Abstract
Niger seed agar was used as a primary plating medium for the isolation of Cryptococcus neoformans from cerebrospinal fluid specimens from AIDS patients with untreated primary cryptococcosis. The medium was used as the primary means to detect variations in the colony morphology of the yeast. To search for phenotypic and genetic variations, nine patients individually harboring two or three types of colony morphology were studied. Intraindividual isolates from nine patients had minor variations in the API 20C profile, and the MICs of one or more antifungal agents (amphotericin B, fluconazole, and itraconazole) for isolates from three patients were significantly different. Intraindividual isolates from three patients had minor karyotype differences, and one showed a dramatic chromosomal length polymorphism. In addition, three serial isolates from a patient with two episodes of infection showed similar karyotypes, confirming persistent infection by the same strain. Random amplified polymorphic DNA products were identical for all isolates (including three isolates from a relapse case). Our results provided evidence suggesting that (i) in humans, C. neoformans may undergo phenotypic and genetic changes during early infection prior to antifungal agent administration; (ii) dramatic variations in electrophoretic karyotypes and in phenotypes, as demonstrated during the early infection of one patient, may be due to infection by different strains; and (iii) the use of niger seed agar as a primary plating medium is useful for studying antifungal susceptibility, phenotypic switching, genetic diversity, and multiple-strain infections.
Cryptococcus neoformans has been the subject of intense study during the last decade because of its importance as a human pathogen. The high incidence of recurrent cryptococcosis in AIDS patients once antifungal drug therapy has ceased (12) has made the recent development and application of techniques to differentiate among individual isolates of particular relevance. It is widely believed that C. neoformans strains exhibit considerable genetic heterogeneity and that recurrent infections are due to the persistence of the original infecting strain (11, 13, 14). Genetic studies require the ability to initially differentiate colony morphologies. In general, colonies of different strains are difficult to distinguish on Sabouraud dextrose agar (SDA), which is used as a primary plating medium by most mycological laboratories. During previous experiments (16), it was observed that C. neoformans isolated from the nasopharynx of AIDS patients exhibited differences in colony morphology when grown on Niger seed agar (NSA) (15). The presence of Guizotia abyssinica in this medium induces the production of melanin by C. neoformans, resulting in distinctive brown colonies. NSA has therefore been used extensively for isolating C. neoformans from environments where many fungal contaminants are present but is not widely used in clinical laboratories.
The objective of this study was to demonstrate the in vivo phenotypic and genetic diversity of C. neoformans by using NSA plates as the primary means to detect variations in colony morphology.
MATERIALS AND METHODS
Isolation, purification, and identification.
NSA was used as a primary plating medium along with SDA for the isolation of C. neoformans from cerebrospinal fluid (CSF) in our clinical research trial with untreated primary cryptococcosis in AIDS patients at Bamrasnaradura Hospital, Nonthaburi, Thailand, between January and July 1998. The medium was used as the primary means to detect variations in the colony morphology of the yeast. To search for phenotypic and genetic variations of C. neoformans, initial CSF specimens were collected from nine AIDS patients with untreated primary cryptococcosis. These patients had no history of taking any antifungal drugs 1 month prior to specimen collection. Colony morphology on NSA was observed (color, texture, and size) during days 5 to 7 of incubation at 30°C. A distinct colony was isolated, and a single cell was cloned with a micromanipulator. In addition, three serial CSF specimens from an AIDS patient with a relapse of cryptococcosis were studied for genetic diversity. These isolates were grown on SDA plates at 30°C for 48 h and subsequently kept at 4°C. They were identified as described by Kwon-Chung and Bennett (8), and serotypes were tested with Crypto Check (Iatron Laboratory, Tokyo, Japan). All isolates were subcultured two more times on SDA before any further experiments.
Phenotypic studies.
