Abstract
Background:
Subacute cutaneous lupus erythematosus (SCLE) manifests with erythematous, nonscarring, annular, or papulosquamous plaques. Proton pump inhibitors (PPIs) are increasingly being incriminated in its causation, but reports of similar nature from India are lacking.
Aims:
To describe the characteristics of seven patients with SCLE induced by PPIs and to review the published cases in order to provide a better perspective of the association.
Materials and Methods:
We describe seven patients of PPI-induced SCLE, seen over a period of 6 years. We also review the literature for additional data on PPI-induced SCLE. The selected publications were reviewed, and relevant clinical and laboratory data were extracted.
Results:
Of the total seven cases, there were four males and three females with a mean age of 60.2 ± 5.5 years (range 53-70 years). Nine episodes of PPI-induced SCLE were recorded in the seven patients. Of the initial episodes, esomeprazole was implicated in four, pantoprazole in two, and rabeprazole in one patient. Latency period ranged from 2 weeks to 1 year (mean 11.4 ± 16.2 weeks). Morphology was described as annular scaly plaques in six and papulosquamous in one. Antinuclear antibodies and anti-Ro antibodies were positive in all patients. Naranjo probability scale was used in all patients; two were categorized as definite and five as probable. Treatments included drug withdrawal in six patients, topical steroids in one, systemic corticosteroids in all seven, and hydroxychloroquine in one patient, used alone or in combinations. Complete remission was achieved in six cases, while one had partial remission.
Limitation:
Retrospective nature of this study and limited number of patients.
Conclusion:
PPIs can trigger SCLE.
Keywords: Drug-Induced Subacute Cutaneous Lupus Erythematosus, proton pump inhibitors, SCLE, subacute cutaneous lupus erythematosus
Introduction
Subacute cutaneous lupus erythematosus (SCLE) is a distinct subset of cutaneous lupus erythematosus and was first described by Sontheimer et al.[1] in 1979. Clinically, SCLE presents with erythematous nonscarring annular or papulosquamous eruptions on photo-exposed areas such as upper back, chest, dorsal arms, and lateral neck. The hallmark feature of this entity is anti-Ro/SSA antibodies.[2] Histopathology shows lupus erythematosus-specific skin changes of interface dermatitis.[2]
Most of the cases of SCLE are idiopathic. In about 30% cases, it may be triggered or induced by drugs. In 1985, Reed et al.[3] were the first to report drug-induced SCLE (DI-SCLE) due to hydrochlorothiazide, and subsequently it was reported in association with various other drugs.[4] DI-SCLE is now recognized as a clinical entity characterized by a more widespread presentation than idiopathic SCLE and with frequent occurrence of malar rash, bullous, erythema multiforme (EM)-like, and vasculitic manifestations.[5] It is immunologically and histopathologically indistinguishable from idiopathic SCLE.[5] The time from drug exposure to development of SCLE varies from 3 days to 11 years with a median of 6 weeks.[6] It is a reversible condition, and most cases resolve in 1-3 months after withdrawal of triggering drug. However, serological resolution takes longer.[2] The exact pathogenesis of DI-SCLE remains unknown.
More than 50 drugs have been implicated for causing DI-SCLE, including thiazides, calcium channel blockers, Acetycholinesterase (ACE) inhibitors, terbinafine, and tumor necrosis factor blockers.[2,6] Recent reports have raised concern about the development of SCLE following intake of proton pump inhibitors (PPIs).[2,6,7] Currently, PPI-induced SCLE has been observed to predominate over other drug classes.[8,9] We report seven cases of DI-SCLE triggered by PPIs and review the literature of SCLE induced by PPIs to provide an up-to-date and comprehensive appraisal of this entity to the physicians and dermatologists.
Materials and Methods
We describe seven patients of PPI-induced SCLE, seen over a period of 6 years from June 2014 to May 2020 in the Department of Dermatology, Venereology, and Leprology of a tertiary care hospital of North India. These cases were identified from the inpatient and outpatient hospital records. The diagnosis of SCLE induced by PPI in our patients was made on the basis of correlation of clinical presentation, histopathologic and immunological investigations, and response to withdrawal of the offending drug. Naranjo scale was used to assess drug causality. Following parameters were studied: age, gender, type of PPI, latency period (time from drug initiation to onset of SCLE), comorbidities, and characteristics of cutaneous lesions, laboratory abnormalities, skin biopsy findings, treatment, and outcome. The cases of SCLE without any preceding drug history or those with SCLE attributed to other drugs were excluded. The study was approved by institutional ethical review committee. A summary of the patients, investigations, and clinical response is presented in Tables 1 and 2.
Table 1.
Epidemiology, etiology, and cutaneous signs seen in patients
| Age/sex | Latent period | PPI | Indication of PPI | Sites | Morphology | Underlying disease |
|---|---|---|---|---|---|---|
| 70/F | 2 months | E | GERD | T+Ex+F | Annular | HTN, hyperlipidemia |
| 60/F | 6 weeks | E | Prophylaxis | T+Ex+F | Papulosquamous, target lesions | Pulmonary TB |
| 55/M | 4 weeks | E | Duodenitis | T+Ex+F | Annular | DLE |
| 62/F | 8 weeks | E | GERD | T+Ex+F | Annular | HTN, COPD |
| 62/M | 2 weeks | R | Prophylaxis | T+Ex+F | Annular | RA, HTN, COPD |
| 53/M | 4 weeks | P | Pain abdomen | T+Ex | Annular | Cirrhosis |
| 60/M | 1 year | P | Prophylaxis | T+Ex | Annular | SLE |
COPD=Chronic obstructive pulmonary disease, DLE=Discoid lupus erythematous, E=Esomeprazole, Ex=extremities, F=face, GERD=Gastroesophageal reflux disease, HTN=Hypertension, P=Pantoprazole, PPI=Proton pump inhibitor, R=rabeprazole, RA=Rheumatoid arthritis, SLE=Systemic lupus erythematous, T=trunk
Table 2.
