Abstract
Objectives
Complicated urinary tract infections (cUTIs) are frequently encountered in hospitals and ICUs. Increasingly, the causative pathogens harbour enzymatic resistance mechanisms. Taniborbactam is a novel β-lactamase inhibitor with activity against Ambler class A, B, C and D β-lactamases. Herein, we assessed the efficacy of cefepime alone and the combination cefepime/taniborbactam in a neutropenic murine cUTI model.
Methods
Eighteen cefepime-resistant clinical isolates (9 Enterobacterales, 3 Pseudomonas aeruginosa and 6 Stenotrophomonas maltophilia; cefepime MIC = 32 to >512 mg/L) were assessed. Cefepime/taniborbactam MICs ranged from 0.06 to 128 mg/L. Human-simulated plasma regimens (HSRs) of cefepime alone and in combination with taniborbactam were developed in the murine cUTI model. The efficacy of cefepime HSR and cefepime/taniborbactam HSR was determined as the change in log10 cfu/kidney at 48 h compared with 48 h controls.
Results
Mean ± SD initial bacterial burden was 5.66 ± 0.56 log10 cfu/kidney, which increased to 9.05 ± 0.39 log10 cfu/kidney at 48 h. The cefepime HSR was ineffective, as bacterial burden was similar to untreated controls (−0.14 ± 0.40 change in log10 cfu/kidney). In contrast, cefepime/taniborbactam exhibited substantial killing, with log10 cfu/kidney changes of −5.48 ± 1.3, −4.79 ± 0.3 and −5.04 ± 0.7 for ESBL/AmpC-, KPC- and OXA-48-harbouring Enterobacterales, respectively. Cefepime/taniborbactam also exhibited robust killing of P. aeruginosa (−6.5 ± 0.26) and S. maltophilia (−5.66 ± 0.71).
Conclusions
Humanized exposures of cefepime/taniborbactam achieved robust killing of Enterobacterales, P. aeruginosa and S. maltophilia harbouring ESBL, AmpC, KPC and/or OXA-48. These data support the role of cefepime/taniborbactam for cUTI treatment for cefepime/taniborbactam MICs up to 32 mg/L.
Introduction
Urinary tract infections (UTIs) continue to be one of the most common bacterial diseases globally, resulting in substantial clinical and financial burden among outpatients and hospitalized patients.1–3 β-Lactam antibiotics are an important option for treating bacterial infections, including UTIs, due to their well-established safety profile and effectiveness; however, the rising incidence of β-lactamase-harbouring pathogens is reducing the clinical utility of these agents.4,5 Taniborbactam is a novel cyclic boronic acid-based β-lactamase inhibitor with potent in vitro activity against many clinically relevant Ambler class A, C and D β-lactamases and select class B MBLs (e.g. NDM and VIM).6–8 Taniborbactam is being developed in combination with cefepime as a broad-spectrum option for the treatment of serious Gram-negative infections and is currently being evaluated for safety and efficacy in a Phase III randomized complicated UTI (cUTI), including acute pyelonephritis, clinical trial (NCT03840148).6,9
In the preclinical arena, the evaluation of dose–response relationships in a variety of infection types (thigh, lung, kidney and sepsis animal models) using clinically relevant exposures has been instrumental for bridging in vitro data to clinical efficacy. To that end, this study sought to develop a human-simulated exposure of cefepime/taniborbactam in the neutropenic murine complicated kidney infection model and assess in vivo efficacy against a variety of clinical isolates.
Methods
Ethics
All murine experiments were conducted in concordance with the National Research Council of the National Academy of Sciences standards. The protocol was approved by the Institutional Animal Care and Use Committee of Hartford Hospital (Assurance #A3185-01).
Isolates and antimicrobial susceptibility testing
Eighteen clinical isolates [9 Enterobacterales and 9 non-fermenting Gram-negative pathogens (Pseudomonas aeruginosa and Stenotrophomonas maltophilia)] were included in this study. Respective isolate genotypes are provided in Table 1. Enterobacterales isolates harboured acquired AmpC, KPC or OXA-48/OXA-48-like enzymes, while P. aeruginosa isolates harboured acquired AmpC or KPC. Notably, two isolates (EC 773 and EC 774) had 4 amino acid insertions in PBP3 (the target of cefepime), in addition to β-lactamase-mediated resistance. WGS analysis was performed using Geneious Prime version 2021.1.1 (Biomatters Inc., CA, USA). DNA extraction, Illumina library preparation and raw WGS data from Illumina HiSeq 2 × 150 bp were provided by Genewiz (NJ, USA).
