Immune checkpoint inhibitors (ICPis) have revolutionized the treatment of cancers by engaging the patient’s own immune system against the tumor (1–3). Checkpoint pathways are innate mechanisms to put the brakes on immune activation. Approved agents target checkpoint pathways mediated by cytotoxic T lymphocyte–associated antigen 4 (CTLA4), programmed cell death protein 1 (PD-1), and programmed death ligand 1 (PDL-1) (1,4–7). Releasing the breaks on the immune system enhances destruction of tumor cells, but can lead to immune-related adverse effects (8,9), including renal toxicity (1,2,10–13).
The incidence of renal toxicity with ICPis ranges from 1% to 5% (12). This risk is higher in patients that are on combination therapy with both anti-CTLA4 and anti–PD-1/PD-L1 with an odds ratio of 3.88 (95% CI, 2.21 to 6.81) (14). Further, a lower baseline GFR before therapy increases risk of kidney injury with an odds ratio of 1.99 (95% CI, 1.43 to 2.76) for every 30 ml/min per 1.73 m2 decline (14). The time to prevent kidney injury starts at the time of ICPi initiation. Based on a recent multicenter review of 138 patients, risk of ICPi-associated AKI (ICPi-AKI) was higher with concomitant use of proton pump inhibitors (PPIs) with an odds ratio of 2.85 (95% CI, 1.81 to 4.48) (14). Cortazar et al. describe that 70% of patients with ICPi-AKI were on potential acute interstitial nephritis (AIN)–causing medications including PPIs (15,16), nonsteroidal anti-inflammatory drugs (NSAIDs) (17), and antibiotics (18) where PPI was present on >50% of patients’ medication lists. Baseline use of PPIs was also associated with ICPi-AKI in a large study by Seethapathy et al. (1), particularly 2.5 months after being on ICPi. Appropriate counseling to stop or substitute these medications should be given before ICPi initiation and these medications should certainly be discontinued in patients with ICPi-AKI.
In patients that develop ICPi-AKI, the most common pathologic finding identified to date is AIN (10–12,19). In 2016, Shirali et al. (11) reviewed six patients on PD-L1 inhibitor therapy and all six patients had AIN on kidney biopsy. Notably, all patients were also receiving either PPIs or NSAIDs. Similarly, Cortazar et al. (12) describe 13 patients on ICPis where AIN was present in 12 of the 13 (92%) patients, and Mamlouk et al. (10) describe 16 patients on ICPis with AIN identified in 14 of the 16 (86%) patients. These case series emphasize that it is reasonable to assume that AIN is the cause for ICPi-AKI in the majority of cases. Finally, in a recent multicenter study of AKI in this setting, 60 patients underwent a kidney biopsy and 93% of samples were consistent with AIN (14).
Glomerular disease is less common in ICPi-AKI and, in the majority of glomerular cases reported, patients had nephrotic range proteinuria of >3 g/g (10,13). Izzedine et al. (13) reported two cases with minimal change disease: one with 3.5 g/g and the other with 6 g/g. Mamlouk et al. (10) identified two cases of IgA nephropathy: one with 7.7 g/g and the other with no protein and IgA as part of the pathology interpretation with a comment that no pathologic indication of active disease present. Of note, the latter patient also had interstitial inflammation on the biopsy report (10). The same case series also identified one case of membranous nephropathy with 9.7 g/g (10).
Acute tubular necrosis (ATN) is another finding reported after biopsy in patients with ICPi-AKI (13,19). Izzedine et al. (13) report ATN in five out of 12 cases and highlight that without kidney biopsy patients would have been inappropriately treated with steroids. However, investigation into these cases suggests other reasons for ATN on kidney biopsy. As an example, two of five patients underwent prior platinum therapy which is nephrotoxic and known to cause ATN (20,21). Further, the majority of these patients had more cardiovascular risk factors and marked histologic vascular lesions on kidney biopsy (13). It would have been interesting to decipher ATN and AIN cases based on proteinuria or oliguria, but these clinical features were not included in the paper. In the case series by Cassol et al. (13), six of 15 patients had ATN on kidney biopsy. One of these patients had nephrotic range proteinuria of 13.1 g/g which would have prompted a glomerular indication for kidney biopsy. More patients in the ATN group were exposed to antibiotics, raising the possibly of infection-associated hypotension leading to the development of ATN. There was also an increased exposure to iodinated contrast in the ATN group. Additionally, authors do not specify if other causes of AKI were ruled out before the kidney biopsy to characterize kidney dysfunction related to immunotherapy. In the more recent multicenter study by Cortazar et al. (14), only one case of ATN was reported in 60 biopsied cases. ATN was also not the sole diagnosis, but in the setting of minimal change disease. Given the low incidence of ATN in this larger study, it is unlikely to be a major cause of ICPi-AKI.
