Abstract
Objective
To determine potential predictive associations between patient-/lesion-specific factors, clinical outcome and anterior ankle impingement in patients that underwent isolated autologous matrix-induced chondrogenesis (AMIC) for an osteochondral lesion of the talus (OLT).
Design
Thirty-five patients with a mean age of 34.7 ± 15 years who underwent isolated cartilage repair with AMIC for OLTs were evaluated at a mean follow-up of 4.5 ± 1.9 years. Patients completed AOFAS (American Orthopaedic Foot and Ankle Society) scores at final follow-up, as well as Tegner scores at final follow-up and retrospectively for preinjury and presurgery time points. Pearson correlation and multivariate regression models were used to distinguish associations between patient-/lesion-specific factors, the need for subsequent surgery due to anterior ankle impingement and patient-reported outcomes.
Results
At final follow-up, AOFAS and Tegner scores averaged 92.6 ± 8.3 and 5.1 ± 1.8, respectively. Both body mass index (BMI) and duration of symptoms were independent predictors for postoperative AOFAS and Δ preinjury to postsurgery Tegner with positive smoking status showing a trend toward worse AOFAS scores, but this did not reach statistical significance (P = 0.054). Nine patients (25.7%) required subsequent surgery due to anterior ankle impingement. Smoking was the only factor that showed significant correlation with postoperative anterior ankle impingement with an odds ratio of 10.61 when adjusted for BMI and duration of symptoms (95% CI, 1.04-108.57; P = 0.047).
Conclusion
In particular, patients with normal BMI and chronic symptoms benefit from AMIC for the treatment of OLTs. Conversely, smoking cessation should be considered before AMIC due to the increased risk of subsequent surgery and possibly worse clinical outcome seen in active smokers.
Keywords: smoking, ankle, autologous matrix-induced chondrogenesis, talus, cartilage lesion, osteoarthritis, cartilage repair
Introduction
Osteochondral lesions of the talus (OLT) are defined as a damage involving both articular cartilage and subchondral bone primarily caused by a single or multiple traumatic events.1,2 Due to the poor regeneration potential of human hyaline cartilage, conservative therapy often fails and thus, operative treatment becomes necessary. 3
It is generally accepted that bone marrow stimulation alone is an adequate treatment for small OLTs of up to 1.5 cm2.4,5 In this procedure, blood cells emerging through small holes in the bone (microfracturing) form a clot in the defect zone, ultimately resulting in a fibrocartilage repair tissue. Though, microfracturing does not allow for bone stock restoration and the repair tissue is biomechanically inferior to native hyaline cartilage.6,7 Therefore, numerous techniques that restore rather the original joint conditions were advocated for larger defects,8,9 including autologous chondrocyte implantation (ACI), matrix-induced autologous chondrocyte implantation (MACI), osteochondral autograft transplantation (OAT), and osteochondral allograft (OCA). Of these procedures, however, none have shown superiority over others. 4 Procedures that transplant cartilage to the defect are generally either coupled with a 2-stage procedure to cultivate the cartilage ex vivo, entail donor site morbidity or use limited resources such as allograft.
Recently, autologous matrix-induced chondrogenesis (AMIC) was described as a single step procedure to treat cartilage defects with good to very good short- and midterm results and high return to sport. 10 Several patient specific risk factors for poor outcome after cartilage repair are controversially discussed in the current literature, including large defect size, increased body mass index (BMI), active smoking, hypertension, and increased patient’s age.11-18 However, little is known about patient- and lesion-specific predictive factors in isolated AMIC for the treatment of OLTs. 10 Additionally, we observed in our clinical practice that some patients who underwent AMIC for OLTs required subsequent ankle arthroscopy due to excessive anterior ankle scar tissue formation resulting in anterior ankle impingement.
Hence, this study sought to determine potential predictive associations between patient- and lesion-specific factors, clinical outcome, and need for reoperation due to anterior ankle impingement in patients that underwent isolated AMIC for OLTs. It was hypothesized that patient-specific factors, especially obesity and smoking, would lead to inferior clinical outcome and an increased rate of subsequent surgery due to anterior ankle impingement.
