Abstract
Objective
Assess how treatment with the viscosupplement hylan G-F 20 relates to opioid prescriptions and intraarticular corticosteroid injections (IACS) in patients with osteoarthritis of the knee (OAK).
Design
Case-crossover; adult patients with OAK identified in a claims database were treated with hylan G-F 20 from July 1, 2007, to June 29, 2017. Opioid or IACS prescriptions in the 6 months before treatment were compared to the 6 months after. Patients with comorbid conditions requiring pain medications were excluded, resulting in a 29,395-patient cohort. Four subgroups were investigated: patients with (1) opioids before hylan G-F 20 (OB; n = 6,609); (2) opioids before and after hylan G-F 20 (OBF; n = 3,320); (3) IACS before hylan G-F 20 (CB; n = 11,162); and (4) IACS before and after hylan G-F 20 (CBF; n = 2,810). All opioids were converted to morphine milligram equivalents (MME).
Results
OB subgroup patients had a significant decrease (P < 0.01) in total MME (−14.0%), MME per day (−14.2%) and opioid prescription days (−12.6%) after treatment versus before. Only 50.2% of patients prescribed opioids before hylan G-F 20 were prescribed an opioid after treatment. OBF subgroup patients had a significant increase (P < 0.01) in opioid prescription days (7.8%) before versus after treatment. There was a significant decrease (P < 0.01) in the number of IACS after versus before treatment for the Total Cohort (−56.1%), and subgroups CB (−72.6%) and CBF (−4.1%). A total of 74.8% of patients receiving an IACS before treatment did not receive an IACS after treatment.
Conclusions
Hylan G-F 20 is associated with a reduction in opioid prescriptions and IACS in OAK patients.
Keywords: osteoarthritis of the knee, opioids, corticosteroids, hyaluronic acid, viscosupplement
Introduction
The global leading cause of disability from 1990 to 2010 was osteoarthritis (OA), contributing 17.1 million patient years with disability over this time. Osteoarthritis of the knee (OAK) accounted for 83% of this OA burden. 1 It is estimated that in the United States over 10% of men and 13% of women aged 60 years or older have symptomatic OAK. 2 Hylan G-F 20 is a hyaluronic acid (HA) viscosupplement used to reduce pain in patients with mild to moderate OAK. The aim of intraarticular HA is to increase the viscoelastic properties of synovial fluid, to maintain intraarticular lubrication, thus reducing joint pain and structural damage,3-6 although recent evidence suggests that anti-inflammatory7-9 and chondroprotective7,10 effects may also play a role. Indeed, results from a number of prospective multicenter, randomized, double-blind, controlled clinical trials involving a total of more than 500 patients have demonstrated that Hylan G-F 20 is more efficacious than placebo in relieving knee OA pain.11-14 Other pharmacologic therapeutic options for treating pain associated with OAK include acetaminophen, nonsteroidal anti-inflammatory medications (NSAIDs), intraarticular corticosteroid injections (IACS), and/or opioid prescriptions. These alternative treatment options, however, are not suitable for long-term use. Opioids are known to have addictive properties 15 with up to 1 in 4 patients with long-term opioid use for noncancer pain struggling with addiction. 16 There are also adverse events associated with opioids which include nausea, vomiting, constipation, urinary retention, and respiratory depression, which may result in death. 17 Long-term use of IACS has side effects that include nerve damage, thinning of nearby bone, 8 and gross cartilage damage. 18 Chronic acetaminophen use has been associated with a risk of gastrointestinal bleeding, hypertension, and hepatotoxicity in certain patient populations. 19 Long-term use of NSAIDs is associated with cardiovascular20-22 and gastrointestinal risk. 23 Adverse events associated with HA include local injection site reactions such as pain, swelling, and arthralgia. 24
Although there are several different nonoperative treatment options for OAK, 2 recent meta-analyses concluded that HA (along with platelet-rich plasma) is one of the most efficacious treatments for OAK-related pain,25,26 and a third found that patients treated with hylan G-F 20 had improved Western Ontario and McMaster Universities Osteoarthritis Index physical function compared to patients with appropriate care or corticosteroids 27 ; however, another meta-analysis revealed large uncertainty around the effect sizes for change in pain with various OAK treatments, including HA, and concluded that larger prospective randomized controlled trials were needed. 28 A recent study from a large claims database investigated the number of NSAID prescriptions, corticosteroid injections, and opioid prescriptions in OAK patients treated with a nonavian high-molecular-weight (MW) hyaluronan. 29 This study found a significant decrease in the utilization of NSAIDs, corticosteroid injections, and opioids, 29 but did not include patients over 65 years of age or with Medicare. 29 The current study utilizes a national claims database to examine the relationship between treatment with hylan G-F 20 and opioid prescriptions/days on opioids, and the number of IACS received among patients diagnosed with OAK. This study includes patients >65 years of age and with Medicare Supplement, and compared prescribed opioid amounts and IACS received in the 6 months before versus the 6 months after initiating hylan G-F 20 treatment.
Methods
Data Source
This retrospective study utilized the Health Insurance Portability and Accountability Act (HIPAA)-compliant US Truven Marketscan claims database, which contains 170 million de-identified patients and is eligible for exemption from institutional review board approval. 30 This database captures the full continuum of care in all health care settings and provides the amount of opioids prescribed and the number of IACS administered, key metrics for this study.
Exposure and Time Frame
Exposure to hylan G-F 20 was determined through Healthcare Common Procedure Coding System (HCPCS) J-codes. Opioid prescriptions were identified through National Drug Codes (NDC; see Supplemental Table 1 for list of opioid generic names) for opioid medications as per recommended milligram morphine equivalent (MME) by the Centers for Medicare and Medicaid Services (CMS) 31 or the Centers for Disease Control and Prevention (CDC). 32 IACS administration was identified by HCPCS J-codes (see Supplemental Table 2). The index date was defined as the date of initial hylan G-F 20 treatment, as either a single injection or 1 to 3 injections over the course of 21 days. The baseline period was defined as the 6 months prior to the index date. The start of the 6-month follow-up period was defined either as the date of the third injection or the last injection (if <3 injections) within a 21-day period after the initial injection.
