Abstract
Objective
To compare the efficacy and safety of costal chondrocyte–derived pellet-type autologous chondrocyte implantation (CCP-ACI) with microfracture (MFx) for repair of articular cartilage defects of the knee.
Design
Thirty subjects with an International Cartilage Repair Society (ICRS) grade 3 to 4 chondral defect (2-10 cm2 in area; ≤4 cm3 in volume) were randomized at a ratio of 2:1 (CCP-ACI:MFx). Twenty patients were allocated in the CCP-ACI group and 10 patients in the MFx group. CCP-ACI was performed by harvesting costal cartilage at least 4 weeks before surgery. Implantation was performed without any marrow stimulation. Efficacy and safety were assessed at weeks 8, 24, and 48 after surgery according to the magnetic resonance observation of cartilage repair tissue (MOCART) score and clinical outcomes.
Results
MOCART scores improved from baseline to 24 and 48 weeks postoperatively in both treatment groups. The improvement in MOCART scores in the CCP-ACI group was significantly greater than that in the MFx group at 24 and 48 weeks (39.1 vs 21.8 and 43.0 vs 24.8, respectively). The proportions of complete defect repair and complete integration were significantly higher in the CCP-ACI group than the MFx group at 48 weeks. Improvement in Lysholm score and KOOS subscores, including Function (Sports and Recreational Activity) and knee-related quality of life was significantly greater in the CCP-ACI group than the MFx group at 48 weeks (35.4 vs 31.5, 35.7 vs 28.5, and 27.9 vs 11.6, respectively).
Conclusion
Treatment of cartilage defects with CCP-ACI yielded satisfactory cartilage tissue repair outcomes, with good structural integration with native cartilage tissue shown by magnetic resonance imaging at 24 and 48 weeks after surgery.
Level of Evidence
Level 1: Randomized controlled study.
Keywords: costal cartilage, autologous chondrocyte implantation, chondral lesion, cartilage repair
Introduction
The articular cartilage has limited intrinsic regenerative capacity due to its low cellularity and lack of blood supply. 1 Chondrocytes are arranged within the articular cartilage and are surrounded by compact extracellular matrix (ECM), which prevents their migration to sites of injury. Therefore, injuries in the articular cartilage are associated with impairment of joint function and pain. It may further result in osteoarthritis.1-3
Following the initial report of Brittberg et al., 4 there have been several other reports of the clinical outcomes of articular chondrocyte-based autologous chondrocyte implantation (ACI) treatment.5-8 The reproducibility and durability of restored cartilage structure and function, as well as the cost-effectiveness of this procedure is controversial. 9 Moreover, the possibility of donor site morbidity has always been an issue in articular chondrocyte–based ACI treatment. Nonarticular cartilage, including nasoseptal, auricular, and costal cartilage, has been suggested as an alternative cell source for articular cartilage regeneration.10-12 Donor site morbidity is low when harvesting costal cartilage, which has been used widely as a graft source for rhinoplasty. 13 Also. costal cartilage is the largest permanent hyaline cartilage in the mammalian body, and it contains active chondrocytes. 14 Both costal and articular cartilage are derived from the somites, which appear as bilaterally paired blocks of paraxial mesoderm in the embryo. Due to its good surgical accessibility and expandability in vitro, Lee et al. 15 and Isogai et al. 16 previously reported that costal cartilage was a promising source of chondrocytes for hyaline cartilage regeneration. Chondrocytes derived from costal cartilage were comparable to those obtained from articular cartilage. Moreover, they showed superior expandability and better capacity to generate hyaline-like cartilage tissues. Costal chondrocytes could produce superficial zone proteins in vitro and showed the capacity for articular cartilage regeneration.
CartiLife (Biosolution Co., Ltd., Seoul, Korea) is a small pellet-like ACI manufactured from chondrocytes in the subject’s own costal cartilage, followed by expansion culture and 3-dimensional pellet culture. In the first human study (phase I clinical trial), the feasibility, safety, and efficacy of costal chondrocyte–derived pellet-type autologous chondrocyte implantation (CCP-ACI) for treatment of full-thickness cartilage defects of the knee were analyzed. 17 Costal chondrocytes of all subjects formed pellets of homogeneous size, which showed similar characteristics to hyaline cartilaginous tissue, that is, lacunae-occupying chondrocytes surrounded by glycosaminoglycan and type II collagen-rich ECM. There were no serious treatment-related adverse events during the 5-year follow-up period and significant improvements were seen in all clinical scores compared with the preoperative baseline.
Microfracture (MFx) has gained popularity because of its less invasive approach in addition to its low cost and technical simplicity. 18 MFx surgery consists of performing multiple perforations to the subchondral bone which allows access of reparative progenitor cells to the cartilage lesion. 19 The repaired cartilage consists of fibrocartilaginous tissue. There is accumulating evidence suggesting degeneration and deterioration of the fibrocartilaginous tissue 1.5 to 5 years, postoperatively. 20 MFx, despite its drawbacks, is the most popular treatment for articular cartilage defects of the knee. 21
This randomized controlled trial was performed to compare the efficacy and safety of CCP-ACI and MFx for treatment of cartilage lesions in subjects with injured or defective articular cartilage of the knee.
Materials and Methods
Study Design
This prospective, randomized, open-label, multicenter study was designed to evaluate the efficacy of CCP-ACI as compared with that of MFx. A total of 30 subjects with an International Cartilage Repair Society (ICRS) grade 3 to 4 chondral defect (2-10 cm2 in area; ≤4 cm3 in volume) were randomized at a ratio of 2:1 (CCP-ACI:MFx). Safety and efficacy were assessed on hospital visits at weeks 8, 24, and 48 after surgery. Subjects were randomized at a ratio of 2:1 because an imbalanced randomization provided adequate subject exposure to CCP-ACI for assessment of safety. In order to reduce the predictability of assigned groups, block randomization was applied with permuted blocks of 6, and the treatment group and the control group were assigned in 2:1 based on the block size. Allocations were kept in sequentially numbered and sealed envelopes.
The study was approved by Korea’s Ministry of Food and Drug Safety, as well as the institutional review boards of the institutions that participated in this study. The study was registered at ClinicalTrials.gov (NCT03545269), and all subjects provided written informed consent.
Subjects
A screening test and magnetic resonance imaging (MRI) evaluation were conducted in subjects with articular cartilage damage or defects of the knee who provided written informed consent to participate in the study, to determine their eligibility according to the inclusion/exclusion criteria. Eligible subjects were assigned randomization numbers, and donor tissues were collected from the CCP-ACI group.
The inclusion criteria were as follows: (1) age 19 to 65 years; (2) 2- to 10-cm2 lesion in the knee joint cartilage due to acute or repetitive damage (total volume ≤4 cm3); (3) full-thickness, local ICRS grade 3 to 4 lesion on the medial or lateral femoral condyle or trochlea, with relatively healthy cartilage adjacent to the defect (ICRS grade 1-2); (4) joint space of the subject knee maintained over 50%; (5) independently mobile; (6) agreed to follow the recommended physiotherapy, including home exercises; and (7) provided informed consent to participate in this clinical study.
