Abstract
Objective. Osteochondral allograft (OCA) transplantation has demonstrated good long-term outcomes in treatment of cartilage defects. Viability, a key factor in clinical success, decreases with peri-implantation storage at 4°C during pathogen testing, matching logistics, and transportation. Modern, physiologic storage conditions may improve viability and enhance outcomes. Design. Osteochondral specimens from total knee arthroplasty patients (6 males, 5 females, age 56.4 ± 2.2 years) were stored in media and incubated at normoxia (21% O2) at 22°C or 37°C, and hypoxia (2% O2) at 37°C. Histology, live-dead staining, and quantitative polymerase chain reaction (qPCR) was performed 24 hours after harvest and following 7 days of incubation. Tissue architecture, cell viability, and gene expression were analyzed. Results. No significant viability or gene expression deterioration of cartilage was observed 1-week postincubation at 37°C, with or without hypoxia. Baseline viable cell density (VCD) was 94.0% ± 2.7% at day 1. At day 7, VCD was 95.1% (37°C) with normoxic storage and 92.2% (37°C) with hypoxic storage (P ≥ 0.27). Day 7 VCD (22°C) incubation was significantly lower than both the baseline and 37°C storage values (65.6%; P < 0.01). COL1A1, COL1A2, and ACAN qPCR expression was unchanged from baseline (P < 0.05) for all storage conditions at day 7, while CD163 expression, indicative of inflammatory macrophages and monocytes, was significantly lower in the 37°C groups (P < 0.01). Conclusion. Physiologic storage at 37°C demonstrates improved chondrocyte viability and metabolism, and maintained collagen expression compared with storage at 22°C. These novel findings guide development of a method to optimize short-term fresh OCA storage, which may lead to improved clinical results.
Keywords: cartilage, osteochondral allograft, storage, defect, osteoarthritis, donor
Introduction
Osteoarthritis (OA) is a painful and debilitating joint disease, affecting more than 46 million Americans older than 25 years1,2 and resulting in annual costs of more than $60 billion dollars.3,4 Loss of articular cartilage is considered a hallmark of OA. The inflammation, degradation, and dysfunction caused by a focal articular cartilage injury affects all tissues that comprise the joint organ, leading to loss of homeostasis and generalized degenerative changes throughout the joint. 5
Articular cartilage defects, resulting from acute joint trauma, are common in younger patients with active lifestyles, 6 and, left untreated, can cause generalized posttraumatic OA, thereby severely impairing quality of life. 7 Such lesions have limited spontaneous reparative potential due to the poor regenerative capacity and avascular nature of cartilage. 5 Relief of symptoms therefore often requires operative intervention including micro-fracture, cell-based therapies such as autologous chondrocyte implantation (ACI), or osteochondral allograft transplantation (OCA).8-11
OCA transplantation has been associated with consistently positive functional outcomes, with 69% graft survival at 20 years 12 and established long-term clinical safety.8,13,14 Furthermore, this technique enables transplantation of both cartilage and underlying bone, enabling surgeons to address pathology extending beyond the subchondral plate. Although OCA has a proven record of clinical efficacy, the limited availability and logistical difficulties of procuring suitable grafts from deceased donors restricts widespread implementation of this technique.15,16 This is due in part to concerns related to contamination and disease transmission.17,18 Current cartilage banking and testing protocols, recommended and mandated by the American Association of Tissue Banks (AATB) according to the United States Pharmacopeia (USP) <71>, requires implementation of a 14-day culturing and disease-screening period before release of grafts for clinical use.12,14,15,19,20
To confound matters, cartilage allograft viability has been shown to decrease in storage over time. 17 Using the standard tissue bank practice of 4°C storage, chondrocyte viability deteriorates significantly within the initial 14-day tissue clearance period12,15,19,21-29 with cellular demise driven by both apoptosis30,31 and stress response. 32 Because chondrocyte viability influences the long-term clinical success of OCA,1,22,24,33-37 optimization of storage conditions during the early period after harvesting is crucial. Proposed preservation goals suggest maintaining a minimum viable chondrocyte density (VCD) consisting of 70% living chondrocytes.8,22 Key variables of interest have included type of preservation solution,18,23,29,31,38 choice of supplementation (see Bian et al. 39 and other factors12,27,28), temperature,15,18,24,31,40,41 and level of oxygenation. 15 Conditions have been assessed across several animal species 18 (including rabbit, 42 pig, 41 goat,15,31 canine22,24,43), but there is only a very limited number of human studies8,40. While both room temperature storage and physiologic storage at 37°C have demonstrated promise in animal models, no comparisons between nonrefrigerated conditions have been made. Furthermore, validation of animal models, in which variables such as species-specific core temperature and cartilage morphology may differ from humans, is necessary using clinical human tissues.
Even with improvements to preservation techniques, healthy cadaveric cartilage candidates remain scarce, and there is significant interspecimen and intrastudy variability in chondrocyte viability of OCAs at the time of implantation.24,44 Differences in harvest timing, technique, location, and the initial condition of the cartilage are all expected to have significant influence on the quality and performance of the graft. 45 To alleviate this shortage, we propose the implementation of a Living Donor Cartilage Program (LDCP). This approach would improve availability of grafts, provide grafts with high cell viability, allow for prescreening of donors for safety, facilitate more convenient scheduling of surgery, and offer a potentially less expensive alternative. In fact, the successful transplantation of OCAs from living donors has been previously reported. 46 Given that much of the long-term data regarding OCAs is based on patients that had grafts implanted within 7 days of harvest, 24 the clinical goal for this pilot program is to preserve osteochondral allografts for only a short time (approximately a week) at temperatures greater than 4°C, in an effort to avoid deterioration in chondrocyte viability and to preserve extracellular matrix (ECM) integrity.
Therefore, the purpose of this study was to evaluate the quality of macroscopically healthy cartilage from relatively young middle-aged patients on storage by testing chondrocyte histology, viability, and gene expression after 1 week of incubation in a number of modern storage conditions (i.e. in chondroprotective media incubated at normoxia (21% O2) at 22°C and 37°C or hypoxia [2% O2] at 37°C). In addition, we aimed to perform a limited validation experiment to compare physiologic storage (37°C with hypoxia) to the current industrial standard of 4°C to further evaluate and compare modern storage conditions in light of currently used clinical protocols. We hypothesized that storage at physiologic conditions (37°C, hypoxia) would lead to improved tissue viability and gene expression as compared with storage at room temperature or refrigerated storage at 4°C. The short-term storage of healthy OCAs from donors has the potential to provide efficacious treatment through the novel utilization of this postoperative product, with high chondrocyte viability, to overcome the persistent global shortage of cartilage allografts.
Methods
Tissue Collection and Processing
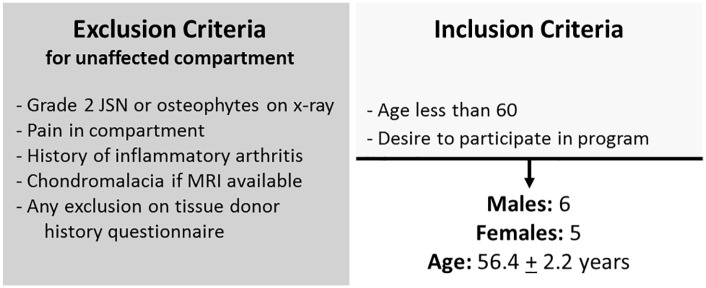
Joint resections were collected from young patients (<60 years) undergoing total knee replacement surgery for varus or valgus pathology with well-preserved contralateral compartments demonstrating Kellgren-Lawrence grade 0 or 1 pathology. Eleven young total knee arthroplasty (TKA) donors (6 males, 5 females, age 56.4 ± 2.2 years) were screened using standardized inclusion/exclusion criteria ( Fig. 1 ) and eligible for inclusion in this study.
