Abstract
Abstract
The American Diabetes Association guidelines for the management of type 2 diabetes mellitus recommends treating patients with atherosclerotic cardiovascular diseases, heart failure or diabetic kidney disease with sodium-glucose co-transporter-2 inhibitors or glucagon-like peptide-1 receptor agonists, irrespective of the baseline HbA1c, to reduce adverse renal and cardiovascular outcomes. Initiation of such therapies have a significant cost impact on health economies. Cost of gain in quality-adjusted life-years is normally used for cost effectiveness for a particular drug. In the absence of head-to-head comparisons, prescribers may go for the cheapest option, which may not necessarily be the right decision. We propose using the calculated ‘YoDa’ (Years of Drug administration) as an easily comparable metric between the drug accrual cost and clinical outcomes. YoDa is calculated as a product of numbers needed to treat and the median duration in years that the trial ran over, to accrue the positive clinical outcomes. Clinical phenotyping of the patient to the specific inclusion and exclusion criteria of relevant clinical trials could guide the clinician to choose the most appropriate therapy. We also propose a series of steps or ‘deliberations’, which a clinician should consider in making a final choice of sodium-glucose co-transporter-2 inhibitor therapy. A comprehensive summary of the sodium-glucose co-transporter-2 inhibitor trials, clinical phenotyping and YoDa calculations for various significant clinical outcomes could assist making evidence-based, patient-individualised and cost-effective management plans for diabetes care.
Video abstract
Informing and Empowering Patients and Clinicians to Make Evidence-Supported Outcome-Based Decisions in Relation to SGLT2 Inhibitor Therapies: The Use of The Novel Years of Drug administration (YoDa) Concept
Supplementary Information
The online version contains supplementary material available at 10.1007/s40261-021-01105-7.
Key Points
| The sodium-glucose co-transporter-2 inhibitor class of drugs has shown consistent benefits in cardiovascular events, hospitalisation for heart failure and renal outcomes in type 2 diabetes mellitus but choosing between the drugs can be difficult. |
| A comprehensive summary of the relevant cardiovascular outcome trials published and a stepwise systematic approach (‘deliberations’) to every consultation to the introduction of sodium-glucose co-transporter-2 inhibitors can help this process. Based on the inclusion and exclusion criteria of trials, a patient can be reasonably matched (‘clinical phenotype’) to a trial to support evidence-based therapeutic decision making. |
| A novel concept of Years of Drug administration (YoDa), which gives a simple clinical measure of cumulative drug exposure needed to derive a clinical outcome benefit, can help the clinician and patient decide the most evidence-based and clinically effective treatment available. |
Introduction
Sodium-glucose co-transporter-2 inhibitors (SGLT2i) have shown significant efficacy in reducing the risk of major adverse cardiovascular events (MACE), heart failure (HF) and renal outcomes in patients with type 2 diabetes mellitus (T2D) in several randomised, placebo-controlled cardiovascular outcome trials (CVOTs). These studies were primarily conducted to show non-inferiority and cardiovascular (CV) safety to comply with US Food and Drug Administration regulatory requirements for new glucose-lowering agents in T2D. The European Association for the Study of Diabetes-American Diabetes Association (EASD-ADA) consensus guidelines proposed the use of a SGLT2i as a preferred second-line drug, in patients with established atherosclerotic cardiovascular disease (ASCVD), HF with reduced ejection fraction or chronic kidney disease (CKD) with significant proteinuria [1]. The recently published ADA guidelines recommend introducing SGLT2i or glucagon-like peptide-1 receptor agonists (GLP-1RA) therapy early in diabetes to offer CV protection, even in patients with good glycaemic control, irrespective of HbA1c [2, 3].
Multiple SGLT2i drugs are available for clinical use but there is no head-to-head comparison between them to inform the choice of use [4]. Healthcare professionals might default to using the drug with the lowest acquisition cost, risking an inappropriate therapeutic choice. The cost associated with the gain in quality-adjusted life-years might be an alternate metric but this information may not be easily accessible and is based on the overall efficacy of a particular drug [5]. There is therefore a risk that clinicians may resort to using a headline summary of trials, based on risk reduction values to make drug choices. It is therefore imperative that the trial data are presented in an easily accessible comparable format with relevant cost-effectiveness data, tailored to individual clinical endpoints desired in a specific patient cohort.
Though the design of all these trials appear similar, there are important differences in the characteristics of patients used (i.e. inclusion and exclusion criteria) and clinical endpoints (i.e. primary and secondary outcomes). Such heterogeneity needs to be taken into consideration when making a clinical choice, but equally provides an opportunity to ‘match’ a given patient to a clinical trial and individualise a treatment option (‘clinical phenotyping’). Patient-accessible clinical information would help to facilitate this endeavour and actively involve the patient in the decision-making process, including the choice of agent to use. Individual drug choice should therefore depend on patient preferences for the potential beneficial outcomes; a clinician’s informed decision making, comparable cost effectiveness and the evidence base.
The objective of this article is to provide a clinical summary of the various SGLT2i CVOTs published and discuss how the positive clinical outcomes from the trial data can be applied to specific patient groups with T2D. The EASD-ADA guideline step of considering SGLT2i in patients with established atherosclerotic disease, HF or diabetic kidney disease (DKD) will be examined from a clinical efficacy and cost-efficiency perspective to guide choosing between the drugs [3]. We used the data in the studies to calculate the numbers of years of treatment that is needed to accrue specific outcome benefits in relation to specific patient groups. The analyses can be used by clinicians and patients to ‘clinically phenotype’ the patient, prioritise the clinical outcome, and devise a cost-effective and evidence-based therapeutic plan.
Years of Drug Administration: YoDa Concept
The number needed to treat (NNT) value informs on the number of patients needed to be treated for the duration of the study to accrue that specific benefit. To give a theoretical example, an NNT of 22 for the outcome of mortality informs a clinician that 22 people need to be treated. Though this is a useful result, a study of 5 years duration vs 3 years would result in very different years of treatment, which could be translated into potential costs to accrue a benefit. A product of NNT and duration of the trial can be represented as person-years of treatment and can help to reasonably equate this. This concept is named, Years of Drug administration (YoDa).
The YoDa can then be multiplied by the annual cost of the drug, (which is commonly readily available from sources such as the British National Formulary and ADA Guidelines 2021) to provide an indication of the cost of deferring or saving an event from occurring, at least for the duration equal to the length of the trial from which such evidence is derived [3, 6]. To provide an example of this concept, the NNT for all-cause mortality was 41 in the EMPA-REG OUTCOME Trial [7]. The median trial duration was 3.1 years. The YoDa is therefore 41 × 3.1 years or 127 treatment-years, meaning that, as a theoretical consideration, 127 years of treatment have to be purchased to save one mortality event. A higher YoDa would therefore imply a higher drug acquisition cost to derive the intended clinical benefit.
