Abstract
Immune checkpoint inhibitors (ICPIs) have transformed the landscape of oncology, but are associated with a variety of autoimmune adverse events, including AKI. ICPI-associated AKI (ICPI-AKI) is emerging as an increasingly frequent cause of AKI in patients with cancer, and poses unique diagnostic and management challenges to clinicians who care for these patients. In this review, we describe the incidence and risk factors for ICPI-AKI, including proton pump inhibitor use, CKD, and combination immunotherapy. We discuss the limitations of the various definitions used for ICPI-AKI in prior studies, and propose a novel classification system (definite, probable, and possible ICPI-AKI) that recognizes the diagnostic uncertainty inherent in many cases. We discuss the key clinicopathologic features and treatment strategies for ICPI-AKI, including the role of kidney biopsy versus empirical treatment with steroids. We also explore the under-studied area of ICPI use in the setting of solid organ transplantation, where nephrologists and oncologists must balance the risk of rejection versus treating the underlying malignancy. Finally, we summarize existing data on the role of ICPI rechallenge after an episode of ICPI-AKI.
Keywords: Clinical Nephrology, Acute Kidney Injury and ICU Nephrology, Nephro-Pharmacology, acute interstitial nephritis, Acute kidney injury, acute tubulointerstitial nephritis, immune-related adverse event, immunotherapy
Introduction
Targeted immune therapies have transformed the landscape of oncology, with immune checkpoint inhibitors (ICPIs) at the forefront. ICPIs are monoclonal antibodies that target inhibitory receptors expressed on T cells, other immune cells, and tumor cells. These receptors include cytotoxic T lymphocyte–associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and PD-ligand 1 (PD-L1). By inhibiting these receptors, ICPIs “remove the brakes” on the immune system, allowing T cells to become activated and exert antitumor activity (1,2).
ICPIs have been demonstrated to prolong overall- and progression-free survival in patients with melanoma (3,4), nonsmall-cell lung cancer (5,6), urothelial cancer (7,8), renal cell cancer (9,10), and many other malignancies (11,12), becoming first-line therapies for many types of cancer. As of 2019, one CTLA-4 inhibitor and five PD-1 and PD-L1 inhibitors have been approved by the Food and Drug Administration (Table 1), and others are undergoing testing in phase 3 clinical trials (13).
Table 1.
Food and Drug Administration–approved immune checkpoint inhibitors and timing of approval
| Immune Checkpoint Inhibitor | Year of Approval | Indication |
| CTLA-4 inhibitor | ||
| Ipilimumab | 2011 | Melanoma, renal cell carcinoma; in combination with nivolumab in CRC |
| PD-1 inhibitor | ||
| Nivolumab | 2015 | Melanoma, nonsmall-cell lung cancer, small cell lung cancer, renal cell carcinoma, urothelial carcinoma, squamous cell carcinoma of the head and neck, Hodgkin lymphoma, hepatocellular carcinoma, colorectal carcinoma with microsatellite instability/mismatch repair |
| Pembrolizumab | 2015 | Melanoma, nonsmall-cell lung cancer, squamous cell carcinoma of the head and neck, Hodgkin lymphoma, renal cell carcinoma, urothelial carcinoma, gastric cancer, cancers with microsatellite instability, mismatch repair, cervical cancer, primary mediastinal B cell lymphoma |
| Cemiplimab | 2018 | Cutaneous squamous cell cancer |
| PD-L1 inhibitor | ||
| Atezolizumab | 2016 | Nonsmall-cell lung cancer, urothelial carcinoma |
| Avelumab | 2017 | Merkel-cell carcinoma, urothelial carcinoma |
| Durvalumab | 2018 | Urothelial carcinoma, nonsmall-cell lung cancer |
CRC, colorectal carcinoma; CTLA-4, cytotoxic T lymphocyte–associated protein 4; PD-1, programmed cell death protein 1; PD-L1, PD-ligand 1.
Despite their survival benefits, ICPIs can cause a unique spectrum of autoimmune phenomena known as immune-related adverse events (irAEs). The most common tissues involved are the skin, gastrointestinal tract, and endocrine system (14). The kidneys are less commonly involved; however, ICPI-associated AKI (ICPI-AKI) poses unique diagnostic and management challenges, including differentiation of ICPI-AKI from other causes of AKI, the requirement for often prolonged courses of steroids and/or interruptions of ICPI therapy, and the potential for irreversible organ damage. Here we highlight ten burning questions on ICPI-AKI. We discuss our current understanding of the incidence, risk factors, clinicopathologic features, and treatment strategies for ICPI-AKI, along with gaps in knowledge that require future study.
How Is ICPI-AKI Defined?
Initial case reports and small case series of ICPI-AKI were based exclusively on biopsy-proven findings (15–17). However, because patients are increasingly diagnosed and treated for ICPI-AKI according to clinical features alone, there is a need for standardization of definitions across studies. The National Cancer Institute’s Common Terminology Criteria for Adverse Events defines AKI, in part, by comparing changes in serum creatinine (SCr) to the “upper limit of normal” (18). However, patients with cancer often have decreased muscle mass and these definitions may therefore be insensitive to relatively large increases in SCr that fall within the “normal range.” In contrast, the Kidney Disease: Improving Global Outcomes Work Group (KDIGO) consensus criteria define AKI according to relative changes in SCr (Table 2) (19). Even among studies that use the KDIGO criteria to define ICPI-AKI, some define ICPI-AKI as any stage (20), whereas others include only stage 2 or higher (21). Thus, there is a need for harmonization of definitions of ICPI-AKI across studies (discussed further below). Additionally, for all definitions of ICPI-AKI, it is critical that the renal injury be directly attributable to the ICPI and not to an alternative etiology.
Table 2.
Comparison of grading systems for AKI
| System | Grade | Description |
| CTCAE | Grade 1 | Higher than ULN to 1.5× ULN |
| Grade 2 | >1.5–3.0× baseline; >1.5–3.0× ULN | |
| Grade 3 | >3.0× baseline; >3.0–6.0× ULN; hospitalization indicated | |
| Grade 4 | >6.0× ULN; life-threatening consequences; RRT indicated | |
| Grade 5 | Death | |
| KDIGO | Stage 1 | Increase in SCr of ≥0.3 mg/dl within 48 h or 1.5–1.9× baseline |
| Stage 2 | Increase in SCr to 2–2.9× baseline | |
| Stage 3 | Increase in Cr to 3× baseline or to ≥4.0 mg/dl or initiation of RRT |
CTCAE, Common Terminology Criteria for Adverse Events; ULN, upper limit of normal; SCr, serum creatinine.
What Is the Incidence of ICPI-AKI?
Reliable data on the incidence of ICPI-AKI are scarce. We estimated the incidence of ICPI-AKI using pooled data from all phase 2 and 3 clinical trials published between 2014 and 2015 that included at least 100 patients treated with ICPIs (15). In this combined analysis of 3695 patients, the overall incidence of ICPI-AKI was 3%; the incidence among patients receiving combination ICPI therapy was 5%; and the incidence of severe AKI, defined as an increase in SCr more than threefold above baseline, an increase in SCr to >4.0 mg/dl, or the need for RRT, was 0.6% (15). A more recent meta-analysis of 48 clinical trials that included 11,482 patients reported similar findings, with an estimated incidence of ICPI-AKI of 2% (22). Although clinical trial data may not reflect the true incidence of ICPI-AKI, Seethapathy et al. (20) examined the incidence of ICPI-AKI in a real-world setting of 1843 patients treated at a single large academic center, and determined the incidence to be 3%, consistent with earlier estimates.
