Abstract
Objective
To create a treatment algorithm for focal grade 3 or 4 cartilage defects of the knee using both classic and novel cartilage restoration techniques.
Design
A comprehensive review of the literature was performed highlighting classic as well as novel cartilage restoration techniques supported by clinical and/or basic science research and currently being employed by orthopedic surgeons.
Results
There is a high level of evidence to support the treatment of small to medium size lesions (<2-4 cm2) without subchondral bone involvement with traditional techniques such as marrow stimulation, osteochondral autograft transplant (OAT), or osteochondral allograft transplant (OCA). Newer techniques such as autologous matrix-induced chondrogenesis and bone marrow aspirate concentrate implantation have also been shown to be effective in select studies. If subchondral bone loss is present OAT or OCA should be performed. For large lesions (>4 cm2), OCA or matrix autologous chondrocyte implantation (MACI) may be performed. OCA is preferred over MACI in the setting of subchondral bone involvement while cell-based modalities such as MACI or particulated juvenile allograft cartilage are preferred in the patellofemoral joint.
Conclusions
Numerous techniques exist for the orthopedic surgeon treating focal cartilage defects of the knee. Treatment strategies should be based on lesion size, lesion location, subchondral bone involvement, and the level of evidence supporting each technique in the literature.
Keywords: articular cartilage, cartilage repair, cartilage transplantation
Introduction
Cartilage surgery is indicated in patients with symptomatic defects, with the short-term goal of improvements in pain and function, and the long-term hope of delaying the need for arthroplasty. 1 While focal cartilage defects are common and may be present in as much as 63% of the general population and 36% of athletes, the majority are not symptomatic.2,3 Larger defects, however, can become problematic, especially in young patients who hope to maintain a physically active lifestyle without debilitating pain. Cartilage defects may also lead to accelerated wear, worsening pain, and potential arthritis progression. For example, competitive athletes (high school or collegiate) may be at increased risk of high-grade multicompartment or anterior compartment defects in comparison to recreational athletes. 4 The high prevalence and high cost of chondral disease, which ranges from small focal defects to end-stage osteoarthritis, has significant financial implications for the health care system. The responsible application of resources for the treatment of cartilage disease therefore should take into account lifetime costs, opposed to limiting ones focus on per procedure cost.
Classic cartilage repair and restoration techniques including marrow-stimulation techniques (MST) such as microfracture (MFx) and subchondral drilling, osteochondral allograft transplantation (OCA), osteochondral autograft transfer (OAT), and autologous chondrocyte implantation (ACI) have been have been performed for decades;5,6 however, in recent years, numerous novel strategies and products for the treatment of cartilage defects have been brought on to the market. The purpose of this article is to provide an overview of both classic and new cartilage restoration procedures including their mechanisms of action, surgical techniques, overview of results, advantages and disadvantages, costs, and commercial availabilities. Finally, based on the current evidence presented and the authors’ experience, we propose an up-to-date comprehensive algorithm that integrates new technologies to help guide treatment choices when dealing with focal cartilage defects of the knee.
Overview of the Techniques
The classic procedures described herein include the following: MST, matrix-induced autologous chondrocyte implantation (MACI), OAT, and OCA. Please see the appendix for a detailed description of each classic cartilage procedure with supporting evidence. The novel procedures we will describe below include micronized cartilage extracellular matrix (Biocartilage), Autologous Matrix-Induced Chondrogenesis (AMIC), bone marrow aspirate concentrate (BMAC) implantation, particulated juvenile allograft cartilage (PJAC, DeNovo), particulated autologous cartilage implantation (PACI), viable cartilage allograft putty (CartiMax), cryopreserved viable osteochondral allograft (CVOCA; Cartiform and Prochondrix), aragonite biphasic osteochondral scaffolds (Agili-C), and human umbilical cord blood-derived mesenchymal stem cells (CARTISEM).
Micronized Cartilage Extracellular Matrix (Biocartilage)
Micronized cartilage extracellular matrix, known commercially as Biocartilage, is frequently utilized as an augment to MST. Biocartilage is a desiccated acellular particulated cartilage allograft used as a bioactive scaffold to treat focal cartilage defects. It contains type II collagen, proteoglycans, and chondral growth factors. The material is generally mixed with platelet-rich plasma (PRP) or bone marrow aspirate concentrate (BMAC), applied to the prepared contained cartilage defect and sealed with fibrin glue. It has been postulated that the extracellular matrix elements in the Biocartilage promote the mesenchymal progenitor cells released from the MST and/or present in BMAC to differentiate along a chondral lineage resulting in new hyaline cartilage. 7 Furthermore, a recent in vitro study by Commins et al. showed that Biocartilage supported mesenchymal progenitor cell and chondrocyte adhesion and contained 254 proteins, many of which were anabolic toward cartilage. 8
The procedure can be performed either open or arthroscopically. An initial diagnostic arthroscopy is often performed followed by defect preparation and MST according to the standard protocol previously described. 9 The defect must be thoroughly dried. The implant is then mixed with an autologous blood solution in a 1:0.8 ratio, and the slurry is then introduced into the defect either manually if open or by an arthroscopic device that is commercially available with the device kit. The mixture is spread over the entirety of the defect and a fibrin sealant is used to seal the entire defect, especially at the edges. It takes approximately 5 minutes for the mixture to cure, after which the knee is cycled through a range of motion to ensure stability.
To our knowledge, there is no long-term clinical outcome data available regarding the results of Biocartilage use to date, but animal studies have yielded promising results.10,11 Furthermore, small case series in human subjects have reported good to excellent results at 12 months postoperatively for osteochondral defects of the talus and distal tibia.12,13
Autologous Matrix Induced Chondrogenesis
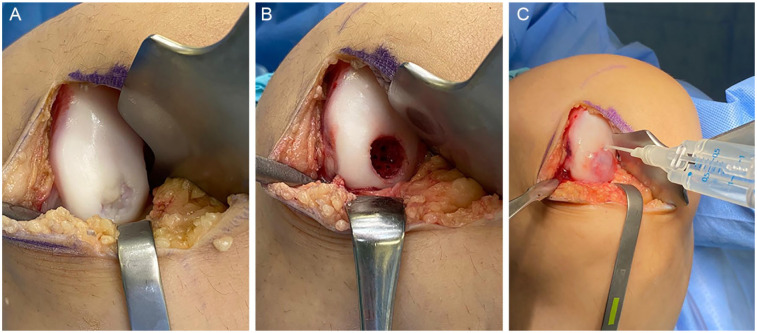
AMIC utilizes MST in combination with a type I/III porcine collagen- or hyaluronan-based membrane (Fig. 1). The patch lies over the MST defect and acts as a scaffold to retain the bone marrow elements and allow them to mature, facilitating their differentiation into chondrogenic cells.14-17
Figure 1.
Autologous matrix-induced chondrogenesis (AMIC). (A) Chondral lesion in the lateral femoral condyle. (B) After debridement, microfracture is performed. (C) Lesion is covered with a collagen membrane and fixed with sutures or fibrin glue.
To perform AMIC, a diagnostic arthroscopy can be performed, but is not required. The procedure is typically performed via a small arthrotomy and MST is performed in standard fashion (Fig. 1). The defect is sized and a template is created from foil or glove paper. The cut-out template is then transferred to the patch, which is sized accordingly. The patch should be slightly undersized so the edges are not proud, which could risk dislodging the patch with knee range of motion. The patch is placed over the defect with the rough, porous side facing the bone, and the patch is fixed with either absorbable sutures or fibrin glue (Fig. 1). After the patch is secured, the knee should be ranged to ensure stability of the patch under direct visualization.
In a recent systematic review, Gao et al. concluded that patients who underwent AMIC for isolated grade IV cartilage defects of the knee (average size 3.6 cm2) reported decreased pain and improved functional outcome scores up to 5 years postoperatively. 18 In a randomized controlled trial (RCT) comparing patients treated with AMIC, to AMIC augmented with BMAC, both groups demonstrated significant improvement in pain and functional outcome scores up to 9 years postoperatively. 19 Zhang et al. reviewed “one-step” cartilage repair techniques and noted improved symptom relief and improved functional outcomes at short-term follow-up and regeneration of hyaline-like cartilage tissue. 20 Though results of AMIC have been promising, current evidence is insufficient to recommend a range of joint-specific defect sizes that may be treated with AMIC based on the limited data available from clinical trials to date.
Bone Marrow Aspirate Concentrate Implantation
The BMAC method entails the harvesting and concentration of mesenchymal progenitor cells, growth factors, and cytokines from autologous bone marrow collected from the patient which is then injected into a focal chondral defect forming a bioactive clot. Clot stabilization can be augmented via combination with fibrin glue or by placement of a membrane which can be either synthetic, porcine I/III collagen, or hyaluronan based. 21 The membrane acts as a scaffold containing the BMAC and providing an anchor within the defect on which the medicinal signaling cells (MSCs) can attach, proliferate, and differentiate. Alternatively, when performed in conjunction with AMIC the collagen scaffold may be soaked in BMAC for 10 to 15 minutes prior to implantation. 22
A diagnostic arthroscopy can be performed but is not required. The BMAC implantation procedure is performed via an open or arthroscopic approach. Bone marrow aspirate can be harvested from a variety of sites including the iliac crest (anterior or posterior), the distal femur (supracondylar region or arthroscopically through the intercondylar notch), the proximal tibia, or the calcaneus. Once obtained, the aspirate is prepared (concentrated) according to each system’s protocol. Starting volumes may vary between systems, but typically approximately 60 mL is desired, which is concentrated to 5 to 10 mL. An arthrotomy or a mini-arthrotomy is performed as required by the defect, and the defect is prepared in the standard manner for cartilage restoration procedures. If an osteochondral defect is present, autogenous bone grafting may be performed prior to BMAC implantation. If performed arthroscopically, it is important that all fluid be removed from the knee prior to BMAC implantation. Once the defect site is dry, the activated clot is transferred into the defect and secured with fibrin glue. A membrane may be placed over the defect (rough/porous side facing the bone) and secured with absorbable suture or fibrin glue. After curing of the glue, the knee should be taken through a range of motion to ensure stability of the BMAC clot and/or patch.
The use of BMAC for the treatment of focal articular cartilage defects has yielded inconsistent clinical results in the literature, some of which may be due to the different preparations that yield different BMAC products. Dragoo and Guzman compared 3 separate commercially available BMAC preparation systems (Harvest, Biomet, Arthrex) and showed that each produced BMAC with similar overall cellular consistency (white blood cell count, platelet count, CD34+ cell count, etc.) but in varying concentrations. 23
In their systematic review of BMAC for the treatment of cartilage defects, which included both animal and clinical human studies, Cavinatto et al. concluded that BMAC treatment leads to improved short-term and midterm outcomes. 24 BMAC has demonstrated improved outcomes when compared to MFx and equivalent outcomes to MACI. 24 However, the quality and quantity of data on the use of BMAC for chondral defects is limited.
Particulated Juvenile Allograft Cartilage
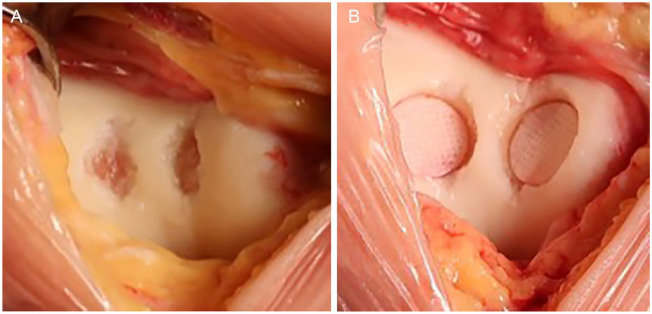
PJAC, known commercially as DeNovo NT, utilizes juvenile hyaline cartilage obtained from young donors (Fig. 2). Immature chondrocytes have been shown to have increased proliferative activity, cell density, and metabolic activity when compared to adult chondrocytes.25-29 Once packaged, the cells are viable for up to 45 days.
Figure 2.
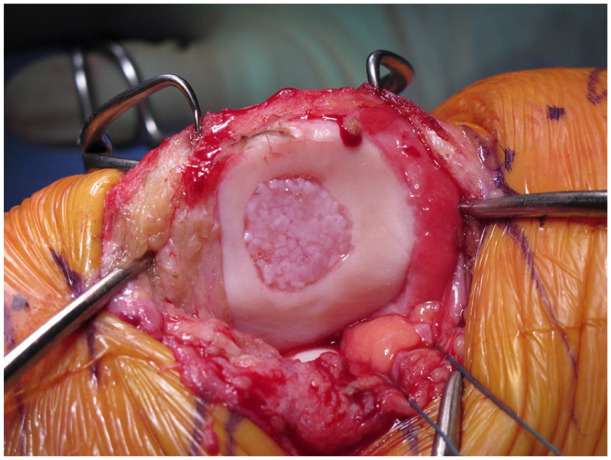
Particulated juvenile allograft cartilage (DeNovo NT) applied directly to a patellar lesion. The pieces are arranged in one layer and close together (touching or almost touching). Fibrin glue is added. The whole implant is recessed below the margins of the defect (1 mm).