The idea may also help compare the cost effectiveness of drugs across various studies; for instance, the MICRO-HOPE study showed that the NNT for ramipril, for MACE prevention, was 22 for 4.5 years. This gives a YoDa of 99; ramipril being generic, costs approximately £25 a year [8]. This means that only £2500 needs to be spent to prevent a mortality event. Similar calculations can therefore allow comparisons between potential costs within a class (SGLT2i in this paper) or between classes for similar outcomes, to choose the most cost-efficient option.
SGLT2i Trials
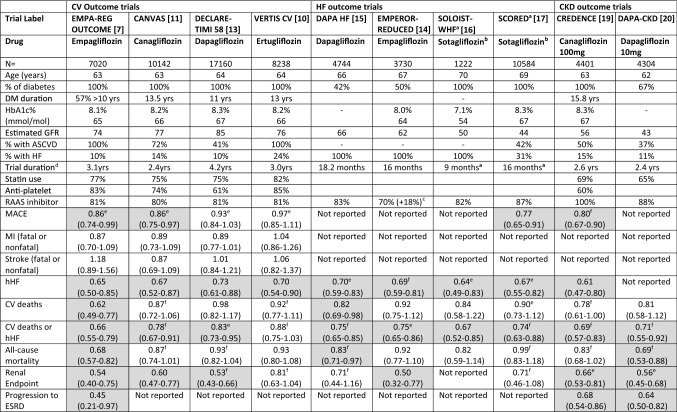
Multiple CVOTs and independent randomised controlled trials have been published on the safety and outcomes of SGLT2i in patients with T2D, specifically looking at MACE (combination of CV death, non-fatal myocardial infarction or non-fatal stroke), but also reporting on benefits with hospitalisation for HF (hHF), CKD progression and all-cause mortality. Further, dedicated trials have since been conducted on specific cohorts of patients with HF and DKD. The clinical summary of the relevant trials, with cohorts stratified according to clinical phenotypes are shown in Table 1.
Table 1.
Summary of cardiovascular outcome, heart failure and renal trials published on SGLT2i drugs
All outcome statistics shown as hazard ratio (95% confidence interval); values in boxes shaded boxes denote hazard ratio not crossing 1
Trial Labels: EMPA-REG OUTCOME, Empagliflozin Cardiovascular Outcome Event trial in Type 2 Diabetes Mellitus Patients; CANVAS/CANVAS-R, Canagliflozin Cardiovascular Assessment Study; DECLARE-TIMI 58, Dapagliflozin Effect on Cardiovascular Events-Thrombolysis in Myocardial Infarction; VERTIS CV, Cardiovascular Outcome following Ertugliflozin treatment in Type 2 Diabetes Mellitus Participants with Vascular Disease; DAPA-HF, Dapagliflozin and Prevention of Adverse-outcomes in Heart Failure; EMPEROR-REDUCED, Empagliflozin Outcome trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction; SOLOIST-WHF, Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure; SCORED, Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2 Diabetes and Moderate Renal impairment who are at Cardiovascular Risk; CREDENCE, Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy; DAPA-CKD, Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease
ASCVD atherosclerotic cardiovascular disease, CV cardiovascular, CKD chronic kidney disease, DM type 2 diabetes mellitus, ESRD end-stage renal disease, GFR glomerular filtration rate (mL/min/1.73 m2), HF heart failure, hHF hospitalisation for heart failure, MI myocardial infarction, RAAS renin angiotensin aldosterone system, SGLT1 sodium-glucose co-transporter 1 inhibitor, SGLT2 sodium-glucose co-transporter 2 inhibitor
aStudy terminated because of funding withdrawal and drug not available in the UK
bSotagliflozin is an SGLT1 and 2 inhibitor
cEMPEROR-Reduced, a further 18% were on a sicabutril+valsartan combination
dMedian duration
ePrimary outcomes as documented in the trials
fSecondary outcomes as protocolled in the trials (some differences accommodated to allow comparability, renal endpoints are different between trials; some trials included urgent visits as hHF)
The ADA guidelines advise the initiation of SGLT2i or GLP-1RA in patients with established ASCVD or patients with very high risk of such events [3]. Atherosclerotic cardiovascular disease is broadly defined in the guidelines as: Coronary artery disease—known history of myocardial infarction, coronary artery bypass grafting, coronary stents, unstable angina, imaging-proven myocardial ischaemia; Cerebrovascular disease—stroke or transient ischaemic attack; Peripheral arterial disease—requiring revascularization, foot or above amputation [3]. The inclusion criteria of many of the trials had an overlap of established or high risk for a CV event labelled under the umbrella of ASCVD. Some of these trials also included cohorts of patients without such ASCVD but with risk factors for CV disease such as hypertension or dyslipidaemia (Table 4 of the Electronic Supplementary Material [ESM]).
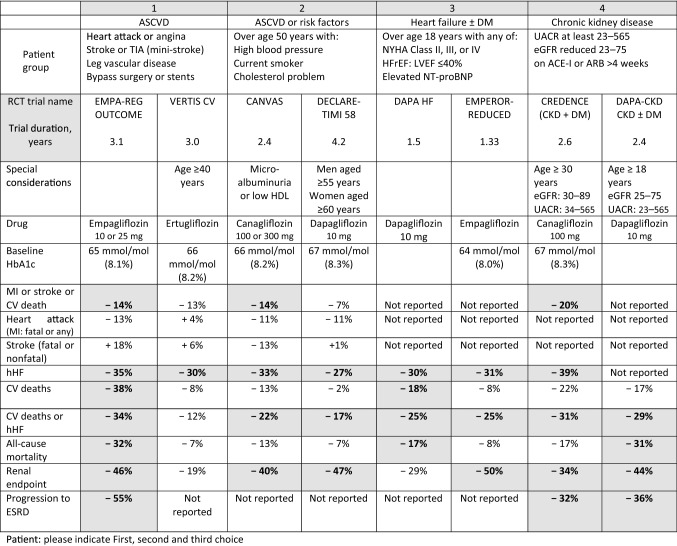
The outcomes’ statistics of these studies are generally published as relative risk reductions, hazard ratios (HRs) with 95% confidence intervals (CIs). The percentage reduction (or indeed increase) in an outcome can be calculated from 1 minus the HR multiplied by 100. For example, an HR of 0.53 will then equate to a 47% reduction in that outcome. The NNT to prevent one event is not often included for all outcomes but can be calculated from the absolute risk reduction data presented in the relevant papers. Table 2 shows the calculated YoDa for relevant clinical endpoints where the CI for HR did not cross 1 (Table 2 of the ESM provides a comparison of cost in US dollars, based on NADAC (National Average Drug Acquisition Cost) data published in ADA guidelines). A patient-friendly version of the trial data, with the concept of ‘clinical phenotyping’ is provided in Table 3 (Tables 1 and 3 of the ESM provide additional information on the calculated NNT and relative risk reduction for the trial outcomes).