Table 1.
Isolates included in neutropenic murine direct kidney infection model studies and corresponding MICs of tested agents (in mg/L)
| CAIRD ID | Genotype | Cefepime | Cefepime/ taniborbactam | Ceftazidime/ avibactam | Meropenem/ vaborbactam |
|---|---|---|---|---|---|
| KP 390 | KPC | 512 | 0.06 | 1 | ≤0.06 |
| ECL 123 | TEM-OSBL, CTX-M-15, ACT-New Variant, OXA-48 | >512 | 0.5 | 0.25 | 2 |
| KP 905 (CDC: #0453) | KPC-3, SHV-11, TEM-1B, OMPK35 | >512 | 1 | 16c | 4 |
| KP 737 | SHV-OSBL, CTX-M-15, OXA-48 | >512 | 2 | 1 | 32 |
| KP 738 | KPC-3 | >512 | 4 | 8 | 4 |
| ECL 124 | TEM-OSBL, CTX-M-15, ACT-7 | >512 | 8 | 4 | ≤0.06 |
| KP 579 | SHV-11, TEM-1, CTX-M-15, OXA-48 | >512 | 16 | 1 | 64 |
| EC 774 | OXA-48, CTX-M-15; PBP3 4-aa (YRIK) insertion | >512 | 16b | 8 | 8 |
| EC 773 | CTX-M-15, OXA-1, OXA-181; PBP3 4-aa (YRIN) insertion | >512 | 16b | 2 | 64 |
| PSA 1602 (CDC: #0090) | KPC-5, OXA-50, PAO | 512 | 4 | 4 | 2 |
| PSA 1681 | AmpC | >512 | 8 | 4 | 32 |
| PSA 1711 (CDC: #0598) | PDC-103, KPC-2 | 128 | 16 | 4 | >64 |
| STM 109 | L1, L2a | 256 | 32 | >128 | >64 |
| STM 110 | L1, L2a | 128 | 32 | 128 | >64 |
| STM 111 | L1, L2a | 128 | 128 | >128 | >64 |
| STM 112 | L1, L2a | 128 | 2 | 2 | >64 |
| STM 113 | L1, L2a | 128 | 1 | 8 | >64 |
| STM 115 | L1, L2a | 256 | 1 | 4 | >64 |
EC, Escherichia coli; KP, Klebsiella pneumoniae; ECL, Enterobacter cloacae; PSA, Pseudomonas aeruginosa; STM, Stenotrophomonas maltophilia.
No additional β-lactamase detected by WGS and genome analysis.
Broth microdilution conducted at International Health Management Association (IHMA).
Broth microdilution reported by the Centers for Disease Control (CDC).
Cefepime, cefepime/taniborbactam, meropenem/vaborbactam and ceftazidime/avibactam MICs were determined in at least triplicate by broth microdilution.10,11
Neutropenic cUTI model
Female, specific-pathogen-free, CD-1 mice (weight = 20–22 g) were obtained from Charles River Laboratories, Inc. (NC, USA). All animals were allowed to acclimatize for 48 h. During acclimatization, animals were housed in groups of six mice at controlled room temperature in HEPA-filtered cages (Innovive, CA, USA). Cages were supplemented with nesting material for enrichment purposes. Study rooms were maintained with diurnal cycles (12 h light/12 h dark). Food and water was provided ad libitum. Monitoring was conducted at least three times per day for signs of morbidity. A 48 h cUTI model using a direct kidney inoculation technique was conducted as previously described (Figure S1, available as Supplementary data at JAC Online).12,13
Pharmacokinetic studies
Dosing regimens were developed that resulted in murine exposures similar to Phase I healthy volunteer mean plasma exposures of cefepime 2 g administered over 2 h every 8 h and cefepime/taniborbactam 2/0.5 g administered over 2 h every 8 h.14 To guide regimen development, 48 h pharmacokinetic studies (n = 4 studies) of cefepime (6.5 mg/kg at 0 h and 3.5 mg/kg q8h thereafter; 8 mg/kg at 0 h and 4.5 mg/kg q8h thereafter; 10 mg/kg at 0 h and 5.5 mg/kg q8h thereafter; and 15 mg/kg at 0 h and 7.5 mg/kg q8h thereafter) were conducted in the cUTI model. Similarly, pharmacokinetic studies (n = 2) with concomitant administration of cefepime and taniborbactam (i.e. cefepime/taniborbactam) were conducted: (10/5 mg/kg at 0 h and 5.5/2.5 mg/kg q8h thereafter; and 15/7.5 mg/kg at 0 h and 7.5/3.75 mg/kg q8h thereafter). Blood was collected at eight sampling points over a 48 h period (1, 2, 4, 7.5, 25, 26, 28 and 31.5 h) by cardiac puncture following CO2 asphyxiation. Blood samples were placed in K2EDTA vials and centrifuged at 10 000 rpm for 10 min at 8°C. Plasma samples were collected and stored at −80°C until drug concentration analysis.