Investigations, therapeutic and clinical profile of patients
| Age/sex | Investigations | ANA (IF/ELISA)/ANA profile | Skin biopsy (H and E/DIF) | Treatment | Outcome | Relapse (if any) | Naranjo score |
|---|---|---|---|---|---|---|---|
| 70/F | ESR- 115 mm/1st hr Mild proteinuria | ANA (IF)- 1:320 Anti-Ro/SSA++Anti-La/SSB +/− |
Hyperkeratosis, epidermal atrophy, vacuolar degeneration of DEJ, necrotic keratinocytes, dense collection of neutrophils and lymphocytes in papillary dermis consistent with SCLE DIF- negative | DW + SCS | CR | + (On re-exposure to P) | Definite |
| 60/F | WNL | ANA (ELISA) +++ Anti-Ro/SSA ++ |
Hyperkeratosis, parakeratosis, irregular acanthosis, spongiosis, intraepidermal bulla, vacuolar degeneration of basal layer with increased dermal collagenization, few necrotic keratinocytes with perivascular lymphocytic infiltrate- reported as EM-like | DW + TCS + SCS | CR | - | Probable |
| 55/M | WNL | ANA (ELISA) +++ Anti-Ro/SSA++Anti-La/SSB ++ |
Epidermal atrophy, basal cell vacuolization, mild perivascular lymphocytic infiltrate- SCLE | SCS + HCQS | PR | LTF | Probable |
| 62/F | WNL | ANA (ELISA) ++ Anti-Ro/SSA ++ | Hyperkeratosis, epidermal atrophy, basal cell vacuolization, perivascular lymphocytic infiltrate, thickened basement membrane, and myxoid degeneration- SCLE | DW + SCS | CR | - | Probable |
| 62/M | WNL | ANA (ELISA) +++ Anti-Ro/SSA++Anti-La/SSB++ |
Basal cell vacuolization, dermal edema, mucin deposition, chronic perivascular infiltrate- SCLE | DW + SCS | CR | + (On re-exposure to E) | Definite |
| 53/M | Thrombocytopenia (13,000 mL/dL) | ANA (IF)- 1:80 Anti-Ro/SSA ++ |
Hyperkeratosis, perivascular and periadnexal lymphocytic infiltrate, basal cell vacuolization- SCLE
DIF- negative |
DW + SCS | CR | - | Probable |
| 60/M | WNL | ANA (ELISA) +++ Anti-Ro/SSA++Anti-La/SSB ++ |
Hyperkeratosis, epidermal atrophy, basal cell vacuolization, perivascular chronic inflammatory infiltrate- SCLE
>DIF- negative |
DW + SCS | CR | - | Probable |
ANA=Antinuclear antibody, CR=Clinical remission, DEJ=Dermo-epidermal junction, DIF=Direct immunofluorescence, DW=Drug withdrawal, E=Esomeprazole, ELISA=Enzyme-linked immunosorbent assay, EM=erythema multiforme, H and E=Hemotoxylin and eosin, IF=Immunofluorescence, LTF=Lost to follow-up, P=Pantoprazole, PR=Partial remission, SCLE=Subcutaneous lupus erythematosus, SCS=Systemic corticosteroids, TCS=Topical corticosteroids, WNL=within normal limit
Case Reports
Case 1
A 70-year-old female with hypertension, hyperlipidemia, and gastroesophageal reflux presented with skin rash over face, trunk, and limbs for 2 months. She had history of oral erosions, but no fever, joint pain, hair loss, or malar rash. Her medications included telmisartan, clonidipine, atorvastatin, and clopidogrel. Six months earlier, she had been prescribed esomeprazole.
Examination revealed multiple symmetrical, erythematous, annular, polycyclic, scaly plaques over face, neck, trunk, upper limbs, and thighs with central scaling and peripheral hyperpigmentation [Figure 1]. Purpuric lesions over extremities and targetoid EM-like lesions over palms and soles were also noted. Oral cavity, hair, and nails were normal. General physical examination was unremarkable.
Figure 1.
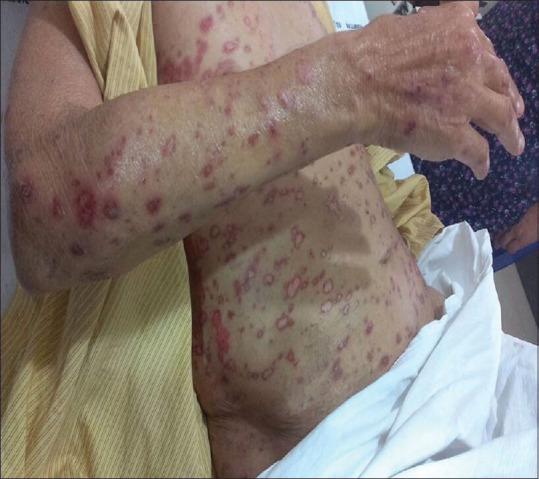
Erythematous to brownish annular scaly plaques over entire trunk and arms
Laboratory investigations are presented in Table 2. On urine examination, mild proteinuria was observed, while all other relevant investigations including complete hemogram (hemoglobin- 9.3 g/dL, total leukocyte count (TLC- 5.8 × 103/mm3), liver and renal function tests were within normal limits.