Subjects with inflammatory arthropathic disease (e.g., rheumatoid arthritis or gouty arthritis), Kellgren and Lawrence (KL) grade of 3 or higher, arthritis associated with autoimmune disease, history of hypersensitivity to bovine protein or gentamycin, hemophilia, other tumors in addition to articular cartilage defects of the knee, past history of radiotherapy or anticancer treatment within the past 2 years, or pregnant or breast-feeding women were excluded.
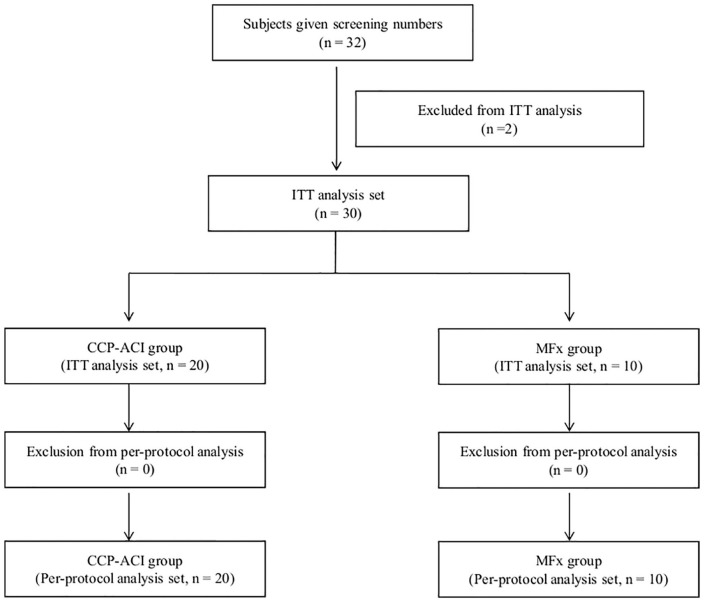
A total of 32 subjects were screened and enrolled in this clinical trial, of whom 2 were randomized but then excluded from the intention-to-treat (ITT) analysis; 1 withdrew consent and the other did not fulfill the inclusion/exclusion criteria ( Fig. 1 ).
Figure 1.
Flowchart of study population recruitment.
There were no significant differences between the 2 groups in any of the baseline characteristics. The ICRS grade and defect size were assessed by MRI during screening, including the defect area and volume. The average area was 3.5 ± 1.4 cm2 for the CCP-ACI group and 2.5 ± 0.4 cm2 for the MFx group. The average volume was 1.6 ± 0.8 cm3 for the CCP-ACI group and 0.8 ± 0.4 cm3 for the MFx group; these values were significantly different (P = 0.001). There were no statistically significant differences in other ICRS items between the 2 groups ( Table 1 ).
Table 1.
Demographic Data and Baseline Characteristics.
| CCP-ACI Group | MFx Group | P | |
|---|---|---|---|
| Patients, n | 20 | 10 | |
| Gender, male/female, n (%) | 14 (70)/6 (30) | 3 (30)/7 (70) | n.s. |
| Age, y, mean ± SD | 41.5 ± 13.0 | 47.2 ± 10.8 | n.s. |
| <50 y, n (%) | 11 (55) | 6 (60) | n.s. |
| ≥50 y, n (%) | 9 (45) | 4 (40) | |
| BMI, kg/m2, mean ± SD | 24.7 ± 3.5 | 25.1 ± 2.4 | n.s. |
| <25 kg/m2, n (%) | 12 (60) | 3 (30) | n.s. |
| 25-30 kg/m2, n (%) | 6 (30) | 7 (70) | |
| ≥30 kg/m2, n (%) | 2 (10) | 0 (0) | |
| Activity level, n (%) | |||
| Nonathletes | 15 (75) | 9 (90) | n.s. |
| Athletes | 5 (25) | 1 (10) | |
| Lesion site, n (%) | |||
| Condyle | 9 (45) | 7 (70) | n.s. |
| Trochlear | 11 (55) | 3 (30) | |
| Etiology, n (%) | |||
| Trauma | 10 (50) | 0 (0) | 0.007 |
| Osteoarthritis a | 7 (35) | 4 (40) | |
| Unknown | 3 (15) | 6 (60) | |
| KL grade, n (%) | |||
| 1 | 13 (65) | 6 (60) | n.s. |
| 2 | 7 (35) | 4 (40) | |
| ICRS grade, n (%) | |||
| 3 | 5 (25) | 1 (10) | n.s. |
| 4 | 15 (75) | 9 (90) | |
| Defect area, cm2, mean ± SD | 3.5 ± 1.4 | 2.5 ± 0.4 | n.s. |
| <4 cm2, n (%) | 15 (75) | 9 (90) | n.s. |
| ≥4 cm2, n (%) | 5 (25) | 1 (10) | |
| Defect depth, mm, mean ± SD | 4.6 ± 1.7 | 3.2 ± 1.4 | n.s. |
| <6 mm, n (%) | 16 (80) | 10 (100) | n.s. |
| ≥6 mm, n (%) | 4 (20) | 0 (0) | |
| Defect volume, cm3, mean ± SD | 1.6 ± 0.8 | 0.8 ± 0.4 | 0.001 |
| <2 cm3, n (%) | 14 (70) | 10 (100) | n.s. |
| ≥2 cm3, n (%) | 6 (30) | 0 (0) | |
CCP-ACI = costal chondrocyte–derived pellet-type autologous chondrocyte implantation; MFx = microfracture; BMI = body mass index; KL = Kellgren and Lawrence; ICRS = International Cartilage Repair Society; n.s. = not significant.
Symptomatic and radiographic osteoarthritis according to American College of Rheumatology clinical classification criteria for osteoarthritis of the knee.
Preparation of the Costal Chondrocyte–Derived Pellet-Type ACI
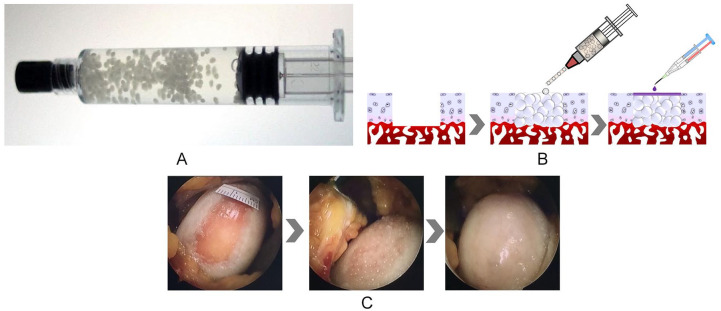
In the CCP-ACI group, a cartilage biopsy specimen was obtained from the costal cartilage at least 4 weeks before surgery. Under general anesthesia, costal cartilage biopsy samples of approximately 200 to 500 mg were obtained from the 8th, 9th, or 10th costal cartilage region, according to the size of the articular cartilage defect. The cartilage biopsy was stored in sterile Dulbecco’s modified Eagle’s medium, which was sent to the Good Manufacturing Practice facility at Biosolution Co., Ltd. for isolation and culture of autologous cells. During pellet culture, chondrocytes are initially aggregated, and induced to differentiate into cartilage to produce their own ECM that is similar to the natural ECM of hyaline cartilage. The final product was packaged into 5-mL prefilled syringes ( Fig. 2A ). Detailed description on the preparation is presented in the previous phase 1 clinical trial of CCP-ACI. 17
Figure 2.