Cefepime and taniborbactam concentrations were analysed using a qualified LC-MS/MS method (AB SCIEX Triple Quad™ 5500 System, Keystone Bioanalytical, PA, USA). Intra-batch precision (coefficient of variation) and accuracy (relative error) for all quality control (QC) plasma samples for both cefepime and taniborbactam ranged from 3.18% to 5.05% and −8.14% to 7.15%, respectively. Inter-batch precision and accuracy for all QC plasma samples for both compounds ranged from 3.88% to 9.21% and −4.83% to 4.02%, respectively. Pharmacokinetic parameters were calculated and regimens were simulated using Phoenix 64 (WinNonlin 6.4; Pharsight Corp., CA, USA). Human and murine drug concentrations were corrected for cefepime (humans, 20%; mice, 0%) and taniborbactam (humans, 0%; mice, 19.4%) protein binding.15
In vivo efficacy studies
Three hours after inoculation, one group of six mice was sacrificed via CO2 asphyxiation followed by cervical dislocation and their kidneys (n = 12) harvested aseptically (0 h control group). The remaining groups (six mice per group) were administered one of the following interventions: vehicle (saline), cefepime human-simulated plasma regimen (HSR) or cefepime/taniborbactam HSR. After 48 h of treatment, the mice were sacrificed and their kidneys were harvested aseptically. All kidneys were homogenized in 0.9% normal saline and serially diluted onto 5% sheep’s blood agar plates and incubated overnight for enumeration. For consistency with previous murine cUTI studies,12,13 efficacy was assessed by the change in log10 cfu/kidney from 48 h controls and reported as mean ± SD. To compare antimicrobial efficacy between cefepime and cefepime/taniborbactam, a Student’s t-test was used and a P value ≤0.05 was considered statistically significantly different.
Results
All isolates were cefepime resistant and cefepime/taniborbactam MICs ranged from 0.06 to 128 mg/L (Table 1). Ceftazidime/avibactam and meropenem/vaborbactam MICs ranged from 1 to >128 mg/L and ≤0.06 to >64 mg/L, respectively, spanning their clinical breakpoints.
Pharmacokinetic studies
A one-compartment model was used to fit the data (Table S1). The cefepime and cefepime/taniborbactam combination murine concentration–time profiles, as well as %fT>MIC and fAUC0–8 values, were comparable to the human profiles (Figure S2 and Table S2). When the cefepime and taniborbactam murine HSRs were administered concomitantly, cefepime elimination was enhanced and the plasma exposure was observed to be slightly reduced, which necessitated a cefepime dose modification for animals receiving the combination, in order to attain the target human plasma exposure. As a result, the cefepime HSR dosing regimen was as follows: 8 mg/kg at 0 h followed by 4.5 mg/kg every 8 h. For the combination HSR therapy, the cefepime doses utilized were: 15 mg/kg at 0 h followed by 7.5 mg/kg every 8 h thereafter when co-administered with taniborbactam (7.5 mg/kg at 0 h followed by 3.75 mg/kg every 8 h thereafter).