Study Population
Adult patients with an OAK diagnosis who received hylan G-F 20 treatment between July 1, 2007, and June 29, 2017, were included in the study. Patients were included if they had at least one medical claim with an OAK diagnosis (based on International Classification of Diseases [ICD]-9 and ICD-10 codes; see Supplemental Table 3) prior to hylan G-F 20 treatment, at least one claim for hylan G-F 20 using injectable HCPCS J-codes (see Supplemental Table 4), and had 6 months of continuous enrollment in the baseline period. Patients were excluded from this study if they were diagnosed with fibromyalgia, rheumatoid arthritis, spondyloarthropathy, avascular necrosis, other arthritis, or collagen disease (determined via presence of relevant medical codes within the record; see Supplemental Table 5) to reduce the likelihood that pain medications were prescribed related to these conditions and not OAK. Patients were excluded if they received a non-hylan G-F 20 viscosupplement, underwent a major surgical procedure 33 within the baseline or follow-up periods, were prescribed an antidepressant, or did not have 6 months of data in the follow-up period.
Hylan G-F 20 was chosen due to its high molecular weight (MW, 6,000 kDa), 34 which is similar to the MW of hyaluronate in synovial fluid (7,000 kDa). 35 HA products with an average MW ≥3,000 kDa also have improved efficacy over HA products with an average MW <3,000 kDa, while having better safety than HA products with an average MW ≤1,500 kDa. 36
After applying selection criteria, several patient subgroups were created who received prescriptions or were administered the medications of interest for further analyses: (1) patients prescribed opioid(s) in the baseline period, regardless of opioid prescriptions in the follow-up period (OB); (2) patients prescribed opioid(s) in both baseline and follow-up periods (OBF); (3) patients who received IACS in the baseline period, regardless of IACS receipt in the follow-up period (CB); (4) patients who received IACS in both baseline and follow-up periods (CBF). All patients in the OBF subgroup were included in the OB subgroup, and all patients in the CBF subgroup were included in the CB subgroup. Analyses of the total cohort were conducted to present the overall opioid prescriptions and IACS usage for patients, after versus before treatment with hylan G-F 20. Patients in the total cohort may have received opioids, IACS, other medications, any combination of treatments, or no medication. The OB and CB subgroups were investigated to determine how many patients who received ≥1 opioid prescription or IACS before hylan G-F 20, still needed an opioid prescription (and average MME) or IACS after hylan G-F 20 treatment. The OBF and CBF subgroups were investigated to determine if there was a change in opioids or IACS for those who did require opioid prescription(s) or IACS at baseline and after hylan G-F 20 treatment.
Outcomes
Opioid prescription amounts were converted to MME,31,32 which allowed for comparison of different prescribed opioid medication types in the baseline and follow-up periods. This study incorporated a 400 MME per day threshold to guard against data errors, above which values were truncated to this value (affected <0.5% of patients). This threshold is double the CMS recommended hard threshold of 200 MME per day 37 that is corroborated by the CDC 38 since some physicians may prescribe higher opioid amounts based on individual patient history. Primary outcomes were total average MME (average of total opioid amounts prescribed for the same patient within each study time period, pre- and post-hylan G-F 20 treatment); MME per day (total MME divided by 180 days [6 months]) covered in each the baseline or follow-up periods; and number of IACS (count of IACS) received in the baseline or follow-up periods. The secondary outcome was the total number of days covered by opioid prescriptions.
Statistical Analyses
The analysis compared opioid prescriptions and IACS over the baseline versus the follow-up period after hylan G-F 20 treatment. For both the primary and secondary outcomes, a paired t test with a P value <0.05 between the baseline and follow-up periods was utilized to determine significance of results. For all outcomes (total MME, MME per day, number of IACS, and number of opioid prescription days), the mean and standard error of the mean were reported for baseline and follow-up periods. Percent change of the mean for the primary and secondary outcomes, between the baseline and follow-up periods, was calculated as [mean(Follow-up) − mean(Baseline)]/mean(Baseline) × 100.
Results
Patient Selection
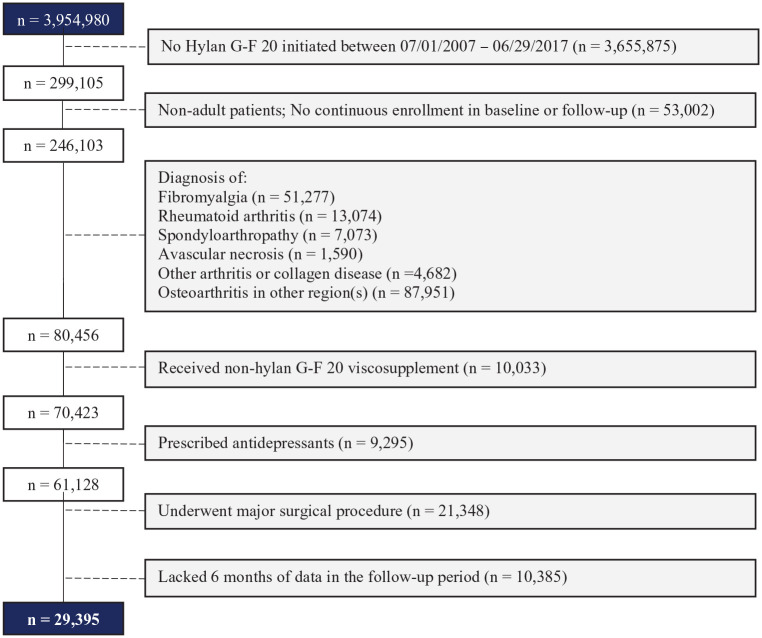
There were 3,954,980 patients diagnosed with OAK in the database. After applying all inclusion and exclusion criteria, 29,395 patients were included in the study cohort ( Fig. 1 ).
Figure 1.
Patient attrition table.
The total cohort included patients who received hylan G-F 20. A total of 19,113 patients (65%) received a course of a single hylan G-F 20 injection, while the remaining 10,282 patients (35%) received a course of 3 weekly hylan G-F 20 injections. These patients may have been treated with opioids, IACS, other medications, any combination of treatments, or no medication. From the study cohort, 4 subgroups were analyzed: (1) OB—patients prescribed opioid(s) in the baseline period, n = 6,609; (2) OBF—patients prescribed opioid(s) in the baseline period and prescribed opioid(s) in the follow-up period, n = 3,320; (3) CB—patients who received at least one IACS in the baseline period, n = 11,162; (4) CBF—patients who received at least one IACS in the baseline period and at least one IACS in the follow-up period, n = 2,810.