All drugs were discontinued, and she was treated with intravenous methylprednisolone 750 mg for 3 days along with topical steroids. Skin lesions resolved over the next 4 weeks. Her lesions recurred 1 year later following inadvertent introduction of pantoprazole by another physician, which improved following its discontinuation. Naranjo causality scale concluded PPIs as a definite cause of the adverse effect. Two years later, anti-Ro antibodies were still positive and she was found to have developed systemic lupus erythematosus (SLE) during follow-up, for which she was given systemic steroids along with colchicine.
Case 2
A 60-year-old female was diagnosed with pulmonary tuberculosis and was prescribed rifampicin, isoniazid, ethambutol, and pyrazinamide along with esomeprazole. Six weeks later, she developed a generalized skin eruption and malaise. She had no fever, joint pain, or photosensitivity. Examination revealed multiple crusted plaques over face, trunk, bilateral upper and lower limbs involving photo-protected sites too [Figure 2]. Confluent erythematous macules and multiple target lesions were identified on palms, soles, and lower limbs [Figures 3 and 4]. Hemogram, serum biochemistry, and urinalysis were normal. Findings on skin biopsy and immunological workup are presented in Table 2.
Figure 2.
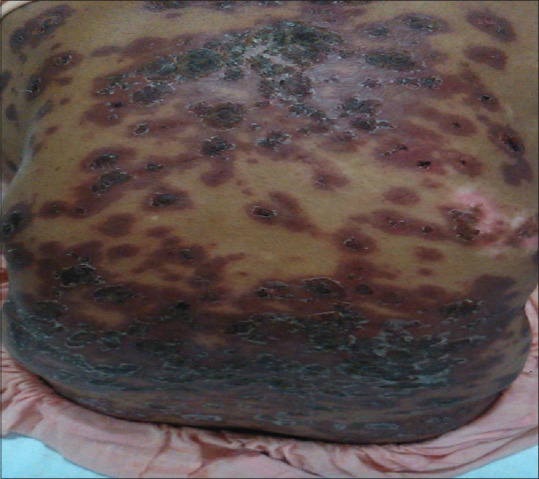
Necrotic and crusted erythematous plaques with peripheral scaling on the entire back
Figure 3.
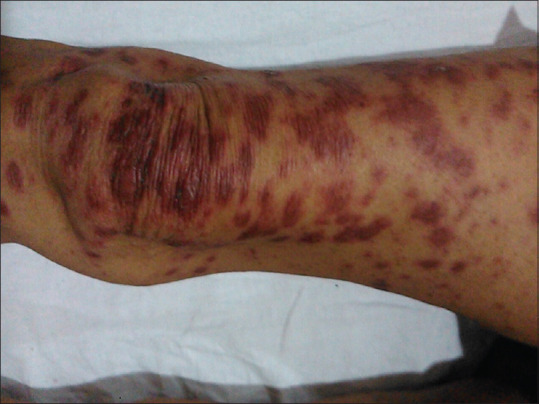
Targetoid and purpuric lesions on the right leg with blistering over knee
Figure 4.
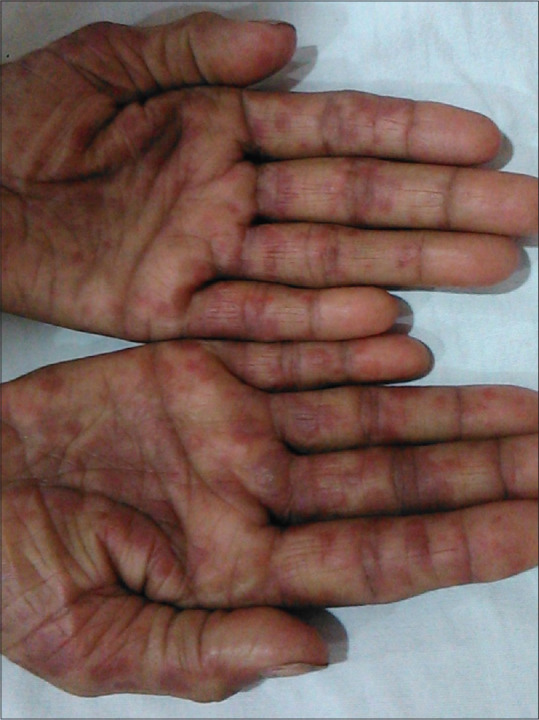
Erythema multiforme-like lesions on palms
Esomeprazole was stopped and she was treated with oral prednisolone for 2 weeks (0.5 mg/kg/day) resulting in improvement of skin lesions in 3 weeks. Anti-tubercular therapy was continued without recurrence of the lesions. Naranjo causality scale concluded esomeprazole as a probable cause of adverse effect.
Case 3
A 55-year-old male, a known case of discoid lupus erythematosus since last 2 years, presented with a widespread rash over trunk for 1 month. His medications included hydroxychloroquine (HCQS), levocetirizine, and esomeprazole that he was taking for past 8 weeks for duodenitis. Examination revealed multiple annular polycyclic plaques over the entire trunk, extending to proximal upper and lower limbs. Systemic examination was normal. Investigation results are tabulated in Table 2. Esomeprazole was not discontinued, and the patient continued to have persistent lesions despite methylprednisolone and HCQS treatment. He was subsequently lost to follow-up. Naranjo causality assessment yielded a probable association with esomeprazole.