Some surgeons may prefer to perform PJAC implantation as a staged procedure with an initial diagnostic arthroscopy to thoroughly assess the joint, whereas others may elect to have the graft available at the time of the initial surgery. The decision to proceed with open versus arthroscopic implantation is up to the surgeon’s discretion depending on defect location and size. The defect is prepared in the standard manner, sized, and the appropriate number of allograft packets is determined. After the defect area has been calculated, the number of packets can be estimated, as one packet of PJAC covers approximately 2.0 to 2.5 cm2. If significant bone loss is present, bone grafting should be performed so that the subchondral bone bed is within 5 mm of the level of the adjacent healthy subchondral bone. The allograft can be prepared for implantation either directly inside the defect or on the back table through the use of a mold. The pieces of PJAC should be arranged close together in a single layer (Fig. 2). The fluid medium should be removed from the allograft taking care to not discard any pieces of juvenile cartilage. Next, fibrin glue is added to the base of the defect and the PJAC is added to the defect. The allograft should fill at least 50% of the total surface area of the defect and should be recessed below the shoulders of the defect by approximately 1 mm. Thumb pressure is used to hold the implant firmly against the glue to ensure good adherence. A Freer elevator may be used to help spread the allograft evenly over the defect as it continues to harden. Five to 10 minutes should be allowed for the fibrin glue to cure. When prepared on the back table, fibrin glue is added to the cartilage fragments and allowed to cure. The implant is then glued into the defect with fibrin glue placed at the base. Implantation can also be performed arthroscopically using the same concepts as previously described in the open method. An arthroscopic introducer can be used to deliver the allograft tissue to the defect. If performed arthroscopically, all fluid should be removed from the joint so that implantation may be performed in a dry environment.
No long-term or randomized clinical data are currently available following PJAC use in the human knee. However, short-term results in the patella, trochlea, and femoral condyle have demonstrated significantly improved pain and function, good fill of the defect with normal appearing articular cartilage on MRI (magnetic resonance imaging), and a combination of hyaline and fibrocartilage on histological analysis.30-32
Particulated Autologous Cartilage Implantation
Use of PACI is similar to PJAC in that it uses particulated cartilage; however, in this technique, the tissue used is obtained autologously from the patient during the same surgical procedure. The first report of this technique was in the German literature in 1983. 33 Later in the United States, DePuy-Mitek created a proprietary cartilage autograft implantation system (CAIS) to automate the mincing process. An initial study approved by the Food and Drug Administration (FDA) comparing CAIS to MFx showed superior results in the CAIS group. 34 However, further studies were discontinued due to slow patient enrollment, and the device was eventually removed from development. 31 However, the technique can still be utilized without the use of a proprietary system.
A first stage diagnostic arthroscopy can be performed but is not required. Using curettes and/or gouges, 200 to 300 mg of cartilage without bone is harvested from the surgeon’s location of choice—typically the intercondylar notch, medial femoral trochlear ridge, or lateral femoral trochlear ridge. 35 The harvested cartilage is fragmented on the back table with a fresh blade, creating fragments no larger than 1 mm in each dimension. This is made into a paste-like consistency. An arthrotomy is performed to expose the defect, which is then prepared in the standard fashion. The defect is templated, and a commercially available patch (either synthetic, porcine I/III collagen, or hyaluronan based) is cut according to the defect. A thin layer of fibrin glue is placed into the defect after hemostasis has been achieved and the cartilage paste is applied evenly across the defect. A second layer of fibrin glue is applied to secure the cartilage, and then this construct is secured with the patch to the top of the defect using small absorbable sutures and fibrin glue around the periphery. The knee is cycled to ensure stability, and then the arthrotomy is closed in standard fashion.
As with PJAC, no long-term clinical data are currently available following PACI in the human knee. However, as mentioned above, an initial pilot study of PACI (in the form of CAIS) demonstrated favorable outcomes compared to MFx, including better patient-reported outcome scores with decreased formation of intralesional osteophytes at 2 years. 34 While the commercial form of PACI (CAIS) is no longer available, this technique can still be utilized without the use of a proprietary system. In fact, Massen et al. in a study of 27 patients who underwent PACI using a freehand scalpel mincing technique for small to medium sized (1-6 cm2) chondral or osteochondral lesions of the femoral condyles, trochlea, or patella showed significant decreases in pain and increases in physical function compared to baseline at a mean follow-up of 28.3 ± 3.8 months. 36 Furthermore, 18 out of the 27 patients had cartilage lesions >2 cm2 with the same number having lesions of the patella meaning that PACI may be a good alternative to MACI/ACI in patients with large patellar lesions who are restricted by cost or health insurance coverage. 36
Viable Cartilage Allograft Putty (CartiMax)
Viable cartilage allograft putty, available commercially as CartiMax, is a new technology that has been created from a joint effort between MTF Biologics and CONMED. This technology utilizes viable cartilage flakes from allograft donor tissue and combines these with lyophilized extracellular matrix, resulting in a putty that can be molded to fit defects of any shape. Per the manufacturers, CartiMax can be utilized for full-thickness cartilage defects up to 5 cm2 in size and has a shelf-life of up to 12 months after cryopreservation.
CartiMax can be utilized in a single-stage procedure. Typically, an arthrotomy is made to expose the articular cartilage defect via an open approach. The defect is prepared in the standard fashion. The solution containing the chondrocytes is thawed, the liquid is drained, and the fibers are washed with normal saline. The cartilage allograft matrix (CAM) is then mixed with the cartilage fibers containing the live chondrocytes to form a putty. The defect is dried, and the putty is added, which can be molded to fit the appropriate defect shape and depth, and typically no sealant or fibrin glue is required.
CartiMax is a new technology, and no clinical outcomes regarding its efficacy have been published to date.
Cryopreserved Viable Osteochondral Allograft (Cartiform and Prochondrix)
A CVOCA is composed of a thin disc that contains hyaline cartilage with its native architecture on top of a microscopic layer of bone. Chondrogenic growth factors, extracellular matrix, and viable chondrocytes are contained in the scaffold. The scaffold is perforated, making it both pliable for contouring along curved surfaces, as well as increasing the surface area for outgrowth of chondrocytes from the matrix (Fig. 3). It is preserved at −80 °C. 37 CVOCA can be used in conjunction with MST or BMAC. 38 There are currently 2 commercially available options, Cartiform and Prochondrix.
Figure 3.
Cryopreserved viable osteochondral allograft (CVOCA; Cartiform). (A) Cartilage defect in the trochlea after debridement. (B) Cartiform implant. (C) The implant is fixed with small suture anchors on the edges of the defect.
As with other cartilage restoration procedures, a diagnostic arthroscopy can be performed, but is not required. The procedure is performed via an open approach. The defect is prepared in the standard manner. Prochondrix has a special kit for preparation. The size of the defect is determined, and the appropriately sized CVOCA packet(s) is (are) selected and allowed to thaw. Cartiform is available in 10 mm and 20 mm diameter discs and 12 × 19 mm and 20 × 25 mm wafers. Prochondrix is available in discs of 2 mm increments from 9 to 17 mm and 20 mm. The allograft is thawed in saline. A template is created and the CVOCA is cut to size. The bone layer side should be placed toward the bony bed of the defect. MST can be performed if desired. The whole implant needs to be directly opposed to the bony surface; thus, defects on a concave surface, such as the trochlea, need a centrally placed anchor to draw the implant into contact with the bone. Conversely, on convex surfaces, the periphery should be fixed with sutures in order to anchor the implant into the surrounding cartilage (Fig. 3). BMAC can be applied if desired. Once adequate fixation is achieved, a thin layer of fibrin glue is applied to the periphery. This should be allowed to cure for a minimum of 5 minutes, and then the knee is taken through a range of motion to ensure stability of the implant.
Outcomes of Cartiform and Prochondrix are limited, and the current literature consists primarily of laboratory data and small case series. Small case series for both have demonstrated good outcomes at 2 years without adverse outcomes or early failures.38,39 Further clinical studies are required to better understand the long-term outcomes of CVOCA.
Aragonite Biphasic Osteochondral Scaffolds (Agili-C)
Agili-C is a crystalline osteoconductive aragonite biphasic scaffold, produced from a coralline exoskeleton, that is currently under clinical trials in the United States for the treatment of focal osteochondral lesions of the knee (femoral condyles/trochlea) (relation to the cartilaginous surface (Fig. 4). It is available in sizes from 6 to 20 mm in diameter, and 8 to 15 mm in length. Preparation is similar to that seen in OCA transplantation. A coring device corresponding to the diameter of the lesion is placed perpendicular to the articular surface and drilled to the desired depth. The tapered Agili-C implant is then inserted into the recipient site in a press-fit manner making sure that the top of the implant is recessed in relation to the cartilaginous surface (Fig. 4). The implant may be soaked in BMAC prior to implantation as an augment.
Figure 4.
Aragonite biphasic osteochondral scaffolds (Agili-C). (A) Chondral defect in the medial and lateral trochlear facets. (B) Implants inserted into the recipient site in a press-fit manner making sure that the top of the implant is recessed in relation to the cartilaginous surface.
In vitro studies using human derived mesenchymal progenitor cells seeded onto the Agili-C scaffold showed increased osteogenic differentiation and proliferation of mesenchymal progenitor cells at the histologic and molecular levels. 40 Furthermore, an ex vivo model comparing donut-shaped cartilage explants of cadaver human articular defects with and without Agili-C implants cultured for 60 days showed that the Agili-C implant contributed to chondrocyte migration and deposition of an extracellular matrix rich in type II collagen and aggrecan. 41 An early clinical study by Kon et al. comparing tapered versus cylindrical Agili-C implants showed improved functional outcome scores (IKDC [International Knee Documentation Committee], Lysholm, and KOOS [Knee Injury and Osteoarthritis Outcome Score]) and MRI findings of bony integration and cartilage regeneration at 12 months postoperative. 42 However, this same study showed improved longevity with 0 revisions at 12-month follow-up in tapered implants compared to 10.5% in cylindrical implants. 42 The Agili-C implant is currently unavailable for use in the United States, Europe, and Asia pending the results of a recently completed clinical trial in order to support its utility and safety in clinical practice.
Human Umbilical Cord Blood–Derived Mesenchymal Stem Cells (CARTISTEM)
CARTISTEM is allogeneic umbilical cord blood–derived mesenchymal stems cells (hUCB-MSCs) used to treat degenerative or traumatic grade IV cartilage lesions of the knee. The hUCB-MSCs come in a 1.5-mL liquid suspension. This vial is gently tilted in order to evenly distribute the stem cells within the preservative fluid. All 1.5 mL are then injected into a 1.5-mL sodium hyaluronate (HA) suspension. The combined solution is then left to cure for 30 minutes at room temperature. The mixed hUCB-MSCs-HA solution is then transferred to a sterile 5-mL syringe for administration. Multiple 5-mm drill holes, 5 mm in depth and 2 to 3 mm apart, are then created in the area of the cartilage defect. 43 The hUCB-MSC-HA solution is then transplanted into each of the newly created drill holes. Recommended dosage for hUCB-MSCs is 500 µL/cm2 with a cell concentration of 0.5 × 107 cells per milliliter based on an early animal study. 44
There is currently one published study out of Korea demonstrating safety and efficacy of CARTISTEM for the treatment of Kellgren-Lawrence grade 3 full-thickness cartilage lesions of the knee greater than 2 cm2 without subchondral bone involvement. 43 In this study, 7 patients were divided into 2 groups, A (4 patients) and B (3 patients), based upon the dose of hUCB-MSCs used (high or low) in the combined HA solution. Mild adverse reactions including back pain, arthralgias, and elevated antithyroglobulin levels were noted in 5 patients but all resolved. Three-month repeat arthroscopy showed evidence of maturing repair tissue at the hUCB-MSCs-HA application site. Significant improvement in functional outcome scores including the IKDC and VAS (Visual Analogue Scale) were noted at 24 weeks postoperative and were maintained at 7-year follow-up. CARTISEM appears to be a safe and efficacy treatment for full-thickness cartilage lesions of the knee greater than 2 cm2 and is currently approved for use in Korea.
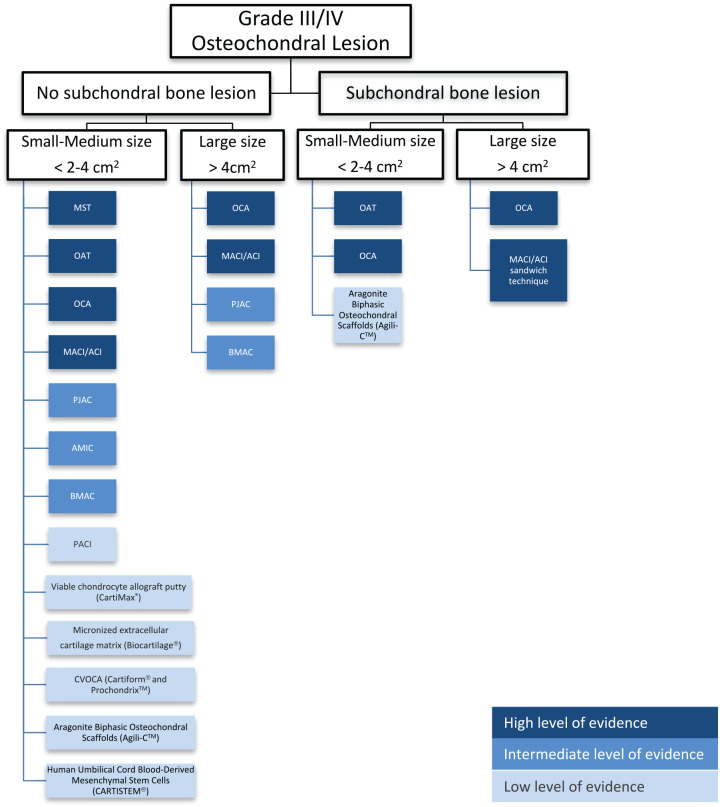
Treatment Algorithm
Prior to any cartilage restoration procedure, it is imperative to define that the lesion is responsible for the patient’s symptoms. In addition, a course of nonoperative treatment should be considered depending on the pathology. For example, chronic degenerative defects in older patients should fail conservative management prior to cartilage repair, while acute large defects in young patients can be considered for repair initially. Conservative care includes weight loss, ice, anti-inflammatories, bracing, physical therapy, and intraarticular injections (corticosteroids, hyaluronic acid, and biologics). Surgery is typically indicated in patients with persistent pain, swelling, or functional limitation in the setting of a focal chondral or osteochondral lesion. Prior to proceeding with surgical intervention, it is important that realistic expectations be set with the patient with regard to surgery, length of recovery, and ultimate functional outcome. Furthermore, the physician should assess the ability of the patient to comply with a lengthy rehabilitation prior to performing surgery.