The biochemical profiles of all isolates from nine patients were determined with API 20C AUX (Biomérieux, Marcy l'Etoile, France), and the MICs of amphotericin B, fluconazole, and itraconazole were determined using a modified Etest. To minimize the variations observed in MICs, pairs of isolates recovered from the same patient were inoculated along each side of the same strip (Fig. 1a), as in the Stokes method (3). The inoculum size of C. neoformans was equal to a McFarland standard no. 1 (as recommended in Etest technical guide 4), as measured with a Biomerieux Vitek colorimeter at 71 to 73% transmission. RPMI medium was used on a 15-cm diameter plastic plate, and the MIC was read at 48 h. Candida parapsilosis ATCC 22019 and C. krusei ATCC 6258 were used as controls. Differences in susceptibilities to antifungal agents were marked as relevant when the MICs of one or more of the antifungal agents for a pair of test isolates varied by two dilutions or more.
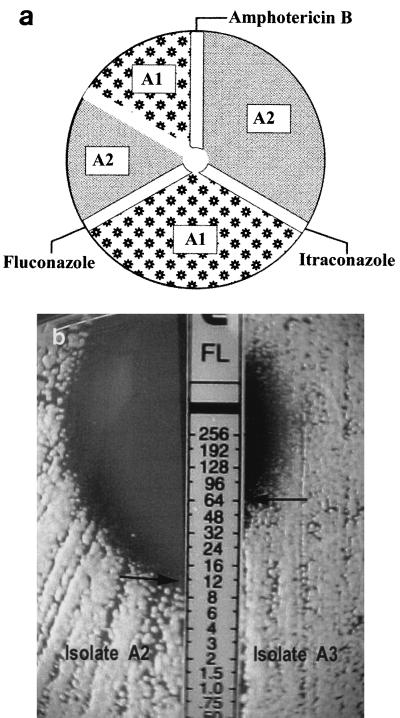
FIG. 1.
(a) Diagram showing inoculation procedure used in the modified Etest. Areas marked with A1 and A2 indicate inoculation areas for isolates A1 and A2, respectively, from patient A. (b) Results of the modified Etest, showing different MICs of fluconazole for isolates A2 and A3.
PCR fingerprinting.
Cryptococcal DNA was extracted from all single-cell isolates and prepared as described previously (18). Briefly, DNA was purified by phenol-chloroform extraction (9) and precipitated with 0.5 volume of 7.5 M ammonium acetate and 2 volumes of absolute ethanol. The resulting DNA was dissolved in 40 μl of 10 mM Tris–1 mM EDTA buffer.
PCR amplifications were performed by the random amplified polymorphic DNA (RAPD) technique with two arbitrary 16-mer oligonucleotide primers: (GACA)4 and M13 + 1, a modified wild-type phage M13 core sequence (GAGGGTGGCCGGTTCT) (10). Amplification reactions were performed with volumes of 50 μl containing PCR buffer, 100 ng of template DNA, 0.2 mM each deoxynucleoside triphosphate, 10 pmol of primer, 3 mM magnesium acetate, and 1.5 U of Taq DNA polymerase (Qiagen, Hilden, Germany). PCR was carried out with a Perkin-Elmer thermal cycler (model 2400) and consisted of 1 cycle of 93°C for 5 min; 35 cycles of 20 s at 93°C, 60 s at 50°C, and 20 s at 72°C; and a final extension for 6 min at 72°C. Amplification products were separated by electrophoresis on 2% agarose gels in 40 mM Tris–20 mM acetic acid–1 mM EDTA buffer for 90 min at 100 V. A 100-bp DNA ladder (New England BioLabs) was used as a molecular size marker. The DNA was stained with ethidium bromide, visualized with UV transillumination, and photographed.
Electrophoretic karyotyping (EK).
All isolates were analyzed by pulsed-field gel electrophoresis. Chromosomal DNA was prepared using a modification of existing protocols (1, 2, 11). The isolates were grown on SDA plates for 48 h at 25°C. Approximately 5 × 108 cells were washed twice in SCE buffer (100 mM sodium citrate [pH 5.8], 1 M sorbitol, 10 mM EDTA [pH 8.0]) and then resuspended in 1 ml of protoplasting solution (10 mg of SP299 [Novo Nordisk, Sydney, Australia], 1 ml of SCE buffer). The samples were incubated at 37°C for 1.5 h and pelleted by centrifugation. The supernatant was removed, and the protoplasts were carefully resuspended in an equal volume of molten 2% low-melting-temperature agarose (Progen, Brisbane, Australia) in 125 mM EDTA (pH 7.5) which had been kept at 37°C. The molten agarose containing the protoplasts was pipetted into prechilled reusable plug molds (Bio-Rad, Richmond, Calif.). Once solidified, the plugs were placed in overlay solution (0.45 M EDTA [pH 9.0], 1% sarcosyl, 1 mg of proteinase K/ml, 10 mM Tris-HCl [pH 8.0]) and incubated at 55°C for 24 h. The overlay solution was removed, and the plugs were stored at 4°C in a solution containing 0.5 M EDTA [pH 9.0], 1% sarcosyl, and 1 mg of proteinase K/ml until use.