Figure 1.
Screening for inclusion/exclusion criteria. Volunteer total knee arthroplasty donors were screened for the given criteria. Eleven donors were included in the pilot study. JSN, joint space narrowing.
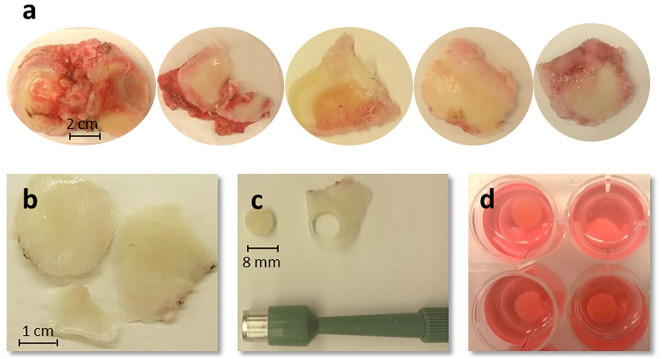
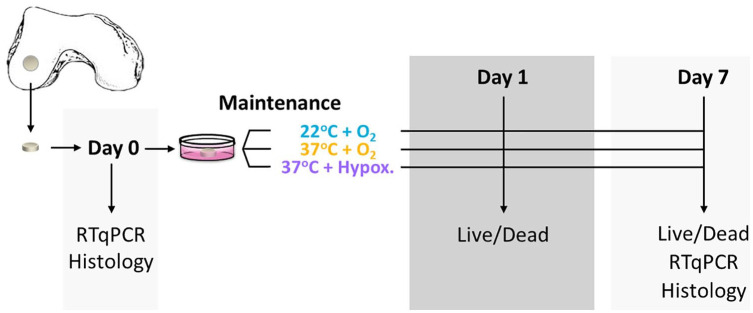
On collection of joint resections, photos were taken to enable gross observation and scoring of the tissues according to the ICRS (International Cartilage Repair Society) grading system ( Fig. 2a ). Subsequently, osteochondral samples were harvested from each resection by the creation of 4- and 8-mm discs using biopsy punches ( Fig. 2b-d ), with a scalpel introduced below the calcified layer and within the superficial bone to include subchondral bone along with the cartilage and create true osteochondral discs. Discs of 4 mm from each tissue section were transferred immediately to 10% formalin for preservation. These served as baseline samples for histology at the time of tissue harvest. All other cartilage discs were transferred into serum-free media and maintained for 7 days at room temperature (22°C ± 0.5°C, normoxia [21%O2]), incubated at 37°C with normoxia, or incubated at 37°C under hypoxic conditions (2.0% ± 0.5% O2; Fig. 3 for timeline). Excess cartilage was frozen in liquid nitrogen for RNA preservation for real-time quantitative polymerase chain reaction (RT-qPCR) analysis.
Figure 2.
Collection of tissue resections and creation of cartilage discs. (a) Representative images of the joint resections. Bone and cartilage joint resections are collected from patients undergoing total knee replacement surgery and scored for disease severity. (b) Healthy cartilage pieces are harvested and cartilage discs are created using a biopsy punch (shown here: 8-mm disc) (c) before transfer to storage cocktail (d).
Figure 3.
Timeline for storage and tissue characterization. Schematic representation of punched cartilage discs, the 3 storage conditions, and the timeline for the different levels of tissue characterization.
Histologic Tissue Characterization
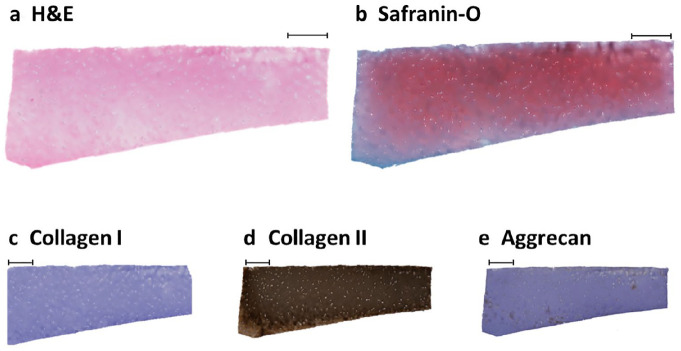
Four-millimeter discs from the time of tissue harvest and following 7 days of incubation for each treatment group were transferred to 10% neutral buffered formalin for preservation. After 24 hours of fixation, samples were transferred to 70% ethanol. Tissues were bisected and embedded in paraffin and sectioned (5 μm) along a vertical plane to get a cross-sectional view of the different cartilage zones. Slides were stained for hematoxylin-eosin (H&E) and safranin-O/fast green using standard methods ( Fig. 4a and b ). Additional sections of each sample underwent immunohistochemical staining for aggrecan (mouse anti-aggrecan antibody, Novusbio NB110-6524, dilution 1:150 in phosphate buffered saline/bovine serm albumin [PBS/BSA] 5%), collagen type I (rabbit monoclonal anti-collagen 1/COL1A1, Abcam EPR7785, dilution: 1:400 in PBS/BSA 5%), and collagen type II (mouse monoclonal anti-collagen II/COL2A1, DSHB, University of Iowa, dilution 1:100 in PBS/BSA 5%) with normal mouse or rabbit IgG used as a negative controls ( Fig. 4c-e ). For the 11 patients studied, 3 blinded and independent reviewers scored histologic sections from each of the samples at each of the 4 conditional time points: day 0 and day 7 at 22°C, 37°C + O2, and 37°C with hypoxia, for a total of n = 246. Samples were scored using the Modified histological-histochemical grading system by Modified Mankin Score (MMS) for histological quality and histomorphometry.47,48
Figure 4.
Histology staining. Representative joint resection sample demonstrating good cartilage architecture by hematoxylin and eosin (H&E) (a) and safranin-O (b) staining. Immunohistochemisty demonstrates an absence of collagen I staining (c), strongly positive collagen II staining throughout (d), and scattered areas of positive aggrecan staining (e) within the cartilage disc. Scale bar = 500 µm.
