Abstract
Background
Lipid metabolism has been proved to be related to the prognosis of breast cancer patients in previous studies, and the tumor immune microenvironment (TIME) plays an important role in tumorigenesis and development, but the dynamic regulation of these is still a challenge.
Material/Methods
This study used lipid metabolism-related pathways to score the gene expression of 980 breast cancer patients in the TCGA database. We used 4 pathways in HALLMARK related to lipid metabolism to score the genes in the database. The differentially expressed genes (DEGs) were further analyzed through survival analysis and Cox regression analysis, and MS4A1, which is associated with better prognosis, was finally determined to be a predictor. In-depth analysis found that MS4A1 was negatively correlated with patient age, clinical stage, tumor size, and distant metastasis. In the MS4A1 high-expression group, most genes were enriched in immune-related pathways, and CIBERSORT analysis found that MS4A1 expression was positively correlated with the abundance of 10 kinds of immune cells, such as CD8+T cells, which are related to the active immune status.
Results
Our results suggest that MS4A1 expression can indicate the situation of lipid metabolism in breast cancer patients and reflect the status of the immune microenvironment.
Conclusions
MS4A1 has the potential to be an independent indicator of prognosis. Since the expression of MS4A1 is also related to the immune checkpoint mutation burden, detecting its expression level can also provide guidance for choosing treatment options.
Keywords: Biomarkers, Breast Neoplasms, Lipid Metabolism, Tumor Microenvironment
Background
The incidence of breast cancer (BRCA) continues to increase. Breast cancer has a poor prognosis and is difficult to detect early, seriously endangering women’s physical and mental health. According to statistics [1], breast cancer has surpassed lung cancer in 2020, becoming the world’s deadliest cancer. In that year, the number of new breast cancer cases was 2.26 million worldwide. The number of female deaths because of cancer was 4.43 million, and 680 000 of them died from breast cancer. The current effective treatment methods mainly include surgery, chemotherapy, and endocrine therapy, but new biomarkers are needed to classify patients to facilitate individualized intervention and survival prediction, and provide new potential targets for future drug development.
Dysregulated lipid metabolism can lead to tumors, and it can also affect tumor cell proliferation, apoptosis, invasion, and metastasis [2–4]. Lipid metabolism is closely related to the occurrence and development of breast cancer: people with hypercholesterolemia and obesity have a significantly higher risk of estrogen receptor-alpha (ERα)-positive breast cancers [5–7]. 27-Hydroxycholesterol (27HC), an important factor in regulating the homeostasis of cholesterol and a selective estrogen receptor, has been experimentally verified to promote the occurrence and progression of breast cancer [8–11]. Clinical studies have further found that the use of statin therapy can improve the prognosis of breast cancer patients [12]. Classifying patients by assessing lipid metabolism and implementing interventions for lipid metabolism disorders can improve the prognosis. Furthermore, studies have found that disorders of lipid metabolism are also related to drug resistance during treatment [13].
The tumor microenvironment (TME) includes endothelial cells, pericytes, fibroblasts, adipocytes, resident and infiltrating immune cells, and extracellular matrix (ECM). ECM is mainly composed of collagen, elastin, fibronectin, laminin, and proteoglycan (PG). TME contains lipid metabolism components such as fatty acids and cholesterol, and these factors can promote tumor progression [14]. The tumor immune microenvironment (TIME) plays an important role in tumor treatment and tumor progression, which can reflect the immune conditions of tumor tissues, and provide potential targets for tumor treatment [15]. In TME, ECM is an important component, and ECM remodeling plays an important role in tumorigenesis and progression. The lysyl oxidase gene (LOX) acts in the cross-linking of elastin and collagen fibers as well as in the modulation of the structure and stiffness of tumor ECM. Studies have shown that in mouse models of breast cancer, drug therapy combined with LOX knockdown or suppression therapy can reduce tumor growth and metastasis and prolong survival [16–18]. LOX has been suggested as a promising option for the treatment of triple-negative breast cancer [19]. Moreover, LOX has prognostic value in breast cancer patients and plays a role in the formation of pre-metastatic niches in bone in ER-negative breast tumors, and patients with high levels of LOX often have a worse prognosis [20,21].
Similarly, in recent years it has been proposed the potential activity of Trabectedin (an anti-tumor compound already registered as second-line treatment of soft tissue sarcoma and for ovarian cancer) in breast cancer treatment, due to its ability to act as modulator of TME by reducing the number of tumor-associated macrophages [22,23]. Moreover, Trabectedin also showed the ability to promote the increase of timp1 expression (a matrix metalloproteases inhibitor), which can affect the activity and contributing to inhibition of cell invasiveness [24]. Studies have shown [25] that the active lipid metabolism can lead to local immunosuppression in TME and promote tumor growth.
Therefore, in the present study, we first used the 4 lipid metabolism-related pathways in HALLMARK (hallmark gene set) to score 980 patients in The Cancer Genome Atlas (TCGA), screened out differentially expressed genes, then comprehensively considered the survival prognosis, and screened out a biomarker, MS4A1, whose higher expression was related to lower lipid metabolism and better survival situation. The patients were grouped according to the level of MS4A1 expression, and enrichment analysis was performed. It was found that high expression of MS4A1 is closely related to the immune active microenvironment and activation of immune cell activation-related pathways. The MS4A family was initially determined based on the observations of pan-B cell markers, and MS4A1 (membrane-spanning 4-domain family, subfamily A) shared a high amino-acid sequence identity [26]. Previous studies have found [27] that MS4A1, which encodes a B cell surface marker, is also expressed in T cell subsets. The high expression of MS4A1 in colon cancer patients is associated with better prognosis and treatment response. However, in breast cancer, MS4A1 as a potential prognostic predictor has not been reported.
Material and Methods
Data Collection
Transcriptome RNA-seq data of 1222 BRCA cases (excluding the duplicate cases and para-cancerous cases, leaving 1091 remaining cases), single-nucleotide variation data of 986 BRCA cases and the corresponding clinical data of 1097 cases were downloaded from the TCGA database (https://portal.gdc.cancer.gov/). We chose all 980 cases with complete transcriptome, single-nucleotide variation and clinical data through the R package “venn” (Figure 1). The clinical data of the 980 cases are summarized in Table 1. The mean age of these patients was 58.46 years old.
Figure 1.
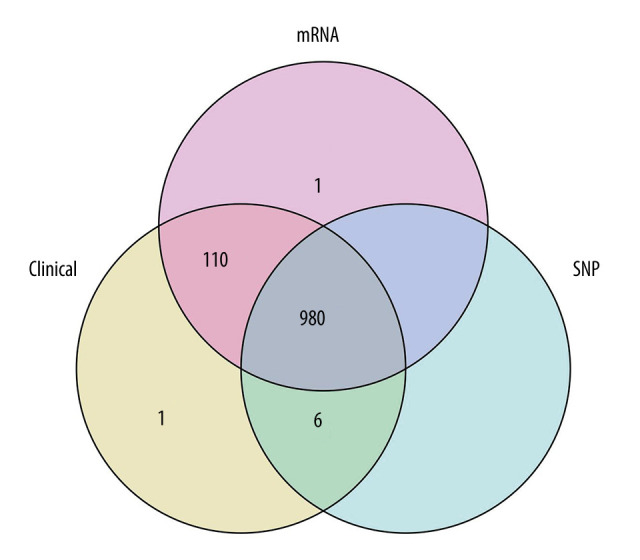
Looking for samples with all 3 pieces of information (mRNA, clinical features, single-nucleotide mutations [SNP]), a total of 980 cases were obtained.
Table 1.
Clinical features of 980 patients.
| Clinical index | MS4A1 high | MS4A1 low | Statistics | p Value | |
|---|---|---|---|---|---|
| (n=726) | (n=254) | ||||
| Age | ≤65 | 536 | 163 | χ2=8.578 | 0.004 |
| >65 | 190 | 91 | |||
| Sex | Female | 720 | 249 | —— | 0.165 |
| Male | 6 | 5 | |||
| T | T1 | 211 | 45 | Z=−3.635 | <0.001 |
| T2 | 413 | 161 | |||
| T3 | 80 | 31 | |||
| T4 | 20 | 16 | |||
| Unknow | 2 | 1 | |||
| N | N0 | 341 | 122 | Z=1.169 | 0.242 |
| N1 | 238 | 86 | |||
| N2 | 86 | 26 | |||
| N3 | 52 | 10 | |||
| Unknow | 9 | 10 | |||
| M | M0 | 611 | 203 | χ2=8.089 | 0.007 |
| M1 | 10 | 11 | |||
| Unknow | 105 | 40 | |||
| Stage | I | 135 | 30 | Z=−1.352 | 0.177 |
| II | 405 | 158 | |||
| III | 163 | 49 | |||
| IV | 10 | 9 | |||
| Unknown | 13 | 8 | |||
Lipid Metabolism-Related Pathways-Score and Screening for Differentially Expressed Genes (DEGs)
The 4 lipid metabolism-related pathways in HALLMARK were downloaded from http://www.gsea-msigdb.org/gsea/msigdb/index.jsp.
We used the R (version 3.6.2) package “GSVA” to score 980 patients for representing the activity level of each patient in the 4 lipid metabolism-related pathways of HALLMARK (HALLMARK_CHOLESTEROL_HOMEOSTASIS, HALLMARK_ADIPOGENESIS, HALLMARK_FATTY_ACID_METABOLISM, and HALLMARK_BILE_ACID_METABOLISM). The median score calculated by GSVA package and the 4 median scores were: HALLMARK_CHOLESTEROL_HOMEOSTASIS: −0.043, HALLMARK_ADIPOGENESIS: −0.028, HALLMARK_FATTY_ACID_METABOLISM: −0.019, and HALLMARK_BILE_ACID_METABOLISM: 0.0004 (lower than or equal to the median value was defined as the low-expression group).
