Abstract
Compared with the available drugs for the treatment of fibrosis in other organs, the development of intestinal anti-fibrosis drugs is limited. Therefore, it is of practical significance to examine novel drugs to delay or block the development of intestinal fibrosis. The present study aimed to investigate the effect of atractylenolide III (ATL-III) on intestinal fibrosis. An MTT assay was used to detect the effect of ATL-III on the activity of IEC-6 cells. The migration and invasion of fibrotic cells stimulated with TGF-β were determined via wound healing and Transwell assays. An immunofluorescence assay and western blotting were conducted to assess the expression levels of protein associated with epithelial-mesenchymal transition (EMT). The role of the AMP-activated protein kinase (AMPK) pathway was verified using compound C (an AMPK inhibitor) treatment. The results of the present study indicated that ATL-III had no effect on the cells at a dose of 1–20 µmol/l. Moreover, ATL-III can inhibit the invasion and migration of cells induced by TGF-β1, as well as block the EMT process. It was found that ATL-III could also activate the AMPK pathway. Furthermore, compound C reduced the inhibitory effect of ATL-III on stimulated cells, which indicated that the AMPK pathway plays a role in the inhibition process. In conclusion, ATL-III may inhibit the EMT of IEC-6 cells stimulated with TGF-β1 by activating the AMPK signaling pathway.
Keywords: atractylenolide III, IEC-6 cells, TBF-β, AMP-activated protein kinase, epithelial-mesenchymal transition
Introduction
Fibrosis is an inevitable outcome after chronic damage to an organ or tissue, and is the main pathological basis that ultimately causes organ dysfunction (1). According to a population-based cohort study, >1/2 of patients with inflammatory bowel disease have diseased intestinal segments that will progress to intestinal fibrosis, ultimately leading to intestinal stenosis (2). In addition, due to repeated flare-ups and an increased risk of bowel cancer, Crohn's disease is a lifelong, incurable inflammatory bowel disease (3). During the course of intestinal fibrosis development, fibrosis will gradually occur in the intestinal wall (4), and can lead to narrowing of the intestinal lumen and intestinal obstruction (5,6). These patients will eventually require surgery to relieve the obstruction caused by fibrotic stenosis (7,8).
Compared with studies on liver, lung and skin fibrosis, which have been a hotspot of research, it has only been in recent years that intestinal fibrosis in inflammatory bowel disease has gained increased attention from the academic community (2). At present, the etiology of inflammatory bowel disease remains largely unknown; however, the generally accepted hypothesis is that under the influence of certain genetic susceptibility and environmental factors, an intestinal microecological imbalance can occur, which then causes intestinal mucosal immune disorders (9). Early genetic to intestinal microecology research, as well as mucosal immune-related studies, have been key in understanding the underlying mechanism of inflammatory bowel disease, and biologics targeting pathogenesis have achieved a precise effect for disease control or remission (10–12). However, the long-term outcome of patients with inflammatory bowel disease has not been significantly improved (13,14), and it remains difficult to identify a solution based on the pathogenesis (15). Therefore, it is a reasonable to focus on the final pathological state, which is fibrosis, and develop strategies that can potentially alter the natural disease course and long-term outcome of patients.
Extracellular matrix (ECM) is overproduced and deposited during the process of intestinal fibrosis (16). There are a variety of growth factors, such as transforming growth factor (TGF)-β, epidermal growth factor and insulin like growth factor (17), in the ECM that can upregulate and activate epithelial-mesenchymal transition (EMT) transcription factors, thereby initiating the EMT process. At present, the development of intestinal anti-fibrosis drugs is lacking (18). Therefore, it is of practical significance to investigate novel drugs to delay or block the development of fibrosis.
Atractylenolide III (ATL-III), the main bioactive component of Atractylodes macrocephala, also exists in other medicinal plants, such as Codonopsis and cocklebur (19). It has been shown to exert a variety of pharmacological activities, including anti-allergic, anti-inflammatory, gastroprotective and neuroprotective effects (20,21). For example, ATL-III was found to reduce depression and anxiety-like behaviors in rat depression models (22). In addition, a previous study revealed that ATL-III could reduce muscle wasting in chronic kidney disease by activating oxidative stress (23), as well as effectively improve bleomycin-induced lung injury and function in rats with pulmonary fibrosis (24). Moreover, in human breast cancer cell lines, MDA-MB-231 and MDA-MB-468, ATL-III was shown to significantly block the cell migration and invasion induced by TGF-β1, ultimately inhibiting the motility of metastatic breast cancer cells in mice models, thereby indicating that ATL-III inhibits the process of EMT in vitro and in vivo (25). However, whether ATL-III can also inhibit the EMT process of intestinal cells remains to be verified.
It has been reported that ATL-III notably increases the phosphorylation of AMP-activated protein kinase (AMPK) and the expression of sirtuin 1, indicating that ATL-III may have a beneficial effect on obesity and type 2 diabetes mellitus by improving the energy metabolism of skeletal muscle (26). Apigenin inhibits the proliferation, differentiation and function of renal fibroblasts via AMPK activation, as well as reduces ERK1/2 phosphorylation, suggesting that it may have a favorable therapeutic potential for the treatment of renal fibrosis (27). Furthermore, wedelolactone, a major coumarin ingredient of E. prostrata, can cause increases in the expression levels of fibrosis markers (collagen I and α-smooth muscle actin) and a decrease in that of the anti-fibrosis marker (E-cadherin). Wedelolactone also activates AMPK and inhibits the increase in TGF-β1 phosphorylation, thereby inhibiting the EMT of alveolar epithelial cells (28). Thus, it was suggested that activation of the AMPK signaling pathway can inhibit the EMT process.
As IEC-6 cells can be passaged stably and have the typical morphological and growth characteristics of normal intestinal epithelial cells, they have been widely used in multiple research studies related to intestines (29–31). In the present study, the effect of ATL-III on the EMT process of a small intestine epithelial cell line, IEC-6, was investigated, as well as its underlying mechanism. The current findings could provide a theoretical basis for ATL-III application to inhibit organ fibrosis in the future.
