Abstract
Objective
To identify joint structural risk factors, measured using quantitative compositional and semiquantitative magnetic resonance imaging (MRI) scoring, associated with the development of accelerated knee osteoarthritis (AKOA) compared with a more normal rate of knee osteoarthritis (OA) development.
Design
From the Osteoarthritis Initiative we selected knees with no radiographic OA (Kellgren-Lawrence grade [KL] 0/1) that developed advanced-stage OA (KL 3/4; AKOA) within a 4-year timeframe and a comparison group with a more normal rate of OA development (KL 0/1 to KL 2 in 4 years). MRIs at the beginning of the 4-year timeframe were assessed for cartilage T2 values and structural abnormalities using a modified Whole-Organ Magnetic Resonance Imaging Score (WORMS). Associations of MRI findings with AKOA versus normal OA were assessed using multivariable logistic regression models.
Results
A total of 106 AKOA and 168 subjects with normal OA development were included. Mean cartilage T2 values were not significantly associated with AKOA (odds ratio [OR] 1.06; 95% confidence interval [CI] 0.82-1.36). Risk factors for AKOA development included higher meniscus maximum scores (OR 1.37; 95% CI 1.11-1.68), presence of meniscal extrusion (OR 6.30; 95% CI 2.57-15.49), presence of root tears (OR 4.64; 95% CI 1.61-13.34), and higher medial tibia cartilage lesion scores (OR 1.96; 95% CI 1.19-3.24).
Conclusions
We identified meniscal damage, especially meniscal extrusion and meniscal root tears as risk factors for AKOA development. These findings contribute to identifying subjects at risk of AKOA at an early stage when preventative measures targeting modifiable risk factors such as meniscal repair surgery could still be effective.
Keywords: osteoarthritis, MRI, knee, cartilage
Introduction
Knee osteoarthritis (OA) is the most common degenerative disorder of the knee and ranked as the one of the leading causes of global disability. 1 The disease is caused by a range of mechanical and biochemical disorders, collectively leading to structural and functional joint failure.2,3 While disease progression typically occurs gradually, over the course of many years, 4 accelerated knee osteoarthritis (AKOA) is a form of knee OA characterized by rapid OA onset and progression of structural damage, leading to severe to end-stage disease within 4 years or less. 5 AKOA has been described to occur in 3.4% of adults at risk of knee OA and is linked to significant pain and functional limitations.5,6 Clinical risk factors associated with AKOA have been studied previously. Driban et al.7-9 identified higher age, recent knee injury, greater coronal tibial slope, and elevated body mass index (BMI) in subjects younger than 63.5 years as risk factors for AKOA. However, identifying subjects at risk of AKOA remains challenging given that most clinical risk factors are also well-established risk factors for knee OA in general. 10 .
Imaging features frequently observed in knees developing AKOA include meniscal tears such as root and radial tears, meniscal extrusion, and subchondral insufficiency fractures.11-13 Moreover, meniscal tears and specifically medial meniscal root tears were frequently reported in knees developing spontaneous osteonecrosis. 14 Though several studies evaluated MR (magnetic resonance) risk factors for OA development in early structural disease,15-17 there appear to be no studies comparing differences in joint structural factors, between subjects at risk of AKOA, and those at with a more normal rate of OA development. Moreover, biochemical cartilage composition has not been assessed in subjects with AKOA. Water content and collagen architecture can be characterized with T2 relaxation time measurements, which has been shown to be a risk factor for incident knee OA18-22; however, it is unclear if it is also a risk factor for AKOA. Given the rapid pace of cartilage loss and limited opportunity to intervene, this knowledge may contribute to identifying subjects at risk of AKOA and initiate preventative measures.
The primary goal of this study was to assess the association of knee cartilage composition and joint morphological features in knees without radiographic OA, with subsequent development of AKOA compared with a more normal rate of OA development within a 4-year time frame. In addition, we describe differences of demographic and clinical characteristics of subjects with AKOA and a normal rate of OA development.
Methods
Subjects
We conducted a nested case-control study within the Osteoarthritis Initiative (OAI, http://www.oai.ucsf.edu). The OAI is a prospective, longitudinal, multicenter, observational cohort study enrolling 4,796 subjects with or at risk for symptomatic knee OA. Informed consent was obtained from all participants; the study was compliant with the Health Insurance Portability and Accountability Act and approved by the local institutional review boards of all participating centers. Participants were recruited from February 2004 until May 2006.
We used Kellgren-Lawrence (KL) grades, from the OAI baseline visit, 24, 48, 72, and 96 months to identify subjects with accelerated knee OA development (KL 0/1 to KL 3/4, AKOA group) and normal OA development (KL 0/1 to KL 2) within a 4-year period (normal OA group) at any time point within the observation period of the OAI. The starting time point of this 4-year time frame for those with AKOA and normal OA was defined as the index visit. The subject selection process is shown in Figure 1 .
Figure 1.
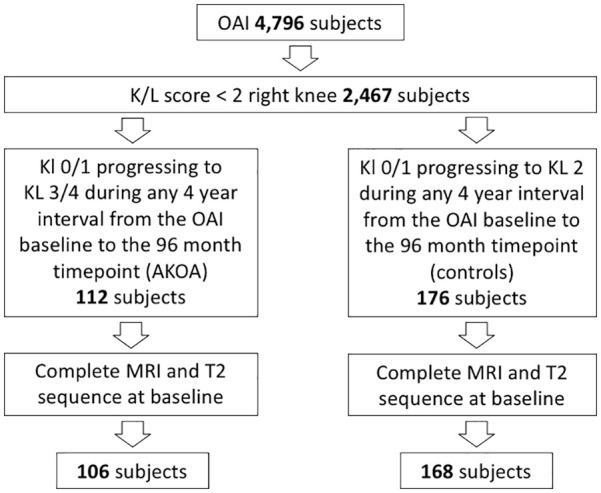
Subject selection flowchart.