(A) Costal chondrocyte–derived pellet-type autologous chondrocyte implantation (CCP-ACI) via a 5-mL prefilled syringe. (B) Animation showing procedures for CCP-ACI. (C) Image showing CCP-ACI applied to the defect site with fibrin glue applied to fix the pellets.
Surgical Procedure and Rehabilitation
In the treatment group, CCP-ACI was implanted into the defect area through miniarthrotomy of the knee. The cartilage defect was prepared by removing all damaged cartilage down to the subchondral bone. No marrow stimulation procedure was performed. Before applying CCP-ACI, as much blood and fluid were removed from the defective region as possible. An intravenous (IV) catheter (14 gauge; BD Medical, Sandy, UT, USA) was connected to the prefilled syringe, and the pellets were implanted into the defect by slowly pushing the plunger of the syringe to the height of the adjacent cartilage ( Fig. 2B and C ). Fibrin glue (Greenplast; Green Cross Corp., Yongin, Korea) was applied to the top of the defect to fix the pellets. The fibrin glue was allowed to set for 5 minutes. A manual range of motion (ROM) test of the knee was performed 5 times before wound closure to ensure pellet fixation.
In the MFx group, after identification of the chondral lesion, unstable cartilage and calcified layer was removed using shaver and curette, including cartilage loosely attached to the adjacent rim. Multiple holes were made on the subchondral bone plate using an MFx awl. The holes were located perpendicular to the subchondral bone surface. Bleeding was observed after reduction of the fluid pressure. The holes were approximately 4 mm apart. Intra-articular drain was not used.
All subjects were encouraged to complete a postoperative rehabilitation program after surgery. Continuous passive motion within 24 hours after surgery was recommended and continued for 4 to 6 weeks. Partial weightbearing walking was recommended 2 weeks after surgery and gradually increased up to 6 weeks posttransplantation, followed by full weightbearing for 6 to 12 weeks to maximize tissue repair.
Efficacy Assessment
The primary efficacy endpoint was the changes in magnetic resonance observation of cartilage repair tissue (MOCART) score at 48 weeks after surgery compared with the time of screening. The MOCART score, used to evaluate the repaired cartilage tissue, is based on the extent of defect repair, integration with border zone, structure, signal intensity and quality of the surface of the repaired tissue, integrity of the subchondral lamina and subchondral bone, degree of adhesion, and degree of synovitis.22-24 The final MOCART score was calculated based on the mean scores obtained by 2 independent orthopedic specialists who were blinded and not involved in the treatment of patients in the present study.
Secondary efficacy endpoints were as follows: (1) changes in the Lysholm score, International Knee Documentation Committee (IKDC) score, Knee Injury and Osteoarthritis Outcome Score (KOOS), and visual analog scale (VAS) pain score (100 mm scale) at each visit (weeks 8, 24, and 48) compared with the date of surgery; (2) changes in the ROM at each visit compared with the date of surgery; (3) weightbearing radiographs were taken at each visit and compared to the date of surgery; (4) MRI assessment (MOCART score) at week 24 compared with the time of screening; and (5) analgesic administration status.
Safety Assessment
Safety was assessed during visits to the hospital at weeks 8, 24, and 48 after the surgery. Safety assessment criteria are as follows: (1) physical assessment; (2) adverse events; (3) vital signs, laboratory tests; and (4) MRI (presence of outgrowth).
Statistical Analysis
Analysis of covariance (ANCOVA) was used to compare the groups regarding the MOCART score, Lysholm score, IKDC score, VAS pain score, KOOS, ROM, and radiography status, with the analgesic administration status during the washout period (before surgery and before the date of outpatient visit) and the baseline scores being used as covariates. Group differences in the mean changes in scores were assessed, after validating the normality of the data, using the independent t test or Wilcoxon’s rank sum test. Descriptive statistics (frequencies, percentages) regarding the analgesic administration status (to manage knee pain during the washout period) are presented. Categorical data were analyzed by the chi-square test or Fisher’s exact test, and frequencies and percentages are presented. To test for group differences in mean MOCART change scores (baseline vs. week 48 after surgery) according to cartilage defect type and size, body mass index, and age, the independent t test or Wilcoxon’s rank sum test was used depending on the normality of the data. In all analyses, P < 0.05 was taken to indicate statistical significance. The present study was evaluated in an exploratory clinical trial (phase 2). Therefore, the number of samples was not calculated through a statistical method.
Results
Changes in MOCART Score at Weeks 24 and 48 after Surgery
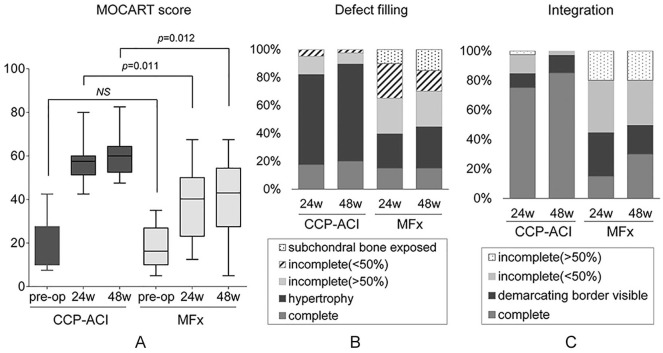
Mean scores and changes in MOCART scores obtained before and after surgery are presented in Table 2 and Figure 3A. At 48 weeks after surgery, the MOCART score increased by 43.0 ± 13.1 in the CCP-ACI group and by 24.8 ± 19.7 in the MFx group, compared with the baseline ( Table 2 ). The extent of change (relative to baseline) was statistically significant in both groups (CCP-ACI group, P < 0.0001; MFx group, P = 0.003), and was significantly greater in the CCP-ACI group than the MFx group (P = 0.002; Table 2 ). At 48 weeks after surgery, the rates of complete defect repair (20% vs. 15%; P = 0.004; Fig. 3B and C , Table 3 ) and complete integration (85% vs. 30%; P < 0.0001; Fig. 4A-C , Table 3 ) in the CCP-ACI group were significantly greater than those in the MFx group ( Fig. 4D and E ).
Table 2.
