Abstract
Patients on Cancer chemotherapeutic agents often develop nail changes most of which are only cosmetic concern and disappear on drug withdrawal. But some nail changes can be painful and disabling thereby affecting quality of life substantially. Different components of the nail unit include the nail matrix, nail bed, nail plate, the hyponychium, lunula, the proximal and lateral nail folds. In this article we review the nail changes induced by chemotherapeutics and targeted anticancer drugs, preventive measures and treatment options available.
Keywords: Anticancer drugs, chemotherapeutic agents, nail changes, nail toxicity, targeted therapies
Nail covers the distal phalanx and the continuously dividing matrix cells generate the nail plate. Cancer chemotherapeutic agents may lead to nail changes as they affect these rapidly dividing nail matrix cells.[1] Nail changes have been reported to be the most common mucocutaneous adverse reactions of cancer chemotherapy and chemoradiation.[2] The nail changes are usually but not always transient and disappear on drug withdrawal. It may affect all the nails or some and shows a temporal relationship to drug intake. Different components of the nail unit include the nail matrix, nail bed, nail plate, the hyponychium, lunula, the proximal, and lateral nail folds. Presentation depends on the nail structure affected and severity of the insult.[3] Some of the nail changes are only a cosmetic concern while others may be symptomatic leading to severe pain. Usually several nails are involved and mostly fingernails are involved more commonly than toe nails except for some changes which are aggravated by pressure and trauma which are commonly observed on big toenail.
Pathophysiology
Finger nails grow at an average rate of 0·1 mm per day (3 mm per month) taking 4 to 6 months for complete regrowth and toenails at 0·03 mm per day (1 mm per month) taking 12 to 18 months for toenails. The nail changes are a past event relative to drug initiation and disappearance of inflicted changes does not coincide with drug interruption.
The clinical presentation of chemotherapeutic induced nail changes depends on the component of nail unit involved and the duration and severity of toxicity.
Pathogenesis of nail changes depending on part of nail unit affected is as follows:
Nail matrix: nail matrix is highly susceptible to damage from chemotherapeutic agents as it is formed of proliferating cells leading to defective nail plate production. Typical nail matrix changes include melanonychia, leukonychia, Beau's lines, and onychomadesis, and melanonychia due to arrest in epithelial proliferation or leukonychia due to abnormal keratinization.
Melanonychia: It refers to brownish or black pigmented bands in nail which can be transverse, longitudinal, or diffuse and mostly appear 1 to 2 months after chemotherapy initiation. Melanonychia occurs due to melanocyte activation in matrix epithelium.[3,4,5] Studies have reported diffuse hyperpigmentation of nails to be most common side effects.[6] Alternating bands of normal and hyperpigmented bands corresponding to the chemotherapy cycles may be seen[7] [Figure 1a] or alternating dark and white bands may also be seen corresponding to intermittent administration of drug [Figure 1b]. The pigmentary change is mostly reversible few months after stoppage of chemotherapeutic agent.
Figure 1.

(a) Alternating dark and normal bands in patient on docetaxel; (b) Alternating dark and white bands on toenail in patient on daunorubicin
Leukonychia: It refers to white coloration of the nail plate which can be a true or apparent leukonychia. True leukonychia develops due to abnormal keratinization of the nail matrix [Figure 2] and apparent leukonychia develops due to abnormalities of the blood flow in the nail bed. Apparent leukonychia can present as half-and-half nail, Muehrcke's nails, or Terry's nails.
Figure 2.
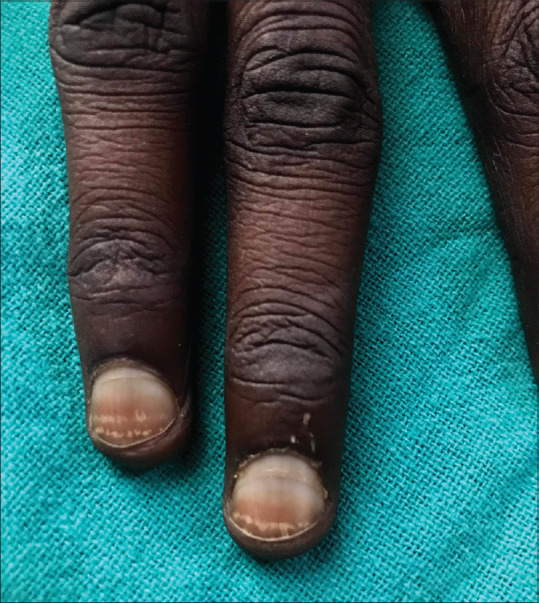
Transverse leuconychia bands corresponding to intermittent chemotherapy
Beau's lines: Beau's lines are depressed linear bands on the dorsum of the nail plate that form due to arrest in mitotic activity of the nail matrix. The distance between Beau's line and proximal nail fold can guide about when the acute arrest in nail proliferation occurred and its depth can guide about the severity of insult. Onychomadesis is its most severe form in which a deep sulcus divides the nail plate into two parts [Figure 3].[7] Repeated chemotherapy cycles can lead to multiple beau's line on all/multiple nails.
Figure 3.