Subject characteristics were assessed at the index time point. Assessed characteristics included age, sex, race, BMI, Physical Activity Score of the Elderly (PASE), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain and stiffness scores, presence of medial or lateral joint line tenderness and presence of knee effusion (bulge and patellar tap sign), and Knee Injury and Osteoarthritis Outcome Score (KOOS) pain scores for twisting/pivoting the knee, straightening or bending the knee fully. We also analyzed self-reported knee injuries limiting subjects’ ability to walk for at least two days and knee surgery.
Magnetic Resonance Imaging
MRIs were acquired on 4 identical 3-T scanners (Siemens Magnetom Trio; Siemens, Erlangen, Germany) using standard transmit-receive knee coils (USA Instruments, Aurora, OH, USA). A sagittal 2-dimensional (2D) multi-slice-multi-echo spin-echo sequence with 7 echo times (10 ms, 20 ms, 30 ms, 40 ms, 50 ms, 60 ms, 70 ms; repetition time = 2700 ms; slice thickness = 3 mm) was used to obtain cartilage T2 relaxation measurements. Sagittal 2D intermediate-weighted fat-suppressed turbo spin-echo sequences (repetition time, 3200 ms; echo time, 30 ms; slice thickness, 3 mm), coronal 2D intermediate-weighted non-fat-suppressed turbo spin-echo sequences (repetition time, 3700 ms; echo time, 29 ms; slice thickness, 3 mm) and sagittal 3D dual-echo in steady state with selective water excitation (repetition time, 16.3 ms; echo time, 4.7 ms; slice thickness, 0.7 mm) were used for the analysis of morphological/structural abnormalities. The acquisition protocol has been published previously. 23
MR Image Analysis
T2 relaxation times were measured at the index visit using an in-house, spline-based algorithm written in MatLab (MathWorks, Natick, MA, USA) for semiautomatic segmentation as described previously. 24 Compartments segmented included the patella, the medial and lateral femur and tibia. The trochlea was not segmented due to flow-artifacts in this region caused by the popliteal artery. T2 values were calculated for each compartment by using a monoexponential decay model as fitting function for the signal intensity using six echoes (echo times 20-70 ms) after excluding the first echo to reduce potential errors resulting from stimulated echoes and using 3 parameter fittings accounting for noise.25,26 A global T2 value was derived using the mean of all compartments.
All index visit images were individually and independently read by 2 radiologists (S.C.F. and Y.L., each with 4 years of experience), blinded to clinical data and group (AKOA or controls), using the modified semiquantitative WORMS score of the knee. 27 In case of disagreement, a consensus reading was performed with a third board-certified musculoskeletal radiologist (T.M.L., 25 years of experience). Morphological abnormalities graded included cartilage lesions and bone marrow edema pattern (BMEP) each graded over 6 subregions (patella, trochlea, medial and lateral femur and tibia) and meniscal abnormalities (3 medial and 3 lateral graded subregions: meniscus body, anterior horn and posterior horn). Maximum scores across all subregions were calculated for each WORMS category. In addition, meniscal tears were graded as vertical, horizontal, flap, complex, bucket handle, or root tears and presence or absence of meniscal extrusion was documented with a cutoff of 3 mm/2 mm for the medial and lateral meniscus, respectively.28,29
Inter-/Intrareader Reproducibility
Intra- and interreader reproducibility of WORMS grading by our group have been validated in multiple previous studies.18,30-33 In these studies, intraclass correlation coefficients were calculated in order to compare WORMS subscores for the meniscus and cartilage. Intraclass correlation coefficients for intrareader reproducibility ranged between 0.80 (0.69-0.95) 32 and 0.96 (0.94-0.97) 31 for the meniscus and between 0.81 (0.68-0.91) 32 and 0.99 (0.98-0.99) 31 for the cartilage. Interreader intraclass correlation coefficients ranged between 0.81 (0.76-0.88) 32 and 0.97 (0.95-0.98) 31 for the meniscus and between 0.79 (0.72-0.868) 33 and 0.97 (0.95-0.98) 31 for the cartilage.
Statistical Analysis
The statistical analysis was performed with Stata v. 14 software (StataCorp, College Station, TX, USA) using a 2-sided 0.05 level of significance. Differences in characteristics between the AKOA and normal OA groups were assessed at the index time point (beginning of the 4-year time frame) using Pearson’s chi-square tests for categorical data (gender, race distribution, risk factors for knee OA) and t tests for numeric variables (age, BMI, PASE, WOMAC). Logistic regression models were used to assess whether cartilage T2 and WORMS were associated with the development of AKOA, adjusting for age, sex, BMI, and race. Standardized T2 values were calculated by subtracting the mean from the variable and dividing it by the standard deviation.
Results
Subject Demographics and Clinical Correlates of AKOA
The final study population selected from the OAI consisted of 106 subjects with AKOA (KL 0/1 to KL3/4 within a 4-year period) and 168 with normal OA (KL 0/1 to KL 2 within a 4-year period). The starting timepoint of the 4-year time frame was defined as the index visit. Subject characteristics are presented in Table 1 .
Table 1.