Materials and methods
Cell culture and reagents
IEC-6 (rat small intestinal epithelial) cells were purchased from Merck-KGaA. ATL-III (purity, >98%) was purchased from Chengdu Pufei De Biotech Co., Ltd. and diluted to 1, 10 or 20 µmol/l (32). TGF-β1 protein (cat. no. TG1-M5218) was purchased from Acro Biosystems Co., Ltd. Compound C (also known as dorsomorphin; cat. no. HY-13418A), a type of selective AMPK inhibitor, was purchased from MedChemExpress.
IEC-6 cells were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 10 U/ml insulin and 100 U/ml penicillin-streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C in an incubator with 5% CO2. Cells were divided into: Control, ATL-III, TGF-β1, TGF-β1 + ATL-III and TGF-β1 + ATL-III + compound C groups.
MTT assay
IEC-6 cells (5×103 cells/well) were seeded onto 96-well plates and cultured in an incubator with 5% CO2 at 37°C. Cells were treated with different doses of ATL-III (0, 1, 10 or 20 µmol/l) at 37°C for 24 h. MTT solution (200 µl) was added to each well and the incubation was continued at 37°C for 4 h. Then, the medium was removed and DMSO was added. The optical density was measured at 490 nm wavelengths using a microplate reader (Thermo Fisher Scientific, Inc.).
Wound healing assay
IEC-6 cells were seeded onto 6-well plates and incubated at 37°C overnight. Sterilized pipette tips (200 µl) were used to scratch the cells. Next, plates were washed three times with PBS to remove the cells, and serum-free medium with TGF-β1 (10 ng/ml), ATL-III (1, 10 or 20 µmol/l) and compound C (20 µmol/l) was added for the continuing culture in the incubator at 37°C. At 0 and 24 h, images were captured under an inverted microscope (magnification, ×100; Olympus Corporation) and evaluated using ImageJ software (1.52v; National Institutes of Health). Cell migration rate = wound area difference between 0 and 24 h/wound area at 0 h.
Transwell assay
Matrigel (BD Biosciences) was thawed overnight at 4°C and diluted with serum-free medium, following which the diluent was inoculated into the upper chamber for 30 min at 37°C. IEC-6 cells (5×104) were seeded into the upper chamber (Corning, Inc.) with 100 µl serum-free medium containing TGF-β1 (10 ng/ml), ATL-III (1, 10 or 20 µmol/l) and compound C (20 µmol/l). The lower chamber was filled with 600 µl medium containing 10% FBS. Following 24 h of incubation, the cells on the lower side were fixed with 4% formaldehyde for 20 min at room temperature and stained with 0.1% crystal violet solution for 20 min at room temperature. Images were captured under an inverted microscope (magnification, ×100; Olympus Corporation). Cell invasion rate = the number of invasive cells/number of inoculated cells.
Western blot analysis
Upon IEC-6 cells reaching 60% confluence, TGF-β1 (10 ng/ml), ATL-III (1, 10 or 20 µmol/l) and compound C (20 µmol/l) were added and incubated at 37°C for 48 h. Proteins were extracted and homogenized in RIPA lysis buffer (Beyotime Institute of Biotechnology). Proteins were determined using a BCA kit (Beyotime Institute of Biotechnology), and then 30 µg samples/lane were separated on 10% SDS-polyacrylamide gels, followed by transfer to a PVDF membrane. The membrane was washed with TBS-0.01% Tween-20 (TBST) and then incubated in blocking fluid containing 5% non-fat milk at 25°C for 1 h. Strips were cut and incubated with the following primary antibodies at 4°C overnight: MMP9 (cat. no. ab76003; 1:1,000), vimentin (cat. no. ab92547; 1:1,000), N-cadherin (cat. no. ab18203; 1:1,000), E-cadherin (cat. no. ab40772; 10,000), zonula occludens (ZO)-1 (cat. no. ab216880; 1:1,000), phosphorylated (p)-AMPK (cat. no. ab133448; 1:1,000) and AMPK (cat. no. ab32047; 1:1,000; all from Abcam). Next, strips were incubated with HRP-conjugated goat anti-rabbit secondary antibody (cat. no. ab6721; 1:3,000; Abcam) at room temperature for 1 h after washing with TBST. An ECL chromogenic substrate (Beyotime Institute of Biotechnology) was used for visualization and data were analyzed using Image Lab v4.0 software (Bio-Rad Laboratories, Inc.).
Immunofluorescence assay
IEC-6 cells were seeded onto 6-well plates and incubated. When cells grew to 90% confluence, TGF-β1 (10 ng/ml), ATL-III (1, 10 or 20 µmol/l) and compound C (20 µmol/l) were added at 37°C. After 48 h of incubation, cells were fixed with 4% formaldehyde for 15 min at room temperature and washed with PBS. Then, 0.2% Triton X-100 was added, and subsequently removed using PBS after 15 min. Next, 5% BSA (Beijing Solarbio Science & Technology Co., Ltd.) was added for 30 min at room temperature for the blocking step, following which cells were incubated with N-cadherin (cat. no. ab18203; 1:200) or E-cadherin antibody (cat. no. ab40772; 1:500) at room temperature for 2 h. Then, FITC-labeled goat anti-rabbit secondary antibody (cat. no. ab6717; 1:100; all from Abcam) was applied at room temperature for 1 h. The nuclei were stained with DAPI for 10 min at room temperature and images were captured under a fluorescence microscope (magnification, ×200; Nikon Corporation).
Statistical analysis
GraphPad Prism 8.0 statistical software (GraphPad Software, Inc.) was utilized to analyze the experimental data. All data are presented as the mean ± SD of three replicate experiments. P<0.05 was considered to indicate a statistically significant difference. A unpaired Student's t-test was used to evaluate differences between two groups, while one-way ANOVA and Tukey's post hoc test were used for multiple groups.