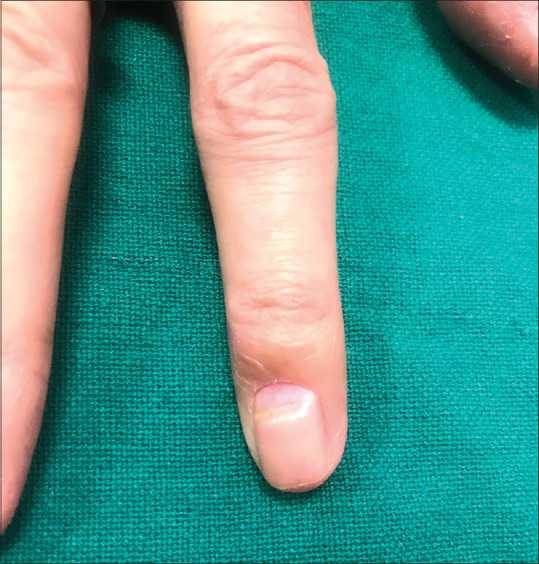
Onychomadesis
Nail bed: Nail bed epithelium is responsible for adhesion of the nail plate to the underlying structures. Onycholysis refers to separation of nail plate from the nail bed. It results due to damage by toxins to the nail bed leading to nail plate detachment.[3,8]
Nail fold: Some drugs may interfere with proximal nail fold integrity exposing the nail matrix, leading to disordered nail growth. It may lead to granulation tissue formation with bleeding. It may manifest as paronychia or pyogenic granuloma like lesion [Figures 4a and 4b]. Its pathogenesis is largely unknown. It may be formed because of activation of angiogenic factors by the drugs.[4,8,9] The periungual lesions are initially sterile but bacterial infections (and rarely candida infection) may develop leading to purulent discharge. Pyogenic granuloma-like lesions are dose dependent, thereby regressing on dose reduction or treatment interruption, but in some patients may take upto several months to heal completely.
Figure 4.

(a) Proximal nail fold paronychia with pyogenic granuloma like lesions secondary to Gefitinib; (b) Granulation tissue at lateral nail fold with EGFR inhibitors
Vessels: Damage to the vascular network of nail bed may lead to splinter hemorrhages and subungual hematoma.[9] Splinter hemorrhages are more commonly observed in fingernails whereas hematomas are more common in toes and occur due to trauma. Thrombocytopenia secondary to cancer chemotherapy may also lead to the above changes.[3,5] Cancer chemotherapeutics may also lead to ischemic changes like Raynaud's phenomenon to digital gangrene.[4]
Nail bed and nail plate changes are observed to occur more commonly with cytotoxic chemotherapeutic agents than targeted anticancer therapies, whereas periungual lesions and paronychia are observed more with targeted anticancer therapies.
Grading of nail toxicity
The nail toxicities are classified using the NCI Common Terminology Criteria for Adverse Events version 4.03 (CTCAE v4.03) which includes five nail changes: paronychia, nail loss, nail ridging, nail discoloration, and nail infection.[10] Although this classification takes into account the impact on quality of life (QOL), it ignores some of the important chemotherapeutic agent induced nail changes, like painful nail bed hemorrhages. The Multinational Association of Supportive Care in Cancer Skin Toxicity Study Group [Table 1] has proposed grading scale for nail changes due to epidermal growth factor receptor (EGFR) inhibitors which takes into account the nail plate, nail folds, and digital tip changes.[11]
Table 1.
MASCC grading for nail toxicity due to EGFR inhibitors[10]
| Grade | Nail plate changes | Nail fold changes | Digit tip changes |
|---|---|---|---|
| Grade 1 | Onycholysis or ridging without pain | Disruption or absence of cuticle | Xerosis and/or erythema without pain |
| Orerythema | |||
| Grade 2 | Onycholysis with mild/moderate pain; | Erythematous/tender/painful nail fold changes | Xerosis and/or erythema with mild/moderate pain, stinging or fingertip fissures |
| Orpyogenic granuloma or crusted lesions | |||
| Any lesion interfering with instrumental ADL | |||
| Grade 3 | Any changes interfering with self-care ADL |
ADL- activities of daily living
Each nail changes observed with different chemotherapeutic agents [Table 2] are summarized in table below.
Table 2.
| Nail changes | Drug implicated | Remarks |
|---|---|---|
| Nail matrix changes | ||
| Melanonychia | Cyclophosphamide | Diffuse black pigmentation, longitudinal melanonychia, and dark grey pigmentation of the proximal part of the nail plate |
| Doxorubicin | Alternating bands of dark brown and white lines and dark brown pigmentation in transverse bands | |
| Hydroxycarbamide (hydroxyurea) | Distal, diffuse dark brown pigmentation | |
| Taxanes | Orange discoloration due to hamorrhage in nail bed | |
| Busulfan, capecitabine, cisplatin, and bleomycin | ||
| Imatinib | Longitudinal, transverse, or diffuse melanonychia | |
| True leukonychia | Doxorubicin, cyclophosphamide, and vincristine | |
| Beau's lines | Almost all cytotoxic agents | |
| Nail bed changes | ||
| Onycholysis | Capecitabine, etoposide, mitoxantrone, or doxorubicin, targeted therapies (anti EGFR, MEK, and mTOR inhibitors) | |
| Taxanes | Haemorrhagic onycholysis, may be associated with paronychia | |
| Anti-EGFR and MEK inhibitors | ||
| Nail fold changes | ||
| Paronychia | Anti-EGFR inhibitors, MEK inhibitors, mTOR inhibitors, capecitabine, methotrexate, and doxorubicin | |
| Vascular changes | ||
| Raynaud's phenomenon/digital gangrene | Bleomycin | May warrant drug cessation |
| Subungual splinter hemorhage | VEGR inhibitors |
Cytotoxic agents
Alkylating agents
Classical alkylating agents (like Cyclophosphamide, ifosfamide and thiotepa): nail changes secondary to classical alkylating changes include nail pigmentation, Beau's lines, and onycholysis.[12,13] Nail pigmentation can occur in all age groups after oral or intravenous (IV) medication and can affect both finger and toe nails. It may vary from horizontal or longitudinal streaks to diffuse pigmentation.[14]
Platinum agents (like Cisplatin, carboplatin, and oxaliplatin)
Leukonychia, Beau's lines, and diffuse hyperpigmentation have been reported after cisplatin chemotherapy.[15] Pus discharge, onycholysis, and nail plate discoloration has been reported with weekly paclitaxel and carboplatin[16] and transverse leukonychia affecting fingernails described in a patient receiving paclitaxel and carboplatin on a 3-week basis.[17]
Antimetabolites
Pemetrexed: Report of longitudinal melanonychia after 2 months of treatment with pemetrexed which further evolved into complex pattern of nail pigmentation as well as onycholysis after further 3 months has been reported in a patient with non-small cell lung cancer.[18]
5-fluorouracil: Nail changes attributed to fluorouracil include Onycholysis, nail plate dystrophy, onychomadesis, transverse bands, paronychial pain and inflammation, and hyperpigmentation [Figure 5].[13,15]
Figure 5.