Concomitant pathology (malalignment, meniscus deficiency, and instability) should be identified and properly addressed prior to or at the same time as the cartilage restoration procedure. Once all other factors have been optimized (or a plan is set for the optimization of these factors), the next step is identifying which cartilage restoration strategy to pursue.
When determining what type of cartilage restoration technique to perform, the most important variables are the condition of the underlying subchondral bone, the size of the defect, and the location of the defect. The subchondral bone is considered deficient when there is bone loss (e.g., traumatic osteochondral defects or osteochondritis dissecans [OCD]), a significant bone lesion (e.g., extensive edema or cystic formation), or if there is previous surgical violation of the subchondral plate (e.g., MST or osteochondral transplantations).
Goals of surgical intervention, including which sports and/or activities the patient wishes to return to and at what level (recreational vs. professional), should also be considered. Unfortunately, the realities of cost and health insurance coverage should also be addressed as this may influence which cartilage restoration procedures are available to the provider and patient. A proposed treatment algorithm is presented in Fig. 5.
Figure 5.
Cartilage restoration algorithm: traditional and new technologies.
MST = bone-marrow stimulation; OAT = osteochondral autograft transfer; AMIC = autologous matrix-induced chondrogenesis, BMAC = bone marrow aspirate concentrate implantation; PJAC = particulated juvenile allograft cartilage; PACI = particulated autologous cartilage implantation; CVOCA = cryopreserved viable osteochondral allograft.
Small-Sized (<2 cm2) and Medium-Sized Defects (2-4 cm2) with Bone Loss/Involvement
OAT and OCA are the osteochondral procedures with the most evidence to support their use in the treatment of small (<2 cm2) and medium (2-4 cm2) sized chondral defects with bony involvement.45-47 Osteochondral procedures such as OCA and OAT replace subchondral bone and are therefore preferred in patients with subchondral bone lesions, particularly when the lesions are located in the femoral condyles. The decision to use OAT versus OCA in the setting of subchondral bone loss/damage is determined by defect size and location. OAT is better suited for small defects (<2 cm2), where 1 or 2 donor plugs are sufficient to cover the defect because using more than 2 donor plugs decreases clinical outcomes and increases donor site morbidity.48,49 OCA can be used in both small and large defects, and is therefore mainly limited by insurance payer guidelines that generally require a minimum size of 2 cm2. However, pre-cut OCA plugs (available in 10 mm, 15 mm, 20 mm diameters) are an option as they are cheaper, more readily available, and result in less wasted tissue than plugs harvested from size- and side-matched hemicondyle allografts. There are currently no studies comparing functional outcomes between patients treated with pre-cut OCA plugs versus those plugs harvested at the time of surgery from size- and side-matched hemicondyles.
In the patellofemoral joint, OCA may be preferable over OAT because OCA enables utilization of a patellar graft from a similar location with a better matching of contour and cartilage thickness. This also avoids the creation of additional donor site morbidity in the treated compartment as the trochlea notch is the primary site for graft harvest of autograft plugs. 50
If OCA or OAT are not possible, ACI/MACI or PJAC can be considered for osteochondral lesions. Osteochondral lesions deeper than 7 to 8 mm should be treated with concomitant bone grafting (sandwich procedure), while shallow lesions such as small OCD lesions can be treated with cartilage resurfacing only. 51
Small-Sized (<2 cm2) and Medium-Sized Defects (2-4 cm2) without Bone Loss/Involvement
In the case of small (<2 cm2) and medium defects (2-4 cm2) without bone involvement, options for treatment include the bone marrow–based techniques (MST, AMIC, and BMAC implantation), ACI/MACI, PJAC, OAT, and OCA (both pre-cut plugs and those harvested from a whole hemicondyle, patella, or trochlea).
Classic MST is the least expensive and a cost-effective treatment in small lesions, <2 cm2.52-55 It has been shown to provide clinical improvements in the short term, but frequently, results deteriorate after 2 years.56-59 Furthermore, studies comparing OAT to MST show that OAT results in better long-term outcomes and increased patient activity levels compared to MST. 45 Evidence from RCTs comparing ACI/MACI and MST are contradictory. Typically, no difference is observed when the study is performed for small defects (<2 cm2) and short-term follow-up (<2 years),56,60-63 whereas better clinical outcomes for ACI/MACI are usually associated with larger defects, patellofemoral lesions, and longer follow-up period (>2 years).58,64-68 Therefore, a subset of young patients with acute, small (<2 cm2), well-shouldered defects in the femoral condyles may be the appropriate candidates for MST treatment.69-71 However, for most patients, we would not recommend MST alone, especially on the patella, which has worse outcomes than other locations. 72 AMIC may also be considered in these patients as it has shown improved pain and functional outcome scores in patients up to 9 years postoperatively compared to MST alone; however, additional RCTs comparing AMIC to other cartilage therapies are needed.19,20 Furthermore, it is important to acknowledge the controversial effect of previous MST on patient outcomes after revision ACI or OCA. Previous studies have shown that failed MST increases the risk of ACI failure but does not influence the results of OCA.73-75 However, a recent study comparing primary and secondary (after failed MST) ACI and OCA showed no difference on outcomes scores between primary ACI and secondary ACI, between primary OCA and secondary OCA, and between secondary ACI and secondary OCA. 76 Therefore, MST can potentially “burn the bridge” of future cell-based therapy treatment in clinical failures resulting in OCA as the preferred treatment in the event of a revision.
For medium-sized lesions (2-4 cm2) of the trochlea and femoral condyles, OCA, OAT, and ACI/MACI are the preferred first choice procedures given the significant level of evidence supporting their use in the literature.32,68,77,78 In RCTs, ACI versus OAT demonstrated similar results in some studies, with slightly better results for ACI in larger defects and better results for OAT in smaller defects.64,79-81Advantages to OAT over ACI/MACI include reduced cost, earlier weight-bearing/mobilization, and faster return to sport.45,52,53 Advantages of ACI/MACI over OAT, even in small lesions, include no donor site morbidity and preservation of the underlying subchondral bone. In addition, the surgeon may consider OCA using pre-cut cores or cores harvested from an allograft hemicondyle. Disadvantages to OCA include the risks associated with the use of allograft tissue as well as the violation of the underlying subchondral bone.
With regard to the patellofemoral joint, OAT can be used in small (<2 cm2) lesions but for the reasons previously mentioned OCA may be preferred in the patellofemoral (PF) joint. First-line treatment options for medium lesions (2-4 cm2) include ACI/MACI and OCA. ACI/MACI has been shown to have excellent short-term, midterm, and long-term clinical outcomes.32,82 The shape of the patella and trochlea are more highly variable than the shape of the femoral condyles and tibial plateaus, which complicates morphology matching, particularly with the involvement of the central trochlear groove and median patellar ridge. For these reasons, ACI/MACI are the most common cartilage restoration procedure performed in the PF joint. 83 Additionally, a meta-analysis revealed that failure rates of OCA (22.7%) are higher than chondrocyte-based therapies (6.8%) in the PF joint. 32
Other options of surface treatments, such as PJAC and BMAC implantation, have good short-term outcomes (2-5 years), but they lack the long-term outcomes evidence that ACI/MACI, OAT, and OCA have. These treatments have the advantages of preserving the subchondral bone and can be used in all knee compartments (femoral condyles, tibia, and PF joint).22,26,30,84-89 Successful results have been reported with >2 years follow-up after PJAC and >5 years follow-up after BMAC implantation in defects with a mean size of 3.5 cm2 and 4 to 6 cm2, respectively.7,20,22,26,30,84
Large-Sized Defects (>4 cm2) with Bone Loss/Involvement
OCA is the preferred cartilage procedure for large chondral defects (>4 cm2) with underlying bone involvement. OCA of the femoral condyles has been shown to be durable in high-demand patients with good to excellent functional outcomes at long-term follow up.90,91 OCA has also been shown to be effective in the treatment of large patellofemoral osteochondral lesions and is preferred over ACI/MACI with or without the sandwich technique. 92 If OCA is not available, ACI/MACI with a sandwich technique (bone grafting of the bony defect and an overlying ACI/MACI) is an alternative solution; however, when subchondral bone lesions are present (e.g., cysts and intralesional osteophytes) cell-based treatments are likely to be less effective.75,93,94 In addition, even when good clinical results are achieved, subchondral changes may still persist for a prolonged period of time.95,96
Large-Sized Defects (>4 cm2) without Bone Loss/Involvement
For the treatment of large chondral defects (>4 cm2) with no bone involvement, surgical options include ACI/MACI, OCA, PJAC, and BMAC implantation. Focal cartilage defects have been treated successfully with long-term follow-up with both OCA and ACI/MACI, especially in the femoral condyles.78,97-101 Results of OCA and ACI/MACI are successful, even in very large defects (>8-10 cm2).98,102 Therefore, in femoral condyle defects, the decision is based on the patient’s activity level and the surgeon’s preference. Multifocal defects in different compartments, defects that need more than one plug (with a “snowman” technique), and PF defects lend themselves to treatment with cell-based modalities (ACI/MACI and PJAC). Multifocal defects in different compartments significantly increase the cost of the procedure since multiple grafts sources will be needed. While there is a financial disadvantage, the clinical outcomes are still favorable. 103 Furthermore, OCA transplantation with the “snowman” technique has had worse outcomes and higher failure rates. 103 Bipolar defects have poorer results than unifocal defects across all treatment options.104-106 More studies are necessary to demonstrate a definitive advantage of one modality over the other; however, ACI/MACI appears to have better outcomes than OCA for bipolar defects.99,104-106 If ACI/MACI and OCA are not available there is some evidence to support the use of surface treatments such as PJAC and BMAC implantation. PJAC has shown early favorable outcomes in its application to chondral lesions of the patellofemoral joint; however, it has a comparatively high cost and is not covered by most insurance providers.25,26 BMAC implantation supplemented with a hyaluronic membrane has also been shown in multiple studies to have favorable clinical outcomes for large chondral lesions (4-6 cm2) of the patellofemoral joint and can be considered an option if the previously discussed treatments are not available.22,107
Additional Treatment Options with No/Minimal Clinical Data Requiring Further Evidence
There are a number of additional cartilage treatment options available on the market to the orthopedic surgeon treating focal cartilage lesions of the knee but with limited data to support their clinical efficacy. These treatments include PACI, micronized cartilage extracellular matrix (Biocartilage), viable cartilage allograft putty (CartiMax), cryopreserved osteochondral allografts (Cartiform/ProChondrix), aragonite biphasic osteochondral scaffolds (Agili-C), and human umbilical cord blood–derived mesenchymal stem cells (CARTISEM).
PACI is cheaper and readily available option for defects <4 cm2; however, clinical data are restricted to one case series.34,36 Biocartilage can potentially improve the quality of cartilage repair as both an augment (i.e., in conjunction with OAT or OCA) and stand-alone treatment but there is currently only one case report to support its use.10,13 MST augmented with Cartiform or ProChondrix not only intends to improve the quality of cartilage repair and midterm results, but also to extend the indications for MST-based procedures to larger defects (between 2 and 4 cm2).37-39 Potential advantages to these grafts compared to traditional fresh OCAs include increased graft availability, reduced graft cost, and reduced retained allograft bone marrow elements. Disadvantages include decreased viable chondrocytes, osteocytes, and chondrogenic growth factors. Currently, there is no literature showing clinical results for the use of CartiMax in focal grade III/IV chondral defects; however, it still remains a potential treatment option based on its promising preclinical data. Potential advantages to this treatment include reduced cost compared to OCA and ACI/MACI, the ability to treat cartilage lesions of variable shapes and sizes due to its moldability, and its “off the shelf” availability. Agili-C has promising initial clinical data from case series but is awaiting more robust results from RCT performed internationally. It has the advantages of being suited to treat chondral and osteochondral lesions with ready availability and avoiding the wait for a matching donor. CARTISEM has the advantage of being a one-time procedure but has very limited preclinical and clinical data.
The authors do not discourage the use of the above-mentioned treatments but the currently limited data to support their use should be considered by the surgeon before implementing them in routine patient care. See Table 1 for a chart depicting the pros and cons of various cartilage procedures as well as their commercial vendors.
Table 1.
