Abstract
Objective
To develop patient-focused consensus guidelines on the indications for the use of scaffolds to address chondral and osteochondral femoral condyle lesions.
Design
The RAND/UCLA Appropriateness Method (RAM) was used to develop patient-specific recommendations by combining the best available scientific evidence with the collective judgement of a panel of experts guided by a core panel and multidisciplinary discussers. A list of specific clinical scenarios was produced regarding adult patients with symptomatic lesions without instability, malalignment, or meniscal deficiency. Each scenario underwent discussion and a 2-round vote on a 9-point Likert-type scale (range 1-3 “inappropriate,” 4-6 “uncertain,” 7-9 “appropriate”). Scores were pooled to generate expert recommendations.
Results
Scaffold (chondral vs. osteochondral), patient characteristics (age and sport activity level), and lesion characteristics (etiology, size, and the presence of osteoarthritis [OA]) were considered to define 144 scenarios. The use of scaffold-based procedures was considered appropriate in all cases of chondral or osteochondral lesions when joints are not affected by OA, while OA joints presented more controversial results. The analysis of the evaluated factors showed a different weight in influencing treatment appropriateness: the presence of OA influenced 58.3% of the indications, while etiology, size, and age were discriminating factors in 54.2%, 29.2%, and 16.7% of recommendations, respectively.
Conclusions
The consensus identified indications still requiring investigation, but also the convergence of the experts in several scenarios defined appropriate or inappropriate, which could support decision making in the daily clinical practice, guiding the use of scaffold-based procedures for the treatment of chondral and osteochondral knee defects.
Keywords: scaffolds, cartilage, osteochondral, knee, osteoarthritis, guidelines
Introduction
Chondral and osteochondral lesions are common and debilitating conditions, 1 which, if not properly treated, may lead to the development of sever articular defects and, ultimately, of osteoarthritis (OA). 2 In the past 20 years, regenerative scaffold-based procedures have been developed to address this kind of lesions. The rationale for using a scaffold is to offer a temporary 3-dimensional structure of biodegradable polymers to mimic chondral or osteochondral architecture and favor cell and tissue growth. 3 From their first use in autologous chondrocyte implantation techniques, to their more recent cell-free use, scaffolds keep playing a central role in cartilage regenerative strategies.4-7
Scaffolds were initially proposed within autologous chondrocyte implantation techniques, either as a replacement for the periosteum patch to contain the chondrocyte culture, or as matrix-assisted autologous chondrocyte transplantation, where the harvested cells were cultured on the 3-dimensional biomaterial, which favored their redifferentiation, homogeneous distribution, and easier handling for the surgical implantation.3,8 As autologous chondrocyte implantation and matrix-assisted autologous chondrocyte transplantation suffer from some drawbacks, such as the need for 2 operations, high costs, and regulatory restrictions, alternative solutions have been explored to regenerate the articular surface. 6 The most recent developments reaching the clinical application could be summarized in 2 major strategies: the use of different cell sources for 1-step solutions or the application of biomaterials as a cell-free approach.9,10 From one side, mesenchymal stem cells or blood-derived growth factors obtained from concentration processes are gaining popularity in the field of cartilage regeneration as a new powerful tool for scaffold augmentation with minimal manipulation. 9 Conversely, there is an increasing awareness of the role of scaffolds, which are not just carrier systems for cell delivery but may also present an intrinsic ability to promote chondral or osteochondral regeneration by exploiting the self-regenerative potential of the body. 10 Several scaffolds (natural or synthetic) in different physical forms (fibers, meshes, and gels)11,12 reached the clinical practice, and studies are now being published with good mid- and long-term results.9,13-18 However, beside an overall clinical improvement, the current literature lacks evidence for a clear patient profiling,14,19,20 and it offers limited indications to guide the use of scaffolds according to the specific clinical conditions encountered by physicians in their clinical practice.
Thus, the International Cartilage Regenerative & Joint Preservation Society (ICRS) set up a consensus process to establish indications for the appropriateness of scaffolds in different clinical scenarios. The aim of this consensus was to develop patient-focused, up-to-date, evidence-based, expert consensus guidelines on the indications for the use of scaffolds for the treatment of chondral and osteochondral knee lesions.
Methods
Consensus Design
The RAND/UCLA Appropriateness Method (RAM) was used to develop patient-specific recommendations on the appropriateness of use of scaffolds for the treatment of chondral and osteochondral lesions affecting the femoral condyles. 21 The RAM is a method of group consensus developed to produce, through a highly structured approach, patient-specific recommendations by combining the best available scientific evidence with the collective judgement of a panel of experts. Several studies support the reliability, internally consistency, and clinical validity of the RAM-based recommendations. 22
The RAM process involved 3 interdependent groups: a core panel, an expert panel, and a discussers panel. The core panel defined the scenarios of the RAM and guided the expert panel through the RAM tasks providing literature data. The expert panel, composed by 13 voting members, used the data provided by the core panel to come to a consensus. The discussers helped providing a multispecialty point of view to the discussions. The members were selected on the basis of their scientific and clinical expertise in cartilage treatment ( Table 1 ).
Table 1.