Table 2.
YoDa estimates and approximate drug acquisition cost to achieve clinical outcomes
| Patient characteristics | DM with ASCVD | DM with Risk factors or ASCVD | DM with HFrEF | DM with CKD with albuminuria | ||||
|---|---|---|---|---|---|---|---|---|
| Trial Label | EMPA-REG OUTCOME [7] | VERTIS CV [10] | CANVAS [11] | DECLARE-TIMI 58 [13] | DAPA-HF [15] | EMPEROR-Reduced [14] | CREDENCE [19] | DAPA-CKD [20] |
| Drugs | Empagliflozin | Ertugliflozin | Canagliflozin | Dapagliflozin | Dapagliflozin | Empagliflozin | Canagliflozin | Dapagliflozin |
| Annual cost (£) | £477 | £383 | £477 | £477 | £477 | £477 | £477 | £477 |
| MACE |
195 YoDaa £93,015 |
NSa |
511 YoDaa £243,747 |
NSa | Not reported | Not reported |
104 YoDab £49,608 |
Not reported |
| HF hospitalization |
220 YoDa £104,940 |
277 YoDa £106,091 |
751 YoDa £358,227 |
525 YoDa £250,425 |
40 YoDaa £19,080 |
26 YoDab £12,402 |
109 YoDa £51,993 |
Not reported |
| CV Deaths |
143 YoDa £68,211 |
NSb | NSb | NS |
80 YoDab £38,160 |
NS | NS | NS |
| CV deaths or hHF |
174 YoDa £82,998 |
NSb |
533 YoDab £254,241 |
466 YoDaa £222,282 |
32 YoDa £15,264 |
25 YoDaa £11,925 |
75 YoDab £35,775 |
134 YoDab £63,918 |
| All-cause mortality |
127 YoDa £60,579 |
NS | NS | NSb |
66 YoDab £31,482 |
NS | NS |
115 YoDab £54,855 |
| Renal-comp endpoint |
220 YoDa £104,940 |
NSb |
686 YoDa £327,222 |
323 YoDab £154,071 |
NSb |
82 YoDa £39,114 |
57 YoDaa £27,189 |
96 YoDaa £45,792 |
| Progression to ESRD |
1032 YoDa £492,264 |
Not reported | Not reported | Not reported | Not reported | Not reported |
117 YoDa £55,809 |
101 YoDa £48,177 |
YoDa = NNT × duration of study in years; cost = YoDa × annual cost of drug (derived from no. of packs needed per year); all values rounded to nearest £
YoDa not calculated if outcome data was not significant
Sotagliflozin not included as costing not available in the UK
ASCVD atherosclerotic cardiovascular disease, CKD chronic kidney disease, CV cardiovascular, ESRD end-stage renal disease, HF heart failure, HFrEF heart failure with reduced ejection fraction, hHF hospitalisation for heart failure, MACE major adverse cardiovascular events, NS not significant, YoDa years of drug administration
aPrimary outcomes as documented in the trials
bSecondary outcomes as protocolled in the trials
Table 3.
Patient information version: summary of clinical outcomes for the sodium-glucose co-transporter 2 inhibitor class of drugs
ACE-I or ARB angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, ASCVD atherosclerotic cardiovascular disease, CKD chronic kidney disease, CV cardiovascular, eGFR estimated glomerular filtration rate (mL/min/1.73 m2), ESRD end-stage renal disease, HDL high-density lipoprotein, HF heart failure, HFrEF heart failure with reduced ejection fraction, hHF hospitalisation for heart failure, LVEF left-ventricular ejection fraction, MACE major adverse cardiovascular events, MI myocardial infarction, NYHA New York Heart Assocation, RCT randomised controlled trial, TIA transient ischaemic attack, UACR urine albumin creatinine ratio
Bold values in shaded boxes denote significant p-value or hazard ratio < 1; negative value means a reduction in the risk of outcome
Using the SGLT2i Trial Data in Clinical Practice
The ADA guidelines recommend the addition of SGLT2i or GLP-1RA irrespective of the HbA1c to reduce CV outcomes in those with ASCVD, HF or CKD [2, 3]. This is a paradigm shift in how diabetes is managed, moving away from the convention of treating a glycaemic target alone, and taking on the perspective of primary and secondary CV prevention. The use of the most cost-efficient approach is clearly mandated in the National Institute for Health and Care Excellence guidelines and specifically within the Getting it Right First Time (GiRFT Report 2020) within the Medicines Optimisation recommendations in the UK (Table 4 and Table 3 of the ESM) [9].
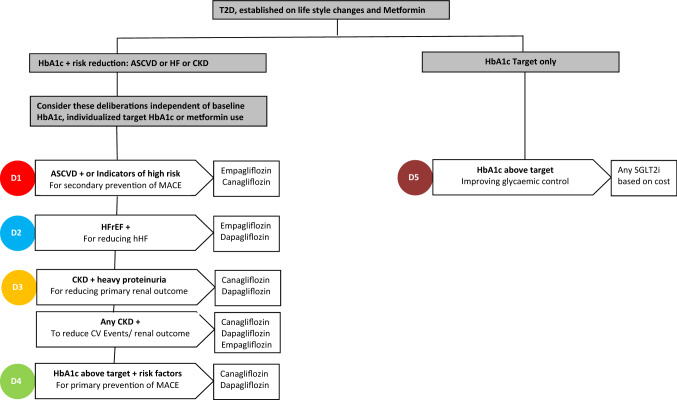
Once a clinical decision to choose SGLT2i has been made between the patient and the clinician, the following stepwise approach might help to facilitate the process of clinical phenotyping, individualising the clinical endpoint target and choosing the appropriate SGLT2i based on the trial outcome and YoDa data (Fig. 1). It is assumed that the patient has been optimised, as far as possible within that individual patient, with respect to lifestyle measures and metformin therapy, which is the first-line treatment in almost all national and international diabetes care guidelines. The recent ADA guidelines, though have the same recommendation, also suggest the need to start considering combination therapy at the outset if the HbA1c is significantly elevated [3].
Fig. 1.
Flow chart showing the clinical deliberations and evidence-based decision to guide the choice of sodium-glucose co-transporter 2 inhibitor (SGLT2i); D stands for deliberations. Glucagon-like peptide-1 receptor agonists would also be alternate choices for deliberations 1–4. Other drug classes such as dipeptidyl peptidase-4 inhibitors offer glycaemic control but without cardiovascular or renal outcome data. ASCVD atherosclerotic cardiovascular disease, CKD chronic kidney disease, CV cardiovascular, HF heart failure, HFrEF heart failure with reduced ejection fraction, hHF hospitalization for heart failure, MACE major adverse cardiovascular events, T2D patients with type 2 diabetes mellitus
First Deliberation: Does the Patient Have Established ASCVD or is High Risk for CVD?