What Are Possible Mechanisms of ICPI-AKI?
The precise mechanisms of ICPI-AKI are poorly understood, with hypotheses about its pathogenesis largely derived from data on extrarenal irAEs or from mouse models. Mutated mice that lack coinhibitory molecules such as PD-1 or CTLA-4 may develop autoimmunity against specific organs, which is driven by the emergence of antigen-specific T cells against self-antigens (23–25). Interestingly, the transfer of infiltrating T cells specific for a certain organ from one mouse to another leads to trafficking of those T cells to the same organ of origin, supporting the notion of antigen specificity of the response (25). Because self-reactive T cells are present in significant numbers in otherwise healthy humans (26,27), blockade of PD-1 or CTLA-4 with ICPIs may break self-tolerance and trigger an autoimmune response against a specific self-antigen on an organ like the kidney. The exact antigen is currently unknown, but is most likely expressed by tubular cells based on the dominant finding of acute tubulointerstitial nephritis (ATIN) on biopsy. An alternative hypothesis is that ICPI-associated ATIN occurs through loss of tolerance to effector T cells primed during prior drug exposure (e.g., proton pump inhibitors [PPIs]). A drug exposure may, in some cases, act as an exogenous antigen and trigger an immune response either by itself or after binding to tubular antigens and acting as haptens (28). Further mechanistic studies in kidney biopsies from patients treated with ICPIs may allow the determination of the specificity of the T-cell response and elucidation of the potential antigen target of the immune response in the kidneys.
What Are the Key Clinical Features of ICPI-AKI?
Unfortunately, no clinical features reliably distinguish ICPI-AKI from alternative etiologies of AKI, although certain features are suggestive. Patients with ICPI-AKI often present with sterile pyuria or subnephrotic-range proteinuria (Table 3) (15,16,28), similar to patients with ATIN from other causes (29), but neither finding is sufficiently sensitive or specific to confirm or rule out ICPI-AKI. Eosinophilia is found only in a minority of patients (15,16,21), but may be helpful when present. Finally, the latency period between ICPI initiation and AKI is often longer than observed with other more commonly reported irAEs, with dermatitis usually occurring within 4 weeks of treatment (4,30,31) and colitis within 6 weeks (32). In our multicenter study, the median time from ICPI initiation to AKI onset was 14 weeks (interquartile range, 6–47 weeks), and many patients presented much later (21).
Table 3.
Summary of clinical characteristics of patients with immune checkpoint inhibitor–associated AKI
| Reference | n | Centers, n | Drugs Received | AKI Criteria | Time to AKI, wka | Concomitant ATIN Medications, n (%) | Combined Therapyb, n (%) | Concomitant or Prior Extrarenal irAEs, n (%) | Pyuria or WBC Casts, n (%) | Biopsy, n (%) | ATIN, n (%) |
| Shirali et al. (16) | 6 | 1 | Nivo, n=3; Pembro, n=2; Ipi and Nivo, n=1 | Biopsy-proven ATIN | 40 | PPIs, n= 33 (83);NSAIDs, n=2 (33) | 1 (17) | 3 (50)c | 5 (83) | 6 (100) | 6 (100) |
| Cortazar et al. (15) | 13 | 7 | Ipi, n=6; Nivo, n=1; Pembro, n=2; Ipi and Nivo, n=4 | Biopsy-proven AKI | 13 | PPIs, n=6 (46);NSAIDs, n=1(8) | 4 (31) | 8 (62)d | 8 (62) | 13 (100) | 12 (92) |
| Izzedine et al. (17) | 12 | 1 | Pembro, n=12 | Biopsy-proven AKI | 36 | 0 | 0e | NR | 4 (33) | 12 (100) | 4 (33) |
| Mamlouk et al. (28) | 16 | 1 | Nivo, n=6; Pembro, n=6; Atezo, n=1; Treme, n=1; Ipi and Nivo, n=2 | Biopsy-proven AKI; AKIN criteria | 14 | PPIs, n=9 (56)NSAIDs, n=3 (19) | 13 (81) | 9 (56)f | 7 (44) | 16 (100) | 14 (88) |
| Seethapathy et al. (20) | 30 | 1 | Ipi, n=12; Nivo, n=9; Pembro, n=7; Durva, n=1; Ipi and Nivo, n=1; | SCr ≥1.5× baseline for ≥3 d; expert adjudicated | 15 | PPIs, n=23 (77);NSAIDs, n=13 (43) | 3 (10) | 26 (87)g | 13 (43) | 1 (3) | 1 (100) |
| Cortazar et al. (21) | 138 | 18 | Nivo, n=40; Pembro, n=47; Durva and pembro, n=1; Ipi, n=4; Atezo, n=5; Ipi and nivo, n=32; Ipi and pembro, n=6; Other combination tx, n=3 | SCr ≥2× baseline or need for RRT | 14 | PPIs, n=75 (54)NSAIDs, n=30 (22) | 28 (20) | 59 (43)h | 76 (55) | 60 (43) | 56 (93) |
| Totali | 215 | Nivo, n=59; Pembro, n=76; Atezo, n=6; Durva, n=1; Ipi, n=22; Treme, n=1; Any combination tx, n=50 | — | 16 | PPIs, n=146 (68)NSAIDs, n=49 (23) | 49 (23) | 105 (49) | 113 (53) | 108 (61) | 93 (91) |
ATIN, acute tubulointerstitial nephritis; irAEs, immune-related adverse events; WBC, white blood cell; nivo, nivolumab; pembro, pembrolizumab; ipi, ipilimumab; PPI, proton pump inhibitor; NSAID, nonsteroidal anti-inflammatory drug; NR, not recorded; atezo, atezolizumab; treme, tremelimumab; AKIN, Acute Kidney Injury Network (70); durva, durvalumab; SCr, serum creatinine; tx, treatment.
From immune checkpoint inhibitor initiation, mean or median.
This refers to combination therapy with a cytotoxic T lymphocyte–associated protein 4 inhibitor and a programmed cell death protein 1 (PD-1)/PD-ligand 1 inhibitor.
Two patients with hypophysitis; one patient with concomitant rash.
Seven patients had an irAE preceding AKI onset, one had one concomitantly.
All patients were treated with pembrolizumab, although one patient had received ipilimumab in the past.
Five irAEs before diagnosis; three at the time of diagnosis; one after the diagnosis.
Concurrent irAEs; thyroiditis most common, occurring in 13 patients.
Concurrent or prior irAE; rash most common.
Weighted average, based on number of patients in each study.
Interestingly, ICPI-AKI often occurs concomitantly or after other irAEs. In our multicenter study, an extrarenal irAE occurred before or concomitantly with AKI in 43% of cases, with rash being the most common manifestation (21). Seethapathy et al. (20) also found a high rate of concomitant extrarenal irAEs in patients with ICPI-AKI, with at least one extrarenal irAE occurring in 26 of 30 (87%) patients. In their study, thyroiditis and colitis were the most common coexisting irAEs. Case series have found variable rates of irAEs occurring before or at the time of AKI, ranging from 33% (16) to 62% (15). Thus, the presence of concomitant or prior extrarenal irAEs is an important clinical clue that should heighten suspicion for ICPI-AKI.