MOCART Scores. a
| CCP-ACI Group (n = 20) | MFx Group (n = 10) | P | |
|---|---|---|---|
| Baseline | 18.1 ± 11.3 | 18.8 ± 10.2 | n.s. |
| Week 24 | |||
| MOCART score | 57.25 ± 8.27 | 40.50 ± 16.41 | 0.011 |
| Change from baseline | 39.13 ± 11.71 | 21.75 ± 18.49 | 0.001 |
| P | <0.0001 | 0.005 | |
| Week 48 | |||
| MOCART score | 61.1 ± 10.5 | 43.5 ± 19.1 | 0.012 |
| Change from baseline | 43.0 ± 13.1 | 24.8 ± 19.7 | 0.002 |
| P | <0.0001 | 0.003 |
MOCART = magnetic resonance observation of cartilage repair tissue; CCP-ACI = costal chondrocyte–derived pellet-type autologous chondrocyte implantation; MFx = microfracture; n.s. = not significant.
Values are means ± SDs.
Figure 3.
(A) Magnetic resonance observation of cartilage repair tissue (MOCART) scores of the costal chondrocyte–derived pellet-type autologous chondrocyte implantation (CCP-ACI) group and microfracture (MFx) group at baseline, 24 weeks, and 48 weeks after surgery.
Table 3.
Radiological Evaluations Using MOCART Score Measured at 48 Weeks after Surgery. a
| Variables | CCP-ACI Group, n (%) | MFx Group, n (%) | P |
|---|---|---|---|
| Degree of defect repair and filling of the defect | |||
| Complete | 8 (20.0) | 3 (15.0) | 0.004 |
| Hypertrophy | 28 (70.0) | 5 (25.0) | |
| Incomplete | 3 (7.5) | 5 (25.0) | |
| >50% of the adjacent cartilage | 1 (2.5) | 2 (25.0) | |
| <50% of the adjacent cartilage | |||
| Subchondral bone exposed | 0 (0.0) | 2 (10.0) | |
| Integration to border zone | |||
| Complete | 34 (85.0) | 6 (30.0) | <0.0001 |
| Incomplete | |||
| Demarcating border visible (split-like) | 5 (12.5) | 4 (20.0) | |
| Defect visible | |||
| <50% of the length of the repair tissue | 1 (2.5) | 6 (30.0) | |
| >50% of the length of the repair tissue | 0 (0.0) | 4 (20.0) | |
| Surface of the repair tissue | |||
| Surface intact (lamina splendens intact) | 13 (32.5) | 1 (5.0) | 0.043 |
| Surface damaged | |||
| <50% of repair tissue depth | 21 (52.5) | 11 (55.0) | |
| >50% of repair tissue depth or total degeneration | 6 (15.0) | 8 (40.0) | |
| Structure of the repair tissue | |||
| Homogenous | 7 (17.5) | 1 (5.0) | n.s. |
| Inhomogeneous or cleft formation | 33 (82.5) | 19 (95.0) | |
| Signal intensity of the repair tissue | |||
| Dual T2-FSE | |||
| Isointense | 7 (17.5) | 2 (10.0) | n.s. |
| Moderately hyperintense | 32 (80.0) | 14 (70.0) | |
| Markedly hyperintense | 1 (2.5) | 4 (20.0) | |
| 3D-GE-FS | |||
| Isointense | 7 (17.5) | 2 (10.0) | n.s. |
| Moderately hypointense | 32 (80.0) | 14 (70.0) | |
| Markedly hypointense | 1 (2.5) | 4 (20.0) | |
| Subchondral lamina | |||
| Intact | 13 (32.5) | 7 (35.0) | n.s. |
| Not intact | 27 (67.5) | 13 (65.0) | |
| Subchondral bone | |||
| Intact | 9 (22.5) | 6 (20.0) | n.s. |
| Edema, granulation tissue, cysts, sclerosis | 31 (77.5) | 16 (80.0) | |
| Adhesions | |||
| No | 40 (100.0) | 20 (100.0) | n.s. |
| Yes | 0 (0.0) | 0 (0.0) | |
| Synovitis | |||
| No synovitis | 36 (90.0) | 15 (75.0) | n.s. |
| Synovitis | 4 (10.0) | 5 (25.0) | |
MOCART = magnetic resonance observation of cartilage repair tissue; CCP-ACI = costal chondrocyte–derived pellet-type autologous chondrocyte implantation; MFx = microfracture; FSE = fast spin echo; FS = fat-suppressed; n.s. = not significant.
Since the MOCART scores were obtained from the 2 clinicians, the n number is twice the number of subjects.
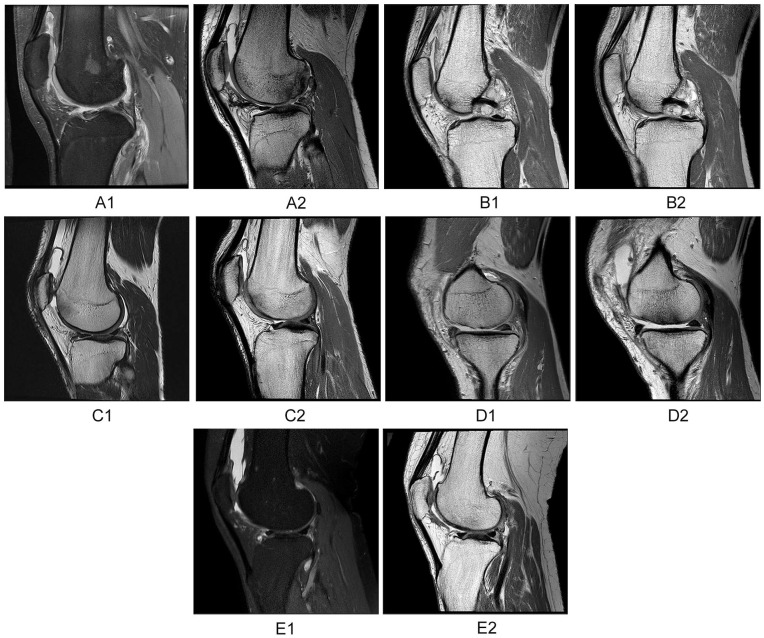
Figure 4.
(A1) Preoperative magnetic resonance imaging (MRI) scan showing osteochondritis dissecans at lateral femoral condyle. (A2) Postoperative costal chondrocyte–derived pellet-type autologous chondrocyte implantation (CCP-ACI) 48-week MRI scan showing less satisfactory defect repair and integration. (B1) Preoperative MRI scan showing cartilage defect at trochlea. (B2) Postoperative CCP-ACI 48-week MRI scan showing satisfactory defect repair and integration. (C1) Preoperative MRI scan showing cartilage defect at trochlea. (C2) Postoperative CCP-ACI 48-week MRI scan showing very satisfactory defect repair and integration. (D1) Preoperative MRI scan showing cartilage defect at medial femoral condyle. (D2) Postoperative microfracture (MFx) 48-week MRI scan showing poor defect repair and integration. (E1). Preoperative MRI scan showing cartilage defect at trochlea. (E2) Postoperative MFx 48-week MRI scan showing satisfactory defect repair and integration.