To find DEGs, we performed Wilson rank sum testing on gene expression. We set the cutoff as: logFc>0 and the median difference between the high expression group and the low expression group>0. At the same time, the statistical significance of FDR <0.05 (P value corrected as FDR).
We found the intersection between the upregulated genes in the high-score group and the low-score group, and performed the same operation in the downregulated genes. Then, we merged the 2 intersections. We finally found 17 common DEGs.
Survival Analysis
We used the R package “survival” and “survminer” to perform survival analysis to determine the prognostic value of these intersected DEGs. The cutoff value was determined through the function surv_cutpoint. Kaplan-Meier method (log rank as the statistical significance test) and univariate and Cox regression analysis were used for survival analysis. P<0.05 was considered statistically significant. We selected genes that were statistically significant in both analysis methods, revealing 12 genes with predictive value for prognosis.
Cox Regression Analysis
Univariate and multivariate Cox regression analysis showed that MS4A1 had the lowest hazard ratio (HR). Therefore, we identified MS4A1 as the object of further analysis.
Enrichment Analysis
According to the cutoff value of expression level (cutoff value was 0.008887257), 980 cases were divided into an MS4A1 high-expression group and an MS4A1 low-expression group
Clusterprofile
R packages “clusterProfiler” and “ggplot2” were used to find DEGs. We performed Wilcoxon signed ranks testing on gene expression, with the cutoff value set as LogFc>0 and the statistical significance of FDR <0.05 (P value corrected as FDR). We then performed gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis.
Gene Set Variation Analysis (GSVA)
We used FDR <0.05 to define the DEGs. Then GO and KEGG enrichment analysis were performed with R packages “GSVA” and “ggplot2”.
Gene Set Enrichment Analysis (GSEA)
GO, HALLMARK, and KEGG gene sets were analyzed using GSEA 4.0.3 software. The whole transcriptome of all tumor cases was used for GSEA, and only gene sets with NOM P<0.05 and FDR <0.25 were considered as significant.
Tumor-Infiltrating Immune Cell (TIC) Profile
We used CIBERSORT [28] computation to estimate the TIC infiltration fraction in 980 cases. Pearson correlation was calculated between the proportions and MS4A1 expression. P<0.05 was selected for the following analysis.
Sub-Network Analysis
We used Cytoscape (3.6.1) with the MCODE plug-in to perform sub-network analysis on the GOBP terms analyzed with GSEA, with the following parameter settings: degree cutoff 15, Mode Score cutoff 0.2, K-Core 15, Max Depth 100. We finally obtained 4 sub-networks.
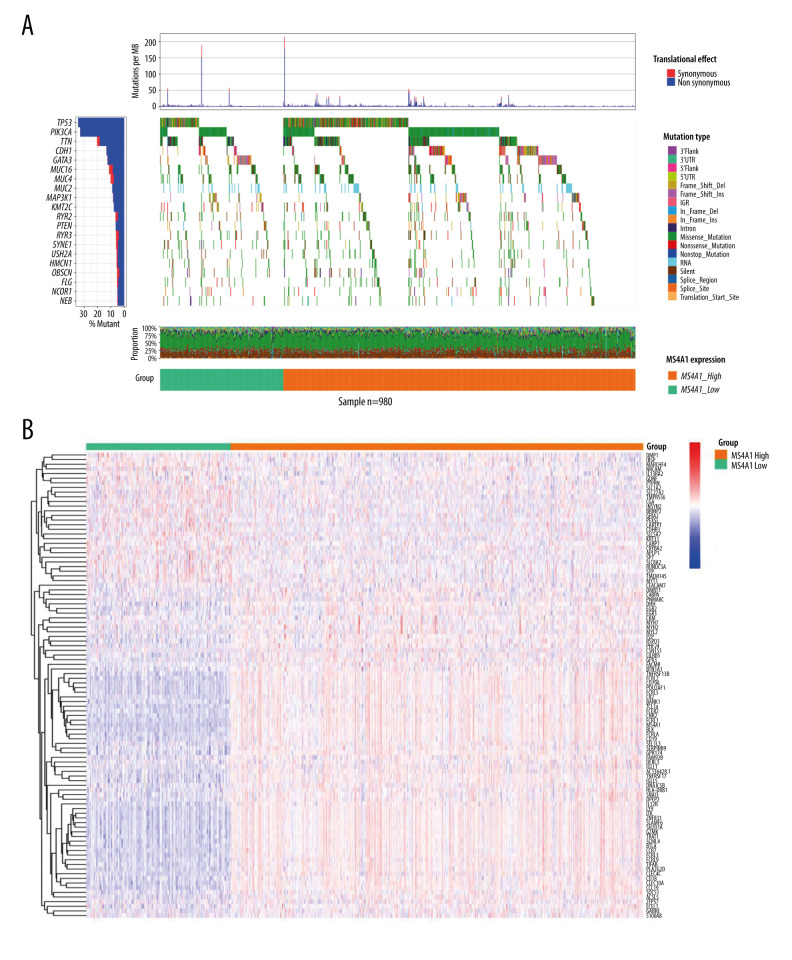
Heatmaps
Heatmaps were used to present DEGs performed in the R package “pheatmap”. The 100 genes with most significant fold change are presented (50 genes with highest LogFc and 50 genes with lowest LogFc).
Volcano Plots
Volcano plots were used to present DEGs performed in the R package “ggplot2”. The genes with |LogFc| >1 are colored and the genes with |LogFc| >2 are labeled.
Results
We Determined The Number of Cases That Needed to Be Analyzed
We chose all 980 cases with complete transcriptome, single-nucleotide variation, and clinical data (Figure 1). The clinical data of the 980 cases are summarized in Table 1.
Scores Were Associated with Lipid Metabolism-Related Pathways in HALLMARK
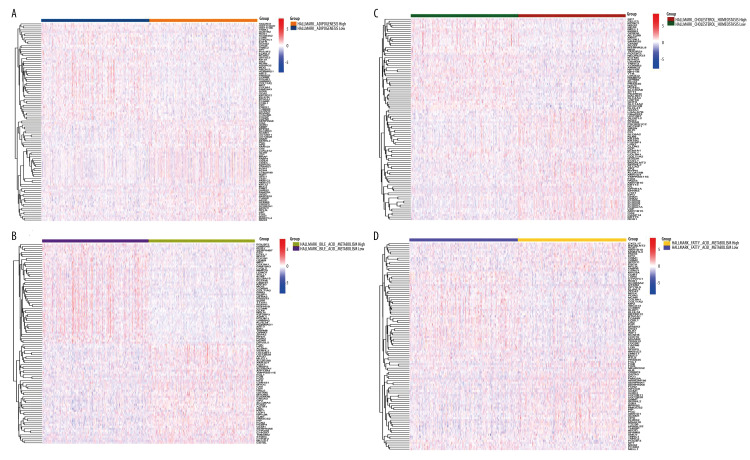
Four lipid metabolism-related pathways in HALLMARK were used to obtain scores of 980 cases, and the median value was used to distinguish the high- and low-score groups. The DEGs between the high-score group and low-score group are presented in heatmaps and volcano plots (Figure 2 and Supplementary Figure 1). The 17 common genes are shown in Figure 3.
Figure 2.
Four lipid metabolism-related pathways in HALLMARK were used as scoring standards. (A) Using the median value of HALLMARK_CHOLESTEROL_HOMEOSTASIS score as the standard, we performed differential gene expression analysis for the high group and the low group. (B) Using the median value of HALLMARK_ADIPOGENESIS score as the standard, we performed differential gene expression analysis for the high group and the low group. (C) Using the median value of HALLMARK_FATTY_ACID_METABOLISM score as the standard, we performed differential gene expression analysis for the high group and the low group. (D) Using the median value of HALLMARK_BILE_ACID_METABOLISM score as the standard, we performed differential gene expression analysis for the high group and the low group.
Figure 3.