Summary of Relevant Factors of Each Cartilage Restoration Technique a .
| Method | Size/Location | Pros | Cons | Commercial Availability |
|---|---|---|---|---|
| Marrow based | ||||
| MFx/Drilling MST | Defect <2 cm2
Femoral condyles |
Single-stage surgery Low cost, easy technique, availability Ability to perform arthroscopically Relatively quick return to play 45 |
Alteration of subchondral architecture Fibrocartilage tissue Conflicting long-term results59,118 Worse results in larger defects58,65,66,67,68,69 Inferior results in the patella 73 |
Manual picks are present in most arthroscopy sets. Also,
available in small versions for small joints. Power options: Arthrex: https://www.arthrex.com/knee/microfracture/products Stryker: http://sportsmedicine.stryker.com/Product/18/0/3/MicroFX Arthrosurface: https://www.arthrosurface.com/products/biologics-overview/nanofx/ |
| Micronized ECM | Defect <2 cm2
Femoral condyles and PF joint |
Single-stage surgery Relatively low cost Ability to perform arthroscopically Off-the-shelf Possibility of better-quality repair tissue versus MST |
Alteration of subchondral architecture Lack of any clinical data |
Arthrex: Biocartilage https://www.arthrex.com/orthobiologics/biocartilage-micronized-cartilage-matrix |
| AMIC | Defect <4 cm2
Femoral condyles and PF joint |
Single-stage surgery Relatively low cost Improved bone marrow elements containment and enhanced cell differentiation versus MST Possibly of better-quality repair tissue versus MFx 16 Good results in the femoral condyles and PF joint 136 |
Disadvantages associated with MFx No long-term data Certain populations may not accept porcine patch due to personal or religious beliefs |
Geistlich Pharma: Chondro-Gide—Porcine I/III
collagen https://www.geistlich-pharma.com/en/orthopaedic/products/chondro-gide/product-range/ BioTissue: Chondrotissue—absorbable polyglycolic acid with hyaluronan http://www.biotissue.de/chondrotissue/patients/chondrotissue/trtreatme-with-chondrotissue/ Anika Therapeutics: Hyalofast—Hyaluronic acid scaffold http://www.anikatherapeutics.com/products/orthobiologics/hyalhyalo/ |
| BMAC implantation | Defect <10 cm2
Femoral condyles and PF joint |
Single-stage surgery Relatively low cost Improved repair tissue compared to microfracture 137 Good results in femoral condyles and PF joint 24 |
Lack of long-term data Questionable results for very large defects 24 Not true hyaline cartilage 137 Certain populations may not accept porcine patch due to personal or religious beliefs |
Geistlich Pharma: Chondro-Gide—Porcine I/III
collagen https://www.geistlich-pharma.com/en/orthopaedic/products/chondro-gide/product-range/ BioTissue: Chondrotissue—absorbable polyglycolic acid with hyaluronan http://www.biotissue.de/chondrotissue/patients/chondrotissue/trtreatme-with-chondrotissue/ Anika Therapeutics: Hyalofast—hyaluronic acid scaffold http://www.anikatherapeutics.com/products/orthobiologics/hyalhyalo/ Arthrex: Angel https://www.arthrex.com/orthobiologics/arthrex-angel-system-for-bone-marrow-concentrate-bmc/products Biomet: BioCUE https://www.zimmerbiomet.com/medicalprofessionals/common/product/biocue-bbma-concentration-system.html Harvest Technologies: Harvest SmartPrep Multicellular Processing System https://www.harvesttech.com/clinician/clinician-home/bmac/product |
| Chondrocyte based | ||||
| ACI/MACI® | No size limits Tibiofemoral joint and PF joint |
Autologous cells No violation of subchondral bone Formation of hyaline-like cartilage 138 Long-term outcome improvement97,98,99 Good results in femoral condyles and PF joint MACI is technically easier in the tibia plateau |
Two-stage surgery High cost Conflicting results in patients with prior marrow stimulation74-76 Certain populations may not accept porcine patch due to personal or religious beliefs |
Vericel: MACI http://www.maci.com/healthcare-professionals/ |
| PJAC | Defect <6 cm2
Femoral condyles and PF joint |
Single-stage surgery Moderate cost Off-the-shelf |
Lack of long-term clinical data Allograft tissue |
Zimmer Biomet: DeNovo NT Natural Tissue
Graft http://www.zimmer.com/medical-professionals/products/biologics-sports-medicine/denovo-nt-natural-tissue.html |
| PACI | Defect <4 cm2
Femoral condyles and trochlea |
Single-stage surgery Relatively low cost Autologous tissue No removal of bone Ability to treat large defects Hyaline-like cartilage |
Lack of long-term clinical data Instrumentation no longer available Technique can still be utilized without the use of a proprietary system |
No proprietary system currently available |
| Viable cartilage allograft | Defect <5 cm2
Femoral condyles and PF joint |
Single-stage surgery Off-the-shelf |
Lack of any clinical data Allograft tissue |
MTF Biologics/CONMED: CartiMax https://www.mtfbiologics.org/cartimax |
| Allogenic stem cell therapy | ||||
| CARTISTEM | Defect <4 cm2
Femoral condyles |
Single-stage surgery | Minimal clinical data Allograft cells |
http://www.medi-post.com/cartistem/ |
| Osteochondral scaffolds | ||||
| CVOCA | Defect <4 cm2
Femoral condyles and trochlea |
Single-stage surgery Moderate cost Off-the-shelf |
Lack of long-term clinical data MST disadvantages if performed |
Osiris Therapeutics: Cartiform https://www.arthrex.com/orthobiologics/cartiform Allosource: Prochondrix https://www.prochondrix.org |
| Agili-C | Defect <4 cm2
Femoral condyles |
Single-stage surgery Moderate cost Off-the-shelf |
Lack of long-term clinical data | https://www.cartiheal.com/agili-c |
| Mature hyaline cartilage | ||||
| OAT | Defect <2 cm2 (1-2 plugs) Femoral condyles |
Ability to perform arthroscopically Relatively low cost Single-stage surgery Autologous Tissue Hyaline cartilage Substitutes damage subchondral bone High rates of return to sports |
Technically demanding to ensure
perpendicularity Donor site morbidity48,49 Restricted to small defects |
Arthrex: OATS https://www.arthrex.com/knee/oats-technique Mitek: COR https://www.depuysynthes.com/hcp/mitek-sports-medicine/products/qs/COR-Precision-Targeting-System |
| OCA | No size limits Femoral condyles and PF joint |
Substitutes damaged subchondral bone No donor site morbidity No size constraint Long-term outcome improvements6,47,91,131Mature hyaline cartilage |
High cost Allograft tissue Usually 2-stage procedure Limited availability of grafts |
Arthrex: Allograft OATS https://www.arthrex.com/orthobiologics/allograft-oats-large RTI: Precision Allograft Cartilage Kit (PACK) http://www.rtix.com/en_us/products/product-implant/precision-allograft-cartilage-kit-pack- MTF: Musculoskeletal Transplant Foundation www.MTF.org JRF: http://jrfortho.org RTI: http://www.rtix.com/en_us/products/category/sports-medicine Lifenet: https://www.lifenethealth.org/fresh-osteochondral-allografts |
| OCA pre-cut plugs | Smaller lesions (<2 cm2) Limited graft sizes 10 × 12 mm 15 × 12 mm 20 × 12 mm Femoral condyle lesions only |
Substitutes damaged subchondral bone No donor site morbidity Mature hyaline cartilage |
Lower cost than traditional OCA Allograft tissue Can be performed as a one-stage procedure Grafts are readily available |
JRF: http://jrfortho.org |
MFx = microfracture; MST = marrow-stimulation techniques; MACI = matrix autologous chondrocyte implantation; OAR = osteochondral autograft transplant; OCA = osteochondral allograft transplant.
The new procedures are micronized cartilage extracellular matrix, autologous matrix induced chondrogenesis (AMIC), bone marrow aspirate concentrate (BMAC) implantation, particulated juvenile allograft cartilage (PJAC), particulated autologous cartilage implantation (PACI), viable cartilage allograft, and cryopreserved viable osteochondral allograft (CVOCA).
Return-to-Sport Expectations
Patient expectations regarding their desired activity level and timeline to return to sport should be considered, especially in patients involved in competitive or professional athletics. In a meta-analysis, Krych et al. found that the return-to-sport rate following cartilage restoration procedures was 76% overall, with the highest rates of return after OAT (93%), followed by OCA (88%), ACI (82%), and MFx (58%). 45 OAT showed the fastest return-to-sport time (5.2 ± 1.8 months), compared to 9.1 ± 2.2 months for MFx, 9.6 ± 3.0 months for OCA, and 11.8 ± 3.8 months for ACI (P < 0.001). It is important to remember that OAT and MST are typically performed for smaller defects, while OCA and ACI are reserved for larger defects. Pestka et al. evaluated the factors that influenced return to sport in 130 patients after ACI. 108 Neither defect location nor size significantly influenced return to physical activity. Both duration of exercise and number of sessions per week significantly decreased from before to after surgery. High-impact as well as start-stop sports were generally abandoned in favor of endurance and low-intensity exercises. A lifetime level of competitiveness was maintained in 31.3% of cases, while return to elite sports at the time of the survey became highly unlikely (0.8%). Nielsen et al. evaluated highly competitive athletes and well-trained and frequently sporting subjects after OCA, and found that 79% were able to participate in a high level of activity (moderate, strenuous, or very strenuous) postoperatively. 109 Patients who did not return to sport were more likely to be female, to have injured their knee in an activity other than sport, and to have a larger graft size.
Cost and Cost-Effectiveness
Cartilage defects and their treatment are associated with a considerable economic burden. 110 The cost of work time lost due to a knee chondral defect over the 10 years prior to surgery has been estimated at approximately $122,000. 111 The cost of surgical cartilage treatments are variable depending on the procedure chosen and the health care system in which they are administered. In the United States, the average costs, for primary and secondary operations in 2018, were $64,593 for MACI, $24,053 for MFx, $32,035 for OAT, and $74,969 for OCA; in comparison to $50,262 for knee arthroplasty. 55 In the United States, ACI treatment has an estimated average cost of $66,752, which includes the initial consult, follow-up visits, and surgical costs. 110 However, even the most expensive option, ACI, can potentially save over $55,000 in the 10 years after surgery compared with expected costs of continued nonoperative care in one health care system. 111 The National Institute for Health Research from the United Kingdom National Health System presented a detailed cost-effectiveness analysis of ACI and other cartilage treatments reported estimated costs for MACI/ACI (biopsy and grafting), mosaicplasty and MFx at $14,083, $2,639, and $1,405, respectively. 112 However, when using a Markov model to compare MFx to ACI as a primary procedure for a focal cartilage defect of the knee, MFx was found to have a total mean cost of £8,028 with a resulting mean 34.16 QALYs (quality-adjusted life years) compared to ACI with a total mean cost of £22,252 and a resulting mean 35.79 QALYs. 112 When this is extrapolated to a time horizon of 20, 30, 40, and even 50 years postoperatively ACI was shown to be more cost-effective than MFx 55% of the time as long as the payer was willing to pay roughly £20,000 intially. 112 The cost-effectiveness of ACI compared to MFx was further demonstrated when simulating scenarios with the initial cell cost reduced by 25%, 50%, and 75%. In a recent systematic review, Everhart et al. found that the most cost-effective treatments in their base model were MFx when applied to defects <3 cm2 (mean size, 2.6 cm2; estimated cost per QALY, $6,808) and OAT when applied to defects small enough to require no more than 2 plugs (mean size, 2.1 cm2; estimated cost per QALY, $7,370). The least cost-effective treatments in the base model were OCA applied to large patellar defects (mean size, 10.1 cm2; estimated cost per QALY, $24,725) and large bipolar defects (mean size, 19.2 cm2; estimated cost per QALY, $27,081). However, if the estimated improvement in symptoms was decreased by a minimum clinically important amount (16.7 points on the IKDC-S) from the values reported in the included clinical studies, several treatments became cost-ineffective. MFx as initial treatment of defects >3 cm2 became highly cost-ineffective and exceeded $100,000 per QALY ($127,782). Therapies that exceeded $50,000 per QALY were ACI with periosteal cover (mean size, 5.5 cm2; cost, $51,379), OCA for large patellar defects (mean size, 10.1 cm2; cost, $66,975), and OCA for large bipolar defects (mean size, 19.2 cm2; cost, $66,255). MACI remained effective (mean size, 4 cm2; cost, $40,122). 55
These data suggests that if surgeons use cartilage restoration tools judiciously and with ideal indications, they can provide the best results for their patients while maximizing value to the healthcare system at large.
Conclusion
Cartilage defects are a source of significant morbidity for patients. The treatment of cartilage defects is multifactorial and should be formulated on a patient-specific basis. We have presented current strategies available for dealing with these defects, and we propose an algorithm to guide the thought process of the orthopedic surgeon in determining a successful treatment plan based upon the literature.
Appendix
Overview of Cartilage Restoration Techniques
Bone Marrow-based Techniques
The foundation of the techniques described in this section is the utilization of bone marrow elements to stimulate cartilage regeneration. In this section, we will describe the traditional MFx technique as well as those newer therapies commonly done in conjunction with MST such as micronized cartilage extracellular matrix, autologous matrix–induced chondrogenesis, and bone marrow aspirate concentrate.
