Abstract
A highly sensitive and specific method has been developed to reproducibly detect and quantitate Toxoplasma gondii burden in animal tissue samples using T. gondii ITS1-derived primers and a fluorogenic probe via real-time PCR. Assay specificity was confirmed against a panel of DNA samples from T. gondii and other common protozoa as well as host animal tissue. This Toxo TaqMan assay was able to detect as little as 0.1 pg of T. gondii genomic DNA, which is equivalent to 1 T. gondii bradyzoite, and has a dynamic range of detection of from 100 ng to 100 fg of T. gondii DNA. Tissues from experimentally infected mice and pigs as well as bradyzoite-spiked pig muscle samples were used to test and standardize this technique. Positive signals were obtained with T. gondii parasite concentrations ranging from 4 to 3.7 × 105 parasites per g of spiked pig tissue, with excellent linearity (R2 = 0.9776). All T. gondii-infected animals were correctly identified by this technique. Results indicate that this assay is applicable to swine carcasses and commercial pig products, is compatible with automation technology for potential slaughterhouse use, and will enable scientists to diagnose and quantitate T. gondii in animal tissues.
Toxoplasmosis caused by Toxoplasma gondii is one of the most prevalent parasitic diseases in human beings and agricultural animals. Mead et al. (27) have estimated that approximately 225,000 new cases are reported each year in the United States and assume that 50% of the cases are due to food-borne transmission of T. gondii. The National Hospital Discharge survey indicated that toxoplasmosis was the first diagnosis for approximately 5,000 discharges each year between 1992 and 1996, including 750 deceased patients. Furthermore, 4,000 persons with AIDS will develop toxoplasmic encephalitis in the United States each year (27). From an economic point of view, T. gondii infection has a negative impact on society, as measured through the increase in the costs of chemotherapy for AIDS patients (13), serological screening for pregnant women, patient care, loss of productivity, and treatment of infected mothers and children (24). T. gondii can be transmitted to humans by ingestion of T. gondii oocysts in food or water or by consumption of tissue cysts in raw or undercooked meat. Infected pork is considered the most important meat source of T. gondii in the United States (4), and infected lamb is a major source worldwide (19). Considering the potentially serious consequences of congenital T. gondii infection in humans, such as birth defects, retinitis, brain damage, and even death, it is essential that efforts be directed at preventing food-borne transmission of T. gondii.
The detection of T. gondii tissue cysts in naturally and experimentally infected pigs and sheep has been reported for years (8, 19), though the prevalence of T. gondii infection in U.S. pig populations has been dramatically reduced as producers modify their management practices (31). However, the true T. gondii burden in any food product has been difficult to measure. To date, the most reliable method of inspecting food for T. gondii has been to demonstrate the presence of T. gondii tissue cysts by in vivo biological assays (12, 15). Because these methods are costly and time-consuming, they are not suitable for slaughterhouse testing or monitoring of commercial meat products (15).
There have been many reports of molecular detection assays for toxoplasmosis, but most have been developed for human diagnostics (2, 9, 21) or phylogenetic studies (1, 10, 22). In veterinary medicine, tests for detection of T. gondii by PCR were reported mostly for companion animals (25, 29) and sheep (11, 28), though Warnekulasuriya et al. (30) reported using PCR to identify T. gondii in cured meat products. Several studies have been directed at quantitating the actual T. gondii burden in biological fluids or tissues, but these involved time-consuming PCR protocols (competitive PCR) followed by agarose gel image analysis (17, 23, 26). Costa et al. (2) addressed the quantitation issue with a real-time PCR analysis of T. gondii in human serum samples in stem cell-transplanted patients; however, this assay was based on the T. gondii B1 gene and not the more abundant rRNA gene.
Herein, we describe a highly sensitive and specific method, the Toxo TaqMan assay, to reproducibly detect and/or quantitate T. gondii burden in animal tissue samples by using real-time PCR. Tissues from experimentally T. gondii-infected mice and pigs as well as bradyzoite-spiked pig muscle samples were used to test and standardize this technique.
MATERIALS AND METHODS
Sequence analysis.
Alignment of the ITS1 region of the 18S rRNA gene sequences from T. gondii (GenBank accession no. X75429.1, RH strain) and other coccidian parasites such as Neospora caninum (GenBank accession no. AF038861, NC-1 isolate), Hammondia hammondi (GenBank accession no. AF096499.1), and Hammondia heydorni (GenBank accession no. AF076858.1) was performed using the Genetics Computer Group (GCG) sequence analysis software package (Wisconsin Package, version 10.0). This region was selected because it has been reported to have a high rate of nucleotide substitutions (1). Primer and probe sequences for detection of T. gondii and for normalization with housekeeping genes are shown in Table 1.
TABLE 1.