Materials and Method
This study was approved by the local research ethics committee before initiation. Data are regularly collected and stored for patients undergoing elective surgery at our institution. This database was used to identify patients who underwent isolated cartilage repair with AMIC for focal osteochondral defects of the talus between October 2009 and August 2015. To avoid heterogeneity of the group due to different surgical techniques, only patients with an open approach and malleolar osteotomy were included. The surgical treatment with AMIC was indicated in patients who were resistant to nonoperative therapies for symptomatic focal full-thickness chondral and osteochondral defects on the talus. Contraindications comprised inflammatory arthritis and/or advanced osteoarthritis.
Patients were included if they underwent isolated AMIC for a focal OLT and completed patient-reported outcome measures including the American Orthopaedic Foot and Ankle Society (AOFAS; 0-100 points) score for ankle function, and the Tegner score (0-10 points) for sports activity. Conversely, patients were excluded if they had any prior or concomitant ankle surgical procedure.
Patient’s age at the time of surgery, BMI, sex, smoking status (active yes/no), lesion etiology, duration of symptoms (DOS), and whether the patient was diagnosed with hypertension were recorded. Osteochondral defect size was collected from surgical notes or preoperative imaging if available. According to Choi et al., 9 lesion size was calculated using the ellipse formula (coronal length × sagittal length × 0.79).
Surgical Technique
The surgical technique recently described by Weigelt et al. 10 was used for all patients in the current study. Briefly, the OLT was addressed via an open approach with malleolar osteotomy. Unstable cartilage and necrotic bone were removed and microfracturing was performed. Subchondral defects or cysts were curetted and filled with autologous cancellous from the medial malleolus through the osteotomy. A moistened collagen I/III bilayer matrix (Chondro-Gide; Geistlich Pharma AG) was adapted to the shape and size of the OLT and fixed with fibrin glue (Tissucol; Baxter) to fill the defect. After passively moving the ankle joint in plantar flexion and dorsal extension, the correct position of the AMIC membrane was verified. The malleolar osteotomy was fixed with two 3.5-cm malleolar screws.
Postoperative Rehabilitation
Patients were mobilized in a lower leg cast for 6 weeks with partial weightbearing of 15 kg. Afterward, protected weightbearing was gradually increased in a lower leg cast to full weightbearing within weeks 10 to 12. Mobilization of the ankle joint was performed after wound healing.
Definition of Anterior Ankle Impingement
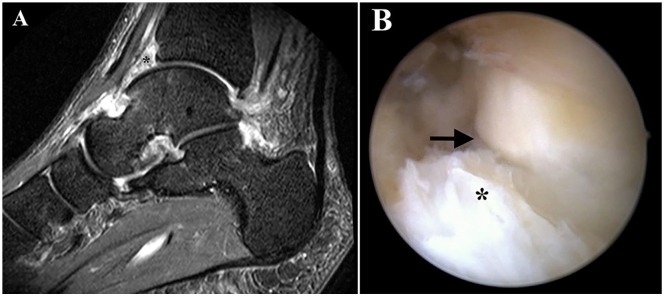
Anterior ankle impingement is defined as impingement of the ankle joint by either an excessive soft tissue (scar tissue) formation or osteophytic changes at the anterior aspect of the tibial platfond or talar neck. 19 In the current study, patients that underwent subsequent surgery due to anterior ankle impingement explicitly complained about anterior ankle joint pain, triggered by full dorsiflexion, which is considered the key clinical element for diagnosis. 19 Patients also received magnetic resonance imaging evaluation, which has shown to be a valuable adjunct to the clinical assessment in patients with anterior ankle impingement. 20 All patients were resistant to conservative treatment including bracing, physiotherapy, and cortisone injections, ultimately requiring anterior ankle debridement by ankle arthroscopy ( Fig. 1 ).
Figure 1.
Magnetic resonance imaging (A) evaluation in a patient with postoperative anterior ankle impingement, who ultimately required subsequent ankle arthroscopy (B) for scar tissue debridement. *scar tissue; → joint line
Statistical Analysis
Descriptive statistics were calculated to determine the sociodemographic and clinical characteristics of patients. Bivariate correlations were assessed by Pearson’s and point-biserial correlation coefficients (r). Potential predictive factors showing significance at a level of P < 0.15 were included in the final clinical outcome regression models. Categorical variables were coded as dummy variables for multivariate linear and logistic regression models (i.e., for sex, 0 represented male and 1 represented female). Models included patient age, sex, BMI, smoking status, hypertension, lesion etiology, DOS, bone grafting, defect size, and defect location. Subsequent surgery due to anterior ankle impingement and patient-reported scores, including AOFAS and Tegner were dependent variables. Regression coefficients and odds ratios are reported. All statistical analyses were performed in SPSS for Mac (Version 23.0, IBM Corp, Armonk, NY). Significance was set at P < 0.05.