Subject Characteristics.
| Characteristics | Controls (n = 168) | AKOA (n = 106) | P a |
|---|---|---|---|
| Age, years, mean ± SD | 59.8 ± 8.2 | 63.3 ± 8.5 | 0.001 b |
| Gender, n (%) | 0.796 c | ||
| Female | 108 (64) | 70 (66) | |
| Male | 60 (36) | 36 (34) | |
| Body mass index, kg/m2, mean ± SD | 29.1±4.5 | 29.4±4.2 | 0.633 b |
| PASE, mean ± SD | 178.4±82.6 | 167.8±91.7 | 0.335 b |
| Race, n (%) | 0.198 c | ||
| Caucasian | 138 (82) | 92 (87) | |
| African American | 23 (14) | 12 (13) | |
| Asian | 4 (2) | 0 (0) | |
| Other | 3 (2) | 0 (0) | |
| Characteristics of OA knees, n (%) | |||
| History of knee injury occurring | |||
| Before index time point | 47 (28) | 30 (28) | 1.000 c |
| During follow-up period | 27 (16) | 46 (43) | <0.001 c |
| History of knee surgery occurring | |||
| Before index time point | 10 (6) | 2 (2) | 0.137 c |
| During follow-up period | 13 (8) | 24 (23) | 0.001 c |
| WOMAC pain scores, mean ± SD | 1.9±2.5 | 2.4±2.9 | 0.151 b |
| WOMAC stiffness scores, mean ± SD | 1.4±1.4 | 1.8±1.6 | 0.038 b |
OA, osteoarthritis; AKOA, accelerated knee osteoarthritis; PASE = Physical Activity Score for the Elderly; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
Significant values are in boldface.
T test.
Pearson’s chi-square test.
The mean age of subjects in this study was 61.1 ± 8.5 years, with a mean BMI of 29.2 ± 4.4 kg/m2 and more females (65%) than males. In comparison to normal OA, the AKOA group was older (63.3 ± 8.5 vs. 59.8 ± 8.2 years; P = 0.001), while the mean BMI (29.5 ± 4.3 vs. 29.3 ± 4.6 kg/m2; P = 0.663) and PASE (167.8 ± 91 vs. 178.4 ± 83; P = 0.335) were similar in both groups. Moreover, both groups had a similar distribution for sex and race.
Incident knee injury or surgery during the four years following the index visit were more frequent in the AKOA group (knee injury: 43% vs. 16%; P < 0.001; knee surgery: 23% vs. 8%; P = 0.001). History of knee injury or surgery before the index visit was not significantly more often present in the AKOA group or the control group (P > 0.137). During the 4 years following the index visit, all surgery types reported were arthroscopic partial meniscectomy surgeries. The surgery types reported before the index visit included n = 2 arthroscopic partial meniscectomy surgeries for the AKOA group; and n = 7 arthroscopic partial meniscectomy surgeries, n = 1 ligament repair surgery, and n = 2 prior arthroscopies for the normal OA group.
We also found differences between both groups comparing clinical symptoms at the index visit: WOMAC knee stiffness scores were significantly higher in the AKOA group, while differences in overall WOMAC pain scores were not significant (P = 0.151). Severe-extreme knee pain when fully bending the knee at the index visit was more often present in the AKOA group (9%, 9/106 vs. 2%, 3/168; P = 0.013). No significant differences were found for pain scores for twisting/pivoting the knee or straightening the knee fully (P > 0.05).
Morphological Knee Abnormalities
Results for WORMS features assessed at the index visit for each group are summarized in Tables 2 and 3 . Meniscal damage was the morphological feature most consistently associated with AKOA development compared with normal OA development. The majority of WORMS meniscus features showed significantly higher scores at the index visit in subjects later developing AKOA. Specifically, knees with higher maximum scores of the medial and lateral meniscus (adjusted odds ratio [OR] per 1 unit increase, 1.37; 95% confidence interval [CI] 1.11-1.68; P = 0.003), higher scores of the medial and lateral meniscus body (adjusted OR per 1 unit increase, 1.37; 95% CI 1.08-1.75; P = 0.010 and adjusted OR per 1 unit increase, 1.42; 95% CI 1.08-1.87; P = 0.011, respectively) and higher scores of the lateral posterior horn (adjusted OR per 1 unit increase, 1.50; 95% CI 1.10-2.05; P = 0.010) were at higher odds of developing AKOA ( Fig. 2 ).
Table 2.