Cell Viability Quantification With Live-Dead Staining
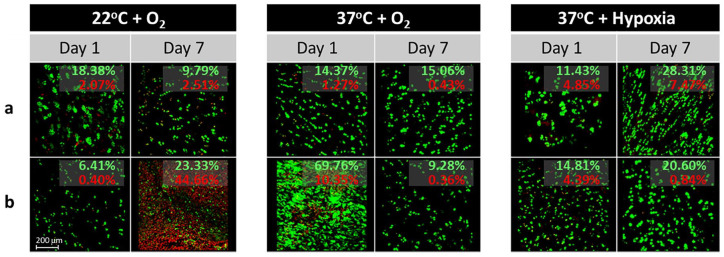
At days 1 and 7, 4-mm pellets were collected from each culture condition (22°C + O2; 37°C + O2; 37°C + hypoxia) and assessed for cell viability using a 2-color fluorescence assay based on the simultaneous determination of live (calcein acetoxymethyl [AM]: green) and dead (ethidium homodimer-1: red) cells (Live-Dead Viability/cytotoxicity kit for mammalian cells; Molecular Probes Fluorescent z-stack images (850 µm × 850 µm × ~100 µm; 7.2-µm slice thickness) were collected for each tissue section and culture condition using an inverted LSM 780 multiphoton laser scanning confocal microscope (488 nm and 561 nm lasers) at 10× magnification. Maximum intensity projections were created in Zen (2.3 SP1, Zeiss 2015; Fig. 5 ). The amount of red and green staining in each image was quantified in MatLab (R2015b, 8.6.0.267246) by setting an intensity threshold to 25 and calculating the percentage of red and green pixels relative to the total number of pixels in the image. Viable cell density (VCD) was calculated by dividing the number of green (live) pixels by the combined number of green (live) and red (dead) pixels in order to provide a measure of percentage viable cells.
Figure 5.
Representative live/dead staining of cartilage discs. Representative images from 5 different cartilage discs (a-e, collected from a single donor) obtained during live/dead microscopy at days 1 and 7 following storage at various temperature and oxygen conditions. Samples stored at 22°C demonstrated significantly decreased proportions of live (green) cells at day 7 compared with baseline (day 1) and matching samples stored at 37°C. Green (red) values represent the percentage of all pixels that are green (red).
Molecular Characterization of Cartilage by Gene Expression Analysis
To ascertain whether the different storage conditions produced distinct molecular responses in the cartilage discs, gene expression was analyzed using reverse transcriptase–based real-time quantitative PCR (RT-qPCR). RNA was isolated from the frozen cartilage samples using a modified Biochain Protocol (Cartilage RNA Isolation Kit, K2031010, Biochain Institute, Newark CA). Detailed methods are provided in the Supplemental Material and Table 1. Gene expression for selected gene markers (ACTB, HPRT1, AKT1, COL1A1, COL2A1, COL10A1, ACAN, HAPLN1, MFAP5, MMP13, CD14, CD117, CD163, CD4) was quantified using RT-qPCR whereby each reaction was performed with 10 ng/µL of cDNA, QuantiTect SYBR Green PCR kit (Qiagen, Hilden, Germany), and a CFX384 RT-qPCR system (Bio-Rad, Hercules, CA, USA). Transcript levels were quantified using the 2ΔΔCt method and normalized to the housekeeping gene AKT1 (set at 100). Technical qPCR triplicates were run for each sample at each condition.
Statistics
Statistical analysis was performed by a formally trained institutional statistician. The total sample size for the study was 11 samples (patients) employed for the core viability, histology, and qPCR analysis performed at baseline (day 0) and at the day 7 22°C, 37°C + O2, and 37°C with hypoxia conditions. Each patient was analyzed at the above timepoints for all 3 outcome measures (viability, histology, and qPCR). An additional n = 3 samples were employed in our validation experiment comparing physiologic storage (37°C with hypoxia) to the current industrial standard of 4°C on the basis of viability.
Comparisons for live-dead quantification and RT-qPCR results were made between groups using generalized linear models utilizing generalized estimating equations (GEE). P values were adjusted for multiple comparisons using the false discovery rate method described by Benjamini and Hochberg. 49 For histology, the weighted kappa statistic with Cicchetti-Allison weights was used to assess the agreement in measures between the observers. Areas and conditions were compared using GEE, taking into account multiple measurements taken from the same person. Power and sample size calculations for the study were chosen based on a primary viability endpoint at alpha = 0.05, power = 0.80 and the viability values provided by Pallante et al 15 in their caprine model for temperature-based osteochondral allograft preservation, resulting in a goal sample size of n = 11 per group. P values <0.05 were considered significant. Statistics were performed using SAS Version 9.4 and JMP Pro 13.0 (SAS Institute, Cary, NC).
Results
Macroscopic and Histologic Tissue Characterization
Eleven osteochondral specimens from relatively young middle-aged TKA patients (6 males, 5 females, age 56.4 ± 2.2 years) were stored in chondroprotective media and incubated at normoxia (21% O2) at 22°C and 37°C and hypoxia (5% O2) at 37°C for 7 days. Cartilage from the unaffected compartments demonstrated healthy macroscopic architecture, with median ICRS grade 0 pathology (range: 0-2) and focal ICRS grade 1-2 changes. In contrast, the median contralateral compartment demonstrated mean grade 4 pathology (range: 3-4).
On histologic embedding and staining, the obtained day 0 samples exhibited excellent collagen II staining and no significant collagen I staining, supporting mature, hyaline cartilage predominance over fibrocartilage, and supporting the use of these TKA resection samples in determining osteochondral tissue viability over time during storage (see Fig. 4 for representative images). Aggrecan staining was present throughout the healthy compartment samples. In addition, all samples were stained for H&E and safranin-O for cartilage morphology grading.
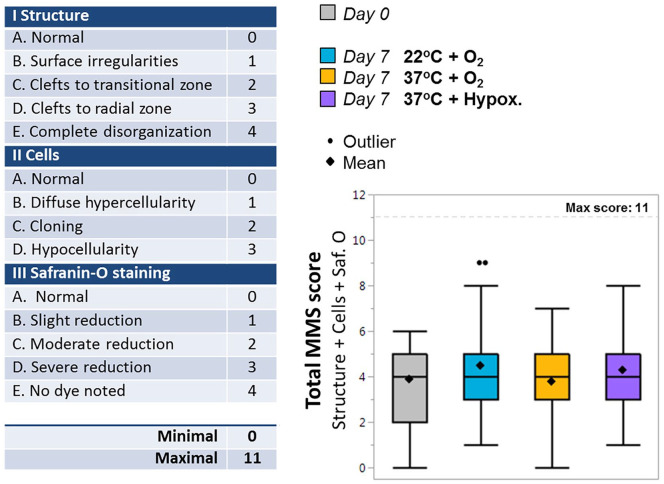
Cartilage morphology was scored according to the MMS ( Fig. 6 ) by 3 independent and blinded reviewers. This system evaluates differences in structure, cell composition, and safranin-O staining, with a minimal score of 0 (normal cartilage) and a maximum score of 11 (complete structure disorganization with hypocellularity and no safranin-O dye staining noted). The mean MMS score of all histological samples at the time of harvest was 3.9 ± 1.8, indicating mild degenerative changes (see Fig. 6 ). Following 7 days of storage, mean MMS scores were 4.5 ± 2.0 for 22°C + O2, 3.8 ± 1.6 for 37°C + O2, and 4.3 ± 1.3 for 37°C + hypoxia with no significant difference noted between groups or as compared with baseline (P ≥ 0.54).
Figure 6.
Histology scoring. (a) Breakdown of Modified Mankin Score (MMS) scoring table. (b) Box (interquartile range [IQR]) and whisker (max, min) plots of the total MMS histological scores (Structure [0-4] + Cells [0-3] + Safranin-O staining [0-4]; 3 independent observers) for day 0 samples harvested immediately at tissue collection, and for samples cultured for 7 days in storage at 22°C + O2, 37°C + O2 or 37°C + hypoxia. Means ± standard deviations are included (diamonds) for each treatment group. No significant difference was found across treatment.