Results
A total of 35 patients with a mean age of 34.7 ± 15 years and a mean BMI of 28.7 ± 5.4 kg/m2 who underwent isolated cartilage repair with AMIC for OLTs and a mean follow-up of 4.5 ± 1.9 years were enrolled in this study. Of these patients, 14 (40%) were female, 17 (48.6%) were active smokers, 6 (17.1%) were diagnosed with hypertension, 27 (77.1%) had symptoms for more than 12 months, and 9 (25.7%) patients presented with osteochondrosis dissecans. The assessed defect size of each patient averaged 0.9 ± 0.6 cm2 (range, 0.2-2.4 cm2) with 27 of 35 lesions (77.1%) requiring autologous bone grafting. Three lesions (8.5%) were located on the lateral talar dome, whereas the majority occurred on the medial talar dome (n = 32, 91.5%). In the study cohort, 9 (25.7%) patients required subsequent surgery due to anterior ankle impingement within the study period. The outcomes for all patient-reported ankle-specific surveys are presented in Table 1 . Both, high preoperative preinjury and pre surgery Tegner scores were negatively correlated with the Δ Tegner of preinjury and presurgery to postsurgery, respectively (r = −0.46, P = 0.005 and r = −0.657, P < 0.001). Patients with higher Δ preinjury to postsurgery Tegner scores (patients that returned to sport on a slightly lower or even higher level than preinjury) also showed better postoperative AOFAS scores (r = 0.383, P = 0.023).
Table 1.
Patient-Reported Outcome Measures.
| Score | Preinjury | Presurgery | Postsurgery |
|---|---|---|---|
| AOFAS | — | — | 92.6 ± 8.3 |
| Tegner | 6.2 ± 1.9 | 3.7 ± 2.0 | 5.1 ± 1.8 |
| Δ Preinjury to postsurgery Tegner | — | — | −1.1 ± 1.6 |
| Δ Presurgery to postsurgery Tegner | — | — | 1.5 ± 2.3 |
AOFAS = American Orthopaedic Foot and Ankle Society.
In the multivariate linear regression models, BMI and DOS over 1 year were independent predictors of Δ preinjury to postsurgery Tegner (P = 0.015 and P = 0.006, respectively) with increased age showing a positive association with the outcome, but this did not reach significance (P = 0.058) ( Table 2 ). BMI and DOS over 1 year also significantly predicted postoperative AOFAS scores (P = 0.002 and P = 0.035, respectively). Positive smoking status showed a trend toward worse postoperative clinical outcome measured by the AOFAS (P = 0.054) ( Table 3 ). Overall, 9 (25.7%) patients underwent subsequent surgery of the ankle due to anterior ankle impingement. Positive smoking status was the only factor correlated to the need for subsequent surgery (r = 0.344; P = 0.043). In fact, 41.2% of active smokers had to undergo subsequent surgery due to anterior ankle impingement compared with 11.2% of nonsmokers. In a multivariate logistic regression model adjusted for BMI and DOS, smokers were 10 times more likely to develop postoperative anterior ankle impingement than nonsmokers (odds ratio [OR] = 10.61; 95% CI, 1.04-108.57; P = 0.047) ( Table 4 ).
Table 2.
Linear Multivariate Regression Model for Δ Preinjury to Postsurgery Tegner.
| Factors | Regression Coefficient | Standard Error | P | 95% Confidence Interval |
|---|---|---|---|---|
| BMI | −0.121 | 0.047 | 0.015 | −0.216 to −0.025 |
| DOS >1 year | 1.570 | 0.535 | 0.006 | 0.478 to 2.662 |
| Gender | −0.083 | 0.464 | 0.859 | −1.031 to 0.864 |
| Age | 0.029 | 0.015 | 0.058 | −0.001 to 0.059 |
BMI = body mass index; DOS = duration of symptoms.
Table 3.