What Are the Risk Factors for Development of ICPI-AKI?
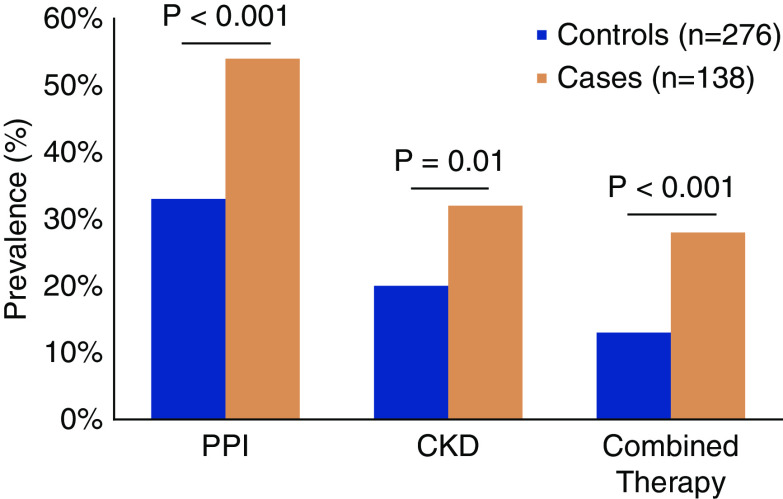
In our multicenter study of 138 patients with ICPI-AKI, which included 276 unmatched control patients who received ICPIs contemporaneously but did not develop AKI, we identified three independent risk factors for development of ICPI-AKI: concomitant use of PPIs, combination treatment with anti–CTLA-4 and anti–PD-1/PD-L1 agents, and lower baseline eGFR (Figure 1) (21). Smaller studies have also implicated PPI use as a risk factor for ICPI-AKI (15,16,20), and PPI use is associated with an increased risk of ATIN in the general population (33). One possible explanation for the finding that PPI use increases the risk of ICPI-AKI is that T cells may be primed by prior exposure to drugs such as PPIs, and ICPIs reactivate these drug-specific latent T cells, leading to loss of tolerance. PPIs, along with other drugs known to cause ATIN such as nonsteroidal anti-inflammatory drugs, should thus be used with caution in patients receiving ICPIs, and should be discontinued in those who develop ICPI-AKI. Our finding that combination therapy is a risk factor for ICPI-AKI is not surprising because combination therapy is known to result in greater immune activation and a higher risk of irAEs in general (3,34–37). Given the heightened risk of ICPI-AKI associated with each of the above risk factors, patients receiving PPIs, those receiving combination ICPI therapy, and those with a lower baseline eGFR may warrant closer renal surveillance to detect ICPI-AKI earlier.
Figure 1.
PPI use lower baseline eGFR and combination treatment with anti–CTLA-4 and anti–PD-1/PD-L1 agents are independently associated with ICPI-AKI. Combined therapy refers to combination therapy with a cytotoxic T lymphocyte–associated protein 4 inhibitor and a programmed cell death protein 1 (PD-1)/PD-ligand 1 inhibitor. Ipilimumab/nivolumab was the combination therapy regimen in 75% of cases and 66% of controls. CKD was defined as a baseline (pretreatment) eGFR <60 ml/min per 1.73 m2. P values were calculated with the chi-squared test. Data from Cortazar et al. (21). PPI, proton pump inhibitor.
Which Patients Should be Biopsied versus Treated Empirically?
Our suggested approach to the evaluation and management of patients with suspected ICPI-AKI is shown in Figure 2. This approach is similar to other published algorithms (38), but with several notable exceptions. Patients who develop stage 1 AKI should be evaluated for reversible causes of renal injury—such as prerenal azotemia, urinary obstruction, or drug-induced injury from agents other than ICPIs—and ICPI therapy should be held until the AKI has resolved. Patients with persistent stage 1 AKI, and those who develop stage 2 or 3 AKI, should be referred to nephrology for consultation and consideration of kidney biopsy.
Figure 2.
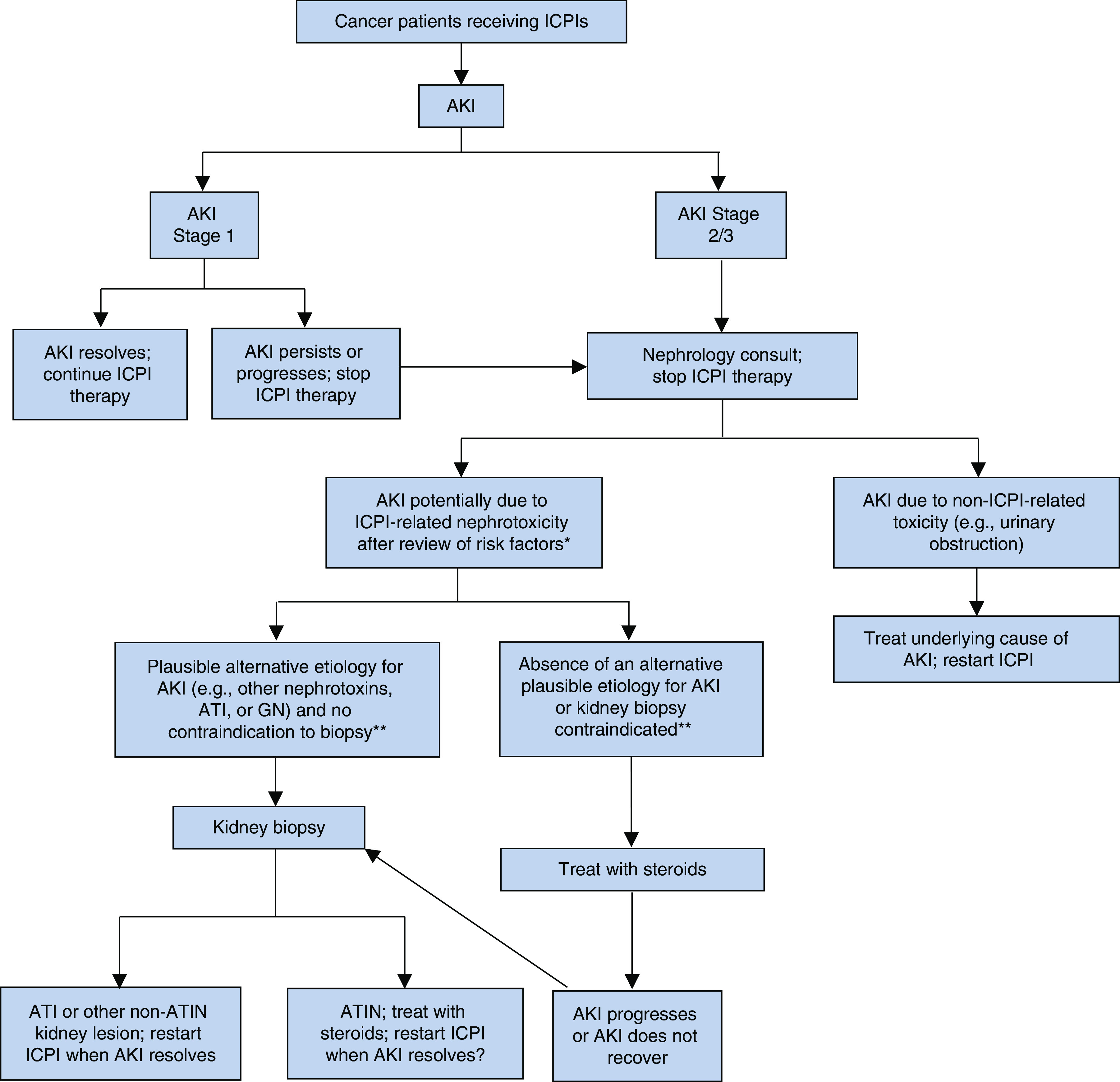
Evaluation and treatment algorithm for suspected immune checkpoint inhibitor–associated AKI. Stages 1, 2, and 3 AKI are based on the Kidney Disease: Improving Global Outcomes AKI staging definition (19). *Clinical review of risk factors (e.g., prior or concomitant PPI use, prior or concomitant extrarenal irAEs). **Contraindications to kidney biopsy include solitary kidney, anticoagulation use that cannot be safely discontinued, or uncontrolled hypertension. ATI, acute tubular injury; ATIN, acute tubulointerstitial nephritis; ICPI, immune checkpoint inhibitor; irAE, immune-related adverse event.
