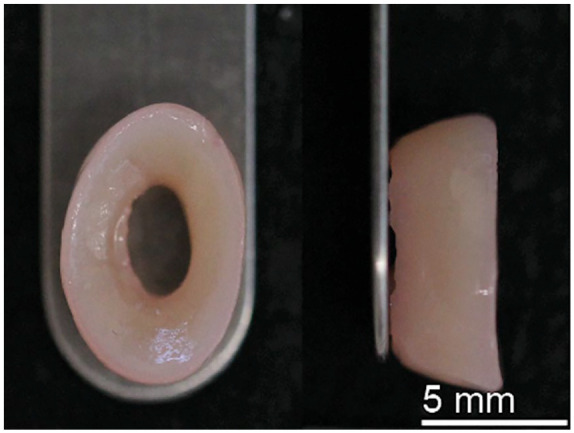
Tissue-engineered neomenisci using the scaffold-free, self-assembling process.
Summary
The meniscus plays an important role in protecting the health of the knee joint. Once meniscus tissue has been torn and removed from the joint, there is little to no capacity for the meniscus to regenerate lost tissue. MAT can be an effective treatment option, with proof of short-term and mid-term clinical functional improvement, although graft durability and return to sport activities remain a challenge. Artificial options, including the CMI, ACTIfit, and NUSurface, are being increasingly used, particularly for segmental defects, although implant durability and third-party insurance reimbursement remain challenges. Investigations of cell-based meniscal tissue replacement options are ongoing. Finally, tissue-engineered options that can generate biomimetic neomeniscus tissue may further optimize patient outcomes after treatment for meniscal tissue deficiency. Even with these advances, surgeons should continue to attempt meniscal repair whenever feasible and resect as little meniscal tissue as possible in tears that are deemed irreparable.
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: E.G.L., and K.A. received support from the following funding sources related to meniscus tissue engineering: National Institutes of Health R01AR071457, HHMI Gilliam Fellowship, National Science Foundation GRFP, and University of California, Irvine Eugene Cota Robles Fellowship. The authors would like to acknowledge support from the funding sources. D.W. and S.R. do not have any disclosures or conflicts of interests related to the proposed manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Dean Wang  https://orcid.org/0000-0002-3005-1154
https://orcid.org/0000-0002-3005-1154
Kyriacos A. Athanasiou  https://orcid.org/0000-0001-5387-8405
https://orcid.org/0000-0001-5387-8405
References
- 1. Koh JL, Zimmerman TA, Patel S, Ren Y, Xu D, Zhang LQ. Tibiofemoral contact mechanics with horizontal cleavage tears and treatment of the lateral meniscus in the human knee: an in vitro cadaver study. Clin Orthop Relat Res. 2018;476(11):2262-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bloch B, Asplin L, Smith N, Thompson P, Spalding T. Higher survivorship following meniscal allograft transplantation in less worn knees justifies earlier referral for symptomatic patients: experience from 240 patients. Knee Surg Sports Traumatol Arthrosc. 2019;27(6):1891-9. [DOI] [PubMed] [Google Scholar]
- 3. Grassi A, Macchiarola L, Lucidi GA, Coco V, Romandini I, Filardo G, et al. Long-term outcomes and survivorship of fresh-frozen meniscal allograft transplant with soft tissue fixation: minimum 10-year follow-up study. Am J Sports Med. 2020;48(10):2360-9. [DOI] [PubMed] [Google Scholar]
- 4. Lee BS, Bin SI, Kim JM, Kim WK, Choi JW. Survivorship after meniscal allograft transplantation according to articular cartilage status. Am J Sports Med. 2017;45(5):1095-101. [DOI] [PubMed] [Google Scholar]
- 5. Searle H, Asopa V, Coleman S, McDermott I. The results of meniscal allograft transplantation surgery: what is success? BMC Musculoskelet Disord. 2020;21(1_suppl):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zaffagnini S, Grassi A, Marcheggiani Muccioli GM, Benzi A, Serra M, Rotini M, et al. Survivorship and clinical outcomes of 147 consecutive isolated or combined arthroscopic bone plug free meniscal allograft transplantation. Knee Surg Sports Traumatol Arthrosc. 2016;24(5):1432-9. [DOI] [PubMed] [Google Scholar]