Electrophoresis was performed with a contour-clamped homogeneous electric field DRIII apparatus (Bio-Rad) under the following running conditions: 34 h with a ramping switch time of 100 to 300 s at 120°C followed by a 450- to 650-s ramp for 40 h at 115°C. A current of 110 V was applied. The gels were made with pulsed-field-grade agarose (Bio-Rad) in 0.5× Tris-borate-EDTA and were electrophoresed at 4°C. Saccharomyces cerevisiae chromosomal DNA (New England BioLabs, Beverly, Mass.) was used as the molecular size standard. After electrophoresis, the gels were stained with ethidium bromide and photographed with UV transillumination. Karyotypes were compared by visual inspection for evidence of microevolution or strain replacement, as indicated by variations in the banding patterns. Isolates were classified as similar if their EK profiles varied by one or two bands and were judged different if their EK profiles differed by more than two bands (5, 7).
RESULTS
Phenotypic studies.
Two or three different colony morphologies were seen for a total of 22 isolates from nine patients. They were stable after undergoing serial subculturing over a period of 6 months. Intraindividual isolates from nine patients had minor variations in the API 20C profile (Table 1), whereas the MICs of one or more antifungal drugs for intraindividual isolates from three patients (patients A, C, and E) were significantly different (Table 1 and Fig. 1b). All discriminative colony morphologies were isolated and identified as being C. neoformans var. neoformans serotype A.
TABLE 1.
API 20C profiles and MIC data for 22 isolates from nine individuals
| Patient code | Colony code | API 20C profile | Etest MIC (μg/ml) of:
|
||
|---|---|---|---|---|---|
| Amphotericin B | Fluconazole | Itraconazole | |||
| A | A1 | 2757173 | 0.125 | >256 | 1 |
| A2 | 2757173 | 0.094 | 64 | 1 | |
| A3 | 2756373 | 0.094 | 12 | 0.094 | |
| B | B1 | 2756373 | 0.125 | 6 | 1 |
| B2 | 2756373 | 0.125 | 6 | 1 | |
| B3 | 2556733 | 0.125 | 6 | 0.75 | |
| C | C1 | 2557373 | 0.094 | 64 | 3 |
| C2 | 2557373 | 0.064 | 24 | 1 | |
| C3 | 2757373 | 0.047 | 48 | 0.125 | |
| D | D1 | 2757373 | 0.38 | >256 | 0.75 |
| D2 | 2557373 | 0.125 | >256 | 1 | |
| E | E1 | 2557373 | 0.125 | 96 | 3 |
| E2 | 2756173 | 0.094 | 8 | 0.047 | |
| E3 | 2756171 | 0.19 | 64 | 3 | |
| F | F1 | 2556153 | 0.19 | 64 | 1.5 |
| F2 | 2557173 | 0.38 | 128 | 1.5 | |
| G | G1 | 2557173 | 0.19 | >256 | 3 |
| G2 | 2757373 | 0.38 | >256 | 1.5 | |
| H | H1 | 2557373 | 0.094 | 64 | 1.5 |
| H2 | 2557173 | 0.094 | 64 | 1.5 | |
| J | J1 | 2757173 | 0.19 | >256 | 1.5 |
| J2 | 2557173 | 0.19 | >256 | 1.5 | |
EK.