Cellular Level Characterization With Live-Dead Staining
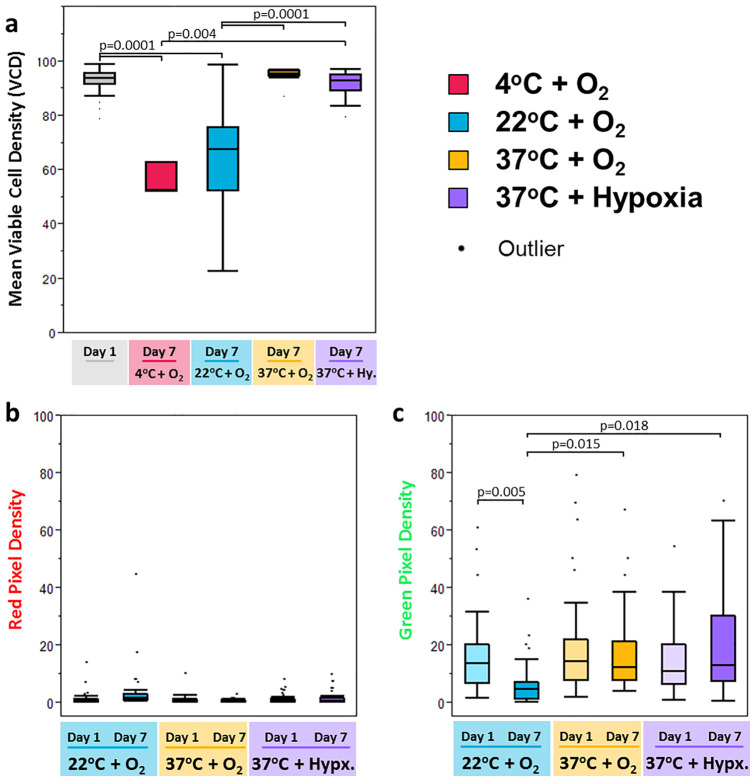
At the time of baseline measurements within the first 24 hours of storage (day 1), all 3 conditions demonstrated similar, high VCDs (94.0% ± 2.7%, range 87.5% to 99.2%,P = 0.13). Following 7 days of storage, incubation at room temperature caused a significant decrease in the presence of viable cells, with a final mean VCD of 65.6% (range: 22.8% to 98.9%, P < 0.01). In comparison, at day 7 the mean VCD for 37°C normoxic storage was 95.1% (range: 87.2% to 97.1) and mean VCD for 37°C hypoxic storage was 92.2% (range: 79.7% to 97.4%), with no significant difference between the two 37°C storage conditions (P = 0.39). No significant difference in VCD was observed when comparing baseline (day 1) VCD to viability at day 7 for the 37°C incubation groups, suggesting that VCD was maintained throughout storage for these groups (P ≥ 0.27). For comparison, the day 7 VCDs for samples incubated at 37°C under either oxygen condition were significantly higher when compared with the day 7 VCD for samples incubated at room temperature (P =0.0001, Fig. 7 ).
Figure 7.
Live/dead staining results. Box and whisker plots of live/dead staining demonstrate significantly better maintenance of cell viability, as measured by percentage of green pixels at 7 days of storage for cells cultured at 37°C as compared with cells cultured at 22°C. (a) Change in cell viability over 1 week is illustrated by plotting the ratios of green pixels: all stained pixels to determine viable cell density [VCD]). All day 1 samples are pooled, while average day 7 results are grouped by condition. There was no significant change in VCD from day 1 to day 7 for cartilage preserved at 37°C, while samples stored at 4°C (validation cohort) and at 22°C had a significant drop. Percentage of (b) red pixels (dead cells) and (c) green pixels (live cells) are shown as a function of the total number of pixels in each image to represents the change in cell density for each condition and time point.
Molecular Level Characterization With RT-qPCR
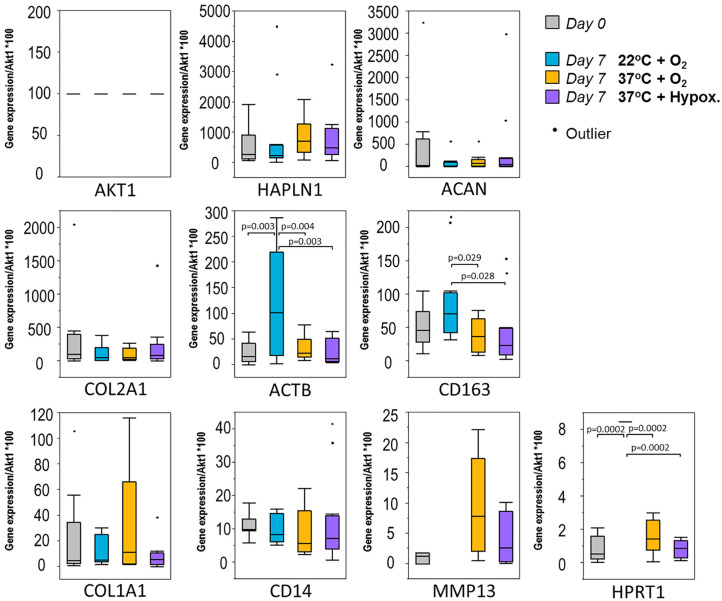
COL1A1, COL1A2, and ACAN qPCR expression following 7 days of storage was unchanged from baseline (P > 0.05) for all 3 storage conditions tested (see Fig. 8 ). CD163 expression, indicative of inflammatory activity including macrophages and monocytes, was significantly lower in the 37°C groups (normoxia and hypoxia) compared with 22°C (P < 0.01). Of the genes tested, MFAP5, COL10A1, CD4 (associated with helper T-cells) and CD117 (KIT; mast cell) demonstrated trace to no detectable expression (data not shown).
Figure 8.
Reverse transcriptase–based real-time quantitative polymerase chain reaction (RTqPCR) data from 3 different week-long maintenance conditions. Box and whisker plots of gene expression levels (obtained by RT-qPCR; normalized to Akt1) that allow comparison of samples collected from different treatment conditions at 2 distinct time points: day 0 samples (gray) and day 7 samples stored at 22°C + O2 (blue), 37°C + O2 (yellow), or 37°C + hypoxia (purple). Samples were assessed for changes in housekeeping genes (AKT1, ACTB), matrix deposition associated markers (COL1A1, COL2A1, COL10A1, ACAN), matrix maintenance–associated markers (HAPLN1, MFAP5, MMP13) and inflammatory infiltrate markers (CD4, CD14, CD163, KIT). Genes with no detectable expression are not plotted. Analysis of variance (ANOVA) with false discovery rate (FDR) adjustments made for multiple comparisons were performed to compare treatments. P values less than 0.05 are significant.
Validation Experiment: Viability Comparisons with Current 4°C Industrial Standard
When comparing physiologic storage at 37°C with hypoxia to the current industrial standard of refrigeration at 4°C, a validation group of n = 3 additional patient samples (3 females, mean age 52.3 ± 1.2 days) demonstrated that the 4°C group had a substantially lower mean VCD of 56.1% ± 6.0% after 7 days as compared with 88.9% ± 4.8% for the 37°C + hypoxia condition (P = 0.004). These results are included in Figure 7a .