Sequences of primers and probes used in this studya
| Gene and primer or probe | Sequence |
|---|---|
| Universal 18S rRNA | |
| Sense primer | 5′CGGCTACCACATCTAAGG 3′ |
| Antisense primer | 5′TATACGCTATTGGAGCTGG 3′ |
| Toxo ITS1 | |
| Sense primer | 5′GATTTGCATTCAAGAAGCGTGATAGTA-t 3′ |
| Antisense primer | 5′AGTTTAGGAAGCAATCTGAAAGCACATC 3′ |
| Probe | 5′(6-FAM)-CTGCGCTGCTTCCAATATTGG-(TAMRA) 3′ |
| Mouse (Hu) beta-actin | |
| Sense primer | 5′TCACCCACACTGTGCCCATCTACGA-3′ |
| Antisense primer | 5′CAGCGGAACCGCTCATTGCCAATGG 3′ |
| Probe | 5′(6-FAM)-ATGCCC-x (TAMRA)-CCCCATGCCATCCTGCGT 3′ |
x, TAMRA dye coupling site.
TaqMan assay.
The TaqMan assay for T. gondii (Toxo TaqMan) uses a specially designed probe and primers (Table 1) and directly relates the amount of initial target gene to the reporter's fluorescence emission (16). The Toxo TaqMan probe sequence was designed using the software Primer Express (version 1; Applied Biosystems, Foster City, Calif.) and synthesized with the reporter dye FAM (6-carboxifluorescein) at the 5′ end and a quencher molecule (TAMRA; 6-carboxytetramethylrhodamine) covalently coupled to the 3′ end (Table 1).
For the Toxo TaqMan assay, a 50-μl mixture containing 1 × TaqMan buffer A (Applied Biosystems), 1.5 mM MgCl2, 1 μM each T. gondii ITS1 primer, 200 nM fluorogenic probe, 200 μM each deoxynucleotide triphosphate, and 1 U of AmpliTaq Gold DNA polymerase (Applied Biosystems) was prepared, and up to 1 μg of the template DNA was tested. After activation of the AmpliTaq Gold DNA polymerase for 10 min at 95°C, PCR was performed for 50 cycles of 15 s at 94°C followed by 1 min at 60°C, and the products were analyzed on an ABI Prism 7700 sequence detector (Applied Biosystems). All the data acquisition and data analyses were performed with Sequence Detector Software (SDS version 1.7; Applied Biosystems) and CT values were recorded for statistical analysis on Excel spreadsheets. CT is defined as the cycle number (C) at which the reporter's fluorescence exceeds the threshold value (T), a parameter defined as 10 standard deviations (SD) above the baseline fluorescence from cycles 3 to 15.
The mouse β-actin housekeeping gene was detected with a commercially available TaqMan kit developed for human β-actin (Applied Biosystems), using 35 cycles. Conditions for mouse β-actin were similar to those described for the Toxo TaqMan assay except the annealing temperature was 52°C, the MgCl2 concentration was 1.5 mM, and the primer and probe concentrations were 180 and 80 nM, respectively.
Sources of T. gondii.
Three strains of T. gondii representing each genotype (RH, genotype I; Me49, genotype II; and VEG, genotype III) were used to infect animals in this study (18). All three infective stages of T. gondii, tachyzoites, bradyzoites, and oocysts, were used to extract genomic DNA or to infect animals (6). To determine minimum infective dose (1 parasite), 10-fold dilutions of tachyzoites, bradyzoites, and oocysts were bioassayed in mice.
Tachyzoites were obtained from peritoneal lavage of mice injected intraperitoneally 3 to 5 days earlier with RH strain tachyzoites. The peritoneal fluid was passed through a 3-μm filter (Nucleopore) to remove host cells. Tachyzoites were pelleted by centrifugation at 1,180 × g for 10 min, and the number of parasites was counted with a hemacytometer. T. gondii genomic DNA was extracted from approximately 108 tachyzoites after overnight incubation with DNA digestion buffer (0.5% sodium dodecyl sulfate [SDS], 25 mM EDTA, 100 mM NaCl, 20 mM Tris-HCl [pH 8.0], and proteinase K [0.1 mg/ml final concentration]). Digested parasites were extracted with phenol-chloroform-isoamyl alcohol (25:24:1), and the DNA was precipitated in 0.3 M sodium acetate (final concentration) with 2.5 volumes of 100% ethanol. DNA pellets were solubilized in TE buffer (10 mM Tris-HCl, 1 mM EDTA) and stored at −20°C. The DNA concentration was estimated by spectrophotometric absorbance at 257 nm.
Oocysts were obtained from feces of cats fed tissue cysts of the VEG strain, as described (7). Bradyzoites were collected from the brains of female Swiss-Webster mice chronically infected with the VEG strain (6). All brains were homogenized, and tissue cysts were isolated by centrifugation through an isotonic Percoll gradient. Bradyzoites were released from tissue cysts by short incubation with pepsin-HCl solution at room temperature, neutralized with sodium bicarbonate, and counted with a hemacytometer (5). Tenfold dilutions were prepared in phosphate-buffered saline (pH 7.2) and added to 1 g of muscle (biceps femoral) samples from T. gondii-free pigs. The spiked tissue samples were pepsin-HCl digested and treated for DNA extraction at a ratio of 1.2 ml/0.1 g of tissue. DNA (500 ng, total input) from each muscle sample was analyzed in duplicate on 4 different days.