Results
ATL-III inhibits the invasion and migration of IEC-6 cells stimulated with TGF-β1
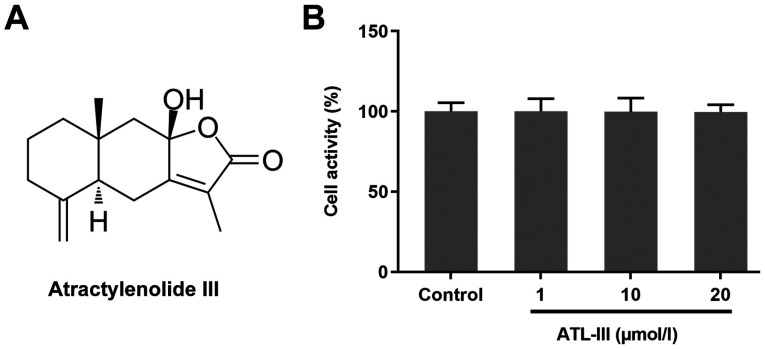
The chemical structure of ATL-III is shown in Fig. 1A. An MTT assay was used to detect the effect of ATL-III on the activity of IEC-6 cells. Cells were treated with different doses of ATL-III [0 (control), 1, 10 or 20 µmol/l]. The addition of ATL-III had no significant effect on normal cultured cells (Fig. 1B), which suggests that ATL-III was not toxic to the normal cultured cells if the concentration was ≤20 µmol/l.
Figure 1.
Effect of ATL-III on the activity of IEC-6 cells. (A) Chemical structure of ATL-III. (B) Effect of ATL-III on the activity of IEC-6 cells was detected using an MTT assay. ATL-III, atractylenolide III.
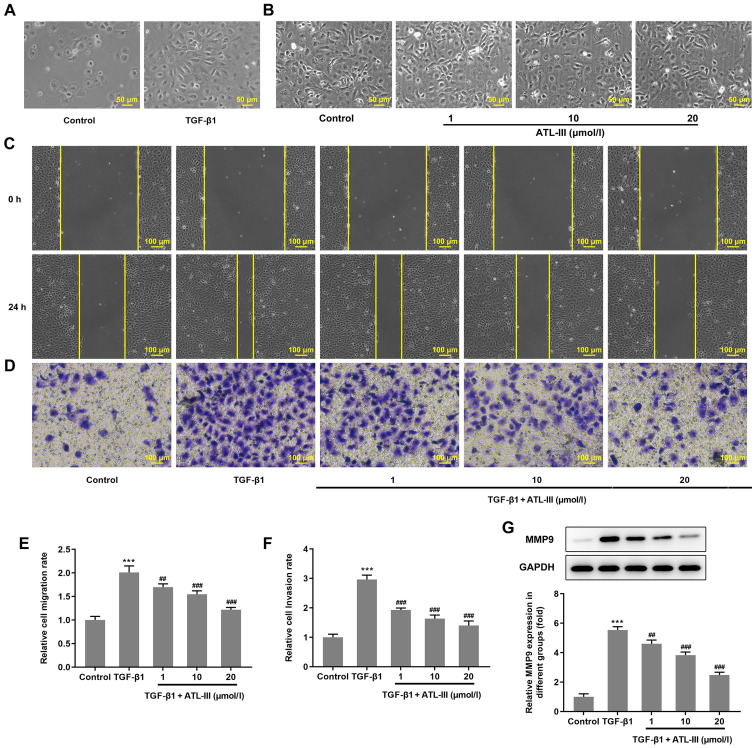
IEC-6 cells were treated with TGF-β1 or ATL-III for 48 h. Using a microscope it was observed that the cells in the TGF-β1 group changed from a cubic shape to a spindle shape (Fig. 2A), whereas the cells in the ATL-III groups had no obvious alteration in morphology (Fig. 2B). The migration of IEC-6 cells was detected using a wound healing assay. Compared with the 0 h group, after 24 h, the cells migrated to the wound area (Fig. 2C). The cell migration rate was notably increased by TGF-β1 stimulation. However, when ATL-III was added, the migration rate was decreased in a concentration-dependent manner (Fig. 2E). Cell invasion was detected using a Transwell assay. The trend observed for the invasive rate was the same as that of the migration rate (Fig. 2D and F). The expression levels of protein associated with migration were detected via western blotting. The expression level of MMP9 was increased when cells were treated with TGF-β1, while the addition of ATL-III reduced this expression in a dose-dependent manner (Fig. 2G). These findings indicate that ATL-III can inhibit the invasion and migration of IEC-6 cells induced by TGF-β1.
Figure 2.
ATL-III inhibits the invasion and migration of IEC-6 cells induced by TGF-β1. (A) Cell morphology after TGF-β1 treatment was observed under a microscope. (B) Cell morphology after ATL-III treatment was observed under a microscope. Magnification, ×200. (C) Migration of IEC-6 cells was detected using a wound healing assay. (D) Cell invasion was detected using a Transwell assay. Histograms of cell (E) migration and (F) invasion rates. (G) Expression level of MMP9, as detected via western blotting. Magnification, ×100. ***P<0.001 vs. control group; ##P<0.01, ###P<0.001 vs. TGF-β1 group; n≥3. ATL-III, atractylenolide III.