Patient Characteristics
Patients with female gender comprised 52.9% of the study cohort and the average (standard deviation [SD]) age was 57.7 (11.9) years. Approximately 36% of the patients in the current study were <55 years of age. This is similar to the median age of 55 years that was reported by Losina et al. from their US study of OAK patients utilizing data from the National Health Interview Survey. 39 Subgroup population characteristics appear similar, with the exception that the OBF subgroup had a slightly earlier most prevalent index year (2010). See Table 1 for detailed patient characteristics for the study cohort and subgroup.
Table 1.
Patient Characteristics by Cohort.
| Variable | Total Cohort (n
= 29,395) |
OB (n =
6,609) |
OBF (n =
3,320) |
CB (n =
11,162) |
CBF (n
=2,810) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients (n) | Patients (%) | Patients (n) | Patients (%) | Patients (n) | Patients (%) | Patients (n) | Patients (%) | Patients (n) | Patients (%) | |
| Gender | ||||||||||
| Female | 15,563 | 52.94% | 3,710 | 56.14% | 1,831 | 55.15% | 6,163 | 55.21% | 1,646 | 58.58% |
| Male | 13,832 | 47.06% | 2,899 | 43.86% | 1,489 | 44.85% | 4,999 | 44.79% | 1,164 | 41.42% |
| Age group | ||||||||||
| 18-34 | 801 | 2.72% | 172 | 2.60% | 89 | 2.68% | 221 | 1.98% | 39 | 1.39% |
| 35-44 | 2,578 | 8.77% | 619 | 9.37% | 316 | 9.52% | 892 | 7.99% | 204 | 7.26% |
| 45-54 | 7,692 | 26.17% | 1,874 | 28.36% | 968 | 29.16% | 2,968 | 26.59% | 723 | 25.73% |
| 55-64 | 12,073 | 41.07% | 2,685 | 40.63% | 1,330 | 40.06% | 4,703 | 42.13% | 1,232 | 43.84% |
| 65-74 | 3,625 | 12.33% | 715 | 10.82% | 334 | 10.06% | 1,364 | 12.22% | 342 | 12.17% |
| 75-84 | 1,933 | 6.58% | 394 | 5.96% | 201 | 6.05% | 722 | 6.47% | 184 | 6.55% |
| 85+ | 693 | 2.36% | 150 | 2.27% | 82 | 2.47% | 292 | 2.62% | 86 | 3.06% |
| Health plan type | ||||||||||
| Consumer-directed Health Plan (CDHP) | 1,068 | 3.63% | 231 | 3.50% | 102 | 3.07% | 419 | 3.75% | 101 | 3.59% |
| Comprehensive (COMP) | 2,854 | 9.71% | 583 | 8.82% | 278 | 8.37% | 1,117 | 10.01% | 298 | 10.60% |
| Exclusive Provider Organization (EPO) | 262 | 0.89% | 59 | 0.89% | 33 | 0.99% | 84 | 0.75% | 17 | 0.60% |
| High Deductible Health Plan (HDHP) | 636 | 2.16% | 118 | 1.79% | 68 | 2.05% | 234 | 2.10% | 65 | 2.31% |
| Health Maintenance Organization (HMO) | 3,368 | 11.46% | 863 | 13.06% | 438 | 13.19% | 1,210 | 10.84% | 279 | 9.93% |
| Point of Service (POS) | 2,182 | 7.42% | 540 | 8.17% | 266 | 8.01% | 811 | 7.27% | 208 | 7.40% |
| Preferred Provider Organization (PPO) | 17,255 | 58.70% | 3,855 | 58.33% | 1,953 | 58.83% | 6,673 | 59.78% | 1,692 | 60.21% |
| Other | 1,770 | 6.02% | 360 | 5.45% | 182 | 5.48% | 614 | 5.50% | 150 | 5.34% |
| Geographic region | ||||||||||
| North central | 7,949 | 27.04% | 1,712 | 25.90% | 828 | 24.94% | 2,954 | 26.46% | 670 | 23.84% |
| Northeast | 6,065 | 20.63% | 1,074 | 16.25% | 484 | 14.58% | 2,070 | 18.55% | 515 | 18.33% |
| South | 10,843 | 36.89% | 2,850 | 43.12% | 1,507 | 45.39% | 4,587 | 41.09% | 1,267 | 45.09% |
| West | 4,175 | 14.20% | 867 | 13.12% | 449 | 13.52% | 1,423 | 12.75% | 328 | 11.67% |
| Unknown | 363 | 1.23% | 106 | 1.60% | 52 | 1.57% | 128 | 1.15% | 30 | 1.07% |
| Index admission year | ||||||||||
| 2007 | 1,587 | 5.40% | 419 | 6.34% | 230 | 6.93% | 512 | 4.59% | 135 | 4.80% |
| 2008 | 3,080 | 10.48% | 756 | 11.44% | 423 | 12.74% | 1,015 | 9.09% | 249 | 8.86% |
| 2009 | 2,660 | 9.05% | 646 | 9.77% | 348 | 10.48% | 939 | 8.41% | 214 | 7.62% |
| 2010 | 4,424 | 15.05% | 1,002 | 15.16% | 530 | 15.96% | 1,544 | 13.83% | 381 | 13.56% |
| 2011 | 4,765 | 16.21% | 1,059 | 16.02% | 512 | 15.42% | 1,775 | 15.90% | 420 | 14.95% |
| 2012 | 4,255 | 14.48% | 889 | 13.45% | 438 | 13.19% | 1,679 | 15.04% | 417 | 14.84% |
| 2013 | 3,236 | 11.01% | 701 | 10.61% | 328 | 9.88% | 1,254 | 11.23% | 305 | 10.85% |
| 2014 | 2,564 | 8.72% | 523 | 7.91% | 231 | 6.96% | 1,089 | 9.76% | 276 | 9.82% |
| 2015 | 2,046 | 6.96% | 448 | 6.78% | 204 | 6.14% | 967 | 8.66% | 290 | 10.32% |
| 2016 | 778 | 2.65% | 166 | 2.51% | 76 | 2.29% | 388 | 3.48% | 123 | 4.38% |
| 2017 a | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% |
| Charlson comorbidities | ||||||||||
| AIDS/HIV | 23 | 0.08% | 7 | 0.12% | 4 | 0.12% | 6 | 0.05% | 1 | 0.04% |
| Cancer | 1,042 | 3.54% | 226 | 3.72% | 109 | 3.28% | 434 | 3.89% | 110 | 3.91% |
| Cerebrovascular disease | 515 | 1.75% | 140 | 2.31% | 70 | 2.11% | 199 | 1.78% | 46 | 1.64% |