Case 4
A 62-year-old female presented with photosensitivity and erythematous rash over scalp, face, trunk, and limbs for last 3 weeks. Her medications were aspirin and nebivolol for hypertension for 2 years and esomeprazole for gastroesophageal reflux for last 2 months. Cutaneous examination revealed multiple erythematous annular and scaly plaques over face, trunk, and proximal extremities. The patient was investigated and the results are tabulated in Table 2. Esomeprazole was discontinued, and treatment with oral and topical corticosteroids resulted in complete resolution of skin lesions over 3 weeks. Naranjo causality scale revealed a probable association with esomeprazole.
Case 5
A 62-year-old male with rheumatoid arthritis (RA), hypertension, and ischemic heart disease presented with erythematous scaly rash over body for last 2 weeks. On examination, there were annular erythematous plaques with peripheral scaling on scalp, neck, trunk, bilateral upper and lower limbs [Figure 5]. His medications included methotrexate, nitroglycerine, aspirin, HCQS, acebrophylline, montelukast, levocetirizine, amiodarone, levosulpiride and rabeprazole. Relevant investigations were done [Table 2]. He improved over 6 weeks with oral and topical steroids and discontinuation of rabeprazole, while other medications were continued. Inadvertent exposure to esomeprazole resulted in a recurrence of annular scaly plaques of SCLE over trunk and lower limbs. PPIs were stopped and histamine-2 receptor (H2) blockers were prescribed for reflux symptoms. Naranjo causality assessment revealed a definite association of PPIs with SCLE in this case.
Figure 5.
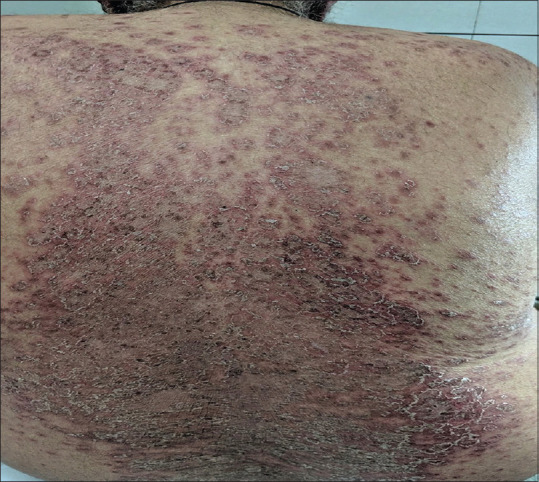
Confluent, annular, and polycyclic erythematous scaly plaques over back
Case 6
A 53-year-old male, a known case of liver cirrhosis and portal hypertension, got admitted with mild pain abdomen and skin rash for 1 month. His regular medications were pantoprazole, spironolactone, torsemide, and rifaximin. Systemic examination revealed ascites and pleural effusion. Cutaneous examination showed erythematous, annular, symmetrical, polycyclic plaques with peripheral pigmentation and scaling over trunk and proximal limbs, sparing face, palms, and soles. Hemogram showed thrombocytopenia (platelet count- 13 × 103/μL) with normal renal and liver function tests. The immunological findings are explained in Table 2. Skin biopsy showed hyperkeratosis and follicular plugging of epidermis, prominent vacuolization of basal cell layer, and perivascular and periadnexal lymphocytic infiltrate in dermis [Figure 6a and b]. Naranjo causality scale concluded a probable causal association with pantoprazole. Pantoprazole was stopped and a short course of oral corticosteroids was given, to which the patient responded well.
Figure 6.
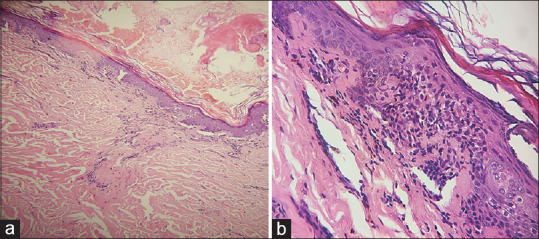
(a) Skin biopsy shows hyperkeratosis, mild atrophy, perivascular and periadnexal chronic inflammatory infiltrate (H and E, ×100). (b) Prominent vacuolization of basal cell layer of epidermis (H and E, ×400)
Case 7
A 60-year-old male with SLE for last 6 years presented with scaly rash over back for 2 weeks. His medications were low-dose prednisolone, azathioprine, HCQS, and pantoprazole for gastroesophageal reflux disease (GERD). Systemic examination was normal. Cutaneous examination revealed multiple brightly erythematous, annular plaques distributed prominently over trunk, proximal upper and lower limbs. Erythematous plaques studded with coalescing pustules over bilateral thenar eminences were noted. Mucosal ulceration was evident over lower lip. The investigations performed are tabulated in Table 2. Pantoprazole was withdrawn and corticosteroids dose was increased, while azathioprine and HCQSs were continued. Complete remission was observed over next 4 weeks. Naranjo causality scale concluded a probable causal association with pantoprazole.
Results
There were four (57%) males and three (43%) females with M: F ratio of 4:3. The mean age overall was 60.2 ± 5.5 years (range 53-70 years), while the mean age for females was 64 ± 5.3 years and for males was 57.5 ± 4.2 years and this difference was not statistically significant [Table 1].
A total of nine (including two recurrences) episodes of PPI-induced SCLE were observed in seven patients. In the initial episodes of SCLE, esomeprazole was responsible for four (57%), pantoprazole for two (28.50%), and rabeprazole for one (14.50%) episode. Two recurrent episodes of SCLE were recorded even on reintroducing different PPIs (case number 1 and 5) [Table 1].
The latency period (from the initiation of PPI to the onset of SCLE) ranged from 14 days to 1 year (mean 11.4 ± 16.2 weeks). The following autoimmune comorbidities were reported: SLE, discoid lupus erythematosus (DLE), and RA in one patient each [Table 1].