Magnetic Resonance Imaging Features. a
| WORMS Scores | Controls b (n = 168) | AKOA b (n = 106) | Adjusted Odds Ratio per 1 Unit Increase (95% CI) | P c,d |
|---|---|---|---|---|
| Cartilage (range 0-6) | ||||
| Cartilage maximum (max) | 2.5 (2, 3) | 3 (2, 5) | 1.18 (0.99-1.39) | 0.062 |
| PAT | 2 (0, 3) | 2.5 (1, 4.25) | 1.08 (0.94-1.25) | 0.270 |
| TRO | 0 (0, 2) | 0.5 (0, 2.5) | 0.98 (0.83-1.16) | 0.830 |
| MF | 0 (0, 1) | 0 (0, 2) | 1.23 (0.99-1.54) | 0.065 |
| LF | 0 (0, 0) | 0 (0, 0) | 1.18 (0.89-1.59) | 0.272 |
| MT | 0 (0, 0) | 0 (0, 0) | 1.96 (1.19-3.24) | 0.008 |
| LT | 0 (0, 1) | 0 (0, 1) | 1.08 (0.82-1.42) | 0.600 |
| Meniscus (range 0-4) | ||||
| Bilateral meniscus max | 2 (0, 2) | 2 (1, 3) | 1.37 (1.11-1.68) | 0.003 |
| Medial anterior horn | 0 (0, 0) | 0 (0, 0) | 1.81 (0.67-4.83) | 0.239 |
| Medial body | 0 (0, 0) | 0 (0, 1) | 1.37 (1.08-1.75) | 0.010 |
| Medial posterior horn | 0 (0, 1) | 1 (0, 1) | 1.09 (0.85-1.41) | 0.493 |
| Lateral anterior horn | 0 (0, 1) | 0 (0, 1) | 1.15 (0.89-1.47) | 0.288 |
| Lateral body | 0 (0, 0) | 0 (0, 1) | 1.42 (1.08-1.87) | 0.011 |
| Lateral posterior horn | 0 (0, 0) | 0 (0, 1) | 1.50 (1.10-2.05) | 0.010 |
| BMEP (range 0-3) | ||||
| BMEP max | 2 (0, 2) | 2 (1, 2) | 1.28 (1.00-1.64) | 0.048 |
| PAT | 0 (0, 2) | 1 (0, 2) | 1.04 (0.81-1.34) | 0.739 |
| TRO | 0 (0, 1) | 0 (0, 1) | 0.99 (0.75-1.30) | 0.934 |
| MF | 0 (0, 0) | 0 (0, 0) | 1.36 (0.86-2.16) | 0.187 |
| LF | 0 (0, 0) | 0 (0, 0) | 0.90 (0.44-1.87) | 0.782 |
| MT | 0 (0, 0) | 0 (0, 0) | 1.69 (1.10-2.60) | 0.016 |
| LT | 0 (0, 0) | 0 (0, 0) | 1.70 (1.12-2.61) | 0.014 |
AKOA = accelerated knee osteoarthritis; BMEP = bone marrow edema pattern; PAT = patella; TRO = trochlea; MF = medial femur; LF = lateral femur; MT = medial tibia; LT = lateral tibia; WORMS = Whole-Organ Magnetic Resonance Imaging Score.
Assessed at index time point.
Numbers are median (25th and 75th percentiles).
Significant values are in boldface.
Multivariable logistic regression adjusting for age, sex, body mass index, and race.
Table 3.
Meniscal Abnormalities. a
| Parameter (Present/Absent) | Controls b (n = 168) | AKOA a (n = 106) | Adjusted Odds Ratio | P c,d |
|---|---|---|---|---|
| Vertical tear | 7 (4) | 12 (11) | 2.28 (0.84-6.14) | 0.104 |
| Horizontal tear | 41 (24) | 21 (20) | 0.68 (0.36-1.27) | 0.225 |
| Complex tear | 10 (6) | 10 (9) | 1.59 (0.62-4.09) | 0.333 |
| Root tear | 6 (4) | 17 (16) | 4.64 (1.61-13.34) | 0.004 |
| Meniscal extrusion | 8 (5) | 25 (24) | 6.30 (2.57-15.49) | <0.001 |
| Flap tear e | 0 (0) | 3 (3) | ||
| Bucket handle tear e | 0 (0) | 0 (0) |
AKOA = accelerated knee osteoarthritis.
Assessed at index time point.
Types of meniscal abnormalities are listed as number of abnormalities observed, with percentage in parentheses; more than 1 type of abnormality could occur in the same knee therefore percentages do not add up to 100%.
Significant values are in boldface.
Multivariable logistic regression adjusting for age, sex, body mass index, and race.
Case numbers not adequate for statistical test.
Figure 2.
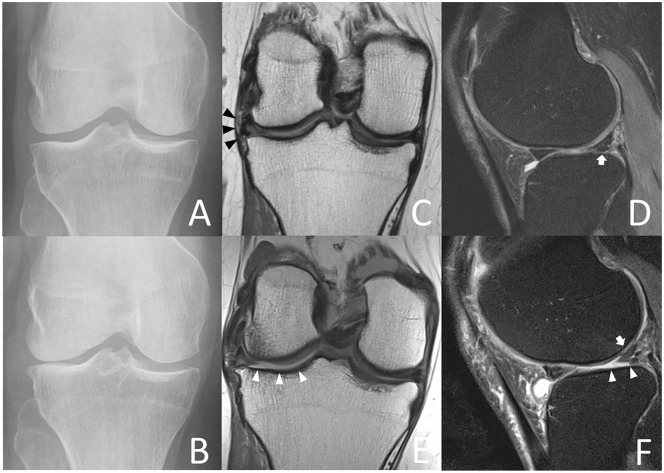
Index visit (A) and 4-year follow-up radiographs (B) of a 69-year-old female accelerate knee osteoarthritis (AKOA) subject show Kellgren-Lawrence (KL) grade progression from KL 0 to KL 4 of the right knee within 4 years. Index visit coronal (C) 3-T magnetic resonance (MR) images depict complex tearing of the lateral meniscus body and extrusion of 4 mm (black arrowheads) and index visit sagittal (D) MR images show a complex tear of the lateral anterior horn (white arrow). At 4-year follow-up (E and F) the lateral meniscus body is extruded by 5 mm (black arrowheads) and there is a small meniscal cyst at the anterior horn (white arrow). Note full-thickness cartilage loss of the lateral tibia and femur (white arrowheads), joint effusion, and loose bodies in the joint (black arrow).
Regarding types of meniscal damage associated with AKOA development, knees with meniscal root tears (adjusted OR 4.64; 95% CI 1.61-13.34; P = 0.004) and medial or lateral meniscal extrusion (adjusted OR 6.30; 95% CI 2.57-15.49; P < 0.001) at the index visit were at higher odds for AKOA development ( Fig. 3 ).
