Abstract
INTRODUCTION:
Coronavirus illness 2019, commonly referred to as COVID-19, is a highly infectious disease brought on by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 was declared a universal pandemic in March 2020 by the World Health Organization and is a severe health issue with unprecedented morbidity and mortality rates. Both surgical and mediastinal emphysema have been seen in cases of critically ill COVID-19 patients in several hospitals in the Eastern Province of Saudi Arabia.
METHODS:
This was a retrospective, cross-sectional, multicentric study involving several hospitals in the Saudi Arabian Eastern Province. Data were collected from intensive care units (ICUs) in these hospitals from March 2 to August 2, 2020. The inclusion criteria consisted of all patients who tested positive for SARS-CoV-2 and were admitted to a critical care unit.
RESULTS:
Thirty patients required thoracic consultation and management, including 26 males (81.3%) and 4 females (12.5%) (1:0.15) who developed surgical and mediastinal emphysema requiring thoracic surgery intervention. Most of the patients were on high ventilation settings, and the mean duration of ventilator support was 16.50 ± 13.98 days. Two patients (6.3%) required reintubation. The median positive end-expiratory pressure (PEEP) was 12 ± 2.80 cmH2O with a median FiO2 of 70% ± 19.73. On average, thoracic complications occurred on day 3 (±6.29 days) postintubation. Ten patients (33.33%) experienced a pneumothorax associated with surgical emphysema (SE), 1 patient (3.33%) presented with only mediastinal emphysema; 17 patients (56.66%) with only SE, and 1 (3.33%) had mediastinal emphysema associated with SE. We noted a correlation between the duration of ventilator support, the length of ICU stay (P < 0.001), and the total length of stay (LOS) in the hospital (P < 0.001). Total length of hospital stay showed significant association with the onset of complications (P = 0.045) and outcomes (P = 0.006). A significant association between PEEP and the duration of ventilator support was also evident with a P value = 0.009 and the onset of complications (P = 0.043). In addition, we found a significant association between the group with pneumothorax in combination with SE, and their outcomes, with a P = 0.002.
CONCLUSION:
Surgical and mediastinal emphysema in the critically ill patients are usually attributed to barotrauma and high ventilations settings. During COVID-19 pandemic, these entities were seen and the pathogenesis was revisited and some attributed its presence to the disease process and destruction on lung parenchyma. The associated with extended LOS and delayed recovery in addition to poor prognosis were seen. Their presence is an indicator to higher morbidity and mortality.
Keywords: COVID-19, emphysema, mediastinal, pneumothorax, surgical
Since the declaration of the COVID-19 pandemic, the health-care system has faced myriad challenges ranging from viral intracellular components to worldwide supply chain issues. Coronavirus illness 2019, commonly referred to as COVID-19, is a highly infectious illness brought on by the severe acute respiratory ailment disease coronavirus 2 (SARS-CoV-2). COVID-19 typically affects the respiratory organs, with the most common symptoms including a cough, fever, and shortness of breath.[1]
A subset of critically ill COVID-19 patients has demonstrated unusual thoracic surgery entities seldom seen in critically ill patients with viral pneumonias. Surgical and/or mediastinal emphysema [Figures 1 and 2] was seen in several critically ill patients admitted to several hospitals throughout the Eastern Province of Saudi Arabia.
Figure 1.
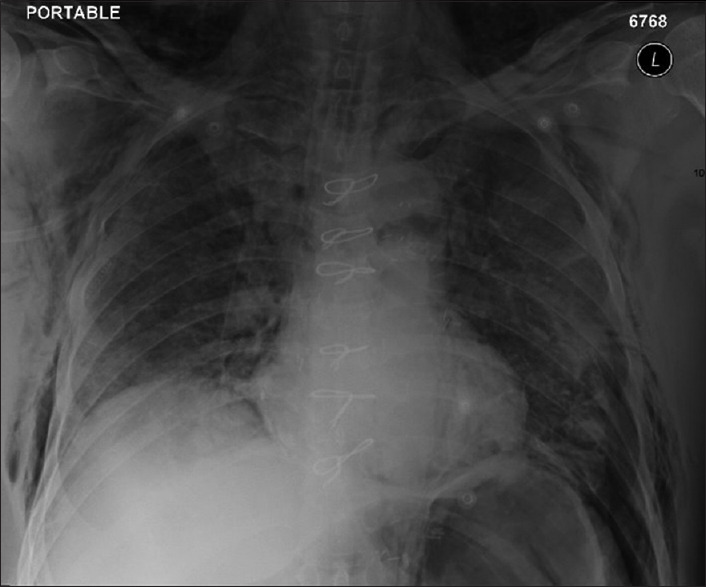
Chest X-ray of bilateral diffuse airspace opacities with bilateral lower zone atelectasis and bilateral subcutaneous emphysema
Figure 2.