Composition of Panels.
| Name | Expertise | Affiliation | Role |
|---|---|---|---|
| Giuseppe Filardo | Orthopedic researcher | ATRc, IRCCS Rizzoli Orthopedic Institute, Bologna, Italy | Core panel (moderator) |
| Luca Andriolo | Orthopedic surgeon | II Orthopedic Clinic, IRCCS Rizzoli Orthopedic Institute, Bologna, Italy | Core panel |
| Massimo Berruto | Orthopedic surgeon | Department of Knee Joint Surgery, ASST PINI-CTO, Milan, Italy | Core panel |
| Peter Angele | Orthopedic surgeon | Clinic for Trauma and Reconstructive Surgery, University Hospital Regensburg, Regensburg, Germany | Voting expert |
| Sporthopaedicum Regensburg, Regensburg, Germany | |||
| Mats Brittberg | Orthopedic surgeon | Cartilage Research Unit, University of Gothenburg, Gothenburg, Sweden | Voting expert |
| Region Halland Orthopaedics, Kungsbacka Hospital, Kungsbacka, Sweden | |||
| Vincenzo Condello | Orthopedic surgeon | Joint Preservation and Reconstructive Surgery and Sports Medicine Unit, Humanitas Castelli Clinic, Bergamo, Italy | Voting expert |
| Alessandro Di Martino | Orthopedic surgeon | II Orthopedic Clinic, IRCCS Rizzoli Orthopedic Institute, Bologna, Italy | Voting expert |
| Berardo Di Matteo | Orthopedic surgeon | Department of Biomedical Sciences, Humanitas University, Rozzano, Milan, Italy | Voting expert |
| Humanitas Clinical and Research Center, Rozzano, Milan, Italy | |||
| Justus Gille | Orthopedic surgeon | Department of Trauma and Orthopaedic Surgery, University Hospital Schleswig-Holstein, Campus Luebeck, Luebeck, Germany | Voting expert |
| Elizaveta Kon | Orthopedic surgeon | Department of Biomedical Sciences, Humanitas University, Rozzano, Milan, Italy | Voting expert |
| Humanitas Clinical and Research Center, Rozzano, Milan, Italy | |||
| First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University), Department of Traumatology, Orthopedics and Disaster Surgery, Moscow, Russian Federation | |||
| Christian Lattermann | Orthopedic surgeon | Department of Orthopaedic Surgery, Division of Sports Medicine, Center for Cartilage Repair, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA | Voting expert |
| Norimasa Nakamura | Orthopedic surgeon | Institute for Medical Science in Sports, Osaka Health Science University, Osaka, Japan | Voting expert |
| Stefan Nehrer | Orthopedic surgeon | Center for Regenerative Medicine, Danube University, Krems-an-der-Donau, Austria | Voting expert |
| Giuseppe M. Peretti | Orthopedic surgeon | IRCCS Istituto Ortopedico Galeazzi, Milan, ItalyDepartment of Biomedical Sciences for Health, University of Milan, Milan, Italy | Voting expert |
| Peter Verdonk | Orthopedic surgeon | ORTHOCA, AZ Monica Hospitals, Antwerp, BelgiumAspetar Hospital, Doha, Qatar | Voting expert |
| Kenneth Zaslav | Orthopedic surgeon | Ortho Virginia, Virginia Commonwealth University, Richmond, VA, USA | Voting expert |
| Susan Chubinskaya | Basic scientist | Department of Pediatrics, Orthopedic Surgery & Medicine (Section of Rheumatology), Rush University Medical Center, Chicago, IL, USA | Discussers |
| Laura de Girolamo | Basic scientist | Orthopaedic Biotechnology Laboratory, IRCCS Istituto Ortopedico Galeazzi, Milano, Italy | Discussers |
| REGAIN—REgenerative GAleazzi Institute, IRCCS Istituto Ortopedico Galeazzi, Milano, Italy | |||
| Nogah Shabshin | Radiologist | Department of radiology, Emek Medical Center, Clalit Healthcare Services, Afula, Israel | Discussers |
| Department of Radiology, PennMedicine, Philadelphia, PA, USA |
Scenarios Development
The RAM process was preceded by an extensive literature overview by the core panel to develop the consensus scenarios and ensure that panelists had access to the body of evidence in view of the rating procedure. To avoid interpretation bias, results were presented as a literature overview rather than as a review. The final document included the results of 38 studies (systematic reviews, randomized controlled trials, and observational studies analyzing possible prognostic factors).
Based on literature results and according to experts’ opinion, a list of specific clinical scenarios/indications was produced. These clinical scenarios described a patient with a set of characteristic features presented in the form of a matrix categorizing patients candidate to scaffold implantation in terms of demographic data and joint and lesion features. These characteristics were chosen according to the available literature evidence suggesting a correlation with the clinical outcome after surgery, and could therefore influence the appropriateness of the procedure. More in detail, the criteria that emerged from the literature search used to define the scenarios were the following:
Age (≤40 years old vs. >40 years old)
Sport activity level (competitive or professional vs. not competitive or not sport active)
Etiology (traumatic/focal vs. degenerative vs. osteochondritis dissecans [OCD])
Size (<2 cm2 vs. 2-4 cm2 vs. >4 cm2)
OA (Kellgren-Lawrence 23 grade 0-I vs. grade II-III)
The scenarios were grouped into “chapters” based on the individual characteristics (age and sport activity level). Each of these chapters presented specific scenarios based on joint and lesion characteristics. Regarding OCD, lesions >5 mm in depth were considered.24,25 Degenerative etiology was intended as a characteristic of the lesion independent from the presence of joint OA defined by X-ray preoperative evaluation. Other factors were excluded from the scenarios. Some of them, like uncorrected malalignment, a joint instability, deficiency of meniscal tissue and function, and advanced OA,26,27 are already known to be contraindications for scaffold-based procedures and thus did not undergo consensus process. Lesions on the patello-femoral joint were considered a different pathological setting compared to the tibiofemoral joint, with peculiar biomechanical and biologic characteristics requiring a separate evaluation, and thus were excluded from the current study.28,29 Finally, some factors were excluded due to the weak evidence about their effect in influencing scaffold-based procedures (e.g., smoking, body mass index, sex, medial or lateral condyle, symptoms duration, etc.).