The morphology and distribution of skin lesions was as follows: annular plaques in six (85.70%) and papulosquamous lesions in one (14.30%); the lesions were widespread (affecting face, trunk, and extremities) in five (71.50%) patients, while two (28.50%) had limited involvement of trunk and extremities [Table 2].
Skin biopsy was performed in all seven (100%) patients. These included epidermal changes like epidermal atrophy in four (57%), hyperkeratosis in five (71.4%), and spongiosis in one (14.30%), while intact intraepidermal bulla was observed in one (14.%) case. Interface dermatitis, vacuolar degeneration of basal layer, and a perivascular/periappendageal dermal lymphocytic infiltrate were seen in all the biopsy specimens. Two biopsies (28%) were showing variable degree of keratinocyte necrosis. Six (85.7%) biopsy reports were reported as consistent with SCLE/cutaneous lupus erythematosus; however, one (14.3%) had EM-like findings. Direct immunofluorescence (DIF) performed on perilesional skin biopsy in three patients failed to reveal deposits in any of these [Table 2].
Antinuclear antibodies (ANA) were positive in all seven (100%) patients; however, five (71.4%) patients had rapid screening enzyme-linked immunosorbent assay (ELISA), while ANA by immunofluorescence (IF) was done in two (28.6%) patients. All (100%) patients were positive for anti-SSA/Ro antibodies and four (57.1%) for anti-SSB/La. Anti-histone antibodies and anti-ds DNA were negative in all patients [Table 2].
Naranjo probability scale for causality assessment was used in all seven (100%) cases, which found the culprit drug to the definite cause in two patients and probable in five cases. Treatments included drug withdrawal in six (85.70%) patients, topical steroids in one (14.30%), systemic corticosteroids in all seven (100%), and HCQS in one (14.30%) patient [Table 1]. Complete remission was seen in six (85.70%) cases, while one (14.30%) had partial remission [Table 2].
Discussion
SCLE is a specific subset of SLE which may be idiopathic or drug induced. In approximately 30% of patients of SCLE, drugs may aggravate or induce their disease.[6] DI-SCLE is probably not uncommon, but is likely to be unrecognized. PPIs are frequently prescribed drugs and generally well tolerated. However, different adverse skin reactions due to PPIs, such as dermatitis, lichen planus, urticaria, angioedema, toxic epidermal necrolysis, and SCLE, can occur.[7,10] PPI-induced SCLE has been reported with omeprazole, lansoprazole, pantoprazole, and esomeprazole.[7]
PPI-induced SCLE was first reported with lansoprazole in 2004.[11] Since then, several case reports and case series of PPI-induced SCLE have appeared in literature.[7,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26] PubMed search (limited to English literature) revealed 43 cases of PPI-induced SCLE, comprising 12 case reports of single patients and five reports of two or more patients.[7,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26] A summary of these five case series compared to current series is tabulated in Table 3.
Table 3.
Summary of case series of patients with PPI-induced SCLE
| Author, year | Bracke et al.[11] 2005 | Dam and Bygum[12] 2008 | Tom Whittle et al.[17] 2011 | Almebayadh et al.[21] 2013 | Sandholdt et al.[7] 2014 | Present series | |
|---|---|---|---|---|---|---|---|
| No. of patients | 2 | 5 | 2 | 3 | 19 | 7 | |
| Sex | Male | 0 | 1 | 0 | 1 | 2 | 4 |
| Female | 2 | 4 | 2 | 2 | 17 | 3 | |
| Age (years) | Range | 63-69 | 50-63 | 78-85 | 30-57 | 28-86 | 53-70 |
| Average | 66 | 56.4 | 81.5 | 39.3 | 61 | 60.2 | |
| Latency period | Range | 3-5 months | 4-8 weeks | 3 months | 7 weeks to several weeks | 1 week-3.5 years | 2 weeks-1 year |
| Average | 4 months | 31.4 days | 3 months | 8 months | 11.4 weeks | ||
| PPI associated | Pantoprazole | 0 | 2 | 0 | 1 | 1 | 2 |
| Omeprazole | 0 | 1 | 2 | 1 | 5 | 0 | |
| Esomeprazole | 0 | 0 | 0 | 1 | 4 | 4 | |
| Lansoprazole | 2 | 2 | 0 | 0 | 9 | 0 | |
| Rabeprazole | 0 | 0 | 0 | 0 | 0 | 1 | |
| Relapse episodes | Nil | Nil | Nil | 1 (pantoprazole) | 5 episodes* | 2 episodes** | |
| Skin biopsy findings | SCLE | 2 | 3 | 2 | 3 | 11 | 6 |
| EM like | 0 | 1 | 0 | 0 | 3 | 1 | |
| Not done | 0 | 0 | 0 | 0 | 5 | 0 | |
| DIF (skin biopsy) | Positive | 1 | 2 | 0 | 1 | 4 | 0 |
| Negative | 0 | 2 | 2 | 2 | 7 | 3 | |
| Not done | 1 | 1 | 0 | 0 | 12 | 4 | |
| Antibodies | ANA | 2 | 5 | 2 | 3 | 11.61% | 7 |
| Anti-Ro | 2 | 4 | 2 | 3 | 13.73% | 7 | |
| Anti-La | 0 | 0 | 1 | 1 | 6.33% | 4 | |
| Others | 1 | 4 | 0 | 2 | 1.8% | 0 | |
| Naranjo scale | Definite | 0 | 0 | 0 | 0 | 3 | 2 |
| Probable | 0 | 0 | 0 | 0 | 14 | 5 | |
| Possible | 0 | 0 | 0 | 0 | 2 | 0 | |
| Not done | 2 | 5 | 2 | 3 | 0 | 0 | |
| Outcome | Complete remission | 2 | 3 | 2 | 3 | 14 | 6 |
| Partial remission | 0 | 0 | 0 | 0 | 2 | 1 | |
| Death | 0 | 2 | 0 | 0 | 3 | 0 | |
DIF=Direct immunofluorescence, EM=Erythema multiforme, PPI=Proton pump inhibitor, SCLE=Subcutaneous lupus erythematosus. *Five relapse episodes, one each from pantoprazole and omeprazole and three episodes from lansoprazole. **Two relapse episodes, one each from pantoprazole and esomeprazole