In vivo efficacy studies
Enterobacterales
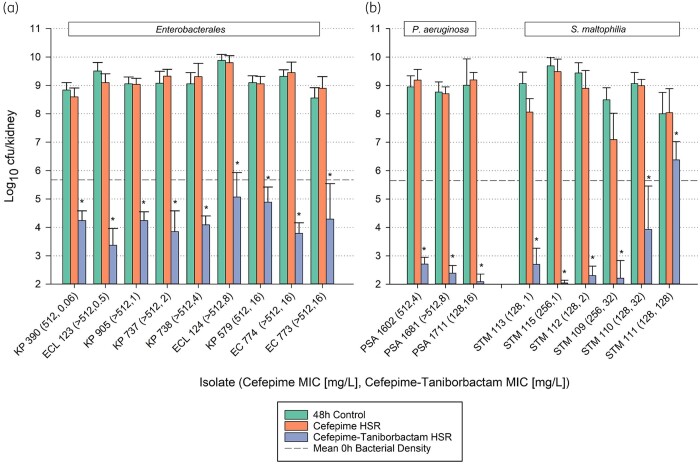
A composite graph of in vivo efficacy in Enterobacterales (n = 9 isolates; cefepime/taniborbactam MIC range = 0.06–16 mg/L) is presented in Figure 1(a) (Figure S3). The average bacterial density was 5.67 ± 0.59 log10 cfu/kidney at 0 h, increasing to 9.16 ± 0.29 log10 cfu/kidney in untreated control animals after 48 h. Among all Enterobacterales isolates evaluated, cefepime/taniborbactam resulted in a statistically significant bacterial density reduction (P < 0.001), i.e. >4 log10 cfu/kidney reduction compared with 48 h controls. In addition, cefepime/taniborbactam exhibited profound average −4.79 ± 0.94 log10 cfu/kidney reduction against the two isolates of Escherichia coli with OXA-48/OXA-48-like β-lactamases, ESBLs and PBP3 variants associated with an elevated cefepime MIC.16
Figure 1.
In vivo efficacy comparison of 48 h control, cefepime HSR and cefepime/taniborbactam HSR against individual (a) Enterobacterales isolates and (b) P. aeruginosa and S. maltophilia isolates. Each bar represents the average bacterial density (±SD) of 12 infected kidneys from six mice. An asterisk indicates a statistically significant decrease in cfu/kidney compared with cefepime HSR. The y-axis starts at the lower limit of detection (2.00 log10 cfu/kidney). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Non-fermenting Gram-negative bacteria
More profound cefepime/taniborbactam activity was observed amongst the non-fermenting isolates (Figure 1b and Figure S3). Cefepime/taniborbactam resulted in an average bacterial burden change of −6.50 ± 0.26 log10 cfu/kidney among all P. aeruginosa isolates (MIC range = 4–16 mg/L) (P < 0.001). For five out of the six S. maltophilia isolates studied (cefepime/taniborbactam MIC range = 1–32 mg/L), cefepime/taniborbactam resulted in an average bacterial burden reduction of −6.47 ± 0.65 log10 cfu/kidney (P < 0.001). Against S. maltophilia isolate STM 111 (MIC = 128 mg/L), cefepime/taniborbactam resulted in expectedly poor activity relative to 48 h controls given its cefepime concentration–time profile (fT>MIC128 = 0%).
Discussion
The ability of taniborbactam to prevent cefepime degradation via reversible covalent inhibition of a variety of clinically relevant serine β-lactamases influenced the isolates and genotypes selected for evaluation in this current animal study. Using clinical exposures of cefepime and taniborbactam, this study demonstrated the enhanced efficacy of this novel β-lactam/β-lactamase inhibitor combination against a variety of cefepime-resistant Gram-negative bacteria harbouring a range of enzyme (ESBL, AmpC, KPC and OXA-48/OXA-48-like)- and cefepime target (PBP3 insertion)-mediated resistance mechanisms in the neutropenic murine cUTI infection model.