- 7. Arnoczky SP, DiCarlo EF, O’Brien SJ, Warren RF. Cellular repopulation of deep-frozen meniscal autografts: an experimental study in the dog. Arthroscopy. 1992;8(4):428-36. [DOI] [PubMed] [Google Scholar]
- 8. Jacquet C, Erivan R, Sharma A, Pithioux M, Parratte S, Argenson JN, et al. Preservation methods influence the biomechanical properties of human lateral menisci: an ex vivo comparative study of 3 methods. Orthop J Sports Med. 2019;7(4):2325967119841622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Binnet MS, Akan B, Kaya A. Lyophilised medial meniscus transplantations in ACL-deficient knees: a 19-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012;20(1_suppl):109-13. [DOI] [PubMed] [Google Scholar]
- 10. Wang H, Gee AO, Hutchinson ID, Stoner K, Warren RF, Chen TO, et al. Bone plug versus suture-only fixation of meniscal grafts: effect on joint contact mechanics during simulated gait. Am J Sports Med. 2014;42(7):1682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ambra LF, Mestriner AB, Ackermann J, Phan AT, Farr J, Gomoll AH. Bone-plug versus soft tissue fixation of medial meniscal allograft transplants: a biomechanical study. Am J Sports Med. 2019;47(12):2960-5. [DOI] [PubMed] [Google Scholar]
- 12. Brial C, McCarthy M, Adebayo O, Wang H, Chen T, Warren R, et al. Lateral meniscal graft transplantation: effect of fixation method on joint contact mechanics during simulated gait. Am J Sports Med. 2019;47(10):2437-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Novaretti JV, Lian J, Sheean AJ, Chan CK, Wang JH, Cohen M, et al. Lateral meniscal allograft transplantation with bone block and suture-only techniques partially restores knee kinematics and forces. Am J Sports Med. 2019;47(10):2427-36. [DOI] [PubMed] [Google Scholar]
- 14. Jauregui JJ, Wu ZD, Meredith S, Griffith C, Packer JD, Henn RF, III. How should we secure our transplanted meniscus? A meta-analysis. Am J Sports Med. 2018;46(9):2285-90. [DOI] [PubMed] [Google Scholar]
- 15. Saltzman BM, Meyer MA, Leroux TS, Gilelis ME, Debot M, Yanke AB, et al. The influence of full-thickness chondral defects on outcomes following meniscal allograft transplantation: a comparative study. Arthroscopy. 2018;34(2):519-29. [DOI] [PubMed] [Google Scholar]
- 16. McCormick F, Harris JD, Abrams GD, Hussey KE, Wilson H, Frank R, et al. Survival and reoperation rates after meniscal allograft transplantation: analysis of failures for 172 consecutive transplants at a minimum 2-year follow-up. Am J Sports Med. 2014;42(4):892-7. [DOI] [PubMed] [Google Scholar]
- 17. Hommen JP, Applegate GR, Del Pizzo W. Meniscus allograft transplantation: ten-year results of cryopreserved allografts. Arthroscopy. 2007;23(4):388-93. [DOI] [PubMed] [Google Scholar]
- 18. van der Wal RJ, Thomassen BJ, van Arkel ER. Long-term clinical outcome of open meniscal allograft transplantation. Am J Sports Med. 2009;37(11):2134-9. [DOI] [PubMed] [Google Scholar]
- 19. Carter TR, Brown MJ. Meniscal allograft survivorship and outcomes 20 years after implantation. Arthroscopy. 2020;36(8):2268-74. [DOI] [PubMed] [Google Scholar]
- 20. van der Wal RJP, Nieuwenhuijse MJ, Spek RWA, Thomassen BJW, van Arkel ERA, Nelissen R. Meniscal allograft transplantation in the Netherlands: long-term survival, patient-reported outcomes, and their association with preoperative complaints and interventions. Knee Surg Sports Traumatol Arthrosc. 