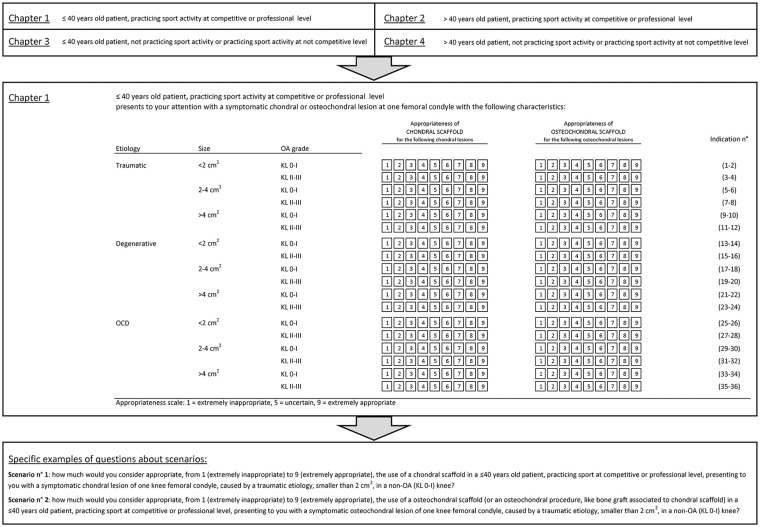
Based on the 5 clinical variables identified as more relevant for the treatment choice, a set of 72 clinical scenarios was developed. These scenarios regarded adult patients (≥18 years old) presenting to the orthopedic surgeon with a symptomatic chondral or osteochondral knee lesion not treated surgically before, without instability, malalignment, or meniscal deficiency. Panelists were asked to individually assess the appropriateness separately for either chondral or osteochondral scaffold (evaluating the use of a chondral scaffold for purely chondral lesions and of an osteochondral scaffold for osteochondral lesions) for all scenarios, for a total of 144 indications. Chondral scaffolds with bone graft augmentation were included among osteochondral procedures. A representation of the scenario organization into chapters, Chapter 1 (young sport active patients), and 2 specific scenarios are reported in detail in Figure 1 as example of scenario presentation to the expert panel and voting form.
Figure 1.
Representation of the scenario organization into chapters, Chapter 1 (young sport active patients), and 2 specific scenarios are reported in detail as example of scenario presentation to the expert panel and voting form.
Consensus Process
The appropriateness of the treatment indications in the different scenarios was rated in 2 rounds. As per RAM method, the 2-round process is designed to sort out whether discrepant ratings are due to real clinical disagreement over the use of the procedure (“real” disagreement) or to fatigue or misunderstanding (“artefactual” disagreement). 21
In the first round, the expert panel received the clinical scenarios by email and was asked to rate the intervention appropriateness. According to the RAM, 21 each panelist ranked, independently from the other panelists, the appropriateness for each scenario on a 9-point Likert-type scale, in which a score in the range 1 to 3 was considered “inappropriate,” 4 to 6 “uncertain,” and 7 to 9 “appropriate.” They were invited to use the synthesized evidence provided by the core panel overseeing the consensus process. The expert panelists were asked not to consider the cost of the procedures in rating the appropriateness of the scenarios.
In the second round, held on December 15, 2018, in Milan, Italy, the experts’ panel and the discussers’ panel met under the leadership of an experienced moderator. Each panelist received an individualized document showing the distribution of all the overall first round rating of the experts, together with his/her own specific ratings. During the meeting, panelists discussed the ratings, focusing on areas of disagreement, and were given the opportunity to modify the original list of indications and/or definitions, if desired. The panel was not forced to consensus, and after discussing each chapter of the list of scenarios, experts rerated each indication individually. 21
Data Analysis and Statistical Methods
The final scores of the 9-point Likert-type scale of each expert were then pooled to generate a median appropriateness score for each scenario. In addition, according to RAM, the presence of voting dispersion was calculated by statistical analysis according to BIOMED Concerted Action on Appropriateness 21 to define the presence of “disagreement” among votes in each scenario. Finally, the use of the treatment for each scenario was classified:
“Appropriate”: median score of ≥7 without disagreement
“Inappropriate”: median vote of ≤3 without disagreement
A scenario receiving a score between 4 and 6, or a scenario with disagreement, was classified as “uncertain.” An “uncertain” recommendation can reflect either the ambiguous state of current evidence or equivocal appropriateness either due to a moderately unfavorable risk profile or to limited efficacy. However, the “uncertain” classification is not intended to be a negative recommendation or to preclude a priori the use of the treatment for the specific scenario, relying on the physician-patient interaction in determining treatment decision in the context of the individual characteristics, comorbidities, and preferences.
Results
The first result of the second-round discussion was the redefinition of the parameters to be considered key for the identification of the clinical scenarios. After discussion and analyzing the first-round data, only 4 of the 5 characteristics were confirmed by the panel to influence the appropriateness of scaffold-based procedures: patient age, lesion etiology, defect size, and OA grade. In fact, the first-round results showed an overlapping between the chapters defined according to the sport activity level. Expert panel and discussers agreed that patient sport activity level can influence more the level of expected improvement than the appropriateness of the indication. Moreover, to weight the indication for a scaffold-based procedure, the sport activity level should be considered together with several other aspects (e.g., sport type, remaining level of high-level career, etc.). As this case-specific definition was outside the aim of the consensus, sport activity level was excluded from the scenario’s definition, leaving 72 scenarios to be discussed as reported in the following paragraphs.