Selection of which patients to biopsy is one of the most complex and subjective decisions in all of nephrology. Particularly in the setting of suspected ICPI-AKI, in which an effective therapy exists for most patients (i.e., glucocorticoids), there is a temptation to treat empirically without a biopsy. Guidelines published by the National Comprehensive Cancer Network similarly lack emphasis on the importance of kidney biopsy in the evaluation of patients with suspected ICPI-AKI, only recommending “consideration” of kidney biopsy in patients with more than a threefold increase in SCr (39). In our view, empirical treatment with steroids should only be considered for patients with an absence of an alternative plausible etiology for AKI and in those with an absolute contraindication to kidney biopsy due to safety concerns (e.g., solitary kidney, uncontrolled hypertension, or anticoagulation that cannot be safely held) (Figure 2). Patients treated empirically with steroids whose kidney function does not improve should undergo kidney biopsy, if feasible, to assess for alternative etiologies of AKI (e.g., glomerulonephritis, which may require additional immunosuppressive therapies). Patients with stage 2 or 3 AKI who have plausible alternative etiologies for AKI other than ICPIs, or in whom there is a concern for GN that may or may not be ICPI related, should proceed directly to kidney biopsy (Figure 2).
What Are the Histopathologic Features of ICPI-AKI?
ATIN is far by the most common histopathologic finding of ICPI-AKI on kidney biopsy (15,16,20,28). In our multicenter study, we defined ICPI-AKI as doubling of SCr or need for RRT (21). Additionally, the AKI had to have been attributed to the ICPI by the treating provider. Kidney biopsy was performed in 60 (43%) patients, 56 (93%) of whom had ATIN as the dominant lesion. Other pathologic entities have also been observed, including various glomerulonephritides (28,40). In one study of 16 patients with biopsy-proven ICPI-AKI, ATIN was present in 14 of the 16 cases, but co-occurred with glomerular disease in nine cases, including IgA nephropathy, pauci-immune GN, and other pathologies (28). Other small series have reported nephrotic syndrome with minimal change disease (41). These observations underscore the heterogeneity of histopathologic patterns of injury from ICPIs, as well as the immune activation seen in patients with ICPI-AKI, including both B cell–driven, autoantibody-mediated disease and T cell–driven lesions. These studies also highlight the importance of performing a kidney biopsy even in patients with a clinical history highly suggestive of ICPI-AKI.
How Should Patients with Suspected or Confirmed ICPI-AKI be Treated?
Glucocorticoids are the mainstay of treatment for ICPI-AKI (Table 4). Although no randomized placebo-controlled trial has ever established the efficacy of steroids for treatment of ICPI-AKI, observational data supporting the efficacy of steroids in this setting are quite strong: in our multicenter study, 103 of 119 patients (87%) treated with steroids had complete or partial renal recovery (21). Interestingly, although the route of administration, dose, and duration of steroid treatment is highly variable across studies (15,16,28), we did not identify any differences in the steroid regimen between patients who achieved complete renal recovery versus those who did not (21). However, defining the optimal glucocorticoid regimen from retrospective data is challenging, because dosing is often confounded by severity of injury and response to treatment.
Table 4.
Renal outcomes among patients with immune checkpoint inhibitor–associated AKI
| Reference | Patients, n | Treatment with Steroids, n (%) | Renal Outcome Data | Rechallenge |
| Shirali et al. (16)a | 6 | 5 (83) | 4/5 patients treated with steroids had return of SCr back to baseline; one had an improvement from a peak SCr of 2.5 mg/dl to 1.3 mg/dl, but not back to baseline. One patient who did not receive steroids had furosemide/PPI discontinued, with improvement of SCr back to baseline. | One patient rechallenged and had recurrence of AKI; one patient with ATIN continued on pembrolizumab |
| Cortazar et al. (15)b | 13 | 11 (92) | 9/10 patients with ATIN treated with steroids had complete or partial renal recovery. Two patients did not receive IS and had no renal recovery. | Two patients rechallenged without AKI |
| Izzedine et al. (17)c | 12 | 7 (58) | 10/12 patients had ICPIs stopped, of whom seven received steroids; one died because of disease progression, the other six had some renal recovery. 2/12 were continued on ICPIs, with some improvement in renal function. | One patient rechallenged with recurrence of ATIN |
| Mamlouk et al. (28)d | 16 | 14 (88) | 3/5 ATIN cases all had partial renal recovery after prednisone, one of whom was also treated with infliximab. Two cases had no renal response; 3/16 died because of disease progression. | NR; 13 survivors continued treatment |
| Seethapathy et al. (20)e | 30 | 21 (70) | Of the 82 patients with sustained AKI, 54 (67%) died in the follow-up period, death occurred a median of 22 d (IQR 6–84) after the sustained AKI episode. | 17 patients (57%) rechallenged, with nine developing recurrent AKI |
| Cortazar et al. (21)f | 138 | 119 (86) | Complete, partial, and no renal recovery after ICPI-AKI occurred in 40%, 45%, and 15% of patients, respectively. Treatment with steroids associated with a 1.7 greater odds of renal recovery in multivariable models among biopsied patients. Patients with no renal recovery had higher mortality than those with complete or partial recovery. | 22% of patients were rechallenged, with recurrent AKI in 23% |
SCr, serum creatinine; PPI, proton pump inhibitor; ATIN, acute tubulointerstitial nephritis; IS, immunosuppression; ICPI, immune checkpoint inhibitor; NR, not recorded; IQR, interquartile range.
Renal recovery not explicitly defined.
Complete recovery defined as return of SCr to <0.35 mg/dl above the baseline value. Partial renal recovery defined as a return of SCr to >0.35 mg/dl but less than twice the baseline value, or liberation from RRT.
Renal recovery not explicitly defined; six patients had a “favorable” renal recovery defined as 50% improvement in eGFR.
