Abstract
Cartilage defects in the knee are being diagnosed with increased frequency and are treated with a variety of techniques. The aim of any cartilage repair procedure is to generate the highest tissue quality, which might correlate with improved clinical outcomes, return-to-sport, and long-term durability. Minced cartilage implantation (MCI) is a relatively simple and cost-effective technique to transplant autologous cartilage fragments in a single-step procedure. Minced cartilage has a strong biologic potential since autologous, activated non-dedifferentiated chondrocytes are utilized. It can be used both for small and large cartilage lesions, as well as for osteochondral lesions. As it is purely an autologous and homologous approach, it lacks a significant regulatory oversight process and can be clinically adopted without such limitations. The aim of this narrative review is to provide an overview of the current evidence supporting autologous minced cartilage implantation.
Keywords: minced cartilage implantation, cartilage regeneration, cartilage defect, articular cartilage, cartilage repair
Introduction
Articular cartilage defects are being detected with increasing frequency due to the increasing use of magnetic resonance imaging (MRI) and higher accuracy and resolution of newer MRI machines.1,2 Operative cartilage restoration techniques in variety and foremost quantity are on the rise. It is well known that existing cartilage defects do not heal postpuberty. 3 The typical natural history of these defects is progression in diameter and depth. During this process, the knee joint is constantly affected by deleterious catabolic cytokines. 4
The generation of pain due to focal cartilage defects is poorly understood. However, studies suggest that pain may arise from exposed subchondral bone when the cartilage lesion reaches a certain depth or from the development of an inflammatory process within the joint.3,5 In most patients both processes occur in parallel. Thus, the aim is to stop both processes as early as possible in order to prevent the evolution of osteoarthritis (OA). 3 As once early-onset osteoarthritis develops, particularly in active young patients, management of biologic joint restoration becomes challenging.
Several techniques are available for the repair of chondral and osteochondral lesions. 6 Only a few cartilage repair techniques have a long track-record and supporting evidence in terms of improvement in patient outcomes. 7 These include marrow stimulation techniques (microfracturing [MFX] and microdrilling), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation system, and osteochondral allograft transplantation. Fresh osteochondral allografts (OCA) are a very attractive and effective tissue source, mainly used in the United States due to relatively high availability of grafts. 8 However, in Europe, only select countries have similar access to donor tissue, yet, most do not and therefore are dependent on autologous tissue. Treatment with microfracture is limited to small diameter purely chondral lesions. 9 Outcome deterioration is expected at 3 to 5 years following surgery due to the low quality of regenerative fibrocartilage. Well-designed randomized studies have shown superiority of ACI over microfracture.7,10 ACI is now in its fourth generation and can be regarded as a highly effective tissue engineering procedure. Lesion diameter and depth does not preclude the use of ACI due to the development of several techniques to address these issues. However, a major limitation of ACI is that currently 2 operations are required. The primary procedure is typically a staging procedure in which a biopsy is harvested. The biopsy must be prepared in a laboratory under strict regulations. This process results in high health-related costs, particularly in the United States. 11 Also, final cellular quality in terms of cell differentiation and the regeneration of hyaline cartilage has been a frequent matter of debate. 12 In light of the limitations of currently available cartilage restoration techniques, 13 novel cartilage restoration procedures are being developed.
The surgical technique of slicing viable cartilage into small pieces and direct reimplantation is not novel. 14 However, it is currently gaining much interest among surgeons worldwide due to several attributes, including a rather simple surgical technique, being a single-step procedure, having a strong biologic potential, and relatively high cost-effectiveness. 15 This principal concept had been, to the best of our knowledge, initially reported by Albrecht et al. 14 in 1982 and 1983 and thereafter by Lu et al. 16 in 2006. These are clearly distinct from a previously described clinical procedure in which autologous osteochondral morselized graft (mixed bone and cartilage) was used as the cell source.17,18 The aim of this narrative review is to provide an overview of the current evidence supporting autologous minced cartilage implantation.
Biologic Background to Fragmented Cartilage Repair Techniques
Postpuberty, articular cartilage cells are postmitotic. Within intact cartilage the chondrocyte ceases to divide. The chondrocyte is responsible for constant matrix breakdown and construction, in reaction to the surrounding biomechanical and biochemical input. 19 Cartilage damage initiates in situ reparative processes, where available chondrocytes aim to refill the defect by proliferation. Since chondral tissue is hypocellular, hypovascular, and aneural, such proliferation processes are ineffective. One can often see clustered chondrocytes within defective regions while the remainder of the defect is filled up by biomechanically inferior connective tissue (fibrocartilage) that was generated by invading fibroblastic cells. As a result, the whole organ system loses homeostasis and catabolic processes ensue. 20
The biologic rationale behind particulated cartilage is to perform an in situ repair using a sufficient amount of activated autologous tissue of the appropriate lineage. When a small piece of articular cartilage is being inserted into an in vitro cell culture flask and surrounded by chondrogenic medium one can observe the process of outgrowth of the chondrocytes off the piece to settle down on plastic. 21 There, the cells divide and proliferate until the cells contact each other and the culture flask is covered with cells. Since an adult chondrocyte cannot divide, it loses its phenotype in order to proliferate—a process called dedifferentiation. 22 As the number of divisions per cell increases, the chondrocyte parts from more of its original characteristics due to the dedifferentiation process. Thus, a chondrocyte that had divided 5 to 8 times in vitro is no longer expressing cartilage-typical proteins (such as collagen 2, aggrecan, and others). 22 The ability to produce differentiated cartilage-typical matrix is therefore lost in favor of cell proliferation. A fibroblast-like cell increases in quantity while producing biomechanically weak type 1 collagen, among other proteins. Simultaneously, telomere length is being shortened as part of the aging process. 23
In summary, chondrocyte proliferation and dedifferentiation constitute a pathologic process within damaged articular cartilage tissue, that results in biomechanically and biologically inferior fibrocartilage. 24 Such processes also take place when ACI products are prepared within in vitro tissue engineering laboratories. It remains unclear whether such cells are capable of redifferentiating when being implanted in vivo.
The environment in vivo may differ significantly from the environment in vitro. A highly complex mix of biomechanical and perhaps foremost biochemical input on the tissue is present in in vivo models. Furthermore, the joint milieu is constantly changing. Such a complex scenario cannot be simulated or readjusted in vitro. Repair of cartilage defects with sufficient cellular quantity may produce coverage of the lesion with high-quality tissue. However, Hansen et al. 25 reported that increasing chondrocyte densities do not positively affect the outcome of cartilage repair in a New Zealand White rabbit animal model. Chondrocytes have the potential to proliferate physiologically without severe dedifferentiation under naive surroundings within a repairing joint. The process of in situ tissue engineering may play a critical role in such interplay. 26 Extracellular surroundings have to be 3-dimensional (3D) and there must be a biochemical input as well as mechanical stimulation for an effective in situ cartilage engineering. 27 Such processes may be key for the minced cartilage procedure, where viable chondrocytes are brought into the defective region at a high quantity. The purpose is to promote outgrowth of embedded chondrocytes through increased tissue surface area that results from tissue fragmentation, a process one may term as “activation.”