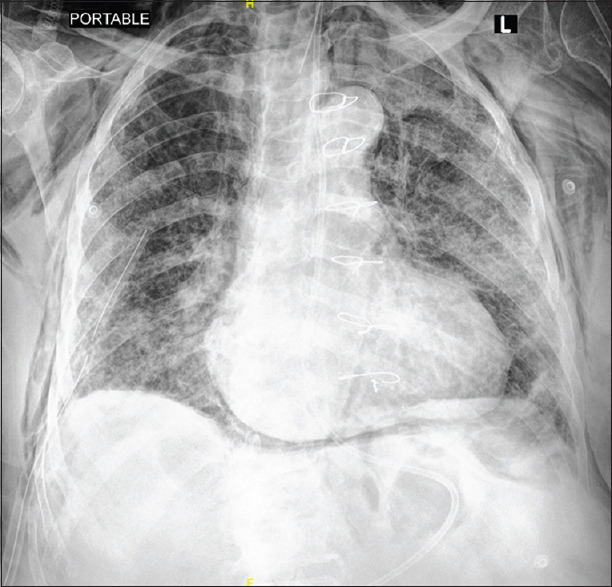
Chest X-ray showing pneumomediastinum with right chest tube
Computerized tomography (CT) plays a critical role in the diagnosis and subsequent follow-up of the coronavirus disease. According to CT findings, this illness presents as a reciprocal, wide ground-glass opacification (GGO) with a posterior or peripheral circulation, usually involving the lower lobes.[1] These observations raised the question: are these entities related to ventilator support complications such as barotrauma, or related to the actual disease process?
Methods
This was a retrospective, cross-sectional, multicentric study involving several hospitals in the Eastern Province of Saudi Arabia. Institutional ethics board approval was obtained, and the data were collected from the intensive care units (ICUs) in these hospitals between March and August, 2020. The inclusion criteria included all patients with a positive SARS-CoV-2 test who were admitted to the critical care units and experienced surgical emphysema (SE), mediastinal emphysema, and/or pneumothorax. The exclusion criteria included iatrogenic pneumothorax, traumatic pneumothorax, and both surgical and mediastinal emphysema due to instrumentation or intubation.
The requirement of written consent was waived by the institutional review board committee due to the retrospective nature of this study.
Data measurement and analysis
Data collected from the hospital system included demographics, radiological data, length of hospital stay, ventilation settings, inflammatory markers, thoracic complications, interventions, outcomes, and the patient's prognosis. Data management was conducted through Statistical Package for the Social Sciences (SPSS Inc. Chicago, IL, USA, version 23). Mann–Whitney U test and Fisher exact tests were used to determine the significant association of variables, and a significant level was considered at P < 0.05.
In addition, multivariate correlation and linear regression were identified, with the Kruskal–Wallis test used for comparisons between the three disease groups.
Results
Chart reviews were performed on all COVID-19-positive patients admitted to the ICUs of multiple hospitals in the Eastern Province of Saudi Arabia between March 2 and August 2, 2020. Thirty patients required thoracic consultation and management, and of those, 26 males (81.3%) and 4 females (12.5%) (1:0.15) developed SE and mediastinum emphysema requiring thoracic surgical intervention. This typically consisted of unilateral or bilateral thoracostomy tube insertion. Patient data are presented in Table 1, and a descriptive analysis of the patient population is demonstrated in Table 2.
Table 1.