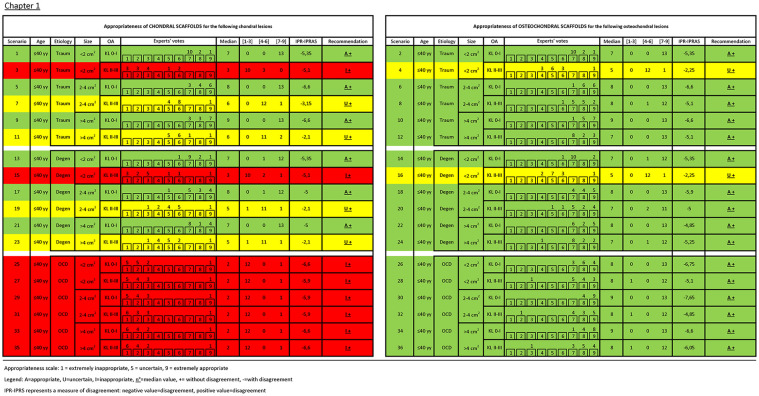
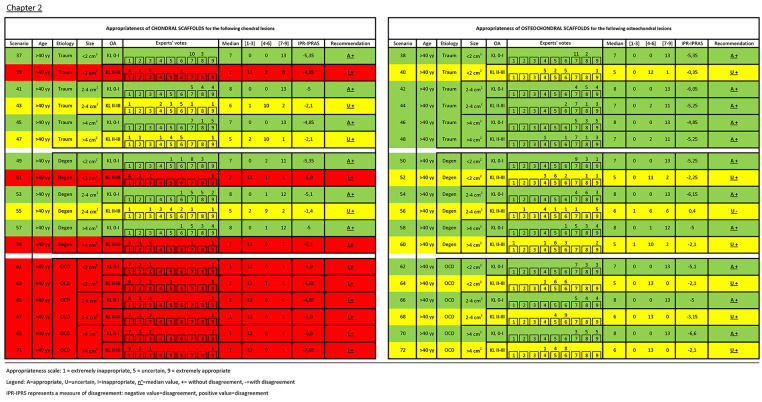
Details of experts’ rating with median, recommendation, and agreement value for each clinical scenario are reported in Figures 2 and 3 . Overall, in 39 scenarios the use of scaffolds was considered appropriate without disagreement, in 17 inappropriate without disagreement, and in 16 uncertain (15 without disagreement and 1 with disagreement). The analysis of the role of the evaluated factors showed a different weight in influencing the treatment indication appropriateness. Beside the nature of the scaffold, either chondral or osteochondral, which influenced 23 out of 36 treatment indications (63.9%), OA was the most discriminating factor: out of the 36 couples of scenarios differing only in terms of OA, the appropriateness changed in 21 scenarios (58.3%) based on the presence or not of knee OA. A different etiology changed appropriateness in 13 of the 24 triplets of scenarios differing only for etiology (54.2%), size changed appropriateness in 7 of the 24 triplets of scenarios differing only for defect size (29.2%), and age changed the appropriateness in 6 of the 36 couples of scenarios differing only for age (16.7%).
Figure 2.
Experts’ rating with median, recommendation, and agreement value for each clinical scenario of Chapter 1.
Figure 3.
Experts’ rating with median, recommendation, and agreement value for each clinical scenario of Chapter 2.
Recommendations can be grouped within each chapter according to the etiology.
- OCD presented a dichotomic outcome with different indications based on the type of scaffold considered:
- A chondral scaffold was considered inappropriate for the treatment of deep OCD lesions (defined by the panel as deeper than 5 mm) of both younger and older patients (scenarios 25, 27, 29, 31, 33, 35, 61, 63, 65, 67, 69, 71: all inappropriate without disagreement).
- On the other hand, the use of osteochondral scaffolds is always appropriate in patients younger than 40 years old affected by OCD, regardless of the size of the lesion and the concomitant presence of OA in the affected joint (scenarios 26, 28, 30, 32, 34, 36: appropriate without disagreement).
- The same was valid also for older patients without OA (scenarios 62, 66, 70: appropriate without disagreement), whereas the appropriateness of using osteochondral scaffolds for OCD lesion in patients older than 40 years in OA joint was uncertain (scenarios 64, 68, 72: uncertain without disagreement).
- Traumatic lesions showed
- The appropriateness of using chondral and osteochondral scaffolds (to address chondral or osteochondral lesions, respectively) when treating patients without OA, regardless of age and lesion size (scenarios 1, 2, 5, 6, 9, 10, 37, 38, 41, 42, 45, 46: all appropriate without disagreement).
- The key role of OA was confirmed also for traumatic lesions. Regardless of patient age, in joint affected by OA chondral scaffolds were deemed inappropriate in small lesions and uncertain in medium/large lesions (scenarios 3, 39 and 7, 11, 43, 47, respectively: all without disagreement).
- Conversely, the indication to use osteochondral scaffolds in joint with OA, regardless of age, was considered uncertain in small lesions and appropriate in medium/large lesions (scenarios 4, 40 and 8, 12, 44, 48, respectively: all without disagreement).
- For degenerative lesions all factors determined treatment appropriateness.
- When treating patients without OA, both chondral and osteochondral scaffolds (to address chondral or osteochondral lesions, respectively) were considered appropriate regardless of age and lesion size (scenarios 13, 14, 17, 18, 21, 22, 49, 50, 53, 54, 57, 58: all without disagreement).
- The situation changed when OA is present. In this case, in patients with degenerative lesions smaller than 2 cm2 the use of scaffolds was never considered indicated regardless of age, being inappropriate for chondral scaffolds (scenarios 15, 51: without disagreement) and uncertain for osteochondral scaffolds (scenarios 16, 52: without disagreement).
- In patients with medium degenerative lesions the indication on chondral scaffolds was considered uncertain, regardless of age (scenarios 19, 55: without disagreement), while the use of osteochondral scaffolds was deemed appropriate in young patients (scenario 20 without disagreement) and uncertain in patients >40 years old (scenario 56 with disagreement).
- Finally, for lesions >4 cm2 the use of chondral scaffolds was considered uncertain in young patients (scenario 23 without disagreement) and inappropriate in older patients (scenario 59 without disagreement), while the use of osteochondral scaffolds was judged appropriate in younger patients (scenario 24 without disagreement) and uncertain in older patients (scenario 60 without disagreement).
Discussion
The main finding of the consensus is that the use of scaffold-based procedures is considered appropriate by the experts in all cases of chondral or osteochondral lesions in knees without instability, malalignment, or meniscal deficiency, when joints are not affected by OA. On the other hand, OA joints presents more controversial results, with appropriateness being affected in different scenarios according to patient and lesion parameters such as age, lesion etiology and size, and the chondral or osteochondral nature of the scaffold.