In a Swedish case-control study of 234 patients with SCLE, 65 had received PPIs and the authors observed an increased odds ratio of 2.9 for PPIs.[6] Sanholdt et al.,[7] in 2014, published the largest case series of 19 patients with 24 episodes of PPI-induced SCLE and also observed cross reactivity between different PPIs. Laurinaviciene et al.[8] identified PPIs as one of the most common culprit drugs for DI-SCLE. A pharmacovigilance analysis of united states food and drug administration (USFDA) adverse event reporting system database identified 120 instances of PPI-associated SCLE over a period of 2 years. The study also found statistically significant association of PPIs with SCLE.[9] Cases of PPI-induced SCLE relative to other medications have shown increase by 34.1% in the last decade.[27] The authors discuss that one of the factors responsible for such a shift could be an increasing popularity and availability of PPIs in USA.[27]
In the current analysis, the mean age of onset of PPI-induced SCLE was 60.2 ± 5.5 years, which is similar to 61 years reported by Sanholdt[7] and 58 years reported by Lowe.[2] This is in contrast to the age of onset of 67 years in DI-SCLE observed by Marzano, but none of the patients in that study had received PPIs.[5] There is male preponderance in this review in contrast to the females as reported by others.[2,7]
The mean latency period from the initiation of PPI to the onset of SCLE ranged from 2 weeks to 1 year (mean 11.4 ± 16.2 weeks), similar to that reported by Sanholdt.[7] However, a latency period from 3 days to 11 years (mean 27.9 weeks) has been reported by Lowe et al.[2]
Nine episodes of PPI-induced SCLE were observed in the current study. Esomeprazole was the culprit drug in four, pantoprazole in two, and rabeprazole in one patient. Two patients developed recurrent episodes from different PPIs. In the largest case series of PPI-induced SCLE from Denmark, the authors reported lansoprazole (nine) to be the commonest PPI, followed by omeprazole (five), esomeprazole (four), and pantoprazole (one).[7] Five episodes of relapse of SCLE, one each from pantoprazole and omeprazole and three from lansoprazole, were noted. Rabeprazole has not been previously reported to cause DI-SCLE.
In the current analysis, five patients (71.4%) had widespread confluent lesions over face, trunk, and extremities. Marzano et al.[5] have also emphasized that compared to idiopathic SCLE, patients with DI-SCLE tended to have cutaneous lesions that were more widespread with predilection of face and legs and with bullous, targetoid, and vasculitic morphology. Sanholdt also described lesions in DI-SCLE to be more widespread and inflammatory. Two of our patients had targetoid EM-like lesions on palms and soles and purpuric vasculitic lesions on legs. This has previously been emphasized by Marzano and colleagues.[5]
Both idiopathic and DI-SCLE are indistinguishable on skin biopsy, as both show interface dermatitis/lichenoid tissue reaction.[2,5] Sanholdt, in addition, described EM-like histopathology in two patients.[7] EM-like histopathology was reported in one patient in our series. Immunologically, DI- and idiopathic SCLE show similar presence of granular deposition of IgM, IgG, and C3 in a linear band-like array along the dermo-epidermal junction.[5] DIF was negative in all three patients tested.
DI-SCLE is characterized by ANA (>80% patients) and anti-Ro/SSA (about 80%) antibodies.[2] All the seven patients in our series had positive ANA. In the current review, anti Ro/SSA positivity was found in 100% cases, while anti-La/SSB was positive in 71.50% cases. Sanholdt et al.[7] reported positive ANA in 61% cases, with speckled pattern being the commonest. Positive anti Ro/SSA was found in 73% and anti-La/SSB in 33%, and anti dsDNA and anti-histone antibodies in one patient each, among those tested in their study.[7]
Anti-histone antibodies, which are important in diagnosing DI-SLE, are found less commonly in DI-SCLE.[28] In DI-SCLE, anti-histone antibody varied from 2% to 33%.[2,5] Even in the current analysis, anti-histone antibodies were not detected in any patient. Therefore, anti-histone antibodies may not be helpful in diagnosis of DI-SCLE.[11,28] The autoantibodies may remain detectable even after resolution of skin lesions and were also observed in one patient.[28]
The diagnosis of DI- or PPI-induced SCLE is difficult. Quite often, patients are on multiple drugs known to cause SCLE. Decision regarding which drugs to discontinue in the setting of DI-SCLE should ideally be based on a drug attributability algorithm, for example, Naranjo probability scale.[29] However, the wide range of lag periods between the start of a drug and onset of SCLE lesions dilutes the value of drug history in such an algorithm.[28] In such cases, the idealistic approach is to discontinue all the drugs. If this is not possible, only the essential drugs should be continued with careful monitoring.