Longitudinal melanonychia in patient on 5 fluorouracil. Also note serpentine pigmentation on forearm
Capecitabine: Onycholysis and onychomadesis are the nail toxicities described after capecitabine therapy.[19] Nail toxicities are observed in about 7% of cases with capecitabine. Sunset appearance of the nail with erythema and edema along with onycholysis has also been described.[20] Periungual inflammation, paronychia, and pyogenic granuloma can develop after capecitabine.[21]
Tegafur: There are reports of blackish discoloration of lunula of fingernails after 4 weeks of oral tegafur administration in a patient with colon cancer.[22]
Topoisomerase inhibitors
Anthracyclines
Daunorubicin: Transverse dark pigmented bands[23] [Figure 6a and b] and transverse leuconychia (Mee's lines)[24] of the fingernails and toenails have been reported following daunorubicin administration. Alternating dark and white bands may be seen corresponding to intermittent administration of Daunorubicin [Figure 1b].
Figure 6.

(a and b) Transverse melanonychia in patient of AML on cytarabin and daunorubicin
Doxorubicin: Beau's lines, longitudinal pigmented bands, onycholysis, nail loss, periungual inflammation, and periungual pyogenic granulomas have been described after doxorubicin.[25,26]
Etoposide: Nail bed pigmentation, Beau's lines, and onycholysis are some reported side effects of etoposide.[4,15]
Topotecan: Topotecan and irinotecan are effective chemotherapeutic drugs for treatment of solid tumors. Topotecan is given as IV infusion lasting five days every three weeks. In a case report, nail pigmentation was observed after topotecan.[27,28]
Antimitotic agents
Taxanes
Taxanes are one of the most common cancer chemotherapeutic agents causing nail toxicity. Most of the cases have been attributed to docetaxel after variable schedule like weekly, 3-weekly, and undefined schedules of administration or combination therapy. Minisini et al.[29] have reported an overall incidence of 44% for taxane-induced nail changes with some series reporting it as 89% with 3 treatment cycles. The nail toxicity may be severe enough to affect QOL, leading to treatment discontinuation.
Docetaxel: Various nail changes have been reported with docetaxel as shown in Table 3. Two hypotheses have been suggested to explain taxane-induced nail toxicity: neurogenic inflammation due to neuropeptides release or the effect of prostaglandins via sympathetic fibers. It was observed in a woman with breast cancer involving right braxial plexus treated with docetaxel that severe nail toxicity affected all nails except the nails of the right hand.[29] It was observed in a study that the nail toxicity improved with treatment of a cyclooxygenase-2 inhibitor, which favors the hypothesis of the role of prostaglandins.[30] The presence of nail toxicity was found to be strongly associated with the number of cycles given, weekly regimen, and its cumulative dose.[31] The cumulative hazard of developing nail toxicity increased above 10% after 2.8 months to upto 40% at 6 months.[32] Winther et al.[33] on studying the patients with metastatic breast cancer treated with docetaxel reported that 58.1% of patients with some nail toxicity increased to 88.5% in those receiving an additional three cycles of docetaxel. Although most of the patients had major cosmetic concerns with the nail changes, about 32% patients had functional difficulty interfering with daily activity. Because studies comparing weekly and three-weekly regimens did not show any difference in the incidence of nail toxicity, nail changes in the weekly treatment arm affected the QOL to the extent that it was the major reason for withdrawal from these studies.[33]
Table 3.