Verification of Toxo TaqMan specificity.
Amplification of the 18S rRNA gene for each sample was performed with universal primers (Table 1) to normalize the quality and quantity of DNA between samples. A panel of DNA samples from the related protozoa N. caninum, H. hammondi, Eimeria acervulina, Eimeria tenella, Cryptosporidium parvum, Sarcocystis muris, Sarcocystis tenella (sheep-dog cycle), Sarcocystis cruzi (cattle-dog cycle), as well as T. gondii were tested for primer specificity. DNA samples from muscle and brain of host animals (pig and mouse) were similarly tested. Approximately 150 ng of each parasite DNA was evaluated with 18S rRNA universal primers and with our T. gondii ITS1 primers.
Analyses of T. gondii-infected animals.
T. gondii DNA was extracted from tissue cysts obtained from chronically infected mice. Five Swiss-Webster mice were injected subcutaneously with 200 counted bradyzoites of the VEG strain. Six weeks later, animals were bled, and serology was performed by a modified agglutination test (MAT) at a 1:50 screening dilution of the serum (3). Animals were subsequently killed, and whole brains were collected, smears were made to verify tissue cysts, and the remaining brain was directly processed with DNA digestion buffer without treatment with pepsin-HCl. DNA was extracted as described above and stored at −20°C for future use. Brain tissues from four mice infected with the Me49 T. gondii strain were included in this experiment and processed in a manner similar to those from VEG-infected mice.
Five seronegative (MAT titers of <1:25) NIH miniature pigs (about 6 months old) from our facilities were orally inoculated with 500 oocysts of the VEG strain and located in an isolated building, as previously described (7). Two seronegative pigs were kept as negative controls. At 35 to 42 days after the infection, all pigs were killed, brain and tongue samples were collected for bioassays, and blood was obtained for serology. Tongue and brain were selected for confirmation of parasite burden because these tissues are the most heavily parasitized among all pig tissues tested (6, 7).
For Toxo TaqMan pig tissue analyses, approximately 50 g of brain or tongue from each pig or 1 g of muscle sample spiked with T. gondii bradyzoites was homogenized in a blender with 5 volumes of sterile saline solution. Samples were then digested with an equal volume of warm (37°C) pepsin-HCl (1.4 mg of pepsin and 10 mg of NaCl per ml in 0.1 N HCl) for 1 h at 37°C in a shaking waterbath. The mixture was neutralized by two washes with 0.1 M Tris buffer (pH 8.0), and aliquots were centrifuged for 10 min at 1,180 × g. The resulting pellet from one aliquot was digested overnight at 55°C with DNA digestion buffer. DNA was extracted as described above and stored at −20°C. In parallel, a second aliquot was used to infect mice for bioassay confirmation of T. gondii infection.
Statistical analyses.
In order to determine the variability of the assay, intra-assay and interassay (repeatability) precision was measured. Ten replicates of three different T. gondii DNA concentrations (10 pg, 100 pg, and 1 ng) and a T. gondii-spiked muscle sample, randomly distributed through the 96 wells of the thermal cycler, were tested simultaneously. The repeatability of the test (precision between runs) was assessed using the previous T. gondii DNA concentrations, testing four replicates of each on five different days. Variability is shown as the mean ± SD in the graphs and reported as coefficient of variation (CV). Statistical analyses were carried out using Microsoft Excel (Redmond, Wash.).
RESULTS
Assay specificity.
DNA (150 ng) from all parasite and host samples included in the panel was amplified with the universal 18S rRNA primers, generating bands of similar intensity (Fig. 1A and data not shown) and confirming the quality and equal amounts of DNA loaded per reaction. The T. gondii ITS1 primers reacted only with T. gondii DNA, generating a DNA fragment of the expected size (∼333 bp) (Fig. 1B and data not shown). No PCR products were observed with any other DNA samples tested. All of these DNA samples were analyzed using the Toxo TaqMan assay, and only the T. gondii DNA gave a strong positive response, with a CT value of ∼16 (Fig. 1C and data not shown).
FIG. 1.
Comparison of amplification of parasite and host DNA samples by direct PCR and Toxo TaqMan assay. Analyses were performed by direct PCR using universal 18S rRNA primers (A) and T. gondii ITS1 primers (B) and by Toxo TaqMan assay (C). Approximately 150 ng of each DNA sample was tested: P, pig; M, mouse; Nc, Neospora caninum; Hh, Hammondia hammondi; Ea, Eimeria acervulina; Et, E. tenella; St, Sarcocystis tenella; Sm, S. muris; Cp, Cryptosporidium parvum, Tg, T. gondii; Neg, no-DNA control. The PCR products were electrophoresed in a 1.5% agarose gel and stained with ethidium bromide, and the image was photographed. For the Toxo TaqMan assay (C), 100 ng of T. gondii DNA was analyzed instead of the 150 ng used for all other DNAs because of the thick band observed with T. gondii DNA in a previous PCR. Samples were assayed on an Applied Biosystems Prism 7700 sequence detector and analyzed using the SDS version 1.7 software. Reporter dye fluorescence is plotted on the y axis (ΔRn) and cycle number on the x axis. The amplification plot shows test results from T. gondii DNA through 50 cycles; other samples are below the threshold line, indicated in bold.