The ADA guidelines recommend starting a SGLT2i or GLP-1RA in patients with ASCVD or with a very high risk for a CV event, irrespective of the HbA1c, with an aim to offer secondary protection. The clinical outcome that needs to be targeted in this cohort would be the improvement in MACE, though other clinical endpoints might also be equally relevant. The EMPA-REG OUTCOME and VERTIS included patients exclusively with ASCVD; other SGLT2i CVOTs included patients with ASCVD or without ASCVD but with CV risk factors alone (Table 1, Fig. 1).
EMPA-REG OUTCOME assessed the safety and efficacy of empagliflozin in patients with ASCVD and showed a modest but significant 14% reduction in MACE (largely driven by a 38% reduction in CV deaths), a 32% reduction in all-cause mortality, a 35% reduction in incident HF or hHF, and a 36% reduction in renal endpoints [7]. The study showed no difference between the 10-mg and 25-mg doses with respect to CV outcomes, though the dose increment would provide some additional metabolic benefit. The reduction in mortality was consistent across all sub-groups of patients, observed quite early on initiation of treatment; the benefit was demonstrated over and above the well-established use of renin-angiotensin-aldosterone system inhibitors, aspirin and statins.
The VERTIS study assessed the safety of ertugliflozin, which is currently the cheapest available SGLT2i [10]. Though the study established CV safety of this drug, it did not show superiority of the drug over placebo in reducing MACE or deaths but showed a benefit in the reduction in hHF (24% of the trial cohort had HF but the diagnostic criteria was not pre-defined).
The CANVAS Program, an integrated analysis of two similarly designed CVOTs, CANVAS and CANVAS-R, assessed the safety of canagliflozin and included 66% of patients with ASCVD [11]. The use of canagliflozin was associated with a significant improvement in MACE, but did not show an individual benefit for CV deaths. The study however showed a higher risk of lower limb amputation rates and fractures compared with placebo. The DECLARE-TIMI 58 trial, which assessed the safety of dapagliflozin, included 41% of patients with ASCVD. This study did not show superiority for this drug for MACE or CV death [12]. Both these studies showed significant reductions in the combination of CV deaths or hHF, driven by HF protection predominantly, and no heterogeneity in outcomes between patients with ASCVD or risk factors, but the benefit for CV deaths or hHF was generally more in the ASCVD cohort. A sub-study published from DECLARE-TIMI 58 showed a lower risk for MACE in patients with ASCVD, especially previous myocardial infarction [13].
The ADA guidelines suggest the use of empagliflozin or canagliflozin in patients with ASCVD for CV benefits, as these two drugs showed reductions in MACE. Based on the research evidence and class effect, all SGLT2i might be able to offer secondary prevention, but the best option might be empagliflozin for secondary protection, with an additional benefit of reducing hHF and renal outcomes in this cohort (Table 2).
Second Deliberation: Does the Patient Have a History of HF?
The next consideration in choosing a drug in a clinical-phenotyped approach would be the presence of HF (Fig. 1). The ADA recommends considering SGLT2i in patients with HF (especially with left-ventricular ejection fraction <45%), again irrespective of HbA1c. The clinically relevant endpoint in this cohort would ideally be a reduction in hHF or CV deaths. The SGLT2i as a class are well known to offer protection against hHF but the inclusion criteria in various CVOT trials are not well characterised and hence difficult to define if the prevention is primary or secondary. The EMPEROR-Reduced and DAPA-HF specifically addressed the efficacy of these drugs in patients with pre-diagnosed HF.
The EMPEROR-Reduced trial studied the effect of empagliflozin on patients with clinically advanced HF, with symptoms of HF (New York Heart Association, NYHA functional class II–IV), elevated B-natriuretic peptide and reduced left-ventricular ejection fraction ≤ 40% [14]. Seventy-three percent of the included cohort had an ejection fraction < 30% and 48% had an estimated glomerular filtration rate (GFR) < 60 mL/min/1.73 m2. Empagliflozin showed a significant 25% reduction in the primary outcome of the combination of hHF or CV deaths and hHF alone by 31%, consistent amongst all subgroups. The study also showed a slower decline in GFR in the empagliflozin cohort, thereby proving the cardio-renal protective effect. The DAPA-HF trial studied the use of dapagliflozin in HF but used marginally lower thresholds for inclusion than the EMPEROR-Reduced [15]. DAPA-HF showed an overall reduction in the combination of hHF and CV deaths, as well as individually with these endpoints, and a reduction in all-cause mortality. The benefit was more pronounced in patients with NYHA class II than with NYHA classes III–IV. Both studies included patients without diabetes and the outcomes were consistently seen across both groups, therefore validating the benefit of SGLT2i beyond glycaemic control. These studies demonstrated the benefits over and above the use of renin-angiotensin-aldosterone system inhibitors and the safety of concurrent use with diuretics.
The SOLOIST-WHF and the SCORED trials looked at the efficacy of the SGLT1 and SGLT2 inhibitor sotagliflozin, in T2D with HF. The studies were terminated at 9 and 16 months, respectively, owing to funding withdrawal. However, an analysis of the available data showed a significant reduction in CV deaths or hHF, largely driven by a reduction in hHF, again demonstrating the very early clinical benefit associated with the use of these drugs [16, 17].
The ADA recommends the use of canagliflozin, empagliflozin or dapagliflozin in this cohort. Dedicated trial evidence is available for the latter two drugs and an informed choice can be made between the clinician and the patient (Table 2).
(A) Third Deliberation: Does the Patient Have DKD with Significant Albuminuria?
The next clinical phenotyping to consider would be the presence of DKD (reduction in estimated GFR and/or albuminuria without other reasons for kidney disease) and the ADA recommends the use of SGLT2i, irrespective of HbA1c, with specific evidence on the reduction of DKD progression; and if this is not possible, at least to choose a drug with evidence of CKD progression on CVOTs. The primary treatment target in this cohort would be to prevent progression to end-stage renal disease and CV events [18]. Though SGLT2i as a class may have a reno-protective effect, the CREDENCE and DAPA-CKD trials specifically recruited patients with DKD (Fig. 1).
The CREDENCE trial evaluated the CV efficacy of canagliflozin 100 mg in patients with T2D with an estimated GFR of 30 but < 90 mL/min/1.73 m2 with a urine albumin creatinine ratio > 300 (expressed as albumin measured in milligrams and creatinine in grams) [19]. Canagliflozin showed a significant 30% reduction in the primary outcome (composite renal endpoint and CV deaths), achieved over and above the use of renin-angiotensin-aldosterone system inhibitors and independent of a glycaemic control difference. The incidence of individual renal endpoints (progression to end-stage renal disease or doubling of serum creatinine) was also significantly reduced. There was a 20% reduction in MACE, 39% in hHF and 34% with renal-specific outcomes, the latter benefit best demonstrated in patients with an estimated GFR between 30 and 60 mL/min/1.73 m2. This is in contrast with its glucose-lowering effect, which diminishes as the GFR drops.