Patient data
| Age | Gender | Comorbidity | Presenting symptoms | LOS unit (days) | LOS ICU (days) | Length of intubation (days) | Reintubation | Ventilator setting | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| PRVC, % | PEEP | ||||||||||
| 34 | Male | Medically free | SOB, cough, fever | NA | 7 | 3 | No | 50 | 16 | ||
| 70 | Male | DM, HTN, CKD, IHD | SOB, cough, fever | 16 | 52 | 52 | No | 70 | 14 | ||
| 45 | Male | Medically free | SOB, cough, fever | 3 | 21 | 3 | No | 40 | 12 | ||
| 52 | Male | HTN, DLP | SOB, cough | 19 | 27 | 27 | No | 50 | 10 | ||
| 66 | Female | DM, HTN | SOB, cough, fever, loss of appetite | 3 | 6 | 4 | No | 50 | 10 | ||
| 65 | Male | DM, HTN, DLP | SOB, cough, fever | 21 | 45 | 45 | Yes | 70 | 18 | ||
| 52 | Male | Medically free | SOB, fever | 3 | 41 | 11 | No | 60 | 12 | ||
| 67 | Male | DM | Asymptomatic | 6 | 17 | 17 | No | 100 | 14 | ||
| 59 | Male | Medically free | SOB | 3 | 9 | 9 | No | 70 | 12 | ||
| 40 | Male | Medically free | SOB, fever | NA | 7 | 7 | No | 70 | 16 | ||
| 62 | Male | CAD, HTN, DSL | SOB, fever | NA | 16 | 16 | No | 100 | 10 | ||
| 48 | Male | DM, HTN | SOB, cough | NA | 15 | 15 | No | 100 | 12 | ||
| 45 | Male | HTN, DSL | SOB, cough, fever | NA | 27 | 27 | No | 70 | 16 | ||
| 63 | Female | DSL, hypothyroidism | SOB, cough, fever | NA | 13 | 13 | No | 90 | 16 | ||
| 68 | Male | HTN | Loss of appetite | NA | 45 | 45 | No | 80 | 16 | ||
| 72 | Male | DM, HTN | Hypotensive, decrease level of conscious | 2 | 26 | 23 | No | 70 | 12 | ||
| 75 | Female | DM, HTN, DSL, hypothyroidism | SOB, cough, fever | 5 | 41 | 38 | Yes | 70 | 14 | ||
| 62 | Female | HTN, BA | SOB, cough, fever | 3 | 8 | 8 | No | 100 | 14 | ||
| 70 | Male | HTN | SOB, cough, fever | NA | 12 | 9 | No | 60 | 10 | ||
| 44 | Male | Medically free | SOB, cough, fever | NA | 38 | 22 | No | 100 | 10 | ||
| 66 | Male | DM, HTN | SOB, cough, fever | 2 | 12 | NA | No | 90 | 7 | ||
| 73 | Male | DM, HTN | SOB, Nausea, Fatigue | NA | 44 | 32 | No | 100 | 12 | ||
| 44 | Male | Medically free | SOB, cough, fever | NA | 2 | 2 | No | 100 | 10 | ||
| 30 | Male | Medically free | SOB, cough, fever, headache | 2 | 2 | NA | No | 70 | 8 | ||
| 61 | Male | DM | SOB, cough, fever | NA | 14 | 14 | No | 70 | 16 | ||
| 54 | Male | SCD | SOB, fever, loss of appetite | NA | 9 | 9 | No | 50 | 10 | ||
| 51 | Male | HTN | SOB, cough, fever | NA | 12 | 12 | No | 100 | 14 | ||
| 81 | Male | DM, HTN, CAD s/p CABG | SOB, cough, loss of appetite | NA | 13 | 13 | No | 60 | 10 | ||
| 59 | Male | Medically free | SOB, fever | NA | 10 | 10 | No | 50 | 10 | ||
| 47 | Male | Medically free | SOB, fever | NA | 9 | 9 | No | 50 | 10 | ||
|
| |||||||||||
| Age | Inflammatory markers | Tdoracic complication | Outcome | ||||||||
|
|
|
||||||||||
| LDH | CRP | Procalcitonin | Absolute lymphocyte | Percentage lymphocyte | Type | Onset from intubation | Intervention | Length of chest tube insertion | Reinsertion | ||
|
| |||||||||||
| 34 | 1225 | 16.48 | 3.79 | 792×103 | Low | ME | 1 | Conservative | NA | No | Died |
| 70 | 616 | 9.5 | 0.3 | 375×103 | Low | Pneumothor.ax and SE | 11 | Thoracostomy | 13 | No | Alive |