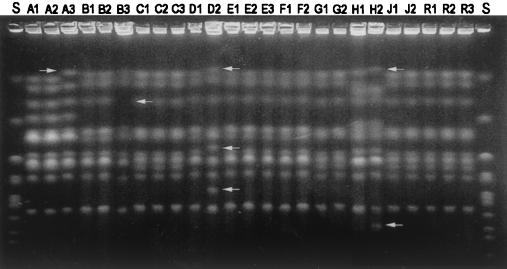
Analysis of the karyotypes of the 25 isolates revealed chromosomal length polymorphisms in intraindividual isolates from four out of nine patients (Fig. 2). Intraindividual isolates from patients A, B, and H were different in one or two band positions, but the two isolates from patient D (D1 and D2) had at least three different bands. The three serial isolates (R1, R2, and R3) from the only relapse case (patient R) were identical.
FIG. 2.
Pulsed-field gel showing the karyotypes of cryptococcal isolates found to have variant morphologies (A to J) or recovered from a cryptococcosis relapse case (R). Arrows indicate chromosomes that differed among isolates from individual patients. S, Saccharomyces cerevisiae chromosomal DNA.
PCR fingerprinting.
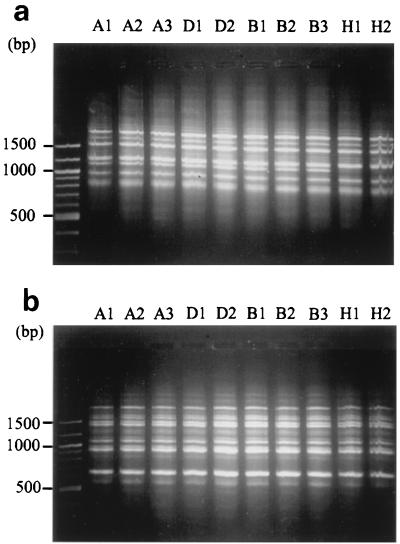
RAPD was performed on the 22 intraindividual isolates from nine patients, the 3 serial isolates from a patient with a relapse of cryptococcosis, and 20 control isolates of C. neoformans serotype A using the M13 + 1 and (GACA)4 primers. The amplification products were identical for all intraindividual isolates, isolates recovered from different patients, and control isolates (Fig. 3).
FIG. 3.
RAPD products. (a) RAPD with primer M13 + 1. (b) RAPD with primer (GAGA)4. Isolate designations are shown above the lanes.
DISCUSSION
Colonies of different isolates of C. neoformans are poorly differentiated on SDA, which is used as a primary plating medium worldwide. Generally, single colonies are chosen at random for molecular studies (6, 7, 17). However, previous studies identified the presence of different colony morphologies when clinical isolates were streaked out to single colonies, which were found to possess minor karyotype differences (4). In this study, NSA was used as a primary plating medium along with SDA. Single and multiple colony morphologies of C. neoformans were more distinguishable on NSA than on SDA (data not shown). Nine patients were found to harbor more than one colony type from each time point in their initial CSF specimens, and the colony morphologies were stable after undergoing serial subculturing for at least 6 months. These results indicate that this approach may be used as a primary tool for identifying the genetic diversity of C. neoformans.
Minor variations in the API 20C profile and multiple colony morphologies among intraindividual isolates from nine patients may be used as the means for further genetic studies as well as antifungal susceptibility studies. We used a modified Etest instead of the conventional tube dilution method, because itraconazole was insoluble in solvents at high concentrations, leading to inaccurate dilutions. The modified Etest was used to minimize influencing factors. Different isolates, based on colony morphology, from an individual patient were inoculated on the same plate along each side of the same strip. The significant MIC variations seen in this study (Table 1 and Fig. 1b) among intraindividual isolates from patients A, C, and E emphasize the care required when interpreting the results of standard antifungal susceptibility tests. Failure to identify a resistant colony from a primary plating medium could affect the outcome of treatment and the prognosis for the patient. It may be better to use a medium such as NSA as the primary plating medium along with SDA for the isolation of C. neoformans, so that distinct colonies can be observed prior to antifungal susceptibility testing. The different colonies may then be isolated and their antifungal drug resistance may be tested individually.