2020;28(11):3551-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Agarwalla A, Liu JN, Christian DR, Garcia GH, Cvetanovich GL, Gowd AK, et al. Return to work following arthroscopic meniscal allograft transplantation. Cartilage. Epub 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Antosh IJ, Cameron KL, Marsh NA, Posner MA, DeBerardino TM, Svoboda SJ, et al. Likelihood of return to duty is low after meniscal allograft transplantation in an active-duty military population. Clin Orthop Relat Res. 2020;478(4):722-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grassi A, Bailey JR, Filardo G, Samuelsson K, Zaffagnini S, Amendola A. Return to sport activity after meniscal allograft transplantation: at what level and at what cost? A systematic review and meta-analysis. Sports Health. 2019;11(2):123-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kelly BT, Potter HG, Deng XH, Pearle AD, Turner AS, Warren RF, et al. Meniscal allograft transplantation in the sheep knee: evaluation of chondroprotective effects. Am J Sports Med. 2006;34(9):1464-77. [DOI] [PubMed] [Google Scholar]
- 25. Kim JG, Lee YS, Bae TS, Ha JK, Lee DH, Kim YJ, et al. Tibiofemoral contact mechanics following posterior root of medial meniscus tear, repair, meniscectomy, and allograft transplantation. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2121-5. [DOI] [PubMed] [Google Scholar]
- 26. Verdonk PC, Verstraete KL, Almqvist KF, De Cuyper K, Veys EM, Verbruggen G, et al. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):694-706. [DOI] [PubMed] [Google Scholar]
- 27. Smith NA, Parkinson B, Hutchinson CE, Costa ML, Spalding T. Is meniscal allograft transplantation chondroprotective? A systematic review of radiological outcomes. Knee Surg Sports Traumatol Arthrosc. 2016;24(9):2923-35. [DOI] [PubMed] [Google Scholar]
- 28. Rodkey WG, DeHaven KE, Montgomery WH, III, Baker CL, Jr, Beck CL, Jr, Hormel SE, et al. Comparison of the collagen meniscus implant with partial meniscectomy. A prospective randomized trial. J Bone Joint Surg Am. 2008;90(7):1413-26. [DOI] [PubMed] [Google Scholar]
- 29. Houck DA, Kraeutler MJ, Belk JW, McCarty EC, Bravman JT. Similar clinical outcomes following collagen or polyurethane meniscal scaffold implantation: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2018;26(8):2259-69. [DOI] [PubMed] [Google Scholar]
- 30. Toanen C, Dhollander A, Bulgheroni P, Filardo G, Zaffagnini S, Spalding T, et al. Polyurethane meniscal scaffold for the treatment of partial meniscal deficiency: 5-year follow-up outcomes: a European multicentric study. Am J Sports Med. 2020;48(6):1347-55. [DOI] [PubMed] [Google Scholar]
- 31. Schenk L, Bethge L, Hirschmann A, Berbig R, Luthi U, Arnold MP, et al. Ongoing MRI remodeling 3-7 years after collagen meniscus implantation in stable knees. Knee Surg Sports Traumatol Arthrosc. 2020;28(4):1099-104. [DOI] [PubMed] [Google Scholar]
- 32. Zaffagnini S, Grassi A, Marcheggiani Muccioli GM, Holsten D, Bulgheroni P, Monllau JC, et al. Two-year clinical results of lateral collagen meniscus implant: a multicenter study. Arthroscopy. 2015;31(7):1269-78. [DOI] [PubMed] [Google Scholar]
- 33. Zaffagnini S, Marcheggiani Muccioli GM, Lopomo N, Bruni D, Giordano G, Ravazzolo G, et al. Prospective long-term outcomes of the medial collagen meniscus implant versus partial medial meniscectomy: a minimum 10-year follow-up study. Am J Sports Med. 2011;39(5):977-85. [DOI] [PubMed] [Google Scholar]
- 34. Verdonk R, Verdonk P, Huysse W, Forsyth R, Heinrichs EL. Tissue ingrowth after implantation of a novel, biodegradable polyurethane scaffold for treatment of partial meniscal lesions. Am J Sports Med. 2011;39(4):774-82. [DOI] [PubMed] [Google Scholar]