Discussion
Articular cartilage defects can cause chronic pain and progression to OA and there is a critical need for safe and cost-effective interventions. OCA transplantation is a safe and effective treatment option for large cartilage defects, with demonstrated positive long-term clinical outcomes.33,50,51 However, OCA suffers from a limited supply of viable tissues. This is compounded by AATB mandates for OCA storage for culture-based infectious disease testing, with this storage classically performed at 4°C with documented deleterious on tissue viability. 16 The purpose of this study was to test macroscopically healthy cartilage from discarded varus and valgus TKA tissue in patients with localized cartilage degeneration and to evaluate and compare chondrocyte viability after 1 week of storage in a number of modern storage conditions. Our hypothesis was confirmed in that storage at physiologic conditions (37°C ± hypoxia) demonstrated improved viability when compared to room temperature storage.
Previous studies have suggested that the success of an OCA is a function of the viability of the graft’s chondrocytes, which diminishes over storage time 16 and that storage at physiological temperatures results in favorable chondrocyte viability12,14,15,24,31 compared with 4°C. The storage conditions examined in this study were selected based on previously published findings, which have compared storage at 4°C to storage at room temperature or 37°C on an individual basis.12,14,15,24,31 Both Pallante et al 15 and McCarty et al, 31 for example, have demonstrated that caprine chondrocyte viability is maintained for up to 28 days at 37°C, while 4°C incubation showed a 30% decrease in viability. In canine cadaver studies, Garrity et al 12 have reported a mean 28-day tissue viability of 40% in samples maintained at 4°C compared with ~76% viability for OCAs stored at 37°C and Day 0 control of 77% viability. Other work has focused on prolonging viability when OCAs are stored at room temperature.8,52 Given that there is considerable evidence pointing to reduced chondrocyte viability following storage at 4°C, we focused on comparing storage of OCAs for 2 of the most commonly proposed storage temperatures, ~ 22°C to 25°C and 37°C, as they have not been compared side by side for human tissues, and this comparison is important for development and future standardization of OCA storage conditions. Furthermore, we have included a validation experiment of an additional 3 patient samples comparing physiologic storage at 37°C to the current industrial practice of storage at 4°C. While limited in sample size, the results, which demonstrate increased viability in the physiologic storage group, provide further human-tissue specific support which favors nonrefrigerated storage at conditions which approximate those temperatures seen in vivo.
The effects of different storage times and different media recipes on cellular viability have been widely studied in the past and have been summarized nicely by De Caro et al 14 and Wright et al. 18 In an effort to limit variables in this study, we tested only a single type of preservation media to enable the comparison of multiple storage conditions while controlling media environment. We focused on the use of a formulation similar to that developed in the MOPS (Missouri Osteochondral Allograft Preservation System) protocol, 8 which avoids the use of fetal bovine serum supplements, as studies have presented conflicting reports as to their benefits.24,39 This proprietary media is able to maintain sufficient (70%) chondrocyte viability for greater than 56 days at room temperature, and is well documented in the literature.8,12,16,22,24,52 We elected to use a serum-free, growth-factor free solution to limit concerns related to disease transmission, batch variability, or contamination.12,24 We also opted to avoid multiple media changes, because media replacement does not result in appreciable improvements in chondrocyte viability. 8 Furthermore, our clinical goal is to preserve cartilage for only a short time (1 week) and regular media changes have not been associated with significant improvements in chondrocyte viability, according to Stoker et al. 8 Our studies replicate a best surgical practice solution for OCA, which has classically been performed within 7 days of donor expiry.
To date, storage at 37°C has not become standard practice, due to preexisting tissue banking regulations and concerns related to cost or microbial contamination at temperatures above 4°C. A recent study by Stoker et al. 8 indicates that storage at room temperature is safe, because all tissue and media samples passed sterility testing, with no presence of microbial growth. Garrity and colleagues 12 have also shown that microbial cultures of the media collected at the end of storage at 37°C caused no increased incidence in bacterial contamination. Of note, storage at 4°C was originally desired and recommended due to theoretic decreased microbiological viability and growth, analogous to the common practice of food refrigeration. However, as demonstrated in previous canine and caprine models, subphysiologic storage has parallel negative consequences on desirable cartilage viability. Considering that it is increasingly well-established that aseptic storage is possible at 37°C, it appears that a microbiological rationale for cold storage is less tenable at present.
Histologically, healthy articular cartilage is composed of smooth collagenous tissue with chondrocytes comprising less than 10% of the total volume.1,53 Collagen type II is abundant, while limited if any collagen type I is present. 54 In the histological staining of our day 0 samples, we observed mild degeneration, suggestive of early osteoarthritic changes, as anticipated in the relatively preserved compartment of varus and valgus total knee arthroplasty specimens. However, tissues were generally healthy, with absence of collagen I staining, strong collagen II staining, and only mild to moderate disease present on mHHGS scoring. Most important, we did not observe significant histologic deterioration of samples during storage at any of the 3 conditions tested. This finding indicates that tissue architecture and composition are well maintained during osteochondral storage, in agreement with similar studies. Stoker et al. 8 have presented data showing no significant changes in OCA material properties after OCA storage in MOPS media at room temperature at 28, 56, or 70 days after procurement. Histological assessments indicated the maintenance of cell morphology, ECM staining for collagen, and articular surface integrity. Others have also suggested that ECM is maintained during graft storage even when chondrocyte viability falls.15,44 This highlights the need for nuanced measures of graft health such as viability (live-dead) and gene expression assays. 8
Most existing OCA studies have focused on quantifying chondrocyte viability at 14 days postharvest or later, presumably to fall in line with AATB testing recommendations; the rate of cell death generally appears to increase after this time point. 24 Numerous factors, including OCA source (human vs. animal), disease state, media composition, level of oxygenation, and time point measured, as well as quantification technique are likely to influence viability measurements. Our analysis of live-dead viability in different storage conditions shows that human OCA tissues stored at 37°C achieved superior VCD results as compared with osteochondral specimens, stored in serum-free media at 22°C, with >90% viability following 7 days of incubation at 37°C as compared to a mean VCD of 66% for storage at 22°C. These results suggest the utility of physiologic storage and highlight that, after only 7 days of incubation, the mean VCD of storage for our human OCAs at room temperature (22°C) falls below the proposed 70% cutoff for OCA viability. Considering that graft viability is considered the leading predictor of clinical success in OCA transplantation, we believe these results strongly support human OCA storage at physiologic 37°C conditions, at least for programs aimed at minimizing storage time and maximizing chondrocyte viability. Of note, a change in oxygen content did not appear to significantly affect the viability of the chondrocytes in our samples. Because physiologic hypoxia is present in the articular environment and may suppress the growth of aerobic bacteria, hypoxic storage may represent a preferred storage environment that could reduce the possibility of infectious disease. Future studies should investigate the potential benefits of reduced oxygen tension by testing a broader range of oxygen conditions for chondrocyte storage.