Via outgrowth, the defect is covered quickly by chondral tissue due to chondrocyte proliferation. Lu et al. 16 have shown that chondrocyte migration and proliferation from the tissue fragments were effectively elicited via tissue morselization or fragmentation. In further studies, outgrowth potential, viability for 28 days in culture, and matrix deposition were demonstrated using human chondrocytes in varying biomaterials. 28 At the same time, differentiation is kept at a certain level, which is related to the fact that the cells are sitting within 3D surroundings. Such architecture may limit dedifferentiation and promote differentiation. Rothdiener et al. 29 reported in an in-vitro hydrogel culture of human OA chondrons versus OA chondrocytes a significantly higher cell viability and increased differentiation pattern in the chondron culture. Additionally, there is an abundance of cytokines and enzymes within the joint in a reparative state following cartilage restoration procedures that may suppress dedifferentiation and promote differentiation. 30 Not all minced cartilage pieces are completely digested, at least initially. Histological data had shown that very small pieces of live chondrocytes and unchanged surrounding matrix are being incorporated into the newly built matrix around the chips. 28
Summary of Biologic Background
Adult human cartilage cells are postmitotic
Fragmentation of healthy cartilage is meant to activate mitogenic activity
Following implantation, outgrowth of autologous chondrocytes is initiated
Outgrowth of chondrocytes from minced pieces results in proliferation
Proliferation to a differentiated state under 3D surroundings in vivo may promote de novo extracellular matrix (ECM) production of naive articular cartilage tissue
Via in situ/in vivo tissue engineering the differentiated state of the chondrocyte is maintained
The time course of healing following implantation is positively influenced by biochemical and biomechanical input of the joint as a whole
Scientific Aspects of Preparation
Autologous minced cartilage implantation ( Fig. 1 ) is composed of cutting healthy and vital hyaline cartilage into small pieces and then directly implanting the minced cartilage into the chondral or osteochondral lesion ( Fig. 2 ). The technique for debridement of cartilage lesions creating a stable vertical wall with a viable rim has been well described. 31 This can be achieved via an arthroscopic or open approach. The removal of the calcified layer remains a matter of debate. 16 Advocates for not performing marrow stimulation emphasize that the aim of the minced cartilage procedure is to promote healing of the cartilage defect via chondrocytes and not via an influx of blood from the subchondral bone, which may be regarded as contamination of the transplanted cells. Some studies suggest that the amount of “stem cells” in the developing blood clot is insignificant and may not be the origin of repair when performing a microfracture procedure. 32
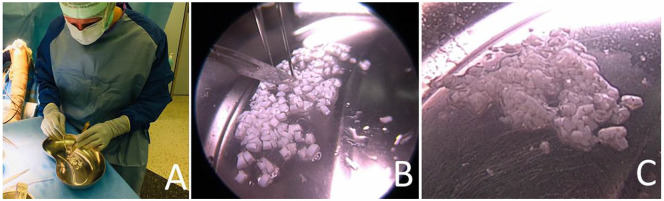
Figure 1.
Scalpel-minced cartilage immediately prior to implantation. While the surgeon is preparing the defect, the assistant is mincing the cartilage using a scalpel within a kidney basin (A). Fragmentation should be performed with caution and optimally in a drop of isotonic solution for better adherence of the chips to the undersurface (B). The chips should have a final paste-like appearance (C).
Figure 2.
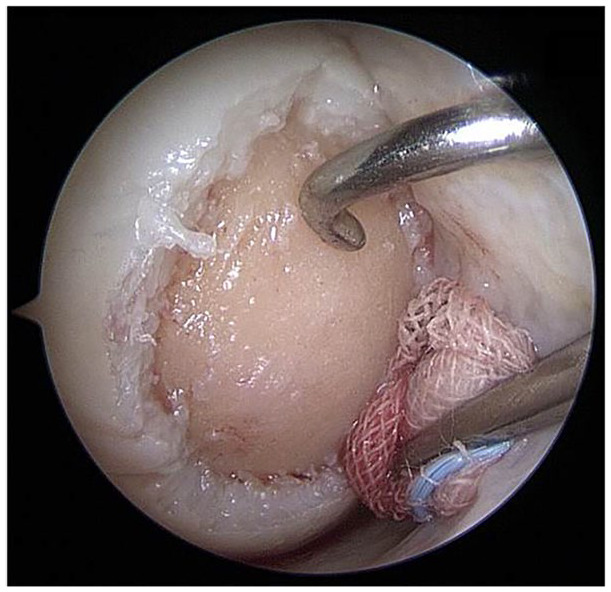
Arthroscopically prepared cartilage defect (medial femoral condyle, right knee joint, dry conditions). Using the proximal medial portal, a probing hook is placed for defect size measurement (application devices can be introduced into the joint via the same portal). In the distal medial portal, a swab is placed to safely keep the joint dry and to tense the joint capsule and Hoffa fat, to distant them from the defect.
Removal of nonhealthy bone is recommended when an osteochondral lesion with more than 3 mm of osseous deficiency is encountered. The defect should then be filled using autologous or allogeneic cancellous bone fragments. 33 For small bony defects, small allogeneic bone chips have shown good results. For larger defects, autologous bone grafting, possibly using the iliac crest, is recommended as recently reported by Grechenig et al. 34 A stable and viable bone bed is key to successful augmentation of the overlying cartilage repair. Autologous bone can also be harvested from nonweightbearing areas such as the distal medial/lateral femur or the intercondylar notch. Osteochondral cylinders harvested from nonweightbearing areas (notch) can provide both the osseous foundation and the cartilage to be minced prior to implantation ( Fig. 3 ).
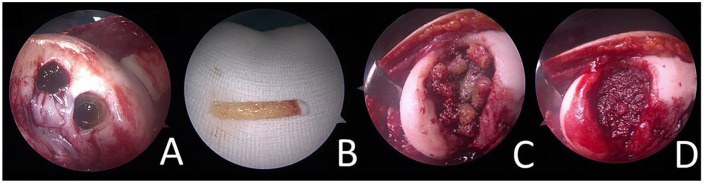
Figure 3.
Harvest site at the lateral femoral intercondylar notch (A). Here, long (2-4 cm) osteochondral cylinders (B) can be easily harvested. The osseous part can be directly implanted (C) into osteochondral lesions and impacted to form a stable, vital, and autologous cancellous bone-plasty (D). Chondrocytes off the top of the harvested cylinders (B) can be applied for a single-step minced cartilage procedure.
There are several sources where cartilage can be harvested from. Two common sources are loose bodies (that are purely chondral and appear healthy macroscopically) or osteochondritis dissecans (OCD) fragments (i.e. when fixation is not a feasible option). In both scenarios, it has been shown that the harvested cartilage is vital. 15 If such options are not feasible, harvesting can be performed from nonweightbearing areas such as the intercondylar notch. Such technique allows for vital cartilage harvesting without significant donor-site morbidity.35,36 Following standard cartilage defect preparation, one can easily harvest cartilage from the healthy cartilage defect edge, using a ringed curette or a shaver. This technique only slightly increases the defect’s diameter, but at the same time ensures the remaining margins of the defect contain only healthy cartilage rim. Aurich et al. 12 demonstrated a superior redifferentiation of chondrocytes harvested from the edge of the defect compared with nonweightbearing regions. Current evidence is limited regarding the effect of defect size on cell-based cartilage repair outcomes. Furthermore, it had been shown via in vitro models that there is a clear difference between weight-bearing and nonweightbearing cartilage. 37 Interestingly, Aurich et al. 12 reported a superior redifferentiation potential of cartilage lesion associated chondrocytes, supporting harvest of cartilage from the defect margins.
The next step of the surgical technique is the fragmentation of cartilage. Cole et al. 38 in their safety trial of cartilage autograft implantation system (CAIS) reported the use of a designated device for mincing the harvested cartilage. Such devices, in some way, may display a black box when cartilage cell vitality is concerned. However, such devices can be effective and fast, and decreased cell viability when compared to other methods has yet to be proven. During fragmentation, the cartilage should be cut sharply and not crushed, in order to limit cell death. Redman et al. 39 demonstrated in a bovine cartilage injury model that blunt trauma performed by trephine resulted in a 100-µm zone of necrosis. In contrast sharp trauma by scalpel did not produce a significant chondrocyte apoptosis rate. Similar results were reported by Tew et al. 40 in a bovine cartilage trephine injury model. Skagen et al. 41 presented a porcine culture model with scalpel produced explants and reported a necrosis zone of 40 to 80 µm after 1 to 4 weeks’ culture. Nevertheless, the main difference to minced cartilage is the additional culture period. Christensen et al. 42 performed a clinical trial with 2-year follow up, where they reported on the use of a scalpel for particulation of cartilage. The goal of mincing cartilage is to mince the cartilage into the smallest pieces possible until a paste-like appearance is achieved. However, this must be achieved without negatively affecting cell viability and further cellular performance. Bonasia et al. 43 performed an experimental trial showing that the degree of fragmentation is affecting proliferation and differentiation capacity in vitro. They reported increased fragmentation increases ECM production. 43 This is, most likely, related to the increased surface of the chips that may promote outgrowth. However, there is no clear evidence of a lower limit of cartilage fragment size. Further research is necessary to analyze fragment size and viability. Levinson et al. 28 performed an in vitro study that compared mincing using a scalpel versus a designated mincing device. They reported that outgrowth potential, differentiation behavior, the viability after 28 days in culture, and the matrix deposition were not different between the mincing techniques. Yet, the mincing device was faster and resulted in significantly smaller cartilage particles, that were more homogenous with regard to size. According to Hunziker et al. 44 approximately 1 million chondrocytes should be implanted per 1 cm2 cartilage defect. This corresponds to 168 cartilage chips of 1 × 1 × 1 mm in size. Currently knife-mincing, device-mincing, and shaver-mincing methods are acceptable. Future studies will need to identify the optimal technique to mince cartilage in clinical practice.