As the secondary efficacy endpoint, MRI scans were performed at week 24 and the MOCART score was obtained. The score increased in both groups compared with baseline, by 39.1 ± 11.7 in the CCP-ACI group (P < 0.0001) and 21.8 ± 18.5 in the MFx group (P = 0.005). The degree of change was significantly greater in the CCP-ACI group than the MFx group (P = 0.001; Table 2).
Subgroup Analysis
Subgroup analysis were performed according to the sex, age, body mass index, activity level, cause of articular cartilage defects, lesion site, KL grade, ICRS grade, defect area, depth, and volume. In most subgroups, the difference of MOCART score at week 48 was superior in the CCP-ACI group compared with the MFx group (Supplemental Material). None in MFx group had defect depth ≥6 mm or volume ≥2 cm3. Despite CCP-ACI group tend to have larger defect size, CCP-ACI group showed superiority in the change of MOCART score at week 48 from baseline over MFx group.
Subject-Reported Clinical Outcomes
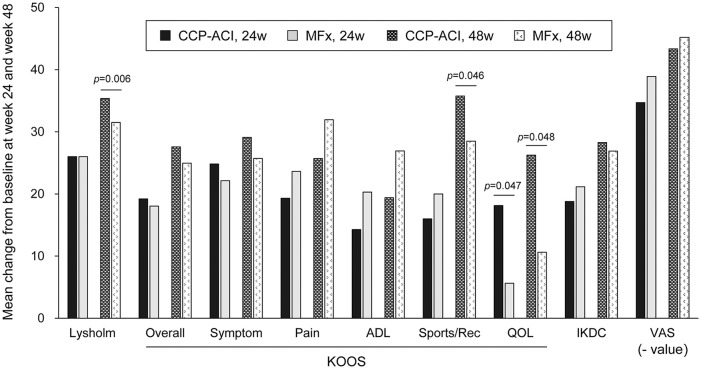
Clinical scores including Lysholm score, KOOS subscale scores, and IKDC score increased at weeks 24, and 48 compared with the baseline in both the CCP-ACI and MFx groups ( Table 4 ). The changes from baseline of the Lysholm score at weeks 48, KOOS-Sport/Recreation score at weeks 48, and KOOS-QOL score at weeks 24 and 48 of the CCP-ACI group were significantly higher than those of the MFx group. There was no significant difference in the extent of change in IKDC score between the 2 groups, at any time point ( Fig. 5 ).
Table 4.
Subject-Reported Clinical Outcomes. a
| CCP-ACI Group (n = 20) | MFx Group (n = 10) | |||||
|---|---|---|---|---|---|---|
| Baseline | 24 Weeks | 48 Weeks | Baseline | 24 Weeks | 48 Weeks | |
| Lysholm score | 47.5 ± 20.3 | 73.5 ± 15.5 | 82.9 ± 8.4 | 39.1 ± 15.4 | 65.1 ± 16.9 | 70.6 ± 12.0 |
| P | 0.0008 | <0.0001 | 0.0006 | 0.0001 | ||
| IKDC score | 38.2 ± 12.5 | 57.0 ± 13.6 | 66.5 ± 15.0 | 30.6 ± 14.7 | 51.7 ± 13.3 | 57.5 ± 14.8 |
| P | 0.0002 | <0.0001 | 0.0003 | <0.0001 | ||
| Overall KOOS | 47.9 ± 17.1 | 67.1 ± 10.4 | 75.5 ± 10.7 | 41.5 ± 17.5 | 59.5 ± 14.3 | 66.4 ± 14.0 |
| P | 0.002 | <0.0001 | 0.001 | 0.0003 | ||
| Symptoms | 54.1 ± 23.0 | 78.9 ± 10.7 | 83.2 ± 11.8 | 50.7 ± 16.0 | 72.9 ± 15.5 | 76.4 ± 14.7 |
| P | 0.0001 | <0.0001 | <0.0001 | 0.001 | ||
| Pain | 58.2 ± 17.3 | 77.5 ± 8.3 | 83.9 ± 9.0 | 47.8 ± 18.8 | 71.4 ± 12.8 | 79.7 ± 14.6 |
| P | 0.0001 | <0.0001 | 0.001 | 0.001 | ||
| ADL | 68.4 ± 14.7 | 82.7 ± 10.9 | 87.8 ± 9.8 | 56.8 ± 18.4 | 77.1 ± 12.3 | 83.7 ± 12.8 |
| P | 0.002 | 0.0001 | 0.002 | 0.0001 | ||
| Sport/Rec | 28.8 ± 26.7 | 44.8 ± 25.2 | 64.5 ± 19.8 | 18.0 ± 16.4 | 38.0 ± 23.5 | 46.5 ± 20.4 |
| P | n.s. | 0.0001 | 0.013 | 0.002 | ||
| QoL | 30.0 ± 18.4 | 51.7 ± 16.1 | 57.9 ± 17.0 | 34.2 ± 17.8 | 38.3 ± 19.3 | 45.8 ± 16.3 |
| P | 0.001 | <0.0001 | n.s. | 0.050 | ||
| 100 mm pain VAS | 59.3 ± 17.5 | 24.6 ± 16.2 | 15.9 ± 15.4 | 65.5 ± 18.2 | 26.6 ± 17.6 | 20.3 ± 12.8 |
| P | <0.0001 | <0.0001 | <0.0001 | 0.002 | ||
CCP-ACI = costal chondrocyte–derived pellet-type autologous chondrocyte implantation; MFx = microfracture; IKDC = International Knee Documentation Committee; KOOS = Knee Injury and Osteoarthritis Outcome Score; ADL = activities of daily living; Sport/Rec = Sports and recreation function; QoL = quality of life; VAS = visual analogue scale; n.s. = not significant
Values are means ± SDs.
Figure 5.
Graph showing clinical outcomes scores.
During the study, all subjects in both groups received acetaminophen as an analgesic. The pain VAS decreased significantly at weeks 24, and 48 compared with the baseline in both groups ( Table 4 ). There was no significant difference in the extent of change in pain score between the two groups, at any time point.
Range of motion
There was no statistically significant difference between the 2 groups in degree of change in ROM compared with baseline.
Radiography
Neither group showed severe deformation or abnormalities after treatment.
Safety Endpoint
There were no abnormal findings related to changes in vital signs or physical measurements during the study period. In physical examinations, all subjects were normal and there were no abnormal findings.
Adverse events occurred in 19 of 30 subjects; 13 (65%, 20 cases) were in the CCP-ACI group and 6 (60%, 14 cases) in the MFx group ( Table 5 ). Adverse events related to treatment were observed in 2 subjects (10%) in the CCP-ACI group (postprocedural hematoma and postoperative adhesion), and were resolved without sequelae.
Table 5.