The first result of this RAM was the definition, based on the literature evidence reviewed for the first consensus step, of the most important factors that might influence the indications for scaffold-based procedures. The first aspect that was underlined is the presence of different types of scaffolds, which have been grouped in chondral and osteochondral strategies. As they have been developed for different indications, mainly based on the depth of the lesions, every scenario was assessed separately for chondral scaffolds in case of superficial lesions and for osteochondral scaffolds in case of deep defects. Within this overall indication, the appropriateness varied significantly according to other factors. Among the different etiologies, OCD has often shown in literature to be the most favorable condition to benefit from scaffold-based procedures, but not for all scaffolds. Chondral scaffolds may suffice to address shallow OCDs, but their use without bone augmentation was considered inappropriate for deep OCDs. 24 On the other hand, osteochondral procedures were always considered appropriate except for OCD lesions in OA knees in older patients, where different kinds of conservative or surgical treatments could be more suitable.
OA actually emerged as the most important factor influencing the expert panel decision on treatment appropriateness according to the different scenarios. In fact, while scaffold-based procedures have been considered appropriate in all cases of chondral or osteochondral lesions (beside chondral strategies for deep OCDs) in non-OA knees, the appropriateness changed in the majority of scenarios in patients with knee OA. The reason lies in the literature evidence with contrasting findings for the use of chondral scaffolds in OA patients,27,30,31 and in the very nature of this pathology, which involves both cartilage and subchondral bone. 32 In this light, chondral scaffolds were judged as either inappropriate or with uncertain indication, while osteochondral scaffolds were instead considered uncertain or appropriate, especially in younger patients aiming to avoid more sacrificing procedures such as metal resurfacing. This underlines another important aspect in the consensus process: recommendations were derived by the convergence of different perspectives, including the evidence on treatment effectiveness, but considering also the risk-benefit ratio and the availability of alternative treatment options in each specific scenario. In this light, the 144 analyzed indications have to be considered a simplification of the specific cases encountered in the clinical practice, with schematization process forcing to include from one side unlikely scenarios, and from the other side a trivialized representation of the multifaceted characteristics of real patients. Nonetheless, despite from one side the consensus process bears the risk of oversimplification, on the other hand it allows to define areas where different aspects can converge. This is the case of the sport activity level, initially considered an important factor for treatment indication. However, although the panel agreed on the importance of sport participation, in the end the usefulness of treating defects of the articular surface was recognized as being similar for less active patients. Sport participation implies the need to meet high functional requests, but restoring the articular surface is important also for daily activities, making the treatment appropriate for both sport-active and sedentary patients. Different considerations characterize high-level/professional athletes, where peculiar aspects have to be considered, such as the expected duration of sport career, not evaluable in the current consensus. Thus, the reached recommendations are not intended for professional players.
The consensus process applied a simplification also with regard to the complex universe of different scaffolds and techniques.3,33 However, it is extremely difficult to find evidences on the superiority of one scaffold-based procedure over the others. Thus, the current consensus did not take in consideration differences in terms of biomaterials, neither of the use of cell-based or cell-free scaffolds. The consensus did not aim at providing indications over the best product, which would require specific comparative studies, but is rather intended to be valid for all available scaffold-based procedures. The focus was rather directed to the definition of recommendations according to different clinical scenarios based on patients and lesion characteristics. In detail, the results of this consensus regard the differences in appropriateness based on the etiology and the size of the lesion, and on the age of the patient, interplaying in determining the recommendations, in particular in the more complex scenario of lesions in OA joints. Lesion size was not a determining factor in non-OA knees; scaffold-based procedures were considered a suitable option even for small lesions, where other effective techniques are available (e.g., OAT, microfractures). However, the presence of OA brought to the table other aspects. While chondral scaffolds were always inappropriate and the use of osteochondral scaffold was uncertain in case of small lesions, as the experts recommended relying on the conservative treatment, the indications changed for bigger lesions. In these cases, a lower improvement can be expected by conservative means, and an attempt to restore the articular surface was considered appropriate, with an osteochondral approach, especially in younger patients. For young patients the attempt to pursue the restoration of the articular surface was supported, but more controversial results emerged discussing degenerative lesions in OA joints of older patients, which resulted in disagreement. This can be explained by the presence of new emerging treatments that hold promise to address also this kind of challenging lesions,32,34 but that are not yet supported by strong evidence due to their recent introduction. More studies need to confirm the potential of scaffolds in this scenario. Moreover, the improvement in the technologies could help pushing the current boundaries for the treatment indications. Currently, all experts agree on the contraindication for the use of scaffolds in advanced OA, which was excluded from the consensus scenarios, and on the importance to define potential and limitations within the earlier phases of OA.32,35 In this context, experts underlined the need to assess the presence and the quality of the cartilage surrounding the area to be treated, and urged the need to better investigate more precise cutoffs for the indications, although this proves to be particularly challenging. This is also the case of age, with the cutoff set at 40 years for the purpose of schematic representation of the consensus process. Obviously, 40 years of age should be only considered a rough limit, 36 as it does not reflect the joint biological age that could differ among patients. Moreover, each category includes different scenarios; within young patients, knee conditions can differ from 18 to 40 years old patients, and the same applies for patients in the older group.