Figure 3.
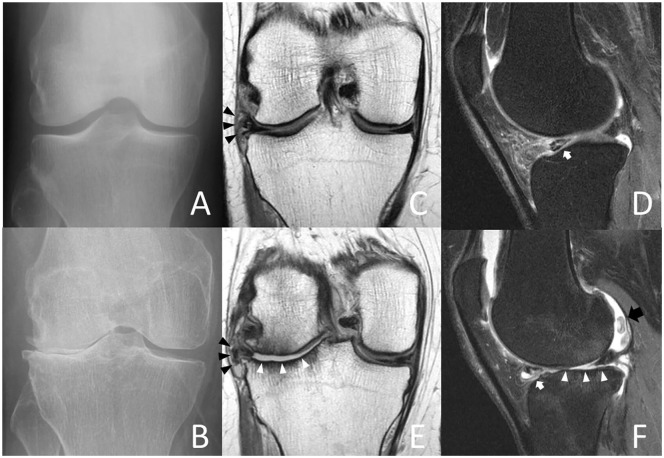
Index visit (A) and 4-year follow-up radiographs (B) of a 53-year-old female accelerated knee osteoarthritis (AKOA) subject with Kellgren-Lawrence (KL) grade progression from KL 1 to 3 of the right knee within 4 years. Coronal (C) and sagittal (D) index visit 3-T magnetic resonance (MR) images show intrasubstance degeneration of the lateral meniscus body with a tear extending to the interior surface and extrusion (3 mm, black arrowheads). Note irregularity, fraying, and abnormal signal of the lateral posterior horn (white arrow) and diffuse thinning of the lateral tibial plateau cartilage. At 4-year follow-up (E and F) extensive cartilage loss of the lateral femur and tibia (white arrowheads), increased degeneration of the lateral posterior horn with tearing (white arrow) and increased intrasubstance degeneration of the anterior horn with a large meniscal cyst (F).
Knees with higher WORMS medial tibia (MT) cartilage lesion scores were at significantly higher odds for developing AKOA. Furthermore, knees with higher medial and lateral tibia BMEP scores at the index visit were at higher odds for AKOA development. One insufficiency fracture was identified in the AKOA and the control group, respectively.
T2 Relaxation Time Measurements
The results for T2 relaxation time measurements assessed at the index visit for both groups are shown in Table 4 . Differences for cartilage T2 values were nonsignificant for all cartilage compartments and global knee T2 values (P > 0.191).
Table 4.
Cartilage T2. a
| Parameter | Controls b (n = 168) | AKOA b (n = 106) | Adjusted Odds Ratio c per SD Increase (95% CI) | P c,d |
|---|---|---|---|---|
| Cartilage T2 | ||||
| Global T2 | 33.83 ± 1.9 | 33.90 ± 1.9 | 1.06 (0.82-1.36) | 0.660 |
| PAT T2 | 33.18 ± 3.1 | 32.56 ± 2.7 | 0.86 (0.66-1.13) | 0.295 |
| MF T2 | 39.18 ± 2.9 | 39.37 ± 2.8 | 0.99 (0.76-1.29) | 0.930 |
| LF T2 | 36.00 ± 2.6 | 35.94 ± 2.4 | 0.89 (0.89-1.16) | 0.401 |
| MT T2 | 31.28 ± 2.6 | 31.50 ± 2.6 | 1.17 (0.90-1.54) | 0.244 |
| LT T2 | 29.62 ± 2.7 | 29.93 ± 2.9 | 1.19 (0.92-1.54) | 0.191 |
AKOA = accelerated knee osteoarthritis; PAT = patella; TRO = trochlea; MF = medial femur; LF = lateral femur; MT = medial tibia; LT = lateral tibia.
Assessed at index time point.
Numbers are mean ± standard deviation.
Multivariable logistic regression adjusting for age, sex, body mass index, and race.
Discussion
In this study, we found meniscal damage, especially meniscal root tears and extrusion to be highly significant risk factors for AKOA development. Moreover, medial tibia cartilage damage and tibial BMEP were also significantly associated with AKOA development, while altered biochemical cartilage composition was not a significant risk factor.