| 45 | 1223 | 39.08 | 5.38 | 1960×103 | Low | Pneumothorax and SE | 20 | Thoracostomy | 1 | No | Died |
| 52 | 1042 | 21.41 | 5.21 | 284×103 | Low | Pneumothorax and SE | 1 | Thoracostomy | 18 | Yes | ** |
| 66 | 976 | 15.04 | 2.02 | 477×103 | Low | SE | 3 | Thoracostomy | 1 | No | Died |
| 65 | 712 | 18.97 | 0.32 | 348×103 | Low | Pneumothorax and SE | 28 | Thoracostomy | 20 | No | Alive |
| 52 | 976 | 15.04 | 2.02 | 477×103 | Low | SE | 1 | Thoracostomy | 10 | No | Died |
| 67 | 732 | 25.79 | 1.24 | 658×103 | Low | SE and ME | 4 | Conservative | NA | No | Died |
| 59 | 746 | 15 | 5 | 389×103 | Low | SE | 7 | Thoracostomy | 3 | No | Died |
| 40 | 649 | 24.87 | 0.17 | 432×103 | Low | SE | 7 | Conservative | NA | No | Died |
| 62 | 1231 | 9.8 | 0.04 | 427×103 | Low | Pneumothorax and SE | 10 | Thoracostomy | 6 | No | Died |
| 48 | 1007 | 10.48 | 1.08 | 1022×103 | Low | SE | 5 | Conservative | NA | No | Died |
| 45 | 3219 | 1.67 | 1.05 | 3760×103 | High | Pneumothorax and SE | 16 | Thoracostomy | 3 | No | ** |
| 63 | 499 | 23.01 | 0.11 | 1588×103 | Low | SE | 9 | Thoracostomy | 1 | No | Died |
| 68 | 409 | 55 | 0.11 | 597×103 | Low | Pneumothorax and SE | 1 | Conservative | NA | NA | ** |
| 72 | 260 | 67 | 0.09 | 1430×103 | High | SE | 1 | Thoracostomy | 1 | No | Died |
| 75 | 219 | 11 | 5.48 | 1550×103 | Low | Pneumothorax and SE | 1 | Conservative | NA | NA | ** |
| 62 | 1185 | 22.3 | NA | 740×103 | Low | SE | 2 | Conservative | NA | NA | Died |
| 70 | 5382 | 47.3 | NA | 320×103 | Low | SE | 1 | Thoracostomy | 1 | No | Died |
| 44 | 1625 | 20.7 | NA | 660×103 | Low | Pneumothorax and SE | 7 | Thoracostomy | 20 | No | Died |
| 66 | 653 | 21.2 | NA | 790×103 | Low | SE | 8* | Conservative | NA | NA | Died |
| 73 | 502 | 43 | NA | 340×103 | Low | SE | 6 | Conservative | NA | NA | Died |
| 44 | NA | 8.5 | NA | 370×103 | High | SE | 2 | Conservative | NA | NA | Died |
| 30 | NA | 23.6 | NA | 2300×103 | Low | Pneumothorax and SE | 2* | Thoracostomy | 1 | No | Died |
| 61 | 2292 | 16.8 | NA | 330x103 | Low | SE | 3 | Thoracostomy | 7 | No | Died |
| 54 | 883 | 12.8 | NA | 330×103 | Low | SE | 2 | Conservative | NA | NA | Alive |
| 51 | 741 | 34.5 | NA | 550×103 | Low | SE | 10 | Conservative | NA | NA | Died |
| 81 | 548 | 55.9 | NA | 520×103 | Low | Pneumothorax and SE | 2 | Thoracostomy | 10 | No | Died |
| 59 | 1498 | 38.9 | NA | 470×103 | Low | SE | 3 | Conservative | NA | NA | ** |
| 47 | 2661 | 20 | NA | 220×103 | Low | SE | 3 | Conservative | NA | NA | ** |
*Day of admission, as the patient not intubated, **Still admitted and slightly improved. ICU=Intensive Care Unit, DM=Diabetes mellitus, HTN=Hypertension, DLP=Dyslipidemia, CKD=Chronic kidney disease, IHD=Ischemic heart disease, CAD=Coronary artery disease, SOB=Shortness of breath, ME=Mediastinal emphysema, SE=Surgical emphysema, LOS=Length of stay, PRVC=Pressure-regulated volume control, PEEP=Positive end-expiratory pressure, LDH=Lactate dehydrogenase, CRP=C-reactive protein, BA=Bronchial asthma, CABG=Coronary artery bypass grafting, NA=Not available, DSL=Dyslipedimia, SCD=Sickle cell disease
Table 2.