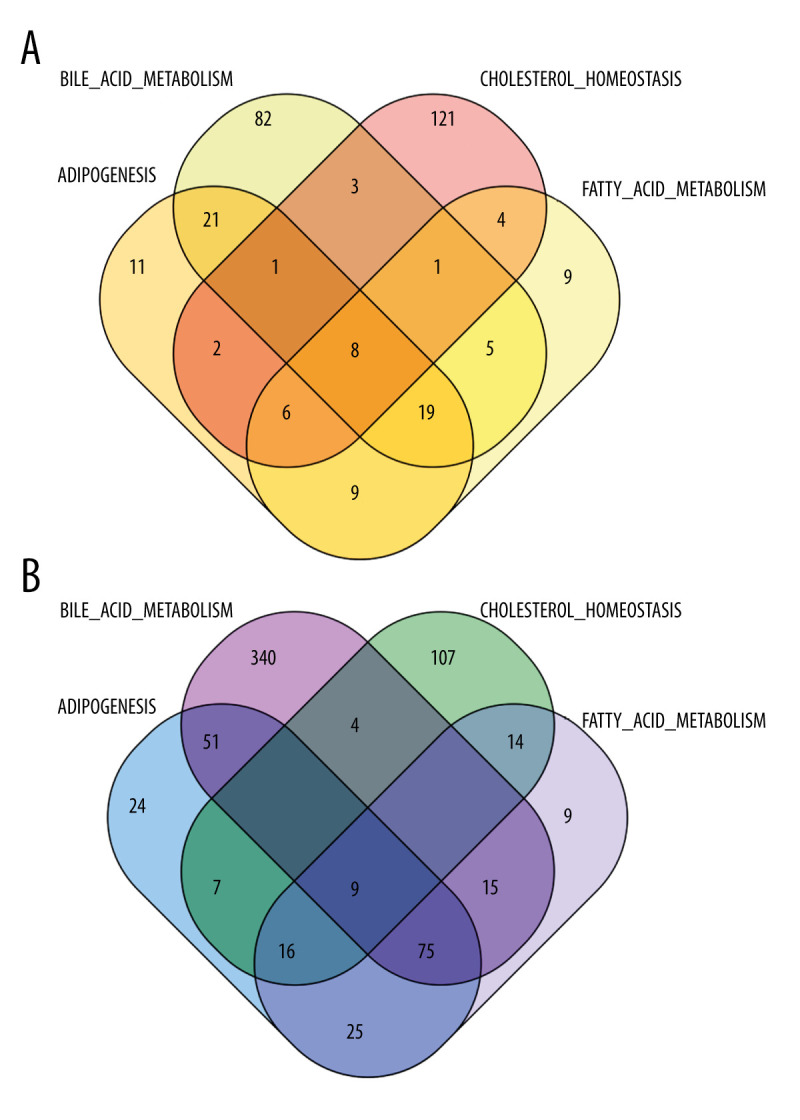
(A) We took the intersection of DEGs in the high-score group and obtained 8 genes. (B) We took the intersection of DEGs in the low-score group and l obtained 9 genes.
Screen Candidate Genes Based On Survival Prognosis
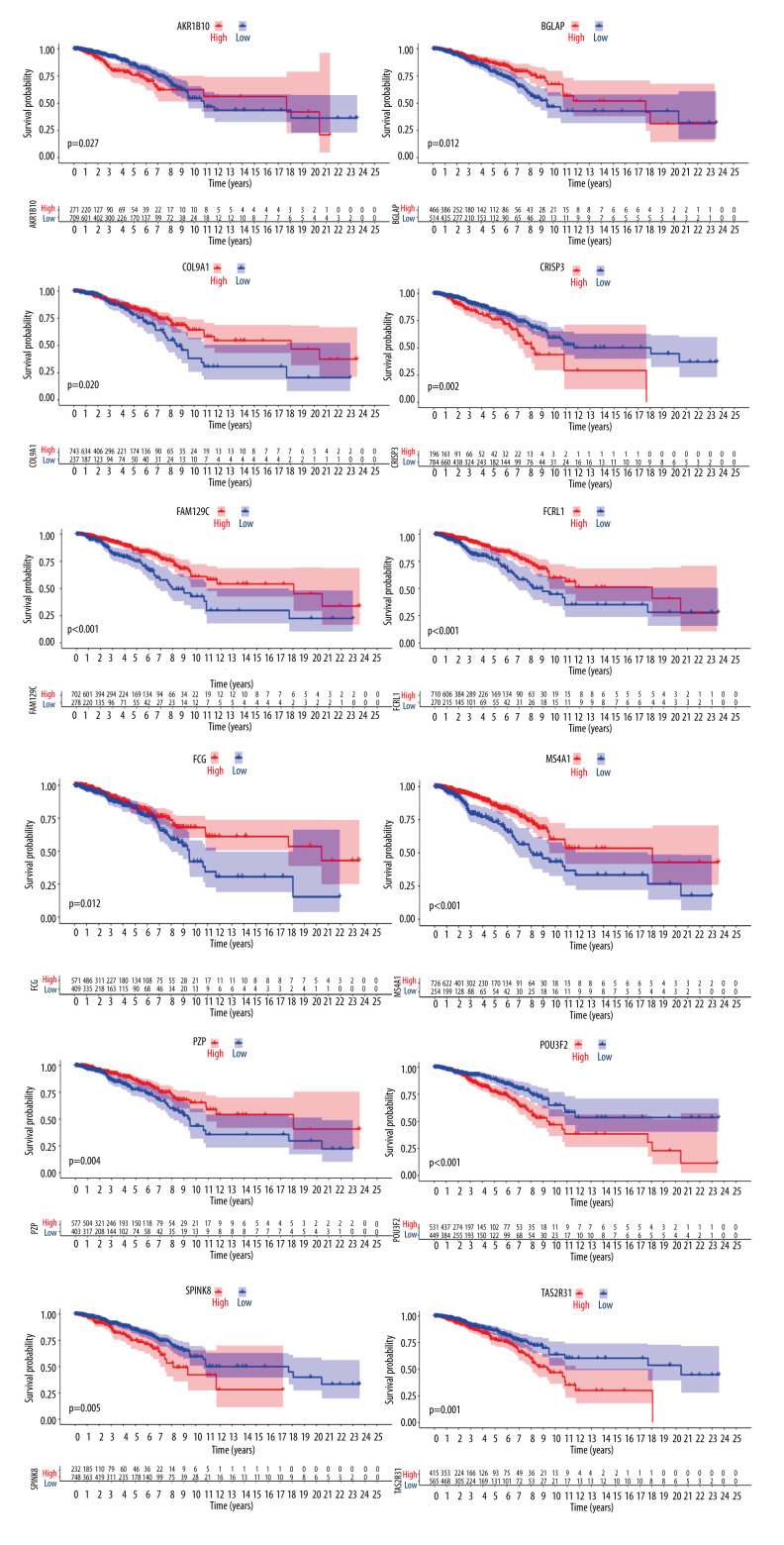
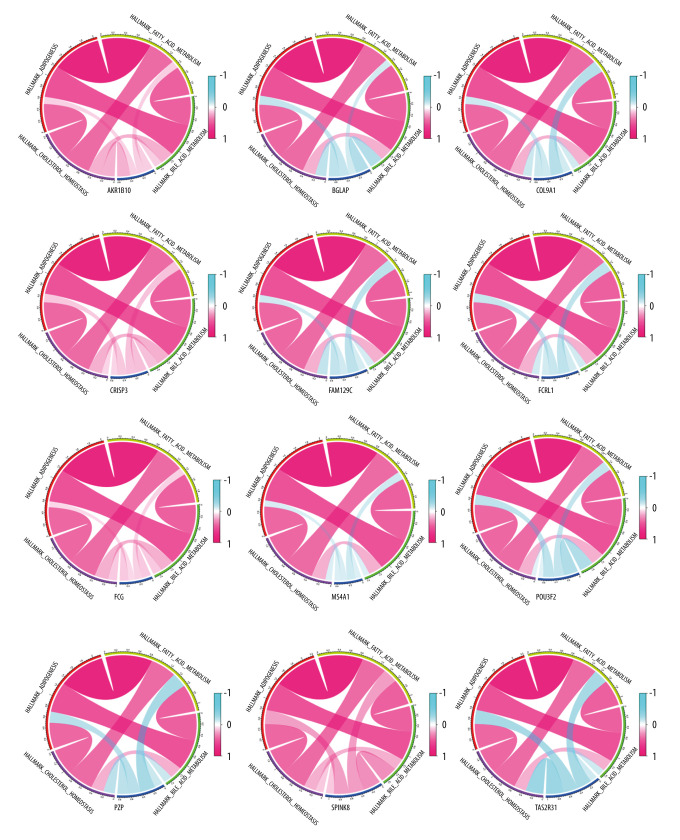
To further screen biomarkers which can predict the prognosis, we analyzed the impact of these 17 DEGs on survival. We obtained 12 genes that were statistically significant in Kaplan-Meier analysis and Cox regression analysis. Survival curves of 12 genes with prognostic value are shown in Figure 4. We used the Spearman rank correlation coefficient to reflect the correlation between gene expression and scoring, displayed in the form of circle graphs (Figure 5). The length of the ribbon represents the value of the correlation coefficient.
Figure 4.
Differences in the expression of 12 genes leading to differences in survival.
Figure 5.
Correlation between 12 genes and 4 scoring pathways. The length of the ribbon indicates the size of the correlation.
Cox Regression Analysis Was Performed To Filter Variables
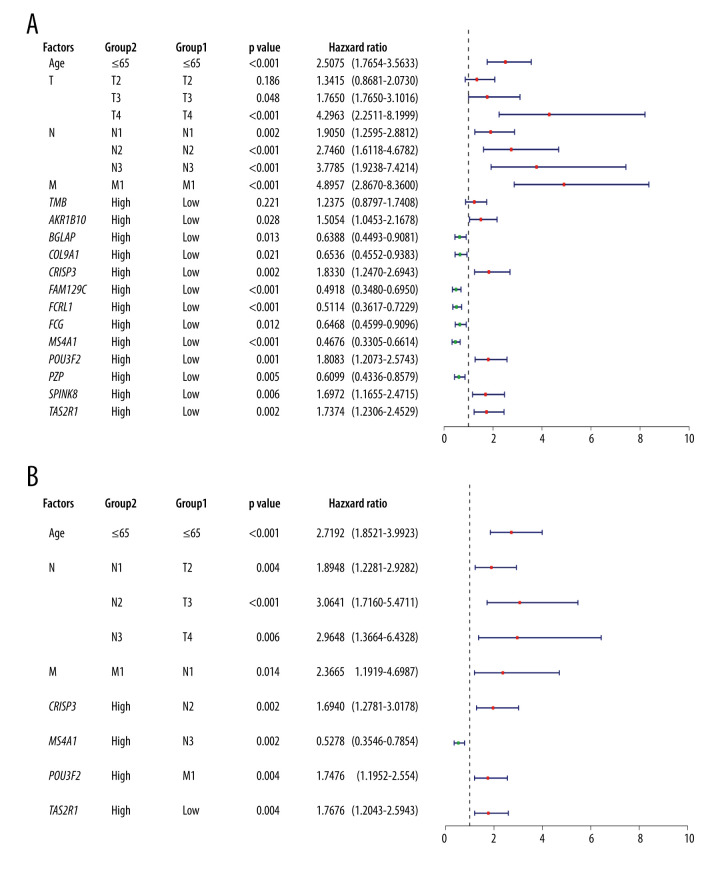
We performed univariate Cox regression analysis of 12 DEGs again, this time incorporating clinical factors into variables: TNM staging and patient age. We found that there were 7 genes with protective effects on the prognosis: BGLAP, COL9A1, FAM129C, FCRL1, FGG, MS4A1 and PZP while AKR1B10, SPINK8, TAS2R31, POU3F2, and CRISP3 were risk factors for prognosis (Figure 6A). Further, we conducted multivariate Cox regression analysis, chose forward selection to filter variables, and drew forest maps (Figure 6B). It was obviously that MS4A1 was an independent protective factor for prognosis. Therefore, we finally determined MS4A1 as the target of further research.