The DAPA-CKD trial evaluated the effect of dapagliflozin in patients with or without T2D with an estimated GFR between 25 and 75 mL/min/1.73 m2 with a urine albumin creatinine ratio > 200 (14% of the trial population had an estimated GFR < 30 mL/min/1.73 m2) [20]. Dapagliflozin showed a significant reduction in progression to the composite renal endpoint and CV death, independent of the presence of T2D and the point estimates for each of the component favoured the use of dapagliflozin.
Based on the research evidence available, a choice can be made between dapagliflozin or canagliflozin in this cohort (Table 2). The established safety of dapagliflozin at a lower GFR would likely be applicable across all drugs in this class.
(B) Does the Patient Have Evidence of CKD?
The ADA recommends the use of SGLT2i (or GLP-1RA) with proven CV benefits in this ‘clinical phenotype’ of patients with CKD (such as an estimated GFR < 60 mL/min/1.73 m2), irrespective of HbA1c, with a primary treatment intention to reduce CV events and reduce progression to end-stage renal disease. A number of SGLT2i CVOTs have also measured renal endpoints and included a small proportion of patients with some proteinuria and/or reduced estimated GFR. The renal composite endpoints used as the outcome are different between the trials (Table 4).
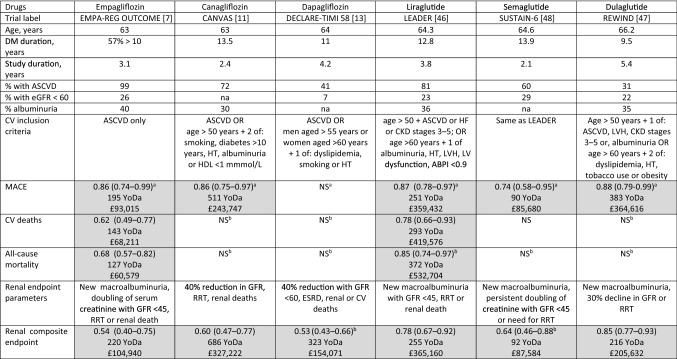
Table 4.
Comparison data of YoDa of sodium-glucose co-transporter 2 inhibitors SGLT2i and glucagon-like peptide-1 receptor agonistsGLP-1RA in achieving similar clinical endpoints and approximate cost implication (rounded to nearest £)
ABPI ankle brachial pressure index, ASCVD atherosclerotic cardiovascular disease, CKD chronic kidney disease, CV cardiovascular, ESRD end-stage renal disease, GFR glomerular filtration rate (mL/min/1.73 m2), HF heart failure, HT hypertension, LV left ventricle, LVH left-ventricular hypertrophy, MACE major adverse cardiovascular events, na not available, NS not significant, RRT renal replacement therapy, YoDa Years of Drug administration
Liraglutide cost based on 1.8 mg/day, thus £1432/year; semaglutide and dulaglutide £952/year
aPrimary outcomes as documented in the trials
bSecondary outcomes as protocolled in the trials
Shaded boxes denote hazard ratio not crossing 1; YoDa not calculated if outcome data not statistically significant
Trial Labels: LEADER, Liraglutide Effect and Action in Diabetes:Evaluation of Cardiovascular Outcome Results; SUSTAIN-6, Trial to Evaluate Cardiovascular and other Long-term Outcomes in Subjects with Type 2 Diabetes; REWIND, Researching Cardiovascular Events with a Weekly Incretin in Diabetes
The EMPA-REG OUTCOME included 26% of patients with an estimated GFR ≤ 59 mL/min/1.73 m2 of whom 47% had normoalbuminuria (urine albumin creatinine ratio < 30), and reported separately on their microvascular outcomes [21]. The use of empaglifozin was associated with a significant reduction in event rates of composite renal endpoints, independent reduction in progression to macroalbuminuria, progression of CKD, and the need for renal replacement therapy. It did not show any protection for incident microalbuminuria, but the renal and CV benefits were consistent across all ranges of GFR and albuminuria [22]. The DECLARE-TIMI 58 trial included only 7% of patients with estimated GFR < 60 mL/min/1.73 m2 and showed an improvement in composite renal outcome with dapagliflozin use, homogenously across all subgroups especially with or without ASCVD [12, 23]. The CANVAS program included 20% with an estimated GFR < 60 mL/min/1.73 m2 with 11% normoalbuminuria. A secondary analysis demonstrated consistent CV and renal outcome benefits across various GFR strata [11, 24].
Though all three SGLT2i drugs have good evidence of reducing renal outcomes, it is important to acknowledge that the EMPA-REG OUTCOME only involved patients with ASCVD. Canagliflozin has evidence for the reduction of MACE in this cohort. Dapagliflozin might be a better option in achieving renal outcomes. The choice between these drugs can be decided based on individualised therapeutic endpoints, taking the evidence base into consideration.
Fourth Deliberation: Does the Patient Need Improvement in Glycaemic Control and Have Risk Factors for CVD
The management in this clinical phenotype (without ASCVD, HF or CKD/DKD but risk factors) would be driven by intent for HbA1c control (Fig. 1). The ADA does not classify this as a separate cohort, but the recommendation is to individualize treatment based on important clinical needs (such as hypoglycaemia risk, weight issues or cost). The CANVAS and DECLARE-TIMI 58 included patients without ASCVD but with major risk factors. The CANVAS programme showed significant improvement with canagliflozin on MACE, with no heterogeneity based on pre-existent ASCVD [11, 25]. The DECLARE-TIMI 58 trial enrolled the largest proportion of patients without ASCVD, did not show a statistically significant reduction in MACE or all-cause mortality, but a reduction in the combination of CV deaths or hHF [12].
A pooled data analysis combining the data of non-ASCVD from CANVAS and DECLARE-TIMI 58 showed a consistent significant reduction in renal outcomes irrespective of the presence of ASCVD, better renal outcomes in patients with preserved renal function and a reduction in hHF irrespective of baseline HF, largely driven by dapagliflozin data [26]. Real-life clinical practice studies have demonstrated comparable benefit for SGLT2i drugs as a class [27]. Based on CV risk factors and the desired clinical endpoint targets in a given patient, an evidence-based best treatment option can be derived from the SGLT2i trials. For instance, a patient who is 60 years old with dyslipidaemia might benefit from the use of dapagliflozin to prevent renal outcomes (Table 3).
It is important to note that all CVOTs had a reasonable proportion of patients taking metformin monotherapy and the average HbA1c in the CVOTs were generally >8.0%, hence the evidence on SGLT2is can help to position the drug appropriately in the treatment pathways [28]. There is certainly also an argument to consider de-prescribing drugs without proven CV benefits, such as dipeptidyl peptidase-4 inhibitors and substitute with SGLT2i [29–31]. However, the primacy of patient preference in relation to outcomes with clinician input applies as a principle in clinical practice.
Final Deliberation: Does the Patient Need Glycaemic Control Improvement Alone?