Descriptive analysis of the patients
| General characteristics | Value |
|---|---|
| Age | |
| Mean±SD (range) | 57.50±12.75 (30-81) |
| Gender, n (%) | |
| Male | 26 (81.3) |
| Female | 4 (12.5) |
| Comorbidities, n (%) | |
| DM | 11 (34.4) |
| HTN | 16 (50.0) |
| DLP | 6 (18.8) |
| CKD | 1 (3.1) |
| Heart disease | 3 (9.4) |
| Presenting symptoms, n (%) | |
| SOB | 27 (84.4) |
| Cough | 19 (59.4) |
| Fever | 22 (68.8) |
| Asymptomatic | 1 (3.1) |
| Loss of appetite | 4 (12.5) |
| Hospital course, mean±SD (range) | |
| Length of unit admission (days) | 2.93±5.62 (0-21) |
| Length of ICU admission (days) | 20.00±14.80 (2-52) |
| Ventilation | |
| Length of intubation (days), mean±SD (range) | 16.50±13.98 (0-52) |
| Reintubation, n (%) | |
| Yes | 2 (6.3) |
| No | 28 (87.5) |
| PEEP, median±SD (range) | 12±2.80 (7-18) |
| PRVC, median±SD (range) | 70±19.73 (40-100) |
| Laboratory, mean±SD (range) | |
| LDH | 1190.39±1078.96 (219-5382) |
| CRP | 25.63±15.99 (1.67-67) |
| Procalcitonin | 1.88±2.14 (0.04-5.48) |
| Absolute lymphocyte | 748.73±719.43 (220-3760) |
| Thoracic complications | |
| Onset of complication after intubation (days), median±SD (range) | 3±6.29 (1-28) |
| Type, n (%) | |
| Mediastinum emphysema | 1 (3.33) |
| SE | 17 (56.6) |
| Pneumothorax and SE | 11 (33.3) |
| SE and ME | 1 (3.33) |
| Interventions | |
| Conservative, n (%) | 14 (46.7) |
| Thoracostomy, n (%) | 16 (53.3) |
| Reinsertion of thoracostomy, n (%) | |
| Yes | 1 (3.1) |
| No | 29 (90.6) |
| Duration of chest tube (days), mean±SD (range) | 3.86±6.30 (0−20) |
| Outcomes, n (%) | |
| Discharged | 3 (9.4) |
| Deceased | 21 (65.6) |
| Still admitted and slightly improved | 6 (18.8) |
SD=Standard deviation, HTN=Hypertension, CKD=Chronic kidney disease, SOB=Shortness of breath, CRP=C-reactive protein, LDH=Lactate dehydrogenase, PEEP=Positive end-expiratory pressure, PRVC=Pressure-regulated volume control, ICU=Intensive care unit, DM=Diabetes mellitus, DLP=Dyslipidemia, SE=Surgical emphysema, ME=Mediastinal emphysema
Over 84% of the patients presented with shortness of breath, which was the most commonly-seen symptom, along with a variety of others including: fever (68.8%), cough (59.4%), and loss of appetite and fatigability. One patient (3.1%) was asymptomatic.
The mean of length of stay (LOS) in the regular unit was 2.9 ± 5.62 days (ranging from 0 to 21 days), and the mean of the ICU LOS was 20.00 ± 14.80 days (ranging from 2 to 52 days).
Most of the patients were on maximum ventilation settings programmed on pressure-regulated volume control. The mean duration of ventilator support was 16.50 ± 13.98 days (range from 0 to 52 days). Two patients (6.3%) required reintubation. The median positive end-expiratory pressure (PEEP) was set at 12 ± 2.80 cmH2O (range from 7 to 18), and the median FiO2 was 70% ± 19.73 (range from 40% to 100%). On average, thoracic complications occurred on day 3 (±6.29 days) postintubation (range from 1 to 28 days).