In two cases of our series (cases 1 and 5), inadvertent reintroduction of PPI resulted in recurrence of the symptoms and signs, confirming the diagnosis of PPI-induced SCLE. Although no rechallenge was performed in the other five cases, temporal relation between the use of drugs and the onset of disease, clinical presentation characteristic of SCLE, presence of anti-Ro and anti-La antibodies, and complete clearance of the skin lesions after discontinuation of culprit PPI suggested the diagnosis of PPI-induced SCLE. In the Danish study, four patients had multiple episodes of PPI-induced SCLE, and in three of these, they were due to a different PPI.[7] They suggested a class effect, that an identical feature in different PPIs is responsible for the adverse effect. They further suggested that in patients who develop an episode of DI-SCLE from a PPI, all PPIs should be avoided in future. Also, PPI should be used with caution in patients with known cutaneous lupus erythematosus, photosensitivity, and connective tissue disorders, especially Sjogren's syndrome.[7]
When treatment is indicated, topical and systemic corticosteroids, topical tacrolimus, HCQS, and a combination of these agents are useful.[2,7] Following discontinuation of the causative agent, lesions of DI-SCLE generally resolve over several weeks. Since there is a cross reactivity, inadvertent exposure to another PPI may trigger a relapse of SCLE.
There are no case reports on PPIs inducing SCLE from India; however, there is a report on pantoprazole-induced SLE in a 29-year-old female. She developed skin lesions along with oral ulcers, and joint pain without any systemic involvement, and was diagnosed as PPI-induced SLE.[30]
One of the hypothesized mechanisms of DI-SCLE could be the drug inducing a photosensitivity state, which is a common feature of many drugs involved in DI-SCLE. This can be followed by the induction of SCLE-like skin lesions via an isomorphic response in an immunogenetically predisposed individual.[2] Additional trigger factors for the process could be ultraviolet radiation, smoking, photosensitizing chemicals, and infections, along with an autoimmune response with high titres of anti-Ro/SSA autoantibodies.[31]
Small number of patients and a retrospective nature are the major limitations of this analysis. A high index of suspicion for diagnosis of DI-SCLE and further studies can help better characterization of the condition.
In conclusion, PPIs can be associated with SCLE. Widespread distribution and morphological characteristics like purpuric vasculitic and EM-like lesions should raise suspicion for drug-induced etiology for the skin condition. Withdrawal of the offending drug with or without topical or systemic corticosteroids generally leads to complete resolution of symptoms in majority of the patients. Prescription of PPI should be avoided in these patients to avoid recurrent episodes, and H2 blockers can be substituted instead.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sontheimer RD, Thomas JR, Gilliam JN. Subacute cutaneous lupus erythematosus: A cutaneous marker for a distinct lupus erythematosus subset. Arch Dermatol. 1979;115:1409–15. [PubMed] [Google Scholar]
- 2.Lowe G, Henderson CL, Grau RH, Hansen CB, Sontheimer RD. A systematic review of drug-induced subacute cutaneous lupus erythematosus. Br J Dermatol. 2011;164:465–72. doi: 10.1111/j.1365-2133.2010.10110.x. [DOI] [PubMed] [Google Scholar]
- 3.Reed BR, Huff JC, Jones SK, Orton PW, Lee LA, Norris DA. Subacute cutaneous lupus erythematosus associated with hydrochlorothiazide therapy. Ann Intern Med. 1985;103:49–51. doi: 10.7326/0003-4819-103-1-49. [DOI] [PubMed] [Google Scholar]
- 4.Marzano AV, Vezzoli P, Crosti C. Drug-induced lupus: An update on its dermatologic aspects. Lupus. 2009;18:935–40. doi: 10.1177/0961203309106176. [DOI] [PubMed] [Google Scholar]
- 5.Marzano AV, Lazzari R, Polloni I, Crosti C, Fabbri P, Cugno M. Drug-induced subacute cutaneous lupus erythematosus: Evidence for differences from its idiopathic counterpart. Br J Dermatol. 2011;165:335–41. doi: 10.1111/j.1365-2133.2011.10397.x. [DOI] [PubMed] [Google Scholar]
- 6.Gronhagen CM, Fored CM, Linder M, Granath F, Nyberg F. Subacute cutaneous lupus erythematosus and its association with drugs: A population-based matched case-control study of 234 patients in Sweden. Br J Dermatol. 2012;167:227–8. doi: 10.1111/j.1365-2133.2012.10969.x. [DOI] [PubMed] [Google Scholar]
- 7.Sandholdt LH, Laurinaviciene R, Bygum A. Proton pump inhibitor-induced subacute cutaneous lupus erythematosus. Br J Dermatol. 2014;170:342–51. doi: 10.1111/bjd.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laurinaviciene R, Sandholdt LH, Bygum A. Drug-induced cutaneous lupus erythematosus: 88 new cases. Eur J Dermatol. 2017;27:28–33. doi: 10.1684/ejd.2016.2912. [DOI] [PubMed] [Google Scholar]