| Class of chemotherapeutic agent | Subclass/Drug | Nail changes |
|---|---|---|
| Alkylating agents | Cyclophosphamide, ifosfamide, and thiotepa | Nail pigmentation, Beau's lines, and onycholysis |
| Platinum agents: Cisplatin, carboplatin, and oxaliplatin | Leukonychia, Beau's lines, and diffuse hyperpigmentation, pus discharge, onycholysis, and nail plate discoloration | |
| Dacarbazine | Melanonychia | |
| Antimetabolites | Antifolate: Pemetrexed | Longitudinal melanonychia onycholysis |
| Antifolate: Methotrexate | Acute paronychia pigmented horizontal bands extensive onycholysis | |
| Fluropyrimidine: 5-fluorouracil | Onycholysis, nail plate dystrophy, onychomadesis, transverse bands, paronychial pain and inflammation, and hyperpigmentation | |
| Fluoropyrimidine: Capecitabine | Onycholysis and onychomadesis Periungual inflammation, paronychia, and pyogenic granuloma | |
| Fluoropyrimindine: Tegafur | Blackish discoloration of lunula | |
| Busulphan | Brownish tinge to the lunula and nail plate or inducing longitudinal nail bands | |
| Hydroxyurea | Pigmentation of the nails | |
| Topoisomerase inhibitors | Anthracyclines: Daunorubicin Doxorubicin | Transverse leuconychia (Mee's lines), Beau's lines, longitudinal pigmented bands, onycholysis, nail loss, periungual inflammation, and periungual pyogenic granulomas |
| Dactinomycin | Beau's lines | |
| Topotecan | Nail pigmentation | |
| Etoposide | Nail bed pigmentation, Beau's lines, and onycholysis, nail bed pigmentation, Beau's lines, and onycholysis | |
| Antimitotic agents | Taxanes | Onycholysis, transverse leukonychia, purulent discharge, acute paronychia, nail ridging, beau's lines, subungual hemorrhages, and hyperpigmentation of hyponychium |
| Docetaxel | ||
| Paclitaxel | ||
| Vincristine | Cause leukonychia, Beau's lines, Mees' lines, and onychodermal bands | |
| Etoposide | Nail bed pigmentation, Beau's lines, and onycholysis. | |
| BCR-ABL inhibitors | Imatinib | Hyperpigmentation, affecting all of the fingernails |
| Epidermal growth factor receptor inhibitors | Cetuximab, erlotinib, panitumumab, Gefitinib, and lapatinib | Paronychia, pyogenic granuloma like lesions, onycholysis, melanonychia, brittle nails, nail cracking, onychoschizia, and onychorrhexis |
| MEK inhibitors | Binimetinib | Paronychia, pyogenic granuloma like lesions, onychoschizia, onychorrhexis, brittle nails, nail cracking, and onycholysis |
| Cobimetinib | ||
| Trametinib | ||
| mTOR inhibitors | Everolimus | Paronychia, pyogenic granuloma like lesions, nail plate thinning, onychodystrophy, brittle nails, distal onycholysis, and diffuse yellow nail discoloration |
| Temsirolimus | ||
| VEGFR inhibitors | Sorafenib | Splinter subungual hemorrhages |
| Sunitinib | ||
| Pazopanib Bevacizumab | ||
| Miscellaneous agents | Bleomycin | Hyperpigmentation, onycholysis, Beau's lines, and raynaud's phenomenon |
Haemorrhagic onycholysis is the characteristic nail toxicity induced by taxanes observed more commonly with docetaxel than with paclitaxel.[36] Taxane-related onycholysis may at times be associated with inflammatory erythema of dorsum of hands, perimalleolar, and Achilles areas, known as periarticular thenar erythema with onycholysis (PATEO syndrome).[37] Docetaxel-induced finger and toenail changes can be reduced by using an Elasto-Gel frozen glove and sock during docetaxel infusion, but once toxicity develops, only conservative measures can be taken.[38,39]
Paclitaxel
Nail changes secondary to paclitaxel are similar to docetaxel including onycholysis of finger and toenails which may be extensive, transverse leukonychia, purulent discharge, acute paronychia, nail ridging, Beau's lines, subungual hemorrhages, nail pain, and hyperpigmentation of hyponychium.[17,40] There is an increase in the incidence of paclitaxel-induced nail abnormalities probably due to increased use of weekly schedules and the side effects were not associated with fewer cycles or 3-weekly schedules.[4]
Vincristine: Vincristine has been reported to cause leukonychia, Beau's lines, Mees’ lines, and onychodermal bands.[15]
Miscellaneous agents
Bleomycin: Hyperpigmentation, onycholysis, Beau's lines, raynaud's phenomenon have been reported with bleomycin.[15,41] Intralesional bleomycin used for treatment of periungual warts may lead to nail dystrophy.[42]
Targeted Therapies
Epidermal growth factor receptor inhibitors
EGFR inhibitors are members of type 1 tyrosine kinase receptor family and are used for treatment of solid organ malignancies. These include cetuximab, erlotinib, panitumumab, and lapatinib. They are associated with various cutaneous adverse effects including nail toxicities. The overall incidence of nail toxicities (all grades) with EGFR inhibitors is 17.2% and of high grade nail toxicity is 1.4% with relative risk of 76.94 and 13.11, respectively.[34] Nail changes typically develop after 2 or more months of drug exposure. Almost all patients on EGFR inhibitors are at risk for same irrespective of the tumor type and has been reported with latest EGFR inhibitors (lapatinib, pertuzumab, and afatinib) as well. It may involve multiple or all 20 nails.[35]
The nail changes observed are independent of the type of EGFR inhibitor used. It may affect all. Various nail changes can be observed in the entir]e nail unit which includes-
Nail fold–paronychia and periungual granulation tissue
Nail bed–onycholysis
Nail matrix–melanonychia and brittle nails.
Of these, most common nail changes seen on EGFR inhibitors include paronychia and periungual granulation tissue which may lead to onycholysis and onychodystrophy.[34]
Mechanism for nail changes with EGFR inhibitors is postulated to be through its inhibitory effect on EGFR dependent pathways in keratinocyte leading to altered differentiation and migration of epidermal cells, thereby inhibiting keratinocyte proliferation and inducing apoptosis of keratinocytes in and around nail unit.[43] This leads to thinning of the periungual tissue. The nail plate pierces through the thinned out epithelium, thereby inciting inflammation.