Figure 6.
(A) Univariate Cox regression analysis incorporating age, TNM stage, and 12 DEGs into variables. (B) Multivariate Cox regression analysis. Because of the large number of variables, we used forward selection. MS4A1 was the only protective factor.
Analysis Results of 980 Cases Grouped According to MS4A1 Expression
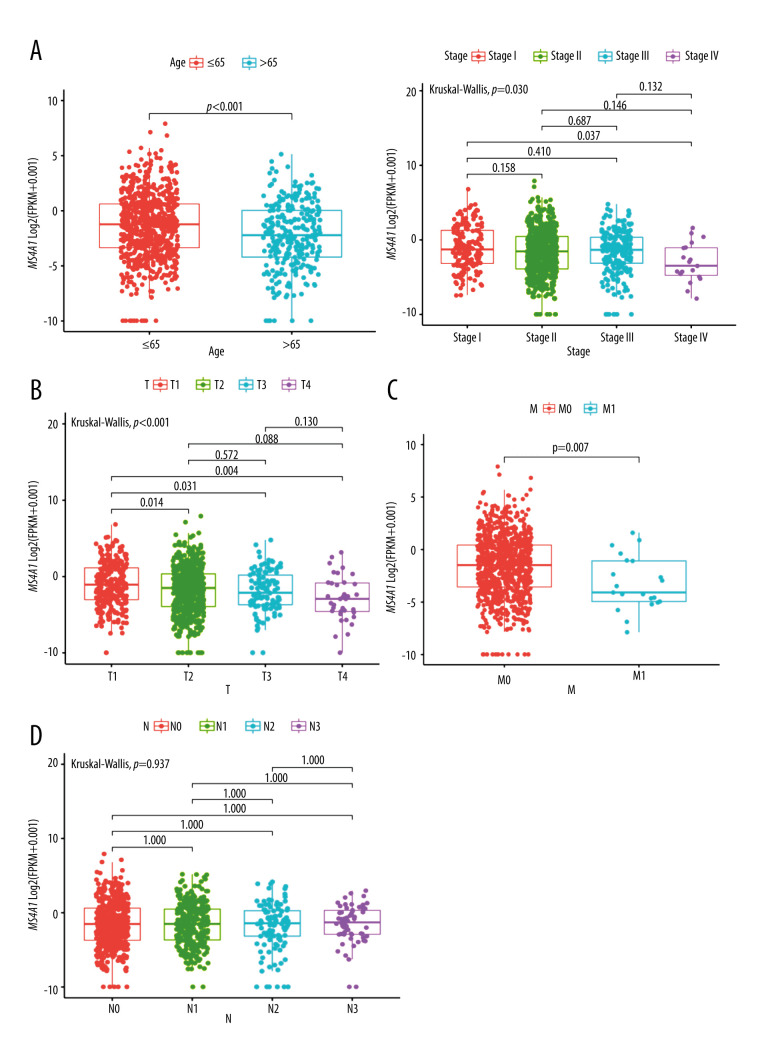
We analyzed SNP, mRNA, and clinical data of 980 cases and the results are showed in Figure 4. According to the DNA data based on 980 cases, TP53 had the highest frequency of mutations (Figure 7A). We found the DEGs between MS4A1 high-expression group and low-expression group and the 100 genes with the most significant fold change value are shown in Figure 7B. We also analyzed the differences of MS4A1 expression in different clinical characters (Figure 8). The cases were divided into 2 groups, one younger than or equal to 65 years old and the other older than 65 years old. The expression of MS4A1 in the younger group was higher than in the older group. The expression of MS4A1 in patients with clinical stage I was higher than in patients with clinical stage IV. For TNM staging, the expression of MS4A1 differed between different T stages and different M stages, meaning that the expression of MS4A1 was higher in the group with smaller tumor diameter and the group without distant metastasis. According to the analysis results, there was no significant difference of MS4A1 expression in patients with different N stages.
Figure 7.
(A) Single-nucleotide variation of MS4A1 high-expression group and low-expression group. (B) Transcriptome level results of MS4A1 high-expression group and low-expression group.
Figure 8.
(A) MS4A1 expression in different age groups and among different stage groups. (B) MS4A1 expression among different T (Topography) groups. (C) MS4A1 expression among different M (Metastasis) groups. (D) MS4A1 expression among different N (Lymph Node) groups.
The Differential Expression of MS4A1 May Be Related To Immune-Related Pathways
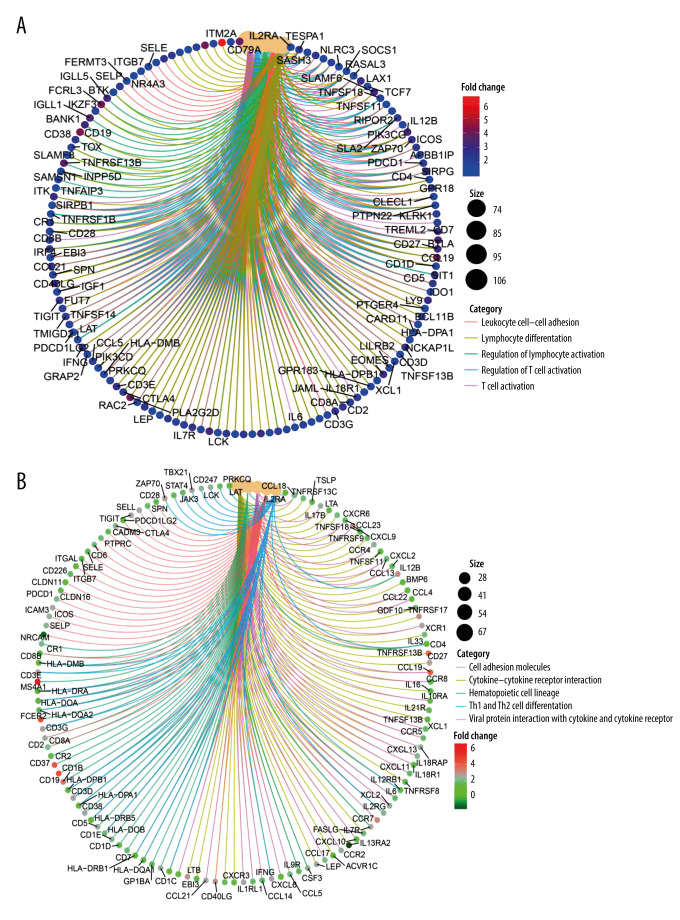
Supplementary Figure 2A shows the differential expression of genes in 980 cases. We used the R package “clusterprofiler”. In GO analysis, the DEGs were enriched to the immune-related GO terms (eg, regulation of T cell activation and lymphocyte differentiation) (Figure 9A and Supplementary Figure 2B). The KEGG analysis also showed enriched immune-related pathways, such as Th1 and Th2 cell differentiation (Figure 9B and Supplementary Figure 2C). Based on the above analysis results, we concluded that most of the DEGs obtained after grouping according to the expression of MS4A1 are enriched in immune-related pathways, which indicated that the differential expression of MS4A1 may be related to immune status.
Figure 9.
(A) We used R language with the clusterProfiler package to perform GO enrichment analysis and created a circle graph. (B)4We used R language with the clusterProfiler package to perform KEGG enrichment analysis and created a circle graph.