In terms of inflammatory markers, lactate dehydrogenase (LDH) levels ranged from 219 to 5382, with a mean of 1190.39 (standard deviation [SD]: 1078.96). C-reactive protein ranged from 1.67 to 67 with a mean of 25.63 (SD 15.99), and procalcitonin ranged from 0.04 to 5.48, with a mean of 1.88 (SD 2.14). Absolute lymphocytes ranged from 220 to 3760 with a mean of 748.73 (SD 719.43), and all lymphocyte percentages were low other than in two cases, which registered high.
Ten patients (33.33%) experienced a pneumothorax associated with SE, one patient (3.33%) presented with mediastinal emphysema, seventeen patients (56.66%) with SE, and one patient (3.33%) with mediastinal emphysema associated with SE. Fourteen patients (46.7%) were treated conservatively and sixteen patients (53.3%) were treated with indwelling thoracostomy tubes. The mean duration of the tube placement was 3.86 ± 6.30 days (ranging from 0 to 20 days). One patient required a reinsertion of the thoracostomy tube after developing SE following a tracheostomy insertion.
From our study group of patients, three (9.4%) were discharged in good condition, six patients (18.8%) were slightly improved and remained admitted pending manuscript preparation, and three (11.1%) were discharged in a good condition. Twenty-one patients (65.6%) died as a result of disease progression and multiple organ failure.
We extracted multivariate correlations and regressions with varying associations. There was a significant correlation between the duration of ventilatory support, the ICU LOS (P < 0.001), and the hospital LOS (P = 0.019). The total length of hospital stay showed a significant association with the onset of complications (P = 0.043) and outcomes (P = 0.043). In addition, a further significant association between PEEP and the duration of ventilatory support was noticed (P = 0.009), as well as with the onset of complications (P = 0.023) as presented in Table 3.
Table 3.
Multivariant correlations
| Variable correlations | LOS ICU | Length of disease | Length of intubation | Reintubation | PEEP | Onset of complication since intubation | Length of tube | Outcome |
|---|---|---|---|---|---|---|---|---|
| LOS ICU | ||||||||
| Pearson Correlation | 1 | 0.963** | 0.881** | −0.422* | 0.340 | 0.298 | 0.526** | −0.425* |
| Significant (two-tailed) | 0.000 | 0.000 | 0.020 | 0.066 | 0.109 | 0.003 | 0.019 | |
| n | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Length of disease | ||||||||
| Pearson Correlation | 0.963** | 1 | 0.889** | −0.494** | 0.338 | 0.367* | 0.631** | −0.486** |
| Significant (two-tailed) | 0.000 | 0.000 | 0.006 | 0.068 | 0.046 | 0.000 | 0.006 | |
| n | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Length of intubation | ||||||||
| Pearson Correlation | 0.881** | 0.889** | 1 | −0.486** | 0.471** | 0.287 | 0.462* | −0.601** |
| Significant (two-tailed) | 0.000 | 0.000 | 0.006 | 0.009 | 0.124 | 0.010 | 0.000 | |
| n | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Reintubation | ||||||||
| Pearson Correlation | −0.422* | −0.494** | −0.486** | 1 | −0.351 | −0.371* | −0.264 | 0.408* |
| Significant (two-tailed) | 0.020 | 0.006 | 0.006 | 0.057 | 0.043 | 0.158 | 0.025 | |
| n | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| PEEP | ||||||||
| Pearson Correlation | 0.340 | 0.338 | 0.471** | −0.351 | 1 | 0.373* | 0.061 | −0.176 |
| Significant (two-tailed) | 0.066 | 0.068 | 0.009 | 0.057 | 0.043 | 0.748 | 0.351 | |
| n | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Onset of complication since intubation | ||||||||
| Pearson Correlation | 0.298 | 0.367* | 0.287 | −0.371* | 0.373* | 1 | 0.341 | −0.152 |
| Significant (two-tailed) | 0.109 | 0.046 | 0.124 | 0.043 | 0.043 | 0.065 | 0.424 | |
| n | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Length of tube | ||||||||
| Pearson Correlation | 0.526** | 0.631** | 0.462* | −0.264 | 0.061 | 0.341 | 1 | −0.225 |
| Significant (two-tailed) | 0.003 | 0.000 | 0.010 | 0.158 | 0.748 | 0.065 | 0.231 | |
| n | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Outcome | ||||||||
| Pearson Correlation | −0.425* | −0.486** | −0.601** | 0.408* | −0.176 | −0.152 | −0.225 | 1 |
| Significant (two-tailed) | 0.019 | 0.006 | 0.000 | 0.025 | 0.351 | 0.424 | 0.231 | |
| n | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
*Correlation is significant at the 0.05 level (two-tailed), **Correlation is significant at the 0.01 level (two-tailed). LOS=Length of stay, ICU=Intensive care units, PEEP=Positive end-expiratory pressure
By applying linear regression, significant associations were confirmed as mentioned above, between the duration of ventilatory support and ICU stay (P < 0.001) and total LOS in the hospital (P < 0.001). Total length of hospital stay showed significant association with the onset of complications (P = 0.045) and outcomes (P = 0.006). A significant association between PEEP and the duration of ventilatory support also was noticed (P = 0.009), with the onset of complications (P = 0.043), as presented in Table 3.