Linear Multivariate Regression Model for AOFAS.
| Factors | Regression Coefficient | Standard Error | P | 95% Confidence Interval |
|---|---|---|---|---|
| BMI | −0.845 | 0.244 | 0.002 | −1.343 to −0.347 |
| Smoking | −4.666 | 2.331 | 0.054 | −9.421 to 0.089 |
| DOS >1 year | 6.062 | 2.742 | 0.035 | 0.470 to 11.654 |
AOFAS = American Orthopaedic Foot and Ankle Society; BMI = body mass index; DOS = duration of symptoms.
Table 4.
Logistic Regression Model for the Need of Subsequent Surgery.
| Factors | Odds Ratio | 95% Confidence Interval | P | |
|---|---|---|---|---|
| Smoking | 10.61 | 1.04 | 108.57 | 0.047 |
| BMI | 1.03 | 0.858 | 1.25 | 0.73 |
| DOS >1 year | 0.17 | 0.015 | 1.91 | 0.15 |
BMI = body mass index; DOS = duration of symptoms.
Discussion
The key finding of the current study is that patients with a positive smoking status are 10 times more likely to undergo subsequent surgery due to anterior ankle impingement after isolated AMIC for the treatment of OLTs. Smoking did also show a trend toward inferior postoperative clinical outcome, yet, without reaching statistical significance (P = 0.054). Furthermore, increased BMI was correlated with less improvement in activity and worse clinical outcome after AMIC. Conversely, chronicity of symptoms (DOS >1 year) was associated with increased improvement of Tegner scores and postoperative AOFAS scores.
Thus far, various patient and lesion specific factors for poor clinical outcome after cartilage repair have been identified, including increased age, lesion size, BMI, and smoking.11,13,21-23 Although smoking was not statistically significantly associated with worse postoperative clinical outcome after AMIC (P = 0.054) in the current study, it substantially increased the odds for subsequent surgery due to anterior ankle impingement by 1 power of 10 (OR = 10.61, P = 0.047). While there is paucity of literature investigating the effect of smoking on cartilage repair in the ankle, it is well known that smoking changes blood supply and healing potential of soft tissue with an increased risk for wound complications and nonunion in foot and ankle surgery.24-28 The present study supports the idea that smoking also alters healing of soft tissue after cartilage repair of the ankle and might therefore lead to postoperative anterior ankle impingement. Kanneganti et al. 17 systematically reviewed the literature to find evidence if smoking influences ligamentous or cartilage repair surgery in the knee from both a basic science and clinical outcome perspective. While 3 studies reported a negative influence of smoking on clinical outcome after cartilage repair,11,29,30 1 study showed that both insulin-like growth factor-I (IGF-I) and basic fibroblast growth factor (bFGF) were significantly diminished in smokers compared with nonsmokers. 31 These markers have been shown to be substantially involved in cartilage repair mechanisms,32,33 suggesting that smoking cessation would benefit patients undergoing cartilage repair surgery, which is supported by the findings of this study.
Generally, studies that determined the predictive value of preoperative parameters in patients undergoing AMIC for the treatment of OLTs are scarce. Yet, only 1 prior study has focused on assessing age as a potential risk factor for AMIC in the treatment of OLTs. The authors evaluated 31 patients that underwent arthroscopic talus AMIC, of which 17 were younger and 14 were older than 33 years. Patients were assessed preoperatively as well as postoperatively at 6, 12, and 24 months. Both groups showed significantly improved clinical and radiological scores at all time points, regardless of group allocation. Younger patients, however, presented with slightly better preoperative, as well as 12- and 24-month postoperative AOFAS scores. The study concluded that arthroscopic talus AMIC is a safe and reliable procedure to treat OLTs in both young and old patients. 34 Similar results were seen in the current study as patient’s age did not predict inferior outcome but showed a tendency toward better improvement of activity after surgery compared with preinjury activity level (P = 0.58). It can be theorized that this finding roots in the rather low preinjury activity level of older patients when compared with younger patients as age was inversely correlated with preinjury Tegner scores (r = −0.595, P < 0.001), thus requiring only a small postoperative benefit in activity level to reach the preinjury activity level. In other words, patients with low sporting activity are more likely to postoperatively reach their preinjury activity level compared with highly active patients. Analogous postulations can be made when analyzing the impact of chronicity of symptoms on the clinical outcome after AMIC. Patients with symptoms that lasted for more than 12 months showed an increased improvement of the individual sports activity level and patient-reported clinical outcome. Generally, patients with chronic symptoms tend to be less active and exhibit a smaller sporting ambition than patients with traumatic injuries (current study: mean preinjury Tegner 5.9 vs. 7.0), which are usually caused by a single traumatic event, often involving high-impact sports. Hence, patient’s expectations on clinical outcome may diverge between groups with return to low-impact sports being sufficient for chronic but not for traumatic patients.