Assay sensitivity and range of detection.
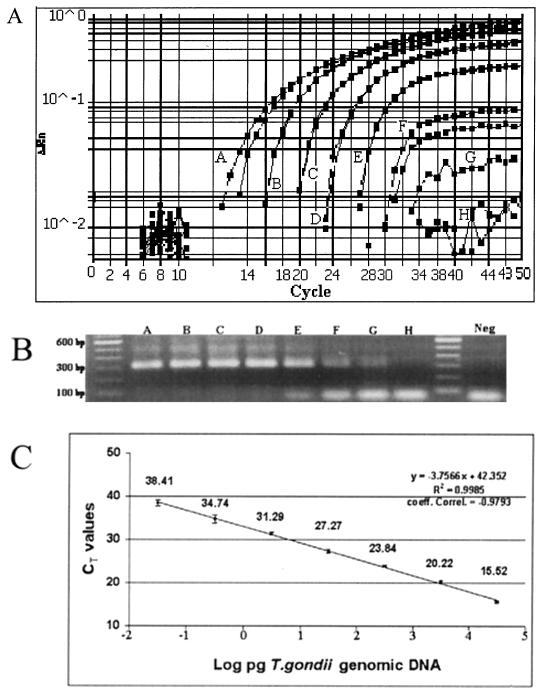
To estimate the sensitivity of the Toxo TaqMan assay, amplification of 10-fold dilutions of T. gondii genomic DNA was performed. Dilution series containing from 100 ng to 10 fg of T. gondii DNA were tested in duplicate. Figure 2A shows a typical display of a Toxo TaqMan assay provided by the ABI Prism 7700 instrument. Positive signals (CT values) were found for all dilutions except 10 fg of T. gondii DNA. Thus, in our hands, a detection limit of 100 fg of T. gondii DNA, or DNA equivalent to 1 bradyzoite, was achievable. The PCR products generated through the Toxo TaqMan assay were electrophoresed in an agarose gel and stained with ethidium bromide. In agreement with the CT values, bands of the expected sizes were detected down to 100 fg of T. gondii DNA, although the bands for 1 pg and 100 fg were dim (Fig. 2B, lanes F and G). Figure 2C shows the mean CT values for eight replicates of the series of T. gondii DNA concentrations. The CT values, ranging from 15.52 ± 0.21 for 100 ng of T. gondii genomic DNA to 38.41 ± 0.64 for 100 fg, showed reproducible linearity over a 10,000,000-fold range (R2 = 0.9984). A significant coefficient of correlation was found for the mean CT values and T. gondii DNA concentrations (r = −0.9793).
FIG. 2.
Sensitivity of the Toxo TaqMan assay. (A) Duplicates of dilutions of T. gondii DNA containing 100 ng (A), 10 ng (B), 1 ng (C), 100 pg (D), 10 pg (E), 1 pg (F), 0.1 pg (G), and 0.01 pg (H) of DNA plus no-template controls (NTC) were assayed. The assay threshold limit is shown as a bold line. Each duplicate sample result is plotted separately. (B) The PCR products were electrophoresed in 1.5% agarose gel and stained with ethidium bromide, and the image was photographed. (C) Plot of mean CT values from eight replicates tested on different days against the T. gondii DNA inputs. Variability is shown as mean CT values ± 1 SD. The plot of the CT values and DNA input fits a linear function (R2 = 0.9985).
Precision of Toxo TaqMan assay.
The intra-assay precision (white bars) (Fig. 3) was measured on 10 replicates of three T. gondii DNA concentrations and spiked pig samples tested on the same day and expressed as mean CT values. Results showed low variability, with a CV of <1.75% for the lowest T. gondii genomic DNA concentration (10 pg) and <1% for the T. gondii-spiked sample. The interassay precision (black bars) (Fig. 3), expressed as mean CT values for 20 replicates (four replicates on five different days), presented a CV of 4.5% for T. gondii genomic DNA and a CV of 1.7% for the T. gondii-spiked sample.
FIG. 3.
Precision of Toxo TaqMan assay for T. gondii DNA. Intra-assay precision for the detection of genomic T. gondii DNA (1 ng, 100 pg, and 10 pg) and a pig muscle sample spiked with 3.7 × 105 bradyzoites (sample A) is represented by the mean CT value for 10 replicates tested simultaneously (white bars). Repeatability of the Toxo TaqMan assay is shown as mean CT values ± 1 SD for four replicates of the same T. gondii DNA samples analyzed on five different days (black bars).
Toxo TaqMan assay results with bradyzoite-spiked pig muscle samples.