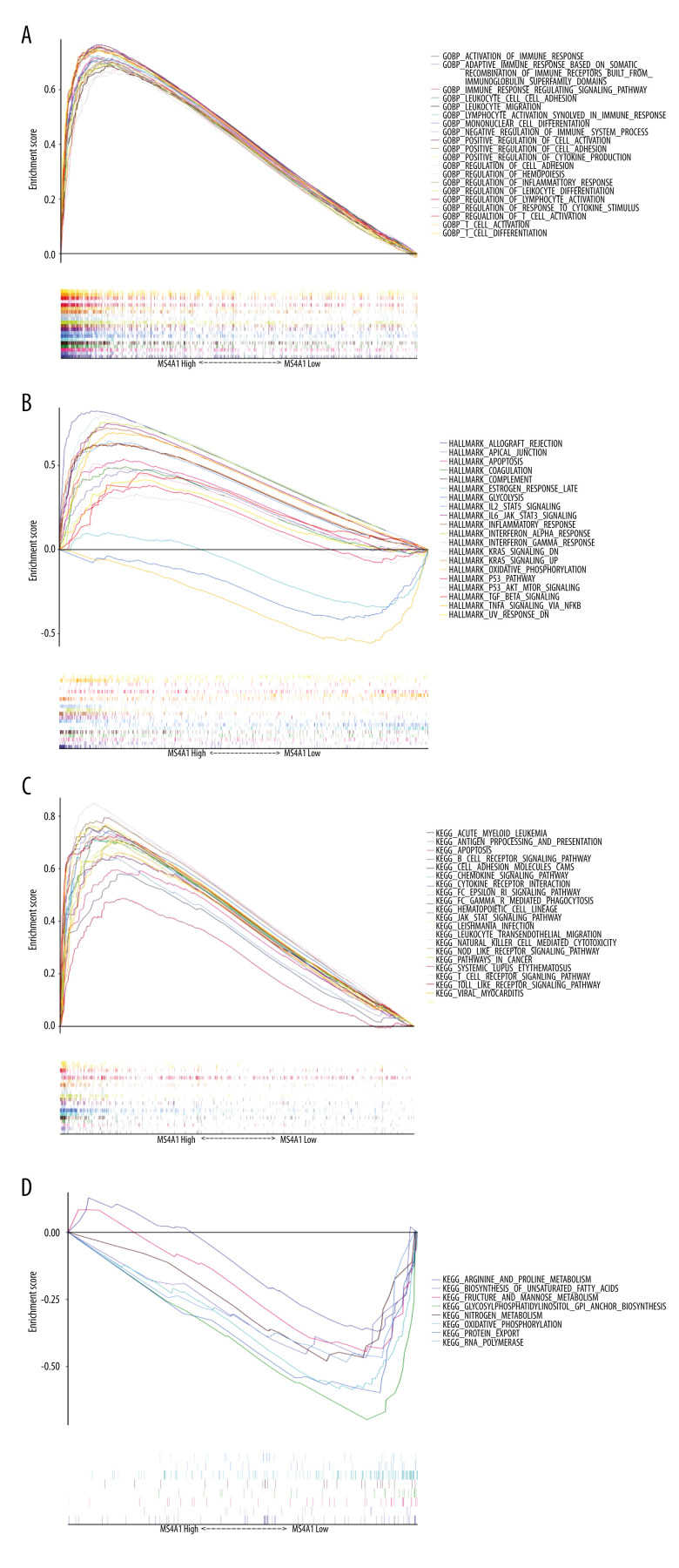
We used the R package “GSVA” for further enrichment analysis. We found that DEGs in GO, HALLMARK, and KEGG gene sets all displayed enrichment of immune-related pathways (Supplementary Figure 3).
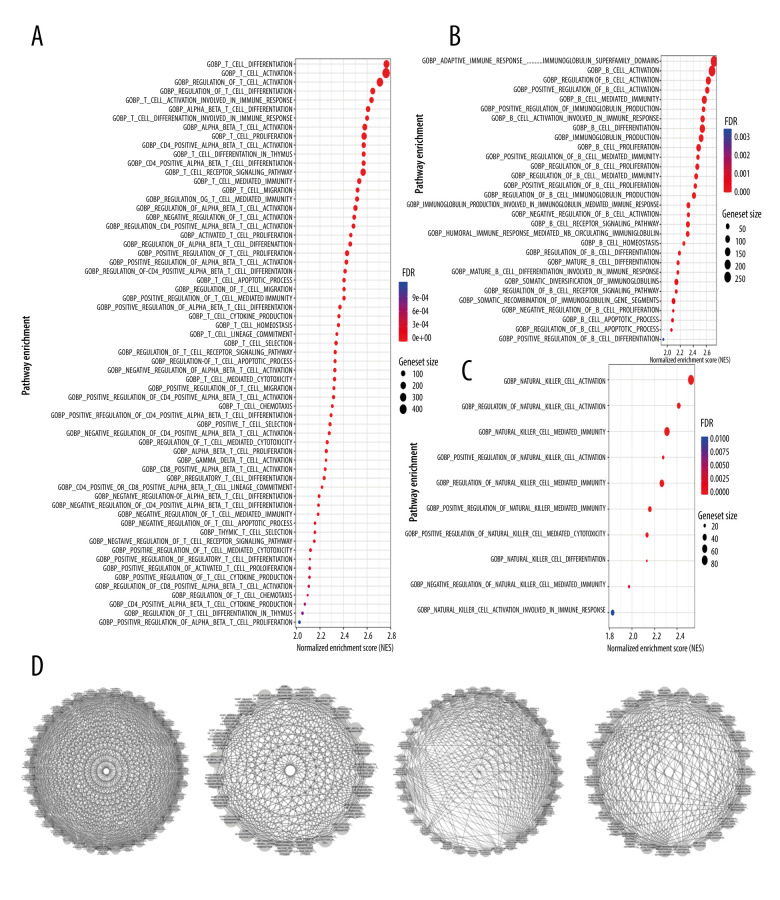
We also performed GSEA, with similar results obtained as in the above 2 analysis methods: immune activity in the MS4A1 high-expression group was active (Figure 10). KEGG and HALLMARK results of GSEA enrichment analysis charts are displayed in Supplementary Figure 4. In GOBP-related pathways enrichment analysis, we particularly selected the terms related to T cell immunity, B cell immunity, and NK (natural killer) cell immunity to display in the chart (Figure 11A–11C). Due to the high level of redundancy in GOBP, we performed a sub-network analysis on the results of the pathway in GOBP. After setting the parameters, 4 sub-networks were finally obtained, 2 of which are related to immunity (one is related to T cells and the other is related to B cells), 1 is related to calcium transportation, and the other is related to the cytoskeleton (Figure 11D). These analysis results show that high expression of MS4A1 is related to active immunity.
Figure 10.
(A) We used R language with the GSEA package to perform GO enrichment analysis. (B, C) We used R language with the GSEA package to perform HALLMARK enrichment analysis. (D) We used R language with the GSEA package to perform KEGG enrichment analysis.
Figure 11.
(A) T cell-related pathways in GOBP enrichment analysis. (B) B cell-related pathways in GOBP enrichment analysis. (C) NK cell-related pathways in GOBP enrichment analysis. (D) We used Cytoscape software to perform sub-network analysis of GOBP enrichment analysis results. The first and third sub-networks are related to immune function, the second sub-network is related to calcium channels, while the fourth is relate to cytoskeleton.
Relationship Between MS4A1 Expression and the Proportion of TICs
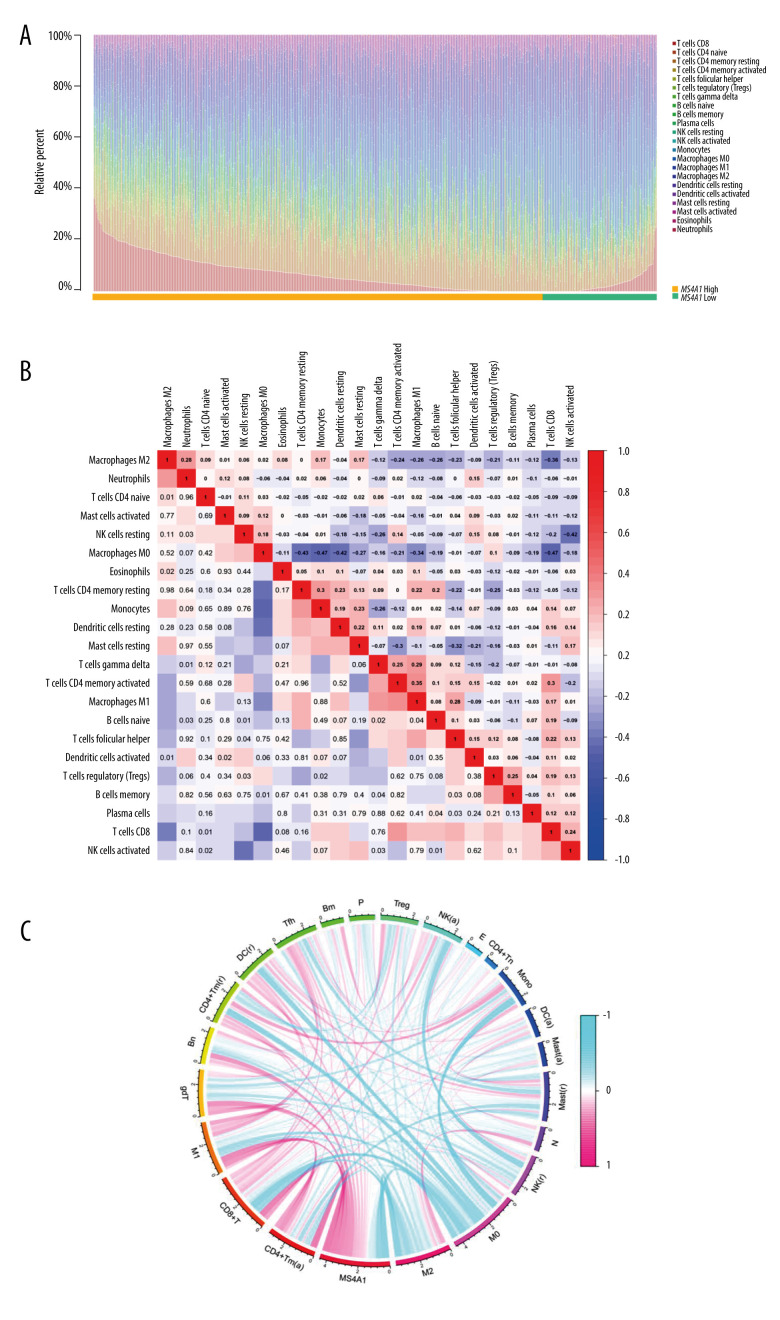
To explore the relationship between MS4A1 and the immune microenvironment, immune subsets were analyzed using the CIBERSORT algorithm. A barplot shows the proportion of 22 kinds of TICs in BRCA cases (Figure 12A). A heatmap shows the correlation between 22 kinds of TICs and Pearson coefficient used for significance testing (Figure 12B). The correlation between MS4A1 and 22 kinds of immune cells is shown in a circle graph. The length of the ribbon represents the correlation coefficient (Figure 12C). The 22 kinds of immune cells are T cells CD8, T cells CD4 naïve, T cells CD4 memory resting, T cells CD4 memory activated, T cells follicular helper, T cells regulatory (Tregs), T cells gamma delta, B cells naïve, B cells memory, plasma cells, NK cells resting, NK cells activated, monocytes, macrophages M0, macrophages M1, macrophages M2, dendritic cells resting, dendritic cells activated, mast cells resting, mast cells activated, eosinophils, and neutrophils. We found that the expression MS4A1 was positively related to macrophages M1 and CD8+T cells, which are related to immune activation, and are negatively related to macrophages M2 and Tregs, which are related to immunosuppression.