These recommendations were reached in a consensus meeting aimed at exploiting the best available scientific evidence together with the collective judgement of experts to help physicians in the daily practice. 21 In fact, randomized controlled trials are often neither available nor able to provide enough detailed evidence to apply to the wide range of patients seen in the clinical practice. In this light, the RAM consensus method was selected to provide recommendations. This method of group consensus was developed in the 1980s by RAND (research and development) Corporation and UCLA (University of California-Los Angeles), 21 and since then it has been extensively used for assessing appropriateness of medical and surgical procedures (e.g., management of vertebral fragility fractures, coronary angiography, carotid endarterectomy, hysterectomy, and upper gastrointestinal tract endoscopy). 22 With respect to other consensus methods (e.g., Delphi method, nominal group technique, and National Institutes of Health consensus development conference), RAM has several advantages: it incorporates current scientific evidence in conjunction with expert opinion, it allows for both confidential ratings as well as group discussion, it has a moderate to excellent reproducibility as determined by different panelists for “appropriate” and “inappropriate” care, and it has an acceptable predictive validity for a recommendation supported by randomized controlled trials. Some disadvantages were also reported for this method: misclassification is possible, 37 it takes time and cost gathering the evidence and performing the multiple steps foreseen, it requires third party (core panel) to construct clinical indications for an intervention and analyze/interpret the results from the expert panel meeting, and the face-to-face confrontation leads to highly opinionated individuals in the field dominating the discussion. 22 On the other hand, the presence of a core panel and of a moderator has the scope to limit such relational biases, and the important support given from scientific evidences maintains some fixed points, which allowed this RAM group consensus to produce several recommendations.
The consensus need for a schematic representation required to force patients’ and lesions’ into categories, which are by definition artificial limits and therefore arguable, as reflected by the voting heterogeneity. Although the purpose of the consensus was to obtain univocal recommendations, even after discussion and the second voting round in most of the scenarios there were outliers, which means that not all the experts agreed on the recommendation. This could be also explained by different perspectives, as different experts could have given more or less weight to the several aspects considered for the recommendation, such as the importance to restore the articular surface, the strength of the available evidence, the presence of specific studies investigating specific conditions, the expected improvement considering the risks, and the alternative available treatment options. Thus, no univocal consensus could be reached on several indications. However, all experts agreed on the importance, especially for scenarios with uncertain recommendation, of applying scaffold-based procedures in specialized center, also considering that often cartilage lesions require a complex management.
The main limitation of the consensus, the simplification of the clinical reality, also implied the focus on isolated lesions, while often patients present comorbidities, such as instability, meniscal loss, and malalignment, which are key for the final outcome, as even small alignment variations have been correlated with the results of cartilage treatment. 38 Beside the articular surface, the entire joint has to be considered, and associated conditions have to be addressed by expert surgeons to optimize the chances of a successful outcome of scaffold-based procedures. In this light, an expert panel including only orthopedic surgeons was chosen, which could be considered another limitation as usually a multidisciplinary panel is recommended to avoid bias from a like-minded group with identical agenda. 21 Nevertheless, the procedures analyzed are strictly surgical, and the expert panel of surgeons was selected from different countries, with different health systems with different scaffolds and alternative treatments availability, to provide a heterogeneous view of the topic. Moreover, discussers skilled in other disciplines (radiology, regenerative medicine, basic science dealing with cartilage issues) were included, while other rehabilitation specialists and physiotherapists were not included due to the strictly surgical nature of the procedure. Another limitation regarded the need to simplify the heterogeneous field of scaffolds by grouping them into 2 main categories, chondral and osteochondral, while each scaffold might present peculiarities, with different potential and limitations. In fact, there are actually several types of scaffold (gels or more structured scaffolds, biologic vs. synthetic materials, more or less biodegradable scaffolds, which can be applied with or without the augmentation of cells, etc.), which probably have different results for the specific patients’ and lesions’ category; thus, this grouping process may be oversimplistic. Nevertheless, it is not the purpose of a RAM process to determine or to shed some lights over the best scaffold to be used in the specific indication. Appositely designed comparative trials may be much more indicated and desirable to clarify these aspects, while a RAM consensus can help in defining more broad treatment indications according to different clinical scenarios.
This RAM process included an expert panel of orthopedic surgeons and multidisciplinary discussers guided by a core panel through a structured process to merge the best available evidence with the collective judgement of experts. In conclusions, this consensus analyzed a broad spectrum of scenarios, underlying the importance to further investigate specific indications which still present unclear recommendations, but also showing the convergence of the experts in defining several scenarios where the use of scaffolds is deemed appropriate or inappropriate. In particular, the use of scaffold-based procedures was considered appropriate in all cases of chondral or osteochondral lesions when joints are not affected by OA, while OA joints presented more controversial results. Etiology, size, and age were also considered discriminating factors. These results may be helpful to support decision making in the daily clinical practice of cartilage surgery, guiding the use of scaffold-based procedures for the treatment of chondral and osteochondral knee defects.
Footnotes
Authors’ Note: Peter Verdonk is also affiliated to Department of Orthopaedic Surgery, Antwerp University Hospital, Belgium.
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Consensus Meeting has been financially supported by International Cartilage Regeneration and Joint Preservation Society.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M. Brittberg: Consultant for Vericel, Episuf AB; S. Chubinskaya: Research support from Jointechlabs, Inc., and Novartis research institute. Nothing to disclose in regards to this publication; L. de Girolamo: Consultant for Lipogems; A. Gobbi: research support from Anika Therapeutics; C. Lattermann: Consultant for Joint Restoration Foundation, Samumed, Vericel; S. Nehrer: Research support from Anika Therapeutics, Braincon, Orthosera GmbH, presenter for Arthrex, Inc., consultant for Orthosera GmbH; N. Shabshin: Consultant for Active Implants Ltd, Cartiheal; P. Verdonk: Consultant for Cartiheal, Conmed, Depuy Synthes, Active Implants Ltd; K. Zaslav: Research support from Active Implants, Aesculap/B. Braun, Organogenesis, Zimmer, presenter for Lifenet, Vericel, consultant for Cartiheal, Lifenet, stock or stock options in Cartiheal, Orthospace; E. Kon: Presenter for Zimmer-Biomet, Fidia, Finceramica, consultant for Cartiheal, Green Bone, stock or stock options in Cartiheal; G. Filardo, L. Andriolo, P. Angele, M. Berruto, V. Condello, A. Di Martino, B. Di Matteo, J. Gille, N. Nakamura, and G. M. Peretti have nothing to disclose.