Scientific Aspects of Implantation
Fixation of the chips into the defective region following implantation is the final step ( Fig. 4 ). Albrecht et al. 14 in 1983 reported on the use of fibrin glue in order to hold the fragmented pieces in place. Lu et al. 16 reported on the use of a membrane that was preloaded with cartilage chips. The membrane was secured into the lesion using resorbable staples. Of note, such staples create perforations in the subchondral bone resulting in an influx of blood into the biomaterial. Furthermore, during degradation, in many cases the staples cause local inflammation, potentially harming the maturing chondrocytes. The aim should be to have the highest possible chips to biomaterial/carrier ratio at the defect site. Articular chondrocytes perform best without any disturbances after implantation and primary fixation. 45 Christensen et al. 46 reported on fixation of the construct using fibrin glue only. This approach may generate enough initial stability so that the cells can attach to the base of the defect and surrounding cartilage walls. Salzmann et al. 33 published a technical note where they reported the combination of fibrin glue with a collagen membrane. This combination might increase initial stability to the developing construct underneath, creating a water-tight chamber as similarly reported by Brittberg et al. 47 during first-generation ACI. Moreover, a secondary coverage might increase resistance to shearing forces in specific areas within the knee, such as the patellofemoral joint.
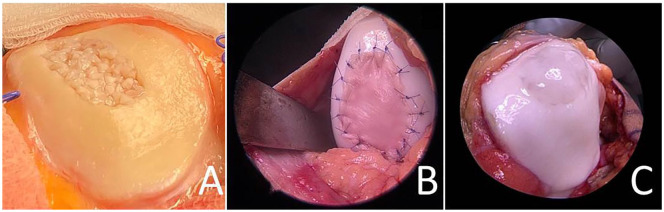
Figure 4.
Minced cartilage implanted at the patella retrosurface before fixation (A). Minced cartilage implanted at the lateral femoral condyle (right knee joint) and covered by collagen membrane (Chondrogide, Geistlich) (B), minced cartilage at the patella undersurface fixed by fibrin glue only (C).
Further developments might result in a purely autologous fixation technique that may combine a biologic agent with a biologic sealant. For example, this can be applied using platelet-rich plasma (PRP) ( Fig. 5 ). However, there is contradictory evidence in PRP effect and cartilage repair, which is not the focus of this review.48-50
Figure 5.
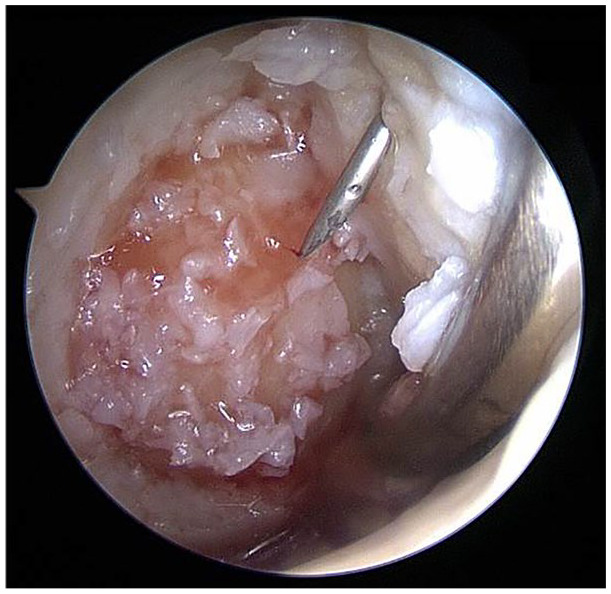
Arthroscopically prepared cartilage defect (medial femoral condyle, right knee joint, dry conditions; defect from Fig. 2 ). The cartilage chips (previously mixed with platelet-rich plasma [PRP]) have been implanted already via arthroscopic techniques. An autologous thrombin solution is currently applied via a needle through the proximal portal for final fixation of the chips. As the last step, a mix of PRP and the autologous thrombin solution is applied to finalize the coagulation cascade in order to provide initial construct stability. In the distal portal, a swab is placed in order to safely keep the joint dry and to tense the joint capsule and Hoffa fat to distance them from the defect.
The authors of this article had reported improved success of the minced cartilage procedure when an anterior cruciate ligament reconstruction ( Fig. 6 ) is being performed at the same time. Influx of bone marrow into the joint cavity may be the explanation for that. A similar benefit had been described in meniscal repair surgery as well. 51 When suturing of the membrane on top of the cartilage chips, an open approach is necessary. If fibrin glue or other hydrogel-based biomaterials are applied, arthroscopic techniques are feasible.
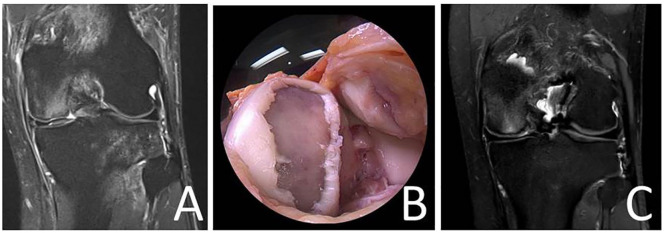
Figure 6.
Magnetic resonance image demonstrating a full-thickness cartilage lesion (and anterior cruciate ligament [ACL] rupture) at the medial femoral condyle of a left knee joint (A). Intraoperative image of the medial femoral condyle displaying a large diameter (7.5 cm2) cartilage lesion at the medial femoral condyle (B). Magnetic resonance Image 6 months following minced cartilage implantation (MCI) at the medial femoral condyle (the patient also received an ACL reconstruction) (C).
Last, postoperative rehabilitation is key to the success of any procedure and the optimal protocol would need to be elucidated. The biomechanical input is of great importance to the generating tissue as it develops into functioning cartilage tissue. Bioreactor models have shown a significant difference between evolving cartilage (minced cartilage) tissue that is mechanically challenged early on versus cartilage tissue that was not subject to any mechanical stimulation. 52 Currently, similar rehabilitation protocols are recommended for ACI and MCI. 53
Summary of Surgical Techniques
Fragmentation should achieve the smallest possible cartilage chips (<1 mm3)—optimally resulting in a paste-like appearance
Sharply cut and avoid crushing the cartilage during particulation (to minimally decrease cell vitality)
Fragmentation should be quick, controllable, and highly standardized
Optimal fragmentation promotes outgrowth and differentiation
Mincing devices hold potential to fragment in a fast, effective, and highly standardized manner
Fixation of minced cartilage can be performed via fibrin/thrombin, hydrogels and/or membrane coverage, potentially augmented by PRP/platelet-poor plasma (PPP)
In Vitro Studies
Lu et al. 16 described initial experiments where cartilage tissue was harvested from the intercondylar notch of human subjects undergoing anterior cruciate ligament reconstruction and from the femoral condyles of adult bovine animals, then the tissue was aseptically rinsed, and transferred into a Petri dish. The tissue was minced into small fragments (approximately 1 mm3) with a surgical scalpel and evenly loaded onto a 3D scaffold. The cell-matrix products were then kept in vitro under standard chondrogenic culturing conditions. They observed that the initial bromodeoxyuridine (BrdU) incorporation was little, but that over time the chondrocytes seemed to get activated and a progressive increase in the BrdU signal was detected in chondrocytes within the cultured cartilage fragments. It was also reported that the BrdU signal in the chondrocytes seemed to localize along the tissue edge or on the surface, suggesting a correlation between tissue mincing and mitogenic activation of the chondrocytes. Furthermore, chondrocyte outgrowth into the scaffolds was reported. Outgrown cells were interconnected with newly deposited extracellular matrix. An inverse relationship between tissue fragment size and the efficiency of the outgrowth was found when cartilage fragments of different sizes were analyzed.