Periungual lesions are observed gradually after about 4 to 8 weeks of starting EGFR inhibitors treatment which is late in comparison with other cutaneous side effects like folliculitis developing within first week of treatment initiation.[44,45,46] First, acute paronychia appears with periungual swelling, tenderness, and oozing which may progress into granulation tissue on the lateral nail folds. The periungual changes are observed most commonly on big toes though any digit may be involved due to repeated microtrauma. Some of the changes are just a cosmetic concern but a few especially the periungual changes can be debilitating severely affecting QOL and essential daily self care activities warranting dose reduction. Drug interruption is not recommended given the long half life of drug and slow growth of the nail plate.[46]
MEK (mitogen-activated protein kinase) inhibitors
Nail changes with MEK inhibitors can be due to inhibition of EGFR in the nail matrix.[43] MEK inhibitors can affect all nails and the nail changes include brittle nails, nail cracking, onychoschizia, onychorrhexis, onycholysis, and perinungual inflammation.[43,44,47,48] There are no prospective studies on incidence of these lesions but authors have reported its frequency and severity to be lower than EGFR inhibitors. MEK inhibitors exhibit side effects similar to EGFR inhibitors like folliculitis, painful fissuring of fingertips, or hair changes probably due to inhibition of the MAP-kinase pathway.[47]
mTOR(mammalian target of rapamycin) inhibitors
In view of the role of mTOR signalling downstream of EGFR, MAPK, and PI3K pathway, its side effect profile closely resembles that of EGFR inhibitors and MEK inhibitors which includes nail plate thinning, onychodystrophy, brittle nails, distal onycholysis, diffuse yellow nail discoloration (xanthochromia), and periungual lesions.[49,50] A study has shown incidence of 22% for periungual lesions with mTOR inhibitors.[51]
BCL-ABL (breakpoint cluster region protein- Aberlson murine leukemia viral gene) inhibitors
BCL-ABL inhibitors like Imatinib can lead to finger as well as toenail melanonychia which can be longitudinal, transverse, or diffuse [Figure 7].[52] The probable mechanism of action is c-KIT blockade by imatinib which has role in melanogenesis.[53] This may also explain pigmentatory alteration observed with imatinib.[44]
Figure 7.

Longitudinal melanonychia secondary to imatinib
VEGFR (Vascular endothelial growth factor) Inhibitors
Splinter hemorrhages have been frequently reported (incidence 25%-75%) with VEGFR inhibitors[54] and have been seen more commonly with sorafenib than sunitinib or pazopanib.[45,55] Fingers nails are affected more commonly than toenails. Splinter hemorrhages develop within first few weeks of therapy and disappear as the nail progressively grows despite continued treatment.[44] The mechanism of development of subungual hemorrhages has been hypothesized as impaird repair of nail bed capillaries from the trauma sustained by repeated micro injuries due to VEGFR inhibition.[54] Splinter subungual hemorrhages do not need any definite treatment.
Management
It is important to counsel the patient before starting chemotherapeutic agent about the potential nail side effects and brief them about preventive strategies. Preventive measures to avoid/decrease chemotherapeutic induced nail toxicity focus on minimizing the pressure, trauma, and friction on the nail unit. These include avoiding irritants like frequent water immersion, wet work, corrosive chemicals, avoiding aggressive manicure, acetone, artificial nails, nail biting, cutting nails too short, and encouraging frequent application of emollients like petroleum jelly to the nail plate, filing the edges to smoothen the corners of nail plate, and promoting wide and comfortable footwear. It is important to maintain appropriate hygiene to avoid secondary infection. Use of frozen glove and socks can be effective preventive measure for taxane-induced toxicity.[38] Dark-colored nail may prevent photo-induced onycholysis, but it is yet to be established. Treatment with biotin may be helpful, but it is yet to be established.[56]
Most of the nail toxicities once developed can be managed conservatively. Since nail growth is a slow process, there is no effective means to reverse nail abnormalities arising secondary to transient arrest of nail matrix mitotic activity. It is important to counsel the patient that the nail changes will disappear with the nail growth but this may take months. Usually, it is not recommended to withdraw the drug as it takes several months for the nail changes to improve but if toxicity is severe, reduction or cessation of the offending drug may be necessary. Management of different nail toxicities has been briefly described in Table 4.
Table 4.
| Drug induced nail toxicity | Management |
|---|---|
| Drug-induced onycholysis | keep nails short debridement for moderate onycholysis local application of antimicrobial solutions avoid contact with irritants if haematoma limited to <25% of the visible nail-drain with sharp scalpel or by hot paper-clip cautery touched at center of dark spot. |