- 35. van Minnen B, van Leeuwen MB, Kors G, Zuidema J, van Kooten TG, Bos RR. In vivo resorption of a biodegradable polyurethane foam, based on 1,4-butanediisocyanate: a three-year subcutaneous implantation study. J Biomed Mater Res A. 2008;85(4):972-82. [DOI] [PubMed] [Google Scholar]
- 36. Sandmann GH, Adamczyk C, Grande Garcia E, Doebele S, Buettner A, Milz S, et al. Biomechanical comparison of menisci from different species and artificial constructs. BMC Musculoskelet Disord. 2013;14:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Welsing RT, van Tienen TG, Ramrattan N, Heijkants R, Schouten AJ, Veth RP, et al. Effect on tissue differentiation and articular cartilage degradation of a polymer meniscus implant: a 2-year follow-up study in dogs. Am J Sports Med. 2008;36(10):1978-89. [DOI] [PubMed] [Google Scholar]
- 38. Reale D, Previtali D, Andriolo L, Grassi A, Candrian C, Zaffagnini S, et al. No differences in clinical outcome between CMI and Actifit meniscal scaffolds: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. Epub 2021 Apr 16. doi: 10.1007/s00167-021-06548-1. [DOI] [PubMed] [Google Scholar]
- 39. Shemesh M, Shefy-Peleg A, Levy A, Shabshin N, Condello V, Arbel R, et al. Effects of a novel medial meniscus implant on the knee compartments: imaging and biomechanical aspects. Biomech Model Mechanobiol. 2020;19(6):2049-59. [DOI] [PubMed] [Google Scholar]
- 40. McKeon BP, Zaslav KR, Alfred RH, Alley RM, Edelson RH, Gersoff WK, et al. Preliminary results from a US clinical trial of a novel synthetic polymer meniscal implant. Orthop J Sports Med. 2020;8(9):2325967120952414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zaslav KR, Farr J, Alfred R, Alley RM, Dyle M, Gomoll AH, et al. Treatment of post-meniscectomy knee symptoms with medial meniscus replacement results in greater pain reduction and functional improvement than non-surgical care. Knee Surg Sports Traumatol Arthrosc. Epub 2021 Apr 21. doi: 10.1007/s00167-021-06573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Whitehouse MR, Howells NR, Parry MC, Austin E, Kafienah W, Brady K, et al. Repair of torn avascular meniscal cartilage using undifferentiated autologous mesenchymal stem cells: from in vitro optimization to a first-in-human study. Stem Cells Transl Med. 2017;6(4):1237-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vangsness CT, Farr J, Boyd J, Dellaero DT, Mills CR, LeRoux-Williams M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy. J Bone Joint Surg Am. 2014;96(2):90-8. [DOI] [PubMed] [Google Scholar]
- 44. Zhang ZZ, Chen YR, Wang SJ, Zhao F, Wang XG, Yang F, et al. Orchestrated biomechanical, structural, and biochemical stimuli for engineering anisotropic meniscus. Sci Transl Med. 2019;11(487):eaao0750. [DOI] [PubMed] [Google Scholar]
- 45. Filardo G, Petretta M, Cavallo C, Roseti L, Durante S, Albisinni U, et al. Patient-specific meniscus prototype based on 3D bioprinting of human cell-laden scaffold. Bone Joint Res. 2019;8(2):101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bandyopadhyay A, Mandal BB. A three-dimensional printed silk-based biomimetic tri-layered meniscus for potential patient-specific implantation. Biofabrication. 2019;12(1_suppl):015003. [DOI] [PubMed] [Google Scholar]
- 47. Gonzalez-Leon EA, Bielajew BJ, Hu JC, Athanasiou KA. Engineering self-assembled neomenisci through combination of matrix augmentation and directional remodeling. Acta Biomater. 2020;109:73-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huey DJ, Athanasiou KA. Tension-compression loading with chemical stimulation results in additive increases to functional properties of anatomic meniscal constructs. PLoS ONE. 2011;6(11):e27857. [DOI] [PMC free article] [PubMed] [Google Scholar]