For patients who need glycaemic control only, the current drug choice would not be guided by empirical evidence but decided based on patient choice, cost, efficacy and side effects as per the ADA. A number of real clinical practice database reviews have shown similar benefits of hHF and renal outcomes in patients without ASCVD or substantial risk factors [27, 32, 33]. Therefore, when there is no specific steer from the clinical risk factors, agents with the lowest acquisition cost should be considered. The treatment choices should be continuously reviewed to assess treatment efficacy. At each review, the patient can be clinically phenotyped again based on the above deliberations and initiated on a different medication based on clinical needs.
Discussion
This review provides a stepwise approach to manage a patient with T2D, incorporating the current guidelines, providing trial data in a comparable format and using a simple YoDa calculation, to provide evidence-based and patient-centred individualised care. This approach is summarised in Fig. 1.
The YoDa calculation provides an easy and comparable parameter to estimate the cost involved in preventing or improving a clinical endpoint and making therapeutic judgements. For instance, clinicians could prefer using ertugliflozin as this is the cheapest SGLT2i, but from the trial data its CV benefits are not similar; similarly from an hHF perspective, empagliflozin might be a better choice based on YoDa [10]. This model of calculation had been previously reported as a teaching tool but this review extends its use as a clinical model to inform practice and decision making [34]. A number of studies have shown the cost effectiveness of SGLT2i as a class, especially when balanced against the expenditure associated with morbidities of vascular complications of diabetes and other therapies [35, 36]. Complex economic modelling studies may help to show the advantage of a particular drug against a specific outcome [37, 38]. The incremental cost-effectiveness ratio (ICER) per quality-adjusted life-year is a commonly used parameter to calculate a cost estimate but this is based on the overall therapeutic efficacy of a particular drug [39, 40]. As per the National Institute for Health and Care Excellence technology appraisal guidance in the UK, the ICERs associated with all three SGLT2i as monotherapy were deemed comparable [5]. Though this does not guide towards a choice, it provides the clinician the opportunity to choose between the drugs freely. The YoDa calculation can further guide to an ‘individualised’ choice based on the most important clinical outcome to be prevented.
A number of meta-analyses have been published showing the efficacy of SGLT2i on HF and renal outcomes, therefore supporting a class effect especially in the high-risk cohort [41–43]. A recently published meta-analysis also showed that the protection offered by this class increases significantly with the presence of multiple ASCVD or increasing CVD risk factors in the patient [44]. The benefits of this class of drugs have also been shown in real-world practice. The EMPRISE study showed a significant reduction in hHF in patients with or without CV disease with the use of empagliflozin [33]. CVD-REAL, a large database comparative study of SGLT2i with other anti-diabetic medications, showed a significant 39% reduction in hHF and a 51% reduction in all-cause mortality, with a large proportion of patients not having ASCVD, and therefore proving that the benefit may also extend to patients with low CV risk [27, 32]. However, it is important to acknowledge that not all the benefits seen in trials can be replicated in real-life clinical practice [45].
The ADA guidelines proposes an “either/or” approach to choose between GLP-1RA and SGLT2i in the ASCVD cohort and three important GLP-1RA CVOTs demonstrated CV benefits. YoDa can be applied to reasonably compare the benefit offered by various classes of drugs for the same clinical outcome (Table 4). The LEADER study demonstrated the safety and efficacy of liraglutide with a significant improvement in MACE, with a particular reduction in CV deaths [46]. The REWIND study showed a significant reduction in MACE with the use of dulaglutide and SUSTAIN-6 with semaglutide, both of which were largely driven by a reduction in non-fatal stroke rather than CV deaths [47, 48]. The GLP-1RA trials had different inclusion characteristics to the SGLT2i CVOTs (Table 4 of the ESM). Lixisenatide (ELIXA trial had 100% patients with ASCVD) or exenatide LAR (EXSCEL trial involving 70% with ASCVD) did not show a significant reduction in MACE compared to placebo [49, 50]. The HARMONY trial had shown evidence of a MACE reduction with the use of albiglutide, predominantly driven by a reduction in myocardial infarction, but this drug has now been withdrawn from clinical use on commercial grounds [51]. A combined meta-analysis of these CVOTs has shown a favourable effect of these drugs on MACE and all-cause mortality [52]. The LEADER, SUSTAIN-6 and REWIND trials had included 23%, 28% and 22% with an estimated GFR of < 60 mL/min/1.73 m2, respectively, and demonstrated reductions in the composite renal endpoint [46–48, 53, 54]. The renal outcomes of GLP-1RA are largely driven by reductions in the onset of macroalbuminuria data whereas SGLT2i offer a more varied protection towards worsening GFR, end-stage renal disease and death [55]. The GLP-1RA CVOTs did not demonstrate any benefit on hHF. The ADA proposes the use of liraglutide, semaglutide or dulaglutide for achieving CV benefits and YoDa can help to support making a choice between these.
The factors that guide the choice between SGLT2i and GLP-1RA in real clinical practice are different. A comparative study combining the various published studies amongst these two classes have demonstrated that SGLT2i, as a class, has significant benefits in hHF and CKD progression compared with GLP-1RA [56]. The outcomes with MACE are reasonably similar between the drug classes. A comparison between these two classes also demonstrated that the adverse CV and renal outcomes were fewer with SGLT2i (apart from strokes) compared with GLP-1RA on similar cohorts of patients [44]. Among drugs with similar outcomes, the duration and cost involved in achieving the clinical endpoint would be significantly different. The cost effectiveness of the drug in achieving these CV outcomes needs to be taken into consideration when a patient and the clinician make the final choice as to which therapy to initiate. The YoDa calculation is useful to provide simple clinical guidance to choose between the drugs and is not meant to directly compare or brand a particular drug as less or more effective than the others in the class. This current era of the COVID-19 pandemic has resulted in almost all national economies experiencing an economic recession of a small or larger magnitude and the need for cost efficiency is abundantly clear. Ultimately the health economies need to prevent diabetes complications by achieving reasonable diabetes ‘remission’ and prevention of diabetes itself, which can have a positive impact on diabetes complications, which would in turn reduce expenditure on medications and healthcare [57].