Marrow-stimulation techniques: microfracture and drilling
The concept of marrow stimulation was first put forth by Pridie in the 1950s, but was expanded upon by Dr. Richard Steadman in the 1980s. 113 The central theory of its mechanism is that a clot forms in the defect from blood and bone marrow released from small perforations created in the subchondral plate of the lesion bed that slowly matures into fibrocartilage. 114
MST can be performed either open or arthroscopically, but the latter has become more common in recent years. The defect is prepared in a standard manner for cartilage restoration. In short, damaged cartilage is debrided in its entirety, along with the calcified cartilage layer, and care is taken to maintain the integrity of the subchondral plate. A knife may be used to create vertical walls in the surrounding healthy cartilage and curettes are used to debride the remaining tissue within the margin of the defect. Following defect preparation, small curved awls or thin wire drills are used to create perforations in the subchondral bone, leaving a bone bridge of 3 to 4 mm between holes. Both awl and drill penetration should be perpendicular to the surface of the defect, progressing from the periphery of the defect toward the center. The perforations in the subchondral bone should extend to a depth of approximately 4 mm for awls, deeper for drilling. If performed arthroscopically, the fluid pressure in the knee should be reduced in order to visualize fat droplets egress from the newly created holes, signifying that the appropriate depth of penetration has been achieved. 9 For MFx, a variety of awl angles can be used ranging from 30° to 90° depending on the geometry of the joint surface. Thirty-degree and 45° awls are typically used in the tibiofemoral compartment and 90° awls can be used for the patella, although the latter has a high risk of creating larger areas of damage to the subchondral plate since the vector of the force is not perpendicular. More recently, studies have demonstrated that smaller diameter perforations using wires create less impaction injury to the surrounding subchondral bone, and therefore, some suggest using wires or narrow proprietary drilling instruments should be used instead of awls. 115
Clinical outcomes after MST are variable in the literature. Short-term outcomes for MST, especially in smaller defects in younger patients, have yielded results comparable to other cartilage restoration techniques. When compared to ACI, MFx patients did not differ significantly in failures or clinical outcomes at 1 to 5 years for lesions between 2.3 and 10 cm2. 116 Riboh et al., in their meta-analysis of cartilage repair procedures, including MFx, also found no difference in reoperation rates at 2 years between procedures. 117 However, MFx had significantly higher reoperation rates at 5 and 10 years compared to advanced repair techniques, including OAT and ACI. 117 In addition, multiple studies have shown that MFx results deteriorate precipitously after 2 years postoperatively especially in elite athletes.59,118 Importantly, a 2016 systematic review by Campbell et al. found that, compared to other cartilage restoration techniques, MFx had the lowest overall rate of return to sport. 119
Chondrocyte-based Techniques
Matrix autologous chondrocyte implantation
In this technique, the patient’s own chondrocytes are harvested, expanded in culture, and applied to the defect on a porcine membrane. The first generation of ACI utilized a periosteal patch harvested from the proximal tibia to cover the chondral defect following chondrocyte implantation; however, this often resulted in patch hypertrophy. Second-generation ACI alleviated this issue by using a porcine type I/III collagen patch. 120 Use of the porcine patch has been shown to be cost-effective compared to using the patient’s own periosteum due to decreased re-operation rates. 110 This second-generation ACI has since been phased out in the United States and replaced by the third generation, MACI®, in which the chondrocytes are seeded on a porcine membrane at the lab prior to clinical use.
A 2-stage surgical approach is necessary to perform MACI. In the first stage, an initial diagnostic arthroscopy and chondral biopsy is performed. About 200 to 300 mg of cartilage is harvested from the intercondylar notch or lateral trochlear ridge and sent to a Good Manufacturing Practice (GMP) laboratory facility for tissue expansion. In vitro cell culture takes place over the course of approximately 3 to 4 weeks (time needed for cell population to reach 15 to 20 million). Alternatively, the cells can be stored for 5 years prior to implantation. The second stage of MACI can be performed open or arthroscopically. The cartilage defects are prepared in the standard manner free-hand or using precontoured guides that come with the system’s instrumentation. Bone grafting can take place using a sandwich technique when bone defects deeper than 7 to 8 mm are present. 51 When removed from its container, a notch in the lower left corner of the implant sheet indicates that the cell side (rough side) is facing up. The patch is sized to fit the chondral defect by using the same precontoured guide used to prepare the cartilaginous surface. If no guide is available, a piece of glove paper or foil may be used to create a template of the defect which can then be used to trim the MACI implant to the appropriate dimensions. Once the bony bed is dried and hemostasis has been achieved, a thin layer of fibrin glue is added to the bottom of the defect and the implant is applied to the defect while ensuring that the cell side is facing the bone. Fibrin glue is then added along the edges of the membrane. Gentle thumb pressure may be applied for 3 to 5 minutes. In certain scenarios, such as uncontained large defects (>10 cm2), small absorbable sutures or anchors can be used to secure the patch to the neighboring cartilaginous surface.
Autologous chondrocyte implantation has demonstrated promising outcomes, particularly in larger cartilage defects. In the Superiority of MACI Implant to Microfracture Treatment (SUMMIT) randomized controlled clinical trial, MACI demonstrated significantly better clinical outcomes in treating larger cartilage defects (>3 cm2) than MFx with a similar safety profile. 69 In their review of the different generations of ACI, Samsudin et al. did not demonstrate superiority of one generation over the others, but suggested that ACI may be most appropriate for patients with larger defects or those who have failed other cartilage restoration procedures. 121 Conversely, a systematic review by Lamplot et al. of treatment of failed articular cartilage procedures demonstrated inferior outcomes in ACI after a failed cartilage procedure compared to primary ACI, especially when the index procedure compromises the subchondral bone as in MST. 76 ACI is the most commonly performed procedure in the PF joint; it is also performed in larger lesions and has similar patient-reported outcomes (PROs) to other cartilage procedures performed in smaller lesions. 32
Hyaline Cartilage
Osteochondral autograft transfer
OAT utilizes a patient’s own bone and native, mature hyaline cartilage, transferring this tissue from a relatively low load-bearing area to repair a defect in a higher load-bearing area.
A first stage diagnostic arthroscopy can be performed but is not required. The procedure can be performed via a mini-open incision or arthroscopically. The defect is visualized and sized with sizing tamps. Ensuring perpendicularity is key to the successful execution of this procedure, and, therefore, is often more successfully achieved through an open approach. 122 The appropriate harvester is used to obtain tissue from the donor site. Donor sites include the lateral trochlea above the sulcus terminalis, the intercondylar notch, and the medial trochlear ridge. The lateral trochlea is most commonly used, even though some studies have shown lower contact pressures medially; however, the medial trochlea is smaller thus providing less tissue for harvest.50,123 In addition, harvesting from the intercondylar notch has been shown to create donor plugs with less perpendicularity between the cartilage and underlying subchondral bone. 124 The donor extractor is malleted to an approximate depth of 10 to 15 mm, which should be confirmed upon extraction. Attention is then turned to the recipient site. The key concept is that perpendicularity is maintained. The socket must exactly match the depth of the harvested plug to allow flush seating. An alignment rod is used to confirm recipient socket depth and to gauge orientation. It may also help dilate the recipient socket. The donor graft is then transferred to the recipient site using the proprietary transfer device. Ideally, gentle pressure (manually with a finger if open, or gently with a tamp if arthroscopic) should be used to seat the graft. The ideal scenario is flush seating. If it is not possible to seat the graft perfectly flush, it is better for the graft to be slightly recessed than proud, as studies have shown that the graft being 1 mm proud equates to a 21% increase in peak contact forces. 125 Conversely, the plug being 2 mm recessed may result in cartilage necrosis or fibrocartilage overgrowth. 126 If the defect is large enough, mosaicplasty can be performed using similar concepts for each plug harvest and transfer. However, use of more than 2 large plugs is less desirable, because it is technically challenging and increases donor site morbidity. For mosaicplasty, surgeons should initially insert the plugs at the periphery of the defect and gradually work toward the center. Recent studies have shown that backfill of the donor site is recommended for plugs >6 mm in diameter. 127 This can be done with commercially available pre-cut osteochondral plugs or fresh osteochondral allograft plugs harvested from a hemicondyle.
OAT has shown overall good longevity, with 10-year survivorship being upwards of 70%.48,49 It has also shown the quickest return to sport of the most popular chondral restorative techniques.45,46 A randomized control trial showed superiority of OAT over MFx in terms of pain and functional outcome scores in young athletes. 54 In a midterm meta-analysis compared to MFx, OAT showed a statistically significant improvement in activity level and lower rates of failure for defects over 3 cm2. 128
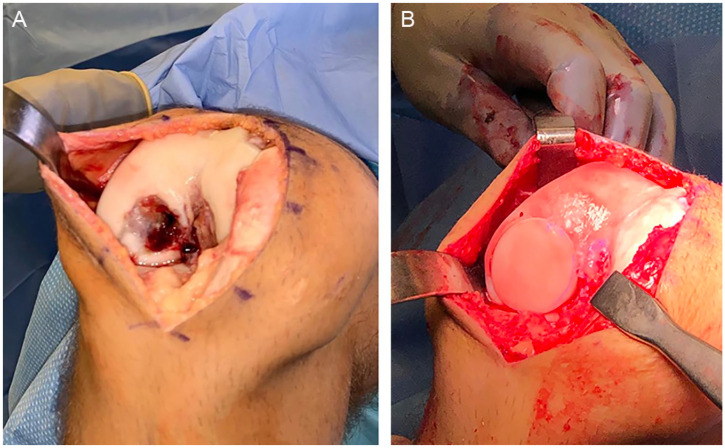
Osteochondral allograft transplantation
OCA utilizes bone and hyaline cartilage from a recently diseased donor within 28 days from harvest (Fig. 6). It can be used in the form of smaller plugs similar to OAT that are pre-cut by the distributing company, or larger plugs fashioned by the surgeon with proprietary instrumentation the day of surgery from a size-matched donor hemicondyle, trochlea, or patella to fill large focal osteochondral defects. Pre-cut cadaver OCA plugs are available in 10 × 12 mm, 15 × 12 mm, and 20 × 12 mm plugs from JRF Ortho (Centennial, CO). The shell technique is an alternative to address very large defects, especially in the patellofemoral joint, in which the surgeon free hand cuts both recipient and donor sites.
A first stage arthroscopy can be utilized for diagnostic purposes and to adequately measure the size of the defect(s). If a pre-cut OCA plug will be used then arthroscopy must be performed to ensure proper measurement of the lesion size and order the appropriate number and sizes of plugs. Pre-cut OCA plugs are implanted per the OAT technique outlined previously. If the needed plug size is in between those that are commercially available a smaller diameter plug may be harvested from a larger diameter plug using a commercially available single-use OAT harvester. 129
Figure 6.
Osteochondral allograft transplantation (OCA). (A) Osteochondral lesion in the lateral femoral condyle. (B) Osteochondral allograft transplanted in the defect.
For larger plugs, an arthrotomy is performed and the defect is exposed and sized with sizing tamps, taking care to ensure perpendicularity. Prior to coring the defect, one should ensure that the available allograft can accommodate the size of the templated defect at the corresponding location. The defect is then cored to an overall (including cartilage thickness) depth of 6 to 8 mm in order to provide the donor plug with a stable recipient site for press-fit fixation. A ruler is used to measure the exact depth at certain positions (typically 12 o’clock, 3, 6, and 9 o’clock) in the prepared recipient site. The donor hemicondyle, trochlea, or patella are then secured in the harvesting jig, and the area corresponding to the defect is marked at the anticipated 12 o’clock position to maintain the proper orientation. This marking should extend from outside the harvested plug to an area onto the plug to ensure that the appropriate contours are restored. A full-thickness plug is harvested while using irrigation to prevent thermal necrosis from reaming. Since the 12 o’clock position is marked, the other positions are determined, and depths are marked at those positions to match the recipient site. A saw is then used to cut along the line created by the depth marks. The edges of the plug can be mildly tapered using a rasp or rongeur to help with seating. Pulse lavage is used to decrease marrow elements in the donor plug. The donor plugs may be soaked in BMAC or PRP prior to implantation. Gentle pressure should be used for seating the graft, ideally with manual pressure and not an impactor to avoid potential chondrocyte death. 130 The plug(s) should not be left proud. As with OAT, it is acceptable to be up to 1 mm recessed, although as flush as possible is ideal (Fig. 6).
In the shell technique, the chondral defect area is removed along with bone using an oscillating saw with a free-hand style and a matching graft is created using a similar cut, again, by a free-hand technique. The graft is secured with metal or resorbable compression screws rather than pins.