An observation that was again underlined by Bonasia et al. 43 years later where cartilage was taken from 5 donors undergoing total hip replacement. The cartilage was minced to obtain 4 groups with different fragment sizes: (1) “fish scale” (diameter, 8 mm; thickness, 0.3 mm); (2) cubes with 2-mm sides; (3) cubes with 1-mm sides; and (4) cartilage paste (<0.3 mm). The cultures were maintained in chondrogenic medium for 6 weeks. The group concluded that human cartilage fragmentation significantly affects ECM production in vitro, suggesting increased fragmentation enhances ECM production. Bonasia et al. 43 assumed a similar behavior in vivo and recommended mincing the cartilage into small pieces when performing the cartilage fragment autograft implantation technique in order to increase ECM production.
In 2012, Marmotti et al. 54 evaluated cultures of rabbit cartilage fragments on Petri dishes. A paste scaffold with injectable hyaluronic acid (HA), and a membrane scaffold with an HA-derivative were saturated with cartilage chips. At 60 days, a time-dependent cell outgrowth from cartilage fragments was observed with both types of scaffolds. A lesser extent of chondrocyte migration was observed with Petri dishes than with scaffolds. After 2 months of in vitro culture, neo-matrix was evident, and the migrated chondrocytes showed a roundish shape. Newly formed tissue was positive for collagen type II immunostaining. In another study published in 2013, Marmotti et al. 55 compared the cell outgrowth from human cartilage fragments of adult and young donors using 2 different types of scaffolds (HA-derivative injectable paste scaffold and HA-derivative membrane scaffold) and evaluated the influence of transforming growth factor–β1 (TGF-β1) and granulocyte colony-stimulating factor (G-CSF) on chondrocyte behavior. The histological analysis showed age- and time-dependent chondrocyte migration. Marmotti et al. 55 later also reported on beneficial effects of PRP on chondrocyte performance. Other authors also reported on beneficial effects of PRP on chondrocyte proliferation and differentiation. 45 Moreover, immunomodulatory effects suppressing inflammation were reported with the use of PRP. It is evident that chondrocyte maturation and correct ECM production is dependent on constant biochemical and biomechanical (physiobiochemical) input.
A reaction to biomechanical input was tested by Wang et al. 56 in 2014 using a knee-joint specific bioreactor with dynamic compression and shear on minced bovine cartilage fragment cultures. The authors noticed that this method of culture was feasible under in vitro free-swelling and dynamic loading conditions, simulating in vivo posttransplantation. Mechanical stimulation significantly provoked cellular outgrowth and long-term chondrogenic maturation at the mRNA level, whereas histology depicted immature neo-tissue (weaker collagen type II and aggrecan expression with an increased collagen type I expression).
Tsuyuguchi et al. 57 in 2018, compared minced cartilage with enzymatically digested chondrocytes (control group) embedded in a 3D carrier in vitro. After 3 weeks, the authors reported, using histology and immunochemistry, that minced cartilage showed cell migration from the cartilage fragments into the gel, with the Bern score and cell count in the minced cartilage group being significantly higher than those in the control group. Minced cartilage exhibited superior cell migration, proliferation, and glycosaminoglycan content than isolated chondrocytes. The authors concluded that minced cartilage has a favorable potential for cell proliferation and matrix production compared with the isolated chondrocytes after enzymatic treatment.
In 2016, Andjelkov et al. 58 cultured human arthritic articular chondrocytes within fibrin in vitro and found that none of the biopsies demonstrated outgrowth of chondrocytes or bone marrow-originated cells into the fibrin matrix. Levinson et al. 28 in 2019 also cultured human arthritic chondrocytes in vitro within fibrin and a collagen-based hydrogel. They also compared hand (scalpel) to device mincing. The group reported that the initial chondrocyte viability in cartilage particles dropped by 25% with the use of a mincing device as compared with no mincing. However, the viability in hand-minced, device-minced, and unminced samples was no longer different after 7 and 28 days in culture. Outgrowth scores were similar among the 3 groups. Fibrin and collagen biomaterials equally supported chondrocyte outgrowth and survival, but neither promoted matrix deposition after in vitro culture.
Zingler et al. 21 also reported on limited outgrowth of minced cartilage chondrocytes within fibrin or collagen matrices. Furthermore, the effect of chemotactic stimuli, including cell lysate, high-mobility-group-protein B1 (HMGB-1), trefoil-factor 3 (TFF3), bone morphogenetic protein-2 (BMP-2), and TGF-β1 were investigated. The occurrence of cellular outgrowth was analyzed by histological examination after a culture period of 4 weeks. Spontaneous cellular outgrowth from cleansed cartilage specimens was not observed at a relevant level and could not significantly be induced by chemotactic stimuli or 3D matrices either. A forming cartilage-adjoining cell layer was only apparent in the case of native cartilage explants with cellular remnants from surgical isolation or in co-culture experiments with synovial membrane. A major drawback of this study is that the authors did not fragment (activate) the cartilage pieces into smaller pieces, but cultured harvested material directly. This approach is opposing the initial idea of mincing cartilage that was initially described by Lu et al. 16 This theory is emphasized by experimental data from Bonasia et al. 43 Multiple other studies, including many animal trials (see below), have shown the functionality of mincing and activating cartilage. Therefore, the reported data from Zingler et al. 21 may not be representative for minced cartilage, since technical execution differs from that recommended for minced cartilage procedures. However, these results may be applicable for cultured cartilage pieces.
In vitro data demonstrated that primary activated chondrocytes establish de novo extracellular matrix via outgrowth, proliferation, and differentiation. Mechanical stimulation is crucial for cartilage regeneration.
Summary of In Vitro Studies
Chondrocyte outgrowth from chondral fragments is feasible in vitro
Chondrocytes are being activated via mincing
Chondrocytes divide and produce de novo ECM after outgrowth
Activation and viability are time-dependent
3D surroundings promote chondrogenic differentiation
Mechanical stimulation promotes chondrogenic differentiation
Biologic stimuli promote proliferation and chondrogenic differentiation
Animal Models
Initial animal data presented by Albrecht et al. 14 reported on 75 knee joints of 46 adult rabbits. Osteochondral defects of 4 mm diameter were created by a drill reaching the cancellous bone. Twenty-three defects were left untreated, or closed by a collagen foam or fibrin adhesive, or a combination of both. Fifty-two defects were closed with very small autologous cartilage fragments and a special fibrin adhesive. In the first group of 23 joints observed over 40 weeks, no hyaline cartilage was found histologically in any of the defects. In the second group, a rapid proliferation of chondrocytes appeared with development of hyaline cartilage with Alcian blue–positive matrix. It resembled juvenile cartilage in its histologic appearance. The phenomenon was interpreted as a “second adolescence” of the adult cartilage induced by the rich nutritional and oxygen supply from the cancellous vessels, which resembles the environmental conditions before the forming of subchondral cortical bone at the end of the growth period. This method enabled the authors to achieve a complete closure of defects by hyaline cartilage on the very level of the surrounding articular surface. 14
Lu et al. 16 were the first to reexamine the topic of cartilage piece implantation in the early 2000s. The group demonstrated that autologous chondrocyte implantation can be delivered without requiring ex vivo cell expansion. The authors proposed that mechanical fragmentation of cartilage tissue is sufficient to mobilize embedded chondrocytes via increased tissue surface area. Fragmented cartilage/chondrocytes outgrown into 3D scaffolds and formed cartilage-like tissue when implanted in severe combined immunodeficient (SCID) mice (subcutaneous pockets located in the lateral thoracic region). The authors described in the same publication on successful treatment of full-thickness chondral defects in goats using cartilage fragments on a resorbable scaffold, that produced hyaline-like repair tissue at 6 months.