Bacterial killing with cefepime/taniborbactam was observed among cefepime-resistant Enterobacterales, P. aeruginosa and S. maltophilia clinical isolates with cefepime/taniborbactam MICs ≤32 mg/L. This finding is likely due to the optimization of the β-lactam backbone [i.e. maximum approved cefepime dose (2 g q8h) and prolonged infusion regimen (2 h)] and taniborbactam’s high potency of β-lactamase inhibition.15 Notably, the observed bacterial growth (i.e. therapeutic failure of the cefepime/taniborbactam HSR) with the S. maltophilia isolate having an elevated cefepime/taniborbactam MIC (128 mg/L) further validates the UTI model as it demonstrates strong correlation between loss of in vitro activity and in vivo efficacy.
There are study limitations worth considering. We acknowledge that the cUTI model required administration of different cefepime doses (mg/kg), but this was necessary to maintain suitable plasma exposures over the range of MICs. While plasma exposures were consistent with human exposures for both regimens, we did not assess urine or kidney tissue drug concentrations. Further studies should be conducted to assess implications of urine or kidney tissue drug concentrations, which may be variable among and within mice over the 48 h dosing period. Secondly, cefepime/taniborbactam has demonstrated in vitro activity against both clinically important serine β-lactamases and MBLs; however, isolates in this current study were limited to serine β-lactamase-harbouring isolates only. This is as a result of β-lactam in vitro–in vivo discordance against MBL-harbouring isolates in the mouse model, necessitating a different study design to appropriately determine that any antimicrobial activity observed in vivo is due to the combination of cefepime and taniborbactam against MBLs.17–19 Resistance development was not assessed in this study and is warranted in future animal studies. Notably, extensive resistance development studies have been performed with cefepime/taniborbactam, including a 7 day hollow fibre model, with no emergence of resistant subpopulations.6,20
In summary, these translational data support the current clinical regimen being evaluated in the cUTI Phase III clinical trial and provide insights into the in vivo potency and spectrum of cefepime/taniborbactam antimicrobial activity over an extended treatment period in the kidney infection model. Ultimately, assessing antimicrobial activity across multiple infection models enhances confidence in the clinical dose required to achieve the pharmacokinetic/pharmacodynamic targets.
Supplementary Material
Acknowledgements
We would like to recognize the team at the Center for Anti-Infective Research for their assistance in the conduct of this study. We would also like to thank David Six from Venatorx for conducting and providing WGS analysis.
Funding
This project was sponsored by Venatorx Pharmaceuticals, Inc. (Malvern, PA, USA) and funded in whole or in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN272201300019C, The Wellcome Trust under Award No. 360G-Wellcome-101999/Z/13/Z, and the Biomedical Advanced Research and Development Authority, Office of the Assistant Secretary for Preparedness and Response, Department of Health and Human Services under Contract No. HHSO100201900007C.
Transparency declarations
M.J.L. has none to declare. D.P.N. has served as a consultant or speaker’s bureau member or has received research funding from Allergan, Cepheid, Merck, Pfizer, Wockhardt, Shionogi, Tetraphase and Venatorx. T.E.A. has received research funding from Venatorx.
The sponsor provided support and did not exercise control over the conduct or reporting of the research except with WGS and genome analysis of S. maltophilia isolates.
Supplementary data
Figures S1 to S3 and Tables S1 and S2 are available as Supplementary data at JAC Online.
Contributor Information
Maxwell J. Lasko, Center for Anti-Infective Research and Development, Hartford Hospital, Hartford, CT, USA
David P. Nicolau, Center for Anti-Infective Research and Development, Hartford Hospital, Hartford, CT, USA Division of Infectious Diseases, Hartford Hospital, Hartford, CT, USA.