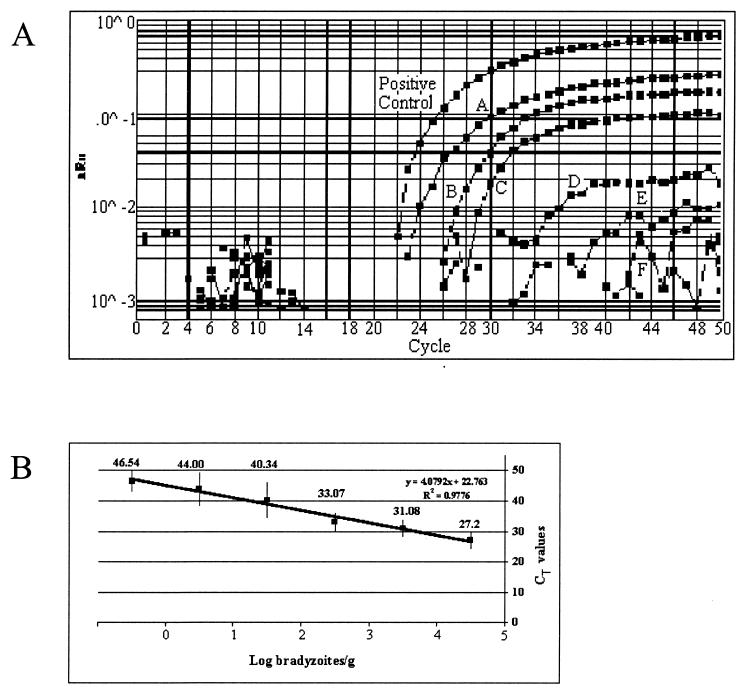
To test whether T. gondii could be detected in pig tissues, DNA (500 ng, total input) isolated from 1-g samples of pig muscle spiked with known numbers of T. gondii bradyzoites was analyzed. All samples gave positive signals, with mean CT values ranging from 27.2 to 46.5 (Fig. 4A). The Toxo TaqMan assay was linear for a 1,000,000-fold range of bradyzoite concentrations (R2 = 0.9776) (Fig. 4B). A significant coefficient of correlation (r = −0.68) between number of bradyzoites and CT values was observed.
FIG. 4.
Detection of T. gondii DNA in bradyzoite-spiked pig muscle samples. (A) Six 1-g muscle samples obtained from a T. gondii-free pig were spiked with known numbers of T. gondii bradyzoites (A, 3.7 × 105; B, 3.7 × 104, C, 3.7 × 103; D, 3.7 × 102; E, 3.7 × 101; F, <4 × 100). Pepsin-HCl digestion and DNA extraction were performed as described in Materials and Methods. Approximately 500 ng of total DNA from each sample was assayed by the Toxo TaqMan assay. (B) The mean CT values ± 1 SD for eight replicates run on four different days are plotted against the number of T. gondii bradyzoites. Linearity is observed over the whole range of bradyzoite numbers (R2 = 0.9776).
Toxo TaqMan assay results from animals experimentally infected with T. gondii.
The Toxo TaqMan assay results on samples from experimentally infected pigs and mice corresponded well with their serological status; all infected and control animals were identified correctly (Table 2). Good correlation between CT values and MAT titers was observed for the pig samples (r = −0.719). All Toxo TaqMan CT values from mouse DNA samples were normalized against CT values generated with the β-actin TaqMan kit (Table 2). These data confirmed that the Toxo TaqMan assay was positive when tested against each of the three T. gondii genotypes.
TABLE 2.
Toxo TaqMan results for samples from T. gondii-infected pigs and micea
| Animal no. | T. gondii strain | Inoculumb | Antibodies to T. gondii (titer) |
CT
|
||
|---|---|---|---|---|---|---|
| T. gondii | Housekeeping gene | NormalizedcT. gondii | ||||
| M 7076 | VEG | 200 B | ≥1:50 | 24.69 | 25.86 | 21.96 |
| M 7077 | VEG | 200 B | ≥1:50 | 24.51 | 27.84 | 23.46 |
| M 7078 | VEG | 200 B | ≥1:50 | 25.49 | 28.16 | 24.68 |
| M 7079 | VEG | 200 B | ≥1:50 | 27.2 | 27.64 | 25.85 |
| M 7080 | VEG | 200 B | ≥1:50 | 25.05 | 27.82 | 23.96 |
| M 8348 | Me49 | 1 T | ≥1:200 | 28.84 | 28.84 | 28.44 |
| M 8349 | Me49 | 1 T | ≥1:200 | 29.41 | 28.23 | 29.19 |
| M 8350 | Me49 | 1 T | ≥1:200 | 29.39 | 27.25 | 28.16 |
| M 8351 | Me49 | 1 T | ≥1:200 | 29.19 | 27.31 | 28.03 |
| MP 521 | VEG | 500 O | 1:800 | 32.26 | N/D | N/D |
| MP 522 | VEG | 500 O | 1:1,600 | 30.33 | N/D | N/D |
| MP 523 | VEG | 500 O | 1:400 | 32.24 | N/D | N/D |
| MP 524 | VEG | 500 O | 1:200 | 33.52 | N/D | N/D |
| MP 526 | VEG | 500 O | 1:800 | 32.09 | N/D | N/D |
| MP 490 | None | None | <1:25 | ≥50 | N/D | N/D |
| MP 532 | None | None | <1:25 | ≥50 | N/D | N/D |
DNA samples from Swiss-Webster mice (M) (500 ng, total input) and NIH minipigs (MP) (1 μg) infected with different T. gondii strains were analyzed by the Toxo TaqMan assay. DNA was obtained from brain tissues as described in Materials and Methods. Tachyzoites and bradyzoites were injected subcutaneously, and oocysts were orally inoculated. Specific antibody detection was performed by MAT as described in Materials and Methods.