We also compared three variations of thoracic complications: pneumothorax in association with SE, SE alone, and mediastinal emphysema, with findings of a significant association between the group presenting with pneumothorax in combination with SE and their outcomes, with a P = 0.002 [Table 4].
Table 4.
Significant differences between pneumothorax in association with surgical emphysema and surgical emphysema regarding outcomes
| Thoracic complications Outcome | n | Mean rank | Sum of ranks | P |
|---|---|---|---|---|
| Pneumothorax and SE | 11 | 10.58 | 137.50 | 0.002 |
| SE | 17 | 18.59 | 297.50 | |
| Total | 28 |
SE=Surgical emphysema
And the question remains; how do COVID-19 patients acquire SE on high ventilator settings in the absence of pneumothorax in most of the cases? Further research is required to clarify this condition.
Discussion
In December 2019, a viral pneumonia resembling SARS was seen in Wuhan, China. This led to the identification of a novel coronavirus, SARS-CoV-2 which causes COVID-19 disease.[1] Since then, much literature has addressed every segment of care pertaining to this disease. Phenomenally, it has been profoundly impacted global economics and presented innumerable political challenges. From earlier experiences with SARS-CoV-1, several observations have been made in regard to both surgical and mediastinal emphysema.[2,3]
Pneumomediastinum is often a self-limiting, benign state, occurring when an excessive amount of luminal gas escapes into the mediastinum. In severe cases of coronavirus, the majority of patients are ventilated mechanically with a tremendous positive strain. This carries a strong risk of dangerous mediastinal emphysema, pneumomediastinum, and pneumothorax with the potential of mimicking cardiac tamponade. Under severe conditions, emphysema has the potential of constricting the primary airway and limiting the flow of blood between the neck vessels and head.[4]
Due to its ease of use and high sensitivity, computerized tomography (CT) of chest is an essential tool in screening potential COVID-19 patients. The most common CT finding in coronavirus pneumonia is a GGO occurring in the subpleural areas of the lower lobes. This is mainly observed during the initial disease stage of patients with suspected COVID-19 pneumonia. Certain cases respond adversely to pharmaceutical therapy, with localized lesions of the lungs advancing to more and diffuse lesions.[5]
The usual pathophysiology is related the onset of increased intra-alveolar pressure. Coughing, sneezing, or ventilator support can lead to such entity. Several patients presented with SE and ME before assisted ventilation, which raises questions regarding the actual pathophysiology of the disease and its complications. COVID-19 causes lung parenchymal and extraparenchymal disease.[1] And as well, inappropriate processes during endotracheal intubation may destroy the trachea walls, potentially resulting in subcutaneous emphysema.