| Chronic pulmonary disease | 1,326 | 4.51% | 394 | 6.49% | 241 | 7.26% | 560 | 5.02% | 162 | 5.77% |
| Congestive heart failure | 306 | 1.04% | 93 | 1.53% | 58 | 1.75% | 105 | 0.94% | 31 | 1.10% |
| Connective tissue disease—Rheumatic disease | 22 | 0.07% | 7 | 0.12% | 6 | 0.18% | 8 | 0.07% | 3 | 0.11% |
| Dementia | 59 | 0.20% | 11 | 0.18% | 9 | 0.27% | 26 | 0.23% | 5 | 0.18% |
| Diabetes with complications | 746 | 2.54% | 213 | 3.51% | 113 | 3.40% | 298 | 2.67% | 84 | 2.99% |
| Diabetes without complications | 3,861 | 13.13% | 1,104 | 18.19% | 598 | 18.01% | 1,483 | 13.29% | 397 | 14.13% |
| Metastatic carcinoma | 34 | 0.12% | 11 | 0.18% | 7 | 0.21% | 14 | 0.13% | 5 | 0.18% |
| Mild liver disease | 62 | 0.21% | 18 | 0.30% | 8 | 0.24% | 24 | 0.22% | 7 | 0.25% |
| Moderate or severe liver disease | 26 | 0.09% | 6 | 0.10% | 3 | 0.09% | 14 | 0.13% | 4 | 0.14% |
| Myocardial infarction | 116 | 0.39% | 33 | 0.54% | 18 | 0.54% | 51 | 0.46% | 13 | 0.46% |
| Paraplegia and hemiplegia | 19 | 0.06% | 7 | 0.12% | 4 | 0.12% | 9 | 0.08% | 2 | 0.07% |
| Peptic ulcer disease | 58 | 0.20% | 22 | 0.36% | 14 | 0.42% | 20 | 0.18% | 7 | 0.25% |
| Peripheral vascular disease | 311 | 1.06% | 99 | 1.63% | 55 | 1.66% | 121 | 1.08% | 36 | 1.28% |
| Renal disease | 490 | 1.67% | 144 | 2.37% | 70 | 2.11% | 207 | 1.85% | 64 | 2.28% |
OB = opioid prescriptions prior to hylan G-F 20 treatment; OBF = opioid prescriptions both before and after hylan G-F 20 treatment; CB = at least one IACS before hylan G-F 20 treatment; CBF = at least one IACS both before and after hylan G-F 20 treatment.
No patients were captured in 2017 due to the required 6 months of follow-up and patient records through June 2017 were considered.
Opioid Prescriptions
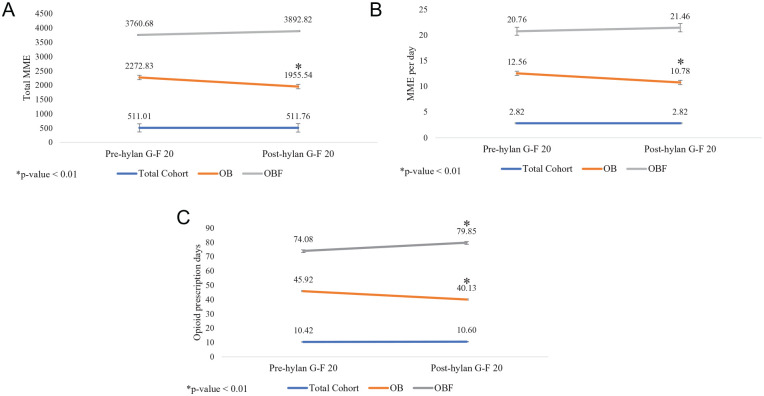
There were no significant differences observed for the total cohort after versus before hylan G-F 20 treatment with regard to prescribed opioids (total MME, MME per day) or opioid prescription days ( Table 2 ; Fig. 2A-C ); however, only 50.2% of patients who were prescribed opioids prior to hylan G-F 20 treatment (OB subgroup), received an opioid prescription post-hylan G-F 20 treatment ( Table 2 ). It is also important to note that 8.1% of patients who did not receive opioids prior to hylan G-F 20 injection received opioid prescription(s) afterwards (data not shown). Among the OB subgroup, there was a significant difference in opioids prescribed before hylan G-F 20 treatment versus after hylan G-F 20 treatment (mean value baseline; mean value follow-up; percentage change; P < 0.01): total MME (2272.83; 1955.54; −13.96%; P < 0.01), MME per day (12.56; 10.78; −14.18%; P < 0.01), and opioid prescription days (45.92; 40.13; −12.61%; P < 0.01; Table 2 ; Fig. 2A-C ). Among patients prescribed opioids both before and after hylan G-F 20 treatment (OBF subgroup), there was no significant difference in the amounts of opioids prescribed before versus after hylan G-F 20 treatment (total MME or MME per day); however, there was a significant increase in the number of opioid prescription days (mean 74.08, baseline; mean 79.85, follow-up; percent change, 7.79%; P < 0.01; Table 2 ; Fig. 2A-C ).
Table 2.
Opioid Prescription Outcomes Before versus After Hylan G-F 20 Treatment a .
| Patient Population: Cohort Description | Patients, n | Primary Outcomes | Secondary Outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Average Total MME per
Patient |
Average MME per Day |
Average Number of Opioid
Prescription Days |
||||||||
| Baseline | Follow-up | Percent Change | Baseline | Follow-up | Percent Change | Baseline | Follow-up | Percent Change | ||
| Total cohort b : Overall medication use | 29,395 | 511.01 | 511.76 | 0.15% | 2.82 | 2.82 | 0.01% | 10.42 | 10.60 | 1.74% |
| Subgroups analyzed | ||||||||||
| OB c : Opioids prescribed in baseline | 6,609 | 2272.83 | 1955.54 | −13.96%* | 12.56 | 10.78 | −14.18%* | 45.92 | 40.13 | −12.61%* |
| OBF d : Opioids prescribed in both baseline and follow-up | 3,320 | 3760.68 | 3892.82 | 3.51% | 20.76 | 21.46 | 3.37% | 74.08 | 79.83 | 7.79%* |
MME = morphine milligram equivalents; Patient subgroups analyzed: OB = patients with opioid prescription(s) before hylan G-F 20 treatment; OBF = patients with opioid prescription(s) both before and after hylan G-F 20 treatment.
Of the 6,609 patients prescribed an opioid before hylan G-F 20 treatment, 3,320 patients were prescribed an opioid after hylan G-F 20 treatment (50.2%).