ATL-III inhibits the EMT of IEC-6 cells induced by TGF-β1
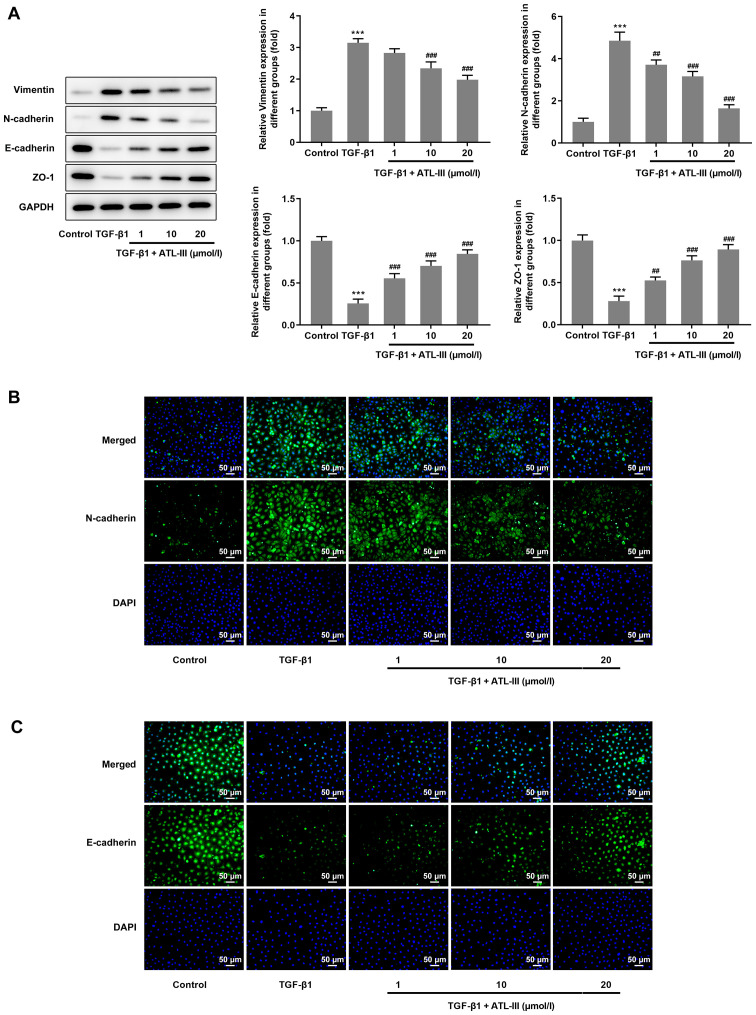
The expression levels of proteins associated with EMT were detected via western blotting. The expression levels of vimentin and N-cadherin were increased when cells were treated with TGF-β1, but the addition of ATL-III reduced this expression in a dose-dependent manner. However, opposite trends were observed with regards to the expression levels of E-cadherin and ZO-1. After stimulation with TGF-β1, the expression levels of E-cadherin and ZO-1 were decreased, but when ATL-III was added, these were increased in a dose-dependent manner (Fig. 3A). Furthermore, an immunofluorescence assay was used for verification. The results for N-cadherin and E-cadherin expression from the fluorescent images matched the results from the western blot analysis (Fig. 3B and C).
Figure 3.
ATL-III inhibits the EMT of IEC-6 cells induced by TGF-β1. (A) Expression levels of protein associated with EMT, as detected via western blotting. (B) Expression levels of N-cadherin and (C) E-cadherin were detected using an immunofluorescence assay. Magnification, ×200. ***P<0.001 vs. control group; ##P<0.01, ###P<0.001 vs. TGF-β1 group; n≥3. ATL-III, atractylenolide III; EMT, epithelial-mesenchymal transition; ZO-1, zonula occludens-1.
ATL-III inhibits the invasion, migration and EMT process of IEC-6 cells induced by TGF-β1 through activating the AMPK signaling pathway
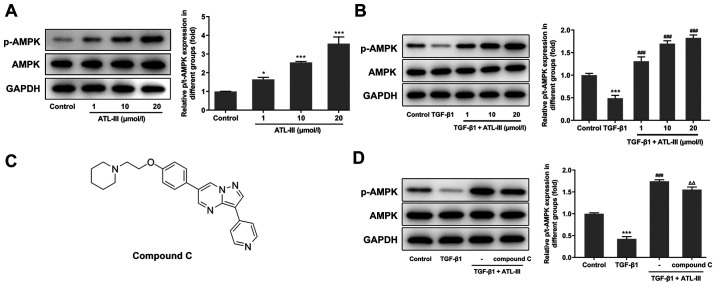
To investigate the effect of ATL-III on the AMPK signaling pathway, the expression levels of p-AMPK and AMPK were detected using western blotting. The expression level of p-AMPK was increased in a concentration-dependent manner after treatment with ATL-III (Fig. 4A). When IEC-6 cells were stimulated by TGF-β1, the expression level of p-AMPK was decreased compared with the control group. However, after the addition of ATL-III, p-AMPK expression was upregulated (Fig. 4B). This indicates that ATL-III can activate the AMPK signaling pathway in IEC-6 cells.
Figure 4.
ATL-III activates the AMPK signaling pathway in IEC-6 cells. (A) Expression levels of p-AMPK and AMPK in the groups treated with different concentrations of ATL-III were measured using western blotting. (B) Expression levels of p-AMPK and AMPK in the groups treated with TGF-β1 and different concentrations of ATL-III were measured using western blotting. (C) Chemical structure of compound C. (D) Expression levels of p-AMPK and AMPK in the groups treated with TGF-β1, ATL-III and compound C were detected using western blotting. *P<0.05, ***P<0.001 vs. control group; ###P<0.001 vs. TGF-β1 group; △△P<0.01 vs. TGF-β1 + ATL-III (20 µmol/l) group; n≥3. ATL-III, atractylenolide III; AMPK, AMP-activated protein kinase; p-, phosphorylated; t-, total.
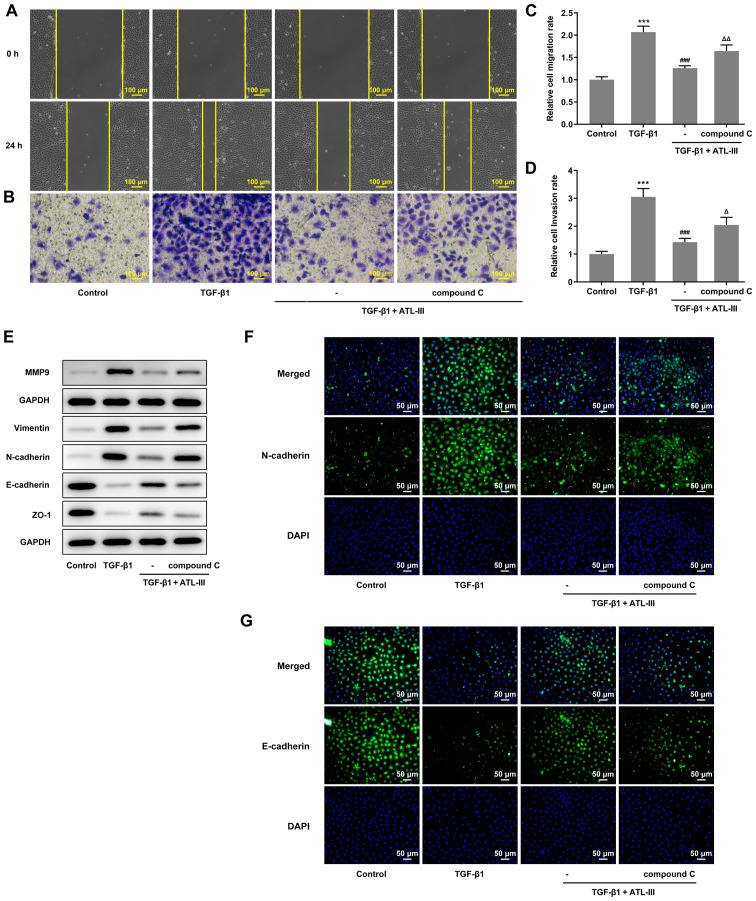
Next, the cells were divided into four groups (control, TGF-β1, TGF-β1 + ATL-III and TGF-β1 + ATL-III + compound C). The concentration of ATL-III used was 20 µmol/l. Western blotting was used to detect the expression levels of AMPK signaling pathway-related proteins after pretreatment with compound C (Fig. 4C). When cells were stimulated with TGF-β1, p-AMPK expression was decreased compared with the control group. After ATL-III was added, this expression rose rapidly. However, when compound C was added, the expression level was decreased. Simultaneously, the expression level of AMPK showed no fluctuation (Fig. 4D).