Among demographic and clinical correlates, subjects later developing AKOA were older compared to subjects with a more gradual OA onset. Higher BMI was not associated with AKOA in univariate or multivariate analyses. While elevated BMI is a well-established risk factor for incident knee OA,10,34 it may be less useful to distinguish subjects at risk of AKOA from subjects with a more gradual OA onset. Baseline WOMAC knee stiffness scores and KOOS pain scores for bending the knee fully were significantly higher in AKOA subjects. Since loss of knee flexion is frequently observed in subjects with meniscal root tears or other meniscal abnormalities, 35 these symptoms could be useful to identify subjects that would benefit from an MRI to identify morphological pathologies associated with rapid cartilage loss. These subjects could then receive treatment for these pathologies to prevent AKOA such as meniscal repair surgery. 36 Recent studies demonstrated promising results using surgical techniques such as arthroscopic pullout suture for meniscal root tears37-39 or arthroscopic direct extrusion reduction to treat meniscal extrusion.40,41 Studies comparing different approaches to treat posterior medial meniscal root tears found that anatomic transtibial pull-out root repair (with and without centralization suture into the posterior medial tibial plateau) best restored contact mechanics in the knee, compared with nonanatomic repair states, 42 while partial meniscectomy surgery or nonoperative management was associated with poor clinical outcomes and high arthroplasty rates.43,44
Previous studies demonstrated that meniscal damage could be an important morphological risk factor for AKOA development: Roemer et al. 13 identified meniscal extrusion and presence of meniscal abnormalities as risk factors for slow and fast tibiofemoral cartilage loss over 30 months comparing both types of cartilage loss to a reference group of knees without cartilage loss. Davis et al. 12 studied the agreement between self-reported knee injury and distinct structural changes in subjects developing AKOA or a more standard rate of OA development. Distinct structural changes most commonly described in AKOA subjects, were medial meniscal lesions such as root or radial tears. 12 We identified several measures of meniscal damage as risk factors for AKOA, including higher scores of the medial and lateral body, and lateral posterior horn. Specifically, root tears and meniscal extrusion were strongly associated with AKOA development. Root tears are assumed to profoundly impact knee health, creating biomechanical changes similar to that of a total meniscectomy.15,45-48 Meniscal extrusion has also previously been identified as a risk factor for cartilage loss and is frequently associated with root tears.13,15,45
Regarding other morphological features, medial tibia cartilage damage was the only cartilage feature significantly associated with AKOA while other cartilage features were nonsignificant. Initial tibial cartilage volume was previously identified as an important factor influencing the overall amount of cartilage loss, 49 though medial and lateral compartment were not analyzed separately. Interestingly, high tibial BMEP scores at baseline were also associated with AKOA development in our study. Since compartments with higher baseline BMEP scores are at risk of greater cartilage loss, 50 higher baseline BMEP scores of the tibia may be related to increased tibial cartilage loss. These findings suggest that tibial cartilage damage, more specifically damage of the medial tibia cartilage could be an important factor initiating, or occurring early in, the development of AKOA. While cartilage composition is a known risk factor for incident knee OA,19-21 biochemical cartilage alteration was not a factor that distinguished between AKOA and a normal rate of OA development. Overall mean T2 values of all compartments exhibited no clear differences between groups, suggesting a limited impact of cartilage composition on AKOA development.
Some limitations are pertinent to this study. Since the patellofemoral joint was not assessed in the KL-based definition used for subject selection, some study knees likely had significant patellofemoral OA at the index visit. However, WORMS patella cartilage and patella BMEP scores were not associated with AKOA. In order to avoid misclassifying AKOA knees as normal OA, those who did not have follow-up radiograph readings 4 years after the index time point were excluded. As a result, a small number of knees were excluded that developed AKOA at an earlier time point but lacked 4-year follow-up readings (5 due to a subsequent knee replacement, 5 due to missing radiographs), and this may have influenced our results.
In conclusion, we identified meniscal damage, especially meniscal extrusion and meniscal root tears as risk factors for AKOA development. Given the rapid pace of cartilage loss in subjects with AKOA and subsequent short opportunity to intervene, our findings contribute to identifying subjects at risk of AKOA at an early stage when preventative measures targeting modifiable risk factors such as meniscal repair surgery could still be effective.
Footnotes
Acknowledgments and Funding: We would like to thank the participants and staff of the Coordinating Center of the OAI for their invaluable assistance with patient selection, statistical analysis, and technical support. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by the OAI, a public-private partnership comprising 5 NIH contracts (National Institute of Arthritis and Musculoskeletal and Skin Diseases contracts N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01-AR-2-2261, and N01-AR-2-2262), with research conducted by the OAI Study Investigators. The study was also funded in part by the Intramural Research Program of the National Institute on Aging, NIH. Private funding partners include Merck Research, Novartis Pharmaceuticals, GlaxoSmithKline, and Pfizer; the private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. The analyses in this study were funded through the NIH/NIAMS (National Institute of Arthritis and Musculoskeletal and Skin Diseases grant R01AR064771).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The study was compliant with the Health Insurance Portability and Accountability Act and was approved by the local institutional review boards of all participating centers.
Informed Consent: Informed consent was obtained from all participants.
Trial Registration: Not applicable.