Figure 12.
(A) We used the CIBERSORT algorithm to explore the relationship between the expression of MS4A1 and the immune microenvironment, and 22 kinds of immune cell profiles in BRCA samples were constructed. (B) Correlation between immune cells. (C) Correlation between 22 kinds of immune cell profiles and MS4A1.
In Supplementary Figure 5, the correlation between 22 kinds of immune cells and MS4A1 are presented. The correlation coefficients (R) were: B cells memory (R=0.16, FDR<0.001), B cells naïve (R=0.35, FDR<0.001), dendritic cells activated (R=−0.01, FDR=−0.867), dendritic cells resting(R=0.21, FDR<0.001), eosinophils (R=0.01, FDR=0.874), macrophages M0 (R=−0.40, FDR<0.001), macrophages M1 (R=0.40, FDR<0.001), macrophages M2 (R=−0.46, FDR<0.001), mast cells resting (R=−0.12, FDR=0.002), monocytes (R>−0.01, FDR=0.952), neutrophils (R=−0.14, FDR<0.001), NK cells activated (R=0.04, FDR=0.389), NK cells activated (R=−0.18, FDR<0.001), plasma cells (R=−0.11, FDR=0.002), T cells CD4 memory activated (R=0.46, FDR<0.001), T cells CD4 memory resting (R=0.23, FDR<0.001), T cells CD4 naïve (R<0.01, FDR=0.979), T cells CD8(R=0.42, FDR<0.001), T cells follicular helper (R=0.46, FDR<0.001), T cells gamma delta (R=0.36, FDR<0.001), and T cells regulatory (Tregs) (R−0.07, FDR=0.057). A total of 16 kind of immune cells were correlated with MS4A1. We found that the expression of MS4A1 was positively correlated with components related to immune activation such as CD8+ T cell and T cells CD4 memory activated and was is negatively correlated with macrophages M2, which are related to immunosuppression. These results indicated that the high expression of MS4A1 was associated with active immunity.
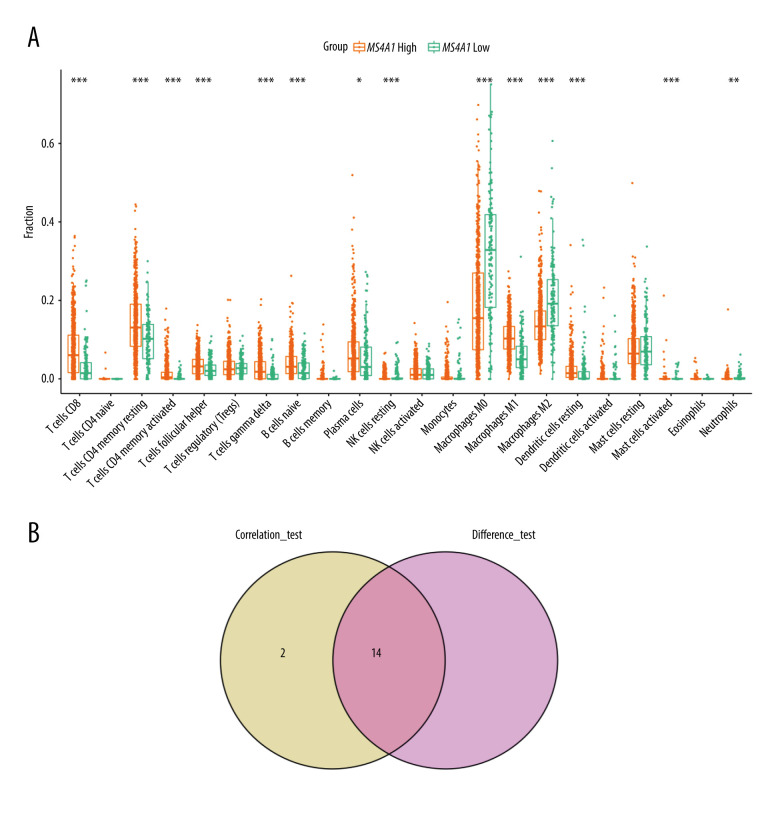
The boxplot (Figure 13A) presents differences in the infiltration of immune cells between MS4A1 high-expression group and low-expression group. There were 14 kinds of cells with different infiltration between the 2 groups. Among them, 10 kinds of cells are positively correlated with MS4A1 expression (eg, CD8+ T cell and T cells CD4 memory activated), while other 4 kinds of cells are negatively correlated with MS4A1 expression (eg, macrophages M0 and macrophages M2). We conclude that the high expression of MS4A1 is related to active immunity. The analysis and correlation analysis results showed a total of 14 common immune cell profiles are related to the expression of MS4A1 (Figure 13B).
Figure 13.
(A) Differences in immune cells infiltrate between MS4A1 high-expression group and low-expression group. (B) Intersection of immune cells related to MS4A1 and differences in expression.
Differential Expression of MS4A1 and Immune Checkpoint-Related Genes
To provide potential drug targets for clinical treatment, we further explored the correlation between MS4A1 expression and immune checkpoint-related genes (Supplementary Figure 5) (genes annotated in gray are not statistically significant). We found that the MS4A1 high-expression group had higher expression of immune checkpoint-related genes, which indicated that the patients with high expression of MS4A1 may be sensitive to immunotherapy.
Discussion
In this study, we tried to find genes from the TCGA database that are related to lipid metabolism and that have prognostic value. MS4A1 stood out from the screening. Interestingly, after a series of bioinformatics analysis, MS4A1 is not only related to lipid metabolism, but may also be an indicator of the immune microenvironment.
In our study, the expression of MS4A1 is correlated with the activity of lipid metabolism. Breasts are mainly composed of fatty tissue. At present, there are many known causes of breast cancer, including obesity and high-fat diet. According to statistics, obesity increases the risk of breast cancer [28–30]. Adipose tissue is the largest endocrine organ of the human body. It not only plays an important role in storing energy, but also in hormone regulation and the development of chronic inflammation, which are closely related to the occurrence and progression of tumors. In the studies of estrogen receptor (+)/progesterone receptor (+)/human epidermal growth factor receptor 2 (−) breast cancer, patients with higher body mass index (BMI) had a lower overall survival rate, and are more prone to recurrence and metastasis, which means worse prognosis [31,32]. In a prospective study, it was found that regardless of BMI, estrogen receptors, and the time interval between blood collection and diagnosis, blood lipids are related to the risk of breast cancer [33]. On the contrary, some studies have concluded that women with hypercholesterolemia have a lower risk of breast cancer and a longer survival period, but the authors believed that this result may also be due to patients with hypercholesterolemia receiving statins treatment, which reduces the risk of breast cancer [34]. If a patient can start to change her lifestyle, including reducing energy intake, adjusting diet structure, avoiding high-fat diets, and increasing exercise, the quality of life and prognosis of the patient can be significantly improved [35–38]. Some other studies found that lipid metabolism disorders are closely related to chemotherapy resistance. Lipid metabolism can be regulated by the JAK/STAT3 pathway. Inhibition of the JAK/STAT3 pathway can decrease the expression of multiple lipid metabolism-related genes, thereby inhibiting breast cancer stem cells and reducing chemotherapy resistance [39].
TME is particularly important in the occurrence and progression of tumors, and metabolic disorders can affect TME [40]. LOX (acting in the cross-linking of elastin and collagen fibers as well as in the modulation of the structure and stiffness of tumor ECM) was also discovered to be an indicator of prognosis and metastasis [20,21]. Use of Trabectedin, which is an anti-tumor compound that can adjust multiple components in TME, including tumor-associated macrophages, has been suggested in breast cancer treatment [22–24]. Many studies have confirmed that the immune components in TME are particularly important in the occurrence and development of tumors. Although breast cancer is not generally considered to be a highly immunogenic cancer, there is still a rich tumor immune microenvironment in some subtypes of breast cancer. The adaptive immune system, macrophages, and other innate immune cells in breast cancer also play important roles [41]. Some studies have found that in triple-negative breast cancer patients, TILs (tumor-infiltrating lymphocytes) are associated with better prognosis, especially prolonging recurrence-free survival, and can be used as tumor prognostic markers [42–44]. Thus, most patients with active immune response will obtain better treatment effect and prognosis.