B, counted bradyzoites; T, tachyzoites, determined by bioassay in mice (the 10−5 dilution was infective to all four mice, the 10−6 dilution was not infective to any of the four mice); O, live oocysts, determined by dilution bioassay in mice.
Normalization factor: T. gondii CT value × (β-actin CT sample/highest β-actin CT value). N/D, not determined.
DISCUSSION
This study presents results for a new molecular Toxo TaqMan assay. Sensitivity at 100 fg of T. gondii DNA is similar to that reported elsewhere despite differences in the target gene (2, 21, 25). The assay exhibits a linear range of detection (R2 = 0.9985) similar to that found by Costa et al. (2). Furthermore, the Toxo TaqMan assay shows a twofold better sensitivity than other tests (20, 28, 29), which detected ∼10 parasites in diverse biological samples. In these other studies, T. gondii DNA extractions were performed from a variety of samples, such as serum (2), aqueous humor (25), amniotic fluid (17), and cell culture (20), where the contaminating host-derived DNA level is low, and therefore cannot be equated to the Toxo TaqMan assay, which was performed on DNA recovered from as much as 50 g of original pig tissue.
When applied to bradyzoite-spiked muscle samples, the Toxo TaqMan test successfully detected as few as ∼4 bradyzoites per g of pig tissue. This sensitivity level is substantially better than that reported by Warnekulasuriya et al. (30) on cured meats (5 × 103 tachyzoites/g), who attributed this low sensitivity to the high salt content of the cured meat. Considering that the Toxo TaqMan assay was linear (R2 = 0.9776) and detected from as few as 4 to as many as 3.7×105 parasites per g of tissue, the range of detection is not a limitation. Other investigators have reported a range of detection of only 102 to 104 T. gondii organisms using a quantitative competitive PCR with the B1 gene (23) or with a highly repetitive DNA sequence as the target gene (17). Costa et al. (2) detected from 1 to ∼400 parasites in 200-μl serum samples of stem cell-transplanted patients using a LightCycler-based PCR test.
It is difficult to find T. gondii tissue cysts in large animal species for several reasons, including sampling bias and preferred parasite sites. Dubey et al. (7) have estimated that less than 1 tissue cyst/50 g of tissue is likely to be found in T. gondii-infected pigs. Thus, it is possible that when performing any test for tissue cyst detection, false-negatives can result from insufficient sample size or improper sample acquisition (12). In our study, it was assumed that the pigs inoculated with T. gondii might harbor low number of tissue cysts despite a moderate infective dose (500 oocysts). Therefore, for the tests in this report, a digestion-concentration method was applied for some samples, such as the 50 g of brain or tongue, to reduce the actual amount of host tissue undergoing DNA extraction. Continuing studies in our laboratory are focusing on how long after infection T. gondii can be detected in pigs or in stored pig tissue samples. Because CT values in the Toxo TaqMan assay correspond to the number of bradyzoites per gram (coefficient of correlation = −0.68), it should be possible to construct a standard curve using samples spiked with known numbers of T. gondii tachyzoites or bradyzoites and from this extrapolate the T. gondii burden in unknown samples once the CT values have been determined.
The ability of our Toxo TaqMan assay to correctly identify T. gondii-infected animals with a high degree of sensitivity was demonstrated for both parasite-infected mice and pigs, and it was also shown to work on multiple stages of infection (Table 2). These results were corroborated by both serological response of all infected animals and presence of tissue cysts in the brain of infected mice. Furthermore, a significant association between antibody titers of pigs and CT values was observed (r = −0.719). Like other quantitative methods (2, 17, 23), the Toxo TaqMan assay is dependent on the initial DNA input; thus, for standardization, the use of a normalization housekeeping gene is strongly recommended. In the future, such analyses could be carried out using multiple TaqMan assays with different fluorogenic probes.
At present, serological testing is the only practical tool to identify T. gondii infection in pigs for either food animal inspections or epidemiological studies (14). Mouse and cat bioassays accurately confirm T. gondii infection, but these bioassays require use of live animals for 6 to 8 weeks and are costly and time-consuming. With the development of the Toxo TaqMan assay, we hope to supplement or even replace current bioassay or indirect serology testing with a more direct assay. With combined tissue digestion, fast DNA preparation, and the Toxo TaqMan assay, it is expected that T. gondii detection could be completed within 24 h or less. This newly developed assay will thus help to monitor T. gondii contamination in commercial meat products, and ultimately reduce food-borne transmission of this harmful parasite. Moreover, the Toxo TaqMan assay will provide an objective tool for quantitating T. gondii burden for vaccination studies and clinical trials for therapeutic treatments.