The phenotypic differences of the isolates and the stability of their morphology did not allow us to decide whether they were different strains or a single strain showing phenotypic switching. Previous studies have focused on the relationship between serial isolates of C. neoformans and successive episodes of meningitis in individual patients. Many investigators have indicated that a relapse of cryptococcal meningitis is due to the persistence of the original infecting strain (7, 11, 13, 14). Minor karyotype differences have been observed for serial isolates during antifungal therapy (4), suggesting that the original infecting strain had undergone microevolution in order to persist. However, RAPD (6) and DNA fingerprinting (17) data from previous studies also provided strong evidence that patients with recurrent cryptococcosis were infected with a different strain of C. neoformans during each episode of infection. Recently, multiple isolates of C. neoformans were identified from 6 out of 30 patients (7). In one patient, two isolates obtained from different body sites had different karyotypes. The investigators did not mention the relationship between the times of specimen collection and antifungal agent administration.
In this study, the natural occurrence of genetic diversity was observed in AIDS patients with cryptococcal meningitis (i.e., without the influence of antifungal agents). The minor karyotype differences among intraindividual isolates from patients A, B, and H suggested either that these isolates underwent microevolution in the patients during the period between the initial infection and the clinical manifestation of symptoms or that the patients were infected with very closely related isolates. The similarity among the karyotypes of the three serial isolates from the relapse case (patient R) would seem to confirm the persistence of the original infecting strain, as seen in previous studies (11, 13, 14). However, for patient D, the karyotype variations seen between isolates D1 and D2 would more strongly suggest the occurrence of infection by two different strains.
In this experiment, PCR fingerprinting with two primers [(GACA)4 and M13 + 1)] was unable to differentiate between either intraindividual or interindividual isolates and control isolates. This evidence may support the previous report that the only RAPD type with minor variations was found among Thai isolates of C. neoformans (10). This method has proven to be sensitive, but its lack of discriminative powers at the subspecies level brings into question its usefulness in epidemiological studies, particularly for C. neoformans. For molecular studies, EK has been shown to be a sensitive tool for distinguishing among C. neoformans isolates (4, 11).
The reason for the genetic diversity observed in this study is unclear, but it is possible that it allows the fungal population to change and adapt in order to escape eradication by the immune system. C. neoformans is capable of microevolution in vivo, and variants exhibiting new genotypic and phenotypic characteristics may emerge in order to allow the organism to persist. In summary, our results provided evidence suggesting that (i) in humans, C. neoformans may undergo phenotypic and genotypic changes during early infection prior to antifungal agent administration; (ii) extensive variations in electrophoretic karyotypes and phenotypes of isolates obtained from early infection may be due to infection by different strains; and (iii) the use of NSA as a primary plating medium would be advantageous for studying antifungal susceptibility, phenotypic switching, genetic diversity, and multiple-strain infections.
ACKNOWLEDGMENTS
This study was supported by Thailand-Tropical Diseases Research Program (T-2); Bamrasnaradura Hospital; and Faculty of Medical Technology, Mahidol University.
We thank Novo Nordisk for the donation of SP299, a yeast-lysing enzyme.