- 9.Aggarwal N. Drug induced subacute cutaneous lupus erythematosus associated with proton pump inhibitors. Drugs- Real World Outcomes. 2016;3:145–54. doi: 10.1007/s40801-016-0067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang YS. Hypersensitivity reactions to proton pump inhibitors. Curr Opin Allergy Immunol. 2012;12:348–53. doi: 10.1097/ACI.0b013e328355b8d3. [DOI] [PubMed] [Google Scholar]
- 11.Bracke A, Nijsten T, Vandermaesen J, Lambert J. Lansoprazole induced subacute cutaneous lupus erythematosus: Two cases. Acta Derm Venereol. 2005;85:353–4. doi: 10.1080/00015550510026668. [DOI] [PubMed] [Google Scholar]
- 12.Dam C, Bygum A. Subacute cutaneous lupus erythematosus induced or exacerbated by proton pump inhibitors. Acta Derm Venereol. 2008;88:87–9. doi: 10.2340/00015555-0335. [DOI] [PubMed] [Google Scholar]
- 13.Popovic K, Wahren-Herlenius M, Nyberg F. Clinical follow-up of 102 anti-Ro/SSA-positive patients with dermatological manifestations. Acta Derm Venereol. 2008;88:370–5. doi: 10.2340/00015555-0473. [DOI] [PubMed] [Google Scholar]
- 14.Panting KJ, Pinto M, Ellison J. Lansoprazole-induced subacute cutaneous lupus erythematosus. Clin Exp Dermatol. 2009;34:731–4. doi: 10.1111/j.1365-2230.2008.03105.x. [DOI] [PubMed] [Google Scholar]
- 15.Mankia SK, Rytina E, Burrows NP. Omeprazole-induced subacute cutaneous lupus erythematosus. Clin Exp Dermatol. 2010;35:e1–2. doi: 10.1111/j.1365-2230.2008.03125.x. [DOI] [PubMed] [Google Scholar]
- 16.McCourt C, Somerville J, McKenna K. Anti-Ro and anti-la antibody positive subacute cutaneous lupus erythematosus (SCLE) induced by lansoprazole. Eur J Dermatol. 2010;20:860–1. doi: 10.1684/ejd.2010.1112. [DOI] [PubMed] [Google Scholar]
- 17.Toms-Whittle LM, John LH, Buckley DA. Drug-induced subacute cutaneous lupus erythematosus associated with omeprazole. Clin Exp Dermatol. 2011;36:281–3. doi: 10.1111/j.1365-2230.2010.03926.x. [DOI] [PubMed] [Google Scholar]
- 18.Alcántara-González J, Truchuelo-Díez MT, González-García C, Jaén Olasolo P. Esomeprazole-induced subacute cutaneous lupus erythematosus. Actas Dermosifiliogr. 2011;102:640–2. doi: 10.1016/j.ad.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Reich A, Maj J. Subacute cutaneous lupus erythematosus due to proton pump inhibitor intake: Case report and literature review. Arch Med Sci. 2012;8:743–7. doi: 10.5114/aoms.2012.30300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wee JS, Natkunarajah J, Marsden RA. A difficult diagnosis: Drug-induced subacute cutaneous lupus erythematosus (SCLE) triggered by omeprazole in a patient with pre-existing idiopathic SCLE. Clin Exp Dermatol. 2012;37:445–6. doi: 10.1111/j.1365-2230.2011.04245.x. [DOI] [PubMed] [Google Scholar]
- 21.Almebayadh M, Regnier-Rosencher E, Carlotti A, Goulvestre C, Le Guem V, Mouthon L, et al. Subacute cutaneous lupus erythematosus induced and exacerbated by proton pump inhibitors. Dermatology. 2013;226:119–23. doi: 10.1159/000346694. [DOI] [PubMed] [Google Scholar]
- 22.Jones EK, Mingioni N, Lee JB. Widespread scaly eruption in a patient with multiple comorbidities. JAMA. 2015;314:1740–1. doi: 10.1001/jama.2015.9391. [DOI] [PubMed] [Google Scholar]
- 23.Hung R, Sangle SR, Benton E, D’Cruz DP, McGibbon D. Proton pump inhibitor-induced subcutaneous lupus erythematosus in a patient with systemic lupus erythematosus. Clin Exp Dermatol. 2015;40:808–9. doi: 10.1111/ced.12592. [DOI] [PubMed] [Google Scholar]
- 24.Drago F, Javor S, Ciccarese G, Cozzani E, Parodi A. Subacute cutaneous lupus erythematosus induced by lansoprazole. Eur J Clin Pharmacol. 2015;71:767–8. doi: 10.1007/s00228-015-1840-0. [DOI] [PubMed] [Google Scholar]
- 25.An I, Demir V, Ibiloğlu İ, Akdeniz S, Ucmak D. Lansoprazole-induced subacute cutaneous lupus erythematosus. Arch Rheumatol. 2017;32:179–80. doi: 10.5606/ArchRheumatol.2017.6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall E, Peravali R, Patel TS. Omeprazole induced subacute cutaneous lupus erythematosus. JAMA Dermatol. 2020;156:1013–4. doi: 10.1001/jamadermatol.2020.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michaelis TC, Sontheimer RD, Lowe GC. An update in drug-induced subacute cutaneous lupus erythematosus. Dermatol Online J. 2017;23 doi: 10.5070/D3233034281. [PubMed] [Google Scholar]
- 28.Sontheimer RD, Henderson CL, Grau RH. Drug-induced subacute cutaneous lupus erythematosus: A paradigm for bedside-to-bench patient-oriented translational clinical investigation. Arch Dermatol Res. 2009;301:65–70. doi: 10.1007/s00403-008-0890-x. [DOI] [PubMed] [Google Scholar]
- 29.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 30.Chinnusamy M, Viswanathan RA, Janakiraman S, Elayidath R. Drug-induced lupus erythematosus associated with proton pump inhibitor. J Health Allied Sci NU. 2020;10:132–4. [Google Scholar]
- 31.Zandman-Goddard G, Solomon M, Rosman Z, Goulvestre C, Le Guern V, Mouthon L, et al. Environment and lupus-related diseases. Lupus. 2012;21:241–50. doi: 10.1177/0961203311426568. [DOI] [PubMed] [Google Scholar]