Wound healing and Transwell assays were used to detect the invasion and migration of cells. The cell invasive and migratory rates were both increased when compound C was added compared with the TGF-β1 + ATL-III group (Fig. 5A-D). In addition, the expression levels of MMP9, vimentin, N-cadherin, E-cadherin and ZO-1 were detected via western blotting. The expression levels of MMP9, vimentin and N-cadherin were increased, while those of E-cadherin and ZO-1 were decreased after the addition of compound C compared with the TGF-β1 + ATL-III group (Fig. 5E). These results were in line with the aforementioned wound healing and Transwell assay results. Moreover, an immunofluorescence assay was used to detect the expression levels of N-cadherin and E-cadherin. It was found that the N-cadherin expression was inhibited in the TGF-β1 + ATL-III group, while the addition of compound C increased the expression levels of N-cadherin (Fig. 5F). E-cadherin expression was found to be promoted in the TGF-β1 + ATL-III group, while the addition of compound C declined E-cadherin expression (Fig. 5G). Thus, it was suggested that the AMPK pathway plays a role in the EMT process. Overall, ATL-III may inhibit the invasion, migration and EMT process of IEC-6 cells induced by TGF-β1 by activating the AMPK signaling pathway.
Figure 5.
ATL-III inhibits the invasion, migration and EMT process of IEC-6 cells induced by TGF-β1 by activating the AMPK signaling pathway. (A) Cell migration, as determined using a wound healing assay. (B) Cells invasion was detected using a Transwell assay. Magnification, ×100. Histograms of cell (C) migration and (D) invasion rates. (E) Expression levels of MMP9 and proteins associated with EMT, as detected via western blotting. (F) N-cadherin and (G) E-cadherin expression was detected using an immunofluorescence assay. Magnification, ×200. ***P<0.001 vs. control group; ###P<0.001 vs. TGF-β1 group; △P<0.05, △△P<0.01 vs. TGF-β1 + ATL-III (20 µmol/l) group; n≥3. ATL-III, atractylenolide III; EMT, epithelial-mesenchymal transition; AMPK, AMP-activated protein kinase; ZO-1, zonula occludens-1.
Discussion
The concept of EMT was first proposed 40 years ago (33). Later studies have reported that EMT was closely associated with tumor epithelial cell invasion and organ fibrosis (34–36). Over the past 10 years, it was discovered that EMT is also an important mechanism in the process of intestinal fibrosis (37). Intestinal fibrosis can cause serious disease. Most patients can only rely on conservative treatment and fibrosis will remain throughout the patient's lifetime. In severe cases, fibrosis can be relieved through surgery, but patients have a high probability of recurrence (38). In view of the fact that clinical studies have reported that there are effective treatments for organ fibrosis, including lung and skin (39–41), it is a reasonable idea to similarly improve intestinal fibrosis using anti-fibrotic drugs. In the present study, the effect of ATL-III on the EMT process of intestinal cells was examined. First, normal IEC-6 cells were treated with ATL-III, and it was found that ATL-III had no effect on cell activity, as determined using an MTT assay.
Next, TGF-β was used to stimulate IEC-6 cells. TGF-β belongs to the family of growth factors and is a multifunctional polypeptide cytokine. TGF-β is expressed in a variety of cell types and organs in mammals, and is associated with ECM (42). In the intestine, both immune and non-immune cells can secrete TGF-β. In animal experiments, it has been shown that the overexpression of TGF-β can cause mice to develop intestinal fibrosis and obstruction (43). In addition, blocking TGF-β/Smad signal transduction can protect mice from colonic fibrosis (44). There are three subtypes of TGF-β, of which TGF-β1 is the most highly expressed (45). These findings indicate that TGF-β1 is associated with fibrosis, and thus, in the present study it was used to stimulate IEC-6 cells to differentiate into fibroblasts. After 48 h of induction, using a microscope it was observed that IEC-6 cells become fibroblasts.
When cells undergo EMT, they have a strong ability to migrate and invade (30). In the current study, wound healing and Transwell assays were used to detect the migration and invasion of cells. The present results demonstrated that the migratory and invasive rates of TGF-β1-stimulated cells were increased compared with normal cells. After the addition of the different doses of ATL-III, these rates were gradually decreased. Moreover, western blotting was used to detect the expression level of MMP9. It has been shown that MMP9 expression is correlated with metastatic potential (46). In the present study, the expression level of MMP9 in cells treated with TGF-β was notably increased, but was decreased after the addition of ATL-III.
E-cadherin and N-cadherin are two important cadherins expressed in epithelial and mesenchymal cells, respectively. The change in phenotype of cells expressing E-cadherin to N-cadherin is an important mechanism in EMT (47). ZO-1 plays an important role in maintaining the integrity of the tightly linked structure and function of cells (48). Vimentin is positively expressed in mesenchymal cells, but negatively expressed in epithelial cells (49). The current results of the immunofluorescence and western blotting assays were consistent with this previous study, as it was demonstrated that E-cadherin and ZO-1 expression was decreased in cells treated with TGF-β, while N-cadherin and vimentin expression was increased. Moreover, treatment with ATL-III could reduce the differences observed between the control group and the stimulated cells group. This indicates that ATL-III can inhibit TGF-β, thereby blocking the EMT process of IEC-6 cells.