Many studies have shown good long-term outcomes following OCA.47,131,132 Good results have been shown in high-demand populations.91,92,109,133 OCA has also proved very effective in the revision setting.134,135 Recently, OCA has been utilized successfully in the patellofemoral joint when concomitant pathologies (i.e., malalignment, soft tissue imbalances) have been appropriately addressed. 32 One systematic review that reported on results of 19 studies with a total of 1036 patients showed a mean survival rate of 86.7% at 5 years, 78.7% at 10 years, 72.8% at 15 years, and 67.5% at 20 years. 101
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Evan E. Vellios  https://orcid.org/0000-0002-9917-3934
https://orcid.org/0000-0002-9917-3934
Jacob G. Calcei  https://orcid.org/0000-0002-4895-1046
https://orcid.org/0000-0002-4895-1046
Tiago L. Fernandes  https://orcid.org/0000-0002-6665-3608
https://orcid.org/0000-0002-6665-3608
References
- 1. Farr J, Gomoll AH. 2016 barriers to cartilage restoration. J Clin Orthop Trauma. 2016;7(3):183-6. doi: 10.1016/j.jcot.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perera JR, Gikas PD, Bentley G. The present state of treatments for articular cartilage defects in the knee. Ann R Coll Surg Engl. 2012;94(6):381-7. doi: 10.1308/003588412x13171221592573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flanigan DC, Harris JD, Trinh TQ, Siston RA, Brophy RH. Prevalence of chondral defects in athletes’ knees: a systematic review. Med Sci Sports Exerc. 2010;42(10):1795-801. doi: 10.1249/MSS.0b013e3181d9eea0 [DOI] [PubMed] [Google Scholar]
- 4. Everhart JS, Boggs Z, DiBartola AC, Wright B, Flanigan DC. Knee cartilage defect characteristics vary among symptomatic recreational and competitive scholastic athletes eligible for cartilage restoration surgery. Cartilage. Epub Mar 3, 2019. doi: 10.1177/1947603519833144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gomoll AH, Lattermann C, Farr J. General treatment algorithm for cartilage defects. Cartil Restor. 2013:39-49. doi: 10.1007/978-1-4614-0427-9_4 [DOI] [Google Scholar]
- 6. Gomoll AH, Filardo G, de Girolamo L, Espregueira-Mendes J, Marcacci M, Rodkey WG, et al. Surgical treatment for early osteoarthritis. Part I: cartilage repair procedures. Knee Surg Sport Traumatol Arthrosc. 2011;20(3):450-66. doi: 10.1007/s00167-011-1780-x [DOI] [PubMed] [Google Scholar]
- 7. Seow D, Yasui Y, Hurley ET, Ross AW, Murawski CD, Shimozono Y, et al. Extracellular matrix cartilage allograft and particulate cartilage allograft for osteochondral lesions of the knee and ankle joints: a systematic review. Am J Sports Med. 2017;46(7):1758-66. doi: 10.1177/0363546517717494 [DOI] [PubMed] [Google Scholar]
- 8. Commins J, Irwin R, Matuska A, Goodale M, Delco M, Fortier L. Biological mechanisms for cartilage repair using a biocartilage scaffold: cellular adhesion/migration and bioactive proteins. Cartilage. Epub Jan 22, 2020. doi: 10.1177/1947603519900803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steadman JR, Rodkey WG, Briggs KK. Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. J Knee Surg. 2002;15(3):170-6. [PubMed] [Google Scholar]
- 10. Fortier LA, Chapman HS, Pownder SL, Roller BL, Cross JA, Cook JL, et al. BioCartilage improves cartilage repair compared with microfracture alone in an equine model of full-thickness cartilage loss. Am J Sports Med. 2016;44(9):2366-74. doi: 10.1177/0363546516648644 [DOI] [PubMed] [Google Scholar]
- 11. Abrams GD, Mall NA, Fortier LA, Roller BL, Cole BJ. BioCartilage: background and operative technique. Oper Tech Sports Med. 2013;21(2):116-24. doi: 10.1053/j.otsm.2013.03.008 [DOI] [Google Scholar]
- 12. Desai S. Treatment of osteochondral lesions of the talus with marrow stimulation and micronized allograft cartilage matrix. Tech Foot Ankle Surg. 2014;13(3):167-73. doi: 10.1097/btf.0000000000000056 [DOI] [Google Scholar]
- 13. Desai S. Surgical treatment of a tibial osteochondral defect with debridement, marrow stimulation, and micronized allograft cartilage matrix: report of an all-arthroscopic technique. J Foot Ankle Surg. 2016;55(2):279-82. doi: 10.1053/j.jfas.2014.07.011 [DOI] [PubMed] [Google Scholar]
- 14. Gille J, Schuseil E, Wimmer J, Gellissen J, Schulz AP, Behrens P. Mid-term results of autologous matrix-induced chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg Sport Traumatol Arthrosc. 2010;18(11):1456-64. doi: 10.1007/s00167-010-1042-3 [DOI] [PubMed] [Google Scholar]
- 15. Benthien JP, Behrens P. Autologous matrix-induced chondrogenesis (AMIC): combining microfracturing and a collagen I/III matrix for articular cartilage resurfacing. Cartilage. 2010;1(1_suppl):65-8. doi: 10.1177/1947603509360044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gille J, Meisner U, Ehlers EM, Müller A, Russlies M, Behrens P. Migration pattern, morphology and viability of cells suspended in or sealed with fibrin glue: a histomorphologic study. Tissue Cell. 2005;37(5):339-48. doi: 10.1016/j.tice.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 17. Kramer J, Böhrnsen F, Lindner U, Behrens P, Schlenke P, Rohwedel J. In vivo matrix-guided human mesenchymal stem cells. Cell Mol Life Sci. 2006;63(5):616-26. doi: 10.1007/s00018-005-5527-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao L, Orth P, Cucchiarini M, Madry H. Autologous matrix-induced chondrogenesis: a systematic review of the clinical evidence. Am J Sports Med. 2017;47(1_suppl):222-31. doi: 10.1177/0363546517740575 [DOI] [PubMed] [Google Scholar]
- 19. de Girolamo L, Schönhuber H, Viganò M, Bait C, Quaglia A, Thiebat G, et al. Autologous matrix-induced chondrogenesis (AMIC) and AMIC enhanced by autologous concentrated bone marrow aspirate (BMAC) allow for stable clinical and functional improvements at up to 9 years follow-up: results from a randomized controlled study. J Clin Med. 2019;8(3):392. doi: 10.3390/jcm8030392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang C, Cai Y, Lin X. One-step cartilage repair technique as a next generation of cell therapy for cartilage defects: biological characteristics, preclinical application, surgical techniques, and clinical developments. Arthroscopy. 2016;32(7):1444-50. doi: 10.1016/j.arthro.2016.01.061 [DOI] [PubMed] [Google Scholar]
- 21. Murphy EP, Fenelon C, McGoldrick NP, Kearns SR. Bone marrow aspirate concentrate and microfracture technique for talar osteochondral lesions of the ankle. Arthrosc Tech. 2018;7(4):e391-e396. doi: 10.1016/j.eats.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gobbi A, Whyte GP. One-stage cartilage repair using a hyaluronic acid–based scaffold with activated bone marrow–derived mesenchymal stem cells compared with microfracture. Am J Sports Med. 2016;44(11):2846-54. doi: 10.1177/0363546516656179 [DOI] [PubMed] [Google Scholar]
- 23. Dragoo JL, Guzman RA. Evaluation of the consistency and composition of commercially available bone marrow aspirate concentrate systems. Orthop J Sport Med. 2020;8(1_suppl). doi: 10.1177/2325967119893634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cavinatto L, Hinckel BB, Tomlinson RE, Gupta S, Farr J, Bartolozzi AR. The role of bone marrow aspirate concentrate for the treatment of focal chondral lesions of the knee: a systematic review and critical analysis of animal and clinical studies. Arthroscopy. 2019;35:1860-77. doi: 10.1016/j.arthro.2018.11.073 [DOI] [PubMed] [Google Scholar]
- 25. Farr J, Cole B, Sherman S, Karas V. Particulated articular cartilage: CAIS and DeNovo NT. J Knee Surg. 2012;25(1_suppl):23-30. doi: 10.1055/s-0031-1299652 [DOI] [PubMed] [Google Scholar]
- 26. Farr J, Yao JQ. Chondral defect repair with particulated juvenile cartilage allograft. Cartilage. 2011;2(4):346-53. doi: 10.1177/1947603511405838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adkisson HD, 4th, Martin JA, Amendola RL, Milliman C, Mauch KA, Katwal AB, et al. The potential of human allogeneic juvenile chondrocytes for restoration of articular cartilage. Am J Sports Med. 2010;38(7):1324-33. doi: 10.1177/0363546510361950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adkisson HD, Gillis MP, Davis EC, Maloney W, Hruska KA. In vitro generation of scaffold independent neocartilage. Clin Orthop Relat Res. 2001;(391 Suppl):S280-S294. doi: 10.1097/00003086-200110001-00026 [DOI] [PubMed] [Google Scholar]
- 29. Bonasia DE, Martin JA, Marmotti A, Amendola RL, Buckwalter JA, Rossi R, et al. Cocultures of adult and juvenile chondrocytes compared with adult and juvenile chondral fragments. Am J Sports Med. 2011;39(11):2355-61. doi: 10.1177/0363546511417172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang T, Belkin NS, Burge AJ, Chang B, Pais M, Mahony G, et al. Patellofemoral cartilage lesions treated with particulated juvenile allograft cartilage: a prospective study with minimum 2-year clinical and magnetic resonance imaging outcomes. Arthroscopy. 2018;34(5):1498-505. doi: 10.1016/j.arthro.2017.11.021 [DOI] [PubMed] [Google Scholar]
- 31. Riboh JC, Cole BJ, Farr J. Particulated articular cartilage for symptomatic chondral defects of the knee. Curr Rev Musculoskelet Med. 2015;8(4):429-35. doi: 10.1007/s12178-015-9300-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hinckel BB, Pratte EL, Baumann CA, Gowd AK, Farr J, Liu JN, et al. Patellofemoral cartilage restoration: a systematic review and meta-analysis of clinical outcomes. Am J Sports Med. 2020;48(7):1756-72. doi: 10.1177/0363546519886853 [DOI] [PubMed] [Google Scholar]
- 33. Albrecht F, Roessner A, Zimmermann E. Closure of osteochondral lesions using chondral fragments and fibrin adhesive. Arch Orthop Trauma Surg. 1983;101(3):213-7. doi: 10.1007/bf00436773 [DOI] [PubMed] [Google Scholar]
- 34. Cole BJ, Farr J, Winalski CS, Hosea T, Richmond J, Mandelbaum B, et al. Outcomes after a single-stage procedure for cell-based cartilage repair. Am J Sports Med. 2011;39(6):1170-9. doi: 10.1177/0363546511399382 [DOI] [PubMed] [Google Scholar]
- 35. Salzmann GM, Calek AK, Preiss S. Second-generation autologous minced cartilage repair technique. Arthrosc Tech. 2017;6(1_suppl):e127-e131. doi: 10.1016/j.eats.2016.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Massen FK, Inauen CR, Harder LP, Runer A, Preiss S, Salzmann GM. One-step autologous minced cartilage procedure for the treatment of knee joint chondral and osteochondral lesions: a series of 27 patients with 2-year follow-up. Orthop J Sport Med. 2019;7(6). doi: 10.1177/2325967119853773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Geraghty S, Kuang JQ, Yoo D, LeRoux-Williams M, Vangsness CT, Danilkovitch A. A novel, cryopreserved, viable osteochondral allograft designed to augment marrow stimulation for articular cartilage repair. J Orthop Surg Res. 2015;10(1_suppl):66. doi: 10.1186/s13018-015-0209-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vangsness C, Higgs G, Hoffman J, Farr J, Davidson PA, Milstein F, et al. Implantation of a novel cryopreserved viable osteochondral allograft for articular cartilage repair in the knee. J Knee Surg. 2017;31(06):528-35. doi: 10.1055/s-0037-1604138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mehta V. Two year results of a novel 3D fresh osteochondral allograft for focal articular cartilage defects. In: Macau, China; 2018. [Google Scholar]
- 40. Matta C, Szűcs-Somogyi C, Kon E, Robinson D, Neufeld T, Altschuler N, et al. Osteogenic differentiation of human bone marrow-derived mesenchymal stem cells is enhanced by an aragonite scaffold. Differentiation. 2019;107:24-34. doi: 10.1016/j.diff.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 41. Chubinskaya S, Di Matteo B, Lovato L, Iacono F, Robinson D, Kon E. Agili-C implant promotes the regenerative capacity of articular cartilage defects in an ex vivo model. Knee Surg Sport Traumatol Arthrosc. 2019;27(6):1953-64. doi: 10.1007/s00167-018-5263-1 [DOI] [PubMed] [Google Scholar]
- 42. Kon E, Robinson D, Verdonk P, Drobnic M, Patrascu JM, Dulic O, et al. A novel aragonite-based scaffold for osteochondral regeneration: early experience on human implants and technical developments. Injury. 2016;47(Suppl 6):S27-S32. doi: 10.1016/S0020-1383(16)30836-1 [DOI] [PubMed] [Google Scholar]