Presence of new hematuria should also flag concern for the treating provider. To date, three cases of pauci-immune GN have been identified during workup for ICPi-AIN (10). All three cases had presence of red blood cells on urinalysis ranging from 7 to 320 cells/high power field. We therefore recommend new hematuria to be part of the consideration for kidney biopsy. Oliguria in the setting of more severe kidney injury is also concerning and was a clinical finding in a patient later found to have thrombotic microangiopathy (12).
Along with careful review of the clinical data, consideration for kidney biopsy should always include whether management would be affected. Typically, during cases of suspected ICPi-AKI, treatment includes steroids along with consideration for stopping ICPis. The majority of case series show that patients with ICPi-AKI have complete/partial recovery with steroids (10–13). Izzedine et al. (13) showed renal recovery of 50% renal function in six of seven patients that received steroids, where one patient died. Shirali et al. (11) showed return of creatinine to baseline in four of five patients treated with steroids. Mamlouk et al. (10) showed renal recovery in 12 of 15 patients treated with steroids. In the largest multicenter study to date, Cortazar et al. (14) described complete or partial recovery in 85% of patients. The same study also showed that the rates of complete and partial kidney recovery after a course of steroids in the biopsy group and no-biopsy group were remarkably similar as well. These findings highlight the benefit of steroids in most cases of ICPi-AKI, even if tissue diagnosis is lacking.
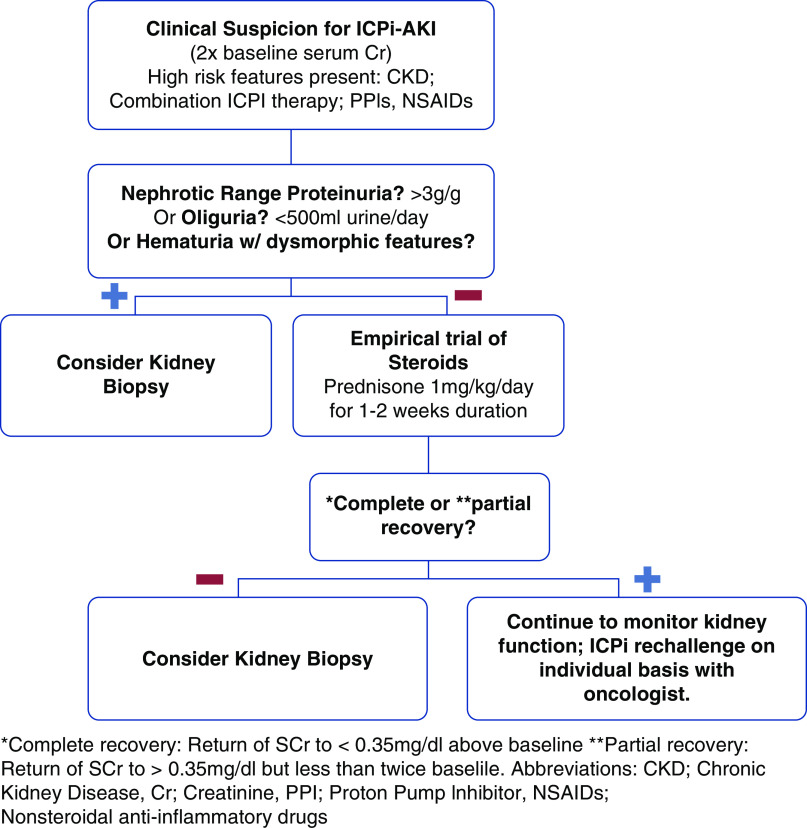
With the expanding use of ICPis (22), it is essential that we establish guidelines to approach ICPi-AKI. Given the increased incidence of ICPi-AKI in patients on agents that cause AIN (14), initial steps must include appropriate counseling to avoid NSAIDs and PPIs while on ICPi therapy. If ICPi-AKI occurs, we suggest an algorithmic approach (Figure 1) to minimize use of kidney biopsy and help risk stratify patients that are more likely to have a non-AIN–mediated ICPi-AKI. The suggested algorithm incorporates the case reports above and is in accordance with the 2018 American Society of Clinical Oncology Clinical Practice Guideline for management of immune-related adverse events in patients on ICPi therapy (23).