The Toxo TaqMan assay is a very sensitive and specific assay that can be used to detect T. gondii in a variety of biological samples using T. gondii-specific ITS1 primers and a fluorogenic probe via real-time PCR. The detection response was linear over a 10,000,000-fold range of T. gondii DNA concentrations (R2 = 0.9985) as well as parasite numbers (R2 = 0.9776), and the assay was able to detect as little as 0.1 pg of T. gondii genomic DNA. The Toxo TaqMan assay presents several important advantages over other methods of detection and quantitation of T. gondii DNA. First, the Toxo TaqMan is performed in a closed tube with no post-PCR manipulations, thereby reducing potential PCR product carryovers and post-PCR processing time. Second, the assay is quick; results can be confirmed within 1 day. Third, the assay response is sensitive and linear over a broad range of DNA concentrations. Finally, sample processing is compatible with current PCR-based automation technology (16).
ACKNOWLEDGMENTS
We thank Diane Hawkins-Cooper, Sam Shen, Oliver Kwok, and Alexander Domingo for excellent technical assistance and Susan Liddell and Mark Jenkins for kindly providing parasite DNA samples and help with DNA sequence analyses.
This work was supported by funds from the ARS, USDA, and grants awarded by the National Pork Producers Council (NPPC 00-132 and 00-024).
REFERENCES
- 1.Carreno R, Barta J R. An eimeriid origin of isosporoid coccidia with Stieda bodies as shown by phylogenetic analysis of small subunit ribosomal RNA gene sequences. J Parasitol. 1999;85:77–83. [PubMed] [Google Scholar]
- 2.Costa J M, Pautas C, Ernault P, Foulet F, Cordonnier C, Bretagne S. Real-time PCR for diagnosis and follow-up of Toxoplasma reactivation after allogeneic stem cell transplantation using fluorescence resonance energy transfer hybridization probes. J Clin Microbiol. 2000;38:2929–2932. doi: 10.1128/jcm.38.8.2929-2932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desmont G, Remington J S. Direct agglutination test for diagnosis of Toxoplasma infection: method for increasing sensitivity and specificity. J Clin Microbiol. 1980;11:562–568. doi: 10.1128/jcm.11.6.562-568.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubey J P. Toxoplasmosis. J Am Vet Med Assoc. 1994;205:1593–1598. [PubMed] [Google Scholar]
- 5.Dubey J P. Oocyst shedding by cats fed isolated bradyzoites and comparison of infectivity of bradyzoites of the VEG strain Toxoplasma gondii to cats and mice. J Parasitol. 2001;87:215–219. doi: 10.1645/0022-3395(2001)087[0215:OSBCFI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Dubey J P, Beattie C P. Toxoplasmosis of animals and man. Boca Raton, Fla: CRC Press; 1988. [Google Scholar]
- 7.Dubey J P, Lunney J K, Shen S K, Kwok O C H, Ashford D A, Thulliez P. Infectivity of low numbers of Toxoplasma gondii oocysts to pigs. J Parasitol. 1996;82:438–443. [PubMed] [Google Scholar]
- 8.Dubey J P, Murrell K D, Fayer R, Schad G A. Distribution of Toxoplasma gondii tissue cysts in commercial cuts of pork. J Am Vet Med Assoc. 1986;188:1035–1037. [PubMed] [Google Scholar]
- 9.Dupon M, Cazenave J, Pellegrin J L, Ragnaud J M, Cheyrou A, Fischer I, Leng B, Lacut J Y. Detection of Toxoplasma gondii by PCR and tissue culture in cerebrospinal fluid and blood of human immunodeficiency virus-seropositive patients. J Clin Microbiol. 1995;33:2421–2426. doi: 10.1128/jcm.33.9.2421-2426.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellis J T, Amoyal G, Ryce C, Harper P A W, Clough K A, Homan W L, Brindley P J. Comparison of the large subunit ribosomal DNA of Neospora and Toxoplasma and development of a new genetic marker for their differentiation based on the D2 domain. Mol Cell Probes. 1998;12:1–13. doi: 10.1006/mcpr.1997.0143. [DOI] [PubMed] [Google Scholar]
- 11.Esteban-Redondo I, Innes E A. Detection of Toxoplasma gondii in tissues of sheep orally challenged with different doses of oocysts. Int J Parasitol. 1998;28:1459–1466. doi: 10.1016/s0020-7519(98)00116-7. [DOI] [PubMed] [Google Scholar]
- 12.Esteban-Redondo I, Maley S W, Thompson K, Nicoll S, Wright S, Buxton D, Innes E A. Detection of T. gondii in tissues of sheep and cattle following oral infection. Vet Parasitol. 1999;86:155–171. doi: 10.1016/s0304-4017(99)00138-7. [DOI] [PubMed] [Google Scholar]