Total cohort included all patients that received hylan G-F 20, regardless of medications received. Patients may have been treated with opioids, IACS, other medications, any combination of treatments, or no medication.
OB subgroup is a subset of patients from the total cohort.
OBF subgroup is a subset of patients from the OB subgroup.
P value <0.05.
Figure 2.
(A) Total MME for opioids prescribed pre-hylan G-F 20 versus post-hylan G-F 20 treatment. (B) Opioids prescribed, MME per day, pre-hylan G-F 20 versus post-hylan G-F 20 treatment. (C) Opioid prescription days pre-hylan G-F 20 versus post-hylan G-F 20 treatment. MME = morphine milligram equivalents; Patient subgroups: OB, patients with opioid prescription(s) before hylan G-F 20 treatment; OBF, patients with opioid prescription(s) both before and after hylan G-F 20 treatment.
Intraarticular Corticosteroid Injections
There was a significant difference in the number of IA corticosteroid injections received before versus after hylan G-F 20 treatment (mean value baseline; mean value follow-up; percent change; P value): total cohort (0.50; 0.22; −56.11%; P < 0.01); among patients who received at least one IACS prior to hylan G-F 20 treatment (CB subgroup; 1.33; 0.36; −72.62%; P < 0.01); and among patients who received at least one IACS both before and after hylan G-F 20 treatment (CBF subgroup; 1.50; 1.44; −4.10%; P < 0.01; Table 3 ; Fig. 3 ). Only 2% of patients who did not receive IACS prior to hylan G-F 20 received at least one injection afterwards; 74.8% of patients who received an IACS prior to hylan G-F 20 treatment did not receive an IACS after hylan G-F 20 treatment ( Table 3 ).
Table 3.
Change in the Number of Intraarticular Corticosteroid Injections Before versus After Hylan G-F 20 Treatment a .
| Patient Population: Cohort Description | Patients, n | Primary Outcome | ||
|---|---|---|---|---|
| Average Number of IACS Injections per Patient | ||||
| Baseline | Follow-up | Percent Change | ||
| Total cohort b : Overall medication use | 29,395 | 0.50 | 0.22 | −56.11%* |
| Subgroups analyzed | ||||
| CB c : IACS in the baseline | 11,162 | 1.33 | 0.36 | −72.62%* |
| CBF d : IACS in both baseline and follow-up | 2,810 | 1.50 | 1.44 | −4.10%* |
IACS = intraarticular corticosteroids; Patient subgroups analyzed: CB = patients at least one intraarticular corticosteroid injection before hylan G-F 20 treatment; CBF = patients with at least one intraarticular corticosteroid injection both before and after hylan G-F 20 treatment.
Of the 11,162 patients who received an IACS before hylan G-F 20, 2,810 received an IACS after hylan G-F 20 (25.2%).
Total cohort included all patients who received hylan G-F 20, regardless of medications. These patients may have been treated with opioids, IACS, other medications, any combination of treatments, or no medication.
CB subgroup is a subset of patients from the total cohort.
CBF subgroup is a subset of patients from the CB subgroup.
P value <0.05.
Figure 3.
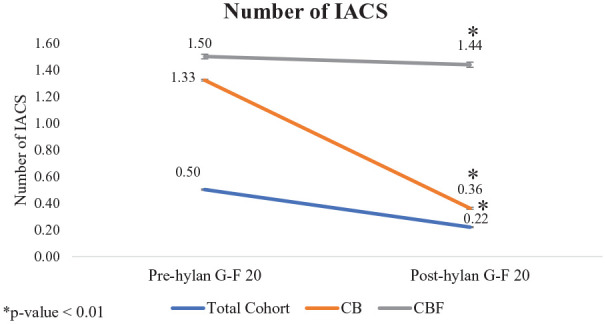
Number of intraarticular corticosteroid injections administered pre-hylan G-F 20 versus post-hylan G-F 20 treatment. IACS = intraarticular corticosteroids; patient subgroups: CB = patients at least one intraarticular corticosteroid injection before hylan G-F 20 treatment; CBF = patients with at least one intraarticular corticosteroid injection both before and after hylan G-F 20 treatment.
Discussion
In light of the US opioid epidemic caused by opioid addiction that can lead to death due to overdose 40 and that 16% of OAK patients were prescribed an opioid from 2007 to 2014, 41 we examined prescription of opioids and corticosteroid injections before and after viscosupplement treatment among patients diagnosed with OAK. Opioids may be prescribed to manage pain associated with OAK, particularly prior to 2010. In 2010, national recommendation(s) to limit opioid prescriptions emerged which were supported by CMS and CDC.37,38 Similarly, the American Association of Hip and Knee Surgeons and the American College of Rheumatology recommend only prescribing opioids for OAK patients that did not respond to initial therapy42,43 while the Osteoarthritis Research Society International does not provide a recommendation for the use of opioids. 44 IACS are commonly administered to treat inflammation associated with OAK.