ORCID iD: Sarah C. Foreman  https://orcid.org/0000-0001-9140-0162
https://orcid.org/0000-0001-9140-0162
References
- 1. Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323-30. [DOI] [PubMed] [Google Scholar]
- 2. Nuki G. Osteoarthritis: a problem of joint failure. Z Rheumatol. 1999;58(3):142-7. [DOI] [PubMed] [Google Scholar]
- 3. Roemer FW, Crema MD, Trattnig S, Guermazi A. Advances in imaging of osteoarthritis and cartilage. Radiology. 2011;260(2):332-54. [DOI] [PubMed] [Google Scholar]
- 4. Hunter DJ. Risk stratification for knee osteoarthritis progression: a narrative review. Osteoarthritis Cartilage. 2009;17(11):1402-7. [DOI] [PubMed] [Google Scholar]
- 5. Driban JB, Stout AC, Lo GH, Eaton CB, Price LL, et al. Best performing definition of accelerated knee osteoarthritis: data from the Osteoarthritis Initiative. Ther Adv Musculoskelet Dis. 2016;8(5):165-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Driban JB, Price LL, Eaton CB, Lu B, Lo GH, Lapane KL, et al. Individuals with incident accelerated knee osteoarthritis have greater pain than those with common knee osteoarthritis progression: data from the Osteoarthritis Initiative. Clin Rheumatol. 2016;35(6):1565-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Driban JB, Stout AC, Duryea J, Lo GH, Harvey WF, Price LL, et al. Coronal tibial slope is associated with accelerated knee osteoarthritis: data from the Osteoarthritis Initiative. BMC Musculoskelet Disord. 2016;17:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Driban JB, McAlindon TE, Amin M, Price LL, Eaton CB, Davis JE, et al. Risk factors can classify individuals who develop accelerated knee osteoarthritis: Data from the osteoarthritis initiative. J Orthop Res. 2018;36(3):876-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davis JE, Price LL, Lo GH, Eaton CB, McAlindon TE, Lu B, et al. A single recent injury is a potent risk factor for the development of accelerated knee osteoarthritis: data from the osteoarthritis initiative. Rheumatol Int. 2017;37(10):1759-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Felson DT. Epidemiology of hip and knee osteoarthritis. Epidemiol Rev. 1988;10:1-28. [DOI] [PubMed] [Google Scholar]
- 11. Driban JB, Ward RJ, Eaton CB, Lo GH, Price LL, Lu B, et al. Meniscal extrusion or subchondral damage characterize incident accelerated osteoarthritis: data from the Osteoarthritis Initiative. Clin Anat. 2015;28(6):792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davis JE, Harkey MS, Ward RJ, Mackay JW, Lu B, Price LL, et al. Characterizing the distinct structural changes associated with self-reported knee injury among individuals with incident knee osteoarthritis: data from the osteoarthritis initiative. Clin Anat. 2018;31(3):330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roemer FW, Zhang Y, Niu J, Lynch JA, Crema MD, Marra MD, et al. Tibiofemoral joint osteoarthritis: risk factors for MR-depicted fast cartilage loss over a 30-month period in the multicenter osteoarthritis study. Radiology. 2009;252(3):772-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hussain ZB, Chahla J, Mandelbaum BR, Gomoll AH, LaPrade RF. the role of meniscal tears in spontaneous osteonecrosis of the knee: a systematic review of suspected etiology and a call to revisit nomenclature. Am J Sports Med. 2019;47(2):501-7. [DOI] [PubMed] [Google Scholar]
- 15. Guermazi A, Hayashi D, Jarraya M, Roemer FW, Zhang Y, Niu J, et al. Medial posterior meniscal root tears are associated with development or worsening of medial tibiofemoral cartilage damage: the multicenter osteoarthritis study. Radiology. 2013;268(3):814-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goldring SR, Goldring MB. Bone and cartilage in osteoarthritis: is what’s best for one good or bad for the other? Arthritis Res Ther. 2010;12(5):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2010;18(1_suppl):24-33. [DOI] [PubMed] [Google Scholar]
- 18. Baum T, Joseph GB, Arulanandan A, Nardo L, Virayavanich W, Carballido-Gamio J, et al. Association of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with knee pain: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2012;64(2):248-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2—preliminary findings at 3 T. Radiology. 2000;214(1_suppl):259-66. [DOI] [PubMed] [Google Scholar]
- 20. Pan J, Pialat JB, Joseph T, Kuo D, Joseph GB, Nevitt MC, et al. Knee cartilage T2 characteristics and evolution in relation to morphologic abnormalities detected at 3-T MR imaging: a longitudinal study of the normal control cohort from the Osteoarthritis Initiative. Radiology. 2011;261(2):507-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stehling C, Liebl H, Krug R, Lane NE, Nevitt MC, Lynch J, et al. Patellar cartilage: T2 values and morphologic abnormalities at 3.0-T MR imaging in relation to physical activity in asymptomatic subjects from the osteoarthritis initiative. Radiology. 2010;254(2):509-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liebl H, Joseph G, Nevitt MC, Singh N, Heilmeier U, Subburaj K, et al. Early T2 changes predict onset of radiographic knee osteoarthritis: data from the osteoarthritis initiative. Ann Rheum Dis. 2015;74(7):1353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16(12):1433-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Joseph GB, Baum T, Alizai H, Carballido-Gamio J, Nardo L, Virayavanich W, et al. Baseline mean and heterogeneity of MR cartilage T2 are associated with morphologic degeneration of cartilage, meniscus, and bone marrow over 3 years—data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2012;20(7):727-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raya JG, Dietrich O, Horng A, Weber J, Reiser MF, Glaser C. T2 measurement in articular cartilage: impact of the fitting method on accuracy and precision at low SNR. Magn Reson Med. 2010;63(1_suppl):181-93. [DOI] [PubMed] [Google Scholar]