Complete recovery defined as return of SCr to <0.35 mg/dl above the baseline value. Partial renal recovery defined as a return of SCr to >0.35 mg/dl but less than twice the baseline value.
Renal recovery not explicitly defined.
Complete recovery was defined as a return of SCr to <0.35 mg/dl of the baseline value, and partial recovery was defined as a return of SCr to >0.35 mg/dl but less than twice the baseline value, or liberation from RRT.
Other immunosuppressive agents have also been investigated in the setting of ICPI-AKI. Mycophenolate mofetil was used in seven patients with ICPI-AKI in our multicenter study: complete renal recovery was achieved in one patient, with partial recovery in the remaining six (21). Other agents that have been studied include rituximab and cyclophosphamide. Mamlouk and colleagues (28) observed a case of ATIN that was refractory to high-dose steroids but responded partially to infliximab. Another patient in the same series with IgA nephropathy also failed to respond to both high-dose steroids and mycophenolate, but responded to infliximab. TNF-α is upregulated in the circulation of patients receiving ICPIs, which may explain why infliximab is successful in treating some patients with ICPI-AKI (42). The use of infliximab to treat extrarenal irAEs has been proposed by others (43), but more data are needed before routine use of infliximab can be recommended for ICPI-AKI.
Our practice is to use prednisone 1 mg/kg daily as a starting dose, with a slow taper over 2–3 months. Rapid tapers may lead to AKI recurrence; however, in some patients with side effects from steroids, shorter tapers can be considered. In patients with severe ICPI-AKI requiring inpatient hospitalization, intravenous steroids (e.g., methylprednisolone 250–500 mg daily for 3 days) may be used as initial therapy.
Regardless of the treatment strategy, we recommend prompt initiation of immunosuppressive therapy in patients with ICPI-AKI. Although we did not detect an association between treatment delay and complete renal recovery in our multicenter study (21), an association between longer treatment delay and worse renal outcomes has been reported in patients with ATIN from other causes (44). Finally, although treatment with immunosuppressive medications could theoretically interfere with the antitumor immune response elicited by ICPIs, most studies have found that such treatment does not appear to negatively affect tumor response or survival (31,45,46).
Which Patients Can be Safely Rechallenged?
When treating patients with severe ICPI-AKI, it is reasonable to temporarily hold the ICPI. However, permanently discontinuing an ICPI in patients who have exhausted other options for chemotherapy can have dramatic implications on overall survival. Deciding which patients should be rechallenged with ICPIs after experiencing a serious irAE such as AKI is extremely challenging and carries enormous weight. According to the American Society of Clinical Oncology guidelines, ICPI therapy should be permanently discontinued in all patients who develop grade 3 or higher AKI (i.e., an increase in SCr more than three times baseline or need for RRT) (39); however, in our view this approach may deprive patients of a potentially life-saving therapy. In our multicenter study, 31 patients were rechallenged with an ICPI after the initial episode of ICPI-AKI, and only seven (23%) experienced recurrent AKI, six of whom had complete or partial renal recovery. Thus, only one of 31 patients (3%) who were rechallenged developed AKI that did not recover. Clearly additional data on the safety of rechallenge after ICPI-AKI are needed, but based on currently available data and the life-saving nature of these medications, rechallenge should not be routinely withheld due to concern for recurrence of AKI. Rather, we recommend reinitiating ICPIs once renal injury has resolved or stabilized.
What Are Special Considerations in Transplant Patients?
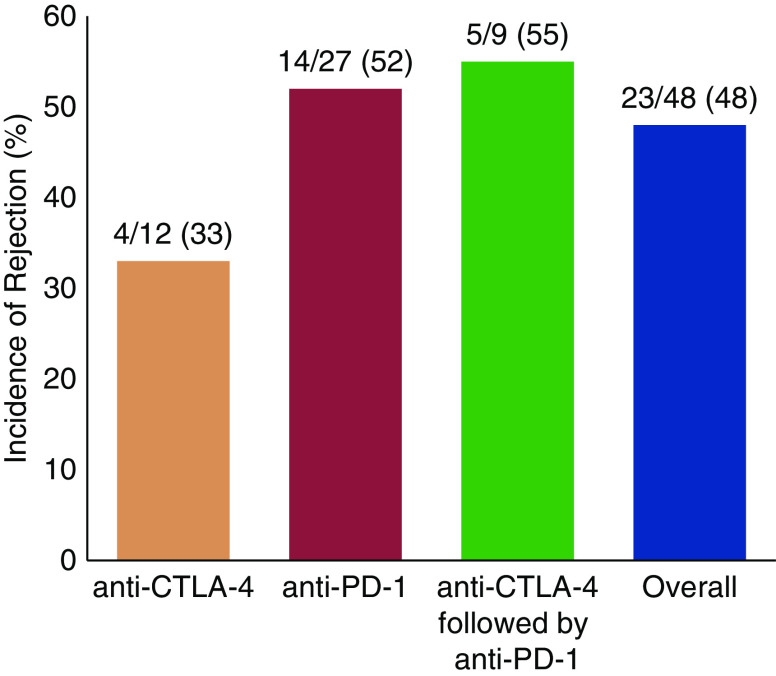
Cancer is the third leading cause of death in solid organ transplant recipients, and the risk of cancer is fourfold higher in transplant recipients compared with matched patients in the general population (47). Immunosuppression may predispose to cancer by inhibiting immune surveillance, suppressing DNA repair, and predisposing to oncogenic viral infections (48–50). Although transplant recipients have been excluded from all clinical trials of ICPIs to date, based on the concern of triggering rejection upon blockade of immune inhibitory signals, case reports and small case series of transplanted recipients receiving ICPIs have begun to emerge with conflicting results (51–62). We reviewed all published case reports and case series and found that the rejection rate with ipilimumab monotherapy was 33% compared to 52% in patients receiving anti–PD-1 monotherapy (Figure 3) (63). Sequential use of ipilimumab followed by anti–PD-1 was associated with rejection in 55% of cases (Figure 3) (63). Only two cases of combined anti–CTLA-4 and anti–PD-1 therapy have been reported, one of whom developed rejection (64). Based on the importance of PD-1 to PD-L1 signaling in peripheral organ transplant homeostasis (65), the higher rate of rejection with PD-1 as compared to CTLA-4 blockade is not surprising. However, it is difficult to draw any definitive conclusion because patients had undergone different reductions or changes of immunosuppression upon metastatic cancer diagnosis, which could strongly influence the rejection rates.
Figure 3.
Incidence of solid organ transplant rejection may be higher after anti–PD-1 therapy. Data are derived from published case series and case reports of rejection after immune checkpoint inhibitors. CTLA-4, cytotoxic T lymphocyte–associated protein 4; PD-1, programmed cell death protein 1; PD-L1, PD-ligand 1.