| Drug-induced paronychia and periungual inflammation | Topical application of corticosteroids and calcineurin inhibitors antibiotics if culture positive/infected vinegar soaks/dilute bleach soaks to prevent secondary infection oral tetracyclines prescribed for folliculitis due to EGFR inhibitors may be helpful.[42] topical adapalene gel has been found promising in few case reports, but further studies needed. |
| Pyogenic granuloma like lesions | pain monitor effect on activities of daily living Topical liquid nitrogen, topical steroids, or weekly 10% aqueous silver nitrate electrodesiccation 88% phenol or 35% trichloroacetic acid topical, or liquid nitrogen applications intralesional triamcinolone acetonide followed by chlorhexidine lotion twice daily topical or oral antibiotic if superinfected surgical treatment - removal of part of the nail together with the matrix may be needed with physical destruction of excessive granulation tissue. dose adjustments needed if lesions are painful, several, or persistent |
| Nail pigmentation | pigmentation may persist unchanged for years even after drug withdrawal only a cosmetic concern Application of colored nail varnish helps to conceal the pigmentation and hardens the nails to prevent fragmentation. |
| Nail plate changes | reduce contact with water wear cotton gloves under plastic or rubber gloves before any wet work Topical applications of nail lacquer containing hydroxypropyl chitosan may increase linear nail growth and improve nail keratin quality (as shown in psoriatic nail dystrophy). |
Conclusion
Nail changes are frequently observed in patients treated with anticancer agents. The cytotoxic chemotherapeutic agents mostly affect the nail plate and bed, whereas the targeted therapies mostly involve the periungual tissue. Patients can be informed about the expected side effects based on the chemotherapeutic agents and explained preventive strategy to minimize the side effects. Nail changes can at times be debilitating affecting the QOL and so treating physician should be aware of changes expected and timely intervention needed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hinds G, Thomas VD. Malignancy and cancer treatment-related hair and nail changes. Dermatol Clin. 2008;26:59–68, viii. doi: 10.1016/j.det.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Naveed S, Thappa DM, Dubashi B, Pandjatcharam J, Munisamy M, Singh N. Mucocutaneous adverse reactions of cancer chemotherapy and chemoradiation. Indian J Dermatol. 2019;64:122–8. doi: 10.4103/ijd.IJD_129_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piraccini BM, Iorizzo M, Tosti A. Drug-induced nail abnormalities. Am J Clin Dermatol. 2003;4:31–7. doi: 10.2165/00128071-200304010-00004. [DOI] [PubMed] [Google Scholar]
- 4.Gilbar P, Hain A, Peereboom V-M. Nail toxicity induced by cancer chemotherapy. J Oncol Pharm Pract. 2009;15:143–55. doi: 10.1177/1078155208100450. [DOI] [PubMed] [Google Scholar]
- 5.Piraccini BM, Iorizzo M, Starace M, Tosti A. Drug-induced nail diseases. Dermatol Clin. 2006;24:387–91. doi: 10.1016/j.det.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Saraswat N, Sood A, Verma R, Kumar D, Kumar S. Nail changes induced by chemotherapeutic agents. Indian J Dermatol. 2020;65:193. doi: 10.4103/ijd.IJD_37_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trivedi M, Mehta RD, Kumar HS, Ghiya BC, Soni P, Meena MK, et al. Nail changes caused by chemotherapy among cancer patients: A cross-sectional study of northwest Rajasthan. Indian Dermatol Online J. 2020;11:953–8. doi: 10.4103/idoj.IDOJ_84_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li A, Li Y, Ge L, Li P, Li W. Onychomadesis associated with chemotherapy: Case report and mini literature review. Drug Des DevelTher. 2017;11:2373–6. doi: 10.2147/DDDT.S139643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piraccini BM, Tosti A. Drug-induced nail disorders: Incidence, management and prognosis. Drug Saf. 1999;21:187–201. doi: 10.2165/00002018-199921030-00004. [DOI] [PubMed] [Google Scholar]
- 10.Uyttendaele H, Geyer A, Scher RK. Drugs and nails. J Eur Acad Dermatol VenereolJEADV. 2004;18:124–5. doi: 10.1111/j.1468-3083.2004.00775.x. [DOI] [PubMed] [Google Scholar]
- 11.Common Terminology Criteria for Adverse Events (CTCAE):196 [Google Scholar]
- 12.Lacouture ME, Maitland ML, Segaert S, Setser A, Baran R, Fox LP, et al. A proposed EGFR inhibitor dermatologic adverse event-specific grading scale from the MASCC skin toxicity study group. Support Care Cancer. 2010;18:509–22. doi: 10.1007/s00520-009-0744-x. [DOI] [PubMed] [Google Scholar]
- 13.Srikant M, Van JV, Raithatha A, Reilly JT. Cyclophosphamide-induced nail pigmentation. Br J Haematol. 2002;117:2. doi: 10.1046/j.1365-2141.2002.03449.x. [DOI] [PubMed] [Google Scholar]
- 14.Griffiths C, Barker J, Bleiker T, Chalmers R, Creamer D, editors. Rook's Textbook of Dermatology. 9 ed. Vol. 4. Hoboken, NJ: Wiley-Blackwell; 2016. p. 4696. Set. Chichester, West Sussex. [Google Scholar]
- 15.Dave S, Thappa DM. Peculiar pattern of nail pigmentation following cyclophosphamide therapy. Dermatol Online J. 2003;9:14. [PubMed] [Google Scholar]