In 2008, Lind et al. 59 investigated the cartilage repair capacity of autologous cartilage chips compared to ACI with a collagen membrane in a goat model. Sixteen full-thickness cartilage defects (6 mm in diameter) were created in the femoral condyles of 8 adult goats. At 4 months, no difference was found in O’Driscoll and Pineda histology scores, tissue filling (35%), or repair tissue stiffness between the 2 groups. In a similar study, Frisbie et al. 60 compared empty control defects, the cartilage autograft implantation (CAIS) technique, and ACI in a horse model (10 skeletally mature horses). Arthroscopic, histologic, and immunohistochemistry results showed superiority of both implantation techniques (ACI and CAIS) compared with control groups, with CAIS achieving the highest score.
In 2012, Marmotti et al. 54 compared the repair tissue of 5 different groups of treatment in a rabbit model (50 adult rabbits). At 6 months postoperatively, cartilage fragment-loaded scaffolds induced significantly better repair tissue (in terms of histological modified ICRS score and a modified O’Driscoll scale) than the scaffold alone groups. In 2013, Marmotti et al. 55 studied a culture-free approach to osteochondral repair with minced autologous cartilage fragments loaded onto a multi-composition scaffold, which were implanted in 15 adult goats. Best hyaline-like repair tissue was evident in the minced cartilage group in terms of morphological, mechanical, and histological assessments.
In 2016, Christensen et al. 46 randomized 12 Göttingen minipigs to either autologous bone graft (ABG) combined with autologous cartilage chips (autologous dual-tissue transplantation [ADTT]) or ABG alone. There was significantly more hyaline cartilage in the ADTT group (25.8%) compared with the ABG group (12.8%) at 6 months after treatment. At 12 months, the fraction of hyaline cartilage in the ABG group had significantly decreased to 4.8%, whereas the fraction of hyaline cartilage in the ADTT group was unchanged (20.1%). The authors concluded that the presence of cartilage chips in an osteochondral defect facilitated the formation of fibrocartilage as opposed to fibrous tissue at both 6 and 12 months posttreatment. The implanted chips were present in the defect and viable after 12 months. In 2017, the same group 61 reported on the direct comparison of microfracture versus autologous cartilage chips (ACC) embedded in fibrin glue within Göttingen minipigs and concluded that the ACC transplant resulted in improved quality of cartilage repair tissue compared with MFX at 6 months postoperatively.
In 2018, Perez et al. 62 showed effective minced cartilage repair plus PPP and PRP in a sheep model with 8 mm diameter condylar defects. The treatment consisted of surgical implantation of an autologous-based matrix of hyaline cartilage chips combined with a clot of PPP and intraarticular injection of plasma rich in growth factors. The 6-month macroscopic evaluation showed nearly normal (11.1 ± 0.7) cartilage repair assessment. The ICRS (International Cartilage Repair Society) score was significantly higher at 6 months compared with 3 months (5.5 ± 1.3; P < 0.0001) and 1 (1.1 ± 0.4; P < 0.0001) month. At 6 months, hyaline cartilage tissue filling the defect was observed with adequate integration of the regenerated cartilage at the surrounding healthy cartilage margin. At 6 months, mature chondrons and cartilage matrix contained collagen fibers with masked fibrillary structure, and the expression of collagen in the newly formed cartilage was similar in intensity and distribution pattern compared to the healthy adjacent cartilage. Matsushita et al. 63 embedded minced cartilage or isolated chondrocytes from rabbits in atelocollagen gel and cultured for 3 weeks. Chondrocyte proliferation and matrix production were evaluated in vitro. An osteochondral defect at the trochlear groove was created in 56 rabbits, which were divided into 4 groups. The defect was left empty (defect group), filled with allogeneic minced cartilage (minced cartilage group), filled with isolated allogeneic chondrocytes embedded in atelocollagen gel (ACI group), or filled with atelocollagen gel (atelocollagen with periosteal flap group). The group concluded that implantation of minced cartilage embedded in atelocollagen gel as a single-step procedure has outcomes similar to those of ACI but is cheaper and more convenient than ACI.
Animal models have demonstrated a clear biologic potential of implanted activated primary chondrocytes similar to that of autologous chondrocyte implantation, the current gold standard in cartilage repair.
Summary of Animal Models
Chondrocyte outgrowth from chondral fragments is feasible in vivo
Minced cartilage shows results superior to control (empty defect, empty membrane)
Minced cartilage shows results superior to microfracture
Minced cartilage shows results similar to ACI
Clinical Data
Cole et al. 38 performed the first clinical trial in 2011, evaluating the safety and outcomes of CAIS using a designated mincing device. Patients (n = 29) were randomized to receive either MFX or the CAIS procedure. Patients were followed at predetermined time points for 2 years using several standardized outcomes assessment tools. General outcome measures indicated an overall improvement in both groups, and no differences in the number of adverse effects were noted between the CAIS and MFX groups. The IKDC (International Knee Documentation Committee) score of the CAIS group was significantly higher at 12 months and at 24 months compared with the MFX group. Qualitative analysis of the imaging data did not demonstrate differences between the 2 groups in fill of the graft bed, tissue integration, or presence of subchondral cysts. The authors concluded that CAIS is a safe, feasible, and effective method.
In 2015, Christensen et al. 42 presented preliminary data on a combined autologous bone and cartilage chips: autologous dual-tissue transplantation (ADTT) technique. Eight patients (age 32 ± 7.5 years) suffering from osteochondritis dissecans (OCD) received a cancellous bone-plasty that was covered by autologous cartilage chips embedded within fibrin glue. Cartilage tissue repair evaluated using the Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) score improved from 22.5 to 52.5 (P < 0.01). Computed tomography imaging demonstrated very good subchondral bone healing, with all 8 patients having a bone filling of >80%. Improvements were found among all clinical scores that were collected (final Knee Injury and Osteoarthritis Outcome Score [KOOS] of 68.1). The authors concluded that treatment of OCD with ADTT resulted in very good subchondral bone restoration and good cartilage repair.
In 2019, Massen et al. 64 reported on 2-year outcome data among 27 consecutive subjects that were treated by the second-generation minced cartilage implantation technique. All patients underwent preoperative and postoperative magnetic resonance imaging for the collection of Area Measurement And Depth and Underlying Structures (AMADEUS) 65 and MOCART scores. Clinical analysis was conducted by a numeric analogure scale (NAS) for pain and knee function before the intervention and at 12 and 24 months postoperatively. There was a significant decrease in pain from 7.2 ± 1.9 preoperatively to 1.8 ± 1.6 (P < 0.001) at 2-year follow-up. Knee function improved from a mean of 7.2 ± 2.0 preoperatively to 2.1 ± 2.3 (P < 0.001) at 2 years after surgery. The mean preoperative AMADEUS score was 57.4 ± 21.4. Postoperatively, the mean MOCART score was 40.6 ± 21.1 at 6-month follow-up. No correlation was observed between the clinical data and the MOCART or AMADEUS scores. The authors concluded that patients undergoing a single-step autologous minced cartilage procedure had a satisfactory outcome at 2-year follow-up ( Figs. 6 , 7 , and 8 ) for varying indications (large chondral, osteochondral, tibia).
Figure 7.
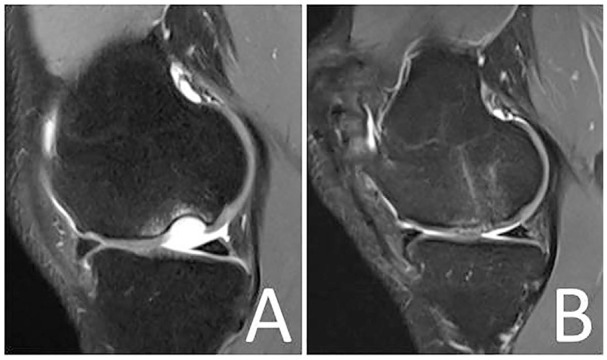
Magnetic resonance image demonstrating a full-thickness osteochondral lesion at the medial femoral condyle of a right knee joint (A). Magnetic resonance image 6 months following minced cartilage implantation (MCI) and underlying cancellous bone-plasty (single-step procedure) at the medial femoral condyle (B).