The high rates of rejection with ICPI use in transplant recipients has led to the development of potential preventative strategies, including the use of pre-emptive steroids and sirolimus at the time of ICPI initiation in one case report (61). The aggressiveness of immunosuppression reduction in the setting of cancer is also under debate. Although immunosuppression reduction is a crucial management strategy in some cancers, such as post-transplant lymphoproliferative disorders and skin cancer, its importance in other solid cancers such as prostate or breast cancer is less clear (66). Based on the high rate of rejection with anti–PD-1 therapy, continuation of calcineurin inhibitors (CNIs) during treatment with anti–PD-1 medications may be warranted. An alternative approach is to convert patients from CNIs to mammalian target of rapamycin (mTOR) inhibitors due to their potential antiangiogenic properties in skin cancer and their reduced immunosuppressive effects as compared to CNIs (49,67). However, mTOR inhibitors are associated with a higher risk of rejection than CNIs, even in the absence of cancer (68). Depending on the individual immunologic risk between donor organ and recipient pair, a decision about reduction or interruption of immunosuppression must be balanced with the higher risk of rejection with ICPIs. Further studies are needed to assess the potential effect of prophylactic steroid mini-pulses with ICPI cycles, and the conversion from CNIs to mTOR inhibitors, on both rejection rate and cancer response.
Proposed Definition and Classification of ICPI-AKI
We propose a definition and classification system for ICPI-AKI that we hope will aid in the harmonization of definitions used in future studies (Table 5). By including definite, probable, and possible ICPI-AKI, this classification system emulates those proposed for extrarenal irAEs (e.g., myocarditis) (69) in recognizing varying degrees of diagnostic uncertainty in the absence of a biopsy (Table 5). We chose a threshold rise in SCr of 50% or need for RRT to be consistent with the KDIGO criteria (19), and we included the requirement that the increase in SCr be sustained on at least two consecutive values to reduce the likelihood of prerenal azotemia being misadjudicated as ICPI-AKI. Similar criteria were used in a recent ICPI-AKI study (20).
Table 5.
Proposed definition and classification of immune checkpoint inhibitor–associated AKI
| Classification | Definition |
| Definite ICPI-AKI | Kidney biopsy confirmed diagnosis compatible with ICPI-AKI, and after clinical review of risk factorsa |
| Probable ICPI-AKI |
BOTH of the following Sustained increase in SCr ≥50% on at least two consecutive values or need for RRT, after clinical review of risk factorsaAbsence of an alternative plausible etiology AND at least one of the following Sterile pyuria (≥5 WBCs/hpf) Concomitant or recent extrarenal irAEEosinophilia (≥500 cells per µl) |
| Possible ICPI-AKI |
BOTH of the following Increase in SCr ≥50% or need for RRTAKI is not readily attributable to alternative causes |
ICPI-AKI, immune checkpoint inhibitor–associated AKI; SCr, serum creatinine; WBC, white blood cell; hpf, high-power field; irAE, immune-related adverse event.
Risk factors include prior or concomitant proton pump inhibitor use, prior or concomitant extrarenal irAEs.
Future Directions
As the indications for ICPI use continue to expand, nephrologists and oncologists will undoubtedly be tasked with caring for a larger number of patients with ICPI-AKI. Differentiation of AKI caused by ICPIs versus other factors remains a key challenge, particularly given the high incidence of AKI in cancer patients, and the lack of sensitive or specific clinical features to reliably diagnose ICPI-AKI in the absence of a kidney biopsy. Development of noninvasive biomarkers—including urinary, blood, and imaging-based biomarkers—is thus urgently needed. Because ICPIs are often life-saving therapies, future studies are also needed to better understand which patients can be safely rechallenged after an episode of ICPI-AKI. Additionally, these studies should include analyses of overall survival after irAEs, rather than solely focusing on renal survival. Finally, studies are needed to better characterize the underlying mechanisms of ICPI-AKI.
Disclosures
F. Cortazar served as a consultant for ChemoCentryx and Momenta Pharmaceuticals. S. Gupta is a scientific coordinator for the ASCEND trial and reports grants from the National Institutes of Health during the conduct of the study and other from GlaskoSmithKline, outside the submitted work. D. Leaf received grant support from BioPorto Diagnostics. L. Riella had received grant support from Bristol-Meyers-Squib and honorarium from Mallinckrodt.
Funding
S. Gupta is supported by the National Institute on Deafness and Other Communication Disorders, grant F32DC017342. D. Leaf is supported by the National Institute of Diabetes and Digestive and Kidney Diseases, grant K23DK106448; and by the National Heart, Lung, and Blood Institute, grant R01HL144566. L. Riella is supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, grant R01AI143887.
Author Contributions
S. Gupta, F. Cortazar, L. Riella, and D. Leaf conceptualized the study, were responsible for data curation, wrote the original manuscript, and reviewed and edited the manuscript; S. Gupta and L Riella were responsible for the visualization; S. Gupta was responsible for the investigation; and F. Cortazar and D. Leaf were responsible for supervision and validation.
References
- 1.Leach DR, Krummel MF, Allison JP: Enhancement of antitumor immunity by CTLA-4 blockade. Science 271: 1734–1736, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Boussiotis VA: Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med 375: 1767–1778, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callahan MK, Kluger H, Postow MA, Segal NH, Lesokhin A, Atkins MB, Kirkwood JM, Krishnan S, Bhore R, Horak C, Wolchok JD, Sznol M: Nivolumab plus ipilimumab in patients with advanced melanoma: updated survival, response, and safety data in a phase i dose-escalation study. J Clin Oncol, Vol. 36, 2018, pp 391–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V, Marquez-Rodas I, Grob JJ, Butler MO, Middleton MR, Maio M, Atkinson V, Queirolo P, Gonzalez R, Kudchadkar RR, Smylie M, Meyer N, Mortier L, Atkins MB, Long GV, Bhatia S, Lebbé C, Rutkowski P, Yokota K, Yamazaki N, Kim TM, de Pril V, Sabater J, Qureshi A, Larkin J, Ascierto PA; CheckMate 238 Collaborators : Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 377: 1824–1835, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L; KEYNOTE-001 Investigators : Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372: 2018–2028, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Faivre-Finn C, Reck M, Vansteenkiste J, Spigel DR, Wadsworth C, Melillo G, Taboada M, Dennis PA, Özgüroğlu M; PACIFIC Investigators : Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 379: 2342–2350, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Massari F, Di Nunno V, Cubelli M, Santoni M, Fiorentino M, Montironi R, Cheng L, Lopez-Beltran A, Battelli N, Ardizzoni A: Immune checkpoint inhibitors for metastatic bladder cancer. Cancer Treat Rev 64: 11–20, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Donin NM, Lenis AT, Holden S, Drakaki A, Pantuck A, Belldegrun A, Chamie K: Immunotherapy for the treatment of urothelial carcinoma. J Urol 197: 14–22, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P; CheckMate 025 Investigators : Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373: 1803–1813, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, Vaishampayan