All Adverse Events.
| Adverse Events | CCP-ACI Group, n (%) | MFx Group, n (%) |
|---|---|---|
| Eye disorders | ||
| Eye pain | 0 (0.0) | 1 (7.1) |
| Gastrointestinal disorders | ||
| Constipation | 4 (20.0) | 0 (0) |
| Dental cyst | 1 (5.0) | 0 (0) |
| Nausea | 3 (15.0) | 2 (14.3) |
| Esophagitis | 0 (0) | 1 (7.1) |
| Vomiting | 1 (5.0) | 2 (14.3) |
| Infections and infestations | ||
| Bacterial vaginosis | 0 (0) | 1 (7.1) |
| Chronic sinusitis | 1 (5.0) | 0 (0.0) |
| Rhinitis | 0 (0.0) | 1 (7.1) |
| Upper respiratory tract infection | 1 (5.0) | 0 (0.0) |
| Viral upper respiratory tract infection | 0 (0.0) | 2 (14.3) |
| Injury, poisoning and procedural complications | ||
| Chemical eye injury | 1 (5.0) | 0 (0) |
| Postprocedural hematoma | 1 (5.0) | 0 (0) |
| Postoperative adhesion | 1 (5.0) | 0 (0) |
| Metabolism and nutrition disorders | ||
| Dyslipidemia | 0 (0) | 1 (7.1) |
| Musculoskeletal and connective tissue disorders | ||
| Back pain | 1 (5.0) | 0 (0) |
| Musculoskeletal pain | 1 (5.0) | 0 (0) |
| Neck pain | 1 (5.0) | 0 (0) |
| Temporomandibular joint syndrome | 0 (0) | 1 (7.1) |
| Nervous system disorders | ||
| Dizziness | 1 (5.0) | 1 (7.1) |
| Headache | 1 (5.0) | 0 (0.0) |
| Renal and urinary disorders | ||
| Calculus urinary | 0 (0) | 1 (7.1) |
| Skin and subcutaneous tissue disorders | ||
| Dermatitis contact | 1 (5.0) | 0 (0) |
| Total cases, n (%) | 20 (100) | 14 (100) |
CCP-ACI, costal chondrocyte–derived pellet-type autologous chondrocyte implantation; MFx, microfracture.
Outgrowth confirmed by MRI occurred in four subjects (20.0%) in the CCP-ACI group and two subjects (20.0%) in the MFx group (P = 1.000). All were confirmed to be mild and ongoing.
Discussion
In this randomized controlled study, CCP-ACI showed significantly higher efficacy compared with the control treatment MFx in terms of structural improvement of cartilage defect area, when cartilage tissue repair was evaluated objectively by MRI (MOCART score). Comprehensive analysis of symptoms and functional improvement of the knee joint confirmed that the extent of change in endpoints was similar between the groups, and higher on some endpoints in the group treated with CCP-ACI compared with the controls.
MOCART scores in the CCP-ACI group showed superior results compared with MFx group. MOCART score subgroup analysis showed that defect filling and degree of integration was superior in CCP-ACI group compared with the MFx group at 48 weeks after surgery. CCP-ACI is engineered cartilage that uses costal cartilage to manufacture a pellet type ACI. Therefore, it is presumed to be far more superior in term of structural integration compared to MFx in which the reparative progenitor cells from the subchondral bone has to regenerate the fibrocartilaginous tissue.
Subject-reported clinical outcomes showed similar results between the 2 groups except for the Lysholm score and KOOS subscores, including Function (Sports and Recreational Activity) and knee-related quality of life. MFx is known to have good subject-reported outcome scores in short term data. 25 However, MFx has been linked to significant detrimental effect on the architecture of subchondral bone. 26 Furthermore, results of MFx has been known to deteriorate over time. 27 In contrast, a recent systematic review reported comparable subject-report outcomes scores of MFx to ACI in up to 5 years of follow-up. 28 A longer follow-up data of subject-reported outcome scores is needed for the present study.
CCP-ACI involves the use of autologous chondrocytes taken from the subject’s own costal cartilage. Costal cartilage, the largest permanent hyaline cartilage in mammals, is derived from the somites, similar to articular cartilage, and includes chondrocytes, water, and a matrix composed of type II collagen and glycosaminoglycan29-31; it has been widely utilized in plastic and orthopedic surgery as a source of autologous cartilage tissues.32-34 The costal cartilage has the advantage of containing chondrocytes, which remain active even after the age of 80 years. 14 Moreover, because of the simple noninvasive tissue collection method, costal cartilage is considered a useful source of cells for treating various articular cartilage abnormalities, such as joint injury, osteoarthritis, and articular cartilage defects in elderly patients. 35
Costal cartilage is also considered an excellent source of grafts for primary and revision rhinoplasty, 13 laryngotracheal reconstruction, 36 canal wall reconstruction, 37 and auricular reconstruction. 38 The incidence rates of long-term complications and donor site morbidity associated with cartilage harvesting are low. A previous study showed that hypertrophic chest scarring was the most common complication after graft harvesting. 39 Harvesting of rib cartilage has been criticized for its high rates of donor site morbidity, especially pneumothorax. 40 However, the incidence of pneumothorax after harvesting of rib cartilage is very low, 39 and unlike harvesting for rhinoplasty, the amount of cartilage needed for ACI is small. Therefore, the risk of pneumothorax is expected to be lower than for other procedures requiring large amounts of cartilage. However, dissection should always be performed carefully to prevent pneumothorax. In the present study, ribs below number 8 have been selected for the harvesting of rib cartilage because ribs below 8 overlies the abdominal cavity.
There have been previous reports of attempts to harvest cartilage for ACI. Mumm et al. 10 utilized chondrocytes from the nasal septum to engineer an autologous nasal chondrocyte-based graft. Nasal chondrocytes can be obtained from a small biopsy specimen under minimally invasive conditions; this previous study showed promising results after a 2-year follow-up. The tissue-like properties of the graft, however, required the surgeon to perform a mini-arthrotomy, whereas CCP-ACI can be administered under arthroscopy.
Autologous costal cartilage cells in CCP-ACI are proliferated in two-dimensional culture, and are then cultured 3-dimensionally to form small pellets surrounded by ECM secreted by the cells themselves, which is similar to natural hyaline cartilage. 17 Due to ethical reasons, the present study did not carry out biopsy and histological analysis of the repaired tissue in the clinical trial. However, animal experiment results support the possibility that the cartilage tissue repaired through CCP-ACI implantation is hyaline cartilage.
Experiments involving CCP-ACI into nude mice, rabbits, and goats confirmed that transplanted chondrocytes secreted ECM and integrated well with the surrounding tissues to form articular cartilage with a hyaline cartilaginous appearance. 41 Transplantation experiments in goats showed that the mechanical strength of the regenerated cartilage reached the level of normal cartilage. Nonclinical studies also confirmed that there were no chromosomal abnormalities in long-term culture, no impact on other organs, and no toxicity.