Opioid Prescriptions
This study demonstrated that only half (50.2%) of patients prescribed an opioid prior to hylan G-F 20 treatment were prescribed an opioid in the posttreatment follow-up period. However, since physician notes are not available within the Truven database, we cannot rule out the possibility that some patients may have presented with adverse effects from opioid use. Our findings are similar to those from a previous study that demonstrated a significant decrease (P < 0.01) in percentage of patents filling an opioid prescription before HA treatment (34.3%) versus after HA treatment (29.2%) among patients lacking a total knee replacement (TKR) in the posttreatment period. 45
Opioid Prescribed Amounts
The total patient cohort in the present study did not show a significant difference in the amount of opioids prescribed after versus before HA treatment. McIntyre et al., however, in a retrospective study examining opioid prescriptions among patients with OAK treated with HA (regardless of eventual TKR status), found that the number of opioid prescriptions per patient increased (P < 0.01) following HA treatment (1.25) versus before treatment (1.12) for all OAK patients. 45 However, when a subgroup of patients who did not go on to receive a TKR in the 6 months following HA treatment were analyzed, there was a significant decrease (P < 0.01) in the number of opioid prescriptions per patient after HA treatment (1.02) versus before HA treatment (1.09). 45
The current study demonstrated a reduction in the amount of opioids prescribed after versus before hylan G-F 20 treatment in terms of total MME and MME per day for patients who were prescribed opioids prior to hylan G-F 20 treatment. As the radiological severity of OAK is not available within the database, it is not possible to determine if the observed decrease in opioid prescriptions is due to improvements in knee function. The reduction in the amount of opioids prescribed after versus before hylan G-F 20 is similar to the trend seen in a smaller US observational study, which demonstrated a reduction in the amount of opioids per patient in the 6 months following hylan G-F 20 treatment compared to before treatment among those taking opioids prior to treatment. 46 Waddell and Bricker also found that patients with OAK reported a reduction in the use of oral pain medications over the 6 months following hylan G-F 20 treatment versus before treatment. 47
Opioid Prescription Days
Patients who were prescribed opioids at baseline (the OB subgroup), exhibited a significant decrease (P < 0.01) in opioid prescription days following hylan G-F 20 treatment compared to before treatment. It is possible that patients did not respond well to opioids and therefore discontinued use following hylan G-F 20 treatment. The reduction in opioid prescription days following hylan G-F 20 treatment compared to before treatment is consistent with the results from a recent retrospective study using the Cerner HealthFacts database. 46 In the subgroup of patients who were prescribed with opioids at baseline and after hylan G-F 20 (OBF subgroup) there was an increase in opioid prescription days by an average 5 days (of 180 days); however, further investigation revealed an increase in opioid prescriptions at the time of hylan G-F 20 treatment (Supplemental Fig. 1). While it is possible that this increase is due to a continuation of care, the Truven database does not provide access to doctor’s notes, disease severity or patient pain scores to contribute to this analysis. Through analysis of opioids prescribed by index treatment year, our cohort demonstrated that the percentage of patients with OAK prescribed opioids (in baseline and/or follow-up periods) significantly decreased over the study period (36% in 2007 vs. 29% in 2016; P < 0.01, Cochrane-Armitage Trend Test; Supplemental Fig. 2).
Intraarticular Corticosteroid Injection Utilization
Long-term repeated use of IACS can lead to nearby bone thinning, 48 nerve damage, 48 and gross cartilage damage, 18 while studies have shown that viscosupplements rarely have systemic effects and adverse events are typically easily treatable.49,50
We found that 74.8% of patients who received an IACS in the baseline period did not receive an IACS in the follow-up period. McIntyre et al. also found a significant decrease (P < 0.01) in the proportion of patients receiving a steroid injection before (42.4%) versus after (18.5%) HA treatment within a subgroup of patients that did not receive a TKR in the 6 months following HA treatment. 45 The observed decreases in steroid injections may be related to the fact that HA may be used due to lack of response in some patients to IACS, leading to discontinuation of use of IACS.
The current study revealed that for all patient groups examined (total cohort, patients who received IACS before hylan G-F 20 treatment, and patients who received IACS both before and after hylan G-F 20 treatment), a significant reduction in IACS administered after versus before HA treatment. It is possible that IACS received post-hylan G-F 20 treatment are administered as bridging treatments until the next hyaluronic acid treatment is covered by insurance. We also cannot rule out that some patients may have had poor response to IACS. Two recent retrospective studies also demonstrated a significant decrease in the number of IACS following hylan G-F 20 or HA treatment compared to before treatment.45,46 A prospective study found that fewer OAK patients received steroid injections when treated with appropriate care and hylan G-F 20 versus patients treated with appropriate care alone. 51 Another study found that IACS are more effective than HA in the 4 weeks following treatment, that they have the same efficacy between 5 and 7 weeks, but that HA is more effective from 8 to 26 weeks after treatment. 52 These findings are corroborated by a study that found HA was more effective in treating OAK pain than IACS, 1 month following treatment up until 6 months after treatment. 53 Hylan G-F 20 appears to be associated with a reduction in the use of IACS, which may be due to hylan G-F 20 having better long-term efficacy than IACS in OAK patients.
Limitations
ICD-9 and ICD-10 diagnosis codes were used to identify OAK; however, since ICD-9 diagnosis codes do not contain information about the bilateral nature of OAK, bilateral information from ICD-10 codes was not included to maintain consistency. The Truven database captures patient medication prescriptions filled and disease details, but does not contain information about disease progression, severity of disease, range of motion, duration of symptoms, or patient pain scores. Therefore, it was not possible to determine the severity of OAK or if the decrease in IACS or opioids was related to improvement in pain. The Truven database also did not capture clinician’s notes or information regarding the reasons for using a particular drug or device (e.g., intolerance to opioids or IACS, patients contraindicated for opioids prescription, patients waiting for TKR). Thus, it is not possible to determine whether the reduction in the use of opioids or corticosteroids may be related to intolerance of these agents in some patients, the reluctance of some physicians to continue prescribing opioids or the lack of IACS effect. Reduction in monitored medications may also be related to natural healing of the body and may not be related to hylan G-F 20, but this could not be determined from the data. Patient compliance is not captured within the Truven database; this study quantified medication prescriptions that were filled, or a claim filed, as it was not possible to quantify medication amounts taken by patients. Therefore, it is possible that patients may take fewer medications than prescribed. The Truven database with Medicare supplement coverage was utilized to capture patients using private insurance and Medicare; however, if a patient used 2 private insurance policies and only one was captured within Truven, the pain medication would be underreported. Also, since the reason for an opioid prescription is not available within the database, therefore, while we attempted to exclude confounding conditions that may require pain/inflammatory medications via study selection criteria, it is possible that opioid prescriptions may not be attributable to OAK. Any over-the-counter pain medications, such as NSAIDs, that may have been used by patients in addition to opioids or IACS injections were not captured in this study.
Conclusions
Physicians typically treat pain and inflammation associated with OAK with acetaminophen, NSAIDs, viscosupplements, IACS, or opioids. This study demonstrated a reduction in opioid prescriptions post-hylan G-F 20 treatment among patients prescribed opioids prior to treatment, and a reduction in IACS utilization after HA treatment among all patients treated. It is known that long-term repeated use of opioids and IACS have harmful effects,15,17,18,48 therefore, hylan G-F 20 may be one of the effective means for decreasing utilization of opioids and IACS for individual patients with OAK. However, this study cannot rule out the fact that some patients may have discontinued IACS due to a lack of response and some may have discontinued opioids due to adverse reactions. Future studies to examine pain and anti-inflammatory medication use surrounding hylan G-F 20 treatment should be conducted in a large population to understand how OAK severity impacts medication utilization, and to understand the true rate of opioid utilization for treatment of OAK in 2019.