- 13.Freedberg K A, Scharfstein J A, Seage III G R, Losina E, Weinstein M C, Craven D E, Paltiel D. The cost-effectiveness of preventing AIDS-related opportunistic infections. J Am Med Assoc. 1998;279:130–136. doi: 10.1001/jama.279.2.130. [DOI] [PubMed] [Google Scholar]
- 14.Gamble H R, Brady R C, Dubey J P. Prevalence of Toxoplasma gondii infection in domestic pigs in the New England states. Vet Parasitol. 1999;82:129–136. doi: 10.1016/s0304-4017(99)00004-7. [DOI] [PubMed] [Google Scholar]
- 15.Gamble H R, Murrell K D. Detection of parasites in food. Parasitology. 1998;117:S97–S111. doi: 10.1017/s0031182099004977. [DOI] [PubMed] [Google Scholar]
- 16.Heid C A, Stevens J, Livak K J, Williams P M. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 17.Homan W L, Vercammen M, De Braekeleer J, Verschueren H. Identification of a 200–300-fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR. Int J Parasitol. 2000;30:69–75. doi: 10.1016/s0020-7519(99)00170-8. [DOI] [PubMed] [Google Scholar]
- 18.Howe D K, Sibley L D. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis. 1995;172:1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs L, Remington J S, Melton M L. A survey of meat samples from swine, cattle, and sheep for the presence of encysted Toxoplasma. J Parasitol. 1960;46:23–28. [PubMed] [Google Scholar]
- 20.James G S, Sintchenko V G, Dickeson D J, Gilbert G L. Comparison of cell culture, mouse inoculation and PCR for detection of Toxoplasma gondii: effects of storage conditions on sensitivity. J Clin Microbiol. 1996;34:1572–1575. doi: 10.1128/jcm.34.6.1572-1575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenum P A, Holberg-Petersen M, Moelby K K, Stray-Pedersen B. Diagnosis of congenital Toxoplasma gondii infection by polymerase chain reaction (PCR) on amniotic fluid samples. Acta Pathol Microbiol Immunol Scand. 1998;106:680–686. [PubMed] [Google Scholar]
- 22.Kaufmann H, Yamage M, Roditi I, Dobbelaere D, Dubey J P, Holmdahl O J M, Trees A, Gottstein B. Discrimination of Neospora caninum from Toxoplasma gondii and other apicomplexan parasites by hybrization and PCR. Mol Cell Probes. 1996;10:289–297. doi: 10.1006/mcpr.1996.0038. [DOI] [PubMed] [Google Scholar]
- 23.Kirisits M J, Mui E, McLeod R. Measurement of the efficacy of vaccines and antimicrobial therapy against infection with Toxoplasma gondii. Int J Parasitol. 2000;30:149–155. doi: 10.1016/s0020-7519(00)00009-6. [DOI] [PubMed] [Google Scholar]
- 24.Lappalainen M, Sintonen H, Koskiniemi M, Hedman K, Hiilesmaa V, Ämälä P, Teramo K, Koskela P. Cost-benefit analysis of screening for toxoplasmosis during pregnancy. Scand J Infect Dis. 1995;27:265–272. doi: 10.3109/00365549509019020. [DOI] [PubMed] [Google Scholar]
- 25.Lappin M R, Burney D P, Dow S W, Potter T A. Polymerase chain reaction for the detection of Toxoplasma gondii in aqueous humor of cats. Am J Vet Res. 1996;57:1589–1593. [PubMed] [Google Scholar]
- 26.Luo W, Aosai F, Ueda M, Yamashita K, Shimizu K, Sekiya S, Yano A. Kinetics in parasite abundance in susceptible and resistant mice infected with an avirulent strain of Toxoplasma gondii by using quantitative competitive PCR. J Parasitol. 1997;83:1070–1074. [PubMed] [Google Scholar]
- 27.Mead P S, Slutsker L, Dietz V, McCaig L F, Bresee J S, Shapiro C, Griffin P M, Tauxe R V. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Owen M R, Clarkson M J, Trees A J. Diagnosis of Toxoplasma abortion in ewes by polymerase chain reaction. Vet Rec. 1998;142:445–448. doi: 10.1136/vr.142.17.445. [DOI] [PubMed] [Google Scholar]
- 29.Stiles J, Prade R, Greene C. Detection of Toxoplasma gondii in feline and canine biological samples by use of the polymerase chain reaction. Am J Vet Res. 1996;57:264–267. [PubMed] [Google Scholar]
- 30.Warnekulasuriya M R, Johnson J D, Holliman R E. Detection of Toxoplasma gondii in cured meats. Int J Food Microbiol. 1998;45:211–215. doi: 10.1016/s0168-1605(98)00158-5. [DOI] [PubMed] [Google Scholar]
- 31.Weigel R M, Dubey J P, Siegel A M, Kitron U D, Mannelli A, Mitchell M A, Mateus-Pinilla N E, Thulliez P, Shen S K, Kwok O C H. Risk factors for transmission of Toxoplasma gondii on swine farms in Illinois. J Parasitol. 1995;81:736–741. [PubMed] [Google Scholar]