REFERENCES
- 1.Barchiesi F, Hollis R J, Messer S A, Scalise G, Rinaldi M G, Pfaller M A. Electrophoretic karyotype and in vitro antifungal susceptibility of Cryptococcus neoformans isolates from AIDS patients. Diagn Microbiol Infect Dis. 1995;23:99–103. doi: 10.1016/0732-8893(95)00169-7. [DOI] [PubMed] [Google Scholar]
- 2.Boekhout T, van Belkum A, Leenders A C, Verbrugh H A, Mukamurangwa P, Swinne D, Scheffers W A. Int. J. Syst. Bacteriol. 47:432–442. 1997. Molecular typing of Cryptococcus neoformans: taxonomic and epidemiological aspects. [DOI] [PubMed] [Google Scholar]
- 3.Brown D, Blowers R. Disc methods of sensitivity testing and other semiquantitative methods. In: Reeves D S, Phillips I, Williums J D, Wise R, editors. Laboratory methods in antimicrobial chemotherapy—1978. Edinburgh, Scotland: Churchill Livingstone, Ltd.; 1978. p. 8. [Google Scholar]
- 4.Fries B C, Casadevall A. Serial isolates of Cryptococcus neoformans from patients with AIDS differ in virulence for mice. J Infect Dis. 1998;178:1761–1766. doi: 10.1086/314521. [DOI] [PubMed] [Google Scholar]
- 5.Fries B C, Chen F, Currie B P, Casadevall A. Karyotype instability in Cryptococcus neoformans infection. J Clin Microbiol. 1996;34:1531–1534. doi: 10.1128/jcm.34.6.1531-1534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haynes K A, Sullivan D J, Coleman D C, Clarke J C K, Emilianus R, Atkinson C, Cann K J. Involvement of multiple Cryptococcus neoformans strains in a single episode of cryptococcosis and reinfection with novel strains in recurrent infection demonstrated by random amplification of polymorphic DNA and DNA fingerprinting. J Clin Microbiol. 1995;33:99–102. doi: 10.1128/jcm.33.1.99-102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klepsper E M, Pfaller M A. Variation in electrophoretic karyotype and antifungal susceptibility of clinical isolates of Cryptococcus neoformans at a university-affiliated teaching hospital from 1987 to 1994. J Clin Microbiol. 1998;36:3653–3656. doi: 10.1128/jcm.36.12.3653-3656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon-Chung K J, Bennett J E. Medical mycology. Philadelphia, Pa: Lea & Febiger; 1992. pp. 397–446. [Google Scholar]
- 9.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 10.Meyer W, Marszewska K, Amirmostofian M, Igreja R P, Hardtke C, Methling K, Viviani M A, Chindamporn A, Sukroongreung S, John M A, Ellis D H, Sorrell T C. Molecular typing of global isolates of Cryptococcus neoformans var. neoformans by polymerase chain reaction fingerprinting and randomly amplified polymorphic DNA—a pilot study to standardize techniques on which to base a detailed epidemiological survey. Electrophoresis. 1999;20:1790–1799. doi: 10.1002/(SICI)1522-2683(19990101)20:8<1790::AID-ELPS1790>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Perfect J R, Ketabchi N, Cox G M, Ingram C W, Beiser C. Karyotyping of Cryptococcus neoformans as an epidemiological tool. J Clin Microbiol. 1993;31:3305–3309. doi: 10.1128/jcm.31.12.3305-3309.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powderly W G. Therapy for cryptococcal meningitis in patients with AIDS. Clin Infect Dis. 1992;14:554–559. doi: 10.1093/clinids/14.supplement_1.s54. [DOI] [PubMed] [Google Scholar]
- 13.Spitzer E D, Spitzer S G. Use of a dispersed repetitive DNA element to distinguish clinical isolates of Cryptococcus neoformans. J Clin Microbiol. 1992;30:1094–1097. doi: 10.1128/jcm.30.5.1094-1097.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spitzer E D, Spitzer S G, Freundlich L F, Casadevall A. Persistence of initial infection in recurrent Cryptococcus neoformans meningitis. Lancet. 1993;341:595–596. doi: 10.1016/0140-6736(93)90354-j. [DOI] [PubMed] [Google Scholar]
- 15.Staib F. Cryptococcus neoformans und Guizotia abyssinica (syn. G. oleifera D.C.) Z Hyg. 1962;148:466–475. [Google Scholar]
- 16.Sukroongreung S, Eampokalap B, Tansuphaswadikul S, Nilakul C, Tantimavanich S. Recovery of Cryptococcus neoformans from nasopharynx of AIDS patients. Mycopathologia. 1999;141:131–134. doi: 10.1023/a:1006909532185. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan D, Haynes K, Moran G, Shanley D, Coleman D. Persistence, replacement, and microevolution of Cryptococcus neoformans strains in recurrent meningitis in AIDS patients. J Clin Microbiol. 1996;34:1739–1744. doi: 10.1128/jcm.34.7.1739-1744.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Burik J A H, Schreckhise R W, White T C, Bowden R A, Myerson D. Comparison of six extraction techniques for isolation of DNA from filamentous fungi. Med Mycol. 1998;36:299–303. [PubMed] [Google Scholar]