This method involves production of small pellets that are fixed to the defect area using biocompatible materials (medical adhesives, etc). Procedures can be carried out through less invasive approaches, such as minimal arthrotomy and arthroscopic procedures. As such procedures involve the use of autologous chondrocytes and autologous ECM secreted by the cells, survival rates of cells are high at the implantation site without an immune response. They are also advantageous in terms of shortening the time required to repair articular cartilage.
CCP-ACI is a safe and effective therapeutic option with no associated issues regarding immune rejection, carcinogenicity, or human immunodeficiency virus (HIV) infection risk, as it regenerates damaged tissue using somatic cells harvested from the patient’s own tissues. Historically, ACI has been known for its high up-front costs and favorable cost-efficacy estimate when view over a long-term period. 42
This study had some limitations, especially with regard to the fairly short follow-up period. Nevertheless, MOCART scores at both 24 and 48 weeks showed superior outcomes of CCP-ACI compared with MFx. To analyze the long-term efficacy of CCP-ACI, MRI data will be acquired at the 5-year follow-up. Second, the number of subjects was small. However, a future phase III, multicenter clinical study is being planned to evaluate the efficacy of CCP-ACI. Third, this study included both trochlear and condylar defects, which may show different clinical results. Fourth, lesion size that was considered for CCP-ACI or MFx was relatively large for MFx, which may have adversely affected the outcomes of MFx.
The findings of this randomized controlled trial showed that CCP-ACI achieved satisfactory repair of cartilage tissue defects; there was good structural integration with native cartilage tissue, as shown by MRI evaluations at 24 and 48 weeks after surgery.
Supplemental Material
Supplemental material, Supplementary_Table for Costal Chondrocyte–Derived Pellet-Type Autologous Chondrocyte Implantation versus Microfracture for Repair of Articular Cartilage Defects: A Prospective Randomized Trial by Kyoung-Ho Yoon, Jae Doo Yoo, Chong-Hyuk Choi, Jungsun Lee, Jin-Yeon Lee, Sang-Gyun Kim and Jae-Young Park in CARTILAGE
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants of the Ministry of Health & Welfare, Republic of Korea (HI15C0963). The funding source had no involvement in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. Biosolution Co., Ltd. provided funding for this study in the form of technical solutions, including the use of their facilities and laboratory equipment.
Supplemental material for this article is available on the Cartilage website at https://journals.sagepub.com/home/car.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Authors J.L. and J.Y.L. have stock and stock options in Biosolution Co., Ltd. The other authors (K.H.Y., J.D.Y., C.H.C., S.G.K., and J.Y.P.) have received no benefits in any form from a commercial party related directly or indirectly to the subject of this article.
Ethical Approval: The study was approved by Korea’s Ministry of Food and Drug Safety, as well as the institutional review boards of the institutions that participated in this study (Kyung-Hee University Hospital: KMC IRB 1338-02, Gangnam Severance Hospital: 4-2015-0210, Ewha Womans University Medical Center: EUMC 2014-11-009-004).
Informed Consent: All subjects provided written informed consent.
Trial Registration: The study was registered at ClinicalTrials.gov (Registration No. NCT03545269).
ORCID iD: Jae-Young Park  https://orcid.org/0000-0001-7635-9157
https://orcid.org/0000-0001-7635-9157
References
- 1. Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338(6109):917-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Caplan AI, Elyaderani M, Mochizuki Y, Wakitani S, Goldberg VM. Principles of cartilage repair and regeneration. Clin Orthop Relat Res. 1997;(342):254-69. [PubMed] [Google Scholar]
- 3. Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487-504. [PubMed] [Google Scholar]
- 4. Brittberg M, Nilsson A, Lindahl A, Ohlsson C, Peterson L. Rabbit articular cartilage defects treated with autologous cultured chondrocytes. Clin Orthop Relat Res. 1996;(326):270-83. [DOI] [PubMed] [Google Scholar]
- 5. Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Isaksen V, Ludvigsen TC, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89(10):2105-12. [DOI] [PubMed] [Google Scholar]
- 6. Kreuz PC, Steinwachs M, Erggelet C, Krause SJ, Ossendorf C, Maier D, et al. Classification of graft hypertrophy after autologous chondrocyte implantation of full-thickness chondral defects in the knee. Osteoarthritis Cartilage. 2007;15(12):1339-47. [DOI] [PubMed] [Google Scholar]
- 7. Ochi M, Uchio Y, Tobita M, Kuriwaka M. Current concepts in tissue engineering technique for repair of cartilage defect. Artif Organs. 2001;25(3):172-9. [DOI] [PubMed] [Google Scholar]
- 8. Popko J, Szeparowicz P, Sawicki B, Wolczynski S, Wojnar J. Rabbit articular cartilage defects treated with cultured costal chondrocytes (preliminary report). Folia Morphol (Warsz). 2003;62(2):107-12. [PubMed] [Google Scholar]
- 9. Mistry H, Connock M, Pink J, Shyangdan D, Clar C, Royle P, et al. Autologous chondrocyte implantation in the knee: systematic review and economic evaluation. Health Technol Assess. 2017;21(6):1-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mumme M, Barbero A, Miot S, Wixmerten A, Feliciano S, Wolf F, et al. Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet. 2016;388(10055):1985-94. [DOI] [PubMed] [Google Scholar]
- 11. El Sayed K, Haisch A, John T, Marzahn U, Lohan A, Müller RD, et al. Heterotopic autologous chondrocyte transplantation—a realistic approach to support articular cartilage repair? Tissue Eng Part B Rev. 2010;16(6):603-16. [DOI] [PubMed] [Google Scholar]
- 12. Wong CC, Chen CH, Chiu LH, Tsuang YH, Bai MY, Chung RJ, et al. Facilitating in vivo articular cartilage repair by tissue-engineered cartilage grafts produced from auricular chondrocytes. Am J Sports Med. 2018;46(3):713-27. [DOI] [PubMed] [Google Scholar]
- 13. Özücer B, Dinç ME, Paltura C, Koçak I, Dizdar D, Çörtük O, et al. Association of autologous costal cartilage harvesting technique with donor-site pain in patients undergoing rhinoplasty. JAMA Facial Plast Surg. 2018;20(2):136-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mori R, Kataoka H, Kuriwaka M, Ochi M. Articular cartilage restoration with costal cartilage previously fused with bone. Clin Orthop Relat Res. 2003;(406):262-74. [DOI] [PubMed] [Google Scholar]