This study also revealed a negative influence of BMI on clinical outcome after AMIC. Thus far, only 1 prior study focused on the relevance of BMI regarding outcome of osteochondral restoration with AMIC in ankle surgery. Usuelli et al. 35 prospectively studied 37 patients undergoing arthroscopic AMIC for OLTs and stratified them based on their preoperative BMI, resulting in mean BMIs of 21.90 ± 1.94 and 27.41 ± 1.98 kg/m2 for each group. The clinical assessment comprised magnetic resonance imaging, computed tomography, visual analogue scale, Short-Form Health Survey (SF-12) and AOFAS. Interestingly, increased BMI was associated with larger preoperative lesion size and postoperative bone marrow edema, yet clinical outcome seemed to not be influenced by patient’s BMI as both groups exhibited significantly improved AOFAS at 6, 12, and 24 months postoperatively. 35 However, the authors did not perform a direct comparison between both groups. In fact, patients with an increased BMI showed a smaller improvement of AOFAS scores from pre- to postoperatively (24.8 and 32.3 points at 1 and 2 years compared with 36.8 and 41.2, respectively). Also, a BMI of 27.41 ± 1.98 kg/m2 in the overweight group may not have been sufficient enough to reveal the true impact of BMI as a prognostic factor. The average BMI of the present study was 28.7 ± 5.4 kg/m2, hence displaying a greater range than the study published by Usuelli et al. 35 (24.3 ± 3.4 kg/m2), which might have contributed to the effect seen in the current study. Consequently, it can be theorized that, in fact, an increased BMI negatively influences clinical outcome after AMIC for OLTs.
The authors acknowledge the following limitations. The study design is retrospective, with Tegner preinjury and presurgery scores collected retrospectively at the postoperative follow-up assessment, potentially introducing recall bias. Yet, this was the reason why preoperative AOFAS was not retrospectively collected in the study cohort, as the complexity of the score would have led to an unreliable preoperative score. Conversely, it can be theorized that the simplicity of the Tegner activity scale reduced recall bias in the assessment of preoperative patient’s activity.
In conclusion, especially patients with normal BMI and chronic symptoms benefit from AMIC for the treatment of OLTs. Conversely, smoking cessation should be considered before AMIC due to the increased risk of anterior ankle impingement and possibly worse clinical outcome seen in active smokers.
Footnotes
Authors’ Note: The study was performed at the University Hospital Balgrist, University of Zurich, Zurich, Switzerland.
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by the local research ethics committee before initiation.
Informed Consent: Written informed consent was obtained from all subjects before the study.
Trial Registration: Not applicable.