Limitations
Our comparative review has important limitations. First, a number of benefits such as overall secondary prevention of MACE and hHF and a reduction in renal outcomes with SGLT2i can be a class effect and might be generalized between the drugs; the trials however had not consistently demonstrated this depending on the patient’s inclusion characteristics and choosing a drug within the SGLT2i class can be difficult and challenging [26, 42]. A combination of clinical phenotyping, accessible trial data to the patient and clinician, and YoDa can guide an appropriate choice. Second, assigning patients to clinical phenotypes would not be as discrete and singular as ASCVD, HF or CKD in clinical practice. Improving HbA1c would always be the overarching priority during clinical encounters. Further, various other factors such as patient characteristics and priorities, individualised clinical outcome targets and other co-morbidities influence decision making. Third, the YoDa calculation is only an approximation of the cost involved based on acquisition cost, risk reduction ratios and NNT. A number of other medico-economic factors such as the cost of hospitalisation or interventions would normally be factored into economic models to accurately assess the cost effectiveness of the drugs. Similarly, a sub-analysis of cohorts in larger trials (such as patients with ASCVD in CANVAS or DECLARE-TIMI 58) may yield a different NNT. Fourth, using the median duration of a trial, as an equivalent of treatment duration to measure cost effectiveness, has its own limitations. The trial duration is determined by numerous factors during trial methodology and does not generally reflect the treatment duration needed to derive a benefit. Additionally, clinical events happen at random time-points during a trial. It is well known that the benefits with SGLT2i can start quite early. Using the end-of-the-trial NNT and the trial duration together may not accurately reflect this. Fifth, though NNT could be a useful clinical parameter, it is only one of the statistical outcomes and its limitations are well recognized [58–60]. Furthermore, making binary decisions on drug choices based on p-values and HRs alone may not be ideal as they might not always identify similarities or differences between the strength of evidence from these trials [61].
Conclusions
The YoDa concept provides a triangulated approach between the evidence base from randomised controlled trials, clinical phenotyping and practical cost consideration to facilitate effective clinical decision making. With the latest diabetes management recommendations moving away from using the HbA1c alone as the primary target and instead focusing on CV outcomes, the deliberation-based stepwise approach and YoDa can serve as an effective approach to guide management planning. The summaries of key SGLT2i landmark studies and the novel approaches used in this paper would help facilitate the process of informed decision making, with the patient as the centre of focus of care. The above deliberations can provide a template to review the risk profile and clinical treatment target of the patient at each consultation to tailor treatment choices.
Supplementary Information
Declarations
Funding
No funding was received for this study.
Conflicts of interest
Vinod Patel has worked with a large number of pharmaceutical companies in the field of diabetes care, including Astra Zeneca, Boehringer Ingelheim, Knapp, MSD, Lilly, Novo, Sanofi, Takeda and Mylan. Sarah Ali is a consultant diabetologist on the National Institute for Health and Care Excellence Guidelines Committee. There are no conflicts of interest for the other authors.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
All authors contributed to the study conception and methods. Material preparation, data collection and analysis were performed by Lakshminarayanan Varadhan and Vinod Patel. The first draft of the manuscript was written by Lakshminarayanan Varadhan and all authors commented and contributed to various versions of the manuscript. All authors read and approved the final manuscript.
References
- 1.Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycemia in type 2 diabetes, 2018: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41(12):2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association 10. Cardiovascular disease and risk management: standards of medical care in diabetes: 2021. Diabetes Care. 2021;44(Suppl. 1):S125–S150. doi: 10.2337/dc21-S010. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association 6. Glycemic targets: standards of medical care in diabetes: 2021. Diabetes Care. 2021;44(Suppl. 1):S73–84. doi: 10.2337/dc21-S006. [DOI] [PubMed] [Google Scholar]
- 4.Das SR, Everett BM, Birtcher KK, Brown JM, Januzzi JL, Kalyani RR, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(9):1117–1145. doi: 10.1016/j.jacc.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence. Canagliflozin, dapagliflozin and empagliflozin as monotherapies for type 2 diabetes (TA390). 2016. www.nice.org.uk/guidance/ta390. Accessed Mar 2021.
- 6.Joint Formulary Committee . British National Formulary. 80. London: BMJ Group and Pharmaceutical Press; 2020. [Google Scholar]
- 7.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 8.Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet. 2000;355(9200):253–9. [PubMed]
- 9.Rayman J, Kar P. Diabetes-GIRFT Programme National Specialty Report. 2020. www.gettingitrightfirsttime.co.uk/wp-content/uploads/2020/11/GIRFT-diabetes-report.pdf. Accessed 21 Feb 2021.
- 10.Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383(15):1425–1435. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 11.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 12.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 13.Furtado RHM, Bonaca MP, Raz I, Zelniker TA, Mosenzon O, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation. 2019;139(22):2516–2527. doi: 10.1161/CIRCULATIONAHA.119.039996. [DOI] [PubMed] [Google Scholar]
- 14.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 15.McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 16.Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384(2):117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 17.Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384(2):129–139. doi: 10.1056/NEJMoa2030186. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association 11. Microvascular complications and foot care: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl. 1):S151–S167. doi: 10.2337/dc21-S011. [DOI] [PubMed] [Google Scholar]
- 19.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 20.Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 21.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 22.Wanner C, Lachin JM, Inzucchi SE, Fitchett D, Mattheus M, George J, et al. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation. 2018;137(2):119–129. doi: 10.1161/CIRCULATIONAHA.117.028268. [DOI] [PubMed] [Google Scholar]
- 23.Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7(8):606–617. doi: 10.1016/S2213-8587(19)30180-9. [DOI] [PubMed] [Google Scholar]