- 43. Park YB, Ha CW, Lee CH, Yoon YC, Park YG. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl Med. 2017;6(2):613-21. doi: 10.5966/sctm.2016-0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Park YB, Ha CW, Kim JA, Ham WJ, Rhim JH, Lee HJ, et al. Single-stage cell-based cartilage repair in a rabbit model: cell tracking and in vivo chondrogenesis of human umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel composite. Osteoarthritis Cartilage. 2017;25(4):570-80. doi: 10.1016/j.joca.2016.10.012 [DOI] [PubMed] [Google Scholar]
- 45. Krych AJ, Pareek A, King AH, Johnson NR, Stuart MJ, Williams RJ. Return to sport after the surgical management of articular cartilage lesions in the knee: a meta-analysis. Knee Surg Sport Traumatol Arthrosc. 2017;25(10):3186-96. doi: 10.1007/s00167-016-4262-3 [DOI] [PubMed] [Google Scholar]
- 46. Werner BC, Cosgrove CT, Gilmore CJ, Lyons ML, Miller MD, Brockmeier SF, et al. Accelerated return to sport after osteochondral autograft plug transfer. Orthop J Sport Med. 2017;5(4):232596711770241. doi: 10.1177/2325967117702418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Assenmacher AT, Pareek A, Reardon PJ, Macalena JA, Stuart MJ, Krych AJ. Long-term outcomes after osteochondral allograft: a systematic review at long-term follow-up of 12.3 years. Arthroscopy. 2016;32(10):2160-8. doi: 10.1016/j.arthro.2016.04.020 [DOI] [PubMed] [Google Scholar]
- 48. Pareek A, Reardon PJ, Maak TG, Levy BA, Stuart MJ, Krych AJ. Long-term outcomes after osteochondral autograft transfer: a systematic review at mean follow-up of 10.2 years. Arthroscopy. 2016;32(6):1174-84. doi: 10.1016/j.arthro.2015.11.037 [DOI] [PubMed] [Google Scholar]
- 49. Richter DL, Tanksley JA, Miller MD. Osteochondral autograft transplantation: a review of the surgical technique and outcomes. Sports Med Arthrosc Rev. 2016;24(2):74-8. doi: 10.1097/jsa.0000000000000099 [DOI] [PubMed] [Google Scholar]
- 50. Garretson RB, Katolik LI, Verma N, Beck PR, Bach BR, Cole BJ. Contact pressure at osteochondral donor sites in the patellofemoral joint. Am J Sports Med. 2004;32(4):967-74. doi: 10.1177/0363546503261706 [DOI] [PubMed] [Google Scholar]
- 51. Peterson L. Chondrocyte transplantation. In: Jackson D, ed. Master Techniques in Orthopaedic Surgery . Lippincott, Williams, & Wilkins; 2003. [Google Scholar]
- 52. Schrock JB, Kraeutler MJ, Houck DA, McQueen MB, McCarty EC. A cost-effectiveness analysis of surgical treatment modalities for chondral lesions of the knee: microfracture, osteochondral autograft transplantation, and autologous chondrocyte implantation. Orthop J Sport Med. 2017;5(5):232596711770463. doi: 10.1177/2325967117704634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Krych AJ, Harnly HW, Rodeo SA, Williams RJ, 3rd. Activity levels are higher after osteochondral autograft transfer mosaicplasty than after microfracture for articular cartilage defects of the knee. J Bone Joint Surg Am. 2012;94(11):971-8. doi: 10.2106/jbjs.k.00815 [DOI] [PubMed] [Google Scholar]
- 54. Gudas R, Kalesinskas RJ, Kimtys V, Stankevicius E, Toliusis V, Bernotavicius G, et al. A prospective randomizedclinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint in young athletes. Arthroscopy. 2005;21(9):1066-75. doi: 10.1016/j.arthro.2005.06.018 [DOI] [PubMed] [Google Scholar]
- 55. Everhart JS, Campbell AB, Abouljoud MM, Kirven JC, Flanigan DC. Cost-efficacy of knee cartilage defect treatments in the United States. Am J Sports Med. 2020;48(1_suppl):242-51. doi: 10.1177/0363546519834557 [DOI] [PubMed] [Google Scholar]
- 56. Van Assche D, Staes F, Van Caspel D, Vanlauwe J, Bellemans J, Saris DB, et al. Autologous chondrocyte implantation versus microfracture for knee cartilage injury: a prospective randomized trial, with 2-year follow-up. Knee Surg Sport Traumatol Arthrosc. 2009;18(4):486-95. doi: 10.1007/s00167-009-0955-1 [DOI] [PubMed] [Google Scholar]
- 57. Vanlauwe J, Saris DBF, Victor J, Almqvist KF, Bellemans J, Luyten FP, TIG/ACT/01/2000&EXT Study Group. Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee. Am J Sports Med. 2011;39(12):2566-74. doi: 10.1177/0363546511422220 [DOI] [PubMed] [Google Scholar]
- 58. Saris DBF, Vanlauwe J, Victor J, Almqvist KF, Verdonk R, Bellemans J, et al. Treatment of symptomatic cartilage defects of the knee. Am J Sports Med. 2009;37(1 suppl):S10-S19. doi: 10.1177/0363546509350694 [DOI] [Google Scholar]
- 59. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee. Am J Sports Med. 2009;37(10):2053-63. doi: 10.1177/0363546508328414 [DOI] [PubMed] [Google Scholar]
- 60. Knutsen G, Drogset JO, Engerbresten L, Isaksen V, Ludvigsen TC, Roberts S, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. J Bone Joint Surg Am. 2007;89(10):2105-12. doi: 10.2106/00004623-200710000-00002 [DOI] [PubMed] [Google Scholar]
- 61. Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Ludvigsen TC, Loken S, et al. A randomized multicenter trial comparing autologous chondrocyte implantation with microfracture. J Bone Joint Surg Am. 2016;98(16):1332-9. doi: 10.2106/jbjs.15.01208 [DOI] [PubMed] [Google Scholar]
- 62. Knutsen G, Isaksen V, Johansen O, Drogset JO, Grontvedt T, Solheim E, et al. Autologous chondrocyte implantation compared with microfracture in the knee: a randomized trial. J Bone Joint Surg Am. 2004;86(3):455-64. doi: 10.2106/00004623-200403000-00001 [DOI] [PubMed] [Google Scholar]
- 63. Lim HC, Bae JH, Song SH, Park YE, Kim SJ. Current treatments of isolated articular cartilage lesions of the knee achieve similar outcomes. Clin Orthop Relat Res. 2012;470(8):2261-7. doi: 10.1007/s11999-012-2304-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Crawford DC, DeBerardino TM, Williams RJ, 3rd. NeoCart, an autologous cartilage tissue implant, compared with microfracture for treatment of distal femoral cartilage lesions. J Bone Joint Surg Am. 2012;94(11):979-89. doi: 10.2106/jbjs.k.00533 [DOI] [PubMed] [Google Scholar]
- 65. Basad E, Ishaque B, Bachmann G, Stürz H, Steinmeyer J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sport Traumatol Arthrosc. 2010;18(4):519-27. doi: 10.1007/s00167-009-1028-1 [DOI] [PubMed] [Google Scholar]
- 66. Brittberg M, Recker D, Ilgenfritz J, Saris DBF. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: five-year follow-up of a prospective randomized trial. Am J Sports Med. 2018;46(6):1343-51. doi: 10.1177/0363546518756976 [DOI] [PubMed] [Google Scholar]
- 67. Kon E, Gobbi A, Filardo G, Delcogliano M, Zaffagnini S, Marcacci M. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee. Am J Sports Med. 2009;37(1_suppl):33-41. doi: 10.1177/0363546508323256 [DOI] [PubMed] [Google Scholar]
- 68. Saris D, Price A, Widuchowski W, Bertrand-Marchand M, Caron J, Drogzet JO, et al. ; SUMMIT Study Group. Matrix-applied characterized autologous cultured chondrocytes versus microfracture. Am J Sports Med. 2014;42(6):1384-94. doi: 10.1177/0363546514528093 [DOI] [PubMed] [Google Scholar]
- 69. Weber AE, Locker PH, Mayer EN, Cventanovich GL, Tilton AK, Erickson BJ, et al. Clinical outcomes after microfracture of the knee: midterm follow-up. Orthop J Sport Med. 2018;6(2):2325967117753572. doi: 10.1177/2325967117753572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Steadman JR, Briggs KK, Matheny LM, Guillet A, Hanson CM, Willimon SC. Outcomes following microfracture of full-thickness articular cartilage lesions of the knee in adolescent patients. J Knee Surg. 2015;28(2):145-50. doi: 10.1055/s-0034-1373737 [DOI] [PubMed] [Google Scholar]
- 71. Steadman JR, Hanson CM, Briggs KK, Matheny LM, James EW, Guillet A. Outcomes after knee microfracture of chondral defects in alpine ski racers. J Knee Surg. 2014;27(5):407-10. doi: 10.1055/s-0034-1376330 [DOI] [PubMed] [Google Scholar]
- 72. Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14(11):1119-25. doi: 10.1016/j.joca.2006.05.003 [DOI] [PubMed] [Google Scholar]
- 73. Gracitelli GC, Meric G, Pulido PA, McCauley JC, Bugbee WD. Osteochondral allograft transplantation for knee lesions after failure of cartilage repair surgery. Cartilage. 2015;6(2):98-105. doi: 10.1177/1947603514566298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37(5):902-8. doi: 10.1177/0363546508330137 [DOI] [PubMed] [Google Scholar]
- 75. Lamplot JD, Schafer KA, Matava MJ. Treatment of failed articular cartilage reconstructive procedures of the knee: a systematic review. Orthop J Sport Med. 2018;6(3):23253967118761871. doi: 10.1177/2325967118761871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Riff AJ, Huddleston HP, Cole BJ, Yanke AB. Autologous chondrocyte implantation and osteochondral allograft transplantation render comparable outcomes in the setting of failed marrow stimulation. Am J Sports Med. 2020;48(4):861-70. doi: 10.1177/0363546520902434 [DOI] [PubMed] [Google Scholar]
- 77. Cameron JI, Pulido PA, McCauley JC, Bugbee WD. Osteochondral allograft transplantation of the femoral trochlea. Am J Sports Med. 2015;44(3):633-8. doi: 10.1177/0363546515620193 [DOI] [PubMed] [Google Scholar]
- 78. Pareek A, Reardon PJ, Macalena JA, Levy BA, Stuart MJ, Williams RJ, 3rd, et al. Osteochondral autograft transfer versus microfracture in the knee: a meta-analysis of prospective comparative studies at midterm. Arthroscopy. 2016;32(10):2118-30. doi: 10.1016/j.arthro.2016.05.038 [DOI] [PubMed] [Google Scholar]
- 79. Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. J Bone Joint Surg Am. 2003;85(2):185-92. doi: 10.2106/00004623-200302000-00001 [DOI] [PubMed] [Google Scholar]
- 80. Bentley G, Biant LC, Carrington RWJ, Akmal M, Goldberg A, Williams AM, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85-B(2):223-30. doi: 10.1302/0301-620x.85b2.13543 [DOI] [PubMed] [Google Scholar]
- 81. Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RWJ. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. J Bone Joint Surg Br. 2012;94-B(4):504-9. doi: 10.1302/0301-620x.94b4.27495 [DOI] [PubMed] [Google Scholar]
- 82. Ebert JR, Fallon M, Wood DJ, Janes GC. A prospective clinical and radiological evaluation at 5 years after arthroscopic matrix-induced autologous chondrocyte implantation. Am J Sports Med. 2016;45(1_suppl):59-69. doi: 10.1177/0363546516663493 [DOI] [PubMed] [Google Scholar]
- 83. Shanmugaraj A, Coughlin RP, Kuper GN, Ekhtiari S, Simunovic N, Musahl V, et al. Changing trends in the use of cartilage restoration techniques for the patellofemoral joint: a systematic review. Knee Surg Sport Traumatol Arthrosc. 2018;27(3):854-67. doi: 10.1007/s00167-018-5139-4 [DOI] [PubMed] [Google Scholar]
- 84. Skowroński J, Skowroński R, Rutka M. Large cartilage lesions of the knee treated with bone marrow concentrate and collagen membrane—results. Ortop Traumatol Rehabil. 2013;15(1_suppl):69-76. doi: 10.5604/15093492.1012405 [DOI] [PubMed] [Google Scholar]
- 85. Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42(3):648-57. doi: 10.1177/0363546513518007 [DOI] [PubMed] [Google Scholar]
- 86. Gobbi A, Scotti C, Karnatzikos G, Mudhigere A, Castro M, Peretti GM. One-step surgery with multipotent stem cells and hyaluronan-based scaffold for the treatment of full-thickness chondral defects of the knee in patients older than 45 years. Knee Surg Sport Traumatol Arthrosc. 2016;25(8):2494-501. doi: 10.1007/s00167-016-3984-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Grawe B, Burge A, Nguyen J, Strickland S, Warren R, Rodeo S, et al. Cartilage regeneration in full-thickness patellar chondral defects treated with particulated juvenile articular allograft cartilage: an MRI analysis. Cartilage. 2017;8(4):374-83. doi: 10.1177/1947603517710308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Buckwalter JA, Bowman GN, Albright JP, Wolf BR, Bollier M. Clinical outcomes of patellar chondral lesions treated with juvenile particulated cartilage allografts. Iowa Orthop J. 2014;34:44-9. [PMC free article] [PubMed] [Google Scholar]
- 89. Stevens HY, Shockley BE, Willett NJ, Lin ASP, Raji Y, Guldberg RE, et al. Particulated juvenile articular cartilage implantation in the knee. Cartilage. 2013;5(2):74-7. doi: 10.1177/1947603513515483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Shasha N, Aubin PP, Cheah HK, Davis AM, Agnidis Z, Gross AE. Long-term clinical experience with fresh osteochondral allografts for articular knee defects in high demand patients. Cell Tissue Bank. 2002;3(3):175-82. doi: 10.1023/A:1023658832356 [DOI] [PubMed] [Google Scholar]