ORCID iDs: Felix W.A. Waibel  https://orcid.org/0000-0003-3991-8743
https://orcid.org/0000-0003-3991-8743
Jakob Ackermann  https://orcid.org/0000-0002-4063-5815
https://orcid.org/0000-0002-4063-5815
References
- 1. Wiewiorski M, Barg A, Valderrabano V. Chondral and osteochondral reconstruction of local ankle degeneration. Foot Ankle Clin. 2013;18(3):543-54. [DOI] [PubMed] [Google Scholar]
- 2. Zengerink M, Struijs PA, Tol JL, van Dijk CN. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):238-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verhagen RA, Struijs PA, Bossuyt PM, van Dijk CN. Systematic review of treatment strategies for osteochondral defects of the talar dome. Foot Ankle Clin. 2003;8(2):233-42, viii-ix. [DOI] [PubMed] [Google Scholar]
- 4. Dahmen J, Lambers KTA, Reilingh ML, van Bergen CJA, Stufkens SAS, Kerkhoffs G. No superior treatment for primary osteochondral defects of the talus. Knee Surg Sports Traumatol Arthrosc. 2018;26(7):2142-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramponi L, Yasui Y, Murawski CD, Ferkel RD, DiGiovanni CW, Kerkhoffs G, et al. Lesion size is a predictor of clinical outcomes after bone marrow stimulation for osteochondral lesions of the talus: a systematic review. Am J Sports Med. 2017;45(7):1698-705. [DOI] [PubMed] [Google Scholar]
- 6. Buckwalter JA, Mow VC, Ratcliffe A. Restoration of injured or degenerated articular cartilage. J Am Acad Orthop Surg. 1994;2(4):192-201. [DOI] [PubMed] [Google Scholar]
- 7. Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4(1_suppl):30-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Driscoll SW. The healing and regeneration of articular cartilage. J Bone Joint Surg Am. 1998;80(12):1795-812. [PubMed] [Google Scholar]
- 9. Choi WJ, Park KK, Kim BS, Lee JW. Osteochondral lesion of the talus: is there a critical defect size for poor outcome? Am J Sports Med. 2009;37(10):1974-80. [DOI] [PubMed] [Google Scholar]
- 10. Weigelt L, Hartmann R, Pfirrmann C, Espinosa N, Wirth SH. Autologous matrix-induced chondrogenesis for osteochondral lesions of the talus: a clinical and radiological 2- to 8-year follow-up study. Am J Sports Med. 2019;47(7):1679-86. [DOI] [PubMed] [Google Scholar]
- 11. Jaiswal PK, Macmull S, Bentley G, Carrington RW, Skinner JA, Briggs TW. Does smoking influence outcome after autologous chondrocyte implantation? A case-controlled study. J Bone Joint Surg Br. 2009;91(12):1575-8. [DOI] [PubMed] [Google Scholar]
- 12. Bentley G, Bhamra JS, Gikas PD, Skinner JA, Carrington R, Briggs TW. Repair of osteochondral defects in joints—how to achieve success. Injury. 2013;44(Suppl 1):S3-10. [DOI] [PubMed] [Google Scholar]
- 13. Jungmann PM, Salzmann GM, Schmal H, Pestka JM, Sudkamp NP, Niemeyer P. Autologous chondrocyte implantation for treatment of cartilage defects of the knee: what predicts the need for reintervention? Am J Sports Med. 2012;40(1_suppl):58-67. [DOI] [PubMed] [Google Scholar]
- 14. Toale J, Shimozono Y, Mulvin C, Dahmen J, Kerkhoffs G, Kennedy JG. Midterm outcomes of bone marrow stimulation for primary osteochondral lesions of the talus: a systematic review. Orthop J Sports Med. 2019;7(10):2325967119879127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gille J, Schuseil E, Wimmer J, Gellissen J, Schulz AP, Behrens P. Mid-term results of autologous matrix-induced chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1456-64. [DOI] [PubMed] [Google Scholar]
- 16. Chuckpaiwong B, Berkson EM, Theodore GH. Microfracture for osteochondral lesions of the ankle: outcome analysis and outcome predictors of 105 cases. Arthroscopy. 2008;24(1_suppl):106-12. [DOI] [PubMed] [Google Scholar]