UN, Drabkin HA, George S, Logan TF, Margolin KA, Plimack ER, Lambert AM, Waxman IM, Hammers HJ: Nivolumab for metastatic renal cell carcinoma: Results of a randomized phase II trial. J Clin Oncol 33: 1430–1437, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I: Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389: 2492–2502, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauschild A, Schadendorf D: Checkpoint inhibitors: A new standard of care for advanced merkel cell carcinoma? Lancet Oncol 17: 1337–1339, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Darvin P, Toor SM, Sasidharan Nair V, Elkord E: Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp Mol Med 50: 165, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Champiat S, Lambotte O, Barreau E, Belkhir R, Berdelou A, Carbonnel F, Cauquil C, Chanson P, Collins M, Durrbach A, Ederhy S, Feuillet S, François H, Lazarovici J, Le Pavec J, De Martin E, Mateus C, Michot JM, Samuel D, Soria JC, Robert C, Eggermont A, Marabelle A: Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Ann Oncol 27: 559–574, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, Le DT, Lipson EJ, Glezerman IG, Wolchok J, Cornell LD, Feldman P, Stokes MB, Zapata SA, Hodi FS, Ott PA, Yamashita M, Leaf DE: Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 90: 638–647, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirali AC, Perazella MA, Gettinger S: Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis 68: 287–291, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Izzedine H, Mathian A, Champiat S, Picard C, Mateus C, Routier E, Varga A, Malka D, Leary A, Michels J, Michot JM, Marabelle A, Lambotte O, Amoura Z, Soria JC, Kaaki S, Quellard N, Goujon JM, Brocheriou I: Renal toxicities associated with pembrolizumab. Clin Kidney J 12: 81–88, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Department of Health and Human Services . Common terminology criteria for adverse events (CTCAE) Version 5.0. 2017. Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Accessed November 10, 2019
- 19.Kidney Disease Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 20.Seethapathy H, Zhao S, Chute DF, Zubiri L, Oppong Y, Strohbehn I, Cortazar FB, Leaf DE, Mooradian MJ, Villani AC, Sullivan RJ, Reynolds K, Sise ME: The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors. Clin J Am Soc Nephrol 14: 1692–1700, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cortazar FB, Kibbelaar ZA, Glezerman IG, Abudayyeh A, Mamlouk O, Motwani SS, Murakami N, Herrmann SM, Manohar S, Shirali AC, Kitchlu A, Shirazian S, Assal A, Vijayan A, Renaghan AD, Ortiz-Melo DI, Rangarajan S, Malik AB, Hogan JJ, Dinh AR, Shin DS, Marrone KA, Mithani Z, Johnson DB, Hosseini A, Uprety D, Sharma S, Gupta S, Reynolds KL, Sise ME, Leaf DE: Clinical features and outcomes of immune checkpoint inhibitor-associated AKI: A multicenter study. [published online ahead of print January 2, 2020]. J Am Soc Nephrol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manohar S, Kompotiatis P, Thongprayoon C, Cheungpasitporn W, Herrmann J, Herrmann SM: Programmed cell death protein 1 inhibitor treatment is associated with acute kidney injury and hypocalcemia: Meta-analysis. Nephrol Dial Transplant 34: 108–117, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Nishimura H, Nose M, Hiai H, Minato N, Honjo T: Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11: 141–151, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW: Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270: 985–988, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Ise W, Kohyama M, Schraml BU, Zhang T, Schwer B, Basu U, Alt FW, Tang J, Oltz EM, Murphy TL, Murphy KM: The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nat Immunol 12: 536–543, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zehn D, Bevan MJ: T cells with low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity 25: 261–270, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richards DM, Kyewski B, Feuerer M: Re-examining the nature and function of self-reactive T cells. Trends Immunol 37: 114–125, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mamlouk O, Selamet U, Machado S, Abdelrahim M, Glass WF, Tchakarov A, Gaber L, Lahoti A, Workeneh B, Chen S, Lin J, Abdel-Wahab N, Tayar J, Lu H, Suarez-Almazor M, Tannir N, Yee C, Diab A, Abudayyeh A: Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: Single-center experience. J Immunother Cancer 7: 2, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muriithi AK, Leung N, Valeri AM, Cornell LD, Sethi S, Fidler ME, Nasr SH: Biopsy-proven acute interstitial nephritis, 1993-2011: A case series. Am J Kidney Dis 64: 558–566, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Weber JS, Kähler KC, Hauschild A: Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 30: 2691–2697, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, Sznol M, Long GV, Li H, Waxman IM, Jiang J, Robert C: Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol 35: 785–792, 2017 [DOI] [PubMed] [Google Scholar]
- 32.Weber JS, Dummer R, de Pril V, Lebbé C, Hodi FS; MDX010-20 Investigators : Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: Detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer 119: 1675–1682, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Blank ML, Parkin L, Paul C, Herbison P: A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int 86: 837–844, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD: Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373: 23–34, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shoushtari AN, Friedman CF, Navid-Azarbaijani P, Postow MA, Callahan MK, Momtaz P, Panageas KS, Wolchok JD, Chapman PB: Measuring toxic effects and time to treatment failure for nivolumab plus ipilimumab in melanoma. JAMA Oncology, Vol. 4, 2018, pp 98–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sznol M, Ferrucci PF, Hogg D, Atkins MB, Wolter P, Guidoboni M, Lebbé C, Kirkwood JM, Schachter J, Daniels GA, Hassel J, Cebon J, Gerritsen W, Atkinson V, Thomas L, McCaffrey J, Power D, Walker D, Bhore R, Jiang J, Hodi FS, Wolchok JD: Pooled analysis safety profile of nivolumab and ipilimumab combination therapy in patients with advanced melanoma. J Clin Oncol 35: 3815–3822, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M: Safety and clinical activity of combined PD-1 (nivolumab) and CTLA-4 (ipilimumab) blockade in advanced melanoma patients. N Engl J Med, Vol. 369, 2013, pp 122–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perazella MA, Sprangers B: AKI in patients receiving immune checkpoint inhibitors. Clin J Am Soc Nephrol 14: 1077–1079, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA; National Comprehensive Cancer Network : Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol 36: 1714–1768, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gallan AJ, Alexander E, Reid P, Kutuby F, Chang A, Henriksen KJ: Renal vasculitis and pauci-immune glomerulonephritis associated with immune checkpoint inhibitors. Am J Kidney Dis 74: 853–856, 2019 [DOI] [PubMed] [Google Scholar]
- 41.Kitchlu A, Fingrut W, Avila-Casado C, Chan CT, Crump M, Hogg D, Reich HN: Nephrotic syndrome with cancer immunotherapies: A Report of 2 cases. Am J Kidney Dis 70: 581–585, 2017 [DOI] [PubMed] [Google Scholar]
- 42.Murakami N, Borges TJ, Yamashita M, Riella LV: Severe acute interstitial nephritis after combination immune-checkpoint inhibitor therapy for metastatic melanoma [published correction appears in Clin Kidney J 9: 649, 2016]. Clin Kidney J 9: 411–417, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedman CF, Proverbs-Singh TA, Postow MA: Treatment of the immune-related adverse effects of immune checkpoint inhibitors: A review. JAMA Oncol 2: 1346–1353, 2016 [DOI] [PubMed] [Google Scholar]