- 26. Miller AJ, Joseph PM. The use of power images to perform quantitative analysis on low SNR MR images. Magn Reson Imaging. 1993;11(7):1051-6. [DOI] [PubMed] [Google Scholar]
- 27. Rauscher I, Stahl R, Cheng J, Li X, Huber MB, Luke A, et al. Meniscal measurements of T1ρ and T2 at MR imaging in healthy subjects and patients with osteoarthritis. Radiology. 2008;249(2):591-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lerer DB, Umans HR, Hu MX, Jones MH. The role of meniscal root pathology and radial meniscal tear in medial meniscal extrusion. Skeletal Radiol. 2004;33(10):569-74. [DOI] [PubMed] [Google Scholar]
- 29. Brody JM, Lin HM, Hulstyn MJ, Tung GA. Lateral meniscus root tear and meniscus extrusion with anterior cruciate ligament tear. Radiology. 2006;239(3):805-10. [DOI] [PubMed] [Google Scholar]
- 30. Chanchek N, Gersing AS, Schwaiger BJ, Nevitt MC, Neumann J, Joseph GB, et al. Association of diabetes mellitus and biochemical knee cartilage composition assessed by T2 relaxation time measurements: data from the osteoarthritis initiative. J Magn Reson Imaging. 2018;47(2):380-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Neumann J, Guimaraes JB, Heilmeier U, Joseph GB, Nevitt MC, McCulloch CE, et al. Diabetics show accelerated progression of knee cartilage and meniscal lesions: data from the osteoarthritis initiative. Skeletal Radiol. 2018;48(6):919-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gersing AS, Schwaiger BJ, Heilmeier U, Joseph GB, Facchetti L, Kretzschmar M, et al. Evaluation of chondrocalcinosis and associated knee joint degeneration using mr imaging: data from the osteoarthritis initiative. Eur Radiol. 2017;27(6):2497-506. [DOI] [PubMed] [Google Scholar]
- 33. Bucknor MD, Nardo L, Joseph GB, Alizai H, Srikhum W, Nevitt MC, et al. Association of cartilage degeneration with four year weight gain—3T MRI data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2015;23(4):525-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord. 2008;9:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bin SI, Kim JM, Shin SJ. Radial tears of the posterior horn of the medial meniscus. Arthroscopy. 2004;20(4):373-8. [DOI] [PubMed] [Google Scholar]
- 36. Faucett SC, Geisler BP, Chahla J, Krych AJ, Kurzweil PR, Garner AM, et al. Meniscus root repair vs meniscectomy or nonoperative management to prevent knee osteoarthritis after medial meniscus root tears: clinical and economic effectiveness. Am J Sports Med. 2019;47(3):762-9. [DOI] [PubMed] [Google Scholar]
- 37. LaPrade RF, LaPrade CM, James EW. Recent advances in posterior meniscal root repair techniques. J Am Acad Orthop Surg. 2015;23(2):71-6. [DOI] [PubMed] [Google Scholar]
- 38. Lee JH, Lim YJ, Kim KB, Kim KH, Song JH. Arthroscopic pullout suture repair of posterior root tear of the medial meniscus: radiographic and clinical results with a 2-year follow-up. Arthroscopy. 2009;25(9):951-8. [DOI] [PubMed] [Google Scholar]
- 39. LaPrade RF, Matheny LM, Moulton SG, James EW, Dean CS. Posterior meniscal root repairs: outcomes of an anatomic transtibial pull-out technique. Am J Sports Med. 2017;45(4):884-91. [DOI] [PubMed] [Google Scholar]
- 40. Chernchujit B, Prasetia R. Arthroscopic direct meniscal extrusion reduction: surgical tips to reduce persistent meniscal extrusion in meniscal root repair. Eur J Orthop Surg Traumatol. 2018;28(4):727-34. [DOI] [PubMed] [Google Scholar]
- 41. Ozeki N, Muneta T, Kawabata K, Koga H, Nakagawa Y, Saito R, et al. Centralization of extruded medial meniscus delays cartilage degeneration in rats. J Orthop Sci. 2017;22(3):542-8. [DOI] [PubMed] [Google Scholar]
- 42. Daney BT, Aman ZS, Krob JJ, Storaci HW, Brady AW, Nakama G, et al. Utilization of transtibial centralization suture best minimizes extrusion and restores tibiofemoral contact mechanics for anatomic medial meniscal root repairs in a cadaveric model. Am J Sports Med. 2019;47(7):1591-600. [DOI] [PubMed] [Google Scholar]
- 43. Krych AJ, Reardon PJ, Johnson NR, Mohan R, Peter L, Levy BA, et al. Non-operative management of medial meniscus posterior horn root tears is associated with worsening arthritis and poor clinical outcome at 5-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2017;25(2):383-9. [DOI] [PubMed] [Google Scholar]
- 44. Krych AJ, Johnson NR, Mohan R, Dahm DL, Levy BA, Stuart MJ. Partial meniscectomy provides no benefit for symptomatic degenerative medial meniscus posterior root tears. Knee Surg Sports Traumatol Arthrosc. 2018;26(4):1117-22. [DOI] [PubMed] [Google Scholar]
- 45. Crema MD, Roemer FW, Felson DT, Englund M, Wang K, Jarraya M, et al. Factors associated with meniscal extrusion in knees with or at risk for osteoarthritis: the Multicenter Osteoarthritis study. Radiology. 2012;264(2):494-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Allaire R, Muriuki M, Gilbertson L, Harner CD. Biomechanical consequences of a tear of the posterior root of the medial meniscus. Similar to total meniscectomy. J Bone Joint Surg Am. 2008;90(9):1922-31. [DOI] [PubMed] [Google Scholar]
- 47. Bhatia S, LaPrade CM, Ellman MB, LaPrade RF. Meniscal root tears: significance, diagnosis, and treatment. Am J Sports Med. 2014;42(12):3016-30. [DOI] [PubMed] [Google Scholar]
- 48. Krych AJ, Johnson NR, Mohan R, Hevesi M, Stuart MJ, Littrell LA, et al. Arthritis progression on serial MRIs following diagnosis of medial meniscal posterior horn root tear. J Knee Surg. 2018;31(7):698-704. [DOI] [PubMed] [Google Scholar]
- 49. Wluka AE, Stuckey S, Snaddon J, Cicuttini FM. The determinants of change in tibial cartilage volume in osteoarthritic knees. Arthritis Rheum. 2002;46(8):2065-72. [DOI] [PubMed] [Google Scholar]
- 50. Hunter DJ, Zhang Y, Niu J, Goggins J, Amin S, LaValley MP, et al. Increase in bone marrow lesions associated with cartilage loss: a longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis Rheum. 2006;54(5):1529-35. [DOI] [PubMed] [Google Scholar]