In order to perform comprehensive characterization of cartilage under the 3 experimental storage conditions, we performed RT-qPCR on baseline and stored osteochondral discs to ascertain the presence of key matrix deposition and maintenance markers and investigate the presence of inflammatory infiltrates. Following 7 days of storage, there was no change in collagen or aggrecan expression for all 3 nonrefrigerated storage conditions, while high expression of collagen II and aggrecan and low expression of collagen I were maintained. Remarkably, we did observe increased expression of the macrophage/monocyte biomarker CD163 with storage at 22°C. The latter indicates the presence of inflammatory activity, which may reflect the immunological effects of storage below physiologic conditions, consistent with previous literature suggesting that stress-response gene expression can be driven by both supra- and infra-physiologic environments. 32 Increased CD163 expression observed at 22°C samples in our study provides molecular-level support for the potential utility of physiological storage at 37°C. Other groups looking at gene response have observed a significant increase in apoptotic gene expression in human (femur) OCA tissues stored at 4°C, indicating that loss of chondrocyte viability observed during storage is at least partially due to apoptosis. 30 We did not observe matrix degeneration at 7 days, which supports the findings of Robertson et al. 30 who concluded that there was little to no upregulation of genes involved in ECM degradation even at their latest time point of 35 days. Since both histologic and gene expression markers appear to be limited to long-term storage effects, we reiterate the importance of having reliable and robust measurements of chondrocyte viability for short-term storage when assessing OCA quality, when decreases in metabolic activity and increased chondrocyte death may become apparent. 29
Our study is not without limitations. Paired analyses, performed by having all 3 storage conditions tested for each osteochondral donor, enabled better accounting for intersample tissue variance. However, the absolute amount of tissue per donor remains limited and thus, additional temperature conditions such as 4°C could not be tested in parallel to the three methods of storage compared. These limitations were mitigated by the addition of a validation cohort to provide comparisons and a context including standard 4°C storage, next to which our main results can be interpreted. For the same reasons, we did not use clinically relevant sized discs although we note that the size of clinical OCA lesions can vary considerably, with one estimate describing ranges of 2.3 to 11.5 cm2. 55 We also did not measure the bone-to-cartilage ratio in our discs. It is possible that a larger bone presence in the discs could have adversely affected cartilage during storage, although Pennock et al. 56 suggest that the bone-to-cartilage ratio plays little to no role in the degradation of allografts during prolonged storage. We did not attempt to quantify this factor, or OCA size in these experiments. Additionally, we did not include microbiological testing in this cohort in an effort to ensure tissue availability for histologic, live-dead, and qPCR analysis. Finally, further expansion of sample sizes and metabolic comparisons in follow-up studies may provide a more stringent physiologic proof-of-concept.
In conclusion, storage of human osteochondral tissues in serum free media at 37°C, with or without hypoxia, demonstrates maintained macroscopic tissue quality and chondrogenic gene expression, improved chondrocyte viability, and decreased inflammatory CD163 activity when compared to storage at 22°C. These data provide guidance for a novel yet simple method that optimizes tissue viability during short-term storage of fresh OCA and has the potential to improve long-term clinical results of surgical cartilage repair.
Supplemental Material
Supplemental material, 12._SupplementaryMaterial for Modernizing Storage Conditions for Fresh Osteochondral Allografts by Optimizing Viability at Physiologic Temperatures and Conditions by Janet M. Denbeigh, Mario Hevesi, Carlo A. Paggi, Zachary T. Resch, Leila Bagheri, Kristin Mara, Arvin Arani, Chenghao Zhang, A. Noelle Larson, Daniel B.F. Saris, Aaron J. Krych and Andre J. van Wijnen in CARTILAGE
Supplemental material, 13._Supplementary_Table_1_1 for Modernizing Storage Conditions for Fresh Osteochondral Allografts by Optimizing Viability at Physiologic Temperatures and Conditions by Janet M. Denbeigh, Mario Hevesi, Carlo A. Paggi, Zachary T. Resch, Leila Bagheri, Kristin Mara, Arvin Arani, Chenghao Zhang, A. Noelle Larson, Daniel B.F. Saris, Aaron J. Krych and Andre J. van Wijnen in CARTILAGE
Footnotes
Supplementary material for this article is available on the Cartilage website at https://journals.sagepub.com/home/CAR.
Author Contributions: Janet M. Denbeigh: Conception and design, analysis and interpretation of the data, drafting of the article, critical revision of the article for important intellectual content, final approval of the article, statistical expertise, administrative, technical, or logistic support, collection and assembly of data.
Mario Hevesi: Conception and design, analysis and interpretation of the data, drafting of the article, critical revision of the article for important intellectual content, final approval of the article, statistical expertise, administrative, technical, or logistic support, collection and assembly of data.
Carlo A. Paggi: Critical revision of the article for important intellectual content, final approval of the article, collection and assembly of data.
Zachary T. Resch: Critical revision of the article for important intellectual content, final approval of the article, provision of study materials or patients, administrative, technical, or logistic support.
Leila Bagheri: Collection and assembly of data, critical revision of the article for important intellectual content, final approval of the article.
Kristin Mara: Analysis and interpretation of the data, critical revision of the article for important intellectual content, final approval of the article, statistical expertise.
Arvin Forghanian-Arani: Critical revision of the article for important intellectual content, final approval of the article, collection and assembly of data.
Chenghao Zhang: Critical revision of the article for important intellectual content, final approval of the article, collection and assembly of data.
Daniel B.F. Saris: Conception and design, critical revision of the article for important intellectual content, final approval of the article.
Aaron J. Krych: Conception and design, analysis and interpretation of the data, critical revision of the article for important intellectual content, final approval of the article, provision of study materials or patients, obtaining of funding.
Andre van Wijnen: Conception and design, analysis and interpretation of the data, critical revision of the article for important intellectual content, final approval of the article, provision of study materials or patients, obtaining of funding, administrative, technical, or logistic support.
Acknowledgments and Funding: We would like to acknowledge Catherine Gray for assistance with histology, and the teams at the Biomaterials and Histomorphometry Laboratory (Rochester) and Biorepository Cores (Arizona) for processing histology tissues. We also thank Michella H. Hagmeijer, Koen Dijkstra, and Roeland Huitsing for assistance with histological scoring, as well as the members of our research group for stimulating discussions. We are also indebted to the kind support of the Mayo Clinic Bone Bank and the significant efforts of Susan Puffer and Renae Boyum. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was in part supported by NIH R01 AR049069 (to AJvW) for general lab supplies and investigator effort. We also thank William and Karen Eby for their generous support of our program in joint preservation.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M. Hevesi is a paid consultant for Moximed.D. B.F. Saris is a paid consultant for Cartiheal, Ivy Sports, and Smith & Nephew; is on the editorial or governing board, of Cartilage; and receives research support from Ivy Sports and Smith & Nephew. A.J. Krych receives research support from Aesculap/B. Braun, Arthrex, Inc., Arthritis Foundation, Ceterix, and Histogenics; receives IP royalties from Arthrex, Inc.; is a paid consultant from Arthrex, Inc., JRF Ortho, and Vericel; is on the editorial or governing board of the American Journal of Sports Medicine; and is a board or committee member of the International Cartilage Repair Society, International Society of Arthroscopy, Knee Surgery, and Orthopaedic Sports Medicine, Minnesota Orthopedic Society, and Musculoskeletal Transplantation Foundation. The other authors have no disclosures to make.
Ethical Approval: All aspects of this study were performed following institutional review board approval (IRB 13-005619).
Informed Consent: Written informed consent was obtained from all subjects before the study.
Trial Registration: Not applicable.