- 24.Neuen BL, Ohkuma T, Neal B, Matthews DR, de Zeeuw D, Mahaffey KW, et al. Cardiovascular and renal outcomes with canagliflozin according to baseline kidney function. Circulation. 2018;138(15):1537–1550. doi: 10.1161/CIRCULATIONAHA.118.035901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahaffey KW, Neal B, Perkovic V, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS Program (Canagliflozin Cardiovascular Assessment Study) Circulation. 2018;137(4):323–334. doi: 10.1161/CIRCULATIONAHA.117.032038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 27.Kosiborod M, Lam CSP, Kohsaka S, Kim DJ, Karasik A, Shaw J, et al. Cardiovascular events associated with SGLT-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL 2 Study. J Am Coll Cardiol. 2018;71(23):2628–2639. doi: 10.1016/j.jacc.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Marx N, Davies MJ, Grant PJ, Mathieu C, Petrie JR, Cosentino F, et al. Guideline recommendations and the positioning of newer drugs in type 2 diabetes care. Lancet Diabetes Endocrinol. 2021;9(1):46–52. doi: 10.1016/S2213-8587(20)30343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filion KB, Lix LM, Yu OH, Dell'Aniello S, Douros A, Shah BR, et al. Sodium glucose cotransporter 2 inhibitors and risk of major adverse cardiovascular events: multi-database retrospective cohort study. BMJ. 2020;370:m3342. doi: 10.1136/bmj.m3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birkeland KI, Bodegard J, Banerjee A, Kim DJ, Norhammar A, Eriksson JW, et al. Lower cardiorenal risk with sodium-glucose cotransporter-2 inhibitors versus dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes without cardiovascular and renal diseases: a large multinational observational study. Diabetes Obes Metab. 2021;23(1):75–85. doi: 10.1111/dom.14189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohsaka S, Lam CSP, Kim DJ, Cavender MA, Norhammar A, Jorgensen ME, et al. Risk of cardiovascular events and death associated with initiation of SGLT2 inhibitors compared with DPP-4 inhibitors: an analysis from the CVD-REAL 2 multinational cohort study. Lancet Diabetes Endocrinol. 2020;8(7):606–615. doi: 10.1016/S2213-8587(20)30130-3. [DOI] [PubMed] [Google Scholar]
- 32.Kosiborod M, Cavender MA, Fu AZ, Wilding JP, Khunti K, Holl RW, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors) Circulation. 2017;136(3):249–259. doi: 10.1161/CIRCULATIONAHA.117.029190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patorno E, Pawar A, Franklin JM, Najafzadeh M, Deruaz-Luyet A, Brodovicz KG, et al. Empagliflozin and the risk of heart failure hospitalization in routine clinical care. Circulation. 2019;139(25):2822–2830. doi: 10.1161/CIRCULATIONAHA.118.039177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maharaj R. Adding cost to number needed to treat: the COPE statistic. Evid Based Med. 2007;12(4):101–102. doi: 10.1136/ebm.12.4.101. [DOI] [PubMed] [Google Scholar]
- 35.Pawaskar M, Bilir SP, Kowal S, Gonzalez C, Rajpathak S, Davies G. Cost-effectiveness of intensification with sodium-glucose co-transporter-2 inhibitors in patients with type 2 diabetes on metformin and sitagliptin vs direct intensification with insulin in the United Kingdom. Diabetes Obes Metab. 2019;21(4):1010–1017. doi: 10.1111/dom.13618. [DOI] [PubMed] [Google Scholar]
- 36.McEwan P, Bennett H, Khunti K, Wilding J, Edmonds C, Thuresson M, et al. Assessing the cost-effectiveness of sodium-glucose cotransporter-2 inhibitors in type 2 diabetes mellitus: a comprehensive economic evaluation using clinical trial and real-world evidence. Diabetes Obes Metab. 2020;22(12):2364–2374. doi: 10.1111/dom.14162. [DOI] [PubMed] [Google Scholar]
- 37.Kansal A, Reifsnider OS, Proskorovsky I, Zheng Y, Pfarr E, George JT, et al. Cost-effectiveness analysis of empagliflozin treatment in people with type 2 diabetes and established cardiovascular disease in the EMPA-REG OUTCOME trial. Diabet Med. 2019;36(11):1494–1502. doi: 10.1111/dme.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshida Y, Cheng X, Shao H, Fonseca VA, Shi L. A systematic review of cost-effectiveness of sodium-glucose cotransporter inhibitors for type 2 diabetes. Curr Diab Rep. 2020;20(4):12–15. doi: 10.1007/s11892-020-1292-5. [DOI] [PubMed] [Google Scholar]
- 39.Johnston R, Uthman O, Cummins E, Clar C, Royle P, Colquitt J, et al. Canagliflozin, dapagliflozin and empagliflozin monotherapy for treating type 2 diabetes: systematic review and economic evaluation. Health Technol Assess. 2017;21(2):1–218. doi: 10.3310/hta21020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reifsnider OS, Kansal AR, Franke J, Lee J, George JT, Brueckmann M, et al. Cost-effectiveness of empagliflozin in the UK in an EMPA-REG OUTCOME subgroup with type 2 diabetes and heart failure. ESC Heart Fail. 2020;7(6):3910–3918. doi: 10.1002/ehf2.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giugliano D, Maiorino MI, Longo M, Bellastella G, Chiodini P, Esposito K. Type 2 diabetes and risk of heart failure: a systematic review and meta-analysis from cardiovascular outcome trials. Endocrine. 2019;65(1):15–24. doi: 10.1007/s12020-019-01931-y. [DOI] [PubMed] [Google Scholar]
- 42.Lo KB, Gul F, Ram P, Kluger AY, Tecson KM, McCullough PA, et al. The effects of SGLT2 inhibitors on cardiovascular and renal outcomes in diabetic patients: a systematic review and meta-analysis. Cardiorenal Med. 2020;10(1):1–10. doi: 10.1159/000503919. [DOI] [PubMed] [Google Scholar]
- 43.Kluger A, Tecson K, Lee A, Verma E, Rangaswami J, Lepor N, et al. Class effects of SGLT2 inhibitors on cardiorenal outcomes. Cardiovasc Diabetol. 2019;18(1):99. doi: 10.1186/s12933-019-0903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palmer SC, Tendal B, Mustafa RA, Vandvik PO, Li S, Hao Q, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021;372:m4573. doi: 10.1136/bmj.m4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patorno E, Goldfine AB, Schneeweiss S, Everett BM, Glynn RJ, Liu J, et al. Cardiovascular outcomes associated with canagliflozin versus other non-gliflozin antidiabetic drugs: population based cohort study. BMJ. 2018;360:k119. doi: 10.1136/bmj.k119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 48.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 49.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 50.Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hernandez AF, Green JB, Janmohamed S, D'Agostino RBS, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 52.Kristensen SL, Rorth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–785. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
- 53.Mann JFE, Orsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(9):839–848. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 54.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394(10193):131–138. doi: 10.1016/S0140-6736(19)31150-X. [DOI] [PubMed] [Google Scholar]
- 55.Gorriz JL, Soler MJ, Navarro-Gonzalez JF, Garcia-Carro C, Puchades MJ, D'Marco L, et al. GLP-1 receptor agonists and diabetic kidney disease: a call of attention to nephrologists. J Clin Med. 2020 doi: 10.3390/jcm9040947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139(17):2022–2031. doi: 10.1161/CIRCULATIONAHA.118.038868. [DOI] [PubMed] [Google Scholar]
- 57.Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391(10120):541–551. doi: 10.1016/S0140-6736(17)33102-1. [DOI] [PubMed] [Google Scholar]
- 58.Ludwig L, Darmon P, Guerci B. Computing and interpreting the number needed to treat for cardiovascular outcomes trials: perspective on GLP-1 RA and SGLT-2i therapies. Cardiovasc Diabetol. 2020;19(1):65. doi: 10.1186/s12933-020-01034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garg V, Shen X, Cheng Y, Nawarskas JJ, Raisch DW. Use of number needed to treat in cost-effectiveness analyses. Ann Pharmacother. 2013;47(3):380–387. doi: 10.1345/aph.1R417. [DOI] [PubMed] [Google Scholar]
- 60.Kristiansen IS, Gyrd-Hansen D. Cost-effectiveness analysis based on the number-needed-to-treat: common sense or non-sense? Health Econ. 2004;13(1):9–19. doi: 10.1002/hec.797. [DOI] [PubMed] [Google Scholar]
- 61.Kloecker DE, Davies MJ, Khunti K, Zaccardi F. Cardiovascular effects of sodium-glucose co-transporter-2 inhibitors and glucagon-like peptide-1 receptor agonists: the P value and beyond. Diabetes Obes Metab. 2021;23(7):1685–1691. doi: 10.1111/dom.14384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.