Figure 8.
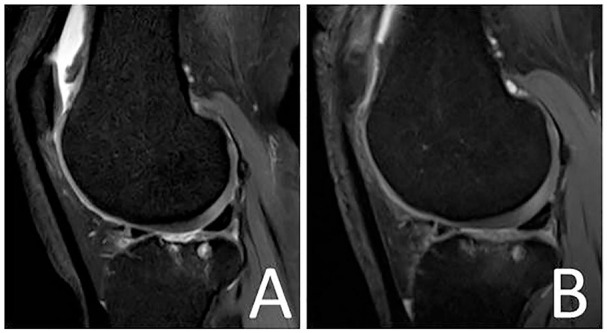
Magnetic resonance image demonstrating a full-thickness cartilage lesion at the lateral tibia plateau of a right knee joint (A). Magnetic resonance image 6 months following minced cartilage implantation (MCI) at the lateral tibia plateau (B).
These results suggest the single-step autologous minced cartilage procedure does represent a possible alternative to standard autologous chondrocyte implantation. Nevertheless, available evidence is limited. To our best knowledge only 3 clinical trials evaluating outcomes of minced cartilage repair are currently available. Longer follow-up and larger cohorts are required to better define the benefits of this procedure. There is frequent use of particulated juvenile articular cartilage (PJAC) as a single-step treatment of chondral lesions in varying musculoskeletal pathologies in the United States. The evidence is still scarce, yet certain investigations have reported good success. 66
Summary of Clinical Data
Clinical evidence on autologous minced cartilage procedures is limited
Available data supports the notion that minced cartilage is a safe procedure
Published studies have shown satisfying clinical outcomes
Failure and revision rates are comparable to other available cartilage repair techniques
More comparative trials are necessary to allow comparison with alternative cartilage repair techniques
Long-term outcomes data are required to determine minced cartilage implant durability
Outlook and Conclusion
There are several aspects that still need to be clarified. Future studies should aim to define lower and upper limits for defect diameter. With regard to small-diameter cartilage defects, minced cartilage can clearly be regarded as an alternative option to marrow stimulation procedures, since minced cartilage implantation can also be applied in a single-step procedure and via arthroscopic techniques. It must be clarified if minced cartilage can also be effectively applied for osteochondral lesions in a standard fashion. Clinical data regarding the efficacy of minced cartilage for different anatomical regions in the knee joint (i.e., lateral femoral condyle versus medial tibial plateau) is needed. Several fragmentation techniques have been described including the use of a scalpel, as well as mincing devices, which have been developed and introduced to the field during the CAIS trial (marketing discontinued) and the newly introduced AutoCart procedure (Arthrex, GmbH, Munich) for an all autologous cartilage regeneration. Experimental studies have shown no significant difference in vitality and proliferation of particulated chondrocytes minced using a scalpel and designated devices. Mincing devices are much faster (seconds) and fragmentation is highly standardized.
Another matter that requires further knowledge is fixation techniques, which currently include the use of staples, a membrane, fibrin glue, and membrane-fibrin glue combination. When aiming for an all-arthroscopic and purely single-step autologous cartilage repair procedure, a hydrogel or similar vehicle might provide higher structural integrity. PRP/PPP may be beneficial for minced cartilage proliferation and differentiation and can be used as an adjunct for minced cartilage repair procedures ( Fig. 5 ). As shown by Irwin et al., 67 autologous thrombin solution may be applied as a sealant to increase stability. Finally, optimal arthroscopic techniques should be defined and published to make the procedure more accessible to surgeons worldwide.
Autologous minced cartilage repair is a single-step approach that does not require manipulation of the specimen in the laboratory or the use of allografts. It is therefore economically attractive and should not require significant regulations, as other procedures might. So far published clinical evidence is limited and further studies are required to establish the clinical efficacy of autologous minced cartilage restoration procedures. Based on the findings of this review, it appears this procedure may have a strong biologic, economic, and clinical potential for future cartilage repair.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Salzmann GM is a consultant for Arthrex.
ORCID iD: Robert Ossendorff  https://orcid.org/0000-0002-6636-8065
https://orcid.org/0000-0002-6636-8065
References
- 1. Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14(3):177-82. doi: 10.1016/j.knee.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 2. Flanigan DC, Harris JD, Trinh TQ, Siston RA, Brophy RH. Prevalence of chondral defects in athletes’ knees: a systematic review. Med Sci Sports Exerc. 2010;42(10):1795-801. doi: 10.1249/MSS.0b013e3181d9eea0 [DOI] [PubMed] [Google Scholar]
- 3. Buckwalter JA, Mankin HJ, Grodzinsky AJ. Articular cartilage and osteoarthritis. Instr Course Lect. 2005;54:465-40. [PubMed] [Google Scholar]
- 4. Ossendorff R, Grad S, Stoddart MJ, Alini M, Schmal H, Sudkamp N, et al. Autologous chondrocyte implantation in osteoarthritic surroundings: TNFα and its inhibition by adalimumab in a knee-specific bioreactor. Am J Sports Med. 2018;46(2):431-40. doi: 10.1177/0363546517737497 [DOI] [PubMed] [Google Scholar]
- 5. Mabey T, Honsawek S. Cytokines as biochemical markers for knee osteoarthritis. World J Orthop. 2015;6(1_suppl):95-105. doi: 10.5312/wjo.v6.i1.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chimutengwende-Gordon M, Donaldson J, Bentley G. Current solutions for the treatment of chronic articular cartilage defects in the knee. EFORT Open Rev. 2020;5(3):156-63. doi: 10.1302/2058-5241.5.190031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Riboh JC, Cvetanovich GL, Cole BJ, Yanke AB. Comparative efficacy of cartilage repair procedures in the knee: a network meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2017;25(12):3786-99. doi: 10.1007/s00167-016-4300-1 [DOI] [PubMed] [Google Scholar]
- 8. Zouzias IC, Bugbee WD. Osteochondral allograft transplantation in the knee. Sports Med Arthrosc Rev. 2016;24(2):79-84. doi: 10.1097/JSA.0000000000000109 [DOI] [PubMed] [Google Scholar]
- 9. Ossendorff R, Franke K, Erdle B, Uhl M, Südkamp NP, Salzmann GM. Clinical and radiographical ten years long-term outcome of microfracture vs. autologous chondrocyte implantation: a matched-pair analysis. Int Orthop. 2019;43(3):553-9. doi: 10.1007/s00264-018-4025-5 [DOI] [PubMed] [Google Scholar]
- 10. Brittberg M, Recker D, Ilgenfritz J, Saris DBF. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: five-year follow-up of a prospective randomized trial. Am J Sports Med. 2018;46(6):1343-51. doi: 10.1177/0363546518756976 [DOI] [PubMed] [Google Scholar]
- 11. Everhart JS, Campbell AB, Abouljoud MM, Kirven JC, Flanigan DC. Cost-efficacy of knee cartilage defect treatments in the United States. Am J Sports Med. 2020;48(1_suppl):242-51. doi: 10.1177/0363546519834557 [DOI] [PubMed] [Google Scholar]
- 12. Aurich M, Hofmann GO, Best N, Rolauffs B. Induced redifferentiation of human chondrocytes from articular cartilage lesion in alginate bead culture after monolayer dedifferentiation: an alternative cell source for cell-based therapies? Tissue Eng Part A. 2018;24(3-4):275-86. doi: 10.1089/ten.TEA.2016.0505 [DOI] [PubMed] [Google Scholar]