ORCID iDs: Aaron J. Krych  https://orcid.org/0000-0003-3248-8007
https://orcid.org/0000-0003-3248-8007
Andre J. van Wijnen  https://orcid.org/0000-0002-5882-709X
https://orcid.org/0000-0002-5882-709X
References
- 1. O’Connell GD, Lima EG, Bian L, Chahine NO, Albro MB, Cook JL, et al. Toward engineering a biological joint replacement. J Knee Surg. 2012;25:187-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. ; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1_suppl):15-25. [DOI] [PubMed] [Google Scholar]
- 3. Roller BL, Monibi F, Stoker AM, Bal BS, Cook JL. Identification of novel synovial fluid biomarkers associated with meniscal pathology. J Knee Surg. 2016;29(1_suppl):47-62. [DOI] [PubMed] [Google Scholar]
- 4. Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research. Clin Orthop Relat Res. 2004;(427 Suppl):S6-S15. [DOI] [PubMed] [Google Scholar]
- 5. Ng KW, Lima EG, Bian L, O’Conor CJ, Jayabalan PS, Stoker AM, et al. Passaged adult chondrocytes can form engineered cartilage with functional mechanical properties: a canine model. Tissue Eng Part A. 2010;16(3):1041-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buckwalter JA, Brown TD. Joint injury, repair, and remodeling: roles in post-traumatic osteoarthritis. Clin Orthop Relat Res. 2004;(423):7-16. [PubMed] [Google Scholar]
- 7. Heir S, Nerhus TK, Røtterud JH, Løken S, Ekeland A, Engebretsen L, et al. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am J Sports Med. 2010;38(2):231-7. [DOI] [PubMed] [Google Scholar]
- 8. Stoker AM, Stannard JP, Kuroki K, Bozynski CC, Pfeiffer FM, Cook JL, et al. Validation of the Missouri Osteochondral Allograft Preservation System for the maintenance of osteochondral allograft quality during prolonged storage. Am J Sports Med. 2018;46(1_suppl):58-65. [DOI] [PubMed] [Google Scholar]
- 9. Erggelet C, Endres M, Neumann K, Morawietz L, Ringe J, Haberstroh K, et al. Formation of cartilage repair tissue in articular cartilage defects pretreated with microfracture and covered with cell-free polymer-based implants. J Orthop Res, 2009;27(10):1353-60. [DOI] [PubMed] [Google Scholar]
- 10. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smyth NA, Ross KA, Haleem AM, Hannon CP, Murawski CD, Do HT, et al. Platelet-rich plasma and hyaluronic acid are not synergistic when used as biological adjuncts with autologous osteochondral transplantation. Cartilage. 2018;9:321-8. doi: 10.1177/1947603517690022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garrity JT, Stoker AM, Sims HJ, Cook JL. Improved osteochondral allograft preservation using serum-free media at body temperature. Am J Sports Med. 2012;40(11):2542-8. [DOI] [PubMed] [Google Scholar]
- 13. Sherman SL, Garrity J, Bauer K, Cook J, Stannard J, Bugbee W. Fresh osteochondral allograft transplantation for the knee: current concepts. J Am Acad Orthop Surg. 2014;22(2):121-33. [DOI] [PubMed] [Google Scholar]
- 14. De Caro F, Bisicchia S, Amendola A, Ding L. Large fresh osteochondral allografts of the knee: a systematic clinical and basic science review of the literature. Arthroscopy. 2015;31(4):757-65. [DOI] [PubMed] [Google Scholar]
- 15. Pallante AL, Bae WC, Chen AC, Görtz S, Bugbee WD, Sah RL. Chondrocyte viability is higher after prolonged storage at 37°C than at 4°C for osteochondral grafts. Am J Sports Med. 2009;37(Suppl 1):24S-32S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nover AB, Stefani RM, Lee SL, Ateshian GA, Stoker AM, Cook JL, et al. Long-term storage and preservation of tissue engineered articular cartilage. J Orthop Res. 2016;34(1_suppl):141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. LaPrade RF, Botker J, Herzog M, Agel J. Refrigerated osteoarticular allografts to treat articular cartilage defects of the femoral condyles. A prospective outcomes study. J Bone Joint Surg Am. 2009;91(4):805-11. [DOI] [PubMed] [Google Scholar]
- 18. Wright GJ, Brockbank KG, Rahn E, Halwani DO, Chen Z, Yao H. Impact of storage solution formulation during refrigerated storage upon chondrocyte viability and cartilage matrix. Cells Tissues Organs. 2014;199(1_suppl):51-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Csönge L, Bravo D, Newman-Gage H, Rigley T, Conrad EU, Bakay A, et al. Banking of osteochondral allografts, Part II. preservation of chondrocyte viability during long-term storage. Cell Tissue Bank. 2002;3(3):161-8. [DOI] [PubMed] [Google Scholar]
- 20. Lattermann C, Romine SE. Osteochondral allografts: state of the art. Clin Sports Med. 2009;28(2):285-301. [DOI] [PubMed] [Google Scholar]
- 21. Bugbee WD, Pallante-Kichura AL, Görtz S, Amiel D, Sah R. Osteochondral allograft transplantation in cartilage repair: graft storage paradigm, translational models, and clinical applications. J Orthop Res. 2016;34(1_suppl):31-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cook JL, Stannard JP, Stoker AM, Bozynski CC, Kuroki K, Cook CR, et al. Importance of donor chondrocyte viability for osteochondral allografts. Am J Sports Med. 2016;44(5):1260-8. [DOI] [PubMed] [Google Scholar]
- 23. Williams SK, Amiel D, Ball ST, Allen RT, Wong VW, Chen AC, et al. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003;85(11):2111-20. [DOI] [PubMed] [Google Scholar]
- 24. Stoker A, Garrity JT, Hung CT, Stannard JP, Cook J. Improved preservation of fresh osteochondral allografts for clinical use. J Knee Surg. 2012;25(2):117-25. [DOI] [PubMed] [Google Scholar]
- 25. Dontchos BN, Coyle CH, Izzo NJ, Didiano DM, Karpie JC, Logar A, et al. Optimizing CO2 normalizes pH and enhances chondrocyte viability during cold storage. J Orthop Res. 2008;26(5):643-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pearsall AW, 4th, Tucker JA, Hester RB, Heitman RJ. Chondrocyte viability in refrigerated osteochondral allografts used for transplantation within the knee. Am J Sports Med. 2004;32(1_suppl):125-31. [DOI] [PubMed] [Google Scholar]
- 27. Pennock AT, Wagner F, Robertson CM, Harwood FL, Bugbee WD, Amiel D. Prolonged storage of osteochondral allografts: does the addition of fetal bovine serum improve chondrocyte viability? J Knee Surg. 2006;19(4):265-72. [DOI] [PubMed] [Google Scholar]
- 28. Teng MS, Yuen AS, Kim HT. Enhancing osteochondral allograft viability: effects of storage media composition. Clin Orthop Relat Res. 2008;466(8):1804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Williams JM, Virdi AS, Pylawka TK, Edwards RB, 3rd, Markel MD, Cole BJ. Prolonged-fresh preservation of intact whole canine femoral condyles for the potential use as osteochondral allografts. J Orthop Res. 2005;23(4):831-7. [DOI] [PubMed] [Google Scholar]
- 30. Robertson CM, Allen RT, Pennock AT, Bugbee WD, Amiel D. Upregulation of apoptotic and matrix-related gene expression during fresh osteochondral allograft storage. Clin Orthop Relat Res. 2006;442:260-6. [DOI] [PubMed] [Google Scholar]