- 16.Susser WS, Whitaker-Worth DL, Grant-Kels JM. Mucocutaneous reactions to chemotherapy. J Am Acad Dermatol. 1999;40:367–98. doi: 10.1016/s0190-9622(99)70488-3. quiz 399–400. [DOI] [PubMed] [Google Scholar]
- 17.Mackay-Wiggan J, Nair KG, Halasz CL. Onycholysis associated with paclitaxel. Cutis. 2003;71:229–32. [PubMed] [Google Scholar]
- 18.Lehoczky O, Pulay T. Transverse leukonychia secondary to paclitaxel-carboplatin chemotherapy in a patient with ovarian cancer. J ObstetGynaecol J Inst ObstetGynaecol. 2002;22:694. doi: 10.1080/014436102762062439. [DOI] [PubMed] [Google Scholar]
- 19.Dasanu CA, Wiernik PH, Vaillant J, Alexandrescu DT. A complex pattern of melanonychia and onycholysis after treatment with pemetrexed for lung cancer. Skinmed. 2007;6:95–6. doi: 10.1111/j.1540-9740.2007.05720.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen GY, Chen YH, Hsu MM, Tsao CJ, Chen WC. Onychomadesis and onycholysis associated with capecitabine. Br J Dermatol. 2001;145:521–2. doi: 10.1046/j.1365-2133.2001.04391.x. [DOI] [PubMed] [Google Scholar]
- 21.Maino KL, Norwood C, Stashower ME. Onycholysis with the appearance of a “sunset” secondary to capecitabine. Cutis. 2003;72:234–6. [PubMed] [Google Scholar]
- 22.Piraccini BM. Nail Disorders: A Practical Guide to Diagnosis and Management. Mailand: Springer-Verlag; 2014. [Last accessed on 2018 Oct 08]. Available from://www.springer.com/in/book/9788847053038 . [Google Scholar]
- 23.Chen G-Y, Chao S-C, Chen W. Black lunula caused by Tegafur. Acta Derm Venereol. 2004;84:238–9. doi: 10.1080/00015550310007715. [DOI] [PubMed] [Google Scholar]
- 24.deMarinis M, Hendricks A, Stoltzner G. Nail pigmentation with daunorubicin therapy. Ann Intern Med. 1978;89:516–7. doi: 10.7326/0003-4819-89-4-516. [DOI] [PubMed] [Google Scholar]
- 25.Shelley WB, Humphrey GB. Transverse leukonychia (Mees’ lines) due to daunorubicin chemotherapy. Pediatr Dermatol. 1997;14:144–5. doi: 10.1111/j.1525-1470.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 26.Curran CF. Onycholysis in doxorubicin-treated patients. Arch Dermatol. 1990;126:1244. [PubMed] [Google Scholar]
- 27.Priestman TJ, James KW. Letter: Adriamycin and longitudinal pigmented banding of fingernails. Lancet LondEngl. 1975;1:1337–8. doi: 10.1016/s0140-6736(75)92341-7. [DOI] [PubMed] [Google Scholar]
- 28.Baykal Y, Baykal C, Ozer S, Tulunay G. Topotecan induced nail pigmentation. J Dermatol. 2004;31:951–2. doi: 10.1111/j.1346-8138.2004.tb00636.x. [DOI] [PubMed] [Google Scholar]
- 29.Minisini AM, Tosti A, Sobrero AF, Mansutti M, Piraccini BM, Sacco C, et al. Taxane-induced nail changes: Incidence, clinical presentation and outcome. Ann Oncol. 2003;14:333–7. doi: 10.1093/annonc/mdg050. [DOI] [PubMed] [Google Scholar]
- 30.Wasner G, Hilpert F, Baron R, Pfisterer J. Clinical picture: Nail changes secondary to docetaxel. Lancet LondEngl. 2001;357:910. doi: 10.1016/s0140-6736(00)04210-0. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura S, Kajita S, Takagi A, Hashimoto Y, Ishida-Yamamoto A, Takahashi H, et al. Improvement in docetaxel-induced nail changes associated with cyclooxygenase-2 inhibitor treatment. Clin Exp Dermatol. 2009;34:e320–1. doi: 10.1111/j.1365-2230.2009.03275.x. [DOI] [PubMed] [Google Scholar]
- 32.Hong J, Park SH, Choi SJ, Lee SH, Lee KC, Lee J-I, et al. Nail toxicity after treatment with docetaxel: Aprospective analysis in patients with advanced non-small cell lung cancer. Jpn J Clin Oncol. 2007;37:424–8. doi: 10.1093/jjco/hym042. [DOI] [PubMed] [Google Scholar]
- 33.Winther D, Saunte DM, Knap M, Haahr V, Jensen AB. Nail changes due to docetaxel--A neglected side effect and nuisance for the patient. Support Care Cancer. 2007;15:1191–7. doi: 10.1007/s00520-007-0232-0. [DOI] [PubMed] [Google Scholar]
- 34.Miller RA. Nail dystrophy following intralesional injections of bleomycin for a periungual wart. Arch Dermatol. 1984;120:963–4. [PubMed] [Google Scholar]
- 35.Garden BC, Wu S, Lacouture ME. The risk of nail changes with epidermal growth factor receptor inhibitors: A systematic review of the literature and meta-analysis. J Am Acad Dermatol. 2012;67:400–8. doi: 10.1016/j.jaad.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Engels FK, Verweij J. Docetaxel administration schedule: From fever to tears? A review of randomised studies. Eur J Cancer OxfEngl 19902005. 41:1117–26. doi: 10.1016/j.ejca.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 37.Lau C-P, Hui P, Chan T-C. Docetaxel-induced nail toxicity: Acase of severe onycholysis and topic review. Chin Med J (Engl) 2011;124:2559–60. [PubMed] [Google Scholar]
- 38.Hussain S, Anderson DN, Salvatti ME, Adamson B, McManus M, Braverman AS. Onycholysis as a complication of systemic chemotherapy: Report of five cases associated with prolonged weekly paclitaxel therapy and review of the literature. Cancer. 2000;88:2367–71. doi: 10.1002/(sici)1097-0142(20000515)88:10<2367::aid-cncr22>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 39.Scotté F, Tourani J-M, Banu E, Peyromaure M, Levy E, Marsan S, et al. Multicenter study of a frozen glove to prevent docetaxel-induced onycholysis and cutaneous toxicity of the hand. J Clin Oncol. 2005;23:4424–9. doi: 10.1200/JCO.2005.15.651. [DOI] [PubMed] [Google Scholar]