AMPK is a recognized cell bioenergy sensor and metabolic master switch (50). A decrease in AMPK activity is associated with diabetes, obesity and aging, which are risk factors for organ fibrosis (51,52). In preclinical studies, AMPK activators have been shown to play a protective role against lung injury and reduce the subsequent development of fibrosis (53,54). Specifically, pharmacological activation of AMPK in lung myofibroblasts of patients with idiopathic pulmonary fibrosis caused low fibrotic activity (55). Using a mouse lung fibrosis model, it was found that the use of metformin (an AMPK activator) to promote the inactivation and apoptosis of myofibroblasts could reverse the established lung fibrosis (56). Thus, the inhibitory effect of AMPK activation on EMT has been previously shown. In the present study, western blotting was used to detect p-AMPK expression, which was found to increase when the dose of ATL-III was enhanced. Moreover, after pretreatment with compound C, p-AMPK expression was decreased. This suggests that ATL-III activates the AMPK signaling pathway in IEC-6 cells. Wound healing and Transwell assays were used to investigate cell migration and invasion after compound C treatment. It was identified that compound C reduced the inhibitory effect of ATL-III on the invasion and migration of stimulated cells. The detection of MMP9 expression using western blotting also verified this result. Finally, an immunofluorescence assay and western blotting were used to detect the expression levels of EMT-related proteins. The results demonstrated that when the AMPK pathway was inhibited, the EMT inhibitory effect of ATL-III on the cells stimulated by TGF-β was reduced. Thus, it was indicated that the AMPK pathway may be essential for ATL-III to inhibit the EMT process of cells stimulated by TGF-β. To the best of our knowledge, the present study was the first to demonstrate that ATL-III could inhibit the EMT process of intestinal cells. At present, compared with the drug treatment for other types of organ fibrosis, the development of intestinal anti-fibrosis drugs is lacking. Therefore, the development of drugs to inhibit or relieve intestinal fibrosis is of great importance. However, in vivo experiments will also need to be performed to verify the findings in the future.
In conclusion, the present study identified the inhibitory effect of ATL-III on in vitro intestinal fibrosis, and the current findings provide a novel idea for future intestinal fibrosis drug research.
Acknowledgements
Not applicable.
Funding Statement
This work was supported by Guizhou Science and Technology Department and Guizhou University Joint Fund Project [grant no. LH [(2017)].
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
MH and WJ designed and performed the experiments. CL and MY participated in experiments and analyzed the data. YR made substantial contributions to conception, design and wrote the manuscript. All authors read and approved the final manuscript. YR and MH confirmed the authenticity of the raw data.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Rockey DC, Bell PD, Hill JA. Fibrosis - a common pathway to organ injury and failure. N Engl J Med. 2015;372:1138–1149. doi: 10.1056/NEJMra1300575. [DOI] [PubMed] [Google Scholar]
- 2.Rieder F, Fiocchi C, Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology. 2017;152:340–350.e6. doi: 10.1053/j.gastro.2016.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sicilia B, Arias L, Hontoria G, García N, Badia E, Gomollón F. Are steroids still useful in immunosuppressed patients with inflammatory bowel disease? A retrospective, population-based study. Front Med (Lausanne) 2021;8:651685. doi: 10.3389/fmed.2021.651685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutgeerts P, Geboes K, Vantrappen G, Kerremans R, Coenegrachts JL, Coremans G. Natural history of recurrent Crohn's disease at the ileocolonic anastomosis after curative surgery. Gut. 1984;25:665–672. doi: 10.1136/gut.25.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman HJ. Natural history and clinical behavior of Crohn's disease extending beyond two decades. J Clin Gastroenterol. 2003;37:216–219. doi: 10.1097/00004836-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Mao R, Chen BL, He Y, Cui Y, Zeng ZR, Chen MH. Factors associated with progression to surgery in Crohn's disease patients with endoscopic stricture. Endoscopy. 2014;46:956–962. doi: 10.1055/s-0034-1390791. [DOI] [PubMed] [Google Scholar]
- 7.Froehlich F, Juillerat P, Mottet C, Pittet V, Felley C, Vader JP, Gonvers JJ, Michetti P. Fibrostenotic Crohn's disease. Digestion. 2007;76:113–115. doi: 10.1159/000111025. [DOI] [PubMed] [Google Scholar]
- 8.Masaki T, Kishiki T, Kojima K, Asou N, Beniya A, Matsuoka H. Recent trends (2016–2017) in the treatment of inflammatory bowel disease. Ann Gastroenterol Surg. 2018;2:282–288. doi: 10.1002/ags3.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelsen JR, Russo P, Sullivan KE. Early-Onset Inflammatory Bowel Disease. Immunol Allergy Clin North Am. 2019;39:63–79. doi: 10.1016/j.iac.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mentella MC, Scaldaferri F, Pizzoferrato M, Gasbarrini A, Miggiano GA. Nutrition, IBD and Gut Microbiota: A Review. Nutrients. 2020;12:12. doi: 10.3390/nu12040944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahluwalia B, Moraes L, Magnusson MK, Öhman L. Immunopathogenesis of inflammatory bowel disease and mechanisms of biological therapies. Scand J Gastroenterol. 2018;53:379–389. doi: 10.1080/00365521.2018.1447597. [DOI] [PubMed] [Google Scholar]