- 91. Thomas D, Shaw KA, Waterman BR. Outcomes after fresh osteochondral allograft transplantation for medium to large chondral defects of the knee. Orthop J Sport Med. 2019;7(3):232596711983229. doi: 10.1177/2325967119832299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chahla J, Hinckel BB, Yanke AB, Farr J, Bugbee WD, Carey JL, et al. An expert consensus statement on the management of large chondral and osteochondral defects in the patellofemoral joint. Orthop J Sport Med. 2020;8(3). doi: 10.1177/2325967120907343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pestka JM, Bode G, Salzmann G, Südkamp NP, Niemeyer P. Clinical outcome of autologous chondrocyte implantation for failed microfracture treatment of full-thickness cartilage defects of the knee joint. Am J Sports Med. 2011;40(2):325-31. doi: 10.1177/0363546511425651 [DOI] [PubMed] [Google Scholar]
- 94. Vijayan S, Bartlett W, Bentley G, Carrington RWJ, Skinner JA, Pollock RC, et al. Autologous chondrocyte implantation for osteochondral lesions in the knee using a bilayer collagen membrane and bone graft. J Bone Joint Surg Br. 2012;94-B(4):488-92. doi: 10.1302/0301-620x.94b4.27117 [DOI] [PubMed] [Google Scholar]
- 95. Marlovits S, Aldrian S, Wondrasch B, Zak L, Albrecht C, Welsch G, et al. Clinical and radiological outcomes 5 years after matrix-induced autologous chondrocyte implantation in patients with symptomatic, traumatic chondral defects. Am J Sports Med. 2012;40(10):2273-80. doi: 10.1177/0363546512457008 [DOI] [PubMed] [Google Scholar]
- 96. Aldrian S, Zak L, Wondrasch B, Albrecht C, Stelzeneder B, Binder H, et al. Clinical and radiological long-term outcomes after matrix-induced autologous chondrocyte transplantation. Am J Sports Med. 2014;42(11):2680-8. doi: 10.1177/0363546514548160 [DOI] [PubMed] [Google Scholar]
- 97. Pareek A, Carey JL, Reardon PJ, Peterson L, Stuart MJ, Krych AJ. Long-term outcomes after autologous chondrocyte implantation. Cartilage. 2016;7(4):298-308. doi: 10.1177/1947603516630786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Minas T, Von Keudell A, Bryant T, Gomoll AH. The John Insall award: a minimum 10-year outcome study of autologous chondrocyte implantation. Clin Orthop Relat Res. 2013;472(1_suppl):41-51. doi: 10.1007/s11999-013-3146-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38(6):1117-24. doi: 10.1177/0363546509357915 [DOI] [PubMed] [Google Scholar]
- 100. Gracitelli GC, Meric G, Pulido PA, Görtz S, De Young AJ, Bugbee WD. Fresh osteochondral allograft transplantation for isolated patellar cartilage injury. Am J Sports Med. 2015;43(4):879-84. doi: 10.1177/0363546514564144 [DOI] [PubMed] [Google Scholar]
- 101. Familiari F, Cinque ME, Chahla J, Godin JA, Olesen ML, Moatshe G, et al. Clinical outcomes and failure rates of osteochondral allograft transplantation in the knee: a systematic review. Am J Sports Med. 2017;46(14):3541-9. doi: 10.1177/0363546517732531 [DOI] [PubMed] [Google Scholar]
- 102. Tírico LEP, McCauley JC, Pulido PA, Bugbee WD. Lesion size does not predict outcomes in fresh osteochondral allograft transplantation. Am J Sports Med. 2018;46(4):900-7. doi: 10.1177/0363546517746106 [DOI] [PubMed] [Google Scholar]
- 103. Cotter EJ, Hannon CP, Christian DR, Wang KC, Lansdown DA, Waterman BR, et al. Clinical outcomes of multifocal osteochondral allograft transplantation of the knee: an analysis of overlapping grafts and multifocal lesions. Am J Sports Med. 2018;46(12):2884-93. doi: 10.1177/0363546518793405 [DOI] [PubMed] [Google Scholar]
- 104. Meric G, Gracitelli GC, Görtz S, De Young AJ, Bugbee WD. Fresh osteochondral allograft transplantation for bipolar reciprocal osteochondral lesions of the knee. Am J Sports Med. 2015;43(3):709-14. doi: 10.1177/0363546514562549 [DOI] [PubMed] [Google Scholar]
- 105. Ogura T, Bryant T, Merkely G, Minas T. Autologous chondrocyte implantation for bipolar chondral lesions in the patellofemoral compartment: clinical outcomes at a mean 9 years’ follow-up. Am J Sports Med. 2019;47(4):837-46. doi: 10.1177/0363546518824600 [DOI] [PubMed] [Google Scholar]
- 106. Ogura T, Bryant T, Mosier BA, Minas T. Autologous chondrocyte implantation for bipolar chondral lesions in the tibiofemoral compartment. Am J Sports Med. 2018;46(6):1371-81. doi: 10.1177/0363546518756977 [DOI] [PubMed] [Google Scholar]
- 107. Gobbi A, Karnatzikos G, Scotti C, Mahajan V, Mazzucco L, Grigolo B. One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions. Cartilage. 2011;2(3):286-99. doi: 10.1177/1947603510392023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pestka JM, Feucht MJ, Porichis S, Bode G, Südkamp NP, Niemeyer P. Return to sports activity and work after autologous chondrocyte implantation of the knee. Am J Sports Med. 2015;44(2):370-7. doi: 10.1177/0363546515614578 [DOI] [PubMed] [Google Scholar]
- 109. Nielsen ES, McCauley JC, Pulido PA, Bugbee WD. Return to sport and recreational activity after osteochondral allograft transplantation in the knee. Am J Sports Med. 2017;45(7):1608-14. doi: 10.1177/0363546517694857 [DOI] [PubMed] [Google Scholar]
- 110. Samuelson EM, Brown DE. Cost-effectiveness analysis of autologous chondrocyte implantation. Am J Sports Med. 2012;40(6):1252-8. doi: 10.1177/0363546512441586 [DOI] [PubMed] [Google Scholar]
- 111. Lindahl A, Brittberg M, Peterson L. Health economics benefits following autologous chondrocyte transplantation for patients with focal chondral lesions of the knee. Knee Surg Sport Traumatol Arthrosc. 2001;9(6):358-63. doi: 10.1007/s001670100209 [DOI] [PubMed] [Google Scholar]
- 112. Mistry H, Connock M, Pink J, Shyangdan D, Clar C, Royle P, et al. Autologous chondrocyte implantation in the knee: systematic review and economic evaluation. Health Technol Assess. 2017;21(6):1-294. doi: 10.3310/hta21060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Pridie KH. A method of resurfacing osteoarthritic knee joints. J Bone Joint Surg. 1959;41:618. doi: 10.1186/ar4301 [DOI] [Google Scholar]
- 114. Steadman JR, Rodkey WG, Briggs KK. Microfracture: its history and experience of the developing surgeon. Cartilage. 2010;1(2):78-86. doi: 10.1177/1947603510365533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zedde P, Cudoni S, Giachetti G, Manunta ML, Masala G, Brunetti A, et al. Subchondral bone remodeling: comparing nanofracture with microfracture. An ovine in vivo study. Joints. 2016;4(2):87-93. doi: 10.11138/jts/2016.4.2.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Gou GH, Tseng FJ, Wang SH, Chen PJ, Shyu JF, Weng CF, et al. Autologous chondrocyte implantation versus microfracture in the knee: a meta-analysis and systematic review. Arthroscopy. 2020;36(1_suppl):289-303. doi: 10.1016/j.arthro.2019.06.033 [DOI] [PubMed] [Google Scholar]
- 117. Riboh JC, Cvetanovich GL, Cole BJ, Yanke AB. Comparative efficacy of cartilage repair procedures in the knee: a network meta-analysis. Knee Surg Sport Traumatol Arthrosc. 2017;25(12):3786-99. doi: 10.1007/s00167-016-4300-1 [DOI] [PubMed] [Google Scholar]
- 118. Gobbi A, Karnatzikos G, Kumar A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg Sport Traumatol Arthrosc. 2014;22(9):1986-96. doi: 10.1007/s00167-013-2676-8 [DOI] [PubMed] [Google Scholar]
- 119. Campbell AB, Pineda M, Harris JD, Flanigan DC. Return to sport after articular cartilage repair in athletes’ knees: a systematic review. Arthroscopy. 2016;32(4):651-68.e1. doi: 10.1016/j.arthro.2015.08.028 [DOI] [PubMed] [Google Scholar]
- 120. Gooding CR, Bartlett W, Bentley G, Skinner JA, Carrington R, Flanagan A. A prospective, ranomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: periosteum covered versus type I/III collagen covered. Knee. 2006;13(3):203-10. doi: 10.1016/j.knee.2006.02.011 [DOI] [PubMed] [Google Scholar]
- 121. Samsudin EZ, Kamarul T. The comparison between the different generations of autologous chondrocyte implantation with other treatment modalities: a systematic review of clinical trials. Knee Surg Sport Traumatol Arthrosc. 2016;24(12):3912-26. doi: 10.1007/s00167-015-3649-x [DOI] [PubMed] [Google Scholar]
- 122. Epstein DM, Choung E, Ashraf I, Greenspan D, Klein D, McHugh M, et al. Comparison of mini-open versus arthroscopic harvesting of osteochondral autografts in the knee: a cadaveric study. Arthroscopy. 2012;28(12):1867-72. doi: 10.1016/j.arthro.2012.06.014 [DOI] [PubMed] [Google Scholar]
- 123. McCoy B, Miniaci A. Osteochondral autograft transplantation/mosaicplasty. J Knee Surg. 2012;25(02):99-108. doi: 10.1055/s-0032-1322508 [DOI] [PubMed] [Google Scholar]
- 124. Diduch DR, Chhabra A, Blessey P, Miller MD. Osteochondral autograft plug transfer: achieving perpendicularity. J Knee Surg. 2003;16(1_suppl):17-20. [PubMed] [Google Scholar]
- 125. Harris JD, Solak KK, Siston RA, Litsky A, Richards J, Flanigan DC. Contact pressure comparison of proud osteochondral autograft plugs versus proud synthetic plugs. Orthopedics. 2011;34(2):97. doi: 10.3928/01477447-20101221-06 [DOI] [PubMed] [Google Scholar]
- 126. Huang FS, Simonian PT, Norman AG, Clark JM. Effects of small incongruities in a sheep model of osteochondral autografting. Am J Sports Med. 2004;32(8):1842-8. doi: 10.1177/0363546504264895 [DOI] [PubMed] [Google Scholar]
- 127. Jackson DW, Lalor PA, Aberman HM, Simon TM. Spontaneous repair of full-thickness defects of articular cartilage in a goat model. J Bone Joint Surg Am. 2001;83(1_suppl):53-64. doi: 10.2106/00004623-200101000-00008 [DOI] [PubMed] [Google Scholar]
- 128. Pareek A, Reardon PJ, Macalena JA, Levy BA, Stuart MJ, Williams RJ, 3rd, et al. Osteochondral autograft transfer versus microfracture in the knee: a meta-analysis of prospective comparative studies at midterm. Arthroscopy. 2016;32(10):2118-30. doi: 10.1016/j.arthro.2016.05.038 [DOI] [PubMed] [Google Scholar]
- 129. Jones KJ, Mosich GM, Williams RJ. Fresh precut osteochondral allograft core transplantation for the treatment of femoral cartilage defects. Arthrosc Tech. 2018;7(8):e791-e795. doi: 10.1016/j.eats.2018.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Borazjani BH, Chen AC, Bae WC, Patil S, Sah RL, Firestein GS, et al. Effect of impact on chondrocyte viability during insertion of human osteochondral grafts. J Bone Joint Surg. 2006;88(9):1934-43. doi: 10.2106/jbjs.e.00992 [DOI] [PubMed] [Google Scholar]
- 131. Gross AE, Kim W, Las Heras F, Backstein D, Safir O, Pritzker KPH. Fresh osteochondral allografts for posttraumatic knee defects: long-term follow-up. Clin Orthop Relat Res. 2008;466(8):1863-70. doi: 10.1007/s11999-008-0282-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Briggs DT, Sadr KN, Pulido PA, Bugbee WD. The use of osteochondral allograft transplantation for primary treatment of cartilage lesions in the knee. Cartilage. 2015;6(4):203-7. doi: 10.1177/1947603515595072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Krych AJ, Robertson CM, Williams RJ. Return to athletic activity after osteochondral allograft transplantation in the knee. Am J Sports Med. 2012;40(5):1053-9. doi: 10.1177/0363546511435780 [DOI] [PubMed] [Google Scholar]
- 134. Gobbi A, Scotti C, Lane JG, Peretti GM. Fresh osteochondral allografts in the knee: only a salvage procedure? Ann Transl Med. 2015;3(12):164. doi: 10.3978/j.issn.2305-5839.2015.06.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Gracitelli GC, Meric G, Briggs DT, Pulido PA, McCauley JC, Belloti JC, et al. Fresh osteochondral allografts in the knee: comparison of primary transplantation versus transplantation after failure of previous subchondral marrow stimulation. Am J Sports Med. 2015;43(4):885-91. doi: 10.1177/0363546514565770 [DOI] [PubMed] [Google Scholar]
- 136. Kusano T, Jakob RP, Gautier E, Magnussen RA, Hoogewoud H, Jacobi M. Treatment of isolated chondral and osteochondral defects in the knee by autologous matrix-induced chondrogenesis (AMIC). Knee Surg Sports Traumatol Arthrosc. 2012;20(10):2109-15. doi: 10.1007/s00167-011-1840-2 [DOI] [PubMed] [Google Scholar]
- 137. Fortier LA, Potter HG, Rickey EJ, Schnabel LV, Foo LF, Chong LR, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92(10):1927-37. doi: 10.2106/JBJS.I.01284 [DOI] [PubMed] [Google Scholar]
- 138. Oussedik S, Tsitskaris K, Parker D. Treatment of articular cartilage lesions of the knee by microfracture or autologous chondrocyte implantation: a systematic review. Arthroscopy. 2015;31(4):732-44. doi: 10.1016/j.arthro.2014.11.023 [DOI] [PubMed] [Google Scholar]