Tomefa E. Asempa, Center for Anti-Infective Research and Development, Hartford Hospital, Hartford, CT, USA
References
- 1. Medina M, Castillo-Pino E. An introduction to the epidemiology and burden of urinary tract infections. Ther Adv Urol 2019; 11: 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cardwell SM, Crandon JL, Nicolau DP et al. Epidemiology and economics of adult patients hospitalized with urinary tract infections. Hosp Pract 2016; 44: 33–40. [DOI] [PubMed] [Google Scholar]
- 3. Öztürk R, Murt A. Epidemiology of urological infections: a global burden. World J Urol 2020; 38: 2669–79. [DOI] [PubMed] [Google Scholar]
- 4. Aboumarzouk OM. Extended spectrum β-lactamase urinary tract infections. Urol Ann 2014; 6: 114–5. [PMC free article] [PubMed] [Google Scholar]
- 5. Hrbacek J, Cermak P, Zachoval R. Current antibiotic resistance trends of uropathogens in Central Europe: survey from a tertiary hospital urology department 2011-2019. Antibiotics (Basel) 2020; 9: 630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hamrick JC, Docquier J-D, Uehara T et al. VNRX-5133 (taniborbactam), a broad-spectrum inhibitor of serine- and metallo-β-lactamases, restores activity of cefepime in Enterobacterales and Pseudomonas aeruginosa. Antimicrob Agents Chemother 2020; 64: e01963–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mushtaq S, Vickers A, Doumith M et al. Activity of β-lactam/taniborbactam (VNRX-5133) combinations against carbapenem-resistant Gram-negative bacteria. J Antimicrob Chemother 2021; 76: 160–70. [DOI] [PubMed] [Google Scholar]
- 8. Liu B, Trout REL, Chu G-H et al. Discovery of taniborbactam (VNRX-5133): a broad-spectrum serine- and metallo-β-lactamase inhibitor for carbapenem-resistant bacterial infections. J Med Chem 2020; 63: 2789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clinical Trials, 2021. https://clinicaltrials.gov/ct2/show/NCT03840148?term=taniborbactam&draw=2&rank=1.
- 10. CLSI. Performance Standards for Antimicrobial Susceptibility Testing—Thirty-First Edition: M100. 2021.
- 11. CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Eleventh Edition: M07. 2018.
- 12. Borgonovi M, Miossec C, Lowther J, The efficacy of ceftazidime combined with NXL104, a novel β-lactamase inhibitor, in a mouse model of kidney infections induced by β-lactamase producing Enterobacteriaceae. Seventeenth European Congress of Clinical Microbiology and Infectious Diseases, Munich, Germany, 2007. Poster P794.
- 13. Monogue ML, Giovagnoli S, Bissantz C et al. In vivo efficacy of meropenem with a novel non-β-lactam–β-lactamase inhibitor, nacubactam, against Gram-negative organisms exhibiting various resistance mechanisms in a murine complicated urinary tract infection model. Antimicrob Agents Chemother 2018; 62: e02596–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Venatorx Clinical Study Report, VNRX-5133-104. “Pharmacokinetic Analysis of VNRX-5133 with VNRX-5022 (Cefepime) in Subjects with Varying Degrees of Renal Impairment”.
- 15. Abdelraouf K, Almarzoky AS, Nicolau DP. In vivo pharmacodynamics of new-generation β-lactamase inhibitor taniborbactam (formerly VNRX-5133) in combination with cefepime against serine-β-lactamase-producing Gram-negative bacteria. J Antimicrob Chemother 2020; 75: 3601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patiño-Navarrete R, Rosinski-Chupin I, Cabanel N et al. Stepwise evolution and convergent recombination underlie the global dissemination of carbapenemase-producing Escherichia coli. Genome Med 2020; 12: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Asempa TE, Abdelraouf K, Nicolau DP. Metallo-β-lactamase resistance in Enterobacteriaceae is an artefact of currently utilized antimicrobial susceptibility testing methods. J Antimicrob Chemother 2020; 75: 997–1005. [DOI] [PubMed] [Google Scholar]
- 18. Abdelraouf K, Reyes S, Nicolau DP. The paradoxical in vivo activity of β-lactams against metallo-β-lactamase-producing Enterobacterales is not restricted to carbapenems. J Antimicrob Chemother 2021; 76: 684–91. [DOI] [PubMed] [Google Scholar]
- 19. Asempa TE, Abdelraouf K, Nicolau DP. Activity of β-lactam antibiotics against metallo-β-lactamase-producing Enterobacterales in animal infection models: a current state of affairs. Antimicrob Agents Chemother 2021; 65: e02271–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Avery LM, Vernacchio SF, McLaughlin L et al. Assessment of cefepime-taniborbactam human exposures to suppress the emergence of resistance among serine- and metallo-β-lactamase-producing Gram-negative bacteria in a hollow fiber infection model. IDWeek 2020, Virtual. Abstract 907145.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.