- 12.Chapman TP, Gomes CF, Louis E, Colombel JF, Satsangi J. De-escalation of immunomodulator and biological therapy in inflammatory bowel disease. Lancet Gastroenterol Hepatol. 2020;5:63–79. doi: 10.1016/S2468-1253(19)30186-4. [DOI] [PubMed] [Google Scholar]
- 13.Fuller MK. Pediatric inflammatory bowel disease: Special considerations. Surg Clin North Am. 2019;99:1177–1183. doi: 10.1016/j.suc.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Colombel JF, D'haens G, Lee WJ, Petersson J, Panaccione R. Outcomes and strategies to support a treat-to-target approach in inflammatory bowel disease: A Systematic Review. J Crohn's Colitis. 2020;14:254–266. doi: 10.1093/ecco-jcc/jjz131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cosnes J, Nion-Larmurier I, Beaugerie L, Afchain P, Tiret E, Gendre JP. Impact of the increasing use of immunosuppressants in Crohn's disease on the need for intestinal surgery. Gut. 2005;54:237–241. doi: 10.1136/gut.2004.045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Speca S, Giusti I, Rieder F, Latella G. Cellular and molecular mechanisms of intestinal fibrosis. World J Gastroenterol. 2012;18:3635–3661. doi: 10.3748/wjg.v18.i28.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rieder F, Fiocchi C. Intestinal fibrosis in IBD - a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol. 2009;6:228–235. doi: 10.1038/nrgastro.2009.31. [DOI] [PubMed] [Google Scholar]
- 18.Holvoet T, Devriese S, Castermans K, Boland S, Leysen D, Vandewynckel YP, Devisscher L, Van den Bossche L, Van Welden S, Dullaers M, et al. Treatment of intestinal fibrosis in experimental inflammatory bowel disease by the pleiotropic actions of a local rho kinase inhibitor. Gastroenterology. 2017;153:1054–1067. doi: 10.1053/j.gastro.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Zhang N, Liu C, Sun TM, Ran XK, Kang TG, Dou DQ. Two new compounds from Atractylodes macrocephala with neuroprotective activity. J Asian Nat Prod Res. 2017;19:35–41. doi: 10.1080/10286020.2016.1247351. [DOI] [PubMed] [Google Scholar]
- 20.Zhao H, Ji ZH, Liu C, Yu XY. Neuroprotection and mechanisms of atractylenolide III in preventing learning and memory impairment induced by chronic high-dose homocysteine administration in rats. Neuroscience. 2015;290:485–491. doi: 10.1016/j.neuroscience.2015.01.060. [DOI] [PubMed] [Google Scholar]
- 21.Kang TH, Han NR, Kim HM, Jeong HJ. Blockade of IL-6 secretion pathway by the sesquiterpenoid atractylenolide III. J Nat Prod. 2011;74:223–227. doi: 10.1021/np100686a. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y, Huang S, Wu F, Zheng Q, Zhang F, Luo Y, Jian X. Atractylenolide III reduces depressive- and anxiogenic-like behaviors in rat depression models. Neurosci Lett. 2021;759:136050. doi: 10.1016/j.neulet.2021.136050. [DOI] [PubMed] [Google Scholar]
- 23.Wang M, Hu R, Wang Y, Liu L, You H, Zhang J, Wu X, Pei T, Wang F, Lu L, et al. Atractylenolide III attenuates muscle wasting in chronic kidney disease via the oxidative stress-mediated PI3K/AKT/mTOR pathway. Oxid Med Cell Longev. 2019;2019:1875471. doi: 10.1155/2019/1875471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huai B, Ding J. Atractylenolide III attenuates bleomycin-induced experimental pulmonary fibrosis and oxidative stress in rat model via Nrf2/NQO1/HO-1 pathway activation. Immunopharmacol Immunotoxicol. 2020;42:436–444. doi: 10.1080/08923973.2020.1806871. [DOI] [PubMed] [Google Scholar]
- 25.Fu J, Ke X, Tan S, Liu T, Wang S, Ma J, Lu H. The natural compound codonolactone attenuates TGF-β1-mediated epithelial-to-mesenchymal transition and motility of breast cancer cells. Oncol Rep. 2016;35:117–126. doi: 10.3892/or.2015.4394. [DOI] [PubMed] [Google Scholar]
- 26.Song MY, Jung HW, Kang SY, Park YK. Atractylenolide III enhances energy metabolism by increasing the SIRT-1 and PGC1α expression with AMPK phosphorylation in C2C12 mouse skeletal muscle cells. Biol Pharm Bull. 2017;40:339–344. doi: 10.1248/bpb.b16-00853. [DOI] [PubMed] [Google Scholar]
- 27.Li N, Wang Z, Sun T, Lei Y, Liu X, Li Z. Apigenin alleviates renal fibroblast activation through AMPK and ERK signaling pathways in vitro. Curr Pharm Biotechnol. 2020;21:1107–1118. doi: 10.2174/1389201021666200320140908. [DOI] [PubMed] [Google Scholar]
- 28.Yang JY, Tao LJ, Liu B, You XY, Zhang CF, Xie HF, Li RS. Wedelolactone attenuates pulmonary fibrosis partly through activating AMPK and regulating Raf-MAPKs signaling pathway. Front Pharmacol. 2019;10:151. doi: 10.3389/fphar.2019.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi J, Sun S, Liao Y, Tang J, Xu X, Qin B, Qin C, Peng L, Luo M, Bai L, et al. Advanced oxidation protein products induce G1 phase arrest in intestinal epithelial cells via a RAGE/CD36-JNK-p27kip1 mediated pathway. Redox Biol. 2019;25:101196. doi: 10.1016/j.redox.2019.101196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang BL, Zhu P, Li YR, Xu MM, Wang H, Qiao LC, Xu HX, Chen HJ. Total flavone of Abelmoschus manihot suppresses epithelial-mesenchymal transition via interfering transforming growth factor-β1 signaling in Crohn's disease intestinal fibrosis. World J Gastroenterol. 2018;24:3414–3425. doi: 10.3748/wjg.v24.i30.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang HJ, Zhang YN, Zhou H, Guan L, Li Y, Sun MJ. IL-17A promotes initiation and development of intestinal fibrosis through EMT. Dig Dis Sci. 2018;63:2898–2909. doi: 10.1007/s10620-018-5234-x. [DOI] [PubMed] [Google Scholar]
- 32.Gong WX, Zhou YZ, Qin XM, Du GH. Involvement of mitochondrial apoptotic pathway and MAPKs/NF-κB inflammatory pathway in the neuroprotective effect of atractylenolide III in corticosterone-induced PC12 cells. Chin J Nat Med. 2019;17:264–274. doi: 10.1016/S1875-5364(19)30030-5. [DOI] [PubMed] [Google Scholar]