- 31. McCarty WJ, Pallante AL, Rone RJ, Bugbee WD, Sah RL. The proteoglycan metabolism of articular cartilage in joint-scale culture. Tissue Eng Part A. 2010;16(5):1717-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin Y, Lewallen EA, Camilleri ET, Bonin CA, Jones DL, Dudakovic A, et al. RNA-seq analysis of clinical-grade osteochondral allografts reveals activation of early response genes. J Orthop Res. 2016;34(11):1950-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gross AE, Kim W, Heras FL, Backstein D, Safir O, Pritzker KP. Fresh osteochondral allografts for posttraumatic knee defects: long-term follow-up. Clin Orthop Relat Res. 2008;466(8):1863-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beaver RJ, Mahomed M, Backstein D, Davis A, Zukor DJ, Gross AE. Fresh osteochondral allografts for post-traumatic defects in the knee. A survivorship analysis. J Bone Joint Surg Br. 1992;74(1_suppl):105-10. [DOI] [PubMed] [Google Scholar]
- 35. Pallante AL, Chen AC, Ball ST, Amiel D, Masuda K, Sah RL, et al. The in vivo performance of osteochondral allografts in the goat is diminished with extended storage and decreased cartilage cellularity. Am J Sports Med. 2012;40(8):1814-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Williams RJ, 3rd, Ranawat AS, Potter HG, Carter T, Warren RF. Fresh stored allografts for the treatment of osteochondral defects of the knee. J Bone Joint Surg Am. 2007;89(4):718-26. [DOI] [PubMed] [Google Scholar]
- 37. Bakay A, Csönge L, Papp G, Fekete L. Osteochondral resurfacing of the knee joint with allograft. Clinical analysis of 33 cases. Int Orthop. 1998;22(5):277-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Onuma K, Urabe K, Naruse K, Park HJ, Uchida K, Itoman M. Cold preservation of rat osteochondral tissues in two types of solid organ preservation solution, culture medium and saline. Cell Tissue Bank. 2009;10(1_suppl):1-9. [DOI] [PubMed] [Google Scholar]
- 39. Bian L, Lima EG, Angione SL, Ng KW, Williams DY, Xu D, et al. Mechanical and biochemical characterization of cartilage explants in serum-free culture. J Biomech. 2008;41(6):1153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lopez C, Ajenjo N, Muñoz-Alonso MJ, Farde P, León J, Gómez-Cimiano J. Determination of viability of human cartilage allografts by a rapid and quantitative method not requiring cartilage digestion. Cell Transplant. 2008;17(7):859-64. [DOI] [PubMed] [Google Scholar]
- 41. Bastian JD, Egli RJ, Ganz R, Hofstetter W, Leunig M. Chondrocytes within osteochondral grafts are more resistant than osteoblasts to tissue culture at 37 degrees C. J Invest Surg, 2011;24(1_suppl):28-34. [DOI] [PubMed] [Google Scholar]
- 42. Thomas VJ, Jimenez SA, Brighton CT, Brown N. Sequential changes in the mechanical properties of viable articular cartilage stored in vitro. J Orthop Res. 1984;2(1_suppl):55-60. [DOI] [PubMed] [Google Scholar]
- 43. Bian L, Stoker AM, Marberry KM, Ateshian GA, Cook JL, Hung CT. Effects of dexamethasone on the functional properties of cartilage explants during long-term culture. Am J Sports Med. 2010;38(1_suppl):78-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Allen RT, Robertson CM, Pennock AT, Bugbee WD, Harwood FL, Wong VW, et al. Analysis of stored osteochondral allografts at the time of surgical implantation. Am J Sports Med. 2005;33(10):1479-84. [DOI] [PubMed] [Google Scholar]
- 45. Waters NP, Stoker AM, Carson WL, Pfeiffer FM, Cook JL. Biomarkers affected by impact velocity and maximum strain of cartilage during injury. J Biomech. 2014;47(12):3185-95. [DOI] [PubMed] [Google Scholar]
- 46. Hangody LR, Gál T, Szűcs A, Vásárhelyi G, Tóth F, Módis L, et al. Osteochondral allograft transplantation from a living donor. Arthroscopy. 2012;28(8):1180-3. [DOI] [PubMed] [Google Scholar]
- 47. Custers RJ, Creemers LB, Verbout AJ, van Rijen MH, Dhert WJ, Saris DB. Reliability, reproducibility and variability of the traditional Histologic/Histochemical Grading System vs the new OARSI Osteoarthritis Cartilage Histopathology Assessment System. Osteoarthritis Cartilage. 2007;15(11):1241-8. [DOI] [PubMed] [Google Scholar]
- 48. Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53(3):523-37. [PubMed] [Google Scholar]
- 49. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1_suppl):289-300. [Google Scholar]
- 50. Raz G, Safir OA, Backstein DJ, Lee PT, Gross AE. Distal femoral fresh osteochondral allografts: follow-up at a mean of twenty-two years. J Bone Joint Surg Am. 2014;96(13):1101-7. [DOI] [PubMed] [Google Scholar]
- 51. McCulloch PC, Kang RW, Sobhy MH, Hayden JK, Cole BJ. Prospective evaluation of prolonged fresh osteochondral allograft transplantation of the femoral condyle: minimum 2-year follow-up. Am J Sports Med. 2007;35(3):411-20. [DOI] [PubMed] [Google Scholar]
- 52. Cook JL, Stoker AM, Stannard JP, Kuroki K, Cook CR, Pfeiffer FM, et al. A novel system improves preservation of osteochondral allografts. Clin Orthop Relat Res. 2014;472(11):3404-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Horton WA, Dwyer C, Goering R, Dean DC. Immunohistochemistry of types I and II collagen in undecalcified skeletal tissues. J Histochem Cytochem. 1983;31(3):417-25. [DOI] [PubMed] [Google Scholar]
- 54. Roberts S, Menage J, Sandell LJ, Evans EH, Richardson JB. Immunohistochemical study of collagen types I and II and procollagen IIA in human cartilage repair tissue following autologous chondrocyte implantation. Knee. 2009;16(5):398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tirico LEP, McCauley JC, Pulido PA, Bugbee WD. Lesion size does not predict outcomes in fresh osteochondral allograft transplantation. Am J Sports Med. 2018;46(4):900-7. [DOI] [PubMed] [Google Scholar]
- 56. Pennock AT, Robertson CM, Wagner F, Harwood FL, Bugbee WD, Amiel D. Does subchondral bone affect the fate of osteochondral allografts during storage? Am J Sports Med. 2006;34(4):586-91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, 12._SupplementaryMaterial for Modernizing Storage Conditions for Fresh Osteochondral Allografts by Optimizing Viability at Physiologic Temperatures and Conditions by Janet M. Denbeigh, Mario Hevesi, Carlo A. Paggi, Zachary T. Resch, Leila Bagheri, Kristin Mara, Arvin Arani, Chenghao Zhang, A. Noelle Larson, Daniel B.F. Saris, Aaron J. Krych and Andre J. van Wijnen in CARTILAGE
Supplemental material, 13._Supplementary_Table_1_1 for Modernizing Storage Conditions for Fresh Osteochondral Allografts by Optimizing Viability at Physiologic Temperatures and Conditions by Janet M. Denbeigh, Mario Hevesi, Carlo A. Paggi, Zachary T. Resch, Leila Bagheri, Kristin Mara, Arvin Arani, Chenghao Zhang, A. Noelle Larson, Daniel B.F. Saris, Aaron J. Krych and Andre J. van Wijnen in CARTILAGE