Ethical Approval: Ethical approval was not required for this study.
Informed Consent: Informed consent was not required for this article.
ORCID iDs: Luca Andriolo  https://orcid.org/0000-0001-6352-9671
https://orcid.org/0000-0001-6352-9671
Alberto Gobbi  https://orcid.org/0000-0002-4868-0119
https://orcid.org/0000-0002-4868-0119
Stefan Nehrer  https://orcid.org/0000-0001-8008-2226
https://orcid.org/0000-0001-8008-2226
References
- 1. Solheim E, Krokeide AM, Melteig P, Larsen A, Strand T, Brittberg M. Symptoms and function in patients with articular cartilage lesions in 1,000 knee arthroscopies. Knee Surg Sports Traumatol Arthrosc. 2016;24(5):1610-6. doi: 10.1007/s00167-014-3472-9 [DOI] [PubMed] [Google Scholar]
- 2. Heijink A, Gomoll AH, Madry H, Drobnič M, Filardo G, Espregueira-Mendes J, et al. Biomechanical considerations in the pathogenesis of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2012;20(3):423-35. doi: 10.1007/s00167-011-1818-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kon E, Roffi A, Filardo G, Tesei G, Marcacci M. Scaffold-based cartilage treatments: with or without cells? A systematic review of preclinical and clinical evidence. Arthroscopy. 2015;31(4):767-75. doi: 10.1016/j.arthro.2014.11.017 [DOI] [PubMed] [Google Scholar]
- 4. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889-95. doi: 10.1056/NEJM199410063311401 [DOI] [PubMed] [Google Scholar]
- 5. Fischer S, Kisser A. Single-step scaffold-based cartilage repair in the knee: a systematic review. J Orthop. 2016;13(4):246-53. doi: 10.1016/j.jor.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dhollander AA, Sánchez VR, Almqvist KF, Verdonk R, Verbruggen G, Verdonk PC. The use of scaffolds in the treatment of osteochondral lesions in the knee: current concepts and future trends. J Knee Surg. 2012;25(3):179-86. [DOI] [PubMed] [Google Scholar]
- 7. Zaslav K, McAdams T, Scopp J, Theosadakis J, Mahajan V, Gobbi A. New frontiers for cartilage repair and protection. Cartilage. 2012;3(1 Suppl):77S-86S. doi: 10.1177/1947603511411050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dunkin BS, Lattermann C. New and emerging techniques in cartilage repair: MACI. Oper Tech Sports Med. 2013;21(2):100-7. doi: 10.1053/j.otsm.2013.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gobbi A, Scotti C, Karnatzikos G, Mudhigere A, Castro M, Peretti GM. One-step surgery with multipotent stem cells and hyaluronan-based scaffold for the treatment of full-thickness chondral defects of the knee in patients older than 45 years. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2494-501. doi: 10.1007/s00167-016-3984-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andriolo L, Reale D, Di Martino A, Boffa A, Zaffagnini S, Filardo G. Cell-free scaffolds in cartilage knee surgery: a systematic review and meta-analysis of clinical evidence. Cartilage. Epub 2019 June 5. doi: 10.1177/1947603519852406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kon E, Filardo G, Di Matteo B, Perdisa F, Marcacci M. Matrix assisted autologous chondrocyte transplantation for cartilage treatment: a systematic review. Bone Joint Res. 2013;2(2):18-25. doi: 10.1302/2046-3758.22.2000092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shimomura K, Moriguchi Y, Murawski CD, Yoshikawa H, Nakamura N. Osteochondral tissue engineering with biphasic scaffold: current strategies and techniques. Tissue Eng Part B Rev. 2014;20(5):468-76. doi: 10.1089/ten.TEB.2013.0543 [DOI] [PubMed] [Google Scholar]
- 13. Aldrian S, Zak L, Wondrasch B, Albrecht C, Stelzeneder B, Binder H, et al. Clinical and radiological long-term outcomes after matrix-induced autologous chondrocyte transplantation: a prospective follow-up at a minimum of 10 years. Am J Sports Med. 2014;42(11):2680-8. doi: 10.1177/0363546514548160 [DOI] [PubMed] [Google Scholar]
- 14. Filardo G, Kon E, Andriolo L, Di Matteo B, Balboni F, Marcacci M. Clinical profiling in cartilage regeneration: prognostic factors for midterm results of matrix-assisted autologous chondrocyte transplantation. Am J Sports Med. 2014;42(4):898-905. doi: 10.1177/0363546513518552 [DOI] [PubMed] [Google Scholar]
- 15. Gille J, Behrens P, Volpi P, de Girolamo L, Reiss E, Zoch W, et al. Outcome of autologous matrix induced chondrogenesis (AMIC) in cartilage knee surgery: data of the AMIC registry. Arch Orthop Trauma Surg. 2013;133(1_suppl):87-93. doi: 10.1007/s00402-012-1621-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kon E, Filardo G, Brittberg M, Busacca M, Condello V, Engebretsen L, et al. A multilayer biomaterial for osteochondral regeneration shows superiority vs microfractures for the treatment of osteochondral lesions in a multicentre randomized trial at 2 years. Knee Surg Sports Traumatol Arthrosc. 2018;26(9):2704-15. doi: 10.1007/s00167-017-4707-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kon E, Robinson D, Verdonk P, Drobnic M, Patrascu JM, Dulic O, et al. A novel aragonite-based scaffold for osteochondral regeneration: early experience on human implants and technical developments. Injury. 2016;47(Suppl 6):S27-S32. doi: 10.1016/S0020-1383(16)30836-1 [DOI] [PubMed] [Google Scholar]
- 18. Berruto M, Delcogliano M, de Caro F, Carimati G, Uboldi F, Ferrua P, et al. Treatment of large knee osteochondral lesions with a biomimetic scaffold: results of a multicenter study of 49 patients at 2-year follow-up. Am J Sports Med. 2014;42(7):1607-17. doi: 10.1177/0363546514530292 [DOI] [PubMed] [Google Scholar]