Figure 1.
Algorithm for management of patients with clinical suspicion for immune check point inhibitor–associated AKI (ICPi-AKI). *Complete recovery: return of serum creatinine (SCr) to <0.35 mg/dl above baseline. **Partial recovery: return of SCr to >0.35 mg/dl but less than twice baseline. Cr, creatinine; NSAIDs, nonsteroidal anti-inflammatory drugs; PPI, proton pump inhibitor.
Based on our review of the literature, more unusual causes of ICPi-AKI such as glomerular or vascular etiologies would be recognized with either nephrotic-range proteinuria (>3 g/g), presence of new hematuria (especially with dysmorphic features), or oliguria (<500 ml urine per day) during evaluation of AKI. The absence of these features makes AIN most likely, as described in the majority of case series.
Laboratory features, however, should not be used independently given that proteinuria, eosinophilia, and pyuria were not different in patients with and without biopsy-proven AIN in a multicenter study (14). We therefore emphasize the use of clinical features in conjunction with consideration of the underlying risk factors described above.
Patients that are selected to undergo an empirical trial of steroids should have complete or partial recovery assessed based on Cortazar et al.’s (14) definition of return of serum creatinine to <0.35 mg/dl above baseline or return of serum creatinine to >0.35 mg/dl but less than twice baseline, respectively. If no recovery occurs, kidney biopsy should be pursued at this point. Partial recovery may also prompt consideration for kidney biopsy given the possibility of an underlying lesion other than AIN. However, decision to proceed to the biopsy in this setting should be made after careful assessment of risk and benefits in this patient population with advanced malignancies and possibly limited life expectancy.
Our goal with the suggested algorithm is to minimize reflexive ordering of kidney biopsies in patients with ICPi-AKI. Kidney biopsies carry risk of bleeding complications (24), apprehension for patients, and increased risk in patients with solitary kidneys. This algorithm should not replace the individual patients shared decision with their treatment team. Rather, we hope this algorithm serves as an accompanying guide to help clinicians risk stratify patients that are more likely to have a rare cause of ICPi-AKI and to determine whether a kidney biopsy would truly change management.
Disclosures
I. Glezerman reports consultant fees from CytoMx Inc. and Pfizer Inc. stock ownership, outside the submitted work. V. Gutgarts has nothing to disclose.
Funding
I. Glezerman and V. Gutgarts were supported by National Institutes of Health/National Cancer Institute Cancer Center Support grant P30 CA008748 during the conduct of the study.
Author Contributions
I. Glezerman conceptualized the study; I. Glezerman and V. Gutgarts wrote the original draft, and reviewed and edited the manuscript.
Footnotes
See related commentary, “Kidney Biopsy Should Be Performed to Document the Cause of Immune Checkpoint Inhibitor–Associated Acute Kidney Injury: Commentary” and debate, “Kidney Biopsy Should Be Performed to Document the Cause of Immune Checkpoint Inhibitor–Associated Acute Kidney Injury: PRO,” on pages 166-168 and 158-161, respectively.