MS4A1 encodes the B cell surface marker CD20, which participates in B cell receptor signaling and interacts with the immune microenvironment. In our analysis using lipid metabolism-related pathways to score and screen out MS4A1, the prognosis of the high-expression group was significantly better than the low-expression group. A study found that the expression of lipid metabolism-related genes in breast tissue, which is susceptible to breast cancer, is active and CD20 expression is decreased [45]. From this we speculate that MS4A1 improves the prognosis of breast cancer.
We grouped 980 cases according to the differential expression of MS4A1 and found DEGs to use in pathways enrichment analysis. The enrichment results showed that the high expression of MS4A1 was related to immune-related pathways.
CD20 encoded by MS4A1 is closely related to B cell proliferation, differentiation, and activation [46–48], and CD20 disorders have also been reported in patients with immunodeficiency diseases [49,50]. Studies have found that the expression of CD20 on tumor-infiltrating lymphocytes in patients with ovarian cancer is associated with a positive prognosis [51]; in colorectal cancer, the expression of MS4A1 is positively correlated with the survival rate of patients, and the MS4A1 expression can be used as a diagnostic marker [52,53]. These results are consistent with our finding that the prognosis of breast cancer patients in the MS4A1 high expression group was better. In addition, we found that the expression of MS4A1 was positively correlated with 10 types of immune cells, which may be one of the reasons for the better prognosis of patients with high MS4A1 expression. A study of more than 12 000 people showed that CD8+ T cells infiltration can significantly reduce the risk of death in patients with ER-negative, ER, and HER2-positive breast cancer [54]. Higher CD8+ T cells scores were associated with better survival of TNBC patients [55], and PARP inhibitor olaparib induces CD8+ T cells recruitment and activation to exert an anti-tumor effect [56].
For the pathways related to calcium channels and cytoskeleton in the enriched sub-network analysis, we speculate it may be due to the calcium ion transportation and cytoskeleton remodeling involved in the process of immune microenvironment regulation. Cytoskeleton changes are related to immune synapse formation. These physiological processes play important roles in the function of the immune system [57–59].
MS4A1 is related to the lipid metabolism and immune microenvironment status of breast cancer patients, and has potential to be an independent prognostic indicator. Since its expression is positively correlated with immune checkpoint mutation-related genes, MS4A1 could provide guidance in choosing appropriate treatment methods.
A limitation of the present study is that we only used data from a single database. In addition, due to the limitation of objective conditions, no relevant experimental verification was carried out. We will address these limitations in future related research and look forward to making further discoveries.
Conclusions
After a series of analyses of relevant data from the TCGA database, we found that MS4A1 not only reflects the lipid metabolism level of breast cancer patients, but also serves as a potential independent prognostic factor. Our research provides a new biomarker for clinical treatment, which helps to distinguish the different conditions of patients, so as to better carry out individualized treatment and maximize the treatment effect.
Supplementary Data
(A) Using the median value of HALLMARK_CHOLESTEROL_HOMEOSTASIS score as the standard, we performed differential gene expression analysis for the high group and the low group. (B) Using the median value of HALLMARK_ADIPOGENESIS score as the standard, we performed differential gene expression analysis for the high group and the low group. (C) Using the median value of HALLMARK_FATTY_ACID_METABOLISM score as the standard, we performed differential gene expression analysis for the high group and the low group. (D) Using the median value of HALLMARK_BILE_ACID_METABOLISM score as the standard, we performed differential gene expression analysis for the high group and the low group.
(A) Gene expression differences of MS4A1 high-expression group and low-expression group. (B) We used R language with the clusterProfiler package to perform GO enrichment analysis and created a bar graph. (C) We used R language with the clusterProfiler package to perform KEGG enrichment analysis and created a bar graph.
(A) We used R language with the GSVA package to perform GO enrichment analysis. (B) We used R language with the GSVA package to perform HALLMARK enrichment analysis. (C) We used R language with the GSVA package to perform KEGG enrichment analysis.
(A) We used R language with the GSEA package to perform KEGG enrichment analysis and created a bubble chart. (B) We used R language with the GSEA package to perform HALLMARK enrichment analysis and created a bubble chart.
Correlation coefficient between 22 immune cell profiles and MS4A1.
Immune checkpoint mutation burden of MS4A1 high-expression group and low-expression group.
Acknowledgement
We thank T. Xie for helping with statistical analysis.
Footnotes
Conflict of interest: None declared
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
All of the figures were drawn by the first author, Shilin Li.
Financial support: None declared
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Röhrig F, Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer. 2016;16(11):732–49. doi: 10.1038/nrc.2016.89. [DOI] [PubMed] [Google Scholar]
- 3.Yu XH, Ren XH, Liang XH, Tang YL. Roles of fatty acid metabolism in tumourigenesis: Beyond providing nutrition (review) Mol Med Rep. 2018;18(6):5307–16. doi: 10.3892/mmr.2018.9577. [DOI] [PubMed] [Google Scholar]
- 4.Esposito M, Mondal N, Greco TM, et al. Bone vascular niche E-selectin induces mesenchymal-epithelial transition and Wnt activation in cancer cells to promote bone metastasis. Nat Cell Biol. 2019;21(5):627–39. doi: 10.1038/s41556-019-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyd NF, McGuire V. Evidence of association between plasma high-density lipoprotein cholesterol and risk factors for breast cancer. J Natl Cancer Inst. 1990;82(6):460–68. doi: 10.1093/jnci/82.6.460. [DOI] [PubMed] [Google Scholar]
- 6.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3(9):565–74. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 7.Kitahara CM, Berrington de González A, Freedman ND, et al. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol. 2011;29(12):1592–98. doi: 10.1200/JCO.2010.31.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Umetani M, Domoto H, Gormley AK, et al. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med. 2007;13(10):1185–92. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- 9.DuSell CD, Umetani M, Shaul PW, Mangelsdorf DJ, McDonnell DP. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol (Baltimore, MD) 2008;22(1):65–77. doi: 10.1210/me.2007-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson ER, Wardell SE, Jasper JS, et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science (New York, NY) 2013;342(6162):1094–98. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Q, Ishikawa T, Sirianni R, et al. 27-Hydroxycholesterol promotes cell-autonomous, ER-positive breast cancer growth. Cell Rep. 2013;5(3):637–45. doi: 10.1016/j.celrep.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimbung S, Chang CY, Bendahl PO, et al. Impact of 27-hydroxylase (CYP27A1) and 27-hydroxycholesterol in breast cancer. Endocr Relat Cancer. 2017;24(7):339–49. doi: 10.1530/ERC-16-0533. [DOI] [PubMed] [Google Scholar]
- 13.Cao Y. Adipocyte and lipid metabolism in cancer drug resistance. J Clin Invest. 2019;129(8):3006–17. doi: 10.1172/JCI127201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomaraschi M. Role of lipoproteins in the microenvironment of hormone-dependent cancers. Trends Endocrinol Metab. 2020;31(3):256–68. doi: 10.1016/j.tem.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Lei X, Lei Y, Li JK, et al. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020;470:126–33. doi: 10.1016/j.canlet.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Rossow L, Veitl S, Vorlová S, et al. LOX-catalyzed collagen stabilization is a proximal cause for intrinsic resistance to chemotherapy. Oncogene. 2018;37:4921–40. doi: 10.1038/s41388-018-0320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller BW, Morton JP, Pinese M, et al. Targeting the LOX/hypoxia axis reverses many of the features that make pancreatic cancer deadly: Inhibition of LOX abrogates metastasis and enhances drug efficacy. EMBO Mol Med. 2015;7:1063–76. doi: 10.15252/emmm.201404827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saatci O, Kaymak A, Raza U, et al. Targeting lysyl oxidase (LOX) overcomes chemotherapy resistance in triple negative breast cancer. Nat Commun. 2020;11:2416. doi: 10.1038/s41467-020-16199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Vita A, Liverani C, Molinaro R, et al. Lysyl oxidase engineered lipid nanovesicles for the treatment of triple negative breast cancer. Sci Rep. 2021;11(1):5107. doi: 10.1038/s41598-021-84492-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Setargew YFI, Wyllie K, Grant RD, et al. Targeting lysyl oxidase family meditated matrix cross-linking as an anti-stromal therapy in solid tumours. Cancers (Basel) 2021;13(3):491. doi: 10.3390/cancers13030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saatci O, Kaymak A, Raza U, et al. Targeting lysyl oxidase (LOX) overcomes chemotherapy resistance in triple negative breast cancer. Nat Commun. 2020;11(1):2416. doi: 10.1038/s41467-020-16199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Incalci M, Zambelli A. Trabectedin for the treatment of breast cancer. Expert Opin Investig Drugs. 2016;25(1):105–15. doi: 10.1517/13543784.2016.1124086. [DOI] [PubMed] [Google Scholar]