- 19. de Windt TS, Concaro S, Lindahl A, Saris DB, Brittberg M. Strategies for patient profiling in articular cartilage repair of the knee: a prospective cohort of patients treated by one experienced cartilage surgeon. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2225-32. doi: 10.1007/s00167-011-1855-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ebert JR, Smith A, Edwards PK, Hambly K, Wood DJ, Ackland TR. Factors predictive of outcome 5 years after matrix-induced autologous chondrocyte implantation in the tibiofemoral joint. Am J Sports Med. 2013;41(6):1245-4. doi: 10.1177/0363546513484696 [DOI] [PubMed] [Google Scholar]
- 21. Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR, Lazaro P, et al. The RAND/UCLA appropriateness method user’s manual. Santa Monica: RAND Corporation; 2001. [Google Scholar]
- 22. Nair R, Aggarwal R, Khanna D. Methods of formal consensus in classification/diagnostic criteria and guideline development. Semin Arthritis Rheum. 2011;41(2):95-105. doi: 10.1016/j.semarthrit.2010.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502. doi: 10.1136/ard.16.4.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85-A(Suppl 2):17-24. doi: 10.2106/00004623-200300002-00003 [DOI] [PubMed] [Google Scholar]
- 25. Ogura T, Merkely G, Bryant T, Winalski CS, Minas T. Autologous chondrocyte implantation “segmental-sandwich” technique for deep osteochondral defects in the knee: clinical outcomes and correlation with magnetic resonance imaging findings. Orthop J Sports Med. 2019;7(5):2325967119847173. doi: 10.1177/2325967119847173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Biant LC, McNicholas MJ, Sprowson AP, Spalding T. The surgical management of symptomatic articular cartilage defects of the knee: consensus statements from United Kingdom knee surgeons. Knee. 2015;22(5):446-9. doi: 10.1016/j.knee.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 27. Andriolo L, Reale D, Di Martino A, Zaffagnini S, Vannini F, Ferruzzi A, et al. High rate of failure after matrix-assisted autologous chondrocyte transplantation in osteoarthritic knees at 15 years of follow-up. Am J Sports Med. 2019;47(9):2116-22. doi: 10.1177/0363546519855029 [DOI] [PubMed] [Google Scholar]
- 28. Kon E, Filardo G, Gobbi A, Berruto M, Andriolo L, Ferrua P, et al. Long-term results after hyaluronan-based MACT for the treatment of cartilage lesions of the patellofemoral joint. Am J Sports Med. 2016;44(3):602-8. doi: 10.1177/0363546515620194 [DOI] [PubMed] [Google Scholar]
- 29. Filardo G, Kon E, Andriolo L, Di Martino A, Zaffagnini S, Marcacci M. Treatment of “patellofemoral” cartilage lesions with matrix-assisted autologous chondrocyte transplantation: a comparison of patellar and trochlear lesions. Am J Sports Med. 2014;42(3):626-34. doi: 10.1177/0363546513510884 [DOI] [PubMed] [Google Scholar]
- 30. Brix MO, Stelzeneder D, Chiari C, Koller U, Nehrer S, Dorotka R, et al. Treatment of full-thickness chondral defects with hyalograft C in the knee: long-term results. Am J Sports Med. 2014;42(6):1426-32. doi: 10.1177/0363546514526695 [DOI] [PubMed] [Google Scholar]
- 31. Nawaz SZ, Bentley G, Briggs TW, Carrington RW, Skinner JA, Gallagher KR, et al. Autologous chondrocyte implantation in the knee: mid-term to long-term results. J Bone Joint Surg Am. 2014;96(10):824-30. doi: 10.2106/JBJS.L.01695 [DOI] [PubMed] [Google Scholar]
- 32. Angele P, Niemeyer P, Steinwachs M, Filardo G, Gomoll AH, Kon E, et al. Chondral and osteochondral operative treatment in early osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1743-52. doi: 10.1007/s00167-016-4047-8 [DOI] [PubMed] [Google Scholar]
- 33. Deng Z, Jin J, Zhao J, Xu H. Cartilage defect treatments: with or without cells? Mesenchymal stem cells or chondrocytes? Traditional or matrix-assisted? A systematic review and meta-analyses. Stem Cells Int. 2016;2016:9201492. doi: 10.1155/2016/9201492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Condello V, Filardo G, Madonna V, Andriolo L, Screpis D, Bonomo M, et al. Use of a biomimetic scaffold for the treatment of osteochondral lesions in early osteoarthritis. Biomed Res Int. 2018;2018:7937089. doi: 10.1155/2018/7937089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Madry H, Kon E, Condello V, Peretti GM, Steinwachs M, Seil R, et al. Early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1753-62. doi: 10.1007/s00167-016-4068-3 [DOI] [PubMed] [Google Scholar]
- 36. Filardo G, Andriolo L, Sessa A, Vannini F, Ferruzzi A, Marcacci M, et al. Age is not a contraindication for cartilage surgery: a critical analysis of standardized outcomes at long-term follow-up. Am J Sports Med. 2017;45(8):1822-28. doi: 10.1177/0363546517695088 [DOI] [PubMed] [Google Scholar]
- 37. Fraser GM, Pilpel D, Kosecoff J, Brook RH. Effect of panel composition on appropriateness ratings. Int J Qual Health Care. 1994;6(3):251-5. doi: 10.1093/intqhc/6.3.251 [DOI] [PubMed] [Google Scholar]
- 38. Niemeyer P, Albrecht D, Andereya S, Angele P, Ateschrang A, Aurich M, et al. Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: a guideline by the working group “Clinical Tissue Regeneration” of the German Society of Orthopaedics and Trauma (DGOU). Knee. 2016;23(3):426-35. doi: 10.1016/j.knee.2016.02.001 [DOI] [PubMed] [Google Scholar]