Supplemental Material
Supplemental material, Supplementary_material for Evaluation of Hylan G-F 20 Treatment with Opioid Prescriptions and Intraarticular Corticosteroid Injections in Patients with Osteoarthrosis of the Knee Using a Claims Database by Michael J. Langworthy, Charles D. Hummer, Wilson Ngai, Lichen Hao and David Webner in CARTILAGE
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this project was provided by Sanofi. Writing support was provided by Xavier F. Marinaro, MEng, and Sibyl H. Munson, PhD, of Boston Strategic Partners, Inc, and editorial support was provided by Gill Sperrin, CBiol, MRSB, CMPP, of Elevate Medical Affairs, funded by Sanofi.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MJL has no potential conflicts of interest to disclose; CDH and DW have received consulting fees from Sanofi; WN is an employee of Sanofi and owned Sanofi stock; LH is an employee of Sanofi.
Ethical Approval: Ethical approval was not sought for the present study because a HIPAA compliant claims database was utilized which does not meet the definition of human subject research as defined in 45 Code of Federal Regulations 46.102.
Informed Consent: Informed consent was not sought for the present study because a HIPAA compliant claims database was utilized which does not meet the definition of human subject research as defined in 45 Code of Federal Regulations 46.102.
ORCID iD: Michael J. Langworthy  https://orcid.org/0000-0003-3985-0283
https://orcid.org/0000-0003-3985-0283
Supplementary material for this article is available on the Cartilage website at https://journals.sagepub.com/home/car.
References
- 1. Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26(3):355-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Strauss EJ, Hart JA, Miller MD, Altman RD, Rosen JE. Hyaluronic acid viscosupplementation and osteoarthritis: current uses and future directions. Am J Sports Med. 2009; 37(8):1636-44. [DOI] [PubMed] [Google Scholar]
- 4. Altman RD, Dasa V, Takeuchi J. Review of the mechanism of action for Supartz FX in knee osteoarthritis. Cartilage. 2018;9(1_suppl):11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pai SK, Allgar V, Giannoudis PV. Are intra-articular injections of Hylan G-F 20 efficacious in painful osteoarthritis of the knee? A systematic review & meta-analysis. Int J Clin Pract. 2014;68(8):1041-7. [DOI] [PubMed] [Google Scholar]
- 6. Hunter DJ. Viscosupplementation for osteoarthritis of the knee. N Engl J Med. 2015;372(11):1040-7. [DOI] [PubMed] [Google Scholar]
- 7. Nicholls MA, Fierlinger A, Niazi F, Bhandari M. The disease-modifying effects of hyaluronan in the osteoarthritic disease state. Clin Med Insights Arthritis Musculoskelet Disord. 2017;10:1179544117723611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Migliore A, Procopio S. Effectiveness and utility of hyaluronic acid in osteoarthritis. Clin Cases Miner Bone Metab. 2015;12(1_suppl):31-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015;16:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen LH, Xue JF, Zheng ZY, Shuhaidi M, Thu HE, Hussain Z. Hyaluronic acid, an efficient biomacromolecule for treatment of inflammatory skin and joint diseases: a review of recent developments and critical appraisal of preclinical and clinical investigations. Int J Biol Macromol. 2018;116:572-84. [DOI] [PubMed] [Google Scholar]
- 11. Wobig M, Dickhut A, Maier R, Vetter G. Viscosupplementation with hylan G-F 20: a 26-week controlled trial of efficacy and safety in the osteoarthritic knee. Clin Ther. 1998;20(3):410-23. [DOI] [PubMed] [Google Scholar]
- 12. Scale D, Wobig M, Wolpert W. Viscosupplementation of osteoarthritic knees with hylan: a treatment schedule study. Curr Therapeutic Res. 1994;55(3):220-32. [Google Scholar]
- 13. Chevalier X, Jerosch J, Goupille P, van Dijk N, Luyten FP, Scott DL, et al. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo controlled trial. Ann Rheum Dis. 2010;69(1_suppl):113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moreland L, Arnold W, Saway A, Savory C, Sikes D. Efficacy and safety of intra-articular hylan G-F 20 (Synvisc), a viscoelastic derivative of hyaluronan, in patients with osteoarthritis of the knee. Abstract presented at: American College of Rheumatology, Annual Scientific Meeting; November 7-11, 1993; San Antonio, TX. [Google Scholar]
- 15. Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, Clark TW, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:559-74. [DOI] [PubMed] [Google Scholar]
- 16. Boscarino JA, Rukstalis M, Hoffman SN, Han JJ, Erlich PM, Gerhard GS, et al. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction. 2010;105(10):1776-82. [DOI] [PubMed] [Google Scholar]
- 17. Kane-Gill SL, Rubin EC, Smithburger PL, Buckley MS, Dasta JF. The cost of opioid-related adverse drug events. J Pain Palliat Care Pharmacother. 2014;28(3):282-93. [DOI] [PubMed] [Google Scholar]
- 18. Wernecke C, Braun HJ, Dragoo JL. The effect of intra-articular corticosteroids on articular cartilage: a systematic review. Orthop J Sports Med. 2015;3(5):2325967115581163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCrae JC, Morrison EE, MacIntyre IM, Dear JW, Webb DJ. Long-term adverse effects of paracetamol—a review. Br J Clin Pharmacol. 2018;84(10):2218-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Olsen AM, Fosbol EL, Lindhardsen J, Folke F, Charlot M, Selmer C, et al. Long-term cardiovascular risk of nonsteroidal anti-inflammatory drug use according to time passed after first-time myocardial infarction: a nationwide cohort study. Circulation. 2012;126(16):1955-63. [DOI] [PubMed] [Google Scholar]
- 22. Chan AT, Manson JE, Albert CM, Chae CU, Rexrode KM, Curhan GC, et al. Nonsteroidal anti-inflammatory drugs, acetaminophen, and the risk of cardiovascular events. Circulation. 2006;113(12):1578-87. [DOI] [PubMed] [Google Scholar]
- 23. Dincer D, Ulukal Karanci E, Akin M, Adanir H. NSAID, antiaggregant, and/or anticoagulant-related upper gastrointestinal bleeding: is there any change in prophylaxis rate after a 10-year period? Turk J Gastroenterol. 2019;30(6): 505-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bannuru RR, Osani M, Vaysbrot EE, McAlindon TE. Comparative safety profile of hyaluronic acid products for knee osteoarthritis: a systematic review and network meta-analysis. Osteoarthritis Cartilage. 2016;24(12):2022-41. [DOI] [PubMed] [Google Scholar]