- 33.Greenburg G, Hay ED. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol. 1982;95:333–339. doi: 10.1083/jcb.95.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.López-Novoa JM, Nieto MA. Inflammation and EMT: An alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1:303–314. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang E, Liu S, Xu Z, Huang S, Tan X, Sun C, Lu L. Pituitary tumor-transforming gene 1 (PTTG1) is overexpressed in oral squamous cell carcinoma (OSCC) and promotes migration, invasion and epithelial-mesenchymal transition (EMT) in SCC15 cells. Tumour Biol. 2014;35:8801–8811. doi: 10.1007/s13277-014-2143-2. [DOI] [PubMed] [Google Scholar]
- 36.Aiello NM, Maddipati R, Norgard RJ, Balli D, Li J, Yuan S, Yamazoe T, Black T, Sahmoud A, Furth EE, et al. EMT subtype influences epithelial plasticity and mode of cell migration. Dev Cell. 2018;45:681–695.e4. doi: 10.1016/j.devcel.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flier SN, Tanjore H, Kokkotou EG, Sugimoto H, Zeisberg M, Kalluri R. Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J Biol Chem. 2010;285:20202–20212. doi: 10.1074/jbc.M110.102012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mege D, Panis Y. Unmet therapeutic needs: Focus on intestinal fibrosis surgical approach: Resection, strictureplasty and others. Dig Dis. 2017;35:38–44. doi: 10.1159/000449081. [DOI] [PubMed] [Google Scholar]
- 39.Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, King TE, Jr, Lancaster L, Sahn SA, Szwarcberg J, et al. CAPACITY Study Group Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): Two randomised trials. Lancet. 2011;377:1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 40.Kuhn A, Haust M, Ruland V, Weber R, Verde P, Felder G, Ohmann C, Gensch K, Ruzicka T. Effect of bosentan on skin fibrosis in patients with systemic sclerosis: A prospective, open-label, non-comparative trial. Rheumatology (Oxford) 2010;49:1336–1345. doi: 10.1093/rheumatology/keq077. [DOI] [PubMed] [Google Scholar]
- 41.King TE, Jr, Brown KK, Raghu G, du Bois RM, Lynch DA, Martinez F, Valeyre D, Leconte I, Morganti A, Roux S, et al. BUILD-3: A randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:92–99. doi: 10.1164/rccm.201011-1874OC. [DOI] [PubMed] [Google Scholar]
- 42.Munger JS, Harpel JG, Gleizes PE, Mazzieri R, Nunes I, Rifkin DB. Latent transforming growth factor-beta: Structural features and mechanisms of activation. Kidney Int. 1997;51:1376–1382. doi: 10.1038/ki.1997.188. [DOI] [PubMed] [Google Scholar]
- 43.Vallance BA, Gunawan MI, Hewlett B, Bercik P, Van Kampen C, Galeazzi F, Sime PJ, Gauldie J, Collins SM. TGF-beta1 gene transfer to the mouse colon leads to intestinal fibrosis. Am J Physiol Gastrointest Liver Physiol. 2005;289:G116–G128. doi: 10.1152/ajpgi.00051.2005. [DOI] [PubMed] [Google Scholar]
- 44.Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012;11:230–241. doi: 10.1016/j.arr.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Morikawa M, Derynck R, Miyazono K. TGF-β and the TGF-β family: Context-dependent roles in cell and tissue physiology. Cold Spring Harb Perspect Biol. 2016;8:8. doi: 10.1101/cshperspect.a021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baruch RR, Melinscak H, Lo J, Liu Y, Yeung O, Hurta RA. Altered matrix metalloproteinase expression associated with oncogene-mediated cellular transformation and metastasis formation. Cell Biol Int. 2001;25:411–420. doi: 10.1006/cbir.2000.0647. [DOI] [PubMed] [Google Scholar]
- 47.Wong SHM, Fang CM, Chuah LH, Leong CO, Ngai SC. E-cadherin: Its dysregulation in carcinogenesis and clinical implications. Crit Rev Oncol Hematol. 2018;121:11–22. doi: 10.1016/j.critrevonc.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 48.Hisada M, Hiranuma M, Nakashima M, Goda N, Tenno T, Hiroaki H. High dose of baicalin or baicalein can reduce tight junction integrity by partly targeting the first PDZ domain of zonula occludens-1 (ZO-1) Eur J Pharmacol. 2020;887:173436. doi: 10.1016/j.ejphar.2020.173436. [DOI] [PubMed] [Google Scholar]
- 49.Zhang N. Vimentin and tumor diagnosis. Zhonghua Bing Li Xue Za Zhi. 1990;19:122–124. (In Chinese) [PubMed] [Google Scholar]
- 50.Hardie DG, Ross FA, Hawley SA. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, Meldrum E, Sanders YY, Thannickal VJ. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med. 2014;6:231ra47. doi: 10.1126/scitranslmed.3008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burkewitz K, Zhang Y, Mair WB. AMPK at the nexus of energetics and aging. Cell Metab. 2014;20:10–25. doi: 10.1016/j.cmet.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park CS, Bang BR, Kwon HS, Moon KA, Kim TB, Lee KY, Moon HB, Cho YS. Metformin reduces airway inflammation and remodeling via activation of AMP-activated protein kinase. Biochem Pharmacol. 2012;84:1660–1670. doi: 10.1016/j.bcp.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 54.Liu Z, Bone N, Jiang S, Park DW, Tadie JM, Deshane J, Rodriguez CA, Pittet JF, Abraham E, Zmijewski JW. AMP-activated protein kinase and glycogen synthase kinase 3β modulate the severity of sepsis-induced lung injury. Mol Med. 2016;21:937–950. doi: 10.2119/molmed.2015.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sato N, Takasaka N, Yoshida M, Tsubouchi K, Minagawa S, Araya J, Saito N, Fujita Y, Kurita Y, Kobayashi K, et al. Metformin attenuates lung fibrosis development via NOX4 suppression. Respir Res. 2016;17:107. doi: 10.1186/s12931-016-0420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rangarajan S, Bone NB, Zmijewska AA, Jiang S, Park DW, Bernard K, Locy ML, Ravi S, Deshane J, Mannon RB, et al. Metformin reverses established lung fibrosis in a bleomycin model. Nat Med. 2018;24:1121–1127. doi: 10.1038/s41591-018-0170-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.