- 44.González E, Gutiérrez E, Galeano C, Chevia C, de Sequera P, Bernis C, Parra EG, Delgado R, Sanz M, Ortiz M, Goicoechea M, Quereda C, Olea T, Bouarich H, Hernández Y, Segovia B, Praga M; Grupo Madrileño De Nefritis Intersticiales : Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int 73: 940–946, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Amin A, DePril V, Hamid O, Wolchock J, Maio M, Neyns B, Chin K, Ibrahim R, Hoos A, O’Day S: Evaluation of the effect of systemic corticosteroids for the treatment of immune-related adverse events (irAEs) on the development or maintenance of ipilimumab clinical activity. J Clin Oncol 27[Suppl]: 9037, 2009 [Google Scholar]
- 46.Harmankaya K, Erasim C, Koelblinger C, Ibrahim R, Hoos A, Pehamberger H, Binder M: Continuous systemic corticosteroids do not affect the ongoing regression of metastatic melanoma for more than two years following ipilimumab therapy. Med Oncol 28: 1140–1144, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Engels EA, Pfeiffer RM, Fraumeni JF Jr, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA, Copeland G, Finch JL, Fleissner ML, Goodman MT, Kahn A, Koch L, Lynch CF, Madeleine MM, Pawlish K, Rao C, Williams MA, Castenson D, Curry M, Parsons R, Fant G, Lin M: Spectrum of cancer risk among US solid organ transplant recipients. JAMA 306: 1891–1901, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sprangers B, Nair V, Launay-Vacher V, Riella LV, Jhaveri KD: Risk factors associated with post-kidney transplant malignancies: An article from the Cancer-Kidney International Network. Clin Kidney J 11: 315–329, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Esfahani K, Al-Aubodah TA, Thebault P, Lapointe R, Hudson M, Johnson NA, Baran D, Bhulaiga N, Takano T, Cailhier JF, Piccirillo CA, Miller WH: Targeting the mTOR pathway uncouples the efficacy and toxicity of PD-1 blockade in renal transplantation. Nat Commun 10: 4712, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hurkmans DP, Verhoeven JGHP, de Leur K, Boer K, Joosse A, Baan CC, von der Thüsen JH, van Schaik RHN, Mathijssen RHJ, van der Veldt AAM, Hesselink DA: Donor-derived cell-free DNA detects kidney transplant rejection during nivolumab treatment. J Immunother Cancer 7: 182, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boils CL, Aljadir DN, Cantafio AW: Use of the PD-1 pathway inhibitor nivolumab in a renal transplant patient with malignancy. Am J Transplant 16: 2496–2497, 2016 [DOI] [PubMed] [Google Scholar]
- 52.Zehou O, Leibler C, Arnault JP, Sayegh J, Montaudié H, Rémy P, Glotz D, Cordonnier C, Martin L, Lebbé C: Ipilimumab for the treatment of advanced melanoma in six kidney transplant patients. Am J Transplant 18: 3065–3071, 2018 [DOI] [PubMed] [Google Scholar]
- 53.Herz S, Höfer T, Papapanagiotou M, Leyh JC, Meyenburg S, Schadendorf D, Ugurel S, Roesch A, Livingstone E, Schilling B, Franklin C: Checkpoint inhibitors in chronic kidney failure and an organ transplant recipient. Eur J Cancer 67: 66–72, 2016 [DOI] [PubMed] [Google Scholar]
- 54.Wong K, Shen J, D’Ambruoso S, Stefanoudakis D, Drakaki A: Safety and efficacy of immune checkpoint inhibitors in patients with metastatic cancer post solid organ transplantation: A case report and review of the literature. Transplant Proc 51: 3053–3058, 2019 [DOI] [PubMed] [Google Scholar]
- 55.Alhamad T, Venkatachalam K, Linette GP, Brennan DC: Checkpoint inhibitors in kidney transplant recipients and the potential risk of rejection. Am J Transplant 16: 1332–1333, 2016 [DOI] [PubMed] [Google Scholar]
- 56.Jose A, Yiannoullou P, Bhutani S, Denley H, Morton M, Picton M, Summers A, van Dellen D, Augustine T: Renal allograft failure after ipilimumab therapy for metastatic melanoma: A case report and review of the literature. Transplant Proc 48: 3137–3141, 2016 [DOI] [PubMed] [Google Scholar]
- 57.Lipson EJ, Bagnasco SM, Moore J Jr, Jang S, Patel MJ, Zachary AA, Pardoll DM, Taube JM, Drake CG: Tumor regression and allograft rejection after administration of anti-PD-1. N Engl J Med 374: 896–898, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lipson EJ, Bodell MA, Kraus ES, Sharfman WH: Successful administration of ipilimumab to two kidney transplantation patients with metastatic melanoma. J Clin Oncol 32: e69–e71, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ong M, Ibrahim AM, Bourassa-Blanchette S, Canil C, Fairhead T, Knoll G: Antitumor activity of nivolumab on hemodialysis after renal allograft rejection. J Immunother Cancer 4: 64, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spain L, Higgins R, Gopalakrishnan K, Turajlic S, Gore M, Larkin J: Acute renal allograft rejection after immune checkpoint inhibitor therapy for metastatic melanoma. Ann Oncol 27: 1135–1137, 2016 [DOI] [PubMed] [Google Scholar]
- 61.Barnett R, Barta VS, Jhaveri KD: Preserved renal-allograft function and the PD-1 pathway inhibitor nivolumab. N Engl J Med 376: 191–192, 2017 [DOI] [PubMed] [Google Scholar]
- 62.Goldman JW, Abdalla B, Mendenhall MA, Sisk A, Hunt J, Danovitch GM, Lum EL: PD 1 checkpoint inhibition in solid organ transplants: 2 sides of a coin - case report. BMC Nephrol 19: 210, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Venkatachalam K, Malone AF, Heady B, Delos Santos R, Alhamad T: Poor outcomes with the use of checkpoint inhibitors in kidney transplant recipients [published online ahead of print August 9, 2019]. Transplantation [DOI] [PubMed] [Google Scholar]
- 64.Abdel-Wahab N, Safa H, Abudayyeh A, Johnson DH, Trinh VA, Zobniw CM, Lin H, Wong MK, Abdelrahim M, Gaber AO, Suarez-Almazor ME, Diab A: Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: An institutional experience and a systematic review of the literature [published correction appears in J Immunother Cancer 7: 158, 2019]. J Immunother Cancer 7: 106, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murakami N, Riella LV: Co-inhibitory pathways and their importance in immune regulation. Transplantation 98: 3–14, 2014 [DOI] [PubMed] [Google Scholar]
- 66.van Leeuwen MT, Webster AC, McCredie MRE, Stewart JH, McDonald SP, Amin J, Kaldor JM, Chapman JR, Vajdic CM, Grulich AE: Effect of reduced immunosuppression after kidney transplant failure on risk of cancer: Population based retrospective cohort study. BMJ 340: c570, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Euvrard S, Morelon E, Rostaing L, Goffin E, Brocard A, Tromme I, Broeders N, del Marmol V, Chatelet V, Dompmartin A, Kessler M, Serra AL, Hofbauer GF, Pouteil-Noble C, Campistol JM, Kanitakis J, Roux AS, Decullier E, Dantal J; TUMORAPA Study Group : Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med 367: 329–339, 2012 [DOI] [PubMed] [Google Scholar]
- 68.3C Study Collaborative Group : Campath, calcineurin inhibitor reduction, and chronic allograft nephropathy (the 3C Study) - results of a randomized controlled clinical trial. Am J Transplant 18: 1424–1434, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bonaca MP, Olenchock BA, Salem J-E, Wiviott SD, Ederhy S, Cohen A, Stewart GC, Choueiri TK, Di Carli M, Allenbach Y, Kumbhani DJ, Heinzerling L, Amiri-Kordestani L, Lyon AR, Thavendiranathan P, Padera R, Lichtman A, Liu PP, Johnson DB, Moslehi J: Myocarditis in the setting of cancer therapeutics: Proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation 140: 80–91, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A; Acute Kidney Injury Network : Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]