The airways of elderly patients with chronic obstructive pulmonary disease are often more vulnerable during tracheal intubation compared to younger demographics. It is, therefore, important to note whether such a patient has undergone previous thoracotomies, as our findings suggest that a history of thoracotomy is a leading factor in extensive subcutaneous emphysema seen in such a patient.[6] This is demonstrated radiologically in alveolar and interstitial patterns. Other reported patterns include flagstone, air bronchogram, vascular enlargement, airway changes, pleural changes, and halo and inverted halo signs, along with pericardial and pleural effusions. Thoracic surgery entities are also seen, including SE, ME, and pneumothorax.[2]
The most common pattern observed in COVID-19 patients is ground-glass opacities scattered randomly with preference to the posterior segments of lower lobe.[3] The virus gains access to airways by reaching through the terminal alveolar wall and septal interstitium. Cellular lymphocytic injury results in alveolar damage and an ability to rupture with the slightest increase in pressure, causing interstitial emphysema, mediastinal emphysema, SE, or pneumothorax.[5,7] This alveolar damage seems to be the result of cellular lymphoytic injury rather than an outcome of virus replication. This is evident by the subsequent onset of SE and ME, which typically occurs on or around day 14 postintubation, whereas viral replication usually peaks around day 4. The significance of SE and ME is that they aggravate respiratory failure, resulting in worse outcomes as seen in several studies and case reports.[6,8] The resulting hospital LOS and recovery are much slower and longer in patients with these entities.
A study completed by Obeso Carillo GA et al. revealed that patients with a radiologically identifiable Earth-Heart sign were at a higher risk of developing a tension pneumomediastinum. In those cases, the main differential diagnosis included cardiac tamponade caused by pericardial effusion.[9] Thus, early interventions are recommended for patients who present with this sign.
We did not identify the presence of the Earth-Heart sign in any of the patients in our study.
There are multiple earlier studies examining the relationship between mediastinal emphysema and a surgical presence in patients with coronavirus. For instance, C.M. Chu reported in his 2004 study on SARS patients, that only crest serum LDH was related to the advancement of spontaneous pneumomediastinum (P = 0.001).[5]
Conclusion
Surgical and mediastinal emphysema in the critically ill patients are usually attributed to barotrauma and high ventilations settings. During COVID-19 pandemic, these entities were seen and the pathogenesis was revisited and some attributed its presence to the disease process and destruction on lung parenchyma. The associated with extended LOS and delayed recovery in addition to poor prognosis were seen. Their presence is an indicator to higher morbidity and mortality.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ucpinar BA, Sahin C, Yanc U. Spontaneous pneumothorax and subcutaneous emphysema in COVID-19 patient: Case report. J Infect Public Health. 2020;13:887–9. doi: 10.1016/j.jiph.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chu CM, Leung YY, Hui JY, Hung IF, Chan VL, Leung WS, et al. Spontaneous pneumomediastinum in patients with severe acute respiratory syndrome. Eur Respir J. 2004;23:802–4. doi: 10.1183/09031936.04.00096404. [DOI] [PubMed] [Google Scholar]
- 3.Aydın S, Öz G, Dumanlı A, Balcı A, Gencer A. A case of spontaneous pneumothorax in COVID-19 pneumonia. J Surg Res. 2020;3:096–101. [Google Scholar]
- 4.Wali A, Rizzo V, Bille A, Routledge T, Chambers A. Pneumomediastinum following intubation in COVID-19 patients: A case series. Anaesthesia. 2020;75:1076–81. doi: 10.1111/anae.15113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun R, Liu H, Wang X. Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J Radiol. 2020;21:541–4. doi: 10.3348/kjr.2020.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiang C, Wu G. SARS-CoV-2 pneumonia with subcutaneous emphysema, mediastinal emphysema, and pneumothorax: A case report. Medicine (Baltimore) 2020;99:e20208. doi: 10.1097/MD.0000000000020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolani S, Houari N, Haloua M, Lamrani YA, Boubbou M, Serraj M, et al. Spontaneous pneumomediastinum occurring in the SARS-COV-2 infection. IDCases. 2020;21:e00806. doi: 10.1016/j.idcr.2020.e00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W, Gao R, Zheng Y, Jiang L. COVID-19 with spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema. J Travel Med. 2020;27:taaa062. doi: 10.1093/jtm/taaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obeso Carillo GA, Barge Caballero G, Cañizares Carretero MÁ. The Earth-Heart sign: A new diagnostic finding in a patient with tension pneumomediastinum. Lancet. 2014;383:486. doi: 10.1016/S0140-6736(13)62634-3. [DOI] [PubMed] [Google Scholar]