- 15. Lee J, Lee E, Kim HY, Son Y. Comparison of articular cartilage with costal cartilage in initial cell yield, degree of dedifferentiation during expansion and redifferentiation capacity. Biotechnol Appl Biochem. 2007;48(Pt 3):149-58. [DOI] [PubMed] [Google Scholar]
- 16. Isogai N, Kusuhara H, Ikada Y, Ohtani H, Jacquet R, Hillyer J, et al. Comparison of different chondrocytes for use in tissue engineering of cartilage model structures. Tissue Eng. 2006;12(4):691-703. [DOI] [PubMed] [Google Scholar]
- 17. Yoon KH, Park JY, Lee JY, Lee E, Lee J, Kim SG. Costal chondrocyte–derived pellet-type autologous chondrocyte implantation for treatment of articular cartilage defect. Am J Sports Med. 2020;48(5):1236-45. [DOI] [PubMed] [Google Scholar]
- 18. Bekkers JE, Inklaar M, Saris DB. Treatment selection in articular cartilage lesions of the knee: a systematic review. Am J Sports Med. 2009;37(Suppl 1):148S-155S. [DOI] [PubMed] [Google Scholar]
- 19. Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75(4):532-53. [DOI] [PubMed] [Google Scholar]
- 20. Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14(11):1119-25. [DOI] [PubMed] [Google Scholar]
- 21. Kowalczuk M, Musahl V, Fu FH. Cochrane in CORR(R): surgical interventions (microfracture, drilling, mosaicplasty, and allograft transplantation) for treating isolated cartilage defects of the knee in adults. Clin Orthop Relat Res. 2018;476(1_suppl):16-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marlovits S, Striessnig G, Resinger CT, Aldrian SM, Vecsei V, Imhof H, et al. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol. 2004;52(3):310-9. [DOI] [PubMed] [Google Scholar]
- 23. Trattnig S, Ba-Ssalamah A, Pinker K, Plank C, Vecsei V, Marlovits S. Matrix-based autologous chondrocyte implantation for cartilage repair: noninvasive monitoring by high-resolution magnetic resonance imaging. Magn Reson Imaging. 2005;23(7):779-87. [DOI] [PubMed] [Google Scholar]
- 24. Adachi N, Ochi M, Deie M, Nakamae A, Kamei G, Uchio Y, et al. Implantation of tissue-engineered cartilage-like tissue for the treatment for full-thickness cartilage defects of the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1241-8. [DOI] [PubMed] [Google Scholar]
- 25. Orth P, Gao L, Madry H. Microfracture for cartilage repair in the knee: a systematic review of the contemporary literature. Knee Surg Sports Traumatol Arthrosc. 2020;28(3):670-706. [DOI] [PubMed] [Google Scholar]
- 26. Bert JM. Abandoning microfracture of the knee: has the time come? Arthroscopy. 2015;31(3):501-5. [DOI] [PubMed] [Google Scholar]
- 27. Goyal D, Keyhani S, Lee EH, Hui JH. Evidence-based status of microfracture technique: a systematic review of level I and II studies. Arthroscopy. 2013;29(9):1579-88. [DOI] [PubMed] [Google Scholar]
- 28. Kraeutler MJ, Belk JW, Purcell JM, McCarty EC. Microfracture versus autologous chondrocyte implantation for articular cartilage lesions in the knee: a systematic review of 5-year outcomes. Am J Sports Med. 2018;46(4):995-9. [DOI] [PubMed] [Google Scholar]
- 29. Saadeh PB, Brent B, Mehrara BJ, Steinbrech DS, Ting V, Gittes GK, et al. Human cartilage engineering: chondrocyte extraction, proliferation, and characterization for construct development. Ann Plast Surg. 1999;42(5):509-13. [PubMed] [Google Scholar]
- 30. Pietila K, Kantomaa T, Pirttiniemi P, Poikela A. Comparison of amounts and properties of collagen and proteoglycans in condylar, costal and nasal cartilages. Cells Tissues Organs. 1999;164(1_suppl):30-6. [DOI] [PubMed] [Google Scholar]
- 31. Tay AG, Farhadi J, Suetterlin R, Pierer G, Heberer M, Martin I. Cell yield, proliferation, and postexpansion differentiation capacity of human ear, nasal, and rib chondrocytes. Tissue Eng. 2004;10(5-6):762-70. [DOI] [PubMed] [Google Scholar]
- 32. Figueroa AA, Gans BJ, Pruzansky S. Long-term follow-up of a mandibular costochondral graft. Oral Surg Oral Med Oral Pathol. 1984;58(3):257-68. [DOI] [PubMed] [Google Scholar]
- 33. Hasegawa T, Yamano Y. Arthroplasty of the proximal interphalangeal joint using costal cartilage grafts. J Hand Surg Br. 1992;17(5):583-5. [DOI] [PubMed] [Google Scholar]
- 34. Raustia A, Pernu H, Pyhtinen J, Oikarinen K. Clinical and computed tomographic findings in costochondral grafts replacing the mandibular condyle. J Oral Maxillofac Surg. 1996;54(12):1393-1400. [DOI] [PubMed] [Google Scholar]
- 35. Kitaoka E, Satomura K, Hayashi E, Yamanouchi K, Tobiume S, Kume K, et al. Establishment and characterization of chondrocyte cell lines from the costal cartilage of SV40 large T antigen transgenic mice. J Cell Biochem. 2001;81(4):571-82. [DOI] [PubMed] [Google Scholar]
- 36. Cotton RT, Myer CM, 3rd, O’Connor DM, Smith ME. Pediatric laryngotracheal reconstruction with cartilage grafts and endotracheal tube stenting: the single-stage approach. Laryngoscope. 1995;105(8 Pt 1):818-21. [DOI] [PubMed] [Google Scholar]
- 37. Yang HC, Cho HH, Jo SY, Jang CH, Cho YB. Donor-site morbidity following minimally invasive costal cartilage harvest technique. Clin Exp Otorhinolaryngol. 2015;8(1_suppl):13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Siegert R. Combined reconstruction of congenital auricular atresia and severe microtia. The Laryngoscope. 2003;113(11):2021-7. [DOI] [PubMed] [Google Scholar]
- 39. Wee JH, Park MH, Oh S, Jin HR. Complications associated with autologous rib cartilage use in rhinoplasty: a meta-analysis. JAMA Facial Plast Surg. 2015;17(1_suppl):49-55. [DOI] [PubMed] [Google Scholar]
- 40. Cervelli V, Bottini DJ, Gentile P, Fantozzi L, Arpino A, Cannatà C, et al. Reconstruction of the nasal dorsum with autologous rib cartilage. Ann Plast Surg. 2006;56(3):256-62. [DOI] [PubMed] [Google Scholar]
- 41. Huh SW, Shetty AA, Kim JM, Cho ML, Kim SA, Yang S, et al. Autologous bone marrow mesenchymal cell induced chondrogenesis for the treatment of osteoarthritis of knee. Tissue Eng Regen Med. 2016;13(2):200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Samuelson EM, Brown DE. Cost-effectiveness analysis of autologous chondrocyte implantation: a comparison of periosteal patch versus type I/III collagen membrane. Am J Sports Med. 2012;40(6):1252-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Table for Costal Chondrocyte–Derived Pellet-Type Autologous Chondrocyte Implantation versus Microfracture for Repair of Articular Cartilage Defects: A Prospective Randomized Trial by Kyoung-Ho Yoon, Jae Doo Yoo, Chong-Hyuk Choi, Jungsun Lee, Jin-Yeon Lee, Sang-Gyun Kim and Jae-Young Park in CARTILAGE