- 17. Kanneganti P, Harris JD, Brophy RH, Carey JL, Lattermann C, Flanigan DC. The effect of smoking on ligament and cartilage surgery in the knee: a systematic review. Am J Sports Med. 2012;40(12):2872-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Merkely G, Ackermann J, Gomoll AH. The role of hypertension in cartilage restoration: increased failure rate after autologous chondrocyte implantation but not after osteochondral allograft transplantation. Cartilage. Published online January 22, 2020. doi: 10.1177/1947603519900792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vaseenon T, Amendola A. Update on anterior ankle impingement. Curr Rev Musculoskelet Med. 2012;5(2):145-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duncan D, Mologne T, Hildebrand H, Stanley M, Schreckengaust R, Sitler D. The usefulness of magnetic resonance imaging in the diagnosis of anterolateral impingement of the ankle. J Foot Ankle Surg. 2006;45(5):304-7. [DOI] [PubMed] [Google Scholar]
- 21. Becher C, Driessen A, Hess T, Longo UG, Maffulli N, Thermann H. Microfracture for chondral defects of the talus: maintenance of early results at midterm follow-up. Knee Surg Sports Traumatol Arthrosc. 2010;18(5):656-63. [DOI] [PubMed] [Google Scholar]
- 22. Choi WJ, Jo J, Lee JW. Osteochondral lesion of the talus: prognostic factors affecting the clinical outcome after arthroscopic marrow stimulation technique. Foot Ankle Clin. 2013;18(1_suppl):67-78. [DOI] [PubMed] [Google Scholar]
- 23. Domayer SE, Welsch GH, Stelzeneder D, Hirschfeld C, Quirbach S, Nehrer S, et al. Microfracture in the ankle: clinical results and MRI with T2-mapping at 3.0 T after 1 to 8 years. Cartilage. 2011;2(1_suppl):73-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sorensen LT, Jorgensen S, Petersen LJ, Hemmingsen U, Bulow J, Loft S, et al. Acute effects of nicotine and smoking on blood flow, tissue oxygen, and aerobe metabolism of the skin and subcutis. J Surg Res. 2009;152(2):224-30. [DOI] [PubMed] [Google Scholar]
- 25. Lee DO, Eom JS, Jung HG. The effect of smoking on the outcomes of lateral ankle ligament reconstruction. J Orthop Sci. 2018;23(1_suppl):88-91. [DOI] [PubMed] [Google Scholar]
- 26. Cobb TK, Gabrielsen TA, Campbell DC, 2nd, Wallrichs SL, Ilstrup DM. Cigarette smoking and nonunion after ankle arthrodesis. Foot Ankle Int. 1994;15(2):64-7. [DOI] [PubMed] [Google Scholar]
- 27. Frey C, Halikus NM, Vu-Rose T, Ebramzadeh E. A review of ankle arthrodesis: predisposing factors to nonunion. Foot Ankle Int. 1994;15(11):581-4. [DOI] [PubMed] [Google Scholar]
- 28. Reus WF, Robson MC, Zachary L, Heggers JP. Acute effects of tobacco smoking on blood flow in the cutaneous micro-circulation. Br J Plast Surg. 1984;37(2):213-5. [DOI] [PubMed] [Google Scholar]
- 29. Spahn G, Kahl E, Muckley T, Hofmann GO, Klinger HM. Arthroscopic knee chondroplasty using a bipolar radiofrequency-based device compared to mechanical shaver: results of a prospective, randomized, controlled study. Knee Surg Sports Traumatol Arthrosc. 2008;16(6):565-73. [DOI] [PubMed] [Google Scholar]
- 30. Spahn G, Muckley T, Kahl E, Hofmann GO. Factors affecting the outcome of arthroscopy in medial-compartment osteoarthritis of the knee. Arthroscopy. 2006;22(11):1233-40. [DOI] [PubMed] [Google Scholar]
- 31. Schmal H, Niemeyer P, Sudkamp NP, Gerlach U, Dovi-Akue D, Mehlhorn AT. Pain perception in knees with circumscribed cartilage lesions is associated with intra-articular IGF-1 expression. Am J Sports Med. 2011;39(9):1989-96. [DOI] [PubMed] [Google Scholar]
- 32. Li X, Su G, Wang J, Zhou Z, Li L, Liu L, et al. Exogenous bFGF promotes articular cartilage repair via up-regulation of multiple growth factors. Osteoarthritis Cartilage. 2013;21(10):1567-75. [DOI] [PubMed] [Google Scholar]
- 33. Zhang Z, Li L, Yang W, Cao Y, Shi Y, Li X, et al. The effects of different doses of IGF-1 on cartilage and subchondral bone during the repair of full-thickness articular cartilage defects in rabbits. Osteoarthritis Cartilage. 2017;25(2):309-20. [DOI] [PubMed] [Google Scholar]
- 34. D’Ambrosi R, Maccario C, Serra N, Liuni F, Usuelli FG. Osteochondral lesions of the talus and autologous matrix-induced chondrogenesis: is age a negative predictor outcome? Arthroscopy. 2017;33(2):428-35. [DOI] [PubMed] [Google Scholar]
- 35. Usuelli FG, Maccario C, Ursino C, Serra N, D’Ambrosi R. The impact of weight on arthroscopic osteochondral talar reconstruction. Foot Ankle Int. 2017;38(6):612-20. [DOI] [PubMed] [Google Scholar]