- 13. Mistry H, Connock M, Pink J, Shyangdan D, Clar C, Royle P, et al. Autologous chondrocyte implantation in the knee: systematic review and economic evaluation. Health Technol Assess. 2017;21(6):1-294. doi: 10.3310/hta21060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Albrecht FH. Closure of joint cartilage defects using cartilage fragments and fibrin glue [in German]. Fortschr Med. 1983;101(37):1650-2. [PubMed] [Google Scholar]
- 15. Bonasia DE, Marmotti A, Rosso F, Collo G, Rossi R. Use of chondral fragments for one stage cartilage repair: a systematic review. World J Orthop. 2015;6(11):1006-11. doi: 10.5312/wjo.v6.i11.1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu Y, Dhanaraj S, Wang Z, Bradley DM, Bowman SM, Cole BJ, et al. Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair. J Orthop Res. 2006;24(6):1261-70. doi: 10.1002/jor.20135 [DOI] [PubMed] [Google Scholar]
- 17. Stone KR, Pelsis JR, Na K, Walgenbach AW, Turek TJ. Articular cartilage paste graft for severe osteochondral lesions of the knee: a 10- to 23-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2017;25(12):3824-33. doi: 10.1007/s00167-016-4323-7 [DOI] [PubMed] [Google Scholar]
- 18. Stone KR, Walgenbach AW, Freyer A, Turek TJ, Speer DP. Articular cartilage paste grafting to full-thickness articular cartilage knee joint lesions: a 2- to 12-year follow-up. Arthroscopy. 2006;22(3):291-9. doi: 10.1016/j.arthro.2005.12.051 [DOI] [PubMed] [Google Scholar]
- 19. Darling EM, Athanasiou KA. Articular cartilage bioreactors and bioprocesses. Tissue Eng. 2003;9(1_suppl):9-26. doi: 10.1089/107632703762687492 [DOI] [PubMed] [Google Scholar]
- 20. Haseeb A, Haqqi TM. Immunopathogenesis of osteoarthritis. Clin Immunol. 2013;146(3):185-96. doi: 10.1016/j.clim.2012.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zingler C, Carl HD, Swoboda B, Krinner S, Hennig F, Gelse K. Limited evidence of chondrocyte outgrowth from adult human articular cartilage. Osteoarthritis Cartilage. 2016;24(1_suppl):124-8. doi: 10.1016/j.joca.2015.07.014 [DOI] [PubMed] [Google Scholar]
- 22. Darling EM, Athanasiou KA. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res. 2005;23(2):425-32. doi: 10.1016/j.orthres.2004.08.008 [DOI] [PubMed] [Google Scholar]
- 23. Grad S, Kupcsik L, Gorna K, Gogolewski S, Alini M. The use of biodegradable polyurethane scaffolds for cartilage tissue engineering: potential and limitations. Biomaterials. 2003;24(28):5163-71. doi: 10.1016/s0142-9612(03)00462-9 [DOI] [PubMed] [Google Scholar]
- 24. Graceffa V, Vinatier C, Guicheux J, Stoddart M, Alini M, Zeugolis DI. Chasing chimeras—the elusive stable chondrogenic phenotype. Biomaterials. 2019;192:199-225. doi: 10.1016/j.biomaterials.2018.11.014 [DOI] [PubMed] [Google Scholar]
- 25. Hansen OM, Foldager CB, Christensen BB, Everland H, Lind M. Increased chondrocyte seeding density has no positive effect on cartilage repair in an MPEG-PLGA scaffold. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):485-93. doi: 10.1007/s00167-012-1996-4 [DOI] [PubMed] [Google Scholar]
- 26. Zhao Z, Fan C, Chen F, Sun Y, Xia Y, Ji A, et al. Progress in articular cartilage tissue engineering: a review on therapeutic cells and macromolecular scaffolds. Macromol Biosci. 2020;20(2):e1900278. doi: 10.1002/mabi.201900278 [DOI] [PubMed] [Google Scholar]
- 27. Salzmann GM, Nuernberger B, Schmitz P, Anton M, Stoddart MJ, Grad S, et al. Physicobiochemical synergism through gene therapy and functional tissue engineering for in vitro chondrogenesis. Tissue Eng Part A. 2009;15(9):2513-24. doi: 10.1089/ten.tea.2008.0479 [DOI] [PubMed] [Google Scholar]
- 28. Levinson C, Cavalli E, Sindi DM, Kessel B, Zenobi-Wong M, Preiss S, et al. Chondrocytes from device-minced articular cartilage show potent outgrowth into fibrin and collagen hydrogels. Orthop J Sports Med. 2019;7(9):2325967119867618. doi: 10.1177/2325967119867618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rothdiener M, Uynuk-Ool T, Südkamp N, Aurich M, Grodzinsky AJ, Kurz B, et al. Human osteoarthritic chondrons outnumber patient- and joint-matched chondrocytes in hydrogel culture—future application in autologous cell-based OA cartilage repair? J Tissue Eng Regen Med. 2018;12(2):e1206-e1220. doi: 10.1002/term.2516 [DOI] [PubMed] [Google Scholar]
- 30. Barry F, Murphy M. Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol. 2013;9(10):584-94. doi: 10.1038/nrrheum.2013.109 [DOI] [PubMed] [Google Scholar]
- 31. Jones DG, Peterson L. Autologous chondrocyte implantation. J Bone Joint Surg Am. 2006;88(11):2502-20. [DOI] [PubMed] [Google Scholar]
- 32. De Girolamo L, Bertolini G, Cervellin M, Sozzi G, Volpi P. Treatment of chondral defects of the knee with one step matrix-assisted technique enhanced by autologous concentrated bone marrow: in vitro characterisation of mesenchymal stem cells from iliac crest and subchondral bone. Injury. 2010;41(11):1172-7. doi: 10.1016/j.injury.2010.09.027 [DOI] [PubMed] [Google Scholar]
- 33. Salzmann GM, Calek AK, Preiss S. Second-generation autologous minced cartilage repair technique. Arthrosc Tech. 2017;6(1_suppl):e127-e131. doi: 10.1016/j.eats.2016.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grechenig S, Worlicek M, Penzkofer R, Zeman F, Kujat R, Heiss P, et al. Bone block augmentation from the iliac crest for treatment of deep osteochondral defects of the knee resembles biomechanical properties of the subchondral bone. Knee Surg Sports Traumatol Arthrosc. 2019;27(8):2488-93. doi: 10.1007/s00167-018-5242-6 [DOI] [PubMed] [Google Scholar]
- 35. Niemeyer P, Pestka JM, Kreuz PC, Salzmann GM, Kostler W, Sudkamp NP, et al. Standardized cartilage biopsies from the intercondylar notch for autologous chondrocyte implantation (ACI). Knee Surg Sports Traumatol Arthrosc. 2010;18(8):1122-7. doi: 10.1007/s00167-009-1033-4 [DOI] [PubMed] [Google Scholar]
- 36. McCarthy HS, Richardson JB, Parker JC, Roberts S. Evaluating joint morbidity after chondral harvest for autologous chondrocyte implantation (ACI): a study of aci-treated ankles and hips with a knee chondral harvest. Cartilage. 2016;7(1_suppl):7-15. doi: 10.1177/1947603515607963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Salzmann GM, Buchberger MS, Stoddart MJ, Grad S, Milz S, Niemyer P, et al. Varying regional topology within knee articular chondrocytes under simulated in vivo conditions. Tissue Eng Part A. 2011;17(3-4):451-61. doi: 10.1089/ten.TEA.2009.0819 [DOI] [PubMed] [Google Scholar]
- 38. Cole BJ, Farr J, Winalski CS, Hosea T, Richmond J, Mandelbaum B, et al. Outcomes after a single-stage procedure for cell-based cartilage repair: a prospective clinical safety trial with 2-year follow-up. Am J Sports Med. 2011;39(6):1170-9. doi: 10.1177/0363546511399382 [DOI] [PubMed] [Google Scholar]
- 39. Redman SN, Dowthwaite GP, Thomson BM, Archer CW. The cellular responses of articular cartilage to sharp and blunt trauma. Osteoarthr Cartil. 2004;12(2):106-16. doi: 10.1016/j.joca.2002.12.001 [DOI] [PubMed] [Google Scholar]
- 40. Tew SR, Kwan AP, Hann A, Thomson BM, Archer CW. The reactions of articular cartilage to experimental wounding: role of apoptosis. Arthritis Rheum. 2000;43(1_suppl):215-25. doi: [DOI] [PubMed] [Google Scholar]
- 41. Skagen PS, Kruse HA, Horn T. Repair mechanisms in articular cartilage—a porcine in vitro study. Microsc Res. 2014;2(4):67-80. doi: 10.4236/mr.2014.24009 [DOI] [Google Scholar]