References
- 1.Seethapathy H, Zhao S, Chute DF, Zubiri L, Oppong Y, Strohbehn I, Cortazar FB, Leaf DE, Mooradian MJ, Villani AC, Sullivan RJ, Reynolds K, Sise ME: The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors. Clin J Am Soc Nephrol 14: 1692–1700, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shingarev R, Glezerman IG: Kidney complications of immune checkpoint inhibitors: A review. Am J Kidney Dis 74: 529–537, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wanchoo R, Karam S, Uppal NN, Barta VS, Deray G, Devoe C, Launay-Vacher V, Jhaveri KD; Cancer and Kidney International Network Workgroup on Immune Checkpoint Inhibitors: Adverse renal effects of immune checkpoint inhibitors: A narrative review. Am J Nephrol 45: 160–169, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ: Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363: 711–723, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, Chung CH, Hernandez-Aya L, Lim AM, Chang ALS, Rabinowits G, Thai AA, Dunn LA, Hughes BGM, Khushalani NI, Modi B, Schadendorf D, Gao B, Seebach F, Li S, Li J, Mathias M, Booth J, Mohan K, Stankevich E, Babiker HM, Brana I, Gil-Martin M, Homsi J, Johnson ML, Moreno V, Niu J, Owonikoko TK, Papadopoulos KP, Yancopoulos GD, Lowy I, Fury MG: PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med 379: 341–351, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR; KEYNOTE-024 Investigators: Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375: 1823–1833, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD: Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373: 23–34, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Postow MA, Sidlow R, Hellmann MD: Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378: 158–168, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K; ESMO Guidelines Committee: Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up [published correction appears in Ann Oncol 29[Suppl 4]: iv264–iv266, 2018]. Ann Oncol 28[Suppl 4]: iv119–iv142, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Mamlouk O, Selamet U, Machado S, Abdelrahim M, Glass WF, Tchakarov A, Gaber L, Lahoti A, Workeneh B, Chen S, Lin J, Abdel-Wahab N, Tayar J, Lu H, Suarez-Almazor M, Tannir N, Yee C, Diab A, Abudayyeh A: Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: Single-center experience. J Immunother Cancer 7: 2, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shirali AC, Perazella MA, Gettinger S: Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis 68: 287–291, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, Le DT, Lipson EJ, Glezerman IG, Wolchok J, Cornell LD, Feldman P, Stokes MB, Zapata SA, Hodi FS, Ott PA, Yamashita M, Leaf DE: Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 90: 638–647, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izzedine H, Mathian A, Champiat S, Picard C, Mateus C, Routier E, Varga A, Malka D, Leary A, Michels J, Michot JM, Marabelle A, Lambotte O, Amoura Z, Soria JC, Kaaki S, Quellard N, Goujon JM, Brocheriou I: Renal toxicities associated with pembrolizumab. Clin Kidney J 12: 81–88, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortazar FB, Kibbelaar ZA, Glezerman IG, Abudayyeh A, Mamlouk O, Motwani SS, Murakami N, Hermann S, Monahar S, Shirali AC, Kitchlu A, Shirazian S, Assal A, Vijayan A, Renaghan AD, Ortiz-Melo DI, Rangarajan S, Malik AB, Hogan JJ, Dinh AR, Shin DS, Marrone KA, Mithani Z, Johnson DB, Hosseini A, Uprety D, Sharma S, Gupta S, Reynolds KL, Sise ME, Leaf DE: Clinical features and outcomes of immune checkpoint inhibitor–associated AKI: A multicenter study. J Am Soc Nephrol 31: 435–446, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brewster UC, Perazella MA: Proton pump inhibitors and the kidney: critical review. Clin Nephrol 68: 65–72, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Blank M-L, Parkin L, Paul C, Herbison P: A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int 86: 837–844, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixit M, Doan T, Kirschner R, Dixit N: Significant acute kidney injury due to non-steroidal anti-inflammatory drugs: Inpatient setting. Pharmaceuticals (Basel) 3: 1279–1285, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalili H, Bairami S, Kargar M: Antibiotics induced acute kidney injury: Incidence, risk factors, onset time and outcome. Acta Med Iran 51: 871–878, 2013 [PubMed] [Google Scholar]
- 19.Cassol C, Satoskar A, Lozanski G, Rovin B, Hebert L, Nadasdy T, Brodsky SV: Anti-PD-1 immunotherapy may induce interstitial nephritis with increased tubular epithelial expression of PD-L1. Kidney Int Rep 4: 1152–1160, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J-Q, Cai GY, Wang SY, Song YH, Xia YY, Liang S, Wang WL, Nie SS, Feng Z, Chen XM: The characteristics and risk factors for cisplatin-induced acute kidney injury in the elderly. Ther Clin Risk Manag 14: 1279–1285, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latcha S, Jaimes EA, Patil S, Glezerman IG, Mehta S, Flombaum CD: Long-term renal outcomes after cisplatin treatment. Clin J Am Soc Nephrol 11: 1173–1179, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haslam A, Prasad V: Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open 2: e192535, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA; National Comprehensive Cancer Network: Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 36: 1714–1768, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brachemi S, Bollée G: Renal biopsy practice: What is the gold standard? World J Nephrol 3: 287–294, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]