- 40.Scotté F, Banu E, Medioni J, Levy E, Ebenezer C, Marsan S, et al. Matched case-control phase 2 study to evaluate the use of a frozen sock to prevent docetaxel-induced onycholysis and cutaneous toxicity of the foot. Cancer. 2008;112:1625–31. doi: 10.1002/cncr.23333. [DOI] [PubMed] [Google Scholar]
- 41.Link CJ, Sarosy GA, Kohn EC, Christian MC, Davis P, Adamo DO, et al. Cutaneous manifestations of Taxol therapy. Invest New Drugs. 1995;13:261–3. doi: 10.1007/BF00873811. [DOI] [PubMed] [Google Scholar]
- 42.Vogelzang NJ, BoslGJ , Johnson K, Kennedy BJ. Raynaud's phenomenon: Acommon toxicity after combination chemotherapy for testicular cancer. Ann Intern Med. 1981;95:288–92. doi: 10.7326/0003-4819-95-3-288. [DOI] [PubMed] [Google Scholar]
- 43.Lacouture ME, Schadendorf D, Chu C-Y, Uttenreuther-Fischer M, Stammberger U, O’Brien D, et al. Dermatologic adverse events associated with afatinib: An oral ErbB family blocker. Expert Rev Anticancer Ther. 2013;13:721–8. doi: 10.1586/era.13.30. [DOI] [PubMed] [Google Scholar]
- 44.Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer. 2006;6:803–12. doi: 10.1038/nrc1970. [DOI] [PubMed] [Google Scholar]
- 45.Robert C, Soria J-C, Spatz A, Le Cesne A, Malka D, Pautier P, et al. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol. 2005;6:491–500. doi: 10.1016/S1470-2045(05)70243-6. [DOI] [PubMed] [Google Scholar]
- 46.Robert C, Sibaud V, Mateus C, Cherpelis BS. Advances in the management of cutaneous toxicities of targeted therapies. Semin Oncol. 2012;39:227–40. doi: 10.1053/j.seminoncol.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Robert C, Sibaud V, Mateus C, Verschoore M, Charles C, Lanoy E, et al. Nail toxicities induced by systemic anticancer treatments. Lancet Oncol. 2015;16:e181–9. doi: 10.1016/S1470-2045(14)71133-7. [DOI] [PubMed] [Google Scholar]
- 48.Balagula Y, Barth Huston K, Busam KJ, Lacouture ME, Chapman PB, Myskowski PL. Dermatologic side effects associated with the MEK 1/2 inhibitor selumetinib (AZD6244, ARRY-142886) Invest New Drugs. 2011;29:1114–21. doi: 10.1007/s10637-010-9567-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schad K, Baumann Conzett K, Zipser MC, Enderlin V, Kamarashev J, French LE, et al. Mitogen-activated protein/extracellular signal-regulated kinase kinase inhibition results in biphasic alteration of epidermal homeostasis with keratinocytic apoptosis and pigmentation disorders. Clin Cancer. 2010;16:1058–64. doi: 10.1158/1078-0432.CCR-09-1766. [DOI] [PubMed] [Google Scholar]
- 50.Peuvrel L, Quéreux G, Brocard A, Saint-Jean M, Dréno B. Onychopathy induced by temsirolimus, a mammalian target of rapamycin inhibitor. Dermatol Basel Switz. 2012;224:204–8. doi: 10.1159/000338893. [DOI] [PubMed] [Google Scholar]
- 51.Sibaud V, Dalenc F, Mourey L, Chevreau C. Paronychia and pyogenic granuloma induced by new anticancer mTOR inhibitors. Acta Derm Venereol. 2011;91:584–5. doi: 10.2340/00015555-1097. [DOI] [PubMed] [Google Scholar]
- 52.Voilliot-Trotot C, Granel-Brocard F, Geoffrois L, Tréchot P, Nguyen-Thi P, Schmutz J-L, et al. Adverse cutaneous effects and quality of life in patients treated with mTOR inhibitors for renal carcinoma. Ann Dermatol Venereol. 2013;140:353–62. doi: 10.1016/j.annder.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 53.Sibaud V, Robert C. [Pigmentary disorders induced by anticancer agents.Part II: Targeted therapies] Ann Dermatol Venereol. 2013;140:266–73. doi: 10.1016/j.annder.2013.01.442. [DOI] [PubMed] [Google Scholar]
- 54.Scheinfeld N. Imatinib mesylate and dermatology part 2: Areview of the cutaneous side effects of imatinib mesylate. J Drugs Dermatol. 2006;5:228–31. [PubMed] [Google Scholar]
- 55.Robert C, Faivre S, Raymond E, Armand J-P, Escudier B. Subungual splinter hemorrhages: Aclinical window to inhibition of vascular endothelial growth factor receptors? Ann Intern Med. 2005;143:313–4. doi: 10.7326/0003-4819-143-4-200508160-00021. [DOI] [PubMed] [Google Scholar]
- 56.Autier J, Escudier B, Wechsler J, Spatz A, Robert C. Prospective study of the cutaneous adverse effects of sorafenib, a novel multikinase inhibitor. Arch Dermatol. 2008;144:886–92. doi: 10.1001/archderm.144.7.886. [DOI] [PubMed] [Google Scholar]
- 57.Scheinfeld N, Dahdah MJ, Scher R. Vitamins and minerals: Their role in nail health and disease. J Drugs Dermatol JDD. 2007;6:782–7. [PubMed] [Google Scholar]
- 58.Hachisuka J, Yunotani S, Shidahara S, Moroi Y, Furue M. Effect of adapalene on cetuximab-induced painful periungual inflammation. J Am Acad Dermatol. 2011;64:e20–1. doi: 10.1016/j.jaad.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 59.Kiyohara Y, Yamazaki N, Kishi A. Erlotinib-related skin toxicities: Treatment strategies in patients with metastatic non-small cell lung cancer. J Am Acad Dermatol. 2013;69:463–72. doi: 10.1016/j.jaad.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 60.Shu K-Y, Kindler HL, Medenica M, Lacouture M. Doxycycline for the treatment of paronychia induced by the epidermal growth factor receptor inhibitor cetuximab. Br J Dermatol. 2006;154:191–2. doi: 10.1111/j.1365-2133.2005.07010.x. [DOI] [PubMed] [Google Scholar]