- 42. Christensen BB, Foldager CB, Jensen J, Lind M. Autologous dual-tissue transplantation for osteochondral repair: early clinical and radiological results. Cartilage. 2015;6(3):166-73. doi: 10.1177/1947603515580983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bonasia DE, Marmotti A, Mattia S, Cosentino A, Spolaore S, Governale G, et al. The degree of chondral fragmentation affects extracellular matrix production in cartilage autograft implantation: an in vitro study. Arthroscopy. 2015;31(12):2335-41. doi: 10.1016/j.arthro.2015.06.025 [DOI] [PubMed] [Google Scholar]
- 44. Hunziker EB, Quinn TM, Häuselmann H-J. Quantitative structural organization of normal adult human articular cartilage. Osteoarthr Cartil. 2002;10(7):564-72. doi: 10.1053/joca.2002.0814 [DOI] [PubMed] [Google Scholar]
- 45. Walter SG, Ossendorff R, Schildberg FA. Articular cartilage regeneration and tissue engineering models: a systematic review. Arch Orthop Trauma Surg. 2019;139(3):305-16. doi: 10.1007/s00402-018-3057-z [DOI] [PubMed] [Google Scholar]
- 46. Christensen BB, Foldager CB, Olesen ML, Hede KC, Lind M. Implantation of autologous cartilage chips improves cartilage repair tissue quality in osteochondral defects: a study in Göttingen minipigs. Am J Sports Med. 2016;44(6):1597-604. doi: 10.1177/0363546516630977 [DOI] [PubMed] [Google Scholar]
- 47. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889-95. doi: 10.1056/NEJM199410063311401 [DOI] [PubMed] [Google Scholar]
- 48. Fang J, Jiang W, Zhu Y, Hu Y, Zhao Y, Song X, et al. Platelet-rich plasma therapy in the treatment of diseases associated with orthopedic injuries. Tissue Eng Part B Rev. Epub 2020 May 7. doi: 10.1089/ten.TEB.2019.0292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Olesen ML, Christensen BB, Foldager CB, Hede KC, Jørgensen NL, Lind M. No effect of platelet-rich plasma injections as an adjuvant to autologous cartilage chips implantation for the treatment of chondral defects. Cartilage. 2019:1947603519865318. doi: 10.1177/1947603519865318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Abrams GD, Frank RM, Fortier LA, Cole BJ. Platelet-rich plasma for articular cartilage repair. Sports Med Arthrosc Rev. 2013;21(4):213-9. doi: 10.1097/JSA.0b013e3182999740 [DOI] [PubMed] [Google Scholar]
- 51. De Girolamo L, Galliera E, Volpi P, Denti M, Dogliotti G, Quaglia A, et al. Why menisci show higher healing rate when repaired during ACL reconstruction? Growth factors release can be the explanation. Knee Surg Sports Traumatol Arthrosc. 2015;23(1_suppl):90-6. doi: 10.1007/s00167-013-2712-8 [DOI] [PubMed] [Google Scholar]
- 52. Grad S, Eglin D, Alini M, Stoddart MJ. Physical stimulation of chondrogenic cells in vitro: a review. Clin Orthop Relat Res. 2011;469(10):2764-72. doi: 10.1007/s11999-011-1819-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hurley ET, Davey MS, Jamal MS, Manjunath AK, Alaia MJ, Strauss EJ. Return-to-play and rehabilitation protocols following cartilage restoration procedures of the knee: a systematic review. Cartilage. Epub 2019 Dec 19. doi: 10.1177/1947603519894733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Marmotti A, Bruzzone M, Bonasia DE, Castoldi F, Rossi R, Piras L, et al. One-step osteochondral repair with cartilage fragments in a composite scaffold. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2590-2601. doi: 10.1007/s00167-012-1920-y [DOI] [PubMed] [Google Scholar]
- 55. Marmotti A, Bruzzone M, Bonasia DE, Castoldi F, Von Degerfeld MM, Bigrandi C, et al. Autologous cartilage fragments in a composite scaffold for one stage osteochondral repair in a goat model. Eur Cell Mater. 2013;26:15-32. doi: 10.22203/ecm.v026a02 [DOI] [PubMed] [Google Scholar]
- 56. Wang N, Grad S, Stoddart MJ, Niemeyer P, Reising K, Schmal H, et al. Particulate cartilage under bioreactor-induced compression and shear. Int Orthop. 2014;38(5):1105-11. doi: 10.1007/s00264-013-2194-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tsuyuguchi Y, Nakasa T, Ishikawa M, Miyaki S, Matsushita R, Kanemitsu M, et al. The benefit of minced cartilage over isolated chondrocytes in atelocollagen gel on chondrocyte proliferation and migration. Cartilage. 2018:1947603518805205. doi: 10.1177/1947603518805205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Andjelkov N, Hamberg H, Bjellerup P. No outgrowth of chondrocytes from non-digested particulated articular cartilage embedded in commercially available fibrin matrix: an in vitro study. J Orthop Surg Res. 2016;11:23. doi: 10.1186/s13018-016-0355-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lind M, Larsen A. Equal cartilage repair response between autologous chondrocytes in a collagen scaffold and minced cartilage under a collagen scaffold: an in vivo study in goats. Connect Tissue Res. 2008;49(6):437-42. doi: 10.1080/03008200802325037 [DOI] [PubMed] [Google Scholar]
- 60. Frisbie DD, Lu Y, Kawcak CE, DiCarlo EF, Binette F, McIlwraith CW. In vivo evaluation of autologous cartilage fragment-loaded scaffolds implanted into equine articular defects and compared with autologous chondrocyte implantation. Am J Sports Med. 2009;37(Suppl 1):71S-80S. doi: 10.1177/0363546509348478 [DOI] [PubMed] [Google Scholar]
- 61. Christensen BB, Olesen ML, Lind M, Foldager CB. Autologous cartilage chip transplantation improves repair tissue composition compared with marrow stimulation. Am J Sports Med. 2017;45(7):1490-6. doi: 10.1177/0363546517694617 [DOI] [PubMed] [Google Scholar]
- 62. Domínguez Pérez JM, Fernández-Sarmiento JA, García DA, Machuca MDMG, Rodriguez JM, et al. Cartilage regeneration using a novel autologous growth factors-based matrix for full-thickness defects in sheep. Knee Surg Sports Traumatol Arthrosc. 2019;27(3):950-61. doi: 10.1007/s00167-018-5107-z [DOI] [PubMed] [Google Scholar]
- 63. Matsushita R, Nakasa T, Ishikawa M, Tsuyuguchi Y, Matsubara N, Miyaki S, et al. Repair of an osteochondral defect with minced cartilage embedded in atelocollagen gel: a rabbit model. Am J Sports Med. 2019;47(9):2216-24. doi: 10.1177/0363546519854372 [DOI] [PubMed] [Google Scholar]
- 64. Massen FK, Inauen CR, Harder LP, Runer A, Preiss S, Salzmann GM. One-step autologous minced cartilage procedure for the treatment of knee joint chondral and osteochondral lesions: a series of 27 patients with 2-year follow-up. Orthop J Sports Med. 2019;7(6):2325967119853773. doi: 10.1177/2325967119853773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jungmann PM, Welsch GH, Brittberg M, Trattnig S, Braun S, Imhoff AB, et al. Magnetic resonance imaging score and classification system (amadeus) for assessment of preoperative cartilage defect severity. Cartilage. 2017;8(3):272-82. doi: 10.1177/1947603516665444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Riboh JC, Cole BJ, Farr J. Particulated articular cartilage for symptomatic chondral defects of the knee. Curr Rev Musculoskelet Med. 2015;8(4):429-35. doi: 10.1007/s12178-015-9300-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Irwin RM, Bonassar LJ, Cohen I, Matuska AM, Commins J, Cole B, et al. The clot thickens: autologous and allogeneic fibrin sealants are mechanically equivalent in an ex vivo model of cartilage repair. PLoS One. 2019;14(11):e0224756. doi: 10.1371/journal.pone.0224756 [DOI] [PMC free article] [PubMed] [Google Scholar]