- 25. Vannabouathong C, Bhandari M, Bedi A, Khanna V, Yung P, Shetty V, et al. Nonoperative treatments for knee osteoarthritis: an evaluation of treatment characteristics and the intra-articular placebo effect: a systematic review. JBJS Rev. 2018;6(7):e5. [DOI] [PubMed] [Google Scholar]
- 26. Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1_suppl):46-54. [DOI] [PubMed] [Google Scholar]
- 27. Brander VA, Stadler TS. Functional improvement with hylan G-F 20 in patients with knee osteoarthritis. Phys Sportsmed. 2009;37(3):38-48. [DOI] [PubMed] [Google Scholar]
- 28. Gregori D, Giacovelli G, Minto C, Barbetta B, Gualtieri F, Azzolina D, et al. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: a systematic review and meta-analysis. JAMA. 2018; 320(24):2564-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chitnis AS, Etter K, Holy CE, Gray FS, Manalac FJ, Bisson B, et al. Real-world impact of the high concentration non-avian high molecular weight hyaluronan on pain medication use among osteoarthritis patients. Curr Med Res Opin. 2019; 35(9):1523-7. [DOI] [PubMed] [Google Scholar]
- 30. Zhou J, Nutescu EA, Han J, Calip GS. Clinical trajectories, healthcare resource use, and costs of long-term hematopoietic stem cell transplantation survivors: a latent class analysis. J Cancer Surviv. 2020;14(3):294-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Centers for Medicare & Medicaid Services. Opioid oral morphine milligram equivalent (MME) conversion factors. Available from: https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-April-2017.pdf
- 32. Centers for Disease Control and Prevention. Calculating total daily dose of opioids for safer dosage. Available from: https://www.cdc.gov/drugoverdose/pdf/calculating_total_daily_dose-a.pdf
- 33. Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project. Surgery flags software for services and procedures. Available from: https://www.hcup-us.ahrq.gov/toolssoftware/surgflags/surgeryflags.jsp
- 34. Synvisc® [package insert]. Ridgefield, NJ: Sanofi-aventis Canada Inc; 2014. Available from: http://products.sanofi.ca/en/Synvisc-Information-for-Use.pdf [Google Scholar]
- 35. Dahl LB, Dahl IM, Engstrom-Laurent A, Granath K. Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Ann Rheum Dis. 1985;44(12):817-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Altman RD, Bedi A, Karlsson J, Sancheti P, Schemitsch E. Product differences in intra-articular hyaluronic acids for osteoarthritis of the knee. Am J Sports Med. 2016;44(8):2158-65. [DOI] [PubMed] [Google Scholar]
- 37. Wolters Kluwer. Morphine equivalent dosing. Available from: https://www.wolterskluwercdi.com/sites/default/files/documents/ebooks/morphine-equivalent-dosing-ebook.pdf?v3
- 38. Guy GP, Jr, Zhang K, Bohm MK, Losby J, Lewis B, Young R, et al. Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Losina E, Weinstein AM, Reichmann WM, Burbine SA, Solomon DH, Daigle ME, et al. Lifetime risk and age of diagnosis of symptomatic knee osteoarthritis in the US. Arthritis Care Res (Hoboken). 2013;65(5):703-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wilkerson RG, Kim HK, Windsor TA, Mareiniss DP. The opioid epidemic in the United States. Emerg Med Clin North Am. 2016;34(2):e1-e23. [DOI] [PubMed] [Google Scholar]
- 41. DeMik DE, Bedard NA, Dowdle SB, Burnett RA, McHugh MA, Callaghan JJ. Are we still prescribing opioids for osteoarthritis? J Arthroplasty. 2017;32(12):3578-82.e1. [DOI] [PubMed] [Google Scholar]
- 42. American Association of Hip and Knee Surgeons. Opioid use for the treatment of osteoarthritis of the hip and knee: position of the American Association of Hip and Knee Surgeons. Available from: http://www.aahks.org/position-statements/opioid-use-for-the-treatment-of-osteoarthritis-of-the-hip-and-knee/
- 43. Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465-74. [DOI] [PubMed] [Google Scholar]
- 44. McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363-88. [DOI] [PubMed] [Google Scholar]
- 45. McIntyre LF, Beach W, Bhattacharyya S, Yadalam S, Bisson B, Kim M. Impact of hyaluronic acid injections on utilization of pain management medications. Am J Pharm Benefits. 2017;9(6):195-9. [Google Scholar]
- 46. Khangulov V, Zhang X, Munson S, Peyerl F, Rey F. Opioid and cortisone injection utilization before and after initial hylan G-F 20 treatment. J Manag Care Specialty Pharmacy. 2018;24(4-a):S88. [Google Scholar]
- 47. Waddell DD, Bricker DC. Clinical experience with the effectiveness and tolerability of hylan G-F 20 in 1047 patients with osteoarthritis of the knee. J Knee Surg. 2006;19(1_suppl):19-27. [DOI] [PubMed] [Google Scholar]
- 48. Morelli J. Use of corticosteroids in osteoarthritis. Available from: https://www.arthritis.org/living-with-arthritis/treatments/medication/drug-types/corticosteroids/corticosteroid-injections.php
- 49. Waddell DD. Viscosupplementation with hyaluronans for osteoarthritis of the knee: clinical efficacy and economic implications. Drugs Aging. 2007;24(8):629-42. [DOI] [PubMed] [Google Scholar]
- 50. Bert JM, Waddell DD. Viscosupplementation with hylan g-f 20 in patients with osteoarthrosis of the knee. Ther Adv Musculoskelet Dis. 2010;2(3):127-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Raynauld JP, Torrance GW, Band PA, Goldsmith CH, Tugwell P, Walker V, et al. A prospective, randomized, pragmatic, health outcomes trial evaluating the incorporation of hylan G-F 20 into the treatment paradigm for patients with knee osteoarthritis (part 1 of 2): clinical results. Osteoarthritis Cartilage. 2002;10(7):506-17. [DOI] [PubMed] [Google Scholar]
- 52. Bannuru RR, Natov NS, Obadan IE, Price LL, Schmid CH, McAlindon TE. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(12):1704-11. [DOI] [PubMed] [Google Scholar]
- 53. Wang F, He X. Intra-articular hyaluronic acid and corticosteroids in the treatment of knee osteoarthritis: a meta-analysis. Exp Ther Med. 2015;9(2):493-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_material for Evaluation of Hylan G-F 20 Treatment with Opioid Prescriptions and Intraarticular Corticosteroid Injections in Patients with Osteoarthrosis of the Knee Using a Claims Database by Michael J. Langworthy, Charles D. Hummer, Wilson Ngai, Lichen Hao and David Webner in CARTILAGE