- 23.De Vita A, Recine F, Miserocchi G, et al. The potential role of the extracellular matrix in the activity of trabectedin in UPS and L-sarcoma: Evidences from a patient derived primary culture case series in tridimensional and zebrafish models. J Exp Clin Cancer Res. 2021;40:165. doi: 10.1186/s13046-021-01963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Souid S, Aissaoui D, Srairi-Abid N, Essafi-Benkhadir K. Trabectedin (Yondelis®) as a therapeutic option in gynecological cancers: a focus on its mechanisms of action, clinical activity and genomic predictors of drug response. Curr Drug Targets. 2020;21(10):996–1007. doi: 10.2174/1389450121666200128161733. [DOI] [PubMed] [Google Scholar]
- 25.Hao Y, Li D, Xu Y, et al. Investigation of lipid metabolism dysregulation and the effects on immune microenvironments in pan-cancer using multiple omics data. BMC Bioinformatics. 2019;20(Suppl 7):195. doi: 10.1186/s12859-019-2734-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eon Kuek L, Leffler M, Mackay GA, Hulett MD. The MS4A family: Counting past 1, 2 and 3. Immunol Cell Biol. 2016;94(1):11–23. doi: 10.1038/icb.2015.48. [DOI] [PubMed] [Google Scholar]
- 27.Mudd TW, Jr, Lu C, Klement JD, Liu K. MS4A1 expression and function in T cells in the colorectal cancer tumor microenvironment. Cell Immunol. 2021;360:104260. doi: 10.1016/j.cellimm.2020.104260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: A systematic review. Br J Cancer. 2006;94(9):1221–25. doi: 10.1038/sj.bjc.6603051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keum N, Greenwood DC, Lee DH, et al. Adult weight gain and adiposity-related cancers: A dose-response meta-analysis of prospective observational studies. J Natl Cancer Inst. 2015;107(2):djv088. doi: 10.1093/jnci/djv088. [DOI] [PubMed] [Google Scholar]
- 30.Chen GC, Chen SJ, Zhang R, et al. Central obesity and risks of pre- and postmenopausal breast cancer: A dose-response meta-analysis of prospective studies. Obes Rev. 2016;17(11):1167–77. doi: 10.1111/obr.12443. [DOI] [PubMed] [Google Scholar]
- 31.Robinson PJ, Bell RJ, Davis SR. Obesity is associated with a poorer prognosis in women with hormone receptor positive breast cancer. Maturitas. 2014;79(3):279–86. doi: 10.1016/j.maturitas.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Jeon YW, Kang SH, Park MH, et al. Relationship between body mass index and the expression of hormone receptors or human epidermal growth factor receptor 2 with respect to breast cancer survival. BMC cancer. 2015;15:865. doi: 10.1186/s12885-015-1879-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.His M, Dartois L, Fagherazzi G, et al. Associations between serum lipids and breast cancer incidence and survival in the E3N prospective cohort study. Cancer Causes Control. 2017;28(1):77–88. doi: 10.1007/s10552-016-0832-4. [DOI] [PubMed] [Google Scholar]
- 34.Zhong S, Zhang X, Chen L, et al. Statin use and mortality in cancer patients: Systematic review and meta-analysis of observational studies. Cancer Treat Rev. 2015;41(6):554–67. doi: 10.1016/j.ctrv.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Prentice RL, Caan B, Chlebowski RT, et al. Low-fat dietary pattern and risk of invasive breast cancer: The Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):629–42. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 36.Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: Interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst. 2006;98(24):1767–76. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 37.Scott E, Daley AJ, Doll H, et al. Effects of an exercise and hypocaloric healthy eating program on biomarkers associated with long-term prognosis after early-stage breast cancer: A randomized controlled trial. Cancer Causes Control. 2013;24(1):181–91. doi: 10.1007/s10552-012-0104-x. [DOI] [PubMed] [Google Scholar]
- 38.Swisher AK, Abraham J, Bonner D, et al. Exercise and dietary advice intervention for survivors of triple-negative breast cancer: Effects on body fat, physical function, quality of life, and adipokine profile. Support Care Cancer. 2015;23(10):2995–3003. doi: 10.1007/s00520-015-2667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang T, Fahrmann JF, Lee H, et al. JAK/STAT3-regulated fatty acid β-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab. 2018;27(1):136–50e5. doi: 10.1016/j.cmet.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dias AS, Almeida CR, Helguero LA, Duarte IF. Metabolic crosstalk in the breast cancer microenvironment. Eur J Cancer (Oxford, England: 1990) 2019;121:154–71. doi: 10.1016/j.ejca.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Burugu S, Asleh-Aburaya K, Nielsen TO. Immune infiltrates in the breast cancer microenvironment: Detection, characterization and clinical implication. Breast cancer (Tokyo, Japan) 2017;24(1):3–15. doi: 10.1007/s12282-016-0698-z. [DOI] [PubMed] [Google Scholar]
- 42.Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27):2959–66. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31(7):860–67. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 44.Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: Results from the FinHER trial. Ann Oncol. 2014;25(8):1544–50. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 45.Marino N, German R, Rao X, et al. Upregulation of lipid metabolism genes in the breast prior to cancer diagnosis. NPJ Breast Cancer. 2020;6:50. doi: 10.1038/s41523-020-00191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tedder TF, Boyd AW, Freedman AS, et al. The B cell surface molecule B1 is functionally linked with B cell activation and differentiation. J Immunol (Baltimore, MD: 1950) 1985;135(2):973–79. [PubMed] [Google Scholar]
- 47.Léveillé C, R AL-D, Mourad W. CD20 is physically and functionally coupled to MHC class II and CD40 on human B cell lines. Eur J Immunol. 1999;29(1):65–74. doi: 10.1002/(SICI)1521-4141(199901)29:01<65::AID-IMMU65>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 48.Petrie RJ, Deans JP. Colocalization of the B cell receptor and CD20 followed by activation-dependent dissociation in distinct lipid rafts. J Immunol (Baltimore, MD: 1950) 2002;169(6):2886–91. doi: 10.4049/jimmunol.169.6.2886. [DOI] [PubMed] [Google Scholar]
- 49.Kuijpers TW, Bende RJ, Baars PA, et al. CD20 deficiency in humans results in impaired T cell-independent antibody responses. J Clin Invest. 2010;120(1):214–22. doi: 10.1172/JCI40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van de Ven AA, Compeer EB, Bloem AC, et al. Defective calcium signaling and disrupted CD20-B-cell receptor dissociation in patients with common variable immunodeficiency disorders. J Allergy Clin Immunol. 2012;129(3):755–61e7. doi: 10.1016/j.jaci.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 51.Milne K, Köbel M, Kalloger SE, et al. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One. 2009;4(7):e6412. doi: 10.1371/journal.pone.0006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mudd TW, Jr, Lu C, Klement JD, Liu K. MS4A1 expression and function in T cells in the colorectal cancer tumor microenvironment. Cell Immunol. 2021;360:104260. doi: 10.1016/j.cellimm.2020.104260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han M, Liew CT, Zhang HW, et al. Novel blood-based, five-gene biomarker set for the detection of colorectal cancer. Clin Cancer Res. 2008;14(2):455–60. doi: 10.1158/1078-0432.CCR-07-1801. [DOI] [PubMed] [Google Scholar]
- 54.Ali HR, Provenzano E, Dawson SJ, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol. 2014;25(8):1536–43. doi: 10.1093/annonc/mdu191. [DOI] [PubMed] [Google Scholar]
- 55.Oshi M, Asaoka M, Tokumaru Y, et al. CD8 T cell score as a prognostic biomarker for triple negative breast cancer. Int J Mol Sci. 2020;21(18):6968. doi: 10.3390/ijms21186968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pantelidou C, Sonzogni O, De Oliveria Taveira M, et al. PARP inhibitor efficacy depends on CD8(+) T-cell recruitment via intratumoral STING pathway activation in BRCA-deficient models of triple-negative breast cancer. Cancer Discov. 2019;9(6):722–37. doi: 10.1158/2159-8290.CD-18-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Savic S, Caseley EA, McDermott MF. Moving towards a systems-based classification of innate immune-mediated diseases. Nat Rev Rheumatol. 2020;16(4):222–37. doi: 10.1038/s41584-020-0377-5. [DOI] [PubMed] [Google Scholar]
- 58.Papa R, Penco F, Volpi S, Gattorno M. Actin remodeling defects leading to autoinflammation and immune dysregulation. Front Immunol. 2020;11:604206. doi: 10.3389/fimmu.2020.604206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berry CT, May MJ, Freedman BD. Analysis of calcium control of canonical NF-κB signaling in B lymphocytes. Methods Mol Biol (Clifton, NJ) 2021;2366:145–64. doi: 10.1007/978-1-0716-1669-7_9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Using the median value of HALLMARK_CHOLESTEROL_HOMEOSTASIS score as the standard, we performed differential gene expression analysis for the high group and the low group. (B) Using the median value of HALLMARK_ADIPOGENESIS score as the standard, we performed differential gene expression analysis for the high group and the low group. (C) Using the median value of HALLMARK_FATTY_ACID_METABOLISM score as the standard, we performed differential gene expression analysis for the high group and the low group. (D) Using the median value of HALLMARK_BILE_ACID_METABOLISM score as the standard, we performed differential gene expression analysis for the high group and the low group.
(A) Gene expression differences of MS4A1 high-expression group and low-expression group. (B) We used R language with the clusterProfiler package to perform GO enrichment analysis and created a bar graph. (C) We used R language with the clusterProfiler package to perform KEGG enrichment analysis and created a bar graph.
(A) We used R language with the GSVA package to perform GO enrichment analysis. (B) We used R language with the GSVA package to perform HALLMARK enrichment analysis. (C) We used R language with the GSVA package to perform KEGG enrichment analysis.
(A) We used R language with the GSEA package to perform KEGG enrichment analysis and created a bubble chart. (B) We used R language with the GSEA package to perform HALLMARK enrichment analysis and created a bubble chart.
Correlation coefficient between 22 immune cell profiles and MS4A1.
Immune checkpoint mutation burden of